Introduction
Sevoflurane (Sev) is widely used in pediatric
anesthesia. Unfortunately, it has been demonstrated that Sev has
potential neurotoxic effects on the developing brain and cognition,
even in adolescence (1,2). Sev treatment can harm neural stem
cells, neuronal, lung and cancer cells, Substantial evidence has
accumulated to support the conclusion that Sev leads to neuronal
apoptosis (3,4). The present study sought to elucidate
this important mechanism of neuronal apoptosis induced by Sev.
AMP-activated protein kinase (AMPK) is a key
regulator to maintain the stability of energy metabolism by
modulating various regulatory signaling pathways at the cellular
level. It consists of a catalytic subunit (α) and two regulatory
subunits (β and γ), which are activated by the phosphorylation of
threonine 172 (Thr172) (5,6). AMPK downregulation occurs in animal
models with metabolic syndromes (7). Sirtuin 1 (SIRT1), a NAD+
dependent protein deacetylase, has an important role in the
regulation of physiological and pathological processes such as
apoptosis, metabolism and differentiation, through the
deacetylation of intracellular signaling factors (8,9). AMPK
and SIRT1 have similar roles in cellular metabolism and survival
(10). Past work has revealed that
phosphorylated-(p-)AMPK can activate SIRT1, and the activated SIRT1
regulates the formation of the autophagic vacuole (11–13).
There is a positive feedback loop interaction between AMPK and
SIRT1 (14). It has also been shown
that AMPK and SIRT1 are associated with cell aging and neurological
disorders (15,16). Shah et al (17) revealed that the AMPK/SIRT1 pathway
is involved in the modulation of Aβ deposition and cognitive
functions in Alzheimer's disease rats. Yet, the contribution of
AMPK/SIRT1 in neuronal apoptosis and cognitive impairment induced
by Sev exposure is still not clear.
In the present study, the role of the AMPK/SIRT1
pathway in Sev-induced neuronal apoptosis and cognition impairment
was examined in rats. The study may offer the possibility to
address the question of whether AMPK could be a new drug target for
the prevention of cognitive impairment induced by Sev.
Materials and methods
Materials
Sev, AICAR, Annexin V-FITC Apoptosis Detection Kit
and Hoechst 33342 were purchased from Sigma-Aldrich (Merck KGaA).
Fetal bovine serum (FBS) was purchased from Gibco (Thermo Fisher
Scientific, Inc.). The following primary antibodies were purchased
from Abcam: Anti-actin (cat. no. ab179467), anti-total-(t-) AMPK
(cat. no. ab80039), anti-p-AMPK (cat. no. ab133448), anti-SIRT1
(cat. no. ab189494). AMPK (cat. no. bf30053) and SIRT1 ELISA kits
(cat. no. bf90658) were purchased from Northern Institute of
Biotechnology of China.
Cell culture and Sev exposure
PC12 cells, originally derived from a
pheochromocytoma of rat adrenal medulla, were used to evaluate the
involved mechanism of Sev-induced cell apoptosis. PC12 cells were
obtained from Shanghai Institute for Biological Science Cell Bank,
CAS. The cells were cultured in DMEM with 5% FBS in an incubator at
37°C with 5% CO2. For Sev exposure, the cell culture
plates were placed in the airtight incubator connected to an
anesthesia machine that was used to supply Sev into the incubator.
PC12 cells were exposed to 3% Sev for 24 h (18). For the intervention studies, AICAR,
an AMPK activator, was used to treat PC12 cells at a concentration
of 0.5 mM for 1 h at 37°C prior to Sev treatment.
Annexin V-FITC/PI staining
The cell apoptosis was evaluated by flow cytometry
analysis using Annexin V-FITC/PI apoptosis analysis, according to
the manufacturer's instructions (Shanghai Yeasen Biotechnology Co.,
Ltd.). Briefly, PC12 cells were exposed to Sev for 24 h, and
harvested using trypsin. The harvested cells were resuspended in 1X
binding buffer (Shanghai Yeasen Biotechnology Co., Ltd.) at a
concentration of 1×106 cells/ml. A total of 5 µl of
Annexin V-FITC and 10 µl PI staining solution was subsequently
added to the suspensions. The suspensions were vortexed gently, and
400 µl of 1X binding buffer was added and incubated for 5
min at room temperature in the dark. The fluorescence of FITC and
PI was analyzed by flow cytometry. Both early apoptotic (Annexin
V-FITC+/PI−) and late apoptotic (Annexin
V-FITC+/PI+) cells were included in cell
apoptosis determinations.
Hoechst 33342 staining
A total of 6×105 PC12 cells were seeded
into 6-well plates and exposed to Sev at 37°C for 24 h. A total of
1 ml of 4% paraformaldehyde was added to each well for 10 min for
fixation and washed twice with PBS. A total of 10 mg/l Hoechst
33342 was added and stained for 10 min at room temperature. Cell
apoptosis was observed under a fluorescence microscope. Apoptotic
cells were characterized by rounded cell morphology, half-moon
nuclei with condensed chromatin. The apoptotic cells were counted
the average no. of cells/mm2 in 10 fields.
Western blotting
After incubation with Sev for 24 h, PC12 cells were
collected, rinsed twice with cold PBS and lysed by RIPA buffer
(Thermo Fisher Scientific, Inc.) containing 1 mM PMSF on ice for 30
min, followed by centrifugation at 12,000 × g for 5 min at 4°C. A
total of 40 µg of total protein was loaded in each well and
resolved by 10% SDS-PAGE. After electrophoresis, samples were
transferred onto polyvinylidene fluoride (PVDF) membranes, which
were subsequently blocked with 5% skimmed milk for 1 h and
incubated with anti-t-AMPK (1:800), anti-p-AMPK (1:500), anti-SIRT1
(1:1,000) and anti-actin (1:2,000) primary antibodies at 4°C for 12
h. The membrane was washed three times with PBST (0.1% Tween-20),
and then incubated with the second antibody (goat anti-rabbit
1:2,000 in blocking solution) at 37°C for 2 h. The membrane was
then washed three times with PBST. Then, the signals were
visualized with chemiluminescence reagents (Thermo Fisher
Scientific, Inc.). Results were semi-quantified via Quantity One
software (version 4.4; Bio-Rad Laboratories, Inc.).
Measurement of AMPK and SIRT1
levels
AMPK and SIRT1 levels in cell culture medium and
hippocampus homogenate were measured by commercially available
ELISA kits.
Animals and Sev exposure
A total of 20 male Sprague-Dawley (SD) rats (28±2
days old; weight 100±10 g) were used. Animals were housed with free
access to food and water at the temperature of 22±2°C, humidity of
55±5% and a 12-h light/dark cycle. The rats were randomly assigned
to the control (Con) and Sev groups. The Con group was not exposed
to Sev. Sev group received inhalation of 3% Sevin
O2/N2 (fraction of inspired oxygen 50%) for 2
h daily for 5 days consecutively. The dose of Sev was determined as
previously described (19). For the
intervention studies, AICAR, an AMPK activator, was administered to
the rats via an intraperitoneal injection for 2 h at a dose of 500
mg/kg prior to Sev treatment. The health and behavior of animals
were monitored every day. The present study was performed in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health. All
efforts to improve animal welfare were taken, including to minimize
suffering and distress, use of an aesthetics or special housing
conditions.
Water maze
Rats were trained in a 1.5 m diameter open-field
water maze filled with water (26°C) and made opaque with latex
liquid. Prominent extra-maze visual cues around the room remained
in fixed positions throughout the experiment. During behavioral
testing, animals were required to locate a hidden submerged
platform 10 cm in diameter (1.5 cm below the surface), which
remained in the same position across trials for individual animals
but was counterbalanced across animals. The animals were given four
trials per day for 4 days. Trials began as the rat was placed in
the pool facing the side wall at a start position and ended once
the animal had found the platform; if the rat had not found the
platform within 120 sec, it was guided there by hand. A video
camera mounted to the ceiling directly above the center of the maze
was used in conjunction with the animal tracking system. Test
animals underwent Sev exposure for 5 days followed by the water
maze experiment for 5 days. On day 11, the animals were sacrificed
to explore further mechanisms under anesthesia with mebumal sodium
(50 mg/kg) by intraperitoneal injection.
Hippocampus homogenate
After the last water maze was completed, the rats
were anesthetized and euthanized by mebumal sodium. The hippocampus
was removed and the rats were verified dead. Then, the hippocampus
was weighed, transferred into a glass homogenizer and five volumes
of precooled PBS (0.02 mol/l, pH 7.0–7.2) was added. The tissue was
fully ground and then treated with ultrasonic crushing. After
centrifugation of the prepared homogenate at 10,000 × g for 5 min
at 4°C, the hippocampal homogenate was found in the
supernatant.
Statistical analyses
t-tests and two-way ANOVA, followed by a Bonferroni
post hoc analysis, were performed for comparisons between groups.
Statistical analysis was conducted on a standard software package
(SAS® version 10.0; SAS Institute, Inc.) was used. All
data are presented as the mean ± SEM. Each experiment was repeated
>4 times. P<0.05 was considered to indicate a statistically
significant difference.
Results
Sev induces neuronal apoptosis
Cell apoptosis was assessed by Hoechst 33342
staining and Annexin V-FITC/PI staining. To investigate the effects
of Sev on neuronal apoptosis, the percentage of apoptotic cells was
measured using Annexin V/PI staining. The results showed that the
percentage of apoptotic cells was significantly increased after
treatment with Sev (Fig. 1A and B).
Furthermore, Hoechst 33342 staining also showed that Sev caused
neuronal apoptosis (Fig. 1C and
D).
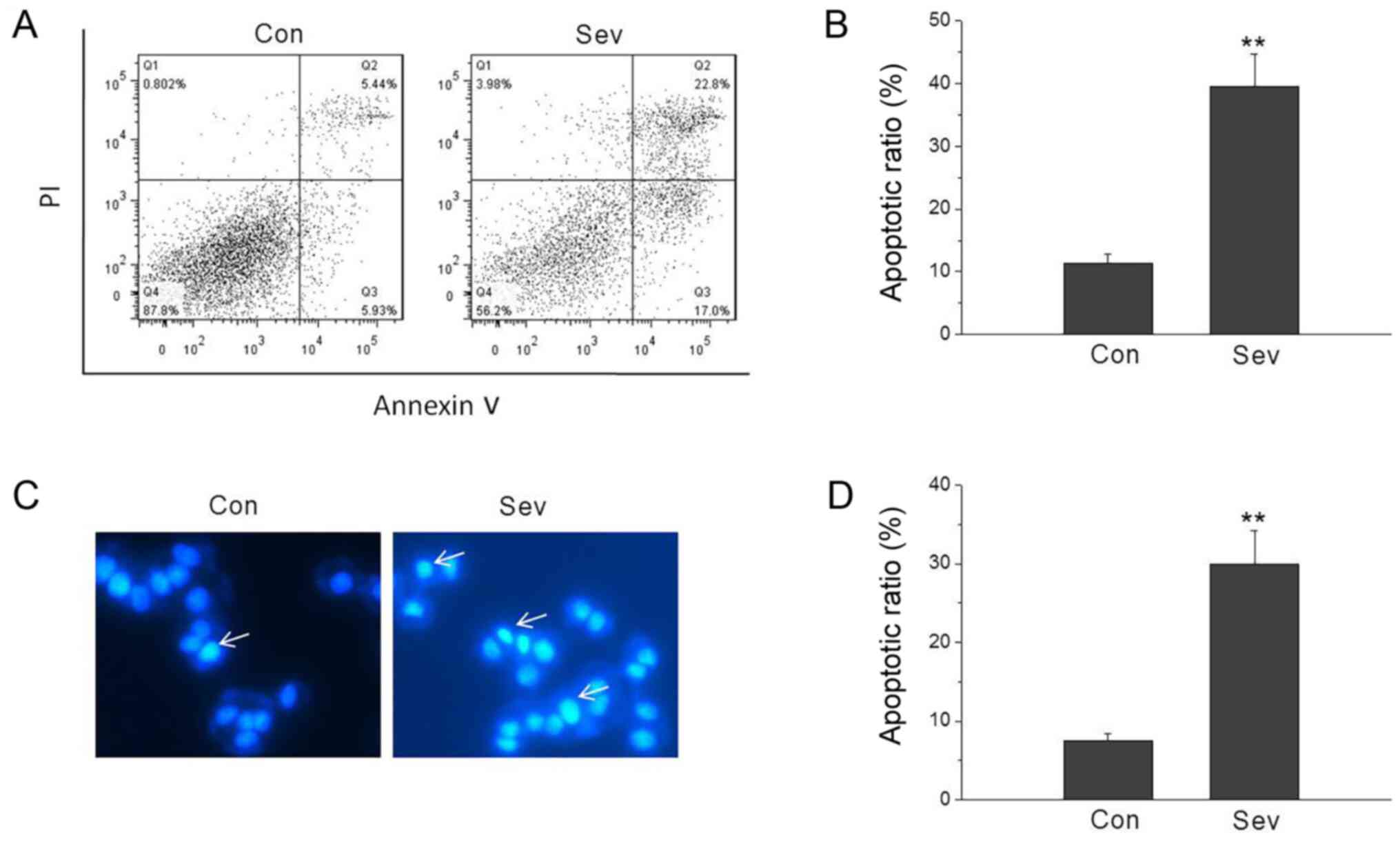 | Figure 1.Sev induces neuronal apoptosis. (A and
B) Sev induced cell apoptosis by Annexin V-FITC assay. The lower
left population of cells in each plot represents viable cells which
excluded PI and did notbind Annexin V, while the upper left
population cells comprise necrotic cells, which did not exclude PI
and were not stained with FITC-labeled Annexin V. The lower and
upper right populations correspond to early apoptotic and late
apoptotic cells, respectively. The percentages of early and late
apoptotic cells, non-apoptotic and necrotic cells are shown in the
right lower quadrant, right upper quadrant, left lower quadrant and
left upper quadrant, respectively. (C and D) Sev induced cell
apoptosis by Hoechst 33342 staining. Apoptotic cells were
characterized by rounded cell morphology, half-moon nuclei with
condensed chromatin. Arrowsindicate apoptotic cells. **P<0.01
vs. Con. n=5. Sev, sevoflurane; Con, control. |
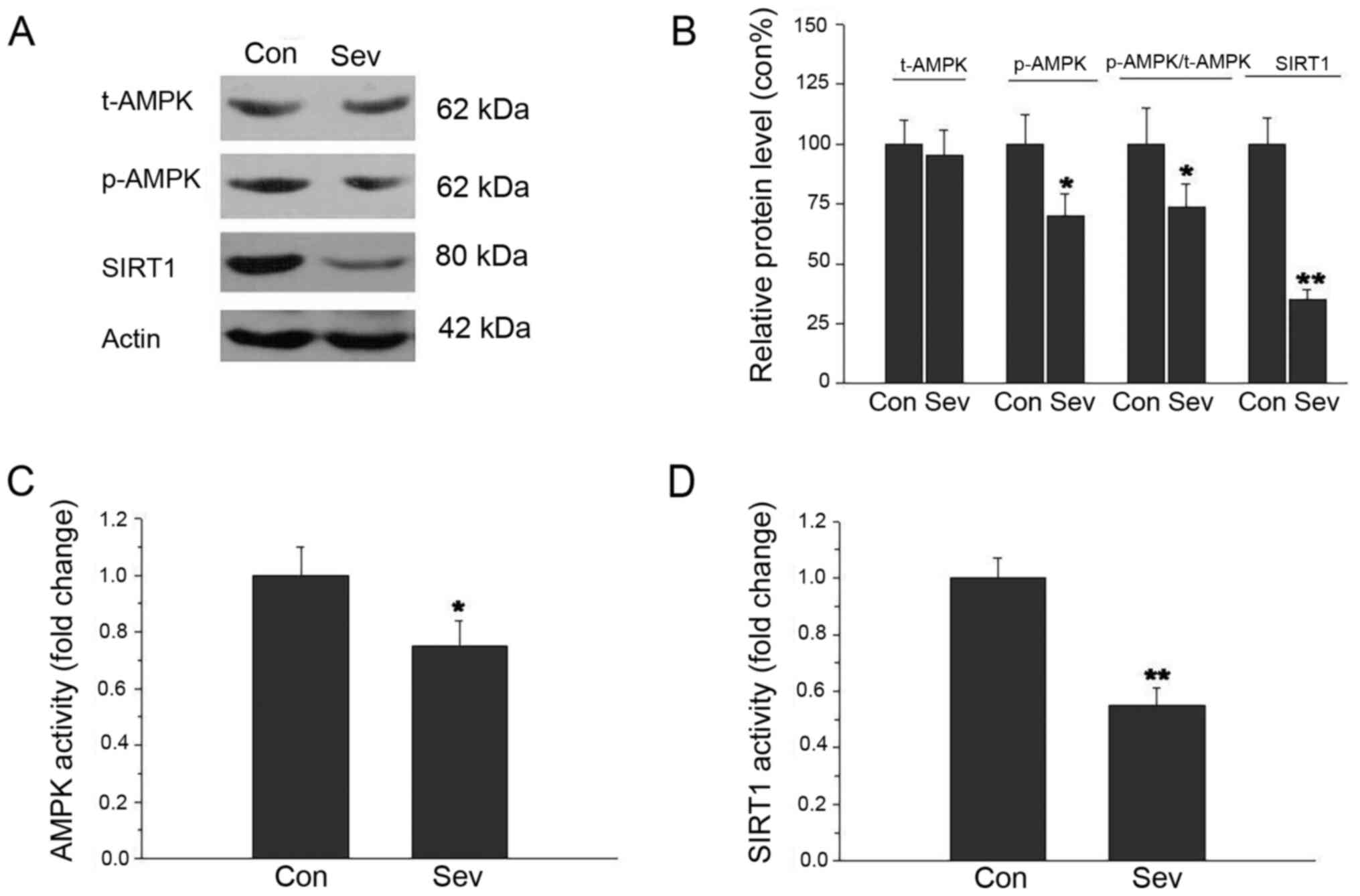
Inhibition of AMPK/SIRT1 signaling is
associated with Sev-mediated neuronal apoptosis
The AMPK and SIRT1 protein expression levels were
assessed by western blot analysis. As shown in Fig. 2A and B, p-AMPK and SIRT1 protein
expression levels were significantly decreased after Sev treatment.
The t-AMPK protein expression had no significant difference between
the two treatment groups. The ratio of p-AMPK/t-AMPK decreased in
the group treated with Sev. Next, AMPK and SIRT1 activity was
examined in cell culture medium by ELISA. There was a significant
inhibitory effect of Sev on AMPK and SIRT1 activity (Fig. 2C and D). These results indicated
that inhibition of AMPK/SIRT1 signaling was related to neuronal
apoptosis in response to Sev.
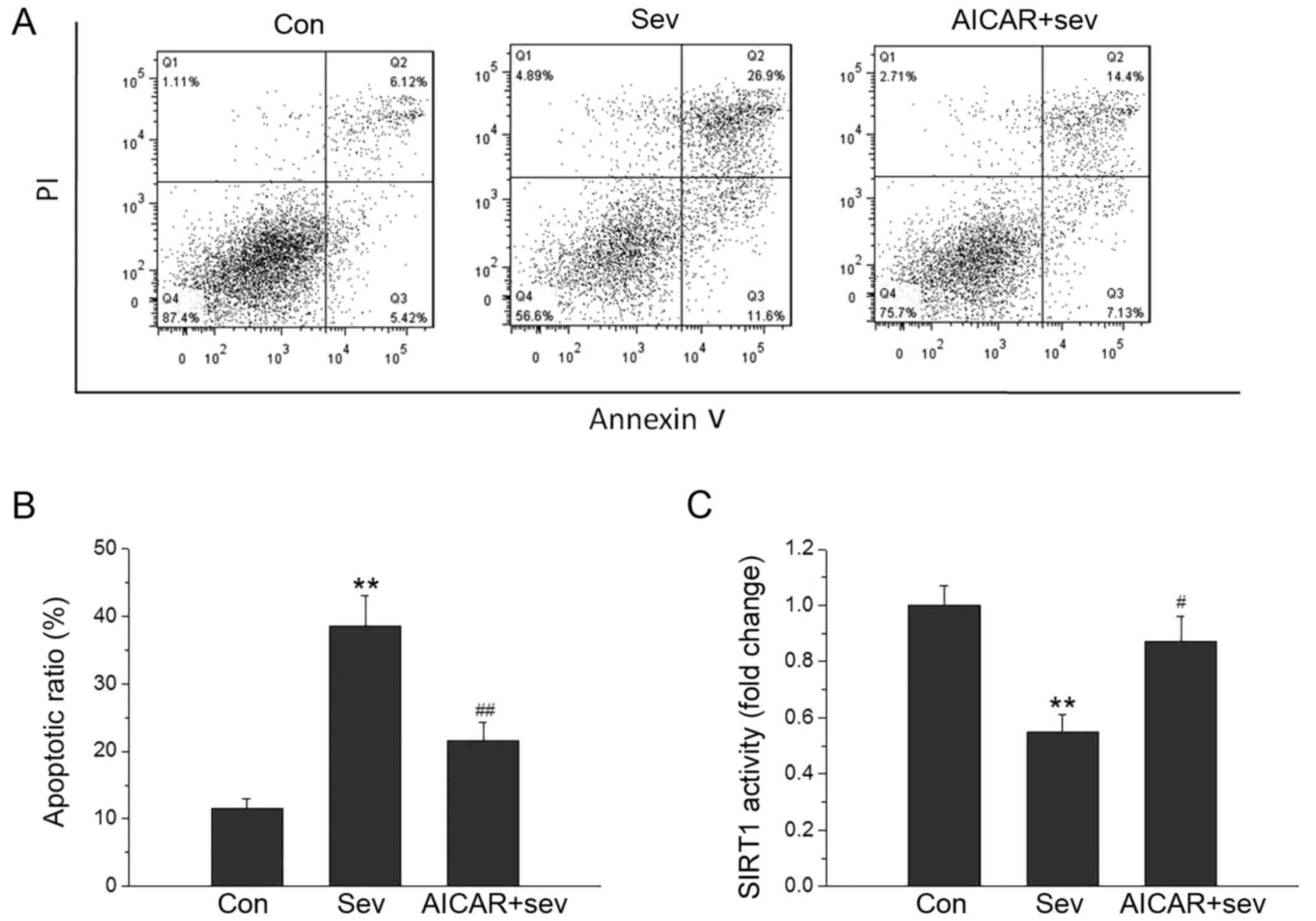
AMPK activation blocks neuronal
apoptosis and the decline in SIRT1 activity induced by Sev
To examine the role of AMPK/SIRT1 signaling in
Sev-induced neuronal apoptosis, the neurons were treated with the
AMPK activator-AICAR at a concentration of 0.5 mM, and the
percentage of apoptotic cells was evaluated using Annexin V/PI
staining. As shown in Fig. 3A and
B, AICAR was able to attenuate the neuronal apoptosis induced
by Sev. AICAR also blocked the decline inSIRT1 activity induced by
Sev (Fig. 3C). These results
suggested that neuronal apoptosis caused by Sev was dependent on
the AMPK/SIRT1 signaling pathway.
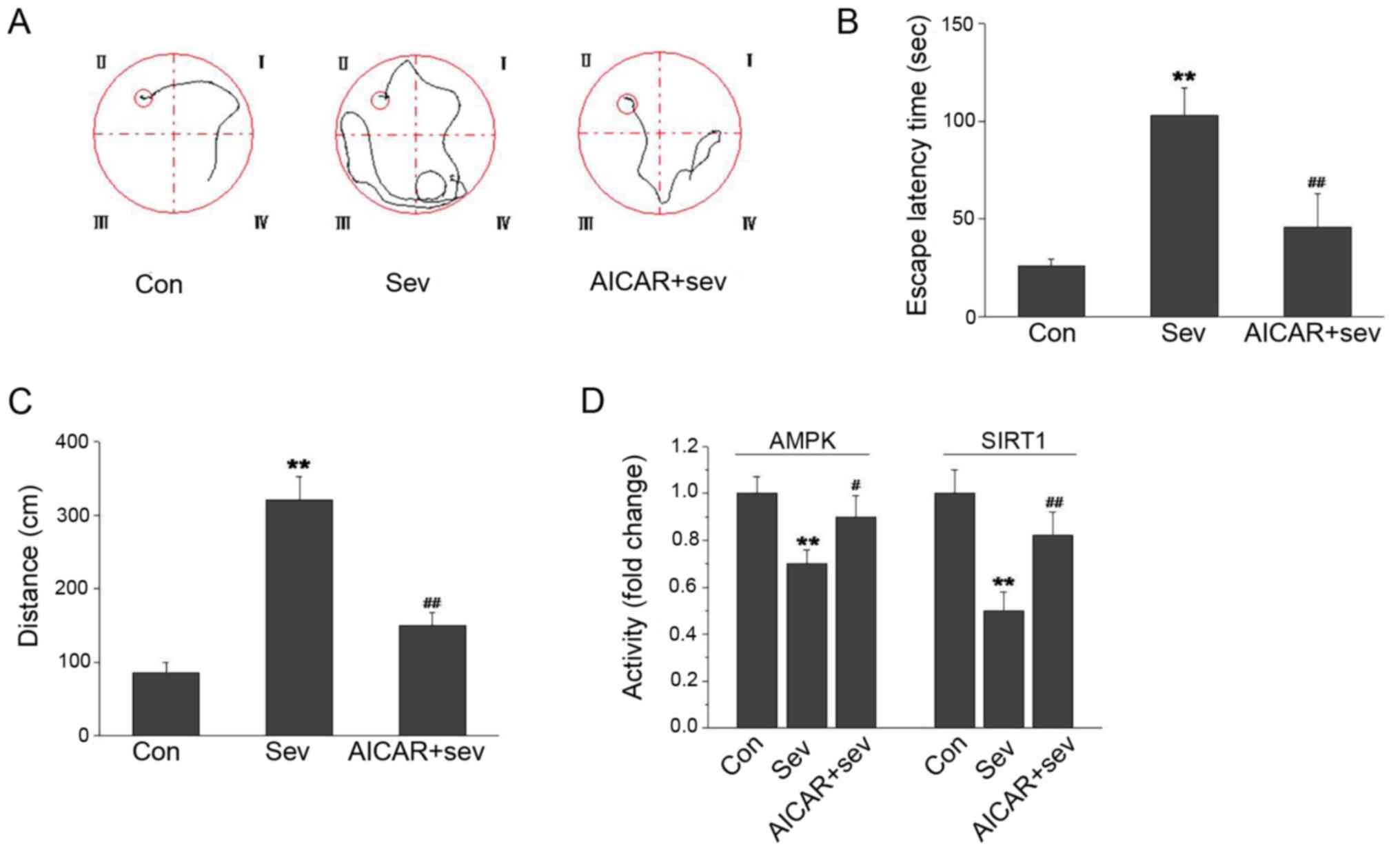
AMPK activation improves cognition and
restores in vivo SIRT1 levels decreased by Sev
To examine the role of AMPK/SIRT1 signaling in
Sev-induced cognition impairment, the rats were treated with the
AMPK activator-AICAR at 500 mg/kg, and cognition was evaluated
using the water maze test. As shown in Fig. 4A-C, Sev increased the escape latency
time and distance in the water maze. The escape latency time and
distance were notably decreased in the AICAR-treated rats.
Furthermore, Sev decreased the AMPK and SIRT1 activity in the
hippocampus. When AICAR was administered to rats, the AMPK and
SIRT1 activity in the hippocampus was significantly increased
(Fig. 4D). These results suggested
that AMPK activation could improve the cognitive impairment caused
by Sev.
Discussion
Sev is delivered via the lungs as a volume percent
of inspired gas. Sev has been described as the agent of choice for
inhalation induction due to its lack of airway irritation,
hemodynamic characteristics and lower pungency (20). Recent studies have reported on
hemodynamic and recovery characteristics following anesthesia
maintenance with Sev (21,22). Children who have received anesthesia
on multiple occasions are more likely to develop cognitive
behavioral disorders, while a single anesthetic exposure may have
no significant effect on neuro-development (23). A topic of interest is the potential
impact of inhalation-based anesthesia in behavioral outcomes such
as postoperative cognitive dysfunction and postoperative delirium
in pediatric, adult and elderly patients (22,24,25).
Neuronal apoptosis is an important contributor to
cognition dysfunction (26). Thus,
investigation of the molecular mechanism of Sev-induced neuronal
apoptosis may provide a new cognition protection strategy. In past
work, numerous genes have been found to be involved in neuronal
apoptosis, such as HMGB1/TLR4, caspase3, bcl-2, ROS and P53
(27–29). At present, much study is still
needed to elucidate the mechanism of neuronal apoptosis induced by
Sev.
AMPK is a major metabolic energy sensor, which may
sense energy deficiency in the form of an increased AMP/ATP ratio
and regulate metabolic homeostasis through the control of several
homeostatic mechanisms, including autophagy and protein degradation
(30). AMPK activation has
previously been identified as required for SIRT1. SIRT1 is a direct
substrate for AMPK phosphorylation (31). The present data has shown that Sev
is an effective inducer of neuronal apoptosis. The present study
also revealed that inhibition of AMPK/SIRT1 was associated with Sev
induced neuronal apoptosis. This was supported with evidence that
AMPK activation restrained the neuronal apoptosis induced by Sev.
The present study in vivo also revealed that AMPK/SIRT1
inhibition was related to the cognition impairment induced by Sev
and that AMPK activation could improve the cognition
dysfunction.
Activation of AMPK by phosphorylation of the α
subunit at Thr172 helps to maintain the energy balance by switching
on a catabolic pathway such as apoptosis induction (32,33). A
decreased protein level of p-AMPK/SIRT1 was confirmed to be
accompanied by an increased number of Sev induced apoptotic
neurons. Furthermore, this effect on apoptosis was blocked by the
AMPK activator AICAR, which indicated that AMPK/SIRT1 inhibition
was involved in the induction of cognitive impairment by Sev. The
protective role of the AMPK activator may be related to the
integrity of the brain-blood barrier (34,35).
In the animal experiments, rats aged 28 days were selected to
simulate human children, as it is desirable to have an experimental
basis for pediatric patients with anesthesia in the clinic.
In the present study, AMPK/SIRT1 inhibition was
shown to be associated with Sev-induced neuronal apoptosis. AMPK
activation restrained the Sev-induced neuronal apoptosis. The
present in vivo results also evaluated that AMPK activation
was related to cognition dysfunction induced by Sev. In summary,
the study revealed the association between the AMPK/SIRT1 pathway,
neuronal apoptosis and Sev-induced cognitive dysfunction. These
data revealed that AMPK/SIRT1 pathway serves a critical role in
Sev-induced neuronal apoptosis. AMPK/SIRT1 pathway may be a
potential drug target for the therapy of cognitive dysfunction
induced by Sev in the future.
Acknowledgements
Not applicable.
Funding
The work was financially supported by the China
Postdoctoral Science Foundation (grant no. 2015M581308).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL designed the study, performed the molecular
biology experiments, acquired data and drafted the manuscript. CL
established the animal model, performed behavioral testing and
molecular biology experiments, collected the behavioral data and
revised the manuscript. LF contributed to the conception of the
work, performed animal behavioral testing and molecular biology
experiments, analyzed and interpreted data, and revised and
approved the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethics approval was provided by the Animal
Experimental Ethical Inspection of Laboratory Animal Center,
Shanghai Tenth People's Hospital. This study was performed in
strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lim BG, Lee IO, Ahn H, Lee DK, Won YJ, Kim
HJ and Kim H: Comparison of the incidence of emergence agitation
and emergence times between desflurane and sevoflurane anesthesia
in children: A systematic review and meta-analysis. Medicine
(Baltimore). 95:e49272016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun L: Early childhood general anaesthesia
exposure and neurocognitive development. Br J Anaesth. 105 (Suppl
1):i61–i8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu X, Wang J, Zhang L, Zhang Q, Duan X and
Zhang Y: Postconditioning with sevoflurane ameliorates spatial
learning and memory deficit via attenuating endoplasmic reticulum
stress induced neuron apoptosis in a rat model of hemorrhage shock
and resuscitation. Brain Res. 1696:49–55. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu L, Shen J, Yu L, Sun J and Yan M:
Autophagy is involved in sevoflurane-induced developmental
neurotoxicity in the developing rat brain. Brain Res Bull.
140:226–232. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Voss CM, Pajęcka K, Stridh MH, Nissen JD,
Schousboe A and Waagepetersen HS: AMPK activation affects glutamate
metabolism in astrocytes. Neurochem Res. 40:2431–2442. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Willows R, Sanders MJ, Xiao B, Patel BR,
Martin SR, Read J, Wilson JR, Hubbard J, Gamblin SJ and Carling D:
Phosphorylation of AMPK by upstream kinases is required for
activity in mammalian cells. Biochem J. 474:3059–3073. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Viollet B, Mounier R, Leclerc J, Yazigi A,
Foretz M and Andreelli F: Targeting AMP-activated protein kinase as
a novel therapeutic approach for the treatment of metabolic
disorders. Diabetes Metab. 33:395–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qian C, Jin J, Chen J, Li J, Yu X, Mo H
and Chen G: SIRT1 activation by resveratrol reduces brain edema and
neuronal apoptosis in an experimental rat subarachnoid hemorrhage
model. Mol Med Rep. 16:9627–9635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan WJ, Wang DB, Ren DQ, Wang LK, Hu ZY,
Ma YB, Huang JW and Ding SL: AMPKα1 overexpression improves
postoperative cognitive dysfunction in aged rats through AMPK-Sirt1
and autophagy signaling. J Cell Biochem. Feb 18–2019.(Epub ahead of
print).
|
|
10
|
Fulco M and Sartorelli V: Comparing and
contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell
Cycle. 7:3669–3679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salminen A and Kaarniranta K: SIRT1:
Regulation of longevity via autophagy. Cell Signal. 21:1356–1360.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Z, Peng IC, Cui X, Li YS, Chien S and
Shyy JY: Shear stress, SIRT1, and vascular homeostasis. Proc Natl
Acad Sci USA. 107:10268–10273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cantó C, Gerhart-Hines Z, Feige JN,
Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P and Auwerx
J: AMPK regulates energy expenditure by modulating NAD+
metabolism and SIRT1 activity. Nature. 458:1056–1060. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
An Y, Wang B, Wang X, Dong G, Jia J and
Yang Q: SIRT1 Inhibits Chemoresistance and cancer Stemness of
gastric cancer by initiating an AMPK/FOXO3 positive feedback loop.
Cell Death Dis. 11:1152020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang J, Wang X, Zhu Y, Li Z, Zhu YT, Wu
JC, Qin ZH, Xiang M and Lin F: Exercise activates lysosomal
function in the brain through AMPK-SIRT1-TFEB pathway. CNS Neurosci
Ther. 25:796–807. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cetrullo S, D'Adamo S, Tantini B, Borzi RM
and Flamigni F: mTOR, AMPK, and Sirt1: Key players in metabolic
stress management. Crit Rev Eukaryot Gene Expr. 25:59–75. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shah SA, Yoon GH, Chung SS, Abid MN, Kim
TH, Lee HY and Kim MO: Novel osmotin inhibits SREBP2 via the
AdipoR1/AMPK/SIRT1 pathway to improve Alzheimer's disease
neuropathological deficits. Mol Psychiatry. 22:407–416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Q, Li K and Yao S: Effect of
inhalational anesthetics on cytotoxicity and intracellular calcium
differently in rat pheochromocytoma cells (PC12). J Huazhong Univ
Sci Technolog Med Sci. 28:104–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bi C, Cai Q, Shan Y, Yang F, Sun S, Wu X
and Liu H: Sevoflurane induces neurotoxicity in the developing rat
hippocampus by upregulating connexin 43 via the JNK/c-Jun/AP-1
pathway. Biomed Pharmacother. 108:1469–1476. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jindal R, Kumra VP, Narani KK and Sood J:
Comparison of maintenance and emergence characteristics after
desflurane or sevoflurane in outpatient anaesthesia. Indian J
Anaesth. 55:36–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi ES, Shin JY, Oh AY, Park HP, Hwang
JW, Lim YJ and Jeon YT: Sevoflurane versus propofol for
interventional neuroradiology: A comparison of the maintenance and
recovery profiles at comparable depths of anesthesia. Korean J
Anesthesiol. 66:290–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parida S and Badhe AS: Comparison of
cognitive, ambulatory, and psychomotor recovery profiles after day
care anesthesia with propofol and sevofurane. J Anesth. 28:833–838.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wilder RT, Flick RP, Sprung J, Katusic SK,
Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL and
Warner DO: Early exposure to anesthesia and learning disabilities
in a population-based birth cohort. Anesthesiology. 110:796–804.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jindal P, Khurana G, Oberoi D and Sharma
JP: Recovery profile and emergence delirium following Sevoflurane
and Isoflurane anesthesia in children posted for cleft lip surgery.
Middle East J Anaesthesiol. 21:679–684. 2012.PubMed/NCBI
|
|
25
|
Chandler JR, Myers D, Mehta D, Whyte E,
Groberman MK, Montgomery CJ and Ansermino JM: Emergence delirium in
children: A randomized trial to compare total intravenous
anesthesia with propofol and remifentanil to inhalational
sevofurane anesthesia. Paediatr Anaesth. 23:309–315. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu Y, Feng L, Li J, Lan X, A L, Lv X,
Zhang M and Chen L: The alteration of autophagy and apoptosis in
the hippocampus of rats with natural aging-dependent cognitive
deficits. Behav Brain Res. 334:155–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu Z, Miao Y, Muhammad I, Tian E, Hu W,
Wang J, Wang B, Li R and Li J: Colistin-induced autophagy and
apoptosis involves the JNK-Bcl2-Bax signaling pathway and
JNK-p53-ROS positive feedback loop in PC-12 cells. Chem Biol
Interact. 277:62–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo X, Shi Y, Du P, Wang J, Han Y, Sun B
and Feng J: HMGB1/TLR4 promotes apoptosis and reduces autophagy of
hippocampal neurons in diabetes combined with OSA. Life Sci.
239:1170202019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fricker M, Tolkovsky AM, Borutaite V,
Coleman M and Brown GC: Neuronal Cell Death. Physiol Rev.
98:813–880. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lau AW, Liu P, Inuzuka H and Gao D: SIRT1
phosphorylation by AMP-activated protein kinase regulates p53
acetylation. Am J Cancer Res. 4:245–255. 2014.PubMed/NCBI
|
|
31
|
Price NL, Gomes AP, Ling AJ, Duarte FV,
Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro
JS, et al: SIRT1 is required for AMPK activation and the beneficial
effects of resveratrol on mitochondrial function. Cell Metab.
15:675–690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Q, Liu S, Zhai A, Zhang B and Tian G:
AMPK-mediated regulation of lipid metabolism by phosphorylation.
Biol Pharm Bull. 41:985–993. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garcia D and Shaw RJ: AMPK: Mechanisms of
cellular energy sensing and restoration of metabolic balance. Mol
Cell. 66:789–800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu HY, Cai YB and Liu Z: Activation of
AMPK improves lipopolysaccharide-induced dysfunction of the
blood-brain barrier in mice. Brain Inj. 29:777–784. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao Z, Hu J, Gao X, Liang H and Liu Z:
Activation of AMPK attenuates lipopolysaccharide-impaired integrity
and function of blood-brain barrier in human brain microvascular
endothelial cells. Exp Mol Pathol. 97:386–392. 2014. View Article : Google Scholar : PubMed/NCBI
|