Introduction
Laryngeal carcinoma is a common type of head and
neck squamous cell carcinoma (SCC), ranking as the sixth most
commonly diagnosed carcinoma worldwide (1). The majority (95%) of laryngeal
carcinoma cases are SCCs (2).
Laryngeal SCC (LSCC) is the most common primary malignant tumor
affecting the laryngeal framework (3). The disease presents with a poor
prognosis, due to the frequent late diagnosis and lymph metastasis
of the disease (4), which can at
least partly be attributed to poor understanding of the molecular
mechanisms underlying its development. Habitual alcohol intake and
tobacco use are known risk factors for LSCC, representing a major
health hazard for humans (5). There
is currently no effective treatment for LSCC (6).
A large number of long non-coding RNAs (lncRNAs)
have been reported to serve important roles in tumorigenesis
(7–9). Lung cancer-associated transcript 1
(LUCAT1), also known as the smoke and cancer-associated lncRNA-1
(SCAL1), is encoded by the RNA gene located on chromosome 5q14.3,
and was first identified as a lncRNA overexpressed in lung cancer
cells (10). Previous studies have
provided evidence of the protumorigenic role of LUCAT1 in a variety
of types of cancer, including oral SCC, breast, colorectal,
papillary thyroid and ovarian cancers (11–15).
However, the possible role of LUCAT1 in the development or
progression of LSCC remains to be determined.
MicroRNAs (miRs/miRNAs) are another subgroup of
non-coding RNAs that interact with lncRNAs to regulate biological
processes, including proliferation, apoptosis and motility
(16). Previous studies have
demonstrated that LUCAT1 promoted the growth, migration and
invasion of cancer cells through the targeted modulation of certain
miRNAs, including miR-5720 and miR-5582 (17,18).
miR-493 was previously reported to serve as a tumor suppressor in
hepatic and pancreatic cancer (19–21);
however, whether and how miR-493 is involved in LSCC remains
unclear. Thus, the present study aimed to investigate the potential
interaction of LUCAT1 with miR-493 and to determine their effects
on LSCC cellular activities, such as cell proliferation, migration,
invasion and apoptosis.
Materials and methods
Cell culture
The normal nasopharyngeal epithelial NP69 cell line
and the corresponding LSCC cancer cell lines AMC-HN-8, Tu177, Tu686
and M4e were obtained from The Cell Bank of Type Culture Collection
of the Chinese Academy of Sciences (22). All cells were cultured in the DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), and maintained at 37°C and
5% CO2.
Prediction of target genes
To further investigate the molecular mechanisms
underlying the role of LUCAT1 in the tumorigenesis of LSCC, the
Encyclopedia of RNA Interactomes (ENCORI) database (http://starbase.sysu.edu.cn) was used. ENCORI is an
open-source platform for studying the miRNA-ncRNA (23), miRNA-mRNA, ncRNA-RNA, RNA-RNA,
RBP-ncRNA and RBP-mRNA interactions from CLIP-sequencing,
degradome-sequencing and RNA-RNA interactome data.
Knockdown plasmids
Two short hairpin RNAs (shRNAs) against LUCAT1
(shRNA-LUCAT1-1 and shRNA-LUCAT1-2; 500 ng/µl) from Guangzhou
RiboBio Co., Ltd. were used for the specific knockdown of LUCAT1,
with use of shRNA containing nonsense shRNA sequences as negative
control (shRNA-NC; 500 ng/µl). Two miR-493 inhibitors (miR-493
inhibitor-1 and miR-493 inhibitor-2; 100 pmol) and their negative
control miR-NC (100 pmol) were purchased from Shanghai GenePharma
Co., Ltd. All shRNAs and inhibitors were separately transfected
into AMC-HN-8 cells (1×105 cells/well) using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C. Subsequent assays were performed at 24 h
after transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was gained from cell samples. First, it
was extracted via TRIzol® reagent (Thermo Fisher
Scientific, Inc.) and then reverse-transcribed into cDNA using a
RevertAid™ cDNA Synthesis kit from Takara Bio, Inc at 42°C for 1 h
and 90°C for just 5 min. The SYBR Premix Ex Taq™ II kit (Thermo
Fisher Scientific, Inc.) was applied for qPCR. The PCR reaction
mixture contained included 3 mM MgCl2, 0.5 µM forward
and reverse primers, 2 µl SYBR Green PCR master mix and 2 µl cDNA.
Samples were run on a QuantStudio 3 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Thermocycling
conditions of the qPCR were: 5 min at 95°C, with 40 cycles for 30
sec at 95°C and 45 sec at 65°C. Relative expression levels were
calculated using the 2−ΔΔCq method and normalized to the
internal reference genes GAPDH or U6 (24). The primers were as follows: LUCAT1
forward, 5′-AGCTCCACCTCCCGGGTTCACG-3′ and reverse,
5′-CGTGAACCCGGGAGGTGGAGCT-3′; miR-493 forward,
5′-TTGTACATGGTAGGCTTTCATT-3′ and reverse,
5′-AACCATTTATTTCTCCCGACC−3; GAPDH forward, 5CACATCGCTCAGACACCATG-3′
and reverse, 5′-TGACGGTGCCATTGGAATTT-3′; U6 forward,
5′-CTCGCTTCGGCAGCACATATA−3′ and reverse,
5′-ACGCTTCACGAATTTGAGTGTC-3′.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was analyzed using a CCK-8 assay
kit following the manufacturer's manual. An AMC-HN-8 cell
suspension (5×103 cells/well) was plated into a 96-well
plate and incubated for 24 h at 37°C. Following the incubation, 10
µl CCK-8 solution was added into each well after transfection and
incubated for 4 h at 37°C. The absorbance was measured at a
wavelength of 450 nm using a microplate reader (Synergy2; BioTek
Instruments, Inc.).
Flow cytometric analysis of the cell
cycle and apoptosis
For cell cycle analysis, AMC-HN-8 cells were
collected in a flow cytometry tube centrifuged at 850 × g at 4°C
for 10 min, washed in PBS and fixed in cold 70% ethanol for 30 min
at 4°C. After washing in PBS, the samples were centrifuged at 850 ×
g at 4°C for 10 min. The pellets were treated with 0.5 ml
ribonuclease and incubated with propidium iodide (Beyotime
Institute of Biotechnology) for 30 min at 37°C. The cells were
measured at an emission wavelength of 605 nm for the forward and
side scatters to identify single cells. For cell cycle analysis,
the number of cells in either the S or G1 phase was counted using
an algorithm to fit Gaussian curves to each phase (ModFit LT,
Version of 4.0).
For the analysis of cell apoptosis, an Apoptosis
Detection kit (Beyotime Institute of Biotechnology) was used. The
cells were collected by centrifugation at 850 × g at 4°C for 5 min,
re-suspended in a binding buffer (195 µl) containing 1% Annexin
V-FITC (5 µl) and 1% PI (10 µl), and incubated at room temperature
for 5 min in the dark. Data were acquired using a flow cytometer
(Accuri C6; BD Biosciences) and analyzed using FlowJo (version of
7.6.5; FlowJo LLC).
Western blotting
Cells were harvested and total protein was extracted
using RIPA lysis buffer (Beyotime Institute of Biotechnology) with
protease inhibitor added to the lysis buffer (Beyotime Institute of
Biotechnology; 1:100). The lysates were centrifuged at 4°C at 850 ×
g for 15 min. The supernatant was collected and mixed with a
loading buffer (Beyotime Institute of Biotechnology) containing 100
mM dithiothreitol. Western blotting was subsequently performed, as
previously described (25).
Briefly, total protein was quantified using Protein Concentration
Determination kit (Beyotime Institute of Biotechnology) and
proteins (30 µg/lane) were separated by SDS-PAGE using 15% gels.
The separated proteins were subsequently transferred onto PVDF
membranes (EMD Millipore) and blocked with 5% BSA (Beyotime
Institute of Biotechnology) at room temperature for 2 h. The
membranes were then incubated with the following primary antibodies
(Abcam) at 4°C overnight: Anti-CDK2 (cat. no. ab32147; 1:1,000
dilution), anti-cyclin E1 (cat. no. ab33911; 1:1,000 dilution),
anti-p21 (cat. no. ab109520; 1:1,000 dilution), anti-matrix
metallopeptidase (MMP)2 (cat. no. ab97779; 1:1,000 dilution),
anti-MMP9 (cat. no. ab38898; 1:1,000 dilution), anti-vascular
endothelial growth factor (VEGF)-C (cat. no. ab9546; 1:1,000
dilution), anti-Bcl-2 (cat. no. ab32124; 1:1,000 dilution),
anti-Bax (cat. no. ab32503; 1:1,000 dilution), anti-cleaved
caspase-3 (cat. no. ab2302; 1:1,000 dilution), anti-pro caspase-3
(cat. no. ab32150; 1:1,000 dilution) and anti-GAPDH (cat. no.
ab8245; 1:2,000 dilution). Following the primary antibody
incubation, the membranes were washed with TBS with 0.1% Tween-20
and incubated at room temperature for 1.5 h with a horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody
(1:5,000; SA00001-9; ProteinTech Group, Inc.) or goat anti-mouse
IgG secondary antibody (1:5,000; SA00001-8; ProteinTech Group,
Inc.). Protein bands were visualized using a luminol reagent (Santa
Cruz Biotechnology, Inc.) and analyzed using ImageJ software
(version 1.48; National Institutes of Health).
Wound healing assay
AMC-HN-8 cells were cultured in six-well plates
(6×104 cells/well) and transiently transfected with
specific plasmids. Following 24 h of transfection, a linear scratch
was created in the cell monolayer using a 200-µl pipette tip. The
cells were subsequently cultured under standard conditions in
serum-free medium (Gibco; Thermo Fisher Scientific, Inc.) at 37°C
in 5% CO2. Using ImageJ software (version 1.8.0;
National Institutes of Health) to calculate the wound width. Images
of the wound were captured at 72 h using a light microscope (Nikon
Corporation), magnification, ×100.
Transwell invasion assay
A Transwell invasion assay was used to analyze the
invasive rate of cells. Briefly, AMC-HN-8 cells in 100 µl
(2×105) serum-free medium (Thermo Fisher Scientific,
Inc.) were plated into the upper chambers of an 8-µm Transwell
plate (Corning, Inc.) precoated with Matrigel™ (BD Biosciences) for
24 h at 37°C after transfection. RPMI-1640 medium containing 20%
FBS was plated in the lower chamber to serve as a chemoattractant.
Following 24-h incubation at 37°C, the invading cells on the bottom
surface of the filter were fixed with 100% methanol at 4°C for 30
min and stained with hematoxylin at room temperature for 20 min.
Cell invasion was analyzed in three randomly selected fields under
a fluorescence microscope (magnification, ×20).
Dual-luciferase reporter assay
The firefly luciferase reporter plasmid
(pGL3-promoter vector; E1761; Promega) and constitutively active
Renilla luciferase control plasmid pRL-TK (pmiR-RB-Report™,
Beijing Baiaolaibo Technology Co., Ltd.) were used. The
LUCAT1-3′-untranslated region (UTR) was cloned into the
pGL3-promoter vector to generate wild-type (WT) LUCAT1-Luc. A
mutant (MUT) LUCAT-1 3′-UTR was cloned into the pGL3-promoter
vector to generate MUT LUCAT1-Luc by site-directed mutagenesis
(QuikChange Lightning Site-Directed Mutagenesis kit; Agilent
Technologies, Inc.). A plasmid containing an miR-493 mimic
(5′-UGAAGGUCUACUGUGUGCCAGG-3′) was purchased from Shanghai
GenePharma Co., Ltd. or empty vector control was co-transfected
using the Lipofectamine® 3000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) with the WT or MUT
LUCAT1-Luc into AMC-HN-8 cells and incubated for 48 h at 37°C
(1.5×105 cells/well). The cells were washed with PBS and
lysed with RIPA lysis solution (Beyotime Institute of
Biotechnology). The relative luciferase activity was analyzed using
a plate reader at 410 nm (BD Biosciences) and normalized to the
activity of a Renilla luciferase activity kit (pRL-TK;
Beijing Baiaolaibo Technology Co., Ltd.). All procedures were
performed according to the manufacturers' protocols.
Ribonucleoprotein immunoprecipitation
(RIP) assay
A total of 1×107 AMC-HN-8 cells were
added to 2 ml PBS (Beyotime, China) to wash, and centrifuged at 850
× g at room temperature for 5 min to collect the cells. A RIP assay
was performed using a Millipore Magna RIP™ RNA-Binding Protein
Immunoprecipitation kit (Active Motif, Inc.), according to the
manufacturer's protocol. Briefly, AMC-HN-8 cells were lysed with
anti-EZH2 or IgG antibody at 4°C for 6 h. A protein-RNA complex was
captured and digested with 0.5 mg/ml proteinase K containing 0.1%
SDS to extract RNA. RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and RT-qPCR
analysis was performed to analyze the expression levels of LUCAT1
and miR-493.
Statistical analysis
All experiments were repeated three times.
Statistical analysis was performed using GraphPad Prism 5 software
(GraphPad Software, Inc.) and all data are presented as the mean ±
SEM, unless otherwise specified. Statistical differences between 2
groups were determined using an unpaired two-tailed Student's
t-test, while a one-way or two-way ANOVA followed by Tukey's post
hoc test were used to analyze data with >2 groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
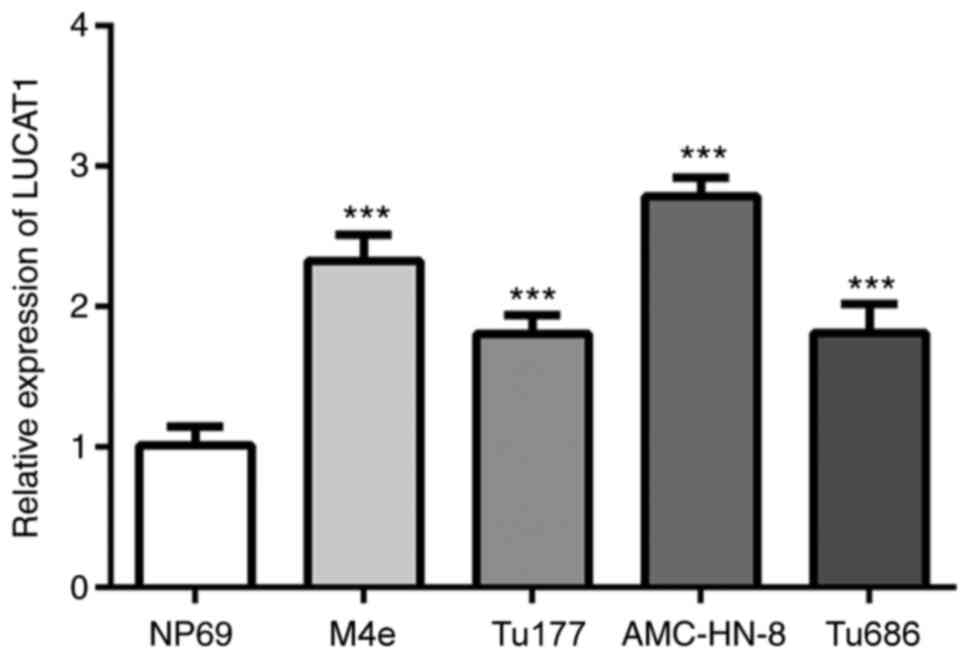
LUCAT1 expression levels are
upregulated in LSCC cells
The expression levels of LUCAT1 in the
nasopharyngeal epithelial NP69 (normal) cell line, and LSCC cell
lines AMC-HN-8, Tu177, Tu686 and M4e were analyzed. The expression
levels of LUCAT1 were significantly upregulated in the LSCC cells
compared with the NP69 cells, with the most prominent upregulation
being observed in the AMC-HN-8 cells (Fig. 1A). Thus, the AMC-HN-8 cell line was
used as the LSCC cell model in subsequent experiments.
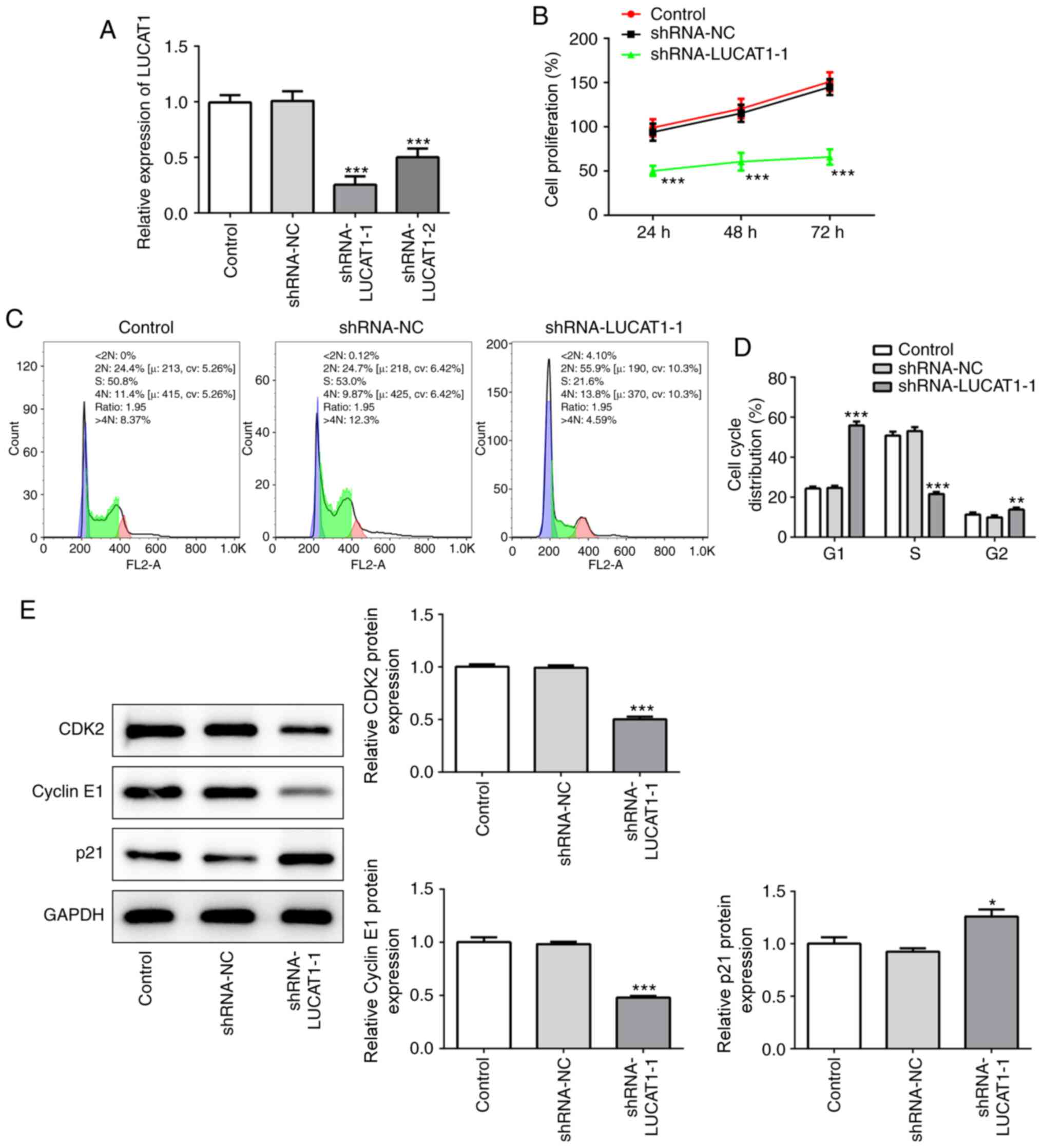
LUCAT1 knockdown suppresses the
proliferation of LSCC cells
To determine how LUCAT1 affects LSCC, LUCAT1
expression levels were knocked down in AMC-HN-8 cells using shRNA.
Out of the two shRNAs used, shRNA-LUCAT1-1 exhibited an enhanced
knockdown efficiency (Fig. 2A); the
expression levels of LUCAT1 were significantly downregulated in
both the shRNA-LUCAT1-1- and shRNA-LUCAT1-2-transfected cells
compared with the shRNA-NC group, but to a greater extent in
shRNA-LUCAT1-1-transfected cells. Therefore, shRNA-LUCAT1-1 was
used for further experiments.
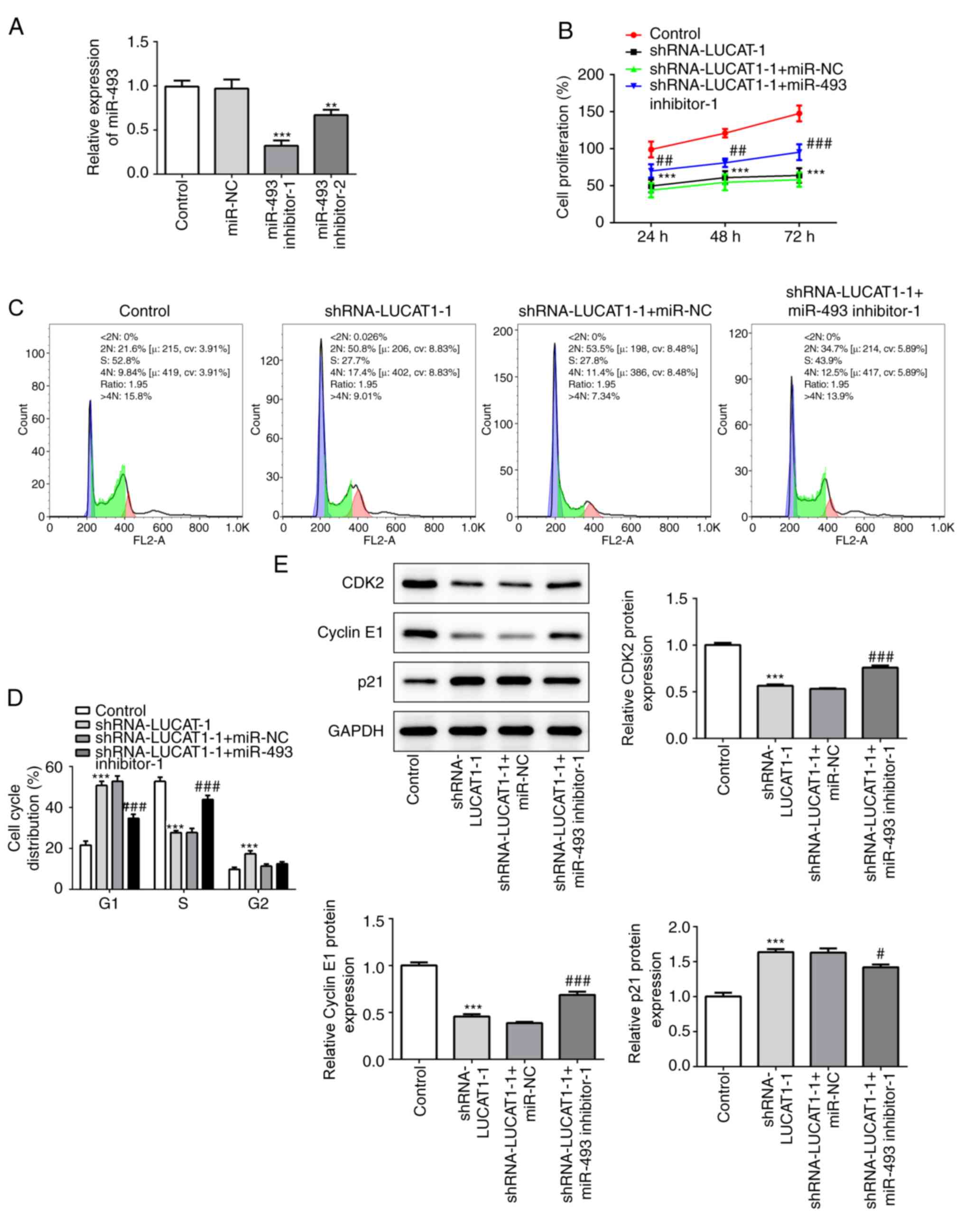
The genetic knockdown of LUCAT1 significantly
decreased the cell proliferation rate of AMC-HN-8 cells compared
with untransfected AMC-HN-8 cells (referred to as control), which
was determined using a CCK-8 assay (Fig. 2B). Moreover, compared with the
control shRNA-LUCAT1-1 also significantly decreased the percentage
of cells in the S phase of the cell cycle, while significantly
increasing the number of G1 phase cells (Fig. 2C and D), indicating that LUCAT1
knockdown may suppress the proliferation of cancer cells.
Consistent with this hypothesis, shRNA-LUCAT1-1 was discovered to
significantly downregulate the expression levels of CDK2 and cyclin
E1 compared with the control and shRNA-NC (Fig. 2E), two proteins which facilitate
cell cycle progression (26,27),
while significantly upregulating the expression levels of p21
compared with the control and shRNA-NC, a cell cycle inhibitor
(28) (Fig. 2E). Therefore, these findings
suggested that LUCAT1 may exert a suppressive effect on cell
proliferation.
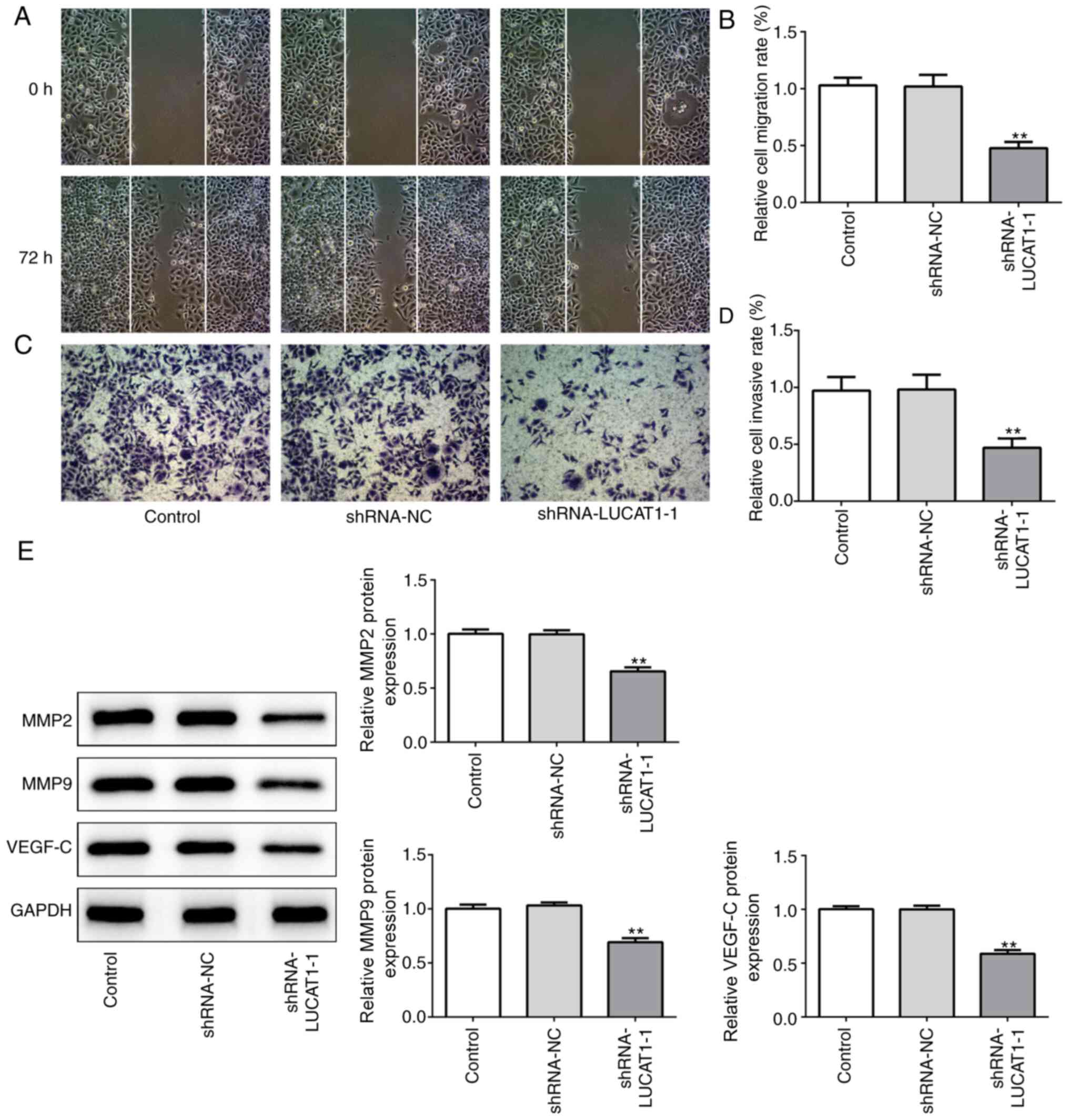
LUCAT1 knockdown inhibits the
migration and invasion of LSCC cells
The effects of the knockdown of LUCAT1 on the
migration and invasion of AMC-HN-8 cells was subsequently
investigated. Compared with the control and shRNA-NC, LUCAT1
knockdown significantly suppressed the migration (Fig. 3A and B) and invasion (Fig. 3C and D) of LSCC cells, as observed
using a wound healing or Transwell assay, respectively. In
addition, shRNA-LUCAT1-1 significantly downregulated the expression
levels of the migration-associated proteins, VEGF-C, MMP2 and MMP9
compared with the control and shRNA-NC (Fig. 3E). These data suggested that the
knockdown of LUCAT1 may inhibit LSCC cell migration and
invasion.
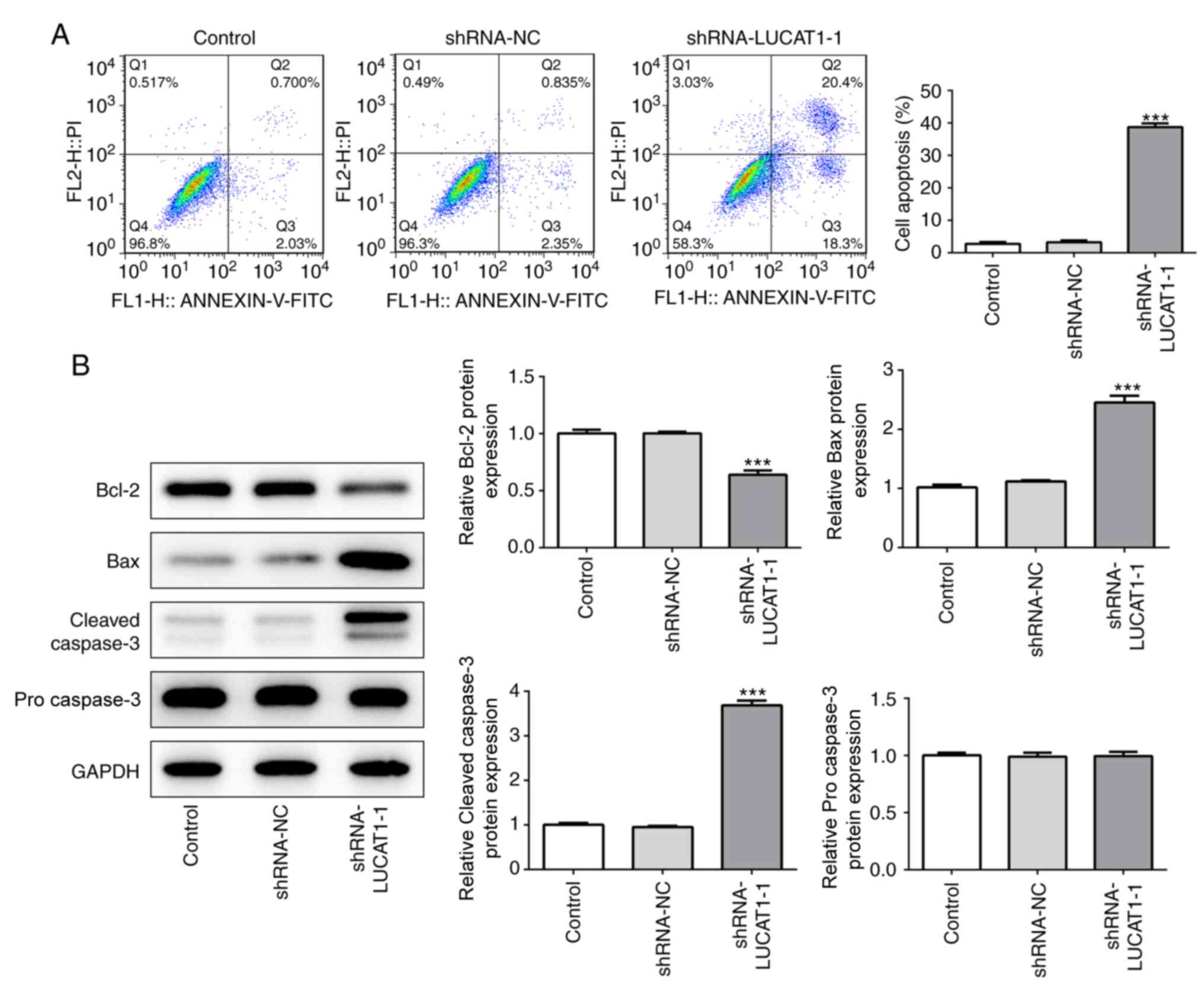
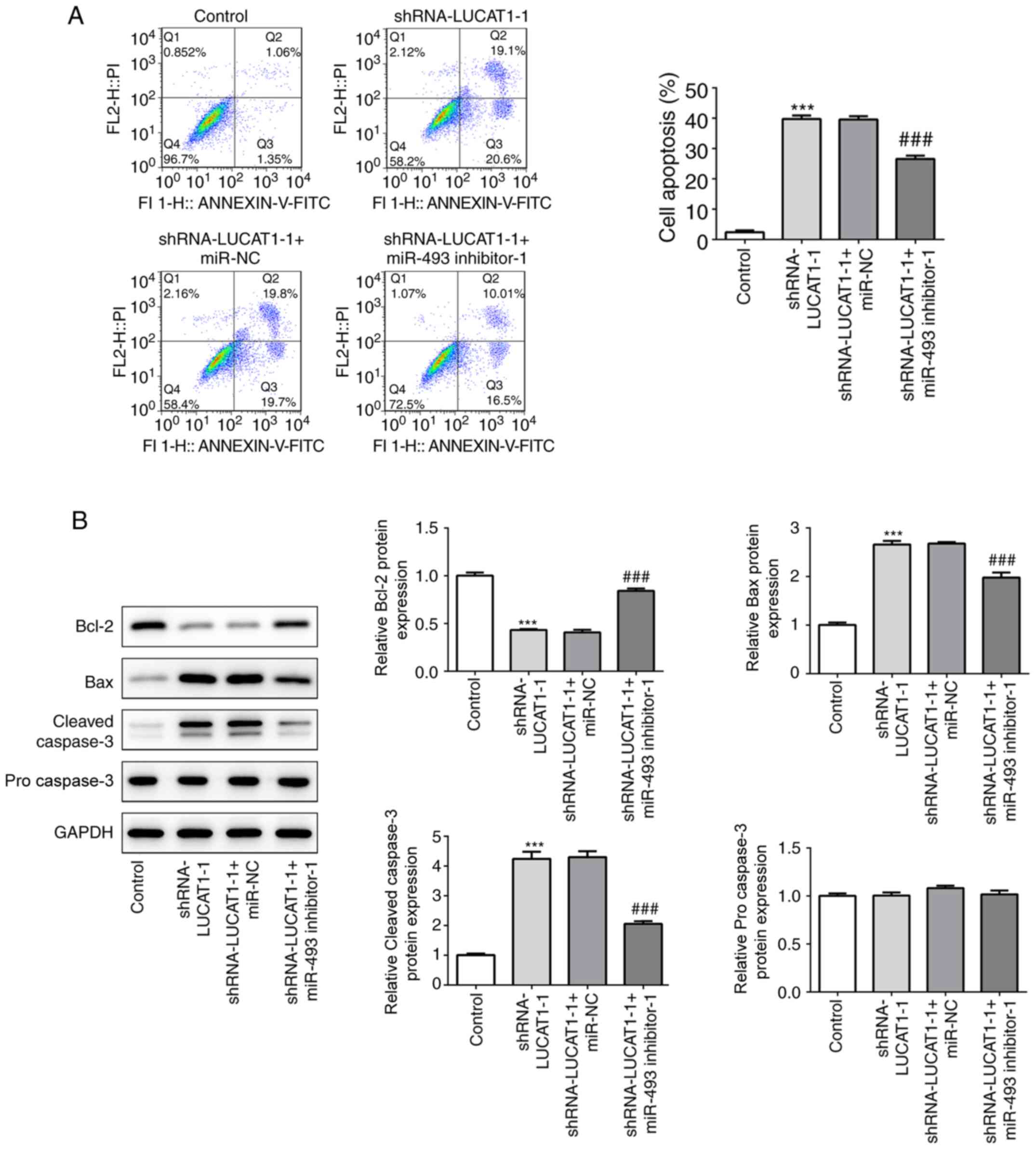
LUCAT1 knockdown promotes the
apoptosis of LSCC cells
Compared with the control and shRNA-NC, LUCAT1-1
knockdown also significantly promoted the apoptosis of cancer cells
(Fig. 4A). Furthermore, compared
with the control and shRNA-NC, the knockdown of LUCAT1-1
significantly downregulated the expression levels of Bcl-2, while
significantly upregulating the expression levels of Bax and cleaved
caspase-3 (Fig. 4B). However, no
statistical differences were observed in the expression levels of
pro caspase-3 between the groups (Fig.
4B). Taken together, these findings indicated that the genetic
knockdown of LUCAT1 may promote the apoptosis of LSCC cells.
Targeted inhibition of miR-493 by
LUCAT1
To further investigate the molecular mechanisms
underlying the role of LUCAT1 in the tumorigenesis of LSCC, the
ENCORI database (http://starbase.sysu.edu.cn) was used. It was
predicted that LUCAT1 directly bound with miR-493, a miRNA
previously reported to inhibit tumorigenesis occurred in the 3′-UTR
(29,30) (Fig.
5A).
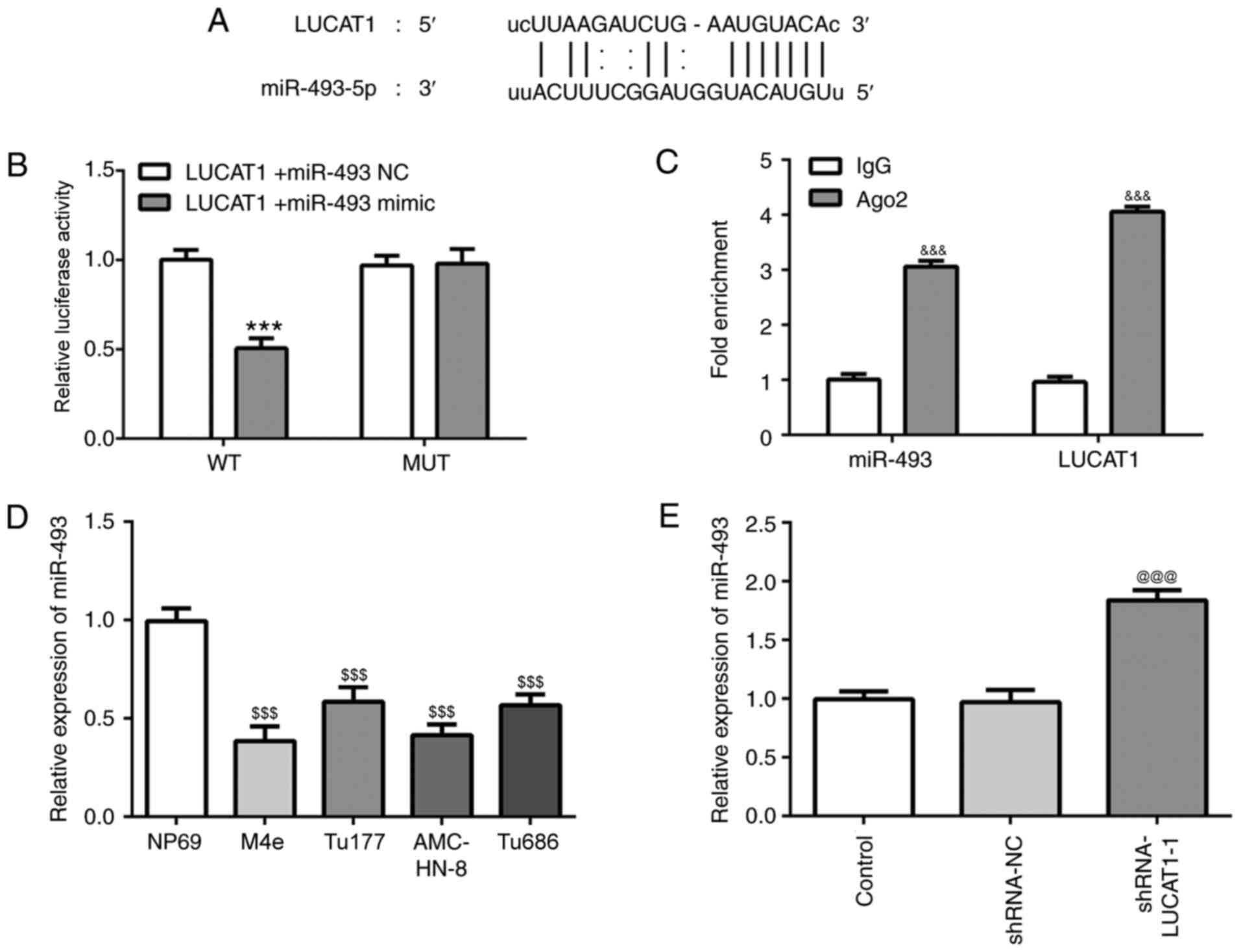 | Figure 5.LUCAT1 targets miR-493. (A)
Complementary binding site between the 3′ untranslated region of
LUCAT1 and miR-493 was predicted using the ENCORI. (B)
Dual-luciferase reporter assay was performed to determine the
relationship between LUCAT1 and miR-493. ***P<0.001, vs.
shRNA-NC group. (C) Ribonucleoprotein immunoprecipitation assay was
used to validate the interaction between LUCAT1 and miR-493.
&&&P<0.001, vs. IgG). (D) miR-493
expression levels in the normal nasopharyngeal epithelial NP69 cell
line and larynx squamous cell carcinoma cells (AMC-HN-8, Tu177,
Tu686 and M4e) were investigated using RT-qPCR.
$$$P<0.001 (Vs NP69). (E) miR-493 expression levels
following the genetic knockdown of LUCAT1 in AMC-HN-8 cells were
analyzed using RT-qPCR. @@@P<0.001, vs. shRNA-NC).
LUCAT1, lung cancer-associated transcript 1; shRNA, short hairpin
RNA; NC, negative control; miR, microRNA; WT, wild-type; MUT,
mutant. |
The targeted binding between LUCAT1 and miR-493 was
confirmed by the significantly reduced relative luciferase activity
observed in cells co-transfected with the WT LUCAT1-Luc and
miR-493-mimic. In addition, no significant differences were
identified in the relative luciferase activity between the cells
co-transfected with the MUT LUCAT1-Luc and miR-493-mimic or miR-493
NC (Fig. 5B). Consistent with these
results, the fold enrichment of LUCAT1 co-immunoprecipitated with
miR-493 was significantly increased in the LUCAT1- and miR-493-Ago2
groups compared with the IgG groups (Fig. 5C).
Notably, significantly downregulated expression
levels of miR-493 were identified in LSCC cells, particularly
AMC-HN-8 cells, compared with the NP69 cells (Fig. 5D), which were significantly reversed
by the specific knockdown of LUCAT1 in AMC-HN-8 cells (Fig. 5E). Taken together, these results
indicated the potential targeted inhibition of miR-493 by LUCAT1 in
LSCC.
miR-493 inhibition blocks the
antitumorigenic effect of LUCAT1 knockdown
To determine whether LUCAT1 promoted tumorigenesis
in LSCC through the targeted suppression of miR-493, miR-493
inhibitor-1 and miR-493 inhibitor-2 were co-transfected with
shRNA-LUCAT1-1 into AMC-HN-8 cells. The transfection efficiency of
the miR-493 inhibitors was verified using RT-qPCR (Fig. 6A). The miR-493 inhibitor-1, which
exhibited an enhanced inference rate on the expression levels of
miR-493, was used for subsequent assays.
The co-inhibition of miR-493 significantly blocked
the inhibitory effect of shRNA-LUCAT1-1 on cell proliferation
(Fig. 6B). Similarly, the
inhibition of miR-493 reversed the reduction in LSCC cell
proliferation induced by shRNA-LUCAT1-1 through significantly
increasing the proportion of S phase cells and significantly
decreasing the proportion of G1 phase cells (Fig. 6C and D). Similarly, the
shRNA-LUCAT1-1+miR-493 inhibitor group demonstrated significantly
upregulated expression levels of CDK2 and cyclin E1, but
downregulated expression levels of p21 compared with the cells
co-transfected with the shRNA-LUCAT1-1 and miR-NC (Fig. 6E).
Furthermore, the co-transfection of
shRNA-LUCAT1-1-transfected AMC-HN-8 cells with the
miR-493-inhibitor promoted the migration and invasion of cells
(Fig. 7A-D). Consistent with these
results, inhibiting miR-493 alongside LUCAT1 also significantly
upregulated the expression levels of MMP2, MMP9 and VEGF-C, which
were downregulated following the silencing of LUCAT1 (Fig. 7E).
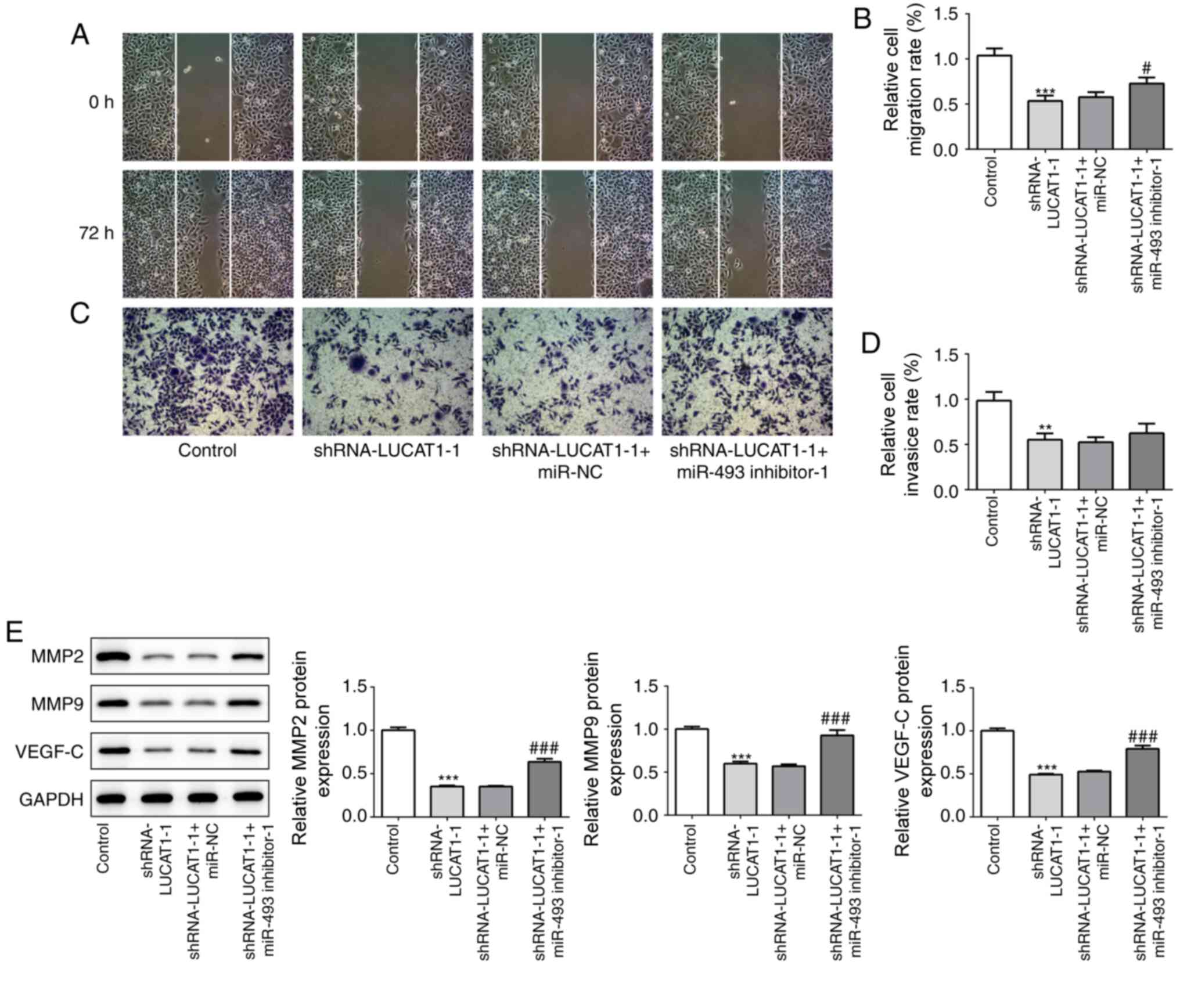 | Figure 7.miR-493 silencing blocks the
anti-migratory and anti-invasive effect of LUCAT1 knockdown. (A)
Cell migration rate was analyzed in AMC-HN-8 cells transfected with
shRNA-LUCAT1-1 with or without the miR-493 inhibitor using a wound
healing assay, magnification ×100. (B) Semi-quantification of the
relative cell migration rate from part (A). (C) Cell invasion rate
was analyzed in AMC-HN-8 cells transfected with shRNA-LUCAT1-1 with
or without miR-493 inhibitor using a Transwell assay (×100). (D)
Semi-quantification of the relative cell invasive rate from part
(C). (E) Protein expression levels of MMP9, MMP13 and VEGF-C were
analyzed using western blotting. **P<0.01 and ***P<0.001, vs.
control; #P<0.05, and ###P<0.001, vs.
shRNA-LUCAT1-1 + miR-NC. LUCAT1, lung cancer-associated transcript
1; shRNA, short hairpin RNA; NC, negative control; miR, microRNA;
MMP, matrix metallopeptidase; VEGF-C, vascular endothelial growth
factor-C. |
In addition, the downregulation of miR-493 and
LUCAT1 partially suppressed the promoting effect of shRNA-LUCAT1-1
on cell apoptosis (Fig. 8A),
upregulated the expression levels of Bcl-2 and downregulated the
expression levels of Bax and cleaved caspase-3 (Fig. 8B) compared with the cells
co-transfected with the shRNA-LUCAT1-1 and miR-NC. Taken together,
these data indicated the potential anticancer role of miR-493,
which may be suppressed by LUCAT1 in LSCC.
Discussion
LSCC accounts for ~90% of head and neck cancers,
which comprise a heterogeneous group of cancers most commonly
located in the oral cavity, oropharynx and larynx (31). LSCC is the second most common subset
of head and neck SCC and poses a major threat to human health. More
than 80,000 throat cancer patients die every year (32,33).
Patients with LSCC presenting with tumor invasion and metastasis
have a poor prognosis and low five-year survival rate is only ~60%
(34), which may be partly
attributed to the poor understanding of LSCC pathogenesis and
limited treatment options. Therefore, determining the molecular
mechanisms underlying the pathogenesis of LSCC may uncover novel
targets for the treatment of this disease. In the present study,
Experiment by genetically knocking down LUCAT1, the expression
levels of LUCAT1 were discovered to be upregulated in LSCC cell
lines, including AMC-HN-8, Tu177, Tu686 and M4e, promoted the
proliferation, migration and invasion of the cancer cells, while
inhibiting apoptosis. Moreover, the findings suggested that LUCAT1
may promote LSCC through the targeted inhibition of miR-493.
LUCAT1, previously referred to as SCAL1, comprises
four exons and three introns, and is located on chromosome 5q14.3,
where it is transcriptionally regulated by nuclear factor erythroid
2-related factor (11). In previous
studies, the expression levels of LUCAT1 were reported to be
upregulated in several types of cancer, and served pro-tumorigenic
roles, including within breast (12), colorectal (13), papillary thyroid (14), ovarian (15) and cervical cancers, among others
(31). In addition, LUCAT1 was
discovered to be overexpressed in oral SCC (11). Therefore, it was hypothesized that
LUCAT1 may also be highly expressed and serve an important role in
LSCC. In the present study, the expression levels of LUCAT1 was
also upregulated in LSCC cells, while the genetic knockdown of
LUCAT1 significantly suppressed the cell proliferation, migration
and invasion, while promoting cell apoptosis.
The possible modulatory mechanism of LUCAT1 in LSCC
was subsequently investigated. lncRNAs are involved in numerous
biological processes such as regulation of transcription,
translation, protein modification, and the formation of RNA-protein
or protein-protein complexes (32,33)
and commonly act by targeted binding and regulation of proteins,
mRNAs or microRNAs (32). Indeed,
LUCAT1 was reported to contribute to the development of
triple-negative breast cancer through promoting cell proliferation,
cell cycle progression and metastasis, and attenuating cell
apoptosis, and its effects were mediated by negatively modulating
miR-5702 (17). Similarly, breast
cancer cell stemness was discovered to be dysregulated by the
LUCAT1/miR-5582-3p/transcription factor 7-like 2 axis, which
increased the activity of the Wnt/β-catenin signaling pathway
(18). Moreover, LUCAT1 was
reported to promote ovarian cancer progression through inhibiting
miR-612 to enhance homeobox protein Hox-A13 expression levels,
thereby promoting cancer cell proliferation, migration and invasion
(15). In the present study, it was
hypothesized that LUCAT1 may exert a role by interacting with
certain miRNAs. By using the ENCORI database, the interaction
between LUCAT1 and miR-493 was predicted in the 3′-UTR. Convincing
evidence supports the tumor-suppressive role of miR-493; for
example, miR-493 served a crucial role in tumor transformation and
the survival of malignant cells, such as renal cell carcinoma
(35). In addition, miR-493 has
been widely reported to suppress diverse subtypes of cancer through
activating or inhibiting a variety of downstream signaling
cascades; for instance, miR-493 decreased the motility and
migratory ability of human bladder cancer cells by downregulating
RhoC and Frizzled-4 expression levels (36). miR-493-5p also reduced the
invasiveness and tumorigenic potential of breast cancer cells via
targeting α-(1,3)-fucosyltransferase 4 (37) and suppressed hepatocellular
carcinoma cell proliferation by targeting Golgi protein 73
(38). Moreover, miR-493 was
reportedly involved in the carcinogenesis of osteosarcoma and
hepatic cancer by targeting different signaling pathways such as
Notch1, Akt, Wnt pathway (39,40).
In the present study, rescue experiments were performed by the
significantly reduced relative luciferase activity observed in
cells co-transfected with the WT LUCAT1-Luc and miR-493-mimic,
revealing that LUCAT1 facilitated LSCC tumorigenesis by inhibiting
miR-493.
In conclusion, the findings of the present study
suggested that LUCAT1 may promote the tumorigenesis of LSCC through
the targeted inhibition of miR-493. To the best of our knowledge,
this was the first study to determine the oncogenic function of
LUCAT1 and detail its underlying regulatory interaction with
miR-493 in LSCC cells. These results identified a novel
LUCAT1/miR-493 axis and highlighted LUCAT1 and miR-493 as potential
therapeutic targets for LSCC treatment. However, there are
limitations to the present study. First, the study was an in
vitro study and no in vivo experiments were performed
and second, the molecular mechanisms underlying the effects of
miR-493 inhibition by LUCAT1 on LSCC cell function were not fully
investigated. These issues require further in-depth investigations
and will be addressed in future studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ designed the study and wrote the manuscript; YX
performed the experiments; YL collected and analyzed the data; and
SJ interpreted the data. The second draft of the manuscript was
revised by ZZ and YX. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pinette A, McMichael E, Courtney NB,
Duggan M, Benner BN, Choueiry F, Yu L, Abood D, Mace TA and Carson
WE 3rd: An IL-15-based superagonist ALT-803 enhances the NK cell
response to cetuximab-treated squamous cell carcinoma of the head
and neck. Cancer Immunol Immunother. 68:1379–1389. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao P, Gong L and Wang X: Induction
chemotherapy in patients with resectable laryngeal cancer: A
meta-analysis. Mol Clin Oncol. 9:155–162. 2018.PubMed/NCBI
|
|
3
|
Yang JQ, Liang Z, Wu M, Sun YM and Liu HX:
Expression of p27 and PTEN and clinical characteristics in early
laryngeal squamous cell carcinoma and their correlation with
recurrence. Int J Clin Exp Pathol. 8:5715–5720. 2015.PubMed/NCBI
|
|
4
|
Zheng Z, Tian R and Wang P: Roles of KAI1
and nm23 in lymphangiogenesis and lymph metastasis of laryngeal
squamous cell carcinoma. World J Surg Oncol. 15:2112017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vijay Parshuram R, Kumar R, Bhatt MLB,
Singh R, Parmar D, Gaur J, Kishan D, Saha M, Roopali, Katepogu P,
et al: To investigate the affiliation of XRCC-1 Gene Arg194Trp
polymorphism in alcohol and tobacco substance users and
loco-regionally progressed Laryngeal squamous cell carcinoma. J
Oral Biol Craniofac Res. 9:77–80. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma Z, Zhang H, Lian M, Yue C, Dong G, Jin
Y, Li R, Wan H, Wang R, Wang Y, et al: SLC7A11, a component of
cysteine/glutamate transporter, is a novel biomarker for the
diagnosis and prognosis in laryngeal squamous cell carcinoma. Oncol
Rep. 38:3019–3029. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He J, Zhou X, Li L and Han Z: Long
noncoding MAGI2-AS3 suppresses several cellular processes of lung
squamous cell carcinoma cells by regulating miR-374a/b-5p/CADM2
axis. Cancer Manag Res. 12:289–302. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu S, Ai H, Zhang K, Yun H and Xie F: Long
non-coding RNA EGOT promotes the malignant phenotypes of
hepatocellular carcinoma cells and increases the expression of
HMGA2 via down-regulating miR-33a-5p. Onco Targets Ther.
12:11623–11635. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao W, Qi CQ, Feng MG, Yang P, Liu L and
Sun SH: SOX2-induced upregulation of lncRNA LINC01561 promotes
non-small-cell lung carcinoma progression by sponging miR-760 to
modulate SHCBP1 expression. J Cell Physiol. 235:6684–6696. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thai P, Statt S, Chen CH, Liang E,
Campbell C and Wu R: Characterization of a novel long noncoding
RNA, SCAL1, induced by cigarette smoke and elevated in lung cancer
cell lines. Am J Respir Cell Mol Biol. 49:204–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kong Y, Feng Y, Xiao YY, Liu SC, Li XG,
Yang QL, Chu WH and Liu JG: LncRNA LUCAT1 promotes rowth,
migration, and invasion of oral squamous cell carcinoma by
upregulating PCNA. Eur Rev Med Pharmacol Sci. 23:4770–4776.
2019.PubMed/NCBI
|
|
12
|
Li YL, Wang XM, Qiao GD, Zhang S, Wang J,
Cong YZ and Zhu SG: Up-regulated lnc-lung cancer associated
transcript 1 enhances cell migration and invasion in breast cancer
progression. Biochem Biophys Res Commun. 521:271–278. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou Q, Hou Z, Zuo S, Zhou X, Feng Y, Sun
Y and Yuan X: The long noncoding RNA LUCAT1 promotes CRC
tumorigenesis by targeting RPL40-MDM2-p53 pathway through binding
with UBA52. Cancer Sci. 110:1194–1207. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luzón-Toro B, Fernández RM,
Martos-Martínez JM, Rubio-Manzanares-Dorado M, Antiñolo G and
Borrego S: LncRNA LUCAT1 as a novel prognostic biomarker for
patients with papillary thyroid cancer. Sci Rep. 9:143742019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu H, Xu Y, Zhang D and Liu G: Long
noncoding RNA LUCAT1 promotes malignancy of ovarian cancer through
regulation of miR-612/HOXA13 pathway. Biochem Biophys Res Commun.
503:2095–2100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dou X, Zhou Q, Wen M, Xu J, Zhu Y, Zhang S
and Xu X: Long noncoding RNA FOXD2-AS1 promotes the malignancy of
cervical cancer by sponging MicroRNA-760 and upregulating
hepatoma-derived growth factor. Front Pharmacol. 10:17002020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mou E and Wang H: LncRNA LUCAT1
facilitates tumorigenesis and metastasis of triple-negative breast
cancer through modulating miR-5702. Biosci. Rep.
39:BSR201904892019.
|
|
18
|
Zheng A, Song X, Zhang L, Zhao L, Mao X,
Wei M and Jin F: Long non-coding RNA LUCAT1/miR-5582-3p/TCF7L2 axis
regulates breast cancer stemness via Wnt/β-catenin pathway. J Exp
Clin Cancer Res. 38:3052019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang G, Fang X, Han M, Wang X and Huang Q:
MicroRNA-493-5p promotes apoptosis and suppresses proliferation and
invasion in liver cancer cells by targeting VAMP2. Int J Mol Med.
41:1740–1748. 2018.PubMed/NCBI
|
|
20
|
Yasukawa K, Liew LC, Hagiwara K,
Hironaka-Mitsuhashi A, Qin XY, Furutani Y, Tanaka Y, Nakagama H,
Kojima S, Kato T, et al: MicroRNA-493-5p-mediated repression of the
MYCN oncogene inhibits hepatic cancer cell growth and invasion.
Cancer Sci. 111:869–880. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhi D, Zhao X, Dong M and Yan C: miR-493
inhibits proliferation and invasion in pancreatic cancer cells and
inversely regulated hERG1 expression. Oncol Lett. 14:7398–7404.
2017.PubMed/NCBI
|
|
22
|
Liu C, Lu Z, Liu H, Zhuang S and Guo P:
LncRNA XIST promotes the progression of laryngeal squamous cell
carcinoma via sponging miR-125b-5p to modulate TRIB2. Biosci Rep.
40:BSR201931722020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yao Z, Li Y, Wang Z, Lan Y, Zeng T, Gong
H, Zhu K, Tang H and Gu S: Research on anti-hepatocellular
carcinoma activity and mechanism of Polygala fallax Hemsl. J
Ethnopharmacol. 260:1130622020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garo Kyurkchiyan S, Miroslavov Popov T,
Stancheva G, Rangachev J, Ivanov Mitev V, Petrova Popova D and
Petrova Kaneva R: Novel insights into laryngeal squamous cell
carcinoma from association study of aberrantly expressed miRNAs,
lncRNAs and clinical features in Bulgarian patients. J BUON.
25:357–366. 2020.PubMed/NCBI
|
|
27
|
Zhao J, Lv K, Li ZH, Wu J, Gao W, Wong TS,
Luo J, Qin H, Wang B, Fu Q and Lei WB: Functional significance of
the long non-coding RNA RP11-169D4.1 as a metastasis suppressor in
laryngeal squamous cell carcinoma by regulating CDH1. Oncol Rep.
38:211–220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Steuer CE, El-Deiry M, Parks JR, Higgins
KA and Saba NF: An update on larynx cancer. CA Cancer J Clin.
67:31–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anschuetz L, Shelan M, Dematté M, Schubert
AD, Giger R and Elicin O: Long-term functional outcome after
laryngeal cancer treatment. Radiat Oncol. 14:1012019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang T and Xia S: Study of the biological
function of LncRNA LUCAT1 on cervical cancer cells by targeting
miR-199b-5p. Biosci Rep. Apr 30–2020.(Epub ahead of print). doi:
10.1042/BSR20200422. View Article : Google Scholar
|
|
31
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peng WX, Koirala P and Mo YY:
LncRNA-mediated regulation of cell signaling in cancer. Oncogene.
36:5661–5667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu J, Chang WH, Fong LWR, Weiss RH, Yu SL
and Chen CH: Targeting the insulin-like growth factor-1 receptor in
MTAP-deficient renal cell carcinoma. Signal Transduct Target Ther.
4:22019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang A, Zhou N, Huang J, Liu Q, Fukuda K,
Ma D, Lu Z, Bai C, Watabe K and Mo YY: The human long non-coding
RNA-RoR is a p53 repressor in response to DNA damage. Cell Res.
23:340–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tao Y, Pinzi V, Bourhis J and Deutsch E:
Mechanisms of disease: Signaling of the insulin-like growth factor
1 receptor pathway-therapeutic perspectives in cancer. Nat Clin
Pract Oncol. 4:591–602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ueno K, Hirata H, Majid S, Yamamura S,
Shahryari V, Tabatabai ZL, Hinoda Y and Dahiya R: Tumor suppressor
microRNA-493 decreases cell motility and migration ability in human
bladder cancer cells by downregulating RhoC and FZD4. Mol Cancer
Ther. 11:244–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao L, Feng X, Song X, Zhou H, Zhao Y,
Cheng L and Jia L: miR-493-5p attenuates the invasiveness and
tumorigenicity in human breast cancer by targeting FUT4. Oncol Rep.
36:1007–1015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao J, Xu T, Wang F, Cai W and Chen L:
miR-493-5p suppresses hepatocellular carcinoma cell proliferation
through targeting GP73. Biomed Pharmacother. 90:744–751. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang Y, Zhou C, Gao Q, Yin ZQ, Wang J, Mu
H and Yan J: FAM64A promotes osteosarcoma cell growth and
metastasis and is mediated by miR-493. J Oncol. 2020:25182972020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gailhouste L, Liew LC, Yasukawa K, Hatada
I, Tanaka Y, Kato T, Nakagama H and Ochiya T: MEG3-derived
miR-493-5p overcomes the oncogenic feature of IGF2-miR-483 loss of
imprinting in hepatic cancer cells. Cell Death Dis. 10:5532019.
View Article : Google Scholar : PubMed/NCBI
|