Introduction
Stromal cell-derived factor-1 (SDF-1), also known as
C-X-C motif chemokine ligand 12 (CXCL12), is a chemokine expressed
in several types of cells and tissues (1). SDF-1 is composed of two forms, SDF-1α
and SDF-1β, by alternate splicing. SDF-1 is a well-characterized
chemokine that induces a chemotactic response in several cell
types, such as hematopoietic cells and mesenchymal stem cells
(MSCs) (1–5). For instance, SDF-1α induces focal
adhesion assembly and turnover through F-actin reorganization in
MSCs (3). Zhu et al
(4) demonstrated that icariin could
induce hypoxia-inducible factor-1α expression and regulate CXCR4
expression, leading to bone marrow stromal cell migration (4). In ectopic ossification of human spinal
ligaments, SDF-1 and its receptor promote chemotaxis in MSCs
(5).
SDF-1 also promotes neurite outgrowth and induces
neurogenesis in bone-marrow derived cells and neural stem cells
(6–8). Shyu et al (6) suggested that intracerebral
administration of SDF-1α in a rat model of stroke exerted a
neuroprotective effect by suppressing neurotoxicity. In addition,
Hu et al (3) demonstrated
that SDF-1α stimulated neuronal differentiation of MSCs by
increasing nestin and β-III tubulin expression. In a rat model of
Parkinson's disease, SDF-1, and its receptor C-X-C motif receptor 4
(CXCR4), exerted a therapeutic effect following neural stem cell
(NSC) transplantation (7).
Interestingly, previous studies have mostly focused on the
endogenous or exogenous expression of the CXCL12 gene, rather than
the SDF-1 protein it encodes. For instance, Kim et al
(9) reported that intermittent
hydrostatic pressure increased endogenous SDF-1 concentration and
promoted migration of mesenchymal stem cells (9). Moreover, endogenous SDF-1/CXCR4
expression is also significantly increased in a Parkinson's disease
rat model following NSC transplantation (7). Similarly, the migration of
transplanted MSCs in cerebral infarction can be improved by
controlling CXCL12/CXCR4 signaling (10). Sheu et al (11) reported that the administration of
SDF-1α increased the migration of CD34+ cells, which
improved peripheral nerve regeneration. Therefore, in light of
these previous findings, the aim of the present study was to
evaluate the effect of a recombinant SDF-1 protein on neuronal
differentiation.
PC-12 is a cell line derived from rat adrenal
medulla and is a mixture of neuroblastic cells and eosinophilic
cells that can differentiate into neuron-like cells (12). Thus, PC-12 cells are widely used for
neuronal differentiation studies. In neuronal differentiation
studies using PC-12 cells, nerve growth factor (NGF) and
dexamethasone are commonly used to induce neuronal differentiation.
Several other compounds have previously been reported to induce
neuronal differentiation of PC-12 cells. For example, Seow et
al (13) suggested that
6-shogaol, a compound derived from ginger (Zingiber officinale
var officinale), induced neurite outgrowth. In another study,
Momordica cochinchinensis seed displayed NGF-mimetic
activity and induced neurite outgrowth in PC-12 cells (14). Moreover, the neuronal
differentiation effect of growth factors has been extensively
studied. For instance, fibroblast growth factor (FGF)-1 and −2 also
induce neuronal differentiation in the PC-12 cell lines (15,16).
In the present study, it was hypothesized that a
recombinant SDF-1 protein might induce neuronal differentiation of
PC-12 cells. A recombinant SDF-1 protein constructed in our
previous study was isolated and purified in Escherichia coli
(E. coli) (17). Cell
toxicity and the proliferative effect of SDF-1 protein in PC-12
cells were measured. Also, the effect of SDF-1 protein on cell
migration was also measured using a wound healing assay. To
investigate the effects of recombinant SDF-1 protein on neurite
outgrowth, neurite number and length was measured. Neurite
morphology was also observed under a confocal laser scanning
microscope (CLSM) and a scanning electron microscope (SEM).
Neurofilament (NF) mRNA levels were quantified by reverse
transcription-quantitative PCR (RT-qPCR). SDF-1 protein was found
to induce neurite growth, thus indicating its potential in nerve
tissue engineering.
Materials and methods
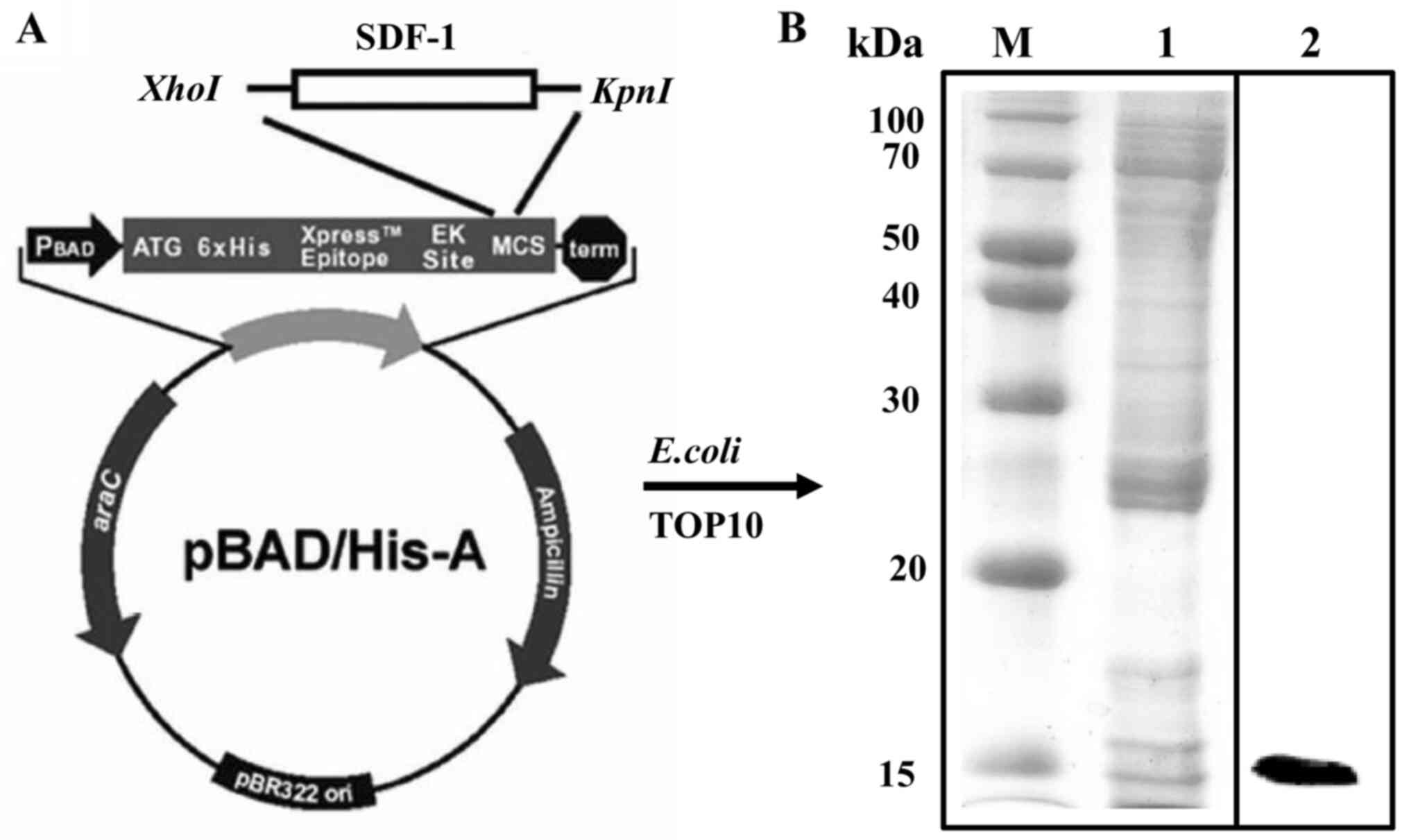
Construction of expression
plasmids
Recombinant SDF-1 protein was constructed as
described previously (17).
Briefly, the human SDF-1 sequence was amplified by PCR (Perkin
Elmer, Inc.) using primers flanked with restriction sites for
XhoI and KpnI (forward,
5′-XhoI-AACCTCGAGAGCGAT GGCAAACCGGTG-3′; reverse,
5′-KpnI-GTTGGTACCTTA TTTGTTCAGCGCT-3′). The thermocycling
conditions consisted of 30 cycles at 94°C for 1 min, 55°C for 1 min
and 72°C for 1 min. Following digestion with XhoI and
KpnI at 37°C for 60 min, ligation with the pBAD/HisA vector
at 20°C for 4 h was performed to produce the pBAD/HisA-SDF-1
construct.
Protein expression and purification of
recombinant SDF-1 protein
Ligation mixture was spread in Luria-Bertani medium
(LPS Solution Co., Ltd.) containing 100 µg/ml ampicillin after
heat-shock at 42°C for 1 min. Following bacterial transformation,
TOP10 E. coli cells (Invitrogen; Thermo Fisher Scientific,
Inc.) were cultured in Luria-Bertani medium containing 100 µg/ml
ampicillin overnight at 37°C. Once the optical density value at 600
nm reached 0.6, bacterial cells were incubated with 0.1% (w/v)
L-arabinose at 20°C for 6 h. Cells were centrifuged at 6,000 × g at
for 10 min at 4°C, then lysed (50 mM NaH2PO4,
300 mM NaCl, 10 mM imidazole, pH 8.0). After centrifugation at
14,000 × g at 4°C for 30 min, the soluble protein was purified from
the supernatant by affinity to a nickel-nitrilotriacetic acid resin
(Invitrogen; Thermo Fisher Scientific, Inc.). The purified
recombinant SDF-1 protein was analyzed via 10% SDS-PAGE followed
either by Coomassie Brilliant Blue staining or western blotting.
The protein concentrations were measured using the Bradford method
(Bio-Rad Laboratories, Inc.). The purity of SDF-1 protein was
determined by Coomassie Brilliant Blue staining at room temperature
for 30 min, and the molecular size was confirmed by western
blotting. For western blotting, SDF-1 protein (50 µg/lane) was
electrophoresed via SDS-PAGE on a 10% gel, and subsequently
transferred to nitrocellulose membrane. Following which, membranes
were blocked with 1% BSA at room temperature for 1 h, washed and
then incubated with the mouse anti-His antibody (1:1,000; cat. no.
sc-8036; Santa Cruz Biotechnology, Inc.) at 4°C overnight. The
blotted membrane was washed with Tris-buffered saline with 0.5%
Tween-20 and incubated with HRP-conjugated anti-mouse secondary IgG
(1:2,000; cat. no. sc-2371; Santa Cruz Biotechnology, Inc.) at room
temperature for 2 h. Bands were detected using ECL detection
reagent (Immobilon® ECL Ultra Western HRP Substrate; EMD
Millipore).
Cell culture
The PC-12 rat pheochromocytoma cell line was
purchased from American Type Culture Collection and cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) containing 10% heat-inactivated fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.) and 5%
penicillin-streptomycin solution in a 5% CO2 atmosphere
at 37°C.
Cell viability and proliferation
assay
Cell viability was evaluated using an MTT assay.
PC-12 cells were seeded at a density of 1×104 cells/well
and incubated for 24 h at 37°C with 5% CO2. The cells
were washed three times with PBS, then incubated for 24 h at 37°C
with 5% CO2 in the presence of 0.5, 1 and 5 µg/ml
recombinant SDF-1 protein. Untreated cells were also used as a
control. Subsequently, 500 µl MTT was added to each well and
incubated at 37°C for 4 h. Then, the media was removed and
dissolved with DMSO. Optical density at 540 nm (OD540)
was read using a microplate reader. Cell proliferation activity was
similarly evaluated. PC-12 cells (1×103 cells/well) were
incubated for 3 and 5 days with various concentration of SDF-1
protein. Cell proliferation was also measured by reading at
OD540.
In vitro wound healing assay
Cell migration was evaluated using an in vitro wound
healing assay (18). PC-12 cells
were seeded at a density of 1×106 cells/well to create a
confluent monolayer. Following overnight serum starvation at 37°C
with 5% CO2, a scratch was made using a 200 µl pipette
tip. Cells were incubated for 6 days in the presence of 5 µg/ml.
Untreated cells were used as a negative control. Cell migration was
evaluated at day 0, 2, 4 and 6 (magnification, ×10) under a CKX41
light microscope (Olympus Corporation).
Neurite outgrowth assay
PC-12 cells were seeded at a density of
1×103 cells/well and incubated with 5 µg/ml SDF-1
protein for 5 days at 37°C with 5% CO2. Untreated cells
were also used as a negative control. On the fifth day, neurite
number and length were examined under a CKX41 light microscope
(magnification, ×10). Three images per well were captured in each
three experiments. To evaluate neurite number, cell images were
captured and the neurite number in the field was counted. Neurite
number was normalized with the control and expressed as a
percentage. To evaluate neurite length, neurite with 1.5 times
longer than the cell body were scored.
CLSM analysis
Neurite morphology was examined under a CLSM. Cells
were treated as aforementioned, and subsequently fixed with 10%
formalin for 30 min and stained with Alexa Fluor 488 conjugated
phalloidin and DAPI for 30 min (Invitrogen; Thermo Fisher
Scientific, Inc.). Then, neurite morphology was observed under an
LSM700 confocal microscope (magnification, ×10; Carl Zeiss AG).
SEM analysis
Neurite morphology was also examined under SEM.
Cells were fixed with 2.5% glutaraldehyde at room temperature for
30 min, and serially dehydrated with ethanol solution (50, 70, 90,
95 and 100%) for 10 min each. Specimens were then treated with
hexamethyldisilazane (Sigma-Aldrich; Merck KGaA) and coated with
platinum (magnification, ×250).
RT-qPCR analysis
PC-12 cells were seeded at density of
1×103 cells/well and incubated for 5 days at 37°C with
5% CO2. Total RNA was extracted using an Easy-spin Total
RNA Extraction kit (Intron Biotechnology, Inc.). Then, cDNA was
synthesized at 50°C for 50 min and 85°C for 5 min using
SuperScript® IV-First Strand Synthesis System
(Invitrogen; Thermo Fisher Scientific, Inc.). The expression of the
NF gene was quantified by RT-qPCR. The reaction mixture contained
0.1 µm of each primer, 10 µl of 2X SYBR-Green PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc), including
AmpliTaq Gold™ DNA Polymerase with Buffer, dNTPs mix, SYBR-Green I
dye, Rox dye and 10 mm MgCl2, and 1 µl template cDNA.
PCR was conducted with activation (94°C for 10 min), followed by 40
cycles of denaturation (94°C for 15 sec), and annealing and
extension (60°C for 1 min). Primer sequences are listed in Table I. The Cq value for each gene was
normalized to GAPDH using the formula: dCq = Cq(GAPDH) -
Cq(NF) (19).
 | Table I.Sequences of primers used for
RT-qPCR. |
Table I.
Sequences of primers used for
RT-qPCR.
| Target gene | Sequence
(5′-3′) |
|---|
| GAPDH | F:
TGGAAGGACTCATGACCACA |
|
| R:
TTCAGCTCAGGGATGACCTT |
| NF | F:
TGGAAGGACTCATGACCACA |
|
| R:
GGAGACGTAGTTGCTGCTTCTT |
Statistical analysis
All experiments were conducted three times. Data are
expressed as the mean ± SD. Date was analyzed using Student's
t-test or one-way ANOVA followed by Duncan's multiple range test.
Statistical analysis was carried out using GraphPad Prism 7
software (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression and purification of
recombinant SDF-1 protein in E. coli
Recombinant SDF-1 protein was constructed and
purified from E. coli. The yield for purified SDF-1 protein
from a 1 L culture was 0.7 mg/ml. The molecular weight of the
purified recombinant SDF-1 protein was ~15 kDa (Fig. 1).
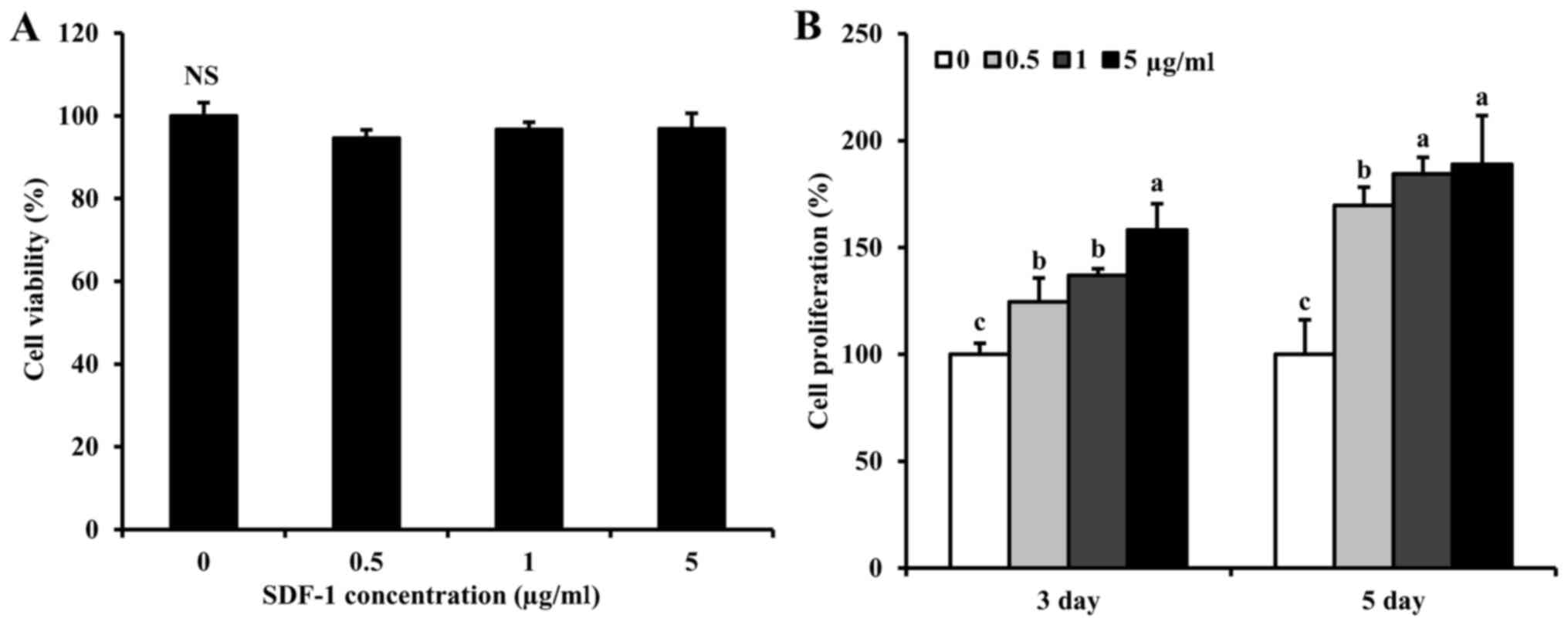
Effect of recombinant SDF-1 protein on
cell viability
Cell viability was measured to evaluate the
potential cytotoxic effects of the recombinant SDF-1 protein on
PC-12 cells. PC-12 cells incubated with SDF-1 concentrations
ranging from 0.5, 1 and 5 µg/ml retained >95% cell viability,
compared with untreated cells (Fig.
2A).
Effect of recombinant SDF-1 protein on
cell proliferation
PC-12 cell proliferation was measured after
incubation for 3 or 5 days in the presence of 0.5, 1 or 5 µg/ml
SDF-1. At both timepoints, cell proliferation significantly
increased in a dose-dependent manner (Fig. 2B). In particular, cell proliferation
in the presence of 5 µg/ml SDF-1 increased ~1.5-fold. Based on cell
viability and proliferation findings, SDF-1 was used at a
concentration of 5 µg/ml in subsequent experiments.
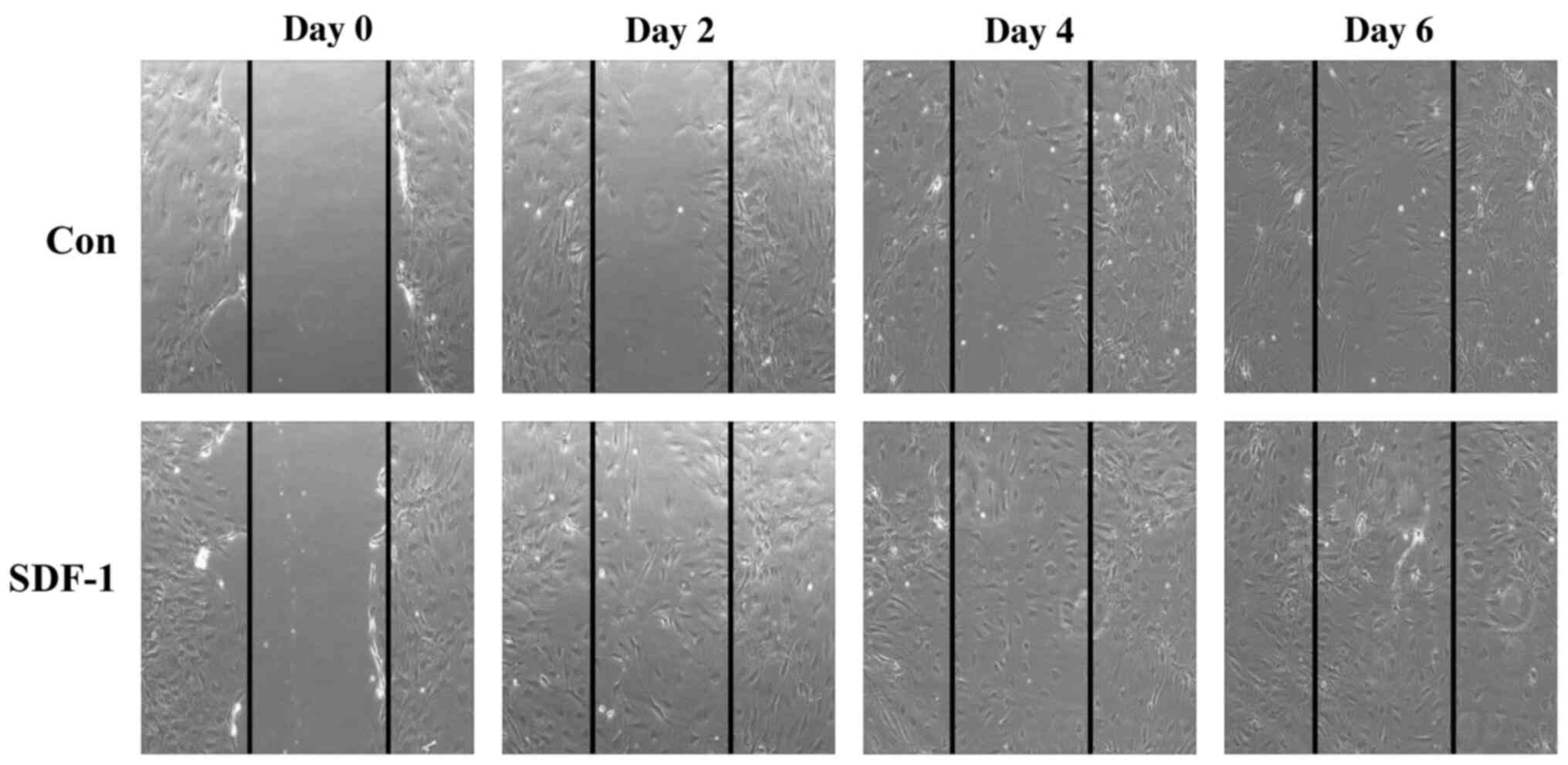
Effect of recombinant SDF-1 protein on
cell migration
Cell migration in PC-12 cells in the presence of
SDF-1 was assessed using an in vitro scratch assay over a 6-day
time course. At day 2, SDF-1 induced PC-12 cell migration from the
edge of the scratch at 2 days, compared with the initial time point
(Fig. 3). However, at day 6, cell
migration in the presence SDF-1 was similar to the control. These
results confirmed the chemoattractant effect of SDF-1 in PC-12
cells.
Effect of recombinant SDF-1 protein on
neurite outgrowth
Neurite number and length in PC-12 cells were
determined from microscopy images (Fig.
4). In all examined microscopic images, SDF-1 induced neurite
outgrowth in PC-12 cells (Fig.
4A-C). This stimulating effect of SDF-1protein on neurite
outgrowth was observed in images obtained from an optical
microscope, as well as CLSM and SEM. In addition, SDF-1
significantly increased neurite number in PC-12 cells 8.97-fold,
compared with the control (Fig.
4D). Moreover, SDF-1 also significantly increased the average
neurite length in the SDF-1-treated cells, compared with the
control (230.56±47.91 vs. 101.39±37.74 µm, respectively; Fig. 4E). Therefore, SDF-1 stimulates
neurite outgrowth in PC-12 cells. Collectively, these results
suggest that recombinant SDF-1 protein might be utilized for nerve
regeneration by inducing neurite outgrowth.
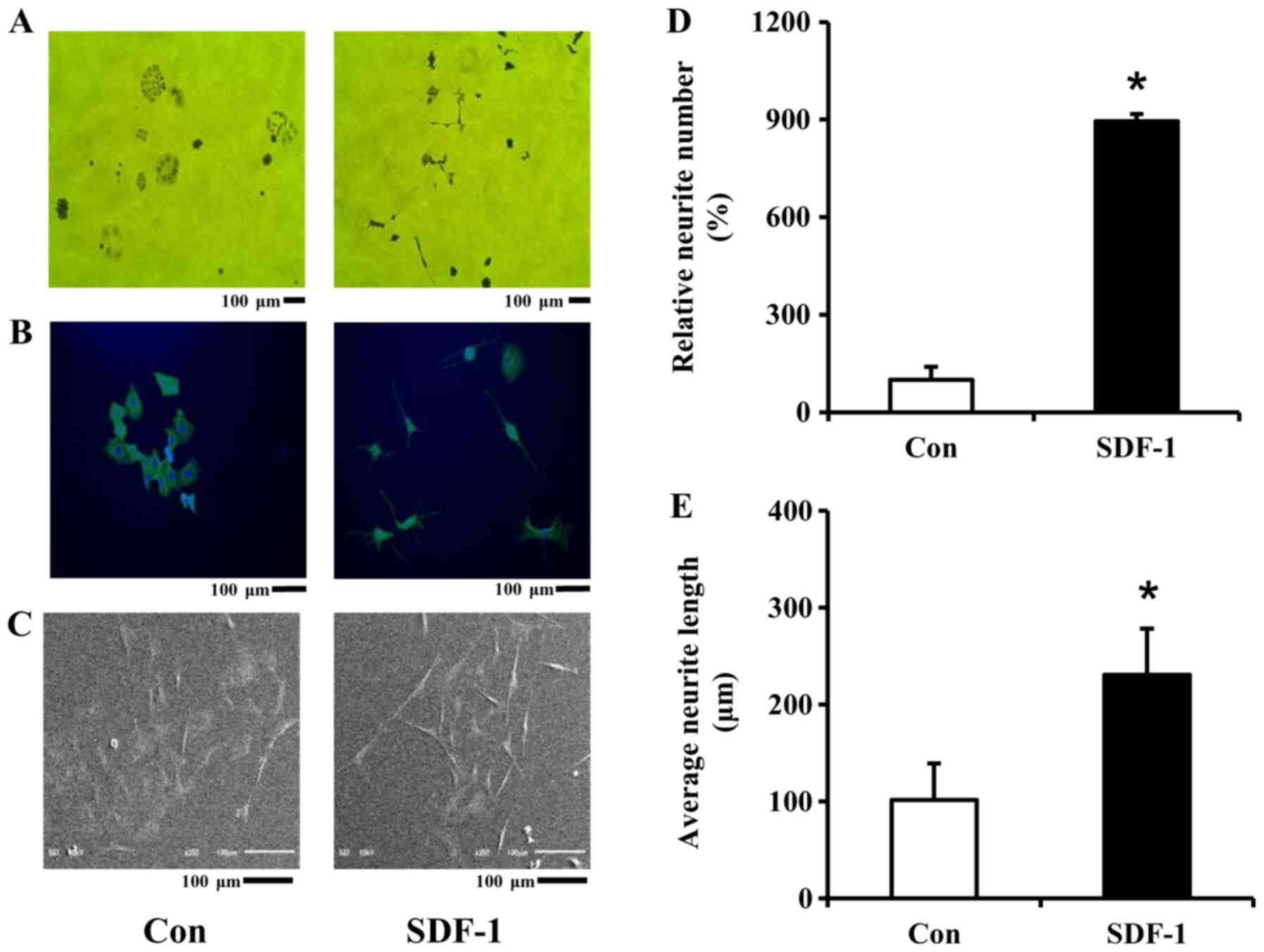 | Figure 4.Effect of SDF-1 protein on neurite
outgrowth in PC-12 cells. (A) Microscopic image (magnification,
×10; scale bar, 100 µm). (B) CLSM image (scale bar, 100 µm). (C)
SEM image (scale bar, 100 µm). (D) Relative neurite number. (E)
Average neurite length. PC-12 cells were incubated with 5 µg/ml
SDF-1 protein for 5 days at 37°C with 5% CO2. Untreated
cells were also used as a negative control. On the fifth day, each
image was obtained under a light microscope, CLSM and SEM. Data are
presented as the mean ± SD (n=3). Different lower letters indicate
significant differences between Con and SDF-1 *P<0.05. SDF-1,
stromal cell-derived factor-1; Con, control; CLSM, confocal laser
scanning microscope; SEM, scanning electron microscope. |
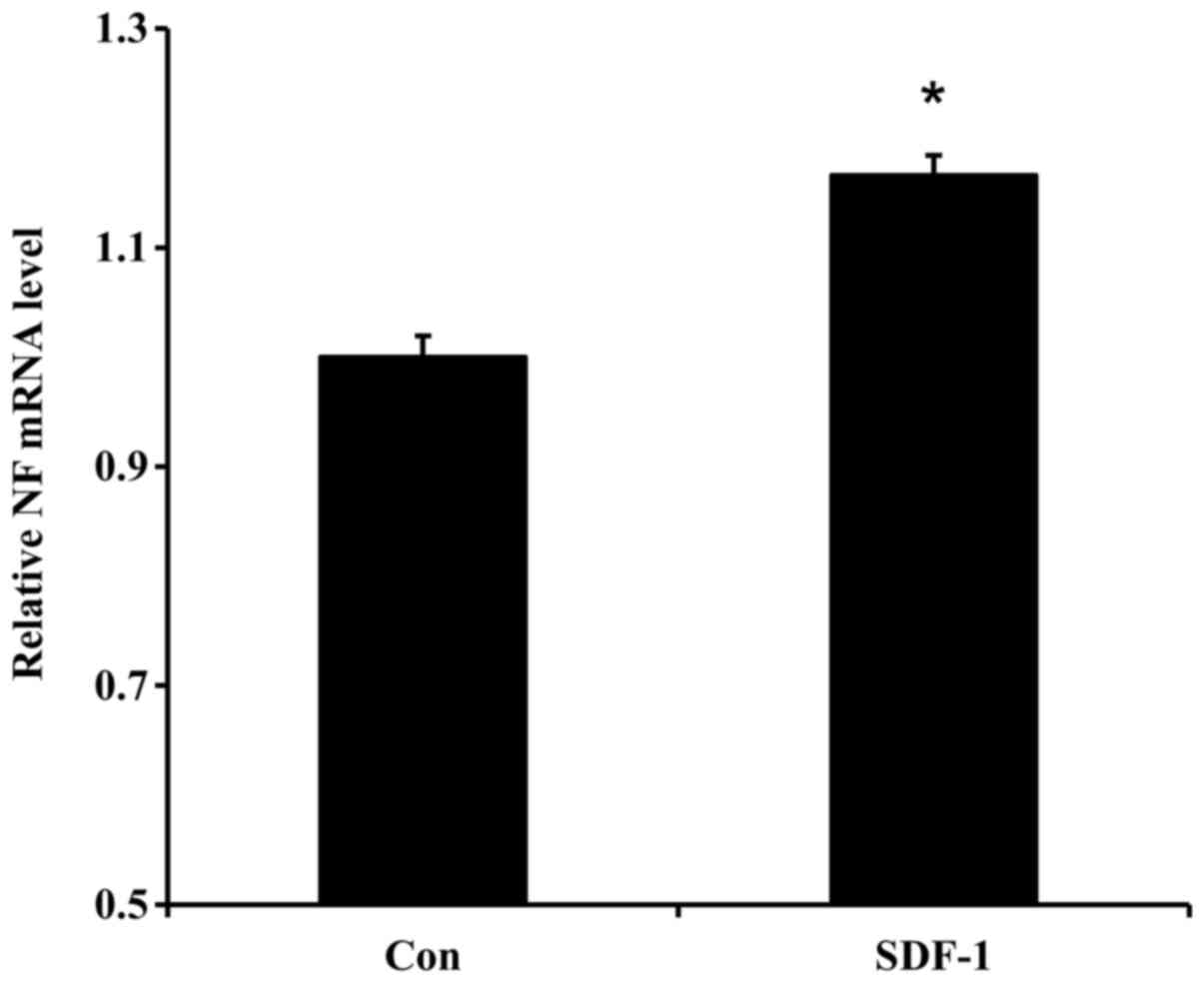
Effect of recombinant SDF-1 protein on
NF gene expression
NF gene expression levels were significantly
upregulated in PC-12 cells treated with 5 µg/ml SDF-1 protein,
compared with control cells (Fig.
5). These results suggested that SDF-1 might influence neurite
outgrowth in part by upregulating the NF gene.
Discussion
Previous studies have documented the use of the
SDF-1 gene, and recombinant SDF-1 protein is well-characterized.
Based on the chemotactic properties of SDF-1, recombinant SDF-1
protein has been extensively utilized as a therapeutic agent in
various tissue engineering studies involving the bones, nerves and
brain (20–22). In the present study, a 15-kDa
recombinant SDF-1 protein was initially purified in E. coli
in order to evaluate its effects on cell viability, proliferation,
migration and neuronal differentiation.
Cytotoxicity analysis is needed because even
purified recombinant proteins can be toxic to cells. However,
similarly engineered recombinant proteins have been demonstrated to
be nontoxic in several studies. For instance, Jo et al
(23) reported that, in the
presence of oxidative-stress, Tat-DJ-1 promoted cell survival in
HepG2 cells without cytotoxicity. Similarly, recombinant human
dentin phosphoprotein also demonstrated low toxicity in our
previous study (24). In the
present study, purified recombinant SDF-1 protein was not toxic to
PC-12 cells.
In the present study, 5 µg/ml SDF-1 significantly
increased cell proliferation. This cell proliferative effect of
SDF-1 is proposed to be due to its chemoattractant properties. Li
et al (25) demonstrated
that endothelial progenitor cells engineered to express the CXCL12
gene promoted the proliferation of oligodendrocyte progenitor cells
in a murine ischemic stroke model. Tang et al (21) also reported that recombinant human
SDF-1α enhanced MSC proliferation and recruited MSCs in injured
eyes for corneal epithelium regeneration.
As aforementioned, SDF-1 is a powerful chemokine,
which directly induces cell migration in various cell types
including NSC, MSC and so on. In our previous study, SDF-1 loaded
scaffolds significantly enhanced cell migration (17). Similarly, SDF-1 significantly
enhanced the migration of PC-12 cells in the present study. These
cell migration and recruiting activities of SDF-1 is expected to be
more effective in damaged tissue. For instance, the combination of
SDF-1α) and transforming growth factor-β1-loaded silk
fibroin-porous gelatin scaffold enhanced MSCs migration, leading to
the promotion of cartilage regeneration and repair (26).
Furthermore, recombinant SDF-1 enhanced neurite
outgrowth by increasing neurite number and length. The neuronal
differentiation effect of SDF-1 was confirmed under SEM and CLSM
examination. These results suggested that recombinant SDF-1 might
replace NGF for inducing neuronal differentiation of PC-12 cells if
comparative studies with NGF in the future were performed. Several
studies have evaluated neurite outgrowth in PC-12 cells (27–29).
For instance, Eik et al (27) reported that Lignosus
rhinocerus aqueous extract increased neurite number and length
in differentiated PC-12 cells. Similarly, Ziziphus jujube
water extract induces neurite outgrowth in PC-12 cells (28). The effect of recombinant SDF-1 on
the neuronal differentiation of PC-12 cells has not yet been
evaluated. Thus, to the best of our knowledge, the present study is
the first to report the effect of SDF-1 on PC-12 neuronal
differentiation.
To confirm the neuronal differentiation of
recombinant SDF-1 protein, NF mRNA levels were measured. NF is
typically expressed in neuronal cells, is closely involved in
neurite outgrowth, and is considered as a leading marker for
neuronal differentiation (30).
Hence, NF expression has been investigated in the neuronal
differentiation study (31–33). In the present study, SDF-1
upregulated NF expression, compared with the control. The neuronal
differentiation effect of SDF-1 on PC-12 cells is similar to that
of NGF, as it increases the number and length of neurites. NGF
binding to the tropomyosin receptor kinase A receptor activates RAS
and RAF, respectively. Subsequently, MEK and ERK are activated,
leading to neurite outgrowth of PC-12 cells (34). In a similar study, 6-shogaol
demonstrated the mimic effect of NGF in the neuronal
differentiation of PC-12 cells (13). Moreover, Yao et al (35) reported that staurosporine (a protein
kinase inhibitor) activated JNK and ERK and contributed to neurite
outgrowth in PC-12 cells. The mechanistic basis of the effects of
SDF-1 in PC-12 cells was not evaluated in the present study and
remains to be determined in the future.
In conclusion, PC-12 cells exposed to a purified
SDF-1 protein retained high cell viability at all concentrations
tested, thus demonstrating low cytotoxicity. In addition, SDF-1
increased cell proliferation and migration. SDF-1 also
significantly induced neurite growth, compared with control cells.
Thus, SDF-1 could be used in nerve tissue engineering.
Acknowledgements
Not applicable.
Funding
This work was supported by an Inha University
Research Grant (grant no. 61545; Incheon, Republic of Korea).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JHJ made substantial contributions to the conception
of the present study. YRY performed the experiments and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guyon A: CXCL12 chemokine and its
receptors as major players in the interactions between immune and
nervous systems. Front Cell Neurosci. 8:652014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takano T, Li YJ, Kukita A, Yamaza T,
Ayukawa Y, Moriyama K, Uehara N, Nomiyama H, Koyano K and Kukita T:
Mesenchymal stem cells markedly suppress inflammatory bone
destruction in rats with adjuvant-induced arthritis. Lab Invest.
94:286–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu Y, Lu J, Xu X, Lyu J and Zhang H:
Regulation of focal adhesion turnover in SDF-1α-stimulated
migration of mesenchymal stem cells in neural differentiation. Sci
Rep. 7:100132017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu H, Wang X, Han Y, Zhang W, Xin W,
Zheng X and Zhang J: Icariin promotes the migration of bone marrow
stromal cells via the SDF-1α/HIF-1α/CXCR4 pathway. Drug Des Devel
Ther. 12:4023–4031. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chin S, Furukawa KI, Kurotaki K, Nagasaki
S, Wada K, Kumagai G, Motomura S and Ishibashi Y: Facilitation of
chemotaxis activity of mesenchymal stem cells via stromal
cell-derived factor-1 and its receptor may promote ectopic
ossification of human spinal ligaments. J Pharmacol Exp Ther.
369:1–8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shyu WC, Lin SZ, Yen PS, Su CY, Chen DC,
Wang HJ and Li H: Stromal cell-derived factor-1 alpha promotes
neuroprotection, angiogenesis, and mobilization/homing of bone
marrow-derived cells in stroke rats. J Pharmacol Exp Ther.
324:834–849. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu JT, Qian Y, Wang W, Chen XX, Li Y, Li
Y, Yang ZY, Song XB, Lu D and Deng XL: Effect of stromal
cell-derived factor-1/CXCR4 axis in neural stem cell
transplantation for Parkinson's disease. Neural Regen Res.
15:112–119. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Addington CP, Pauken CM, Caplan MR and
Stabenfeldt SE: The role of SDF-1α-ECM crosstalk in determining
neural stem cell fate. Biomaterials. 35:3263–3272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SY, Park SH and Shin JW, Kang YG, Jeon
KJ, Hyun JS, Oh MJ and Shin JW: Mechanical stimulation and the
presence of neighboring cells greatly affect migration of human
mesenchymal stem cells. Biotechnol Lett. 35:1817–1822. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu Y, Chen W, Wu L, Jiang L, Qin H and
Tang N: Hypoxic preconditioning improves the survival and neural
effects of transplanted mesenchymal stem cells via CXCL12/CXCR4
signalling in a rat model of cerebral infarction. Cell Biochem
Funct. 37:504–515. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sheu ML, Cheng FC, Su HL, Chen YJ, Chen
CJ, Chiang CM, Chiu WT, Sheehan J and Pan HC: Recruitment by SDF-1α
of CD34-positive cells involved in sciatic nerve regeneration. J
Neurosurg. 116:432–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lindenbaum MH, Carbonetto S, Grosveld F,
Flavell D and Mushynski WE: Transcriptional and
post-transcriptional effects of nerve growth factor on expression
of the three neurofilament subunits in PC-12 cells. J Biol Chem.
263:5662–5667. 1988.PubMed/NCBI
|
|
13
|
Seow SLS, Hong SL, Lee GS, Malek SNA and
Sabaratnam V: 6-shogaol, a neuroactive compound of ginger (jahe
gajah) induced neuritogenic activity via NGF responsive pathways in
PC-12 cells. BMC Complement Altern Med. 17:3342017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mazzio E, Georges B, McTier O and Soliman
KF: Neurotrophic effects of Mu Bie Zi (Momordica
cochinchinensis) seed elucidated by high-throughput screening
of natural products for NGF mimetic effects in PC-12 cells.
Neurochem Res. 40:2102–2112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen JH, Lee DC and Chiu IM: Cytotoxic
effects of acrylamide in nerve growth factor or fibroblast growth
factor 1-induced neurite outgrowth in PC12 cells. Arch Toxicol.
88:769–780. 2014.PubMed/NCBI
|
|
16
|
Vergaño-Vera E, Díaz-Guerra E,
Rodríguez-Traver E, Méndez-Gómez HR, Solís Ó, Pignatelli J, Pickel
J, Lee SH, Moratalla R and Vicario-Abejón C: Nurr1 blocks the
mitogenic effect of FGF-2 and EGF, inducing olfactory bulb neural
stem cells to adopt dopaminergic and dopaminergic-GABAergic
neuronal phenotypes. Dev Neurobiol. 75:823–841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dashnyam K, Perez R, Lee EJ, Yun YR, Jang
JH, Wall IB and Kim HW: Hybrid scaffolds of gelatin-siloxane
releasing stromal derived factor-1 effective for cell recruitment.
J Biomed Mater Res A. 102:1859–1867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yun YR, Lee S, Jeon E, Kang W, Kim KH, Kim
HW and Jang JH: Fibroblast growth factor 2-functionalized collagen
matrices for skeletal muscle tissue engineering. Biotechnol Lett.
34:771–778. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun J, Mou C, Shi Q, Chen B, Hou X, Zhang
W, Li X, Zhuang Y, Shi J, Chen Y, et al: Controlled release of
collagen-binding SDF-1α from the collagen scaffold promoted tendon
regeneration in a rat Achilles tendon defect model. Biomaterials.
162:22–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang Q, Luo C, Lu B, Fu Q, Yin H, Qin Z,
Lyu D, Zhang L, Fang Z, Zhu Y, et al: Thermosensitive
chitosan-based hydrogels releasing stromal cell derived factor-1
alpha recruit MSC for corneal epithelium regeneration. Acta
Biomater. 61:101–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Z, Zhao R, Fang X, Huang Q and Liu J:
Recombinant human SDF-1α administration accelerates aneurysm neck
reendothelialization in rabbit saccular aneurysm after flow
diverter treatment. Acta Biochim Biophys Sin (Shanghai).
49:246–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jo HS, Yeo EJ, Shin MJ, Choi YJ, Yeo HJ,
Cho SB, Park JH, Lee CH, Eum WS and Choi SY: Tat-DJ-1 enhances cell
survival by inhibition of oxidative stress, NF-κB and MAPK
activation in HepG2 cells. Biotechnol Lett. 39:511–521. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yun YR, Jeon E, Lee S, Kang W, Kim SG, Kim
HW, Suh CK and Jang JH: Expression, purification, and
characterization of a dentin phosphoprotein produced by
Escherichia coli, and its odontoblastic differentiation
effects on human dental pulp cells. Protein J. 31:504–510. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Chang S, Li W, Tang G, Ma Y, Liu Y,
Yuan F, Zhang Z, Yang GY and Wang Y: cxcl12-engineered endothelial
progenitor cells enhance neurogenesis and angiogenesis after
ischemic brain injury in mice. Stem Cell Res Ther. 9:1392018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Y, Wu T, Huang S, Suen CW, Cheng X,
Li J, Hou H, She G, Zhang H, Wang H, et al: Sustained release
SDF-1α/TGF-β1-loaded silk fibroin-porous gelatin scaffold promotes
cartilage repair. ACS Appl Mater Interfaces. 11:14608–14618. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eik LF, Naidu M, David P, Wong KH, Tan YS
and Sabaratnam V: Lignosus rhinocerus (Cooke) ryvarden: A
medicinal mushroom that stimulates neurite outgrowth in PC-12
Cells. Evid Based Complement Alternat Med. 2012:3203082012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lam CT, Gong AG, Lam KY, Zhang LM, Chen
JP, Dong TT, Lin HQ and Tsim KW: Jujube-containing herbal
decoctions induce neuronal differentiation and the expression of
anti-oxidant enzymes in cultured PC12 cells. J Ethnopharmacol.
188:275–283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen J, Maiwulanjiang M, Lam KY, Zhang WL,
Zhan JY, Lam CT, Xu SL, Zhu KY, Yao P, Lau DT, et al: A
standardized extract of the fruit of Ziziphus jujuba
(Jujube) induces neuronal differentiation of cultured PC12 cells: A
signaling mediated by protein kinase A. J Agric Food Chem.
62:1890–1897. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murphy A, Breen KC, Long A, Feighery C,
Casey EB and Kelleher D: Neurofilament expression in human T
lymphocytes. Immunology. 79:167–170. 1993.PubMed/NCBI
|
|
31
|
Srivastava A, Singh S, Pandey A, Kumar D,
Rajpurohit CS, Khanna VK and Pant AB: Secretome of differentiated
PC12 cells enhances neuronal differentiation in human mesenchymal
stem cells via NGF-like mechanism. Mol Neurobiol. 55:8293–8305.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan L, Xu SL, Zhu KY, Lam KY, Xin G,
Maiwulanjiang M, Li N, Dong TT, Lin H and Tsim KW: Optimizing the
compatibility of paired-herb in an ancient Chinese herbal decoction
Kai-Xin-San in activating neurofilament expression in cultured PC12
cells. J Ethnopharmacol. 162:155–162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ernsberger U, Esposito L, Partimo S, Huber
K, Franke A, Bixby JL, Kalcheim C and Unsicker K: Expression of
neuronal markers suggests heterogeneity of chick sympathoadrenal
cells prior to invasion of the adrenal anlagen. Cell Tissue Res.
319:1–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vaudry D, Stork PJS, Lazarovici P and
Eiden LE: Signaling pathways for PC12 cell differentiation: Making
the right connections. Science. 296:1648–1649. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yao R, Yoshihara M and Osada H: Specific
activation of a c-Jun NH2-terminal kinase isoform and induction of
neurite outgrowth in PC-12 cells by staurosporine. J Biol Chem.
272:18261–18266. 1997. View Article : Google Scholar : PubMed/NCBI
|