Introduction
Preeclampsia (PE) is a heterogeneous disorder
affecting 3–5% of all pregnancies (1–3). The
disease is often diagnosed by the combination of hypertension and
proteinuria in the second half of pregnancy (4–6). PE
can cause maternal and perinatal death, and has high prevalence in
developing countries (4,7). Currently, delivering the placenta and
neonate by induced labor is a widely used strategy for the
treatment of PE patients, however the timing of delivery, which
will affect perinatal outcomes, is difficult to control (8,9).
Although evidence has demonstrated that calcium, aspirin,
antioxidants, fish oil, vitamin D and zinc, as well as a number of
other substances, play important roles in PE prevention, concrete
scientific proof is still lacking (10,11).
Previous studies have demonstrated that the formation mechanism of
PE is related to placentas (4–11) and
its dysfunction is induced by hypoxia (12). In addition, placental development is
closely associated with the growth of extravillous cytotrophoblast
(EVT) cells (13). Thus, the
pathology and therapy of PE needs to be investigated at a cellular
level.
A previous study indicated that oxidative stress
induced by hypoxia can affect EVT growth through hypoxia-inducible
factor (HIF) response (14). The
HIF transcription factor family (HIF-α and HIF-β) plays pivotal
roles in cellular adaptation, cell migration and signaling under
normoxia. HIF-1α, HIF-2α and HIF-3α are three paralogs of HIF-α in
humans (15). HIF-1α and HIF-2α are
activated in hypoxia and their activities regulate trophoblastic
transcription and promote trophoblast invasion (16). It has been hypothesized that during
hypoxia, increase of HIF-1α and decrease of placental growth factor
(an angiogenic protein) in serum and placentas of pregnant women
contributes to PE development (17). In addition, HIF-2α has been revealed
to mediate the expression of Fms like tyrosine kinase receptor
(Flt) 1, an angiogenic factor, in placental trophoblasts exposed to
hypoxia (18). However, studies
conducted on the effects of HIF-3α on EVT are limited.
The Janus kinase signal transducer and activator of
transcription (JAK/STAT) signaling pathway is involved in a number
of fundamental biological processes, including cell apoptosis,
proliferation and inflammation, and it plays an important role in
regulating metabolic homeostasis (19). The JAK/STAT pathway has also been
revealed to be associated with implantation between trophoblast
cells and receptive endometrium when human chorionic gonadotropin
secretion is reduced (20). The
intercommunication between the JAK/STAT pathway and ERK1/2 has been
demonstrated to promote the invasion of trophoblast cells (21). However, whether HIF-3α is associated
with the JAK/STAT signaling pathway in EVT remains unclear.
The present study is the first, to the best of the
authors' knowledge, demonstrating that the function of HIF-3α in PE
development was realized by mediating EVT migration and invasion
via the Flt-1/JAK/STAT pathways. The findings of the present study
improved the current understanding on the pathological mechanism of
PE and provided a novel treatment possibility for PE patients.
Materials and methods
Clinical specimen collection
Between December 2017 and December 2018, a total of
80 pregnant women (40 normal controls and 40 severe PE patients)
who attended the Affiliated Yantai Yuhuangding Hospital of Qingdao
University were enrolled. PE was diagnosed according to a previous
study (4) as follows: Systolic
blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg on
2 separate readings in gravidae, combined with proteinuria
>300 mg per day. None of the research subjects had pregnancy
complications, such as heart disease or chronic nephritis merger,
and their pregnancy was a singleton. The following data were
collected: Maternal age at delivery, numbers of primigravidae,
gestation, body mass index (BMI) of pregnant women, systolic
pressure, diastolic pressure, 24-h urinary protein and delivery
mode. No significant difference between the two groups of subjects
in age or BMI was observed. All patients had signed informed
consent and agreed that their placentas would be used for clinical
research. The clinical trial program had been reviewed and approved
by the Ethics Committee of the Affiliated Yantai Yuhuangding
Hospital of Qingdao University (YYQ2019012716). The samples
obtained were flash-frozen in liquid nitrogen and stored at −80°C
prior to the experiments.
Cell culture and cell treatment
Human EVT HTR8/SVneo cell line was purchased from
American Type Culture Collection and cultured in high-glucose
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (both from Gibco; Thermo Fisher Scientific, Inc.), 50
µg/ml penicillin and 50 µg/ml streptomycin (Target Molecule Corp.).
Under normoxia culture (Control), the cells were incubated in a
humid incubator at 37°C with 5% CO2. For hypoxia, the
cells were cultured at 37°C in an incubator with a gas mixture of
5% CO2, 2% O2 and 93% N2 for 12,
24, 48 or 72 h. In addition, HTR8/SVneo cells cultured under
hypoxia were treated by 10 mmol/l AG490 (cat. no. A4139; APExBIO)
for 12 h at 37°C to inhibit the phosphorylation of the JAK/STAT
signaling pathway of the cells cultured under hypoxia. Cells
without any treatment served as Control.
Cell transfection
The cells (104 cells/well) were cultured
to a confluence of 90% in 6-well plates before cell transfection.
Opti-MEM™ (Invitrogen; Thermo Fisher Scientific, Inc.) was used to
dilute Lipofectamine® 2000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and 2.5 µg DNA of
HIF-3α-pcDNA3.1 combinant vector (HIF-3α group) or pcDNA3.1 empty
vector (cat. no. sc-403487-HDR, Santa Cruz Biotechnology, Inc.; NC
group). The mixture of diluted DNA and diluted
Lipofectamine® 2000 (1:1 ratio) was incubated for 5 min
at room temperature and then added into each well to co-incubate
with the cells at 37°C for 48 h. Finally, transfection efficiency
was detected by reverse transcription-quantitative (RT-q) PCR.
Cells without any treatment served as Control.
RT-qPCR assay
Cells (1×104 cells/well) were cultured to
90% confluence in 6-well plates before RNA extraction. Total RNA
was extracted from the placental tissues and HTR8/SVneo cells using
TRIzol™ Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
the RNA concentration was detected by DR6000™ UV VIS
Spectrophotometer (HACH) at a wavelength of 280 nm. The first
strand of cDNAs was synthesized using TaqMan™ Reverse Transcription
Reagents (cat. no. 4304134; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions and then subjected to
real-time PCR amplification operated with TaqMan™ Universal PCR
Master Mix (cat. no. 4326708; Thermo Fisher Scientific, Inc.) in
the PCR QuantStudio™ 3 Real-Time PCR System (Thermo Fisher
Scientific, Inc.). The primers used were as follows: HIF-3α
forward, 5′-GCACCCTCAACCTCAAGGC-3′ and reverse,
5′-GCAATCCTGTCGTCACAGTAG-3′; Flt-1 forward,
5′-TGCCGGGTTACGTCACCTA-3′ and reverse,
5′-GTCCCAGATTATGCGTTTTCCAT-3′; GAPDH forward,
5′-CCATCTTCCAGGAGCGAGAT-3′ and reverse, 5′-TGCTGATGATCTTGAGGCTG-3′
(reverse). The conditions were set as follows: At 50°C for 2 min,
then at 95°C for 10 min and 40 cycles at 95°C for 15 sec and at
60°C for 1 min. The relative expression of each mRNA was calculated
by comparative quantification cycle (Cq) method 2−ΔΔCq
(22).
Western blot analysis
The protein expression levels of the treated
HTR8/SVneo cells were detected by western blot analysis. Total
proteins of the HTR8/SVneo cells were isolated by
ProteoPrep® Total Extraction Sample kit (EMD Millipore)
according to the manufacturer's instructions and protein
concentration was detected by Modified BCA Protein Assay kit
(Sangon Biotech Co., Ltd.) in DR6000™ UV VIS Spectrophotometer at a
wavelength of 562 nm. Next, 50 µg of protein samples were separated
on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE; Beijing Solarbio Science & Technology Co., Ltd.) and
then transferred on polyvinylidene fluoride membranes. The
membranes were blocked by 5% non-fat dry milk at 37°C for 1 h and
incubated together with primary antibodies HIF-3α (1:500; product
code ab10134; Abcam), Flt-1 (1:1,000; product code ab2350),
phosphorylated (p-)JAK2 (1:2,000; product code ab32101), JAK2
(1:5,000; product code ab108596), p-STAT3 (1:5,000; product code
ab76315), STAT3 (1:5,000; product code ab119352) and GAPDH
(1:20,000; product code ab8245; all from Abcam) at 4°C overnight.
GAPDH served as an internal reference. After washing the membranes
with phosphate-buffered solution three times, the membranes were
further incubated with corresponding secondary antibodies goat
anti-rabbit IgG H&L (HRP) (1:2,000; product code ab205718) and
goat anti-mouse IgG H&L (HRP) (1:2,000; product code ab205719;
both from Abcam) at 37°C for 1 h. Finally, the different protein
brands were recorded and analyzed with Bio-Rad ChemiDoc™ XRS+
System (Bio-Rad Laboratories, Inc.) with Image Lab software
(version 4.1; Bio-Rad Laboratories, Inc.).
Cell viability
Cell viability of the treated HTR8/SVneo cells was
measured by MTT assay with Cell Proliferation Kit I (Roche
Diagnostics). MTT reagent (0.5 mg/ml; 10 µl) was added to each well
and incubated at 37°C for 4 h. Next, optical density was recorded
using a microplate reader (Bio-Rad Laboratories, Inc.) at a
wavelength of 495 nm.
Flow cytometry
Cell apoptosis was detected by performing flow
cytometry using an Annexin V-FITC Apoptosis Detection kit
(Sigma-Aldrich; Merck KGaA) following the manufacturer's protocol.
HTR8/SVneo cells treated by HIF-3α and/or AG490 were suspended in
buffer solution containing Annexin V-FITC and propidium iodide (50
mg/ml) at 37°C for 1 h in the dark. Then the flow cytometer
CytoFLEX (Beckman Coulter, Inc.) and CytExpert software (version
4.1; Beckman Coulter, Inc.) was used for analysis.
Statistical analysis
All the data were presented as the mean ± standard
deviation and analyzed using SPSS software (version 13.0; SPSS,
Inc.). Chi-square test was applied for clinical sample analysis in
Table I. Statistical differences
between groups were compared using the Student's t-test or one-way
analysis of variance (ANOVA) followed by Tukey's t-test. P<0.05
was considered to indicate a statistically significant
difference.
 | Table I.Clinical data of normal and PE
pregnant women. |
Table I.
Clinical data of normal and PE
pregnant women.
| Variable | Normal, n=40 | PE, n=40 |
|---|
| Maternal age
(years) |
28.65±4.82 | 30.14±6.12 |
| Primigravida
(n) | 31 |
17a |
| Gestation
(weeks) |
39.35±1.41 |
32.46±3.54a |
| BMI (at
delivery) |
27.49±5.43 |
33.81±5.82a |
| Systolic pressure
(mm Hg) | 113.24±8.71 |
163.48±21.57a |
| Diastolic pressure
(mm Hg) |
63.73±7.47 |
107.12±13.34a |
| Urinary protein in
24 h (g) |
0.21±0.06 |
5.82±1.73a |
| Delivery method
(vaginal delivery/cesarean section) |
27/13 |
5/35a |
Results
Clinical data from normal and PE
pregnant women
Clinical data, including maternal age, the number of
primigravida, gestation before delivery, BMI at delivery, blood
pressure, urine protein and delivery mode, were collected from PE
patients and normal controls and are listed in Table I. The maternal age range in the PE
group (30.14±6.12 years old) was higher than the normal group
(28.65±4.82 years old). In addition, compared with the PE group,
primigravidae accounted for the majority of the subjects in the
normal group. Generally, normal pregnant women had a longer
gestation period but had a lower BMI than PE pregnant patients. In
addition, systolic blood pressures, diastolic blood pressures and
24-h urine protein were significantly higher among patients in the
PE group than normal controls. In addition, most of the PE gravidae
underwent a cesarean section, whereas normal pregnant women
commonly delivered vaginally.
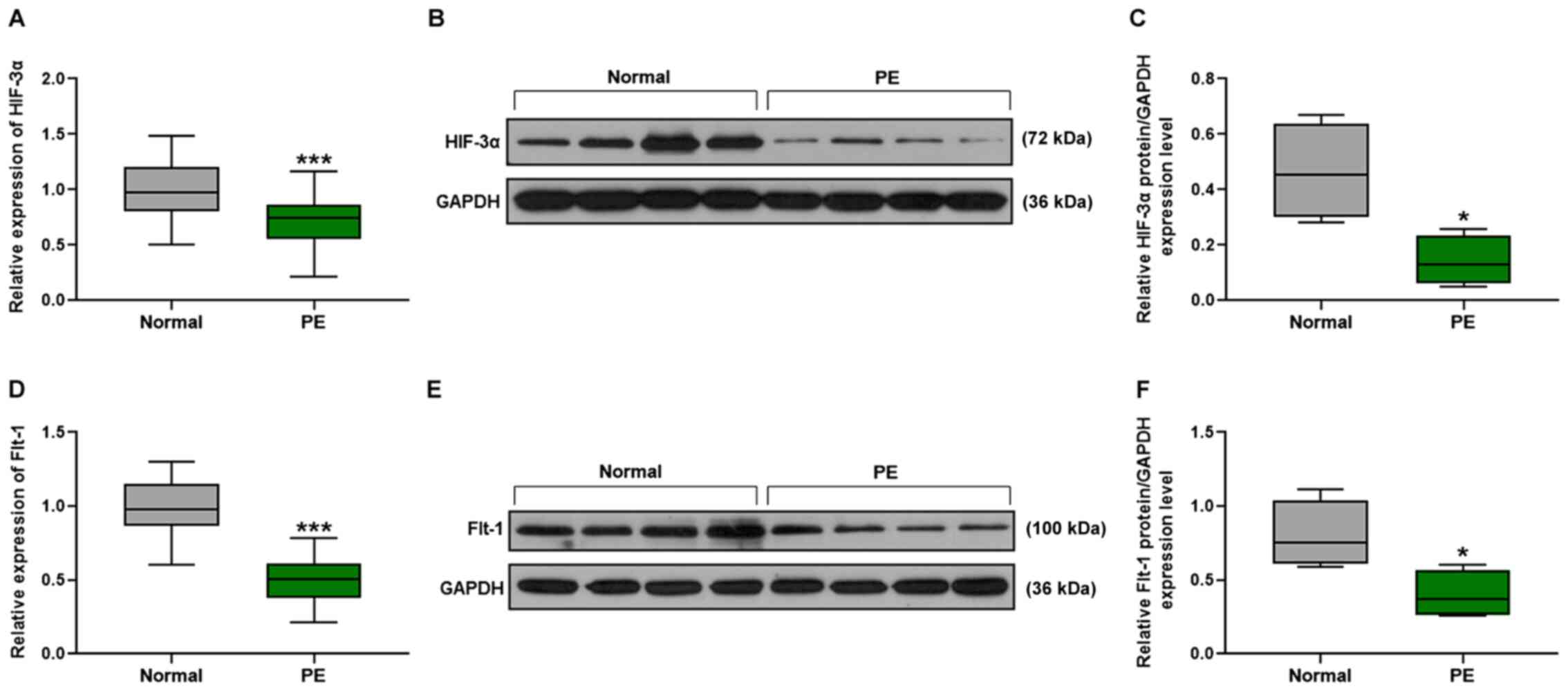
Downregulation of HIF-3α and Flt-1 in
PE patients
The mRNA expression levels of HIF-3α and
Flt-1 were detected by performing RT-qPCR. Compared with the
normal group, the mRNA expression levels of HIF-3α
(P<0.001; Fig. 1A) and
Flt-1 (P<0.001; Fig. 1D)
were significantly reduced in the PE group. Western blot analysis
was performed for the detection of the expression levels of
relative proteins and the results demonstrated that the expression
levels of the HIF-3α protein of PE pregnant woman were decreased
compared with those in normal pregnant women (P<0.05; Fig. 1B and C) and that the relative Flt-1
expression level of the PE group was reduced compared with the
normal group (P<0.05; Fig. 1E and
F).
Hypoxia affects HIF-3α and Flt-1
expression levels, the JAK/STAT3 signaling pathway, cell viability
and cell apoptosis of HTR8/SVneo cells
HTR8/SVneo cells were cultured under hypoxic
conditions for 12, 24, 48 and 72 h and the protein expression
levels were assessed by western blot analysis. The protein levels
of HIF-3α and Flt-1 of HTR8/SVneo cells were markedly increased at
12 h but reduced after 12 h. After 72-h culture in hypoxia, HIF-3α
and Flt-1 protein expression levels were even lower than those in
normoxia (P<0.05; Fig. 2A and
B). Next, RT-qPCR was conducted to detect the relative mRNA
expression levels. Similarly, in comparison with the normoxia
group, the mRNA expression levels of HIF-3α and Flt-1
were first increased at 12 h and then reduced after 12 h
(P<0.05; Fig. 2C). The protein
expression levels of the JAK/STAT signaling pathway were detected
by western blot analysis and the data demonstrated that the
relative protein expression levels of p-JAK2 and p-STAT3 were
significantly promoted at 12 and 24 h, but were suppressed at 72 h,
compared with the normoxia group, and the protein expression levels
of JAK2 and STAT3 remained unchanged over time (P<0.05; Fig. 2D and E). Thus, in hypoxia,
p-JAK2/JAK2 and p-STAT3/STAT3 were significantly increased at 12
and 24 h, but reduced at 72 h compared with those in the normoxia
group (P<0.01; Fig. 2F and G).
Then cell viability of HTR8/SVneo cells was detected by MTT and the
data demonstrated that cell activity was markedly promoted at 12 h
in hypoxia, but inhibited at 72 h compared with the normoxia group
(P<0.05; Fig. 2H). Finally, flow
cytometry was performed to detect cell apoptosis; HTR8/SVneo cell
apoptosis was not altered at 12 and 24 h, but was significantly
increased at 48 and 72 h in hypoxia (P<0.01; Fig. 2I and J).
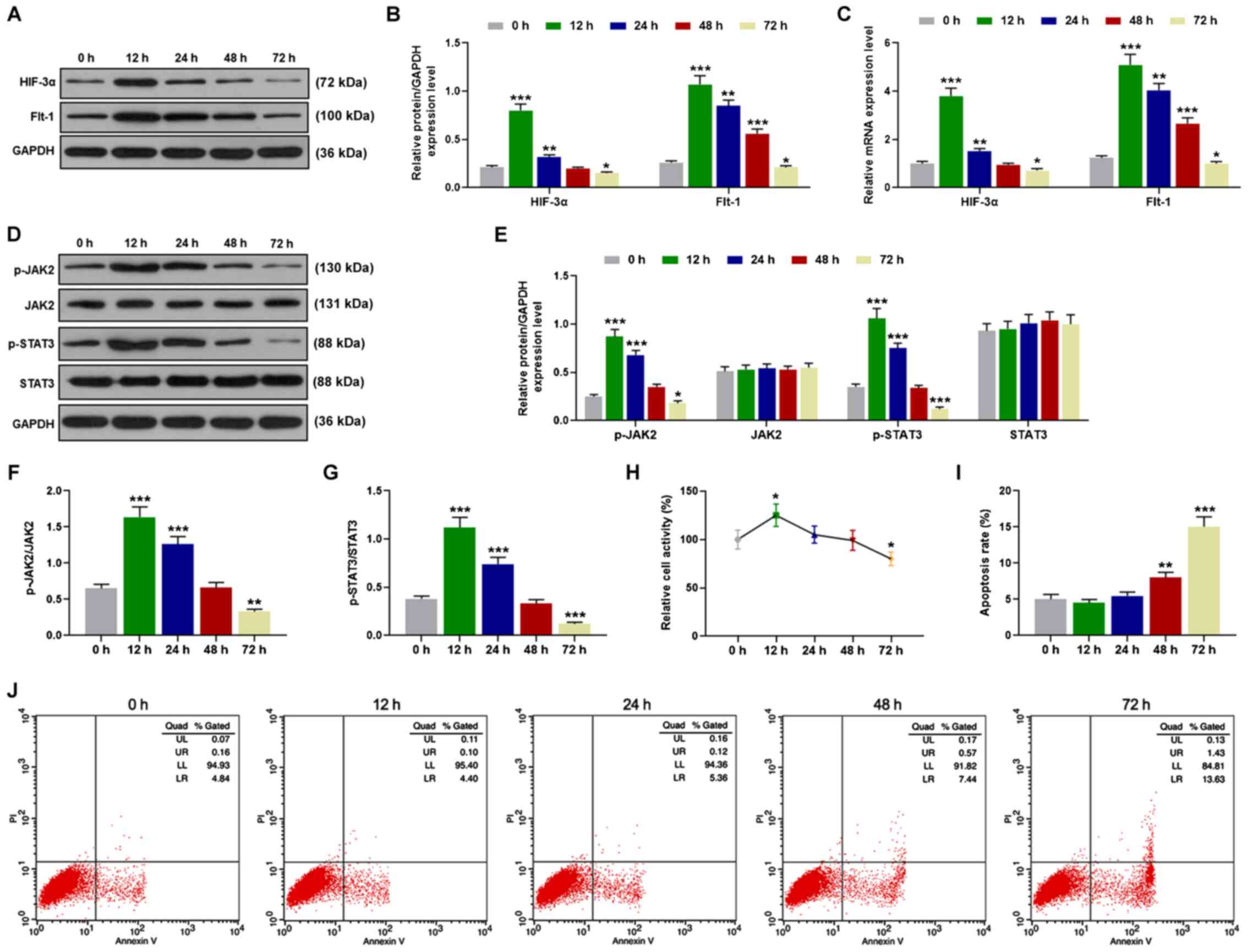 | Figure 2.Hypoxia affects HIF-3α and Flt-1
expression levels, JAK2 and STAT3 phosphorylation, and cell
viability and cell apoptosis of HTR8/SVneo cells. (A and B) Western
blot analysis detected the protein expression levels of HIF-3α and
Flt-1, followed by (C) RT-qPCR to assess the mRNA expression
levels. (D-G) The protein expression levels of p-JAK2, JAK2,
p-STAT3, STAT3, p-JAK2/JAK2 and p-STAT3/STAT3 were detected by
western blot analysis. (H) Cell activity was detected by MTT assay
and (I and J) cell apoptosis was detected by flow cytometry.
*P<0.05, **P<0.01 and ***P<0.001, vs. 0 h, n=3. HIF,
hypoxia-inducible factor; Flt, Fms like tyrosine kinase receptor;
JAK, Janus kinase; STAT, signal transducer and activator of
transcription; RT-qPCR, reverse transcription-quantitative PCR; p-,
phosphorylated. |
HIF-3α regulates cell proliferation
and cell apoptosis via upregulation of the JAK/STAT signaling
pathway of HTR8/SVneo cells
The treated HTR8/SVneo cells were cultured in
hypoxia for 12 h and then subjected to western blot analysis and
RT-qPCR. The transfection efficiency was detected by RT-qPCR and
HIF-3α was significantly over-expressed in the HIF-3α group
(P<0.01; Fig. 3A). Compared with
the control group, hypoxia significantly reduced the protein and
mRNA levels of HIF-3α and Flt-1, which were reversed by HIF-3α
overexpression. In addition, no significant effect of AG490 on
HIF-3α and Flt-1 levels was observed compared with the hypoxia +
HIF-3α group (P<0.05; Fig.
3B-D). Phosphorylation of the JAK/STAT signaling pathway was
assessed by western blot analysis and the data revealed that
chronic hypoxia and AG490 markedly inhibited the protein expression
levels of p-JAK2 and p-STAT3. However, upregulation of HIF-3α
expression significantly increased the phosphorylation of the
JAK/STAT signaling pathway by antagonizing the effect of hypoxia
(P<0.01; Fig. 3E-H). According
to the results of the MTT assay, viability of HTR8/SVneo cells was
suppressed by chronic hypoxia and AG490, but was promoted by the
increase of anti-hypoxic HIF-3α (P<0.05; Fig. 3I). Flow cytometry was performed to
assess cell apoptosis of HTR8/SVneo cells. After 72-h culture in
hypoxia and treatment with AG490, cell apoptosis of HTR8/SVneo
cells was significantly increased, however, overexpression of
HIF-3α abrogated the effects of hypoxia on HTR8/SVneo cells
(P<0.01; Fig. 3J and K).
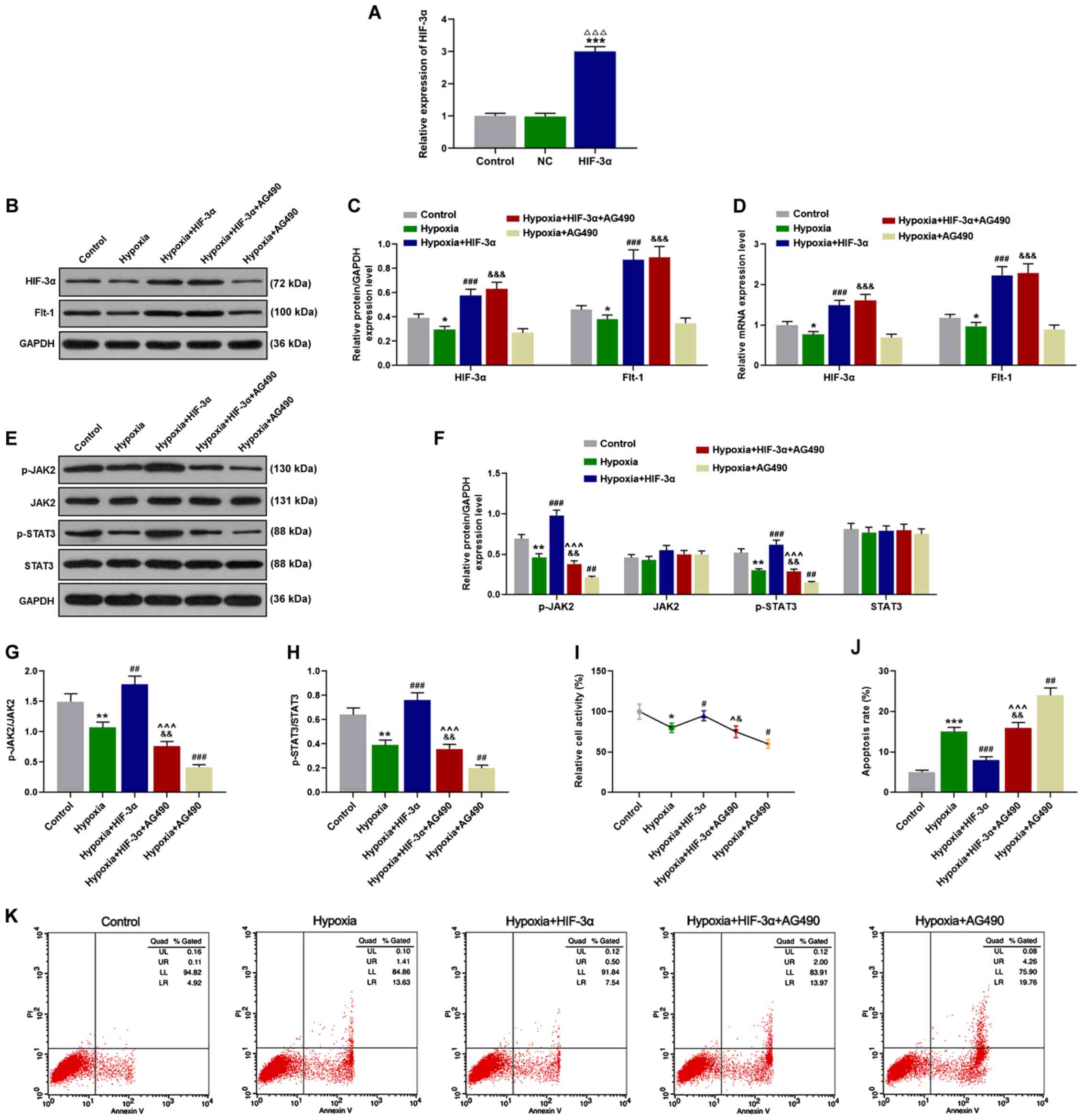 | Figure 3.HIF-3α regulates cell proliferation
and cell apoptosis via upregulation of the JAK/STAT signaling
pathway of HTR8/SVneo cells. (A) The transfection efficiency was
detected by RT-qPCR and HIF-3α was significantly overexpressed in
the HIF-3α group. Cells cultured in hypoxia for 12 h were
transfected with HIF-3α and AG490 alone or in combination, then (B
and C) western blot assays were used to detect the protein
expression levels of HIF-3α and Flt-1. (D) RT-qPCR was performed to
measure HIF-3α and Flt-1 mRNA expression levels.
(E-H) Phosphorylation of the JAK/STAT signaling pathway of the
treated cells was detected by western blot analysis (the expression
levels of p-JAK2, JAK2, p-STAT3, STAT3, p-JAK2/JAK2 and
p-STAT3/STAT3). (I) Cell viability of corresponding treated cells
was detected by MTT, followed by (J and K) flow cytometry for the
detection of cell apoptosis. *P<0.05, **P<0.01 and
***P<0.001 vs. the Control; ΔΔΔP<0.001 vs. NC;
#P<0.05, ##P<0.01 and
###P<0.001, vs. Hypoxia; &P<0.05,
&&P<0.01 and
&&&P<0.001 vs. Hypoxia+AG490;
^P<0.05 and ^^^P<0.001 vs.
Hypoxia+HIF-3α, n=3. HIF, hypoxia-inducible factor; JAK, Janus
kinase; STAT, signal transducer and activator of transcription;
RT-qPCR, reverse transcription-quantitative PCR; p-,
phosphorylated. |
Discussion
In the transcriptional response to hypoxia, the HIF
family plays key roles in the process and HIF-1α and HIF-2α are two
master regulators (15–18). Although the third paralog of HIF-α,
which is oxygen-dependent HIF-3α, can also activate transcriptional
responses to hypoxia and mediate the hypoxia-induced development
retardation, little is known about HIF-3α (23). The present study compared the
protein and mRNA expression levels of HIF-3α extracted from normal
and PE placental tissues. The results demonstrated that HIF-3α gene
expression levels of the PE group were markedly reduced, indicating
that the change of HIF-3α gene expression levels was related to PE.
Flt-1 plays a crucial role in angiopoiesis (24) and is associated with placental
degeneration and invasion (25).
Yamashita et al (25)
demonstrated that the sFLT-1 (an isoform of Flt-1) expression level
is notably high in patients with placenta previa and the study also
hypothesized that PE may be correlated with placenta previa. In the
present study, the gene expression of Flt-1 in PE samples and
normal samples was determined and the data revealed that Flt-1
expression of PE tissues was reduced. A previous study demonstrated
that suppressing HIF-2α can inhibit hypoxia-induced upregulation of
Flt-1 expression of cytotrophoblasts (18). However, whether HIF-3α can regulate
Flt-1 expression of EVT was not clarified. After the transfection
of HIF-3α into HTR8/SVneo cells in hypoxia, the present study
observed that overexpression of HIF-3α resulted in the upregulation
of Flt-1, indicating that upregulated HIF-3α had regulatory effects
on Flt-1 expression.
It has previously been revealed that in hypoxia,
HIF-3α protein and mRNA levels are increased in cultured human lung
epithelial cells based on the protein stability and transcriptional
activation (26). In addition,
HIF-3α expression was increased in the Caki-1 renal carcinoma cells
in response to hypoxia treatment (27). However, in the present study,
HTR8/SVneo cells were cultured in hypoxia and the gene expression
of HIF-3α was significantly increased at 12 h but reduced after 12
h. At 72 h, HIF-3α and Flt-1 gene expression levels were inhibited
compared with the normoxia group. With regard to Flt-1, it has been
demonstrated that the overexpression of soluble Flt-1 exhibited a
cytotoxic effect on BeWo choriocarcinoma cells (28). Flt-1 has been revealed to be
expressed at a higher level in advanced colorectal cancer cells
compared with localized ones and to play an important role in
colorectal cancer progression (29). However, the present study found that
in hypoxia, Flt-1 gene expression was increased at 12 h and then
reduced.
JAK proteins are activated by intracellular
receptors through autophosphorylation, then further phosphorylate
and promote STAT proteins; JAK in combination with STAT proteins
creates a high degree of specificity (19). For instance, the inhibition of JAK1
and JAK2 activity reduced STAT3 phosphorylation and translocation
in prostatic cancer cells (30);
inhibiting miR-210 resulted in the promotion of cell apoptosis and
the suppression of cell proliferation of vascular endothelial cells
via blocking of the JAK/STAT signaling pathway (31); the activation of the JAK/STAT
signaling pathway contributed to the proliferation, migration and
invasion of glioma cells (32).
Nevertheless, in the present study, p-JAK2 and p-STAT3 protein
expression levels were first increased at 12 h and then reduced at
72 h in hypoxia compared with in normoxia and in addition, the cell
viability of HTR8/SVneo cells fluctuated with phosphorylation of
the JAK/STAT signaling pathway, while cell apoptosis of EVT was
steadily increased over time.
To prevent the JAK/STAT signaling pathway from
phosphorylation, AG490 was used to inhibit the activities of JAK2
and STAT3 according to previous studies (32,33).
HTR8/SVneo cells were transfected with HIF-3α and AG490 alone or in
combination and cultured in hypoxia. Chronic hypoxia culture (72-h
hypoxia) significantly reduced the protein and mRNA expression
levels of HIF-3α and Flt-1 as well as the expression levels of
p-JAK2 and p-STAT3, indicating that the phosphorylation of JAK/STAT
was inhibited. AG490 did not affect HIF-3α and Flt-1 gene
expression levels, but it markedly inhibited the protein expression
levels of p-JAK2 and p-STAT3. By upregulating HIF-3α expression
levels in vitro, the HIF-3α and Flt-1 expression levels were
markedly increased and phosphorylation of JAK/STAT was promoted,
suggesting that the abilities of migration and invasion of
HTR8/SVneo cells were increased. In addition, AG490-reduced
viability and AG490-promoted apoptosis in chronic hypoxia were
markedly abrogated by the upregulation of HIF-3α expression. All
these results indicated that HIF-3α overexpression had regulatory
effects on the Flt-1/JAK/STAT pathway in EVT HTR8/SVneo cells.
In conclusion, HIF-3α may be a regulator in the
growth of EVT HTR8/SVneo cells in hypoxia. Upregulation of HIF-3α
promoted the Flt-1 expression and phosphorylation levels of JAK2
and STAT3. In addition, the effects of hypoxia on inhibiting cell
viability and promoting cell apoptosis were reversed to a large
extent. Thus, the present study concluded that HIF-3α affected PE
development by regulating EVT growth via upregulation of the
Flt-1/JAK/STAT signaling pathway in hypoxia. The limitation of the
present study was the small clinical sample size, which should be
improved in future research.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HQ, QY and BJ made substantial contributions to the
conception and design of the study. WZ, YZ and LM performed data
acquisition, data analysis and interpretation. HQ, QY and BJ
drafted the article or critically revised it for important
intellectual content. All authors agreed to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of the work are appropriately investigated
and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. All patients had signed informed consent and agreed that
their placentas would be used for clinical research. The clinical
trial program had been reviewed and approved by the Ethics
Committee of the Affiliated Yantai Yuhuangding Hospital of Qingdao
University (YYQ2019012716).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karumanchi SA and Granger JP: Preeclampsia
and pregnancy-related hypertensive disorders. Hypertension.
67:238–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Sayed AAF: Preeclampsia: A review of
the pathogenesis and possible management strategies based on its
pathophysiological derangements. Taiwan J Obstet Gynecol.
56:593–598. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park JL, Lee SW, Oh KJ and Hong JS: High
mean blood pressure during the first trimester is predictive of
future preeclampsia development in healthy pregnant women: A cohort
study in Korea. Clin Exp Obstet Gynecol. 46:770–775. 2019.
|
|
4
|
Mol BWJ, Roberts CT, Thangaratinam S,
Magee LA, de Groot CJM and Hofmeyr GJ: Pre-eclampsia. Lancet.
387:999–1011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morton A: Imitators of preeclampsia: A
review. Pregnancy Hypertens. 6:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramos JGL, Sass N and Costa SHM:
Preeclampsia. Rev Bras Ginecol Obstet. 39:496–512. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malik A, Jee B and Gupta SK: Preeclampsia:
Disease biology and burden, its management strategies with
reference to India. Pregnancy Hypertens. 15:23–31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bokslag A, van Weissenbruch M, Mol BW and
de Groot CJ: Preeclampsia; short and long-term consequences for
mother and neonate. Early Hum Dev. 102:47–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steegers EA, von Dadelszen P, Duvekot JJ
and Pijnenborg R: Pre-eclampsia. Lancet. 376:631–644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Atallah A, Lecarpentier E, Goffinet F,
Doret-Dion M, Gaucherand P and Tsatsaris V: Aspirin for prevention
of preeclampsia. Drugs. 77:1819–1831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Correa PJ, Palmeiro Y, Soto MJ, Ugarte C
and Illanes SE: Etiopathogenesis, prediction, and prevention of
preeclampsia. Hypertens Pregnancy. 35:280–294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Patot MC, Ebensperger G, Gassmann M
and Llanos AJ: The hypoxic placenta. High Alt Med Biol. 13:176–184.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gude NM, Roberts CT, Kalionis B and King
RG: Growth and function of the normal human placenta. Thromb Res.
114:397–407. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Macklin PS, McAuliffe J, Pugh CW and
Yamamoto A: Hypoxia and HIF pathway in cancer and the placenta.
Placenta. 56:8–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Graham AM and Presnell JS: Hypoxia
Inducible Factor (HIF) transcription factor family expansion,
diversification, divergence and selection in eukaryotes. PLoS One.
12:e01795452017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Highet AR, Khoda SM, Buckberry S, Leemaqz
S, Bianco- Miotto T, Harrington E, Ricciardelli C and Roberts CT:
Hypoxia induced HIF-1/HIF-2 activity alters trophoblast
transcriptional regulation and promotes invasion. Eur J Cell Biol.
94:589–602. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rath G, Aggarwal R, Jawanjal P, Tripathi R
and Batra A: HIF-1 alpha and placental growth factor in pregnancies
complicated with preeclampsia: A qualitative and quantitative
analysis. J Clin Lab Anal. 30:75–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sasagawa T, Nagamatsu T, Morita K, Mimura
N, Iriyama T, Fujii T and Shibuya M: HIF-2α, but not HIF-1α,
mediates hypoxia-induced up-regulation of Flt-1 gene expression in
placental trophoblasts. Sci Rep. 8:173752018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dodington DW, Desai HR and Woo M:
JAK/STAT-emerging players in metabolism. Trends Endocrinol Metab.
29:55–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta SK, Malhotra SS, Malik A, Verma S
and Chaudhary P: Cell signaling pathways involved during invasion
and syncytialization of trophoblast cells. Am J Reprod Immunol.
75:361–371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Malik A, Pal R and Gupta SK:
Interdependence of JAK-STAT and MAPK signaling pathways during
EGF-mediated HTR-8/SVneo cell invasion. PLoS One. 12:e01782692017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guénin S, Mauriat M, Pelloux J, Van
Wuytswinkel O, Bellini C and Gutierrez L: Normalization of qRT-PCR
data: The necessity of adopting a systematic, experimental
conditions-specific, validation of references. J Exp Bot.
60:487–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang P, Yao Q, Lu L, Li Y, Chen PJ and
Duan C: Hypoxia-inducible factor 3 is an oxygen-dependent
transcription activator and regulates a distinct transcriptional
response to hypoxia. Cell Rep. 6:1110–1121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chappell JC, Cluceru JG, Nesmith JE,
Mouillesseaux KP, Bradley VB, Hartland CM, Hashambhoy-Ramsay YL,
Walpole J, Peirce SM, Mac Gabhann F and Bautch VL: Flt-1 (VEGFR-1)
coordinates discrete stages of blood vessel formation. Cardiovasc
Res. 111:84–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamashita M, Kumasawa K, Nakamura H and
Kimura T: Soluble FLT-1 rules placental destiny. Biochem Biophys
Res Commun. 496:1243–1249. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li QF, Wang XR, Yang YW and Lin H: Hypoxia
upregulates hypoxia inducible factor (HIF)-3alpha expression in
lung epithelial cells: Characterization and comparison with
HIF-1alpha. Cell Res. 16:548–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanaka T, Wiesener M, Bernhardt W, Eckardt
KU and Warnecke C: The human HIF (hypoxia-inducible factor)-3alpha
gene is a HIF-1 target gene and may modulate hypoxic gene
induction. Biochem J. 424:143–151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamashita M, Kumasawa K, Miyake T,
Nakamura H and Kimura T: Soluble Flt-1 has cytotoxic effects on
BeWo choriocarcinoma cells. Reprod Sci. 25:830–836. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei SC, Tsao PN, Weng MT, Cao Z and Wong
JM: Flt-1 in colorectal cancer cells is required for the tumor
invasive effect of placental growth factor through a p38-MMP9
pathway. J Biomed Sci. 20:392013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pencik J, Pham HT, Schmoellerl J, Javaheri
T, Schlederer M, Culig Z, Merkel O, Moriggl R, Grebien F and Kenner
L: JAK-STAT signaling in cancer: From cytokines to non-coding
genome. Cytokine. 87:26–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yue JN, Li WM, Hong WZ, Yang J, Zhu T,
Fang Y and Fu WG: MiR-210 inhibits apoptosis of vascular
endothelial cells via JAK-STAT in arteriosclerosis obliterans. Eur
Rev Med Pharmacol Sci. 23 (Suppl 3):S319–S326. 2019.
|
|
32
|
Senft C, Priester M, Polacin M, Schroder
K, Seifert V, Kogel D and Weissenberger J: Inhibition of the
JAK-2/STAT3 signaling pathway impedes the migratory and invasive
potential of human glioblastoma cells. J Neurooncol. 101:393–403.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jane EP, Premkumar DR and Pollack IF:
AG490 influences UCN-01-induced cytotoxicity in glioma cells in a
p53-dependent fashion, correlating with effects on BAX cleavage and
BAD phosphorylation. Cancer Lett. 257:36–46. 2007. View Article : Google Scholar : PubMed/NCBI
|