Introduction
Myocardial cells have limited proliferation, renewal
and repair capabilities, and the loss of myocardial cells followed
by replacement with fibrous tissue is of clinical importance to
ventricular dysfunction and the progression of heart failure
(1). In the past decade, necrosis
has attracted a large amount of attention and has been recognized
as a highly regulated process. Programmed necrosis includes
necroptosis, pyroptosis, ferroptosis and mitochondrial permeability
transition-dependent necrosis (2).
Previous studies have confirmed that necroptosis plays an important
role in the pathogenesis of heart disease, including myocardial
infarction (MI), ischemia/reperfusion and heart failure (3). However, whether necroptosis takes part
in angiotensin II (Ang II)-induced cardiotoxicity is currently
unclear.
Klotho was identified in 1997 as a gene encoding a
novel antiaging protein that is associated with several aging
phenotypes (4). In recent years,
increasing evidence has indicated that Klotho contributes to the
pathophysiology of aging-related disorders, including diabetes,
cancer, arteriosclerosis and chronic kidney disease (5). Guo et al (6) have recently reported that the Klotho
protein plays a protective role through the inactivation of
reactive oxygen species (ROS) and nuclear factor κB
(NF-κB)-mediated inflammation in hyperglycemia-induced injury in
vitro and in vivo (6).
This effect may be attributed to the anti-inflammatory effect of
Klotho, which may also infer that Klotho has an inhibitory effect
on necroptosis. A previous study concluded that Klotho protein
effectively blocked necroptosis, which may be relevant to the
inhibition of oxidative stress to protect tubular epithelial cells
from renal ischemic-reperfusion injury (IRI) (7). Hence, it is necessary to determine
whether Klotho has cardioprotective effects by suppressing
necroptosis.
The present study aimed to investigate the influence
of the anti-inflammatory drug Klotho on Ang II-induced
cardiotoxicity and examine the cellular mechanisms by which Klotho
inhibits necroptosis.
Materials and methods
Cell culture and treatments
Rat ventricular H9c2 cells were obtained from The
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences and cultured at 37°C in a 5% CO2 humidified
incubator using DMEM supplemented with 10% fetal bovine serum, 100
mg/ml streptomycin, and 100 IU/ml penicillin (all HyClone; Cytiva).
H9c2 cells were subjected to serum starvation for 24 h before each
experiment. Ang II (Sigma-Aldrich, Merck KGaA) was incubated with
H9c2 cells for 24 h based on the results of cell viability analysis
(8). Furthermore, in order to
evaluate the role of TLR4/NF-κB p65/necroptosis pathway in the Ang
II-induced injury and inflammation, H9c2 cells were pre-treated
with 25 µmol/l PDTC (Beyotime Institute of Biotechnology) for 1 h
before exposure to Ang II for 24 h, or co-treated with 30 µmol/l
TAK-242 (InvivoGen) or 10 mmol/l Nec-1 (Sigma-Aldrich; Merck KGaA)
and Ang II for 24 h.
Cell viability analysis
The Cell Counting Kit-8 (CCK8) assay (Beijing
Solarbio Science & Technology, Co., Ltd.) was used to evaluate
cell viability. H9c2 cells were seeded into 96-well plates at a
density of 5×103 cells/well. First, cells were incubated
with 0.001 mM Ang II alone or in combination with soluble Klotho
protein (200 ng/ml) (Cloud-Clone Corp.) or Nec-1 (10 mmol/l). Then,
the cells were incubated with 10 µl WST-8 solution for 2 h at 37°C.
The PowerWave XS Microplate Reader (BioTek Instruments, Inc.) was
used to record the absorbance at 450 nm.
Cell viability was also analyzed by staining with
Propidium Iodide ReadyProbes Reagent (Thermo Fisher Scientific,
Inc.) following the protocol previously described in detail
(9). A BD FACSCalibur flow
cytometer (BD Biosciences) was used to detect treated cells and
data was analyzed using FlowJo software (Treestar Inc.). Cells
positive for propidium iodide were defined as dead cells.
Detection of changes in reactive
oxygen species (ROS) in H9c2 cells with a dihydroethidium
(DHE)-derived fluorescence probe
The intracellular ROS assay used depends on the
fluorescent signal of 2,7-dichlorodihydrofluorescein diacetate
(DCFH-DA; Beyotime Institute of Biotechnology), which is a
cell-permeable indicator of ROS. Logarithmic phase H9c2 cells were
harvested and seeded into 6-well plates at a concentration of
105 cells/well. The cells were cultured for 24 h at 37°C
before the experimental treatments. Following treatment, the
culture medium was removed and PBS was used to wash three times.
Subsequently 10 µmol/l DHE was added. The H9c2 cells were incubated
for 30 min at 37°C and viewed under a fluorescence microscope
(magnification, ×20; excitation, 494 nm; emission, 518 nm; Olympus
Corporation). The fluorescence images were analyzed using
Image-Pro® Plus 6 software (Media Cybernetics, Inc.).
Relative fluorescence intensity was used to express the
results.
Mitochondrial membrane potential (ΔΨm)
and apoptosis detection
ΔΨm and apoptosis were measured with a Mitochondrial
Membrane Potential and Apoptosis Detection kit with Mito-Tracker
Red CMXRos and Annexin V-FITC (Beyotime Institute of
Biotechnology). H9c2 cells were incubated in 6-well plates and
incubated with 2 ml culture medium overnight. Then, the H9c2 cells
were serum-starved at 37°C for 24 h and incubated with or without
Ang II (1 µmol/l), Klotho (200 ng/ml) and Nec-1 (10 mmol/l) for 24
h at 37°C. Then, the plates were washed using PBS. After replacing
the culture with 188 µl Annexin V-FITC combination solution, 5 µl
Annexin V-FITC, 2 µl Mito-Tracker Red CMXRos staining solution and
5 µl Hoechst 33342, the plates were incubated at room temperature
for 30 min. Fluorescence microscopy (Leica Microsystems GmbH) was
used to observe H9c2 cells (magnification, ×20).
ELISA to detect serum tumor necrosis
factor (TNF)-α and interleukin (IL)-1β expression
In order to determine whether Klotho pretreatment
affects expression levels of TNF-α and IL-1β induced by Ang II, a
dose gradient of Klotho was used (25–200 ng/ml). The supernatant
from each treatment group was collected and an ELISA kit was used
to measure the levels of TNF-α and IL-1β. After incubation at room
temperature for 30 min, the ELISA kits (TNF-α, cat. no. RK00029;
IL-1β, cat. no. RK00009; ABclonal Biotech Co., Ltd.) were used
according to the manufacturer's instructions. The absorbance
measurements were collected at 450 nm using a microplate reader,
and a standard curve was prepared to calculate the concentration of
TNF-α and IL-1β according to the absorbance of each sample.
Western blot analysis
Cold radioimmunoprecipitation assay (RIPA) lysis
buffer (Beyotime Institute of Biotechnology) was used to lyse the
H9c2 cells according to standard protocols and the Pierce BCA
Protein Assay kit (Beyotime Institute of Biotechnology) was used to
measure protein concentration. A variety of SDS-PAGE (8–12%) was
used to separate 30 mg protein per well. Polyvinylidene difluoride
membranes were used to transfer the protein from the gels. Next the
membranes were blocked using 5% skimmed milk at room temperature
for 2 h and then the membranes were incubated with the following
primary antibodies (all 1:1,000) overnight at 4°C:
Receptor-interacting protein kinase 3 (RIP3; cat. no. A5431),
mixed-lineage kinase domain-like protein (MLKL; cat. no. A17312)
both from ABclonal, phosphate-NF-κB p65 (cat. no. sc-136548), NF-κB
p65 (cat. no. sc-8008) both from Santa Cruz Biotechnology, Inc.,
TLR4 (cat. no. A5258; ABclonal) and β-actin (cat. no. AC026) both
from ABclonal. The membranes were incubated with the corresponding
horseradish peroxidase-conjugated secondary antibodies (ABclonal)
for 2 h and the positive signals were detected at room temperature.
Enhanced chemiluminescence (ECL) western blotting substrate (Thermo
Fisher Scientific, Inc.) was used to visualize the protein bands,
and ImageJ 1.47 software (National Institutes of Health) was used
to semi-quantify the intensity.
Statistical analysis
The mean ± SD was used to express the experimental
results and one-way ANOVA with post hoc comparisons using the
Bonferroni test were used to analyze the differences between
groups. SPSS 22.0 statistical software (IBM Corp.) was used to
perform all statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of Klotho protein on the
viability of Ang II-induced H9c2 cells
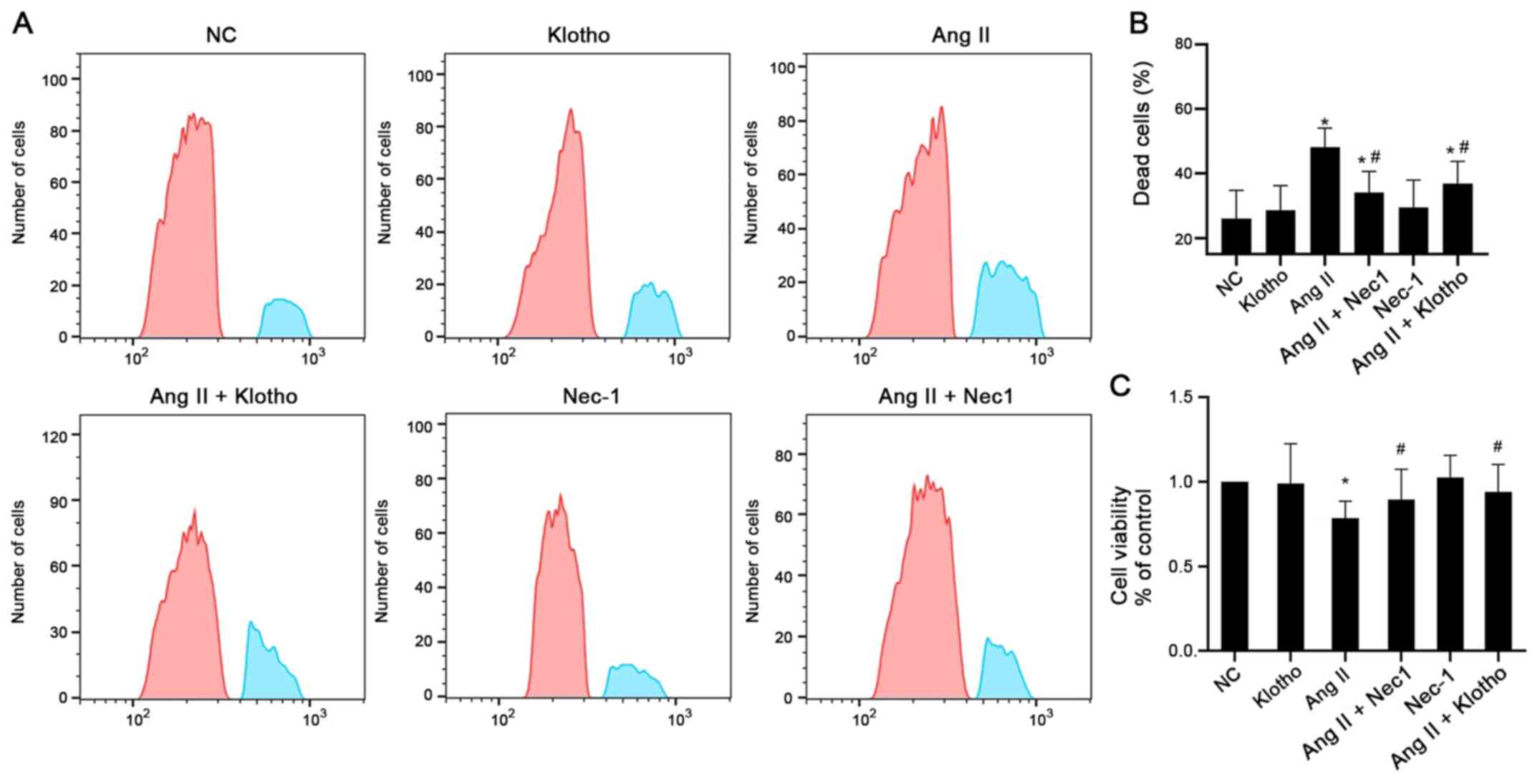
The cell viability was determined by flow cytometry
and CCK-8 assays, as presented in Fig.
1A and B. The results of the CCK-8 assay demonstrated that
pretreatment with Klotho protein before treating with Ang II
significantly increased the viability of H9c2 cells compared with
Ang II alone (Fig. 1A and C). The
cell viability of H9c2 cells in the Ang II-treated group was
significantly decreased compared with that of the control group
(P<0.05). The cell viability of the group treated with Nec-1 (10
mmol/l) together with Ang II was significantly increased compared
with that of the Ang II-only group (P<0.05). These results
indicated that Klotho protein and the necroptosis antagonist Nec-1
enhanced the viability of H9c2 cells after Ang II treatment
(Fig. 1A and C). The flow
cytometric results also confirmed that Ang II-treated H9c2 cells
had a significantly higher proportion of dead cells than the
control cells, whereas both Klotho and Nec-1 pretreatment partially
alleviated the increased number of dead cells (Fig. 1B).
Inflammatory factor expression in Ang
II-treated H9c2 cells and Klotho protein-pretreated cells
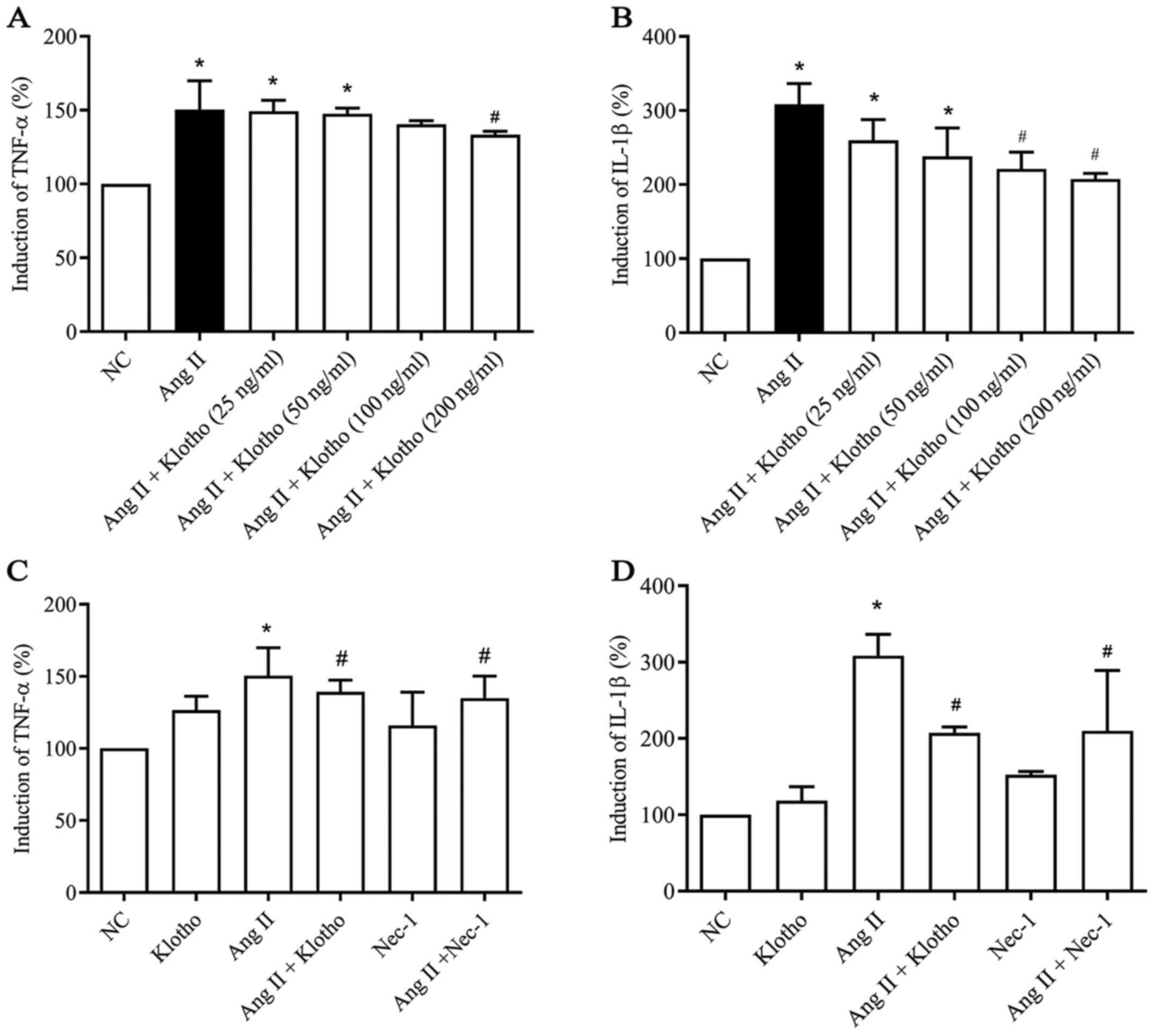
To evaluate whether Klotho protein has a
dose-dependent anti-inflammatory effect, a dose gradient of Klotho
was established (Fig. 2A and B).
The data indicated a dose-dependent effect of Klotho protein on the
concentration of TNF-α and IL-1β secreted by Ang II-treated H9c2
cells. ELISA was used to assess the quantity of TNF-α (Fig. 2A) and IL-1β (Fig. 2B) secreted into the supernatant of
cultured H9c2 cells in each group. The secreted levels of TNF-α and
IL-1β in the H9c2 cell supernatant were significantly increased
(P<0.05) following treatment with Ang II compared with the
negative control group. Furthermore, compared with Ang II
treatment, Klotho protein treatment gradually decreased the
quantity of secreted TNF-α (Fig.
2A) and IL-1β (Fig. 2B) content
in the supernatant in a concentration-dependent manner, which
indicated a concentration-dependent anti-inflammatory effect of
Klotho protein on H9c2 cells (P<0.05). Klotho alleviated
inflammation in the Ang II-induced H9c2 cells at >200 ng/ml in
TNF-α and 100 ng/ml in Il-1β (Fig. 2A
and B). Therefore, 200 ng/ml was selected as the effective
concentration for subsequent experiments. Furthermore, the effect
of Klotho and Nec-1 pretreatment on Ang II-induced H9c2 was
analyzed (Fig. 2C and D). The
content of TNF-α and IL-1β in the supernatant was also
significantly decreased in the Ang II + Nec-1-treated group
compared with the group treated with Ang II alone (Fig. 2C and 2D; P<0.05).
Effect of treatment with Klotho
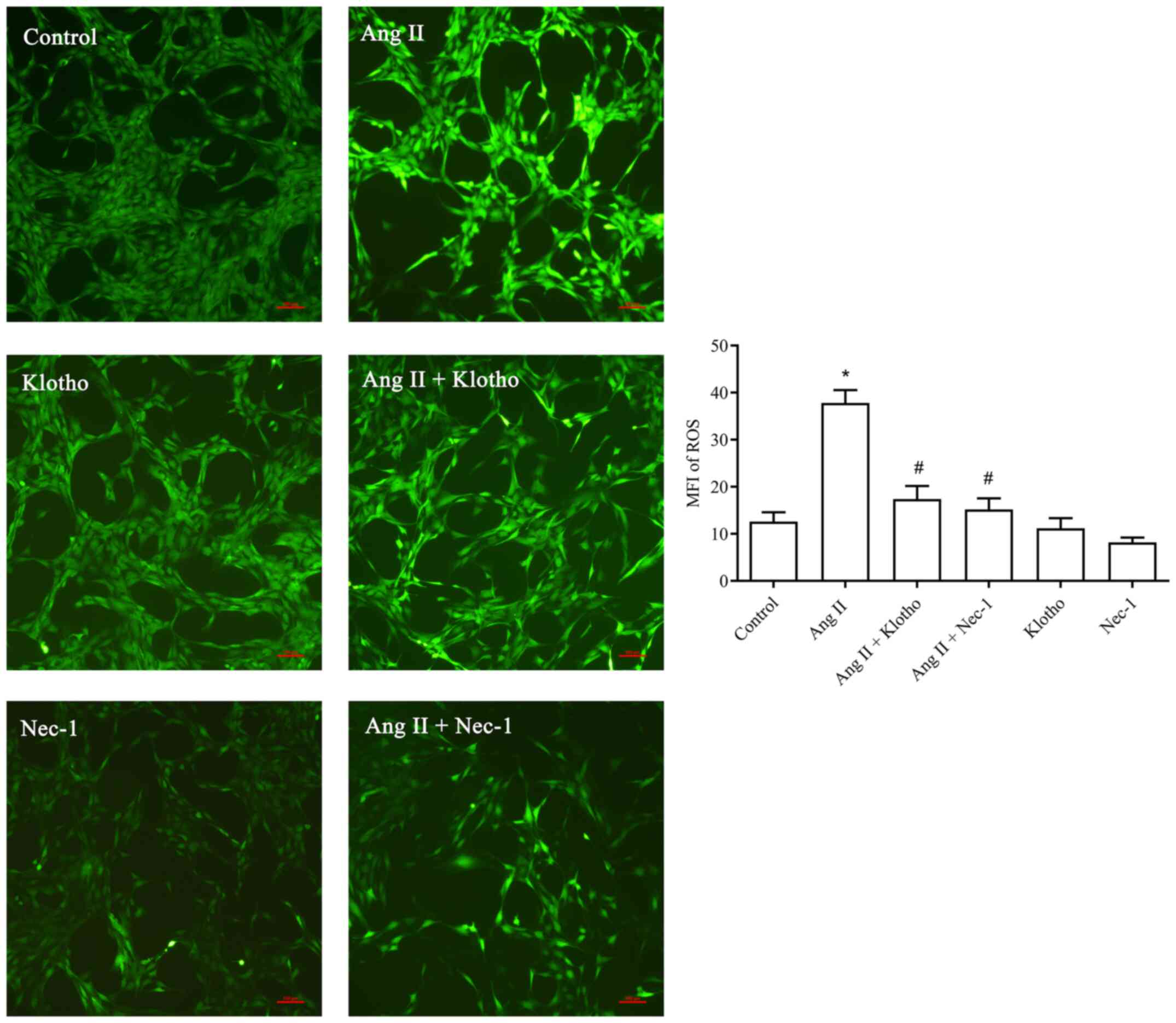
protein on ROS in H9c2 cells after Ang II treatment
The fluorescence probe detection (Fig. 3) revealed that significantly more
ROS were produced in the Ang II-treated group compared with the
control group (P<0.05). Furthermore, the concentration of ROS in
the Ang II + Klotho-treated group was significantly decreased
(P<0.05). Compared with the Ang II-treated group, the Ang II +
Nec-1-treated group (10 mmol/l) exhibited a significantly decreased
ROS content. Klotho protein reduced the production of ROS in H9c2
cells after Ang II-induced oxidative stress, and Nec-1 also served
to inhibit this effect.
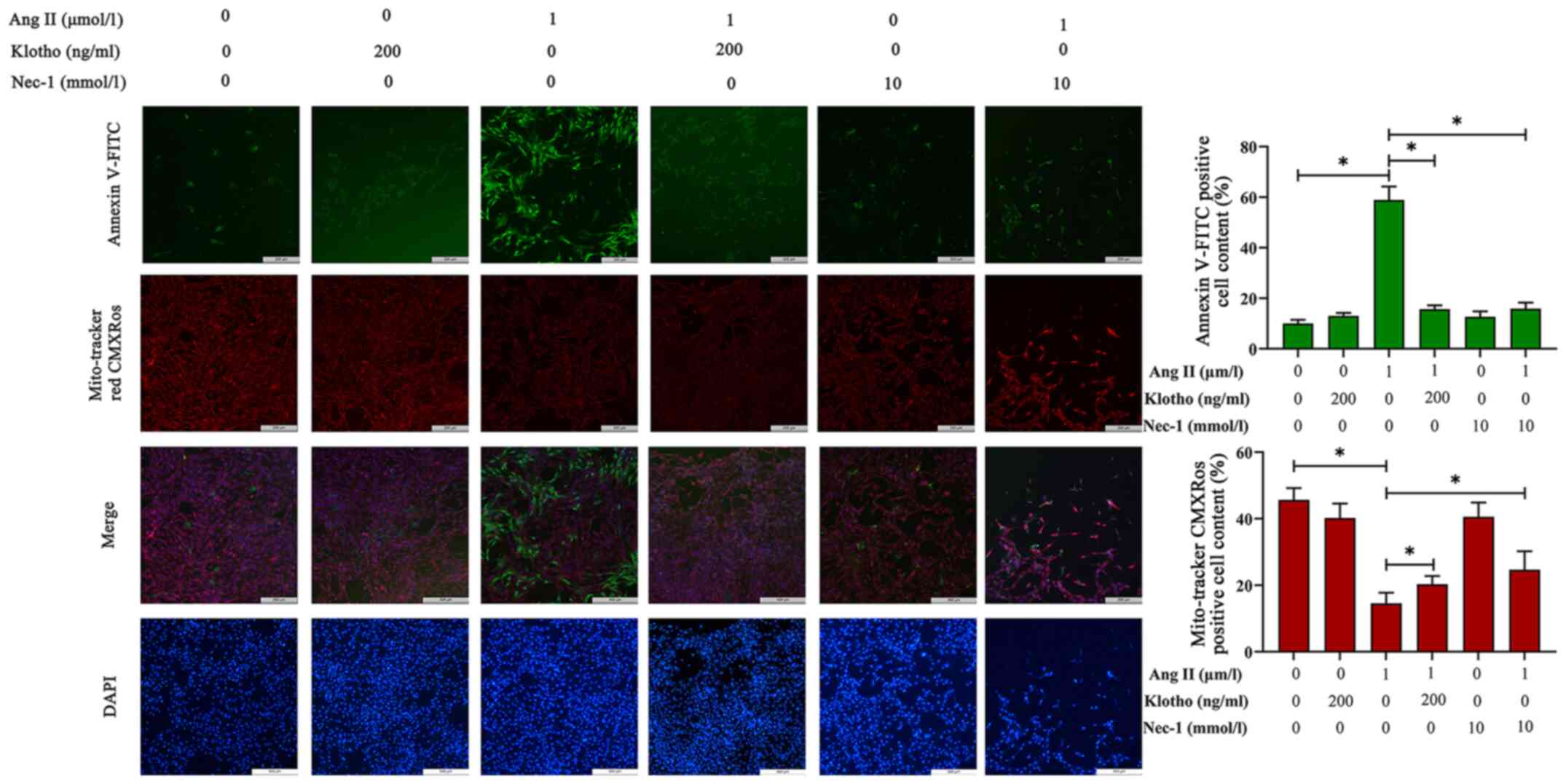
Effects of Klotho protein on H9c2 cell
apoptosis after Ang II treatment
The loss of ΔΨm was used to infer mitochondrial
damage in H9c2 cells pre-treated with Ang II for 24 h, and the
results are presented in Fig. 4.
MitoTracker Red CMXRos can label the functional mitochondria. In
normal cells, MitoTracker Red CMXRos labels mitochondria red, and
while in apoptotic cells, the loss of ΔΨm results in decreased or
even no red fluorescence. Compared with the control group, the Ang
II-treated group exhibited notably decreased red fluorescence, and
pretreatment with either Klotho or Nec-1 blocked this decrease and
proportionally recovered the ΔΨm.
Ang II promotes the expression of
components of the TLR4/NF-κB p65 pathway and increases necroptosis
in H9c2 cells
To estimate the effects of Ang II on TLR4, p-NF-κB
p65, RIP3 and MLKL expression levels in H9c2 cells, initially, a
time-response experiment was performed where the cells were exposed
to Ang II for 0, 6, 12 and 24 h. As revealed in Fig. 5, after exposure to Ang II for 6 h,
the protein expression levels of TLR4 and p-NF-κB p65 gradually
increased, reaching the maximum level at 24 h. Similarly, the
expression levels of RIP3 and MLKL also significantly increased,
the maximum increase in the expression levels observed in this
assay was after exposure to Ang II for 6 h (MLKL) and 24 h (RIP3)
(Fig. 5A). Then, a dose-response
experiment was conducted. Ang II (0.1, 0.01 and 0.001 mM) was
incubated with the cells for 24 h (Fig.
5B). The results demonstrated that incubation of the cells with
0.01 mM Ang II had the most significant effect on the protein
expression levels of TLR4, RIP3 and MLKL. These results confirmed
that Ang II treatment resulted in the activation of the TLR4
pathway and in necroptosis in H9c2 cardiac cells.
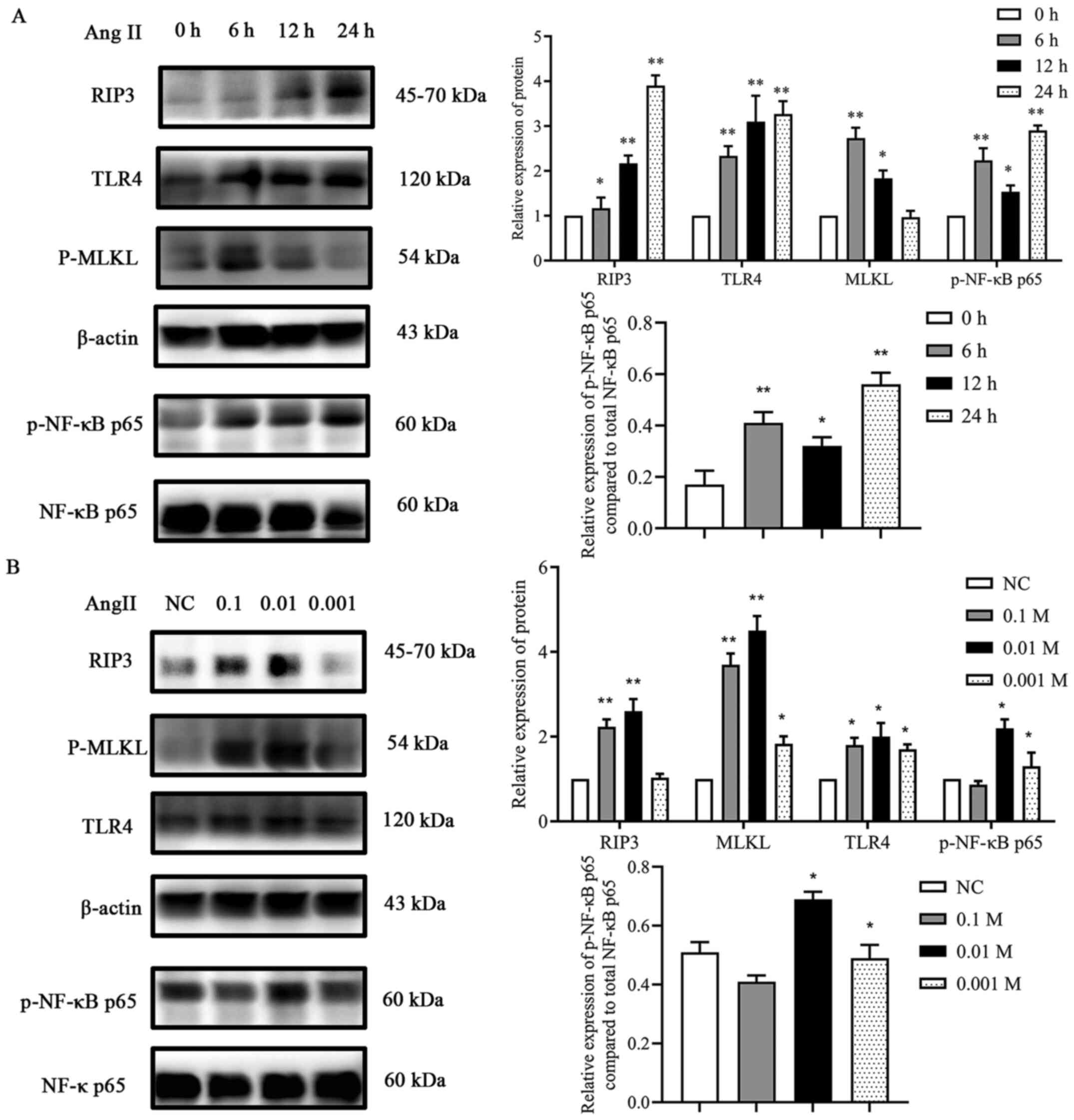 | Figure 5.Effect of Ang II on proteins involved
in necroptosis. (A) Effects of various incubation times of Ang II
on RIP3, TLR4, MLKL and p-NF-κB p65 expression. *P<0.05,
**P<0.01 vs. 0 h. (B) Effects of various concentrations of Ang
II on RIP3, TLR4, MLKL and p-NF-κB p65 expression. *P<0.05,
**P<0.01 vs. NC. Data are expressed as the mean ± SD. n=3. Ang
II, angiotensin II; RIP3, receptor-interacting protein kinase 3;
TLR4, toll-like receptor 4; MLKL, mixed-lineage kinase domain-like
protein; p-, phosphorylated; NF-κB, nuclear factor-κB; NC, negative
control. |
TLR4/NF-κB p65 pathway participates in
the activation of necroptosis in Ang II-induced H9c2 cells
First, H9c2 cells were incubated with or without Ang
II. Ang II was revealed to induce TLR4 protein expression in a
time- and dose-dependent manner (Fig.
5). Next, TLR4 expression was inhibited by using the TLR4
inhibitor TAK-242 and then the cells were cultured with Ang II for
24 h. When compared with the NC group, the TAK-242-treated group
exhibited no difference in the total NF-κB p65 expression, however,
following treatment with Ang II, p-NF-κB p65 expression and
necroptosis-related protein expression levels were significantly
reduced (P<0.05; Fig. 6A). To
further confirm the role of NF-κB p65 in necroptosis in Ang
II-conditioned H9c2 cells, H9c2 cells were preincubated with the
NF-κB p65 inhibitor PDTC (10).
H9c2 cells were pretreated with PDTC at a concentration of 25
µmol/l for 1 h at 37°C and treated with or without Ang II for 24 h.
The data in Fig. 6B inferred that
incubating H9c2 cells with PDTC significantly decreased the
expression of the Ang II-induced necroptosis marker genes RIP3 and
MLKL (P<0.05), whereas PDTC alone had no significant effect on
the basal expression levels. Collectively, the data indicated that
the inhibition of NF-κB p65 activity can reduce the expression of
proteins involved in necroptosis in H9c2 cells grown in the
presence of Ang II in vitro.
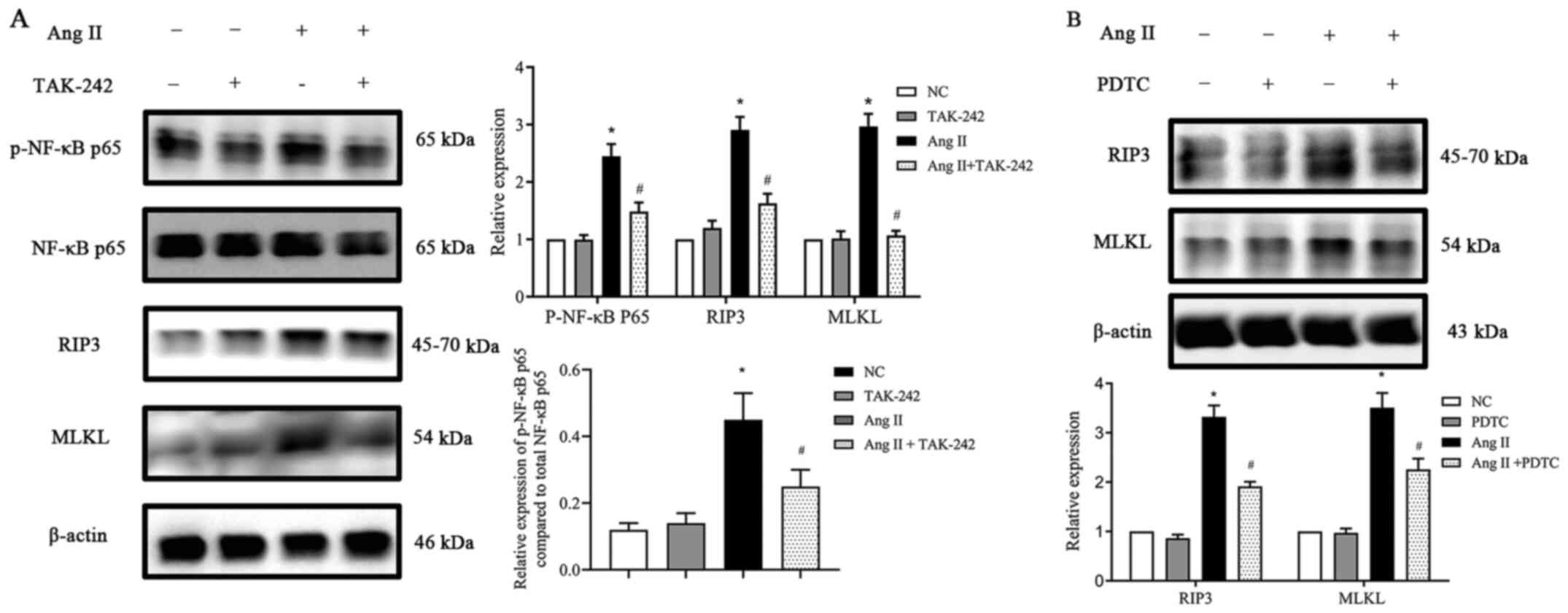 | Figure 6.Effect of TLR4 and NF-κB inhibitors on
RIP3 and MLKL expression (A) TLR4 inhibitor TAK-242 suppressed
p-NF-κB p65, RIP3 and MLKL protein expression upregulation,
stimulated by Ang II in H9c2 cells. (B) NF-κB p65 inhibitor PDTC
suppressed RIP3 and MLKL protein expression upregulation,
stimulated by Ang II in H9c2 cells. Data are expressed as the mean
± SD. n=3. *P<0.05 vs. NC group, #P<0.05 vs. the
Ang II-treated group. TLR4, toll-like receptor 4; NF-κB, nuclear
factor-κB; RIP3, receptor-interacting protein kinase 3; MLKL,
mixed-lineage kinase domain-like protein; p-, phosphorylated; Ang
II, angiotensin II; PDTC, pyrrolidine dithiocarbamate; NC, negative
control. |
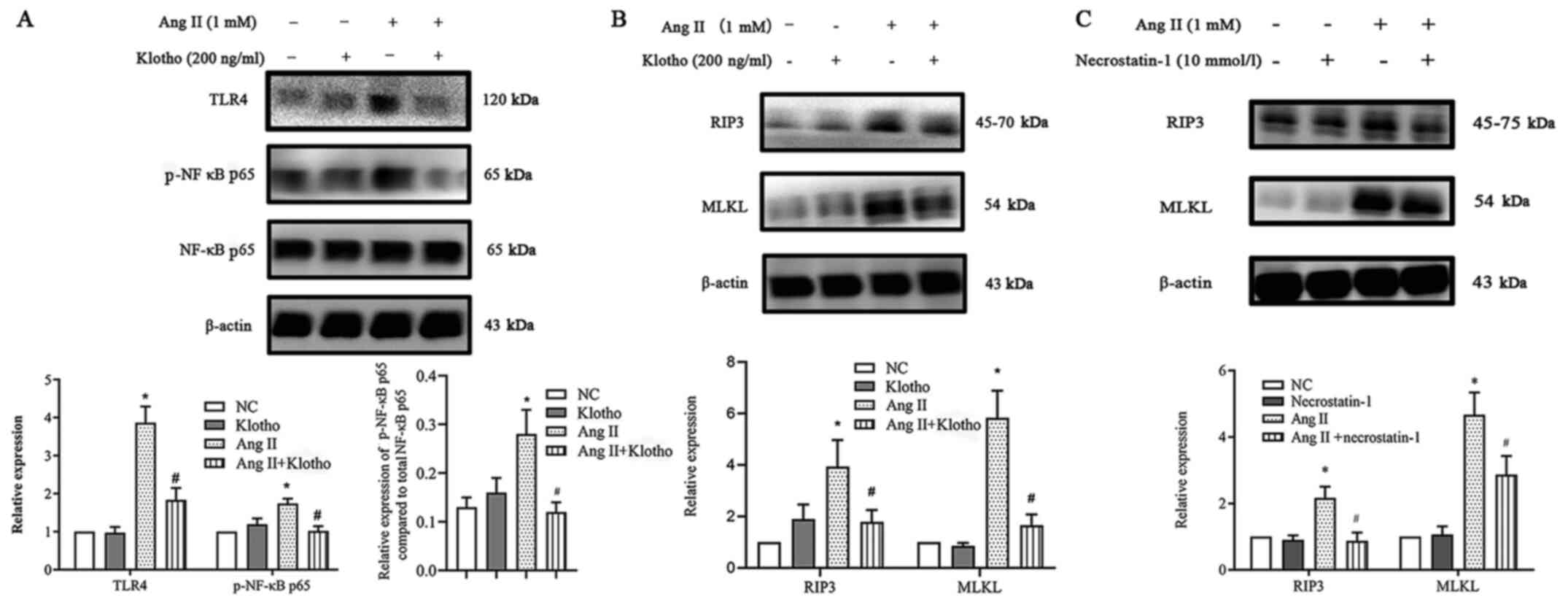
Klotho suppresses necroptosis via the
TLR4/NF-κB p65 pathway, which is induced by Ang II in H9c2
cells
The present research demonstrated that Ang II
upregulated ROS production and necroptosis by promoting the
TLR4/NF-κB p65 pathway. Notably, Klotho negatively regulated the
NF-κB p65 pathway, which is an important molecule in cellular
necroptosis (11). It was
hypothesized that Klotho could regulate the process by which the
NF-κB p65 pathway increases he expression of necroptosis in H9c2
cells treated with Ang II in vitro. To determine whether
Klotho regulated the expression of the TLR4/NF-κB p65 pathway in
Ang II-treated H9c2 cells, H9c2 cells were pretreated with 200 ng/l
Klotho for 1 h before Ang II treatment and were co-treated for 24
h. Western blot analysis revealed that exogenously added Klotho
significantly reduced the Ang II-stimulated expression of TLR4 and
phosphorylation of NF-κB p65 (Fig.
7A). This finding indicated that Klotho restrained the
upregulation of TLR4/NF-κB p65 pathway activation induced by Ang
II. Subsequently, the study aimed to determine whether both Klotho
protein and Nec-1 have an effect on necroptosis. It was revealed
that pretreatment with either Klotho or Nec-1 could alleviate the
increase in the expression of necroptosis gene markers (Fig. 7B and C). Hence, it was concluded
that the Klotho protein may somehow alleviate necroptosis in Ang
II-induced H9c2 cells through the suppression of the TLR4/NF-κB P65
pathway.
Discussion
The present study demonstrated that Ang II could
induce cardiotoxicity through necroptosis, and that Klotho protein
is capable of attenuating Ang II-induced necroptotic cardiotoxicity
via the TLR4/NF-κB p65 pathway in H9c2 cells. To the best of the
authors knowledge, this research is the first to confirm that the
Klotho protein could represent a new therapeutic strategy for Ang
II-induced cardiotoxicity.
Previous studies have reported that necroptosis is
involved in the pathogenesis of post MI heart failure (12,13).
One study previously confirmed the presence and interaction of
necroptotic proteins in advanced heart failure (14). Furthermore, a study by Oerlemans
et al (15) has demonstrated
that using treatments such as genetic knock-out and pharmacological
inhibition of necroptotic signaling molecules, could alleviate
contractile dysfunction, remodeling and inflammation of the cardiac
tissue. In addition to heart failure, Zhang et al (13) identified that the
RIP3-Ca2+/calmodulin-dependent protein kinase II-mPTP
myocardial necroptosis pathway, could be a promising target for the
treatment of ischemia- and oxidative stress-induced myocardial
damage. Furthermore, Zhang et al (16) claimed that the receptor interacting
serine/threonine kinase (RIPK)1/RIPK3/P-MLKL axis induces
necroptosis during MI and that the microRNA-325-3p can effectively
ameliorate symptoms of MI by suppressing necroptosis. Similarly,
Zhao et al (17) confirmed
that necroptosis is involved in the process of cardiac hypertrophy
induced by pressure overload and that the drug losartan can
alleviate cardiac hypertrophy through the inhibition of
necroptosis. These studies have suggested that necroptosis has a
crucial role in myocardial damage and that preventing necroptosis
may be a possible strategy to avoid cardiotoxicity and myocardial
injury. In the present study, Ang II was revealed to induce
necroptosis, which resulted in cardiotoxicity. Furthermore, Nec-1,
an inhibitor of necroptosis, alleviated this cardiotoxicity.
Qin et al (11) reported that the mitogen activated
protein kinase/NF-κB p65 pathway participated in LPS-activated BV-2
microglia-mediated neuronal necroptosis, which allowed the present
study to infer that the NF-κB p65 pathway may also have a role in
Ang II-induced necroptosis in H9c2 cells. Consequently, the present
study further analyzed whether the expression of TLR4/NF-κB p65
increased after treatment with Ang II. It was revealed that TAK-242
(TLR4 antagonist) and PDTC (NF-κB p65 inhibitor) pretreatment
decreased the expression of necroptotic markers in H9c2 cells.
Consequently, it was concluded that the TLR4/NF-κB p65 pathway
participates in inducing necroptosis in Ang II-induced H9c2
cells.
In the present, exogenous Klotho protein was
revealed to decrease Ang II-induced necroptosis and cardiotoxicity.
Qian et al (7) showed that
the levels of necroptotic markers RIP1 and RIP3 increased in renal
IRI and that Klotho protein attenuated the elevation in RIP1 and
RIP3 release induced by hypoxia/reoxygenation (H/R) or
H2O2. Klotho is known to decrease necroptotic
markers by reducing the levels of oxidative stress biomarkers and
elevating superoxide dismutase 2 expression in both in vivo
and in vitro (7). Similarly,
the present study also demonstrated that Klotho proteins could
decrease necroptotic markers in Ang II-induced H9c2 cells, which
was associated with ROS production and inflammation. Numerous
previous studies have reported that Klotho protein can regulate the
TLR4/NF-κB p65 pathway. Wu et al (18) claimed that Klotho regulates the
TLR4/NF-κB p65/NGAL pathway in rat mesangial cells cultured with
high glucose to protect against kidney damage. Jin et al
(19) investigated whether Klotho
ameliorates cyclosporine A-induced nephropathy via the PDLIM2/NF-κB
p65 signaling pathway. It was also revealed that Klotho protein
could reduce the expression of components of the TLR4/NF-κB p65
pathway, which has been indicated to be relevant to
necroptosis.
The present study aimed to evaluate the ability of
Klotho protein to inhibit the Ang II-induced necroptosis of H9c2
cells. For the first time, Klotho was demonstrated to have the
potential to inhibit necroptosis, which caused Ang II-induced
cardiotoxicity. Furthermore, Klotho was revealed to be able to
inhibit Ang II-induced necroptosis through the modulation of the
TLR4/NF-κB p65 pathway in H9c2 cells. The present study provides a
new perspective on exploiting the pathological mechanism of
cardiotoxicity and new findings regarding the mechanism by which
Klotho protects against cardiovascular diseases.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the ‘China
Medical University Youth Backbone Program’ project funds (grant no.
QGZ2018037) and the China Scholarship Council (file no.
201908210044).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY and YS designed the research study. YS and HY
performed the experiments. HY, XG and SY performed the research. SY
and XG analyzed the data. SY and HY contributed essential reagents
or tools. SY wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Adameova A, Goncalvesova E, Szobi A and
Dhalla NS: Necroptotic cell death in failing heart: Relevance and
proposed mechanisms. Heart Fail Rev. 21:213–221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu H and Sun A: Programmed necrosis in
heart disease: Molecular mechanisms and clinical implications. J
Mol Cell Cardiol. 116:125–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo X, Yin H, Li L, Chen Y, Li J, Doan J,
Steinmetz R and Liu Q: Cardioprotective role of tumor necrosis
factor receptor-associated factor 2 by suppressing apoptosis and
necroptosis. Circulation. 136:729–742. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mencke R and Hillebrands JL; NIGRAM
consortium, : The role of the anti-ageing protein Klotho in
vascular physiology and pathophysiology. Ageing Res Rev.
35:124–146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuro-O M: The Klotho proteins in health
and disease. Nat Rev Nephrol. 15:27–44. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo Y, Zhuang X, Huang Z, Zou J, Yang D,
Hu X, Du Z, Wang L and Liao X: Klotho protects the heart from
hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated
inflammation both in vitro and in vivo. Biochim Biophys Acta Mol
Basis Dis. 1864:238–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qian Y, Guo X, Che L, Guan X, Wu B, Lu R,
Zhu M, Pang H, Yan Y, Ni Z and Gu L: Klotho reduces necroptosis by
targeting oxidative stress involved in renal ischemic-reperfusion
injury. Cell Physiol Biochem. 45:2268–2282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Yu S, Zhang N, Li Y, Chen S, Chang
Y, Sun G and Sun Y: Atorvastatin prevents angiotensin II induced
myocardial hypertrophy in vitro via CCAAT/enhancer-binding protein
β. Biochem Biophys Res Commun. 486:423–430. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zavodszky E, Seaman MN, Moreau K,
Jimenez-Sanchez M, Breusegem SY, Harbour ME and Rubinsztein DC:
Mutation in VPS35 associated with Parkinson's disease impairs WASH
complex association and inhibits autophagy. Nat Commun. 5:38282014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin M, Yiu WH, Wu HJ, Chan LY, Leung JC,
Au WS, Chan KW, Lai KN and Tang SC: Toll-like receptor 4 promotes
tubular inflammation in diabetic nephropathy. J Am Soc Nephrol.
23:86–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin S, Yang C, Huang W, Du S, Mai H, Xiao
J and Lü T: Sulforaphane attenuates microglia-mediated neuronal
necroptosis through down-regulation of MAPK/NF-κB signaling
pathways in LPS-activated BV-2 microglia. Pharmacol Res.
133:218–235. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szobi A, Goncalvesova E, Varga ZV, Leszek
P, Kuśmierczyk M, Hulman M, Kyselovič J, Ferdinandy P and Adameová
A: Analysis of necroptotic proteins in failing human hearts. J
Transl Med. 15:862017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv
F, Liu Y, Zheng W, Shang H, Zhang J, et al: CaMKII is a RIP3
substrate mediating ischemia- and oxidative stress-induced
myocardial necroptosis. Nat Med. 22:175–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Chen Y, Doan J, Murray J, Molkentin
JD and Liu Q: Transforming growth factor β-activated kinase 1
signaling pathway critically regulates myocardial survival and
remodeling. Circulation. 130:2162–2172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oerlemans MI, Liu J, Arslan F, den Ouden
K, van Middelaar BJ, Doevendans PA and Sluijter JPG: Inhibition of
RIP1-dependent necrosis prevents adverse cardiac remodeling after
myocardial ischemia-reperfusion in vivo. Basic Res Cardiol.
107:2702012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang DY, Wang BJ, Ma M, Yu K, Zhang Q and
Zhang XW: MicroRNA-325-3p protects the heart after myocardial
infarction by inhibiting RIPK3 and programmed necrosis in mice. BMC
Mol Biol. 20:172019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao M, Qin Y, Lu L, Tang X, Wu W, Fu H
and Liu X: Preliminary study of necroptosis in cardiac hypertrophy
induced by pressure overload. Sheng Wu Yi Xue Gong Cheng Xue Za
Zhi. 32:618–623. 2015.(In Chinese). PubMed/NCBI
|
|
18
|
Wu C, Lv C, Chen F, Ma X, Shao Y and Wang
Q: The function of miR-199a-5p/Klotho regulating TLR4/NF-κB
p65/NGAL pathways in rat mesangial cells cultured with high glucose
and the mechanism. Mol Cell Endocrinol. 417:84–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin M, Lv P, Chen G, Wang P, Zuo Z, Ren L,
Bi J, Yang CW, Mei X and Han D: Klotho ameliorates cyclosporine
A-induced nephropathy via PDLIM2/NF-kB p65 signaling pathway.
Biochem Biophys Res Commun. 486:451–457. 2017. View Article : Google Scholar : PubMed/NCBI
|