Introduction
Osteolysis is a common manifestation of bone
metastasis and skeletal developmental disorders (1). Bone resorption and formation is a
lifelong process that dynamically occurs in the skeletal system and
is essential for regulating skeletal growth, repairing bone damage
and maintaining mineral homeostasis (2). Either temporal or spatial imbalance of
osteoblastic bone formation and osteoclastic bone resorption may
lead to progressive osteopenia and subsequently result in
osteolysis (3,4). Matrix metalloproteinases (MMPs),
including MMP2, have been reported to play key roles in osteolysis
by degrading bone extracellular matrix (ECM) and modulating
osteoclastic bone resorption (5).
MMP2 is crucial to cancer progression, invasion and
metastasis. In many late-stage cancers like breast and prostate
cancers, osteolytic bone metastases are frequently observed
accompanied with aberrant activation of MMP2 expression (6,7).
However, in skeletal developmental disorders, particularly in
multicentric osteolysis, nodulosis and arthropathy (MONA),
defective or reduced enzyme activity of MMP2 is associated with
decreased bone mineralization and generalized osteolysis (8). It is interesting to notice that either
upregulation or downregulation of MMP2 activity can cause bone loss
and osteolysis, suggesting that the mechanism underlying
MMP2-related osteolysis is intricate and may differ in different
situations. In this article, recent studies on the functions of
MMP2 in bone tissues are reviewed and the mechanisms underlying the
role of MMP2 in osteolysis that occur in bone metastases and
skeletal developmental disorders are summarized. Furthermore, the
potential differences between the roles of MMP2 in these two
pathological conditions are discussed.
MMP2 in metastatic osteolysis
Metastasis to the skeletal system is one of the
hallmarks of most malignancies (9).
It occurs in up to 70% of patients with cancer, brings great
difficulties to disease treatment, and seriously affects patient
prognosis (10,11). Due to its ability to cleave and
degrade the ECM and basement membrane, the active form MMP2 plays a
prominent role in tumor progression and metastasis. MMP2 activity
is controlled at multiple levels, from transcriptional regulation,
post-translational modification and secretion, to zymogen
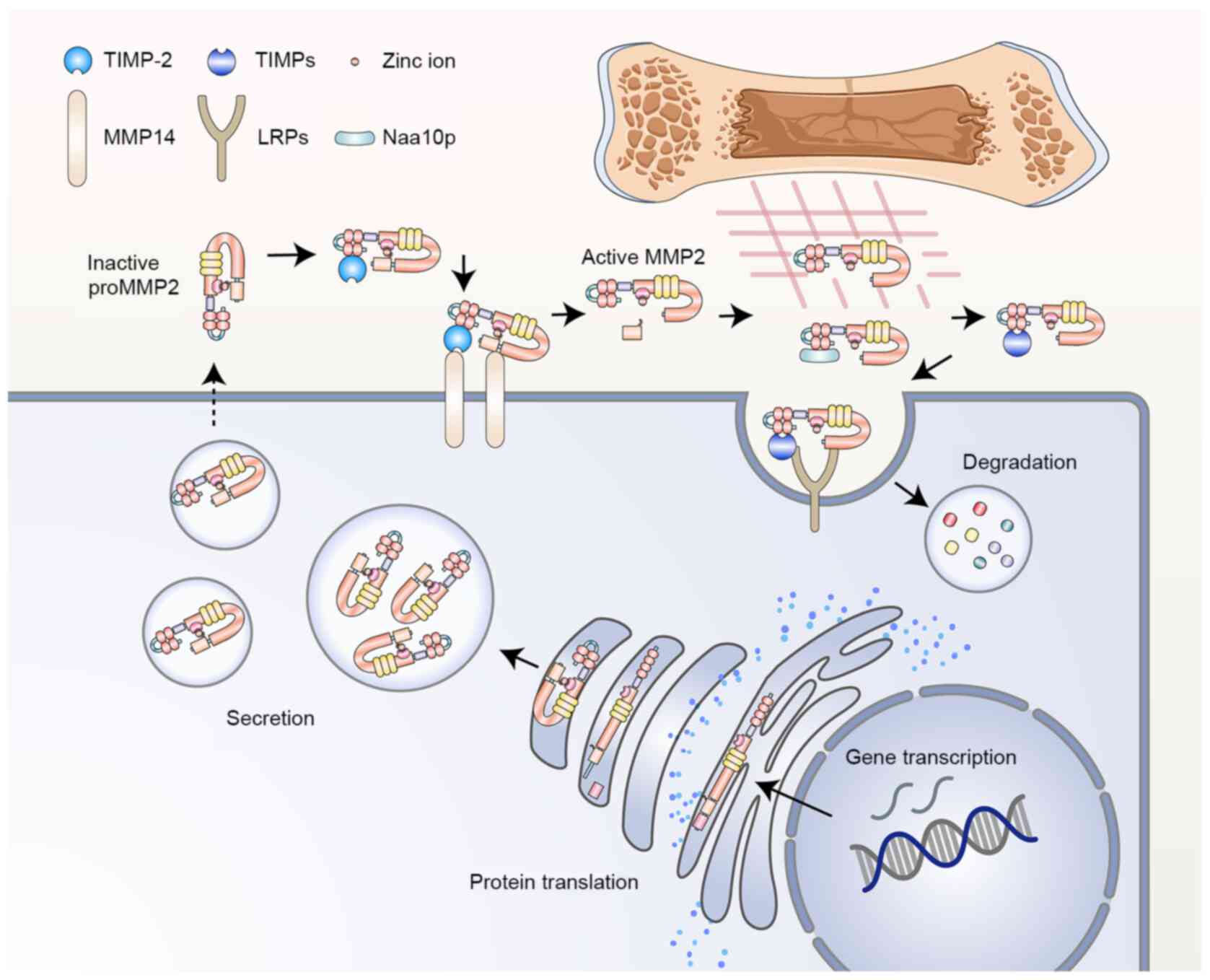
activation, inhibitor modulation and protein degradation (Fig. 1) (12). The activation of MMP2 predominantly
occurs on the cell surface and relies on the coordinated regulation
of type-1 membrane MMP (MT1-MMP, also known as MMP14) and tissue
inhibitor of metalloproteinase 2 (TIMP-2) (13). Overexpression of MMP2 is usually
detected in cancer, and its protein expression levels positively
correlate with factors that indicate a poor prognosis, such as poor
differentiation, metastasis to secondary organs and chemotherapy
resistance (14,15). For instance, in breast cancer,
increased expression of MMP2 protein is associated with
Twist1-induced invasion and metastasis, whereas decreased MMP2
protein expression levels are involved in RNA binding motif
single-stranded interacting protein 3 (RBMS3)-mediated tumor
suppression via the RBMS3/Twist1/MMP2 axis (16). Similarly, the protein expression
level of MMP2 in pulmonary tumors is notably elevated, which
affects the metastatic properties of tumors through a variety of
signaling pathways, such as the sphingolipid and ephrin
receptor-signaling pathway (17).
MMP2 silencing using small interfering RNA, which downregulates the
expression of MMP2 both at the protein and mRNA levels, can
effectively inhibit the growth and metastasis of non-small cell
lung cancer (18). Furthermore,
overexpression and hyperactivation of MMP2 can facilitate multiple
steps of the metastatic cascade (including intravasation,
extravasation, pre-metastatic niche remodeling and regulatory
interaction between tumor cells and bone microenvironment) during
the invasion of malignant tumors to bone, mostly resulting in
osteolytic lesions (19).
Among all manifestations of bone metastasis,
osteolysis is one of the most common features of late-stage cancer
(20). Extension of osteolytic
destruction may lead to pathologic fracture, which occurs in
~10–30% of all bone metastases (4).
Molecules and signaling pathways associated with communication
between tumor cells and bone cells serve a role in the pathogenesis
of osteolytic metastasis, including parathyroid hormone-related
protein (PTHrP), IL-6, IL-11, TNF-α, TGF-β, VEGF and MMPs (21,22).
While most members of the MMP family are involved in tumorigenesis
and metastasis, MMP2 and MMP9 are thought to be closely associated
with bone metastasis and osteolysis. A study on prostate
cancer-related bone metastasis demonstrated that integrin αvβ6
could promote cancer cell-mediated bone destruction by selectively
increasing the catalytic activity of MMP2 and the degradation of
bone matrix (23). Similarly,
thrombospondin-2-induced downregulation of microRNA-376c in
prostate cancer cells increases MMP2 expression and bone metastasis
(24). In breast cancer, MMP2
upregulates ERK signaling and reverses the receptor activator of
NF-κB ligand (RANKL)/osteoprotegerin (OPG) ratio, thus promoting
bone resorption and metastasis (5).
The role of MMP2 in osteolysis during tumor
metastasis has been further confirmed by the application of matrix
metalloproteinase inhibitors (MMPIs) in tumor therapy (25). Insufficient control of MMP2 activity
by TIMPs (especially TIMP-2, −3 and −4), the endogenous inhibitors
of MMPs, would result in dysregulation of tissue remodeling and
tumors (26). When synthesized
MMPIs are utilized, they are expected to disrupt the ‘vicious
cycle’ that occurs in the spread of tumor cells to bone. To date,
four generations of MMP2-targeting inhibitors have been identified
or designed for cancer therapy, namely the first generation of
hydroxamate-based inhibitors, the second generation of
non-hydroxamate-based inhibitors, the third generation of catalytic
domain (non-zinc binding) inhibitors, and the fourth generation of
allosteric and exosite inhibitors (26,27).
Although clinical trials involving MMPIs have not achieved the
expected results in cancer therapy, preclinical results from tumor
experiments using MMP2 inhibitors, such as FYK-1388, chlorotoxin
and the monoclonal antibody 9E8, support the osteolytic effect of
MMP2 in bone metastases (28). For
example, 2-[[(4-phenoxyphenyl) sulfonyl] methyl]-thiirane (SB-3CT),
a selective inhibitor of gelatinase that inhibits MMP2 (also known
as gelatinase A) strongly and MMP9 (gelatinase B) weakly, has been
shown to inhibit intra-bone growth of prostate cancer cells and
bone degradation (29,30).
MMP2 in hereditary skeletal dysplasia
Intriguingly, while numerous studies in cancer have
demonstrated that higher MMP2 activity correlates with more severe
osteolytic destruction in metastatic lesions, MMP2 hypoactivity
also leads to bone loss and osteolysis during skeletal growth and
development (31). The most
well-known evidence for MMP2 deficiency-related osteolysis comes
from inherited developmental diseases, particularly MONA and
Winchester syndrome (WS), which are caused by inactive mutations in
the MMP2 and MMP14 genes, respectively (8,32).
Both of them are autosomal recessive disorders characterized by
highly similar phenotypes of progressive multicentric osteolysis
and arthritis. Although not directly associated with MMP2 mutation,
the pathogenesis of WS is thought to be linked with deficient MMP2
activity, since intact function of MMP14 is critical for activation
of MMP2 (33).
As an extremely rare genetic disease with only 46
cases and 23 mutations from 28 families reported in the literature,
no exact epidemiological statistics of MONA, have been described
(34). Typical clinical
manifestations of MONA consist of extensive osteopenia and
osteolysis (most prominent in carpal and tarsal bones), progressive
osteoarthropathy, and subcutaneous nodules on the palms and soles
(35). In general, most children
with MMP2 mutations are apparently healthy at birth, but signs of
skeletal deformities begin from six months to eleven years old.
Thereafter, features of arthropathy gradually appear, including
joint pain, joint contracture and swelling of distal extremities,
and other symptoms then progressively develop, such as coarse
facies and gum hypertrophy (36).
Vanatka et al (37) reported
a case of MONA with long-term follow-up, in which the pathological
changes of the bones over a 23-year period were striking and the
pattern of bone loss was progressive and periarticular.
Furthermore, ~75% of mutations from the reported MONA cases occur
in the catalytic domain or have detrimental effects on its
catalytic function, resulting in impaired MMP2 activity (34,38).
Consequently, although individual differences in clinical
manifestations exist among MONA patients, almost all of them
contain signs of bone loss and osteolysis, indicating that loss of
MMP2 accounts for impaired bone integrity and mineral homeostasis
during skeletal growth and development.
To date, there is a conflict between the results
obtained in tumor research and developmental research regarding the
relationship between MMP2 and osteolysis. Based on the current
understanding of the structure and function of MMP2 protein, the
finding that MMP2 deficiency causes bone loss and osteolytic
phenotype seems counterintuitive. It was hypothesized that a
reduction in MMP2 activity may lead to bone overgrowth rather than
bone loss (39). However, the role
of MMP2 in osteolysis may be more complex than previously thought,
and studies have been conducted to investigate potential
explanations for how MMP2 functions in opposite ways in these two
pathological conditions.
Mechanisms underlying the role of MMP2 in
metastatic osteolysis
MMP2 promotes tumor-induced osteolysis involving
spatial accessibility achieved through cleavage of collagen on the
bone surface and stimulation of osteoclastic bone resorption
(40). In bone tissue, MMP2 is
mainly expressed by osteoblasts under the regulation of various
factors, such as FasL (41). During
bone metastasis, tumor cells and tumor stromal cells become another
important source of MMP2, and thus markedly promote
osteoclastogenesis and osteoclastic bone resorption (Fig. 2). Expression of MMP2 has been
confirmed in both human breast cancer cell lines and primary breast
tumors, and high protein levels of MMP2 are associated with
increased risk of bone metastasis (5). After being released into the
extracellular compartment, although MMP2 may not directly
participate in degradation of mineralized bone matrix, it can
cleave the collagen and osteoid seam that covers the bone surface,
activate osteolytic factors, and facilitate osteoclasts recruitment
and attachment (4,41). Activated MMP2 can suppress the
expression of OPG in osteoblasts and increase the RANKL/OPG ratio,
thereby contributing to osteoclast differentiation (4). In addition to its role in promoting
osteoclastic resorption, MMP2 can inhibit the later phases of
osteoblast differentiation by shedding the immune costimulatory
molecule B7-H3 from the cell membrane of osteoblasts (42). Moreover, mature osteoclasts may also
synthesize MMP2 and release it along with MMP9 and protons into the
local extracellular compartment to digest bone matrix (43,44).
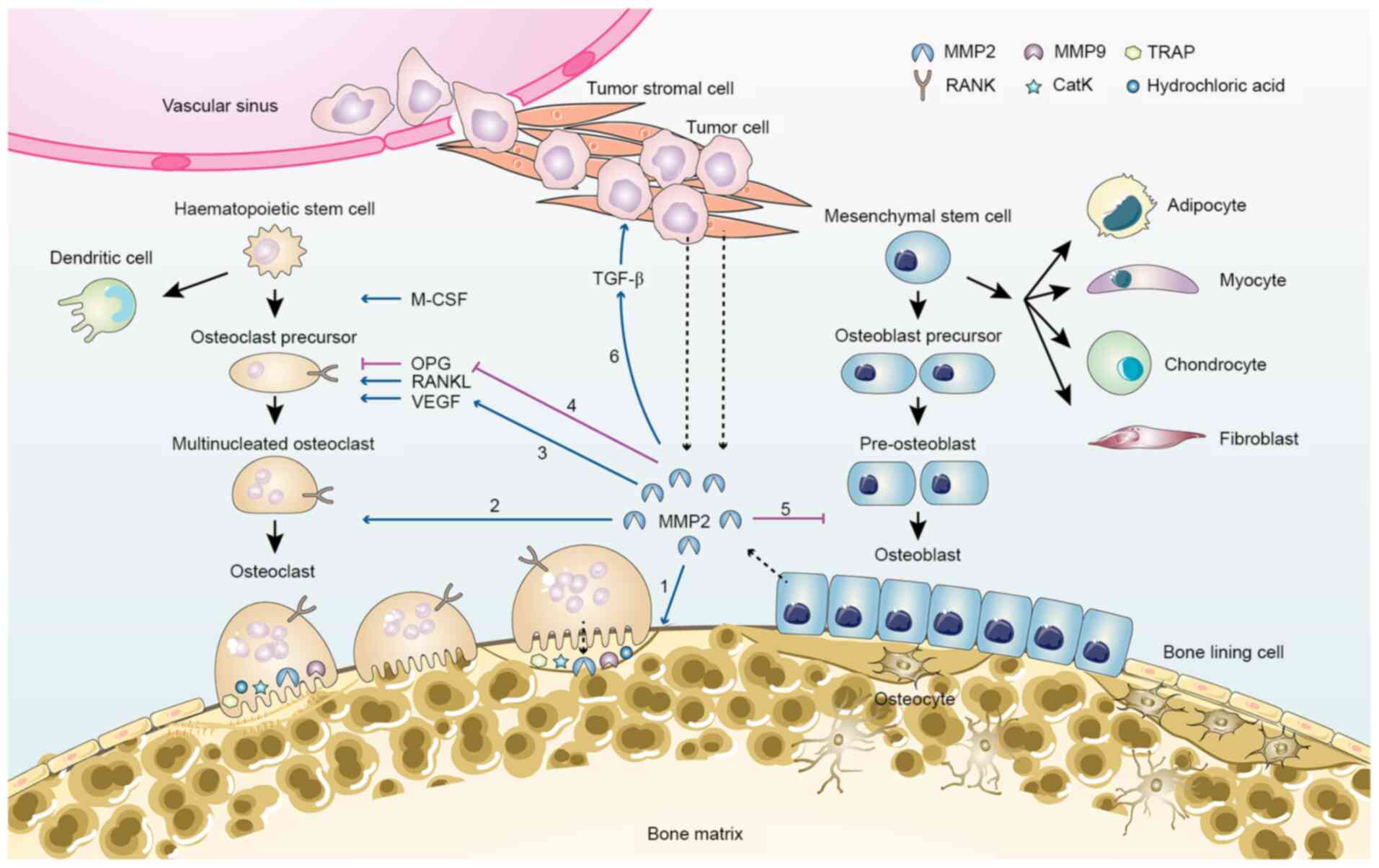 | Figure 2.MMP2 in bone remodelling and tumor
metastasis-related bone loss. MMP2 is primarily secreted by
osteocytes, osteoblasts, tumor stromal cells and tumor cells
(dashed arrow). MMP2 contributes to bone loss via several pathways:
1, Degradation of collagen and osteoid seam on the bone surface; 2,
recruitment of pre-osteoclasts and differentiation of osteoclasts;
3, increased VEGF activation, which promotes osteoclastogenesis; 4,
inhibition of OPG expression and increase in the RANKL/OPG ratio;
5, inhibition of osteoblast differentiation; 6, activation of
growth factors, such as TGF-β. CatK, cathepsin K; M-CSF,
macrophage-colony-stimulating factor; MMP, matrix
metalloproteinase; Naa10p, N-α-acetyltransferase 10 protein; OPG,
osteoprotegerin; RANKL, receptor activator of NF-κB ligand; TRAP,
tartrate-resistant acid phosphatase. |
MMP2 also provides signal amplification for the
‘vicious cycle’ within the tumor-bone microenvironment by
regulating the activity and bioavailability of various growth
factors such as TGF-β, PTHrP and RANKL (19). In particular, the communication
between MMP2 and TGF-β is of particular importance for this
signaling crosstalk between tumor cells and bone cells. Expression
of TGF-β has been confirmed in a large panel of cancer types with
metastatic properties (45).
Furthermore, a large amount of TGF-β could be released from the
bone matrix degraded by MMPs during bone resorption, including MMP2
and MMP9, and this increase in TGF-β from osteolytic lesions after
metastatic destruction has been observed in many mouse models of
osteolytic bone metastasis (45).
Excessive TGF-β signaling in turn stimulates the release of PTHrP
and IL-11 from tumor cells and promotes osteolytic bone destruction
(4,46). TGF-β can regulate bone remodeling
and maintenance of bone mass via its dual effects on osteoblasts
(47). It potentiates the
recruitment of mesenchymal stem cells and promotes the early
differentiation of osteoblast precursors by inducing Runx2
expression, which is required for the initiation of osteoblast
differentiation and inhibition of late differentiation of
osteoblasts (47). In addition to
its effect on RANKL stimulation and OPG expression, hyperactivation
of TGF-β signaling has been reported to directly enhance the
bone-resorptive activity of osteoclasts (48). Moreover, increased TGF-β levels can
promote late-stage cancer by activating surrounding
cancer-associated fibroblasts and stellate cells, stimulating
angiogenesis and inducing immunosuppression, thus facilitating the
proliferation and invasion of cancer cells in osteolytic lesions
(49).
The proangiogenic effect of MMP2 is another critical
mechanism for osteolysis during bone metastasis. Many members of
the MMP family have been implicated in tumor neovascularization and
lymphangiogenesis. For instance, MMP1 induces the expression of
VEGFR2, while MMP7 acts as a regulator for the VEGF pathway and the
degradation of soluble VEGFR1 (50). Specifically, MMP2 and MMP9 cooperate
tightly in neoplastic angiogenesis by modulating the dynamic
remodeling of ECM. Studies have revealed that elevated MMP2
expression is correlated with increased activation of TGF-β and
VEGF in the metastatic lesions (22,51).
The bioactive VEGF, basic fibroblast growth factor (bFGF) and TGF-β
can not only induce angiogenesis by signaling through their
respective receptors on endothelial cells, but also stimulate the
secretion of MMP2 and MMP9-containing vesicles from endothelial
cells, thereby contributing to the upregulation of proteolytic
activity in the metastatic microenvironment (50). Besides, in vivo studies have
confirmed that angiogenesis and tumor progression were inhibited in
mice lacking MMP2, according to a dorsal air sac assay using
melanoma cells and lung carcinoma cells (52).
Mechanisms underlying the role of MMP2 in
developmental osteolysis
How does MMP2 deficiency contribute to developmental
osteolysis? As aforementioned, high activity of MMP2 is associated
with increased bone resorption, suggesting that MMP2 mainly
functions as a negative modulator for bone remodeling. In addition,
there are also some studies supporting that MMP2 positively
associates with bone formation. For instance, Saran et al
(53) demonstrated that MMP2
expression was upregulated in newly formed bone tissue, whereas
MMP9 expression was reduced according to their reamed tibia
medullary canal mouse model. They proposed that MMP2 facilitated
bone remodeling and maturation probably by removing organic bone
content. Since the role of MMP2 in bone remodeling is
controversial, there may be other mechanisms that are responsible
for the pathogenesis of developmental osteolysis resulted from MMP2
deficiency.
Insight from MMP2-deficient mice
In order to understand how MMP2 affects skeletal
development, researchers have developed several mutant mouse models
(12). MMP2 knockout mice were
first generated by Itoh et al (54) via targeted replacement of the
promoter and the first exon of Mmp2 gene. Although the
mutant mice develop normally without any gross anatomical
abnormalities, they have a significantly slower growth rate and
exhibit several attenuated features of human MONA, such as
progressive loss of bone mineral density and articular cartilage
destruction (Fig. 3) (55). Moreover, opposing bone phenotypes of
decreased mineral density in long bones but increased volume in
cranium have also been described (56). In addition, MMP2 deficiency in mice
reduces the connectivity density of trabeculae, and impairs bone
remodeling but not cartilage remodeling during fracture repair
(57,58). Based on the aforementioned
observations, several novel functions of MMP2 in skeletal
development have been proposed, such as maintenance of the number
of osteoblasts and osteoclasts in an age-dependent manner,
supporting the formation of osteocytic canalicular network, and
controlling the transcription of osteopontin (OPN) and bone
sialoprotein (BSP) (39,55,56).
However, evidence from a detailed mechanism that comprehensively
interprets the consequences of MMP2 deficiency is still
insufficient.
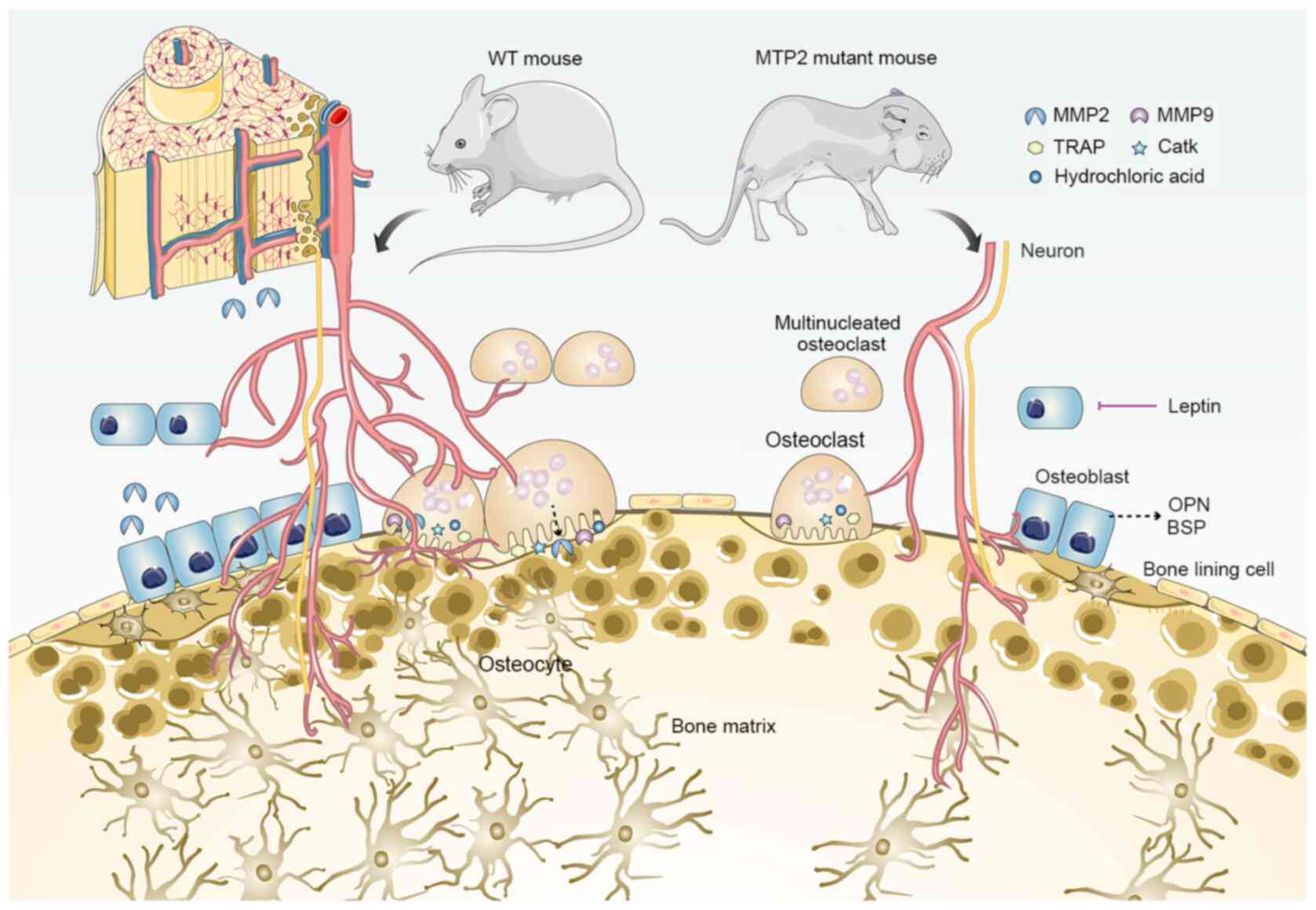 | Figure 3.MMP2 deficiency and bone loss. Based
on studies of MMP2 mutant mouse models, several phenotypes related
to bone loss have been reported, including: 1, Reduced growth rate
during development (although the overall anatomical structure of
mutant mice is normal in adulthood); 2, progressive loss of bone
mineral density and articular cartilage destruction; 3, decreased
connectivity density of trabeculae; 4, age-dependent reduction in
the number of osteoblasts and osteoclasts; 5, suppressed formation
of osteocytic canalicular network; 6, upregulated transcription of
OPN and BSP; 7, enhanced leptin-mediated inhibition of bone
formation. BSP, bone sialoprotein; CatK, cathepsin K; MMP, matrix
metalloproteinase; OPG, osteoprotegerin; OPN, osteopontin; TRAP,
tartrate-resistant acid phosphatase; WT, wild-type. |
Comparative studies of similar diseases have
provided another conclusion regarding the function of MMP2 in
developmental osteolysis. In addition to MONA and WS, there is a
third type of osteolytic skeletal dysplasia called multicentric
carpal-tarsal osteolysis syndrome, which is caused by mutations in
v-maf musculoaponeurotic fibrosarcoma oncogene ortholog B (MAFB)
(59). By investigating the
characteristics of those so-called human carpal-tarsal osteolysis
disorders, Lazarus et al (60) suggested that osteoclast-mediated
resorption was not sufficient to explain the distribution pattern
of developmental osteolysis. Indeed, MMP2, MMP14 and MAFB were
expressed in chondrocytes from the earliest stages of subarticular
endochondral ossification. Based on the hypothesis that the
endochondral ossification in subarticular regions is different from
that in archetypical physeal regions, the authors suggested that
abnormal peri-articular skeletal development and modeling, rather
than the excessive osteoclastic bone resorption, was responsible
for site-specific osteolysis (60).
Proangiogenic function of MMP2
In addition to its direct effect on bone cells, the
role of MMP2 in angiogenesis may be a key mechanism explaining its
different functions in tumor metastasis and bone dysplasia
(Fig. 4). MMP2 can intervene in
angiogenesis in multiple ways. Firstly, MMP2 enables the detachment
of pericytes and migration of endothelial cells by degrading the
vascular basement membrane and ECM and cleaving endothelial
cell-cell adhesions (61).
Secondly, the proteolytic activity of MMP2 can promote the release
and activation of ECM-bound angiogenic growth factors, including
VEGF, bFGF and TGF-β (50,62). The proangiogenic function of MMP2
has been confirmed in MMP2-deficient mice (63,64).
According to a previous study using a hindlimb ischemia model, both
collateral vessel development and capillary formation were
suppressed in MMP2-deficient mice, and the invasive and
proliferative abilities of endothelial cells cultured from mutant
mice were significantly impaired (63). Angiogenesis is a basic condition
necessary to support both tumor progression and bone formation.
Therefore, in spite of the same proangiogenic effect, upregulation
of MMP2 indirectly leads to osteolytic destruction by promoting
tumor cell proliferation and invasion during bone metastasis, while
its downregulation directly impairs bone formation during
osteogenesis, due to reduced blood supply.
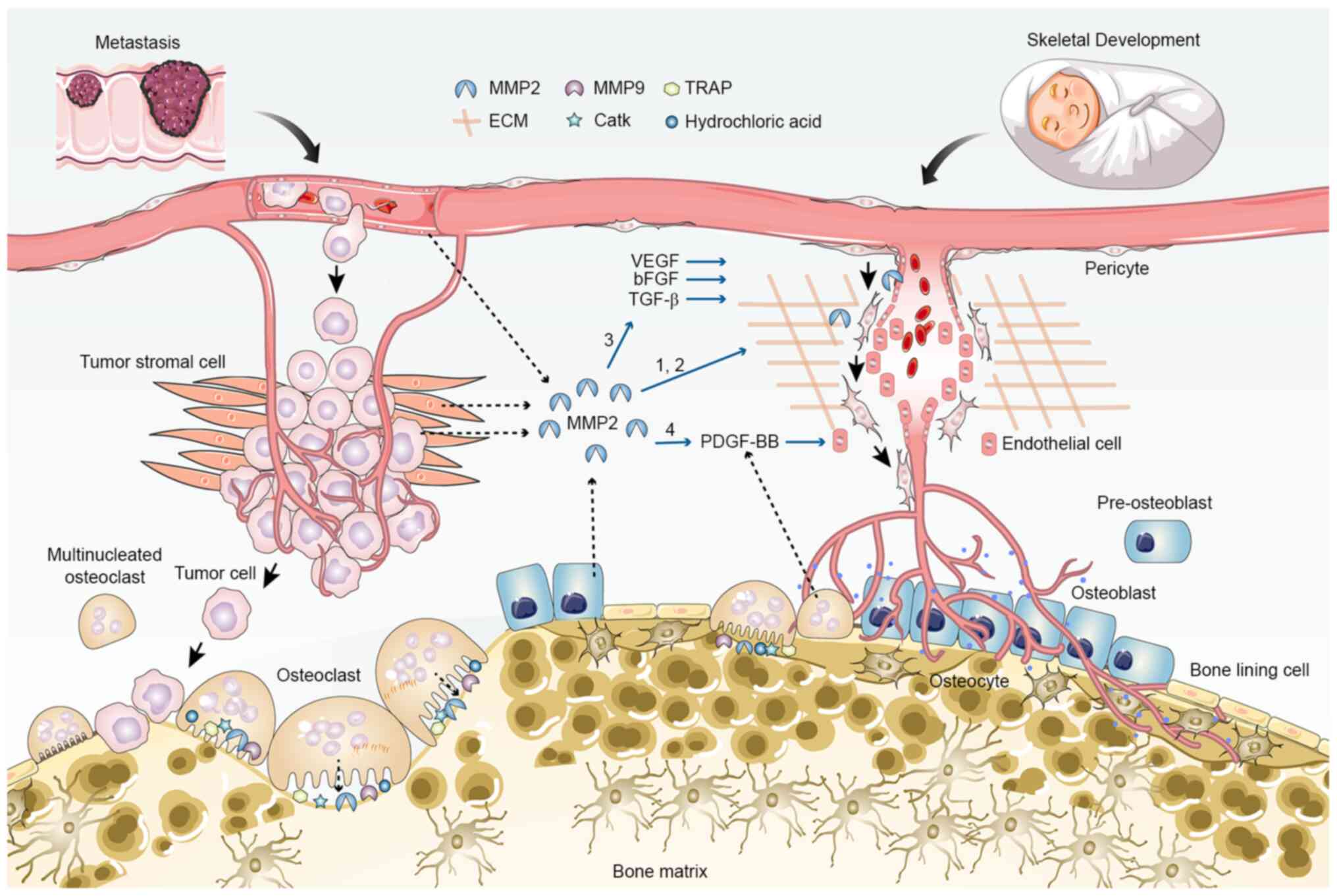 | Figure 4.Proangiogenic role of MMP2 in tumor
metastasis and skeletal development. MMP2 promotes angiogenesis in
multiple ways: 1, Degradation of vascular basement membrane and
extracellular matrix, and cell-cell adhesion cleavage; 2,
Detachment of pericytes and migration of endothelial cells; 3,
increased release and activation of ECM-bound angiogenic growth
factors, such as VEGF, bFGF and TGF-β; 4, potential increase in the
secretion of PDGF-BB through induction of osteoclast
differentiation and maturation. Angiogenesis is coupled with
osteogenesis during skeletal development, but supports tumor
cell-induced osteolysis during metastasis. bFGF, basic fibroblast
growth factor; CatK, cathepsin K; ECM, extracellular matrix; MMP,
matrix metalloproteinase; TRAP, tartrate-resistant acid
phosphatase. |
Recent studies have provided some new insights into
the relationship between MMP2 and angiogenesis. The endothelial
cells of type-H vessels are intimately associated with both bone
modeling and remodeling owing to their ability to support bone
tissues with indispensable nutrients and can secrete MMPs that
modulate ECM and vessel growth (65). MMP9 and MMP2 release participates in
the regulation of cartilage resorption and bone vasculature,
respectively. In particular, the promoting role of MMP2 in
osteoclast differentiation is likely to facilitate the secretion of
platelet-derived growth factor type BB (PDGF-BB), thereby
stimulating the formation of type-H vessels (66). Moreover, non-resorbing
vessel-associated osteoclasts (VAOs), which are critical for
anastomoses of type-H vessels and blood vessel-directed elongation
of bones, are also regulated by RANKL signaling in type-H
endothelial cells expressing high levels of MMP2 (67). It is possible that deficiency in
MMP2 also leads to disrupted orientation of angiogenic blood
vessels and contorted bone shape by affecting type-H endothelial
cells and VAOs.
Other potential mechanisms
Additional factors may be involved in modulating the
function of MMP2 in skeletal development, especially through the
central nervous system. There is increasing evidence for cross-talk
between the brain and bone through several pathways, including
hormonal signals and cell-cell communication between neurons and
bone cells (3). For instance,
leptin is an anorexigenic hormone that is predominantly secreted
from white adipose tissue and is essential for metabolic
regulation. It can activate the sympathetic nervous system through
leptin receptor isoform b, also known as obesity receptor isoform b
(ObRb), on hypothalamic neurons to inhibit bone formation, while
its direct interaction with ObRb-positive bone cells may increase
their growth (68,69). A study on obesity has recently
identified that activated MMP2 in hypothalamus modulates
leptin-mediated signaling by cleaving the extracellular domain of
ObRb leptin receptors (70).
Knockdown of MMP2 restores the expression levels of the leptin
receptor and increases the concentration of leptin in hypothalamic
neurons (70). Therefore, it may be
hypothesized that loss of MMP2 may promote leptin signaling and
enhance the leptin-mediated inhibition of bone formation. Further
studies are required to verify whether leptin signaling or any
other factors of the autonomic system also contribute to skeletal
dysplasia and osteolysis caused by MMP2 deficiency.
In addition to excessive osteoclastic bone
resorption, dysfunction of osteoblastic bone formation can also
give rise to osteopenia and osteolysis during bone development.
Metabolic programming is one of the essential factors that control
the bone-forming function of osteoblasts, and metabolic diseases
such as diabetes mellitus and anorexia nervosa can result in
osteoblast dysfunction and bone loss (71). It has been reported that MMP2
deficiency is associated with metabolic disorders, making the
affected patients prone to osteolysis and growth retardation
(72). In addition to its
extracellular proteolytic functions, increasing evidence indicates
that MMP2 has considerable intracellular functions, including
degradation of intracellular proteins like calcitonin gene-related
peptide and nuclear matrix proteins like poly (ADP-ribose)
polymerase (73,74). Consequently, loss-of-function
mutations in MMP2 may disrupt the energy metabolism of osteoblasts,
thereby impairing their bone formation function.
Conclusions
The molecular and cellular mechanisms involved in
MMP2-related osteolysis during tumor metastasis and skeletal
development are not fully understood yet. In this review, the most
important findings in the field were summarized and classified into
three categories based on their relationships with bone
formation.
The first set of functions answers to a certain
extent how overexpression of MMP2 is associated with osteolysis in
bone metastasis (Fig. 2). Some of
the relevant functions include: i) Degrading the collagen and
osteoid seam on the bone surface; ii) facilitating osteoclast
recruitment and osteoclastogenesis; iii) increasing the RANKL/OPG
ratio; iv) inhibiting osteoblast differentiation; v) activating
growth factors and osteolytic factors, such as TGF-β and IL-6; and
vi) amplifying the mutual regulatory interactions that occur
between tumor cells and the microenvironment. Together, these
functions illustrate the role of MMP2 in promoting osteoclastic
bone resorption, which provides the framework for understanding the
effect of MMP2 on metastatic osteolysis.
The second set of functions is in accord with the
phenomena observed in osteolytic disorders caused by MMP2
deficiency (Fig. 3). The
application of genetically engineered mouse models of MMP2
deficiency has provided insight into the molecular mechanisms that
enable MMP2 to maintain normal bone mass and prevent developmental
abnormalities. The prominent findings for this aspect of MMP2
functions include: i) Maintaining the number of bone cells; ii)
supporting the formation of the osteocytic canalicular network;
iii) controlling the transcription of mineralization-related
factors such as OPN and BSP; iv) fostering subarticular
endochondral ossification and periarticular bone modeling; and v)
suppressing leptin-mediated inhibition of bone formation. However,
the question of whether MMP2 is required for energy metabolism in
osteoblasts remains to be demonstrated.
The last set of functions takes into account the
distinct roles of MMP2 in two pathological conditions. Since both
tumors and bones are highly vascularized tissues and angiogenesis
is a prerequisite for their development, MMP2 can influence bone
metastasis and osteogenesis through its proangiogenic activity
(Fig. 4). As aforementioned, in
terms of molecular mechanisms for neoplastic angiogenesis, MMP2
facilitates the remodeling of ECM, stimulates the migration of
pericytes and endothelial cells, and activates angiogenic growth
factors. During bone metastasis, the pre-metastatic niche developed
by the neovascular network facilitates metastatic colonization of
cancer cells and subsequent bone destruction. These functions of
MMP2 also appear to be of particular importance to vasculogenesis
during skeletal development; formation of neovascular networks
exerts a positive effect on the skeleton. Moreover, several novel
concepts that relate MMP2 with angiogenesis and osteogenesis have
been highlighted, including type-H vessels that are tightly coupled
with periosteal bone formation, as well as VAOs, which are
associated with blood vessel-directed elongation of bones.
Nevertheless, the detailed mechanism underlying how MMP2
contributes to osteolysis and how MMP2 performs distinct roles
under physiological and pathological conditions remains unclear. In
summary, although MMP2 has been shown to be a critical mediator of
bone destruction and resorption, it may also serve additional
functions that remain to be identified. Furthermore, the
mechanistic interactions between MMP2 and other proteases, which
may fine-tune and ultimately determine the end-activity of MMP2,
require further study. A better understanding of the mechanisms
that underlie the functions of MMP2 during osteolytic metastasis
and developmental osteolysis would facilitate the exploitation of
therapeutics for restoring the phenotypes caused by MMP2
hyperactivity or deficiency.
Acknowledgements
Not applicable.
Funding
This study was funded by a grant from the National
Natural Science Foundation of China (grant no. 81702662).
Availability of data and materials
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
Authors' contributions
XL, LJ and YT conceived and designed the study. XL
and LJ researched and performed analysis of the literature. XL
drafted the manuscript, and YT critically revised important
intellectual content of the article. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BSP
|
bone sialoprotein
|
|
CatK
|
cathepsin K
|
|
ECM
|
extracellular matrix
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
LRP
|
lipoprotein receptor-related
protein
|
|
MAFB
|
v-maf musculoaponeurotic fibrosarcoma
oncogene ortholog B
|
|
M-CSF
|
macrophage colony-stimulating
factor
|
|
MMP
|
matrix metalloproteinase
|
|
MMPI
|
matrix metalloproteinase inhibitor
|
|
MONA
|
multicentric osteolysis, nodulosis and
arthropathy
|
|
Naa10p
|
N-α-acetyltransferase 10 protein
|
|
ObRb
|
leptin receptor isoform b
|
|
OPG
|
osteoprotegerin
|
|
OPN
|
osteopontin
|
|
PDGF-BB
|
platelet-derived growth factor type
BB
|
|
PTHrP
|
parathyroid hormone-related
protein
|
|
RANKL
|
receptor activator of NF-κB ligand
|
|
RBMS3
|
RNA binding protein 3
|
|
TIMP
|
tissue inhibitors of
metalloproteinase
|
|
TRAP
|
tartrate-resistant acid
phosphatase
|
|
VAO
|
vessel-associated osteoclast
|
|
WS
|
Winchester syndrome
|
|
WT
|
wild-type
|
References
|
1
|
Zaidi M: Skeletal remodeling in health and
disease. Nat Med. 13:791–801. 2007. View
Article : Google Scholar
|
|
2
|
Hassan B, Baroukh B, Llorens A, Lesieur J,
Ribbes S, Chaussain C, Saffar JL and Gosset M: NAMPT expression in
osteoblasts controls osteoclast recruitment in alveolar bone
remodeling. J Cell Physiol. 233:7402–7414. 2018. View Article : Google Scholar
|
|
3
|
Sato S, Hanada R, Kimura A, Abe T,
Matsumoto T, Iwasaki M, Inose H, Ida T, Mieda M, Takeuchi Y, et al:
Central control of bone remodeling by neuromedin U. Nat Med.
13:1234–1240. 2007. View
Article : Google Scholar
|
|
4
|
Battafarano G, Rossi M, Marampon F and Del
Fattore A: Cellular and molecular mediators of bone metastatic
lesions. Int J Mol Sci. 19:17092018. View Article : Google Scholar
|
|
5
|
Ni X, Xia T, Zhao Y, Zhou W, Wu N, Liu X,
Ding Q, Zha X, Sha J and Wang S: Downregulation of miR-106b induced
breast cancer cell invasion and motility in association with
overexpression of matrix metalloproteinase 2. Cancer Sci.
105:18–25. 2014. View Article : Google Scholar
|
|
6
|
Ell B, Mercatali L, Ibrahim T, Campbell N,
Schwarzenbach H, Pantel K, Amadori D and Kang Y: Tumor-induced
osteoclast miRNA changes as regulators and biomarkers of osteolytic
bone metastasis. Cancer Cell. 24:542–56. 2013. View Article : Google Scholar
|
|
7
|
Huang S, Shao K, Liu Y, Kuang Y, Li J, An
S, Guo Y, Ma H and Jiang C: Tumor-targeting and
microenvironment-responsive smart nanoparticles for combination
therapy of antiangiogenesis and apoptosis. ACS Nano. 7:2860–2871.
2013. View Article : Google Scholar
|
|
8
|
Bhavani GS, Shah H, Shukla A, Gupta N,
Gowrishankar K, Rao AP, Kabra M, Agarwal M, Ranganath P, Ekbote AV,
et al: Clinical and mutation profile of multicentric osteolysis
nodulosis and arthropathy. Am J Med Genet A. 170A:410–417. 2016.
View Article : Google Scholar
|
|
9
|
Ragel BT, Mendez GA, Reddington J, Ferachi
D, Kubicky CD, Philipp TC, Zusman NL, Klimo P, Hart R, Yoo J and
Ching AC: Life Expectancy and metastatic spine scoring systems: An
academic institutional experience. Clin Spine Surg. 30:335–342.
2017. View Article : Google Scholar
|
|
10
|
Croucher PI, McDonald MM and Martin TJ:
Bone metastasis: The importance of the neighbourhood. Nat Rev
Cancer. 16:373–386. 2016. View Article : Google Scholar
|
|
11
|
Vanek P, Bradac O, Trebicky F, Saur K, de
Lacy P and Benes V: Influence of the preoperative neurological
status on survival after the surgical treatment of symptomatic
spinal metastases with spinal cord compression. Spine (Phila Pa
1976). 40:1824–1830. 2015.
|
|
12
|
Page-McCaw A, Ewald AJ and Werb Z: Matrix
metalloproteinases and the regulation of tissue remodelling. Nat
Rev Mol Cell Biol. 8:221–233. 2007. View
Article : Google Scholar
|
|
13
|
Itoh Y: Membrane-type matrix
metalloproteinases: Their functions and regulations. Matrix Biol.
44-46:207–223. 2015. View Article : Google Scholar
|
|
14
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
15
|
Henriet P and Emonard H: Matrix
metalloproteinase-2: Not (just) a ‘hero’ of the past. Biochimie.
166:223–232. 2019. View Article : Google Scholar
|
|
16
|
Zhu L, Xi PW, Li XX, Sun X, Zhou WB, Xia
TS, Shi L, Hu Y, Ding Q and Wei JF: The RNA binding protein RBMS3
inhibits the metastasis of breast cancer by regulating Twist1
expression. J Exp Clin Cancer Res. 38:1052019. View Article : Google Scholar
|
|
17
|
Merchant N, Nagaraju GP, Rajitha B,
Lammata S, Jella KK, Buchwald ZS, Lakka SS and Ali AN: Matrix
metalloproteinases: Their functional role in lung cancer.
Carcinogenesis. 38:766–780. 2017. View Article : Google Scholar
|
|
18
|
Wang H, Guan X, Tu Y, Zheng S, Long J, Li
S, Qi C, Xie X, Zhang H and Zhang Y: MicroRNA-29b attenuates
non-small cell lung cancer metastasis by targeting matrix
metalloproteinase 2 and PTEN. J Exp Clin Cancer Res. 34:592015.
View Article : Google Scholar
|
|
19
|
Tauro M and Lynch CC: Cutting to the
Chase: How matrix metalloproteinase-2 activity controls
breast-cancer-to-bone metastasis. Cancers (Basel). 10:1852018.
View Article : Google Scholar
|
|
20
|
Coleman RE, Croucher PI, Padhani AR,
Clezardin P, Chow E, Fallon M, Guise T, Colangeli S, Capanna R and
Costa L: Bone metastases. Nat Rev Dis Primers. 6:832020. View Article : Google Scholar
|
|
21
|
Kenkre JS and Bassett J: The bone
remodelling cycle. Ann Clin Biochem. 55:308–327. 2018. View Article : Google Scholar
|
|
22
|
Mathis KM, Sturgeon KM, Winkels RM,
Wiskemann J, De Souza MJ and Schmitz KH: Bone resorption and bone
metastasis risk. Med Hypotheses. 118:36–41. 2018. View Article : Google Scholar
|
|
23
|
Dutta A, Li J, Lu H, Akech J, Pratap J,
Wang T, Zerlanko BJ, FitzGerald TJ, Jiang Z, Birbe R, et al:
Integrin αvβ6 promotes an osteolytic program in cancer cells by
upregulating MMP2. Cancer Res. 74:1598–1608. 2014. View Article : Google Scholar
|
|
24
|
Chen PC, Tang CH, Lin LW, Tsai CH, Chu CY,
Lin TH and Huang YL: Thrombospondin-2 promotes prostate cancer bone
metastasis by the up-regulation of matrix metalloproteinase-2
through down-regulating miR-376c expression. J Hematol Oncol.
10:332017. View Article : Google Scholar
|
|
25
|
Zhong Y, Lu YT, Sun Y, Shi ZH, Li NG, Tang
YP and Duan JA: Recent opportunities in matrix metalloproteinase
inhibitor drug design for cancer. Expert Opin Drug Discov.
13:75–87. 2018. View Article : Google Scholar
|
|
26
|
Li K, Tay FR and Yiu CKY: The past,
present and future perspectives of matrix metalloproteinase
inhibitors. Pharmacol Ther. 207:1074652020. View Article : Google Scholar
|
|
27
|
Fields GB: Mechanisms of action of novel
drugs targeting angiogenesis-promoting matrix metalloproteinases.
Front Immunol. 10:12782019. View Article : Google Scholar
|
|
28
|
Winer A, Adams S and Mignatti P: Matrix
metalloproteinase inhibitors in cancer therapy: Turning past
failures into future successes. Mol Cancer Ther. 17:1147–1155.
2018. View Article : Google Scholar
|
|
29
|
Bonfil RD, Sabbota A, Nabha S, Bernardo
MM, Dong Z, Meng H, Yamamoto H, Chinni SR, Lim IT, Chang M, et al:
Inhibition of human prostate cancer growth, osteolysis and
angiogenesis in a bone metastasis model by a novel mechanism-based
selective gelatinase inhibitor. Int J Cancer. 118:2721–276. 2006.
View Article : Google Scholar
|
|
30
|
Tao P, Fisher JF, Mobashery S and Schlegel
HB: DFT studies of the ring-opening mechanism of SB-3CT, a potent
inhibitor of matrix metalloproteinase 2. Org Lett. 11:2559–2562.
2009. View Article : Google Scholar
|
|
31
|
Martignetti JA, Aqeel AA, Sewairi WA,
Boumah CE, Kambouris M, Mayouf SA, Sheth KV, Eid WA, Dowling O,
Harris J, et al: Mutation of the matrix metalloproteinase 2 gene
(MMP2) causes a multicentric osteolysis and arthritis syndrome. Nat
Genet. 28:261–265. 2001. View
Article : Google Scholar
|
|
32
|
Evans BR, Mosig RA, Lobl M, Martignetti
CR, Camacho C, Grum-Tokars V, Glucksman MJ and Martignetti JA:
Mutation of membrane type-1 metalloproteinase, MT1-MMP, causes the
multicentric osteolysis and arthritis disease Winchester syndrome.
Am J Hum Genet. 91:572–576. 2012. View Article : Google Scholar
|
|
33
|
de Vos IJHM, Tao EY, Ong SLM, Goggi JL,
Scerri T, Wilson GR, Low CGM, Wong ASW, Grussu D, Stegmann APA, et
al: Functional analysis of a hypomorphic allele shows that MMP14
catalytic activity is the prime determinant of the Winchester
syndrome phenotype. Hum Mol Genet. 27:2775–2788. 2018. View Article : Google Scholar
|
|
34
|
Kröger L, Löppönen T, Ala-Kokko L, Kröger
H, Jauhonen HM, Lehti K and Jääskeläinen J: A novel mutation in the
matrix metallopeptidase 2 coding gene associated with intrafamilial
variability of multicentric osteolysis, nodulosis, and arthropathy.
Mol Genet Genomic Med. 7:e8022019. View Article : Google Scholar
|
|
35
|
Dagher R, Saliba E, Rizkallah M, Khalife
MCF and Megarbane A: Multicentric osteolysis with nodulosis and
arthropathy (Mona): Report of the first lebanese family. Ann Rheum
Dis. 77:496–497. 2018.
|
|
36
|
de Vos I, Wong ASW, Welting TJM, Coull BJ
and van Steensel MAM: Multicentric osteolytic syndromes represent a
phenotypic spectrum defined by defective collagen remodeling. Am J
Med Genet A. 179:1652–1664. 2019. View Article : Google Scholar
|
|
37
|
Vanatka R, Rouzier C, Lambert JC, Leroux C
and Coussement A: Winchester syndrome: The progression of
radiological findings over a 23-year period. Skeletal Radiol.
40:347–351. 2011. View Article : Google Scholar
|
|
38
|
Azzollini J, Rovina D, Gervasini C,
Parenti I, Fratoni A, Cubellis MV, Cerri A, Pietrogrande L and
Larizza L: Functional characterisation of a novel mutation
affecting the catalytic domain of MMP2 in siblings with
multicentric osteolysis, nodulosis and arthropathy. J Hum Genet.
59:631–637. 2014. View Article : Google Scholar
|
|
39
|
Mosig RA and Martignetti JA: Loss of MMP-2
in murine osteoblasts upregulates osteopontin and bone sialoprotein
expression in a circuit regulating bone homeostasis. Dis Model
Mech. 6:397–403. 2013. View Article : Google Scholar
|
|
40
|
Massague J and Obenauf AC: Metastatic
colonization by circulating tumour cells. Nature. 529:298–306.
2016. View Article : Google Scholar
|
|
41
|
Svandova E, Vesela B, Lesot H, Sadoine J,
Poliard A and Matalova E: FasL modulates expression of Mmp2 in
osteoblasts. Front Physiol. 9:13142018. View Article : Google Scholar
|
|
42
|
Feng P, Zhang H, Zhang Z, Dai X, Mao T,
Fan Y, Xie X, Wen H, Yu P, Hu Y and Yana R: The interaction of
MMP-2/B7-H3 in human osteoporosis. Clin Immunol. 162:118–1124.
2016. View Article : Google Scholar
|
|
43
|
Pesce Viglietti AI, Arriola Benitez PC,
Gentilini MV, Velasquez LN, Fossati CA, Giambartolomei GH and
Delpino MV: Brucella abortus invasion of osteocytes modulates
connexin 43 and integrin expression and induces osteoclastogenesis
via receptor activator of NF-κB ligand and tumor necrosis factor
Alpha Secretion. Infect Immun. 84:11–20. 2016. View Article : Google Scholar
|
|
44
|
Hong G, Zhou L, Shi X, He W, Wang H, Wei
Q, Chen P, Qi L, Tickner J, Lin L and Xu J: Bajijiasu abrogates
osteoclast differentiation via the suppression of RANKL signaling
pathways through NF-κB and NFAT. Int J Mol Sci. 18:2032017.
View Article : Google Scholar
|
|
45
|
Waning DL, Mohammad KS, Reiken S, Xie W,
Andersson DC, John S, Chiechi A, Wright LE, Umanskaya A, Niewolna
M, et al: Excess TGF-β mediates muscle weakness associated with
bone metastases in mice. Nat Med. 21:1262–1271. 2015. View Article : Google Scholar
|
|
46
|
Luis-Ravelo D, Antón I, Zandueta C,
Valencia K, Ormazábal C, Martínez-Canarias S, Guruceaga E, Perurena
N, Vicent S, De Las Rivas J and Lecanda F: A gene signature of bone
metastatic colonization sensitizes for tumor-induced osteolysis and
predicts survival in lung cancer. Oncogene. 33:5090–5099. 2014.
View Article : Google Scholar
|
|
47
|
El-Farrash RA, Ali RH and Barakat NM:
Post-natal bone physiology. Semin Fetal Neonatal Med.
25:1010772020. View Article : Google Scholar
|
|
48
|
Rhodes SD, Wu X, He Y, Chen S, Yang H,
Staser KW, Wang J, Zhang P, Jiang C, Yokota H, et al: Hyperactive
transforming growth factor-β1 signaling potentiates skeletal
defects in a neurofibromatosis type 1 mouse model. J Bone Miner
Res. 28:2476–2489. 2013. View Article : Google Scholar
|
|
49
|
Juarez P and Guise TA: TGF-β in cancer and
bone: Implications for treatment of bone metastases. Bone.
48:23–29. 2011. View Article : Google Scholar
|
|
50
|
Quintero-Fabian S, Arreola R,
Becerril-Villanueva E, Torres-Romero JC, Arana-Argaez V,
Lara-Riegos J, Ramirez-Camacho MA and Alvarez-Sanchez ME: Role of
matrix metalloproteinases in angiogenesis and cancer. Front Oncol.
9:13702019. View Article : Google Scholar
|
|
51
|
Li Q, Yang J, Chen C, Lin X, Zhou M, Zhou
Z and Huang Y: A novel mitochondrial targeted hybrid peptide
modified HPMA copolymers for breast cancer metastasis suppression.
J Control Release. 325:38–51. 2020. View Article : Google Scholar
|
|
52
|
Itoh T, Tanioka M, Yoshida H, Yoshioka T,
Nishimoto H and Itohara S: Reduced angiogenesis and tumor
progression in gelatinase A-deficient mice. Cancer Res.
58:1048–1051. 1998.
|
|
53
|
Saran WR, Chierice GO, da Silva RA, de
Queiroz AM, Paula-Silva FW and da Silva LA: Castor oil polymer
induces bone formation with high matrix metalloproteinase-2
expression. J Biomed Mater Res A. 102:324–331. 2014. View Article : Google Scholar
|
|
54
|
Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T
and Itohara S: Unaltered secretion of beta-amyloid precursor
protein in gelatinase A (matrix metalloproteinase 2)-deficient
mice. J Biol Chem. 272:22389–22392. 1997. View Article : Google Scholar
|
|
55
|
Mosig RA, Dowling O, DiFeo A, Ramirez MC,
Parker IC, Abe E, Diouri J, Aqeel AA, Wylie JD, Oblander SA, et al:
Loss of MMP-2 disrupts skeletal and craniofacial development and
results in decreased bone mineralization, joint erosion and defects
in osteoblast and osteoclast growth. Hum Mol Genet. 16:1113–1123.
2007. View Article : Google Scholar
|
|
56
|
Inoue K, Mikuni-Takagaki Y, Oikawa K, Itoh
T, Inada M, Noguchi T, Park JS, Onodera T, Krane SM, Noda M and
Itohara S: A crucial role for matrix metalloproteinase 2 in
osteocytic canalicular formation and bone metabolism. J Biol Chem.
281:33814–3324. 2006. View Article : Google Scholar
|
|
57
|
Nyman JS, Lynch CC, Perrien DS, Thiolloy
S, O'Quinn EC, Patil CA, Bi X, Pharr GM, Mahadevan-Jansen A and
Mundy GR: Differential effects between the loss of MMP-2 and MMP-9
on structural and tissue-level properties of bone. J Bone Miner
Res. 26:1252–1260. 2011. View Article : Google Scholar
|
|
58
|
Lieu S, Hansen E, Dedini R, Behonick D,
Werb Z, Miclau T, Marcucio R and Colnot C: Impaired remodeling
phase of fracture repair in the absence of matrix
metalloproteinase-2. Dis Model Mech. 4:203–211. 2011. View Article : Google Scholar
|
|
59
|
Mumm S, Huskey M, Duan S, Wenkert D,
Madson KL, Gottesman GS, Nenninger AR, Laxer RM, McAlister WH and
Whyte MP: Multicentric carpotarsal osteolysis syndrome is caused by
only a few domain-specific mutations in MAFB, a negative regulator
of RANKL-induced osteoclastogenesis. Am J Med Genet A.
164A:2287–2293. 2014. View Article : Google Scholar
|
|
60
|
Lazarus S, Tseng HW, Lawrence F, Woodruff
MA, Duncan EL and Pettit AR: Characterization of normal murine
carpal bone development prompts Re-evaluation of pathologic
osteolysis as the cause of human Carpal-tarsal osteolysis
disorders. Am J Pathol. 187:1923–1934. 2017. View Article : Google Scholar
|
|
61
|
Russo MV, Latour LL and McGavern DB:
Distinct myeloid cell subsets promote meningeal remodeling and
vascular repair after mild traumatic brain injury. Nat Immunol.
19:442–452. 2018. View Article : Google Scholar
|
|
62
|
Rundhaug JE: Matrix metalloproteinases and
angiogenesis. J Cell Mol Med. 9:267–285. 2005. View Article : Google Scholar
|
|
63
|
Cheng XW, Kuzuya M, Nakamura K, Maeda K,
Tsuzuki M, Kim W, Sasaki T, Liu Z, Inoue N, Kondo T, et al:
Mechanisms underlying the impairment of ischemia-induced
neovascularization in matrix metalloproteinase 2-deficient mice.
Circ Res. 100:904–913. 2007. View Article : Google Scholar
|
|
64
|
Trivedi A, Zhang H, Ekeledo A, Lee S, Werb
Z, Plant GW and Noble-Haeusslein LJ: Deficiency in matrix
metalloproteinase-2 results in long-term vascular instability and
regression in the injured mouse spinal cord. Exp Neurol. 284:50–62.
2016. View Article : Google Scholar
|
|
65
|
Peng Y, Wu S, Li Y and Crane JL: Type H
blood vessels in bone modeling and remodeling. Theranostics.
10:426–436. 2020. View Article : Google Scholar
|
|
66
|
Yang P, Lv S, Wang Y, Peng Y, Ye Z, Xia Z,
Ding G, Cao X and Crane JL: Preservation of type H vessels and
osteoblasts by enhanced preosteoclast platelet-derived growth
factor type BB attenuates glucocorticoid-induced osteoporosis in
growing mice. Bone. 114:1–13. 2018. View Article : Google Scholar
|
|
67
|
Romeo SG, Alawi KM, Rodrigues J, Singh A,
Kusumbe AP and Ramasamy SK: Endothelial proteolytic activity and
interaction with non-resorbing osteoclasts mediate bone elongation.
Nat Cell Biol. 21:430–441. 2019. View Article : Google Scholar
|
|
68
|
Elefteriou F: Impact of the autonomic
nervous system on the skeleton. Physiol Rev. 98:1083–1112. 2018.
View Article : Google Scholar
|
|
69
|
Reid IR, Baldock PA and Cornish J: Effects
of leptin on the skeleton. Endocr Rev. 39:938–959. 2018. View Article : Google Scholar
|
|
70
|
Mazor R, Friedmann-Morvinski D, Alsaigh T,
Kleifeld O, Kistler EB, Rousso-Noori L, Huang C, Li JB, Verma IM
and Schmid-Schonbein GW: Cleavage of the leptin receptor by matrix
metalloproteinase-2 promotes leptin resistance and obesity in mice.
Sci Transl Med. 10:eaah63242018. View Article : Google Scholar
|
|
71
|
Lee WC, Guntur AR, Long F and Rosen CJ:
Energy metabolism of the osteoblast: Implications for osteoporosis.
Endocr Rev. 38:255–266. 2017. View Article : Google Scholar
|
|
72
|
Fernandez-Patron C, Kassiri Z and Leung D:
Modulation of systemic metabolism by MMP-2: From MMP-2 deficiency
in Mice to MMP-2 deficiency in patients. Compr Physiol.
6:1935–1949. 2016. View Article : Google Scholar
|
|
73
|
Fernandez-Patron C, Stewart KG, Zhang Y,
Koivunen E, Radomski MW and Davidge ST: Vascular matrix
metalloproteinase-2-dependent cleavage of calcitonin gene-related
peptide promotes vasoconstriction. Circ Res. 87:670–676. 2000.
View Article : Google Scholar
|
|
74
|
Kwan JA, Schulze CJ, Wang W, Leon H,
Sariahmetoglu M, Sung M, Sawicka J, Sims DE, Sawicki G and Schulz
R: Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of
cardiac myocytes and is capable of cleaving poly (ADP-ribose)
polymerase (PARP) in vitro. FASEB J. 18:690–692. 2004. View Article : Google Scholar
|