Introduction
Bladder cancer (BCa) is a heterogeneous disease that
commonly presents as a malignant tumor; there are two main
subtypes, non-muscle invasive (NMIBC) and muscle invasive (MIBC)
BCa (1). Although 70% of patients
are diagnosed with NMIBC, the disease can quickly progress to
invade the muscle layer (2). The
current treatment for NMIBC consists of transurethral resection of
the bladder tumor in conjunction with intravesical chemotherapy or
immunotherapy (3). MIBC accounts
for ~30% of all BCa cases at initial presentation, and is
associated with a higher death rate due to distant metastasis
(1). Even after receiving
aggressive radical cystectomy (RC), the 5-year mortality rate of
patients with MIBC remains at 50–70%, with a high recurrence rate
and poor overall prognosis (3).
For decades, RC has been the primary treatment
method for MIBC; however, improved survival rates cannot be
achieved through surgery alone (4).
In the past 20 years, as MIBC treatments have aimed to preserve the
bladder and improve quality of life, radiation therapy (RT),
chemotherapy for radiation sensitization, and immunotherapy have
continuously improved (5). This has
increased the 5-year overall survival (OS) rate to 40–50%,
resulting in improved patient quality of life (6). For patients that are medically
unsuitable for RC, or those who prefer non-surgical alternatives,
RT and concurrent chemotherapy are currently the most effective
treatments. For suitable surgical candidates, bladder conservation
can maintain function and result in similar oncologic outcomes to
RC (7). However, the resistance of
cancer cells to radiation often limits the effectiveness of RT.
Furthermore, patients may still experience local tumor recurrence,
and acquiring radio-resistance (RR) after initial radiotherapy may
exacerbate local tumor recurrence and metastasis (8,9). At
present, the mechanisms by which cancer cells acquire RR remain
unclear. In the present study, obstacles in the treatment of BCa
were considered to be attributed to the radiation tolerance of
cancer stem cells (CSCs) present in the tumor (10,11).
CSCs may be seen as a reservoir of cancer cells due to their
self-renewal and plasticity properties, and the ability to
reconstruct heterogeneous tumor cell populations (12). Although there are a lack of
supporting clinical data, experimental data and preliminary
clinical trials suggest that CSC-targeted treatment has the
potential to improve radiotherapeutic efficacy (13,14).
Signal transduction and transcription activator 3
(STAT3) is a transcription factor with a number of important
biological functions. Increasing evidence suggests that STAT3 is an
important regulator of normal and cancer stem cells (15). It is involved in
epithelial-to-mesenchymal transition (EMT)-associated pathways,
which are hypothesized to be the primary mechanisms for CSC
generation (16), and has the
ability to promote cancer progression by regulating the activity of
CSCs (17). Moreover, our previous
studies revealed that high levels of STAT3 phosphorylation are
closely associated with the acquired radiation resistance (ARR) of
urinary system tumors (18,19). The present study aimed to elucidate
the role of STAT3 in RR and its association with the CSC phenotype
in BCa cells. The data suggested that the aberrant activation of
STAT3 enhanced the migration, invasion and stem-like properties of
BCa cells in response to long-term ionizing radiation (IR)
exposure.
Materials and methods
Cell culture and treatment
Human BCa cell lines (5637 and T24) were purchased
from The Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences and cultured in high glucose Dulbecco's
modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 U/ml streptomycin (Sigma-Aldrich; Merck KGaA). The cells
were maintained at 37°C in a humidified atmosphere with 5%
CO2. Cells were irradiated at room temperature in
ambient air using a 137Cs source (γ-ray; Nordion, Inc.)
at a dose rate of 0.79 Gy/min. Resistant cells were generated by
mimicking clinical radiotherapy treatment as previously described
(19). Subsequently, cells that
survived irradiation with 60 Gy (2 Gy per day, 5 days per week, and
continuous exposure until the cumulative dose reached 60 Gy) were
defined as 5637R and T24R cells.
Colony formation assay
A colony formation assay was used to determine the
sensitivity of cells to IR, as previously described (19). Briefly, the cells were irradiated
with increasing doses of γ-rays 4 h post-IR, and then plated into
60-mm dishes in triplicate. After 14 days of incubation, visible
colonies (>50 cells) were stained and counted. The survival
fraction curve was plotted using SigmaPlot 11.0 (Systat Software,
Inc.).
Cell treatment
The aforementioned cell lines were seeded into 60-mm
dishes at 5×105 cells/dish. At 60–70% confluency, 50 µM STAT3
Inhibitor VI S3I-201 (Santa Cruz Biotechnology, Inc.) was added to
the appropriate dishes, and the cells were cultured once more
(20). After 24 h, the medium was
discarded and the cells were harvested by trypsinization for tumor
sphere formation, soft agar colony formation and western blot
assays.
Small interfering RNA (siRNA)
transfection
siSTAT3 and the negative control siRNA were designed
and constructed by Shanghai GenePharma Co., Ltd. The targeting
sequence for siSTAT3 was 5′-CAGGCTGGTAATTTATATAAT-3′, and that of
the negative control siRNA was 5′-CATTGACTTATAAATTCGTTC-3′.
Transient STAT3 inhibition was achieved by transfection with 20 nM
siRNA (19). Cells
(5×105 cells/dish) in the exponential growth phase were
seeded into 60-mm dishes, and transfection was performed at 70%
confluency. The cells were transfected using
Lipofectamine® 2000 reagent (Thermo Fisher Scientific,
Inc.) in DMEM, according to the manufacturer's instructions. After
24 h, the medium was changed and subsequent experiments were
performed.
Western blot analysis
The expression levels of STAT3, phosphorylated STAT3
(pSTAT3Ser727 and pSTAT3Tyr705), matrix
metalloproteinase (MMP)-2, MMP-9, suppressor of variegation 3–9
homolog 1 (KMT1A), GATA binding protein 3 (GATA3) and Janus kinase
2 (JAK2), epidermal growth factor receptor (EGFR), phosphorylated
EGFR (p-EGFR) were assessed using total cellular protein. The
cellular proteins were extracted using M-PER™ Mammalian Protein
Extraction Reagent (Thermo Fisher Scientific, Inc.) and quantified
using a BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.),
where the ratio of protease inhibitor (Halt™ Protease Inhibitor
Single-Use Cocktail; Thermo Fisher Scientific, Inc.) to protein
lysate was 1:100. Equal amounts of each sample (40 µg) were
fractionated using 8 and 12% SDS-PAGE gels, followed by transfer to
0.45-µm PVDF membranes; the fractionation and transfer times were
controlled according to the molecular weights of the proteins. The
membrane was washed with TBS with 0.05% Tween-20 solution and
blocked with 5% BSA (Beyotime Institute of Biotechnology) at room
temperature for 1 h. The membranes were subsequently probed with
primary antibodies against pSTAT3Ser727 (cat. no.
ET1607-39; 1:1,000; Hangzhou HuaAn Biotechnology Co., Ltd.),
pSTAT3Tyr705 (cat. no. ET1603-40; 1:1,000; Hangzhou
HuaAn Biotechnology Co., Ltd.), STAT3 (cat. no. ET1607-38; 1:1,000;
Hangzhou HuaAn Biotechnology Co., Ltd.), MMP-2 (cat. no. sc-13594;
1:1,000; Santa Cruz Biotechnology, Inc.), MMP-9 (cat. no.
sc-393859; 1:1,000; Santa Cruz Biotechnology, Inc.), KMT1A (cat.
no. sc-377112; 1:1,000; Santa Cruz Biotechnology, Inc.), GATA3
(cat. no. sc-269; 1:1,000; Santa Cruz Biotechnology, Inc.), JAK2
(cat. no. sc-390539; 1:1,000; Santa Cruz Biotechnology, Inc.), EGFR
(cat. no. sc-373746; 1:100; Santa Cruz Biotechnology, Inc.) and
p-EGFR (cat. no. sc-377547; 1:100; Santa Cruz Biotechnology, Inc.)
overnight at 4°C. GAPDH (cat. no. AB-P-R001; 1:1,000; Hangzhou
Goodhere Biotechnology Co., Ltd.) was used as the internal loading
control. The membranes were then incubated with anti-mouse (cat.
no. HA1006; 1:1,000) or anti-rabbit (cat. no. HA1001; 1:1,000)
horseradish peroxidase-conjugated secondary antibodies (both from
Hangzhou HuaAn Biotechnology Co., Ltd.) for 1 h at room
temperature. Finally, the proteins were visualized using the
ChemiDoc™ XRS System (Bio-Rad Laboratories, Inc.).
Tumor sphere formation
A total of 5×103 BCa cells were seeded
into ultra-low attachment surface 6-well plates (Corning, Inc.) and
maintained in DMEM with 20 ng/ml epidermal growth factor
(PeproTech, Inc.), 20 ng/ml basic fibroblast growth factor
(PeproTech, Inc.) and 2% B27 (Thermo Fisher Scientific, Inc.).
After 1 week of cultivation, the number of tumor spheres was
counted in five independent fields (21). The experiments were repeated three
times.
Cell migration assay
5637, 5637R, T24 and T24R cell migration were
determined using 8.0-µm pore size Transwell inserts (BD
Biosciences) placed in 24-well plates. For the 5637 and 5637R
lines, 2×105 cells in 500 µl FBS-free DMEM were seeded
into the upper chambers; for the T24 and T24R, 1×104
cells were used. DMEM containing 10% FBS (1,300 µl) was added to
the lower chambers. Following incubation for 24 h at 37°C, the
upper chambers were carefully removed and washed three times with
phosphate-buffered saline (PBS; pH 7.4). The cells were fixed in
100% methyl alcohol (Sangon Biotech Co., Ltd.) at room temperature
for 15 min, and the chambers were stained with 0.2% crystal violet
(Sangon Biotech Co., Ltd.) for 15–30 min at room temperature. When
the chambers were dry, the cells were analyzed under a light
microscope (DFC450-C; Leica Microsystems GmbH) at ×100
magnification. The experiments were repeated three times.
Cell invasion assay
Transwell chambers (Corning, Inc.) precoated with
Matrigel (BD Biosciences) for 2 h at 37°C were used to assess cell
invasion capacity. The invasiveness of 5637, 5637R, T24 and T24R
cells was determined using 8.0-µm pore size Transwell inserts (BD
Biosciences) in 24-well plates. A total of 2×105 5637
and 5637R cells were seeded into the upper chambers with 400 µl
FBS-free DMEM, and 1×104 T24 and T24R cells were used in
the same manner; 1,300 µl DMEM containing 10% FBS was added to the
lower chambers. Following incubation for 29 h at 37°C, the upper
chambers were removed and washed with PBS as aforementioned. The
cells were then fixed in 100% methyl alcohol for 15 min, and the
chambers were stained with 0.2% crystal violet for 15–30 min, both
at room temperature. After drying, the cells were analyzed using a
light microscope (magnification, ×100), and the experiments were
repeated three times.
Soft agar colony formation
Low-melting agarose solution (Sigma-Aldrich; Merck
KGaA) was prepared using ultrapure water (concentration, 1.2 and
0.8%). Sterile 2X DMEM, (2% 2×PS and 20% FBS) was prepared at room
temperature. For 1.2% agarose, 2X DMEM was added to the agarose
solution at a 1:1 ratio; 3 ml of the mixture was then added to 6-cm
dishes, which was cooled and solidified in an incubator. For 0.8%
agarose, 2X DMEM was mixed with the agar solution (1:1 ratio), and
a single cell suspension was added. The solution was thoroughly
mixed and layered on top of the 1.2% agar in the 6-cm dishes. After
the upper layer of agarose had fully solidified, the dishes were
incubated at 37°C for 12–14 days. The cells were then stained with
0.2% crystal violet (Sangon Biotech Co., Ltd.) for 15–30 min at
room temperature, and images were captured using a digital camera.
The experiments were repeated three times.
Animal model and experimental
protocol
A total of 21 male BALB/c nude mice (age, 35–42
days; weight, 22–25 g) were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. Experimental animals were housed at 24°C, 50–70%
humidity, with a 12-h light/dark cycle and free access to food and
water. All experiments were approved by the Committee for Ethical
Use of Experimental Animals at Fudan University (approval no.
201802144S; Shanghai, China). For the generation of xenografts,
1×106 BCa cells (in 0.1 ml PBS) were subcutaneously
injected into the back of each mouse. The first batch of mice were
randomly divided into the following five groups (n=3/group): i)
Saline control group; ii) 5637 group; iii) 5637R group; iv) T24
group; and v) T24R group. The second batch were randomly divided
into the T24 si control (Ctrl) and T24 siSTAT3 groups (n=3/group).
After 7 days, the xenograft volumes were measured every 2 days.
Tumor volume (mm3) was measured by caliper and
calculated using the following equation: V = (L × W2)/2,
where V is the tumor volume, L is the length and W is the width of
the tumor. On day 30, all mice were placed into the 4% isoflurane
chamber until animals lost consciousness, then cervical dislocation
was performed to euthanize the mice. No toe reflex of muscle tone
should be present at this point. The tumors were dissected
immediately and fixed with 10% formalin overnight at 4°C.
Kaplan-Meier Plotter database
analysis
The survival and gene expression information of 405
patients with BCa was obtained from the Kaplan-Meier Plotter online
database, (https://kmplot.com/analysis/), and the association
between STAT3 mRNA expression and clinical prognosis was evaluated.
All clinical datasets were acquired from published literature, and
written informed consent was previously obtained (22). Specifically, patient samples were
split into two groups according to the median expression of STAT3
mRNA in the tumor tissue: STAT3high vs.
STAT3low (computer optimization). By setting the
threshold as ‘all’, patient OS and relapse-free survival (RFS)
times were plotted using the Kaplan-Meier survival assay. The 95%
confidence interval (CI), hazard ratio (HR) and log rank P-value
were subsequently calculated.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from four types of BCa cell
sublines (5637, 5637R, T24, T24R) using TRIzol® reagent
(Tiangen Biotech Co., Ltd.), according to the manufacturer's
instructions. cDNA was synthesized using the ReverTra Ace™ qPCR RT
Kit (cat. no. FSQ-101; Toyobo Life Science) with the following
conditions: 37°C for 15 min, 98°C for 5 min and stored at 4°C.
Primers for amplification of EGFR and GAPDH were designed by
GenScript. Primer sequences were as follows: EGFR forward,
5′-TCCCTCAGCCACCCATATGTAC-3′ and reverse,
5′-GTCTCGGGCCATTTTGGAGAATCC-3′; GAPDH forward,
5′-CACCAACTGGGACGACAT-3′ and reverse, 5′-ACAGCCTGGATAGCAACG-3′.
RT-qPCR amplification reactions were performed using BioEasy Master
Mix (SYBR-Green) (cat. no. BSB30L1; Hangzhou Bioer Co., Ltd.) with
a Mx3000P Quantitative PCR system (Agilent Technologies, Inc.).
Amplification conditions were as follows: 95°C for 5 min, followed
by 40 cycles of 95°C for 30 sec and 60°C for 45 sec. Each sample
was examined in triplicate and the amount of product was normalized
relative to that of GAPDH. Quantitative values were calculated
according to the 2−ΔΔCq method (23).
Statistical analysis
All data are presented as the mean ± SEM, and all
experiments were repeated at least three times. Statistical
analysis was conducted using GraphPad Prism software (version 8.0;
GraphPad Software, Inc.). Student's two-tailed t-test and the
log-rank test were used for the determination of statistical
relevance between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
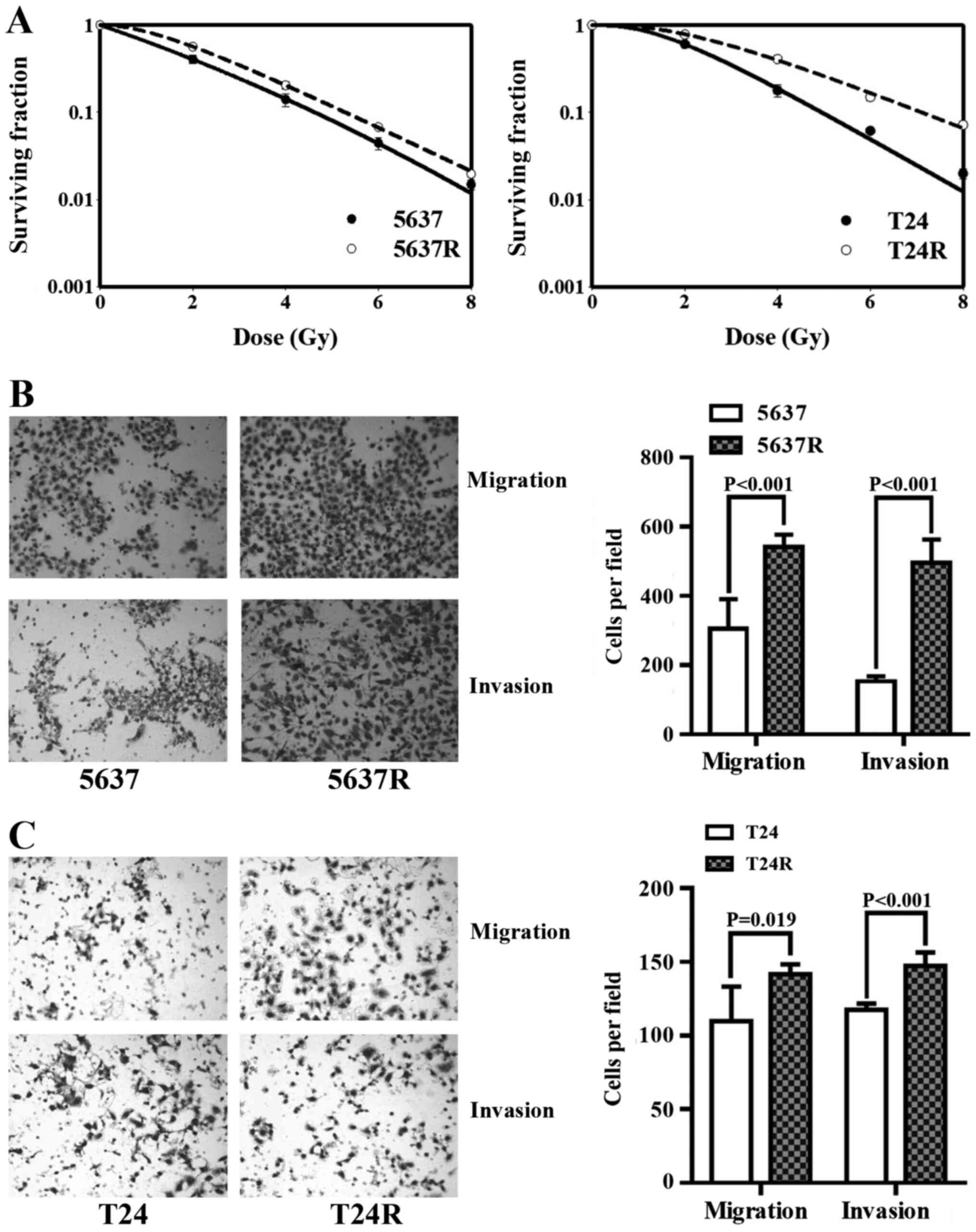
Fractionated irradiation (FI) enhances
the motility of human BCa cells
The human BCa cell lines, 5637 and T24, were
subjected to 30 FI treatments of 2 Gy/day; surviving cells were
named 5637R and T24R, respectively. Initially, the radio-tolerance
of the surviving cells was compared with that of the respective
parental cell lines (Fig. 1A). The
surviving 2-Gy fractions (SF2) in 5637 and 5637R cells
were 0.42±0.05 and 0.57±0.03, respectively (P=0.011), and the
SF2 of the T24 and T24R cells were 0.61±0.04 and
0.78±0.05, respectively (P=0.010). The effects of FI on cell
motility were then investigated. Compared with 5637 and T24 cells,
5637R and T24R cells demonstrated notable increases in both
migration and invasion. The migration rates of 5637 and 5637R cells
were 305.8±85.21 vs. 540.8±36.41, (P<0.001; Fig. 1B), and those of T24 and T24R cells
were 109.8±23.40 and 141.8±6.65 (P=0.019; Fig. 1C), respectively. The invasion rates
of 5637 and 5637R cells were 153.6±13.89 vs. 495.0±68.12
(P<0.001; Fig. 1B), and those of
T24 and T24R cells were 117.6±4.16 and 147.2±9.28, respectively
(P<0.001; Fig. 1C). It is worth
mentioning that, in order to observe the invasiveness of BCa cells,
the end time was set to 29 h after inoculation, and the end time of
migration experiments was 24 h post-inoculation.
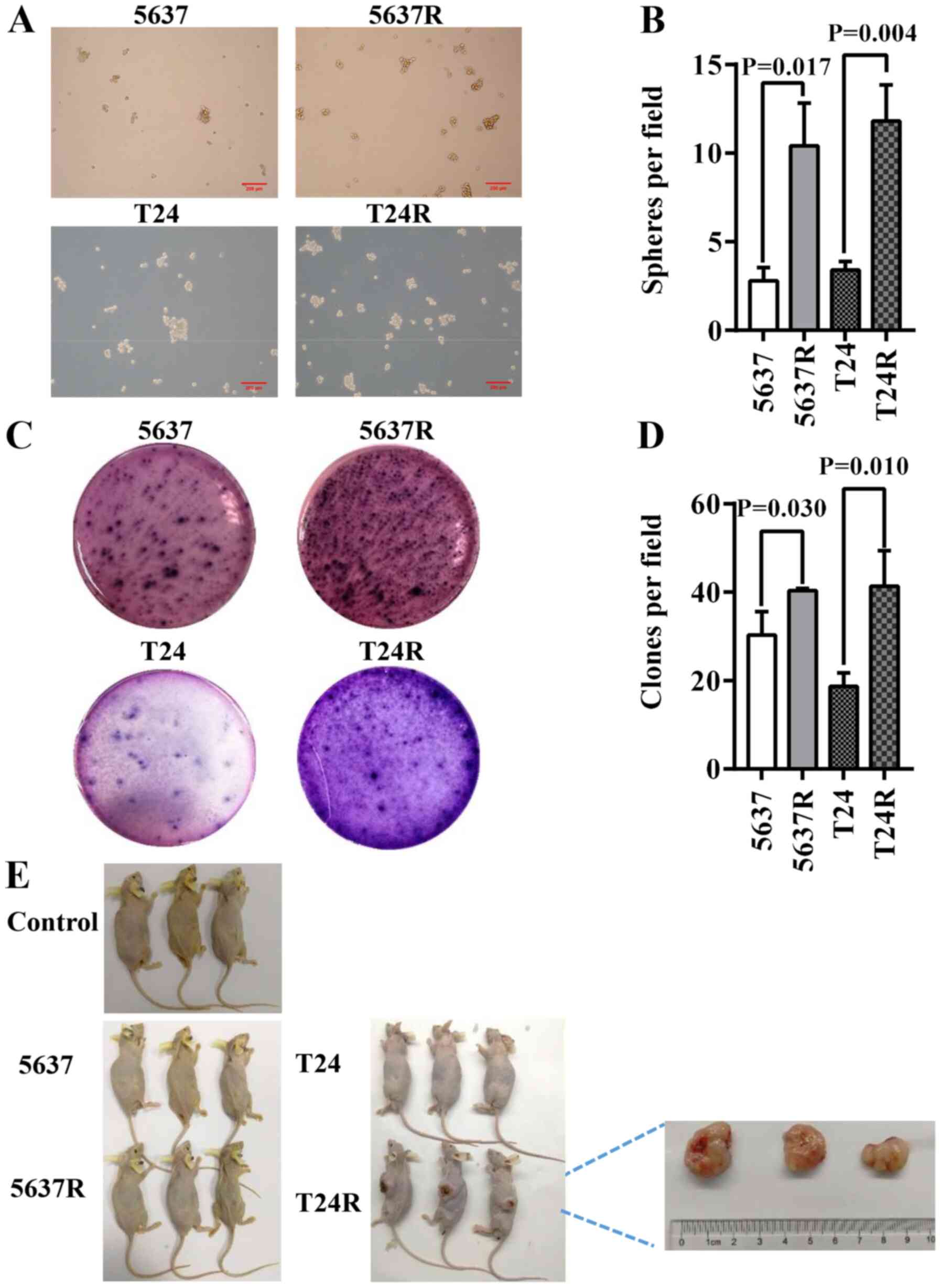
FI enhances the CSC characteristics of
human BCa cells
CSCs primarily reside within tumors and are
responsible for cancer recurrence. They exhibit stem cell-like
characteristics and resistant phenotypes, with self-renewal and
abnormal differentiation potential. As such, the existence of CSCs
is closely associated with tumorigenesis and drug resistance
(24,25). In the present study, 5637R and T24R
cells possessed enhanced CSC characteristics, such as
three-dimensional tumorsphere-forming ability, as well as anchorage
and clonogenic ability in semi-solid culture (Fig. 2A and C). However, as there is still
controversy concerning the existence of bladder CSCs (BCSCs)
(26), 5637R and T24R are referred
to as BCSC-like cells in the present study. An increased number of
spheres (diameter, >50 µM) was observed in both RR cell lines,
compared with the parental cells [5637 vs. 5637R, 2.8±0.75 vs.
10.4±2.42 (P=0.017); and T24 vs. T24R, 3.4±0.49 vs. 11.8±2.04
(P=0.004); Fig. 2B]. However, there
was no notable difference in the size of the tumorspheres formed by
the parental BCa cells or their RR counterparts (Fig. 2A). Another common method of
identifying stem cells is the low-melting soft agar colony
formation assay. Both 5637R and T24R cells formed a greater number
of colonies than 5637 and T24 cells [5637 vs. 5637R, 30.3±5.25 vs.
40.3±0.47 (P=0.030); and T24 vs. T24R, 18.7±3.09 vs. 41.3±8.06
(P=0.010); Fig. 2D]. To further
evaluate the potential CSC properties of RR cells, tumor formation
capacity was assessed by injecting BCa cells into BALB/c nude mice.
Only T24R cells formed tumors in immunodeficient mice and the
maximum diameter reached 2 cm (Fig.
2E).
Elevated STAT3 phosphorylation in RR
BCa cells is associated with an increase in tumor cell motility and
stemness
To further elucidate the mechanism by which FI
promotes BCa recurrence and metastasis, the expression of a series
cell movement- and stemness-associated proteins was investigated
(Fig. 3A). Consistent with previous
reports (18,19), increased levels of pSTAT3 and
MMP-2/9 were detected in both RR cell types. Also, the differences
in two STAT3-based CSC-related signaling pathways,
[KMT1A/GATA3/STAT3 (21) and
JAK/STAT3 (27)] were assessed
between parental BCa cells and their RR counterparts. As predicted,
both signaling pathways were more active in the resistant cells.
Expression levels of KMT1A and JAK2 were upregulated, whereas GATA3
expression was decreased, followed by increased expression of MMP-2
and MMP-9 in both RR cell lines. Next, S3I-201 was used to inhibit
STAT3 activation in BCa cells, which significantly decreased RR
cell migration [5637 Ctrl vs. 5637 S3I-201, 305.8±85.21 vs.
99.4±39.52 (P=0.024); 5637R Ctrl vs. 5637R S3I-201, 540.8±36.41 vs.
95.0±9.38 (P<0.001); T24 Ctrl vs. T24 S3I-201, 109.8±23.40 vs.
50.6±7.06 (P=0.042); and T24R Ctrl vs. T24R S3I-201, 141.8±6.65 vs.
81.4±16.33 (P=0.009); Fig. 3B] and
invasion [5637 Ctrl vs. 5637 S3I-201, 153.6±13.89 vs. 35.4±7.50
(P<0.001); 5637R Ctrl vs. 5637R S3I-201, 495.0±68.12 vs.
97.6±25.42 (P<0.001); T24 Ctrl vs. T24 S3I-201, 117.6±4.16 vs.
77.6±11.22 (P=0.010); and T24R Ctrl vs. T24R S3I-201, 147.2±9.28
vs. 71.0±8.83 (P<0.001); Fig.
3C] compared with untreated control cells. Similarly, the
tumorsphere-forming ability [5637 Ctrl vs. 5637 S3I-201, 2.8±0.75
vs. 0.6±0.49 (P=0.040); 5637R Ctrl vs. 5637R S3I-201, 10.4±2.42 vs.
0.8±0.75 (P=0.005); T24 Ctrl vs. T24 S3I-201, 3.4±0.49 vs. 1.4±0.49
(P=0.020); and T24R Ctrl vs. T24R S3I-201, 11.8±2.04 vs. 1.6±1.02
(P=0.002); Fig. 3D] and soft agar
colony-forming ability [5637 Ctrl vs. 5637 S3I-201, 31.0±4.32 vs.
12.7±1.25 (P=0.002); 5637R Ctrl vs. 5637R S3I-201, 40.3±0.47 vs.
11.7±6.65 (P=0.002); T24 Ctrl vs. T24 S3I-201, 18.7±3.09 vs.
3.7±1.70 (P=0.002); and T24R Ctrl vs. T24R S3I-201, 41.3±8.06 vs.
13.7±1.70 (P=0.004); Fig. 3E] of RR
cells compared with those of the untreated control cells.
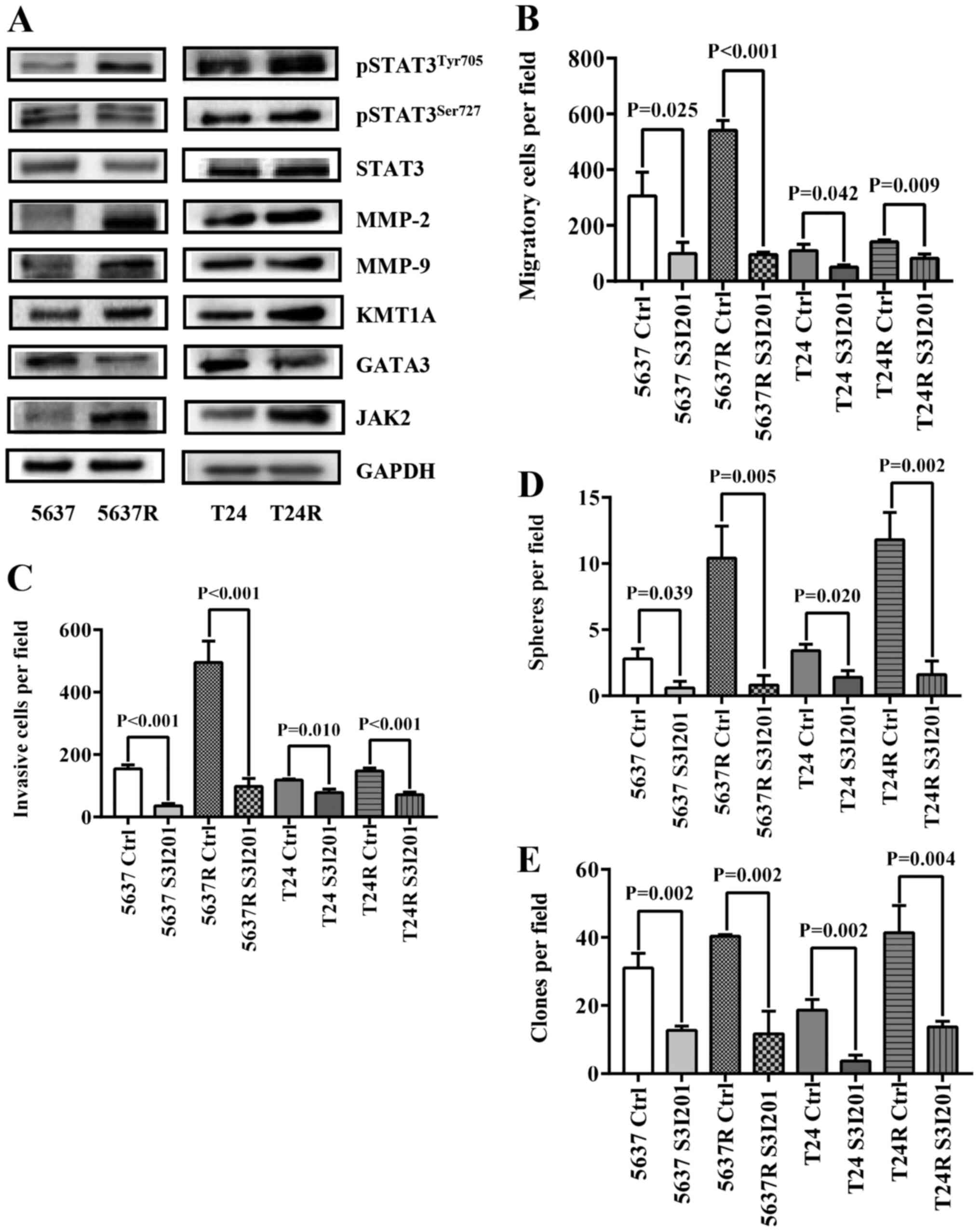 | Figure 3.Elevated STAT3 phosphorylation in
radio-resistant BCa cells is associated with an increase of tumor
cell stemness. (A) Western blot analysis of
pSTAT3Tyr705, pSTAT3Ser727, STAT3, MMP-2,
MMP-9, KMT1A, GATA3 and JAK2, GAPDH served as a loading control.
(B) Quantification of migration analysis of PBS-treated (Ctrl) and
S3I201-treated BCa cells. (C) Quantification of invasion analysis
of Ctrl and S3I201-treated BCa cells. (D) Quantification of
tumorspheres formed by Ctrl and S3I201-treated BCa cells. S3I-201
was added to the culture to the final concentration of 50 µM. (E)
Quantification of soft agar colony formation analysis of Ctrl and
S3I201-treated BCa cells. The data are presented as the mean ± SEM
of triplicate experiments. STAT3, signal transduction and
transcription activator 3; BCa, bladder cancer; p-, phosphorylated;
MMP, matrix metalloproteinase; KMT1A, suppressor of variegation 3–9
homolog 1; GATA3, GATA binding protein 3; JAK2, Janus kinase 2;
Ctrl, control; R, radio-resistant cells. |
STAT3 knockdown abrogates the
self-renewal capacity and tumorigenicity of human BCa cells
In the following experiments, data from 405 patients
with BCa were used to determine the relationship between STAT3
expression and patient prognosis using Kaplan-Meier plot and log
rank test analysis. Although there were no significant differences
in RFS between STAT3high and STAT3low
patients (HR=0.65; P=0.27; 95% CI, 0.3–1.4; Fig. 4A, left), decreased STAT3 mRNA levels
were associated with higher OS in patients with BCa (HR=1.5;
P=0.023; 95% CI, 1.06–2.12; Fig.
4A, right). STAT3high patients exhibited a shorter
mean survival time (13.1 months) compared with STAT3low
patients (18.83 months). Therefore, endogenous STAT3 expression was
knocked down in BCa cells using the RNA interference method
(Fig. 4B). Similar to cells treated
with S3I-201, both parental BCa cells and their RR counterparts
exhibited decreased motility and stemness, as indicated by the
reduced migration [5637 siCtrl vs. 5637 siSTAT3, 273.8±26.11 vs.
89.6±20.53 (P<0.001); 5637R siCtrl vs. 5637R siSTAT3,
495.0±35.92 vs. 85.0±15.23 (P<0.001); T24 siCtrl vs. T24
siSTAT3, 101.2±27.26 vs. 45.2±9.42 (P=0.003); and T24R siCtrl vs.
T24R siSTAT3, 130.2±26.90 vs. 67.8±7.09 (P=0.001); Fig. 4C] and invasion capabilities [5637
siCtrl vs. 5637 siSTAT3, 134.8±18.69 vs. 36.2±12.60 (P<0.001);
5637R siCtrl vs. 5637R siSTAT3, 214.8±51.05 vs. 31.4±12.40
(P<0.001); T24 siCtrl vs. T24 siSTAT3, 108.4±16.13 vs.
44.4±12.93 (P<0.001); and T24R siCtrl vs. T24R siSTAT3,
124.6±4.51 vs. 50.8±10.50 (P<0.001; Fig. 4D] compared with the cells
transfected with the siRNA negative control. The number of tumor
spheres [5637 siCtrl vs. 5637 siSTAT3, 2.0±0.01 vs. 0.80±0.40
(P=0.017); 5637R siCtrl vs. 5637R siSTAT3, 15.2±1.94 vs. 2.4±0.8
(P<0.001); T24 siCtrl vs. T24 siSTAT3, 3.6±1.02 vs. 0.4±0.49
(P=0.022); and T24R siCtrl vs. T24R siSTAT3, 5.4±1.02 vs. 2.2±0.75
(P=0.035); Fig. 4E] and soft agar
colonies [5637 siCtrl vs. 5637 siSTAT3, 23.3±3.68 vs. 6.7±2.05
(P=0.002); 5637R siCtrl vs. 5637R siSTAT3, 52.3±10.40 vs. 5.0±1.41
(P=0.002); T24 siCtrl vs. T24 siSTAT3, 25.0±2.83 vs. 3.3±0.47
(P<0.001); and T24R siCtrl vs. T24R siSTAT3, 36.7±6.80 vs.
3.7±0.47 (P=0.001); Fig. 4F] was
also lower in siSTAT3-treated BCa cells compared with their siCtrl
counterparts. Moreover, T24R cells lost the ability to form tumors
in immunodeficient mice following STAT3 suppression (Fig. 4G). These results indicated that the
expression and activation of STAT3 play indispensable roles in the
self-renewal maintenance and tumorigenicity of BCa cells. It has
been reported that radiation may induce STAT3 phosphorylation via
EGFR in lung cancer cells (28).
Therefore, in the current study, the expression and activation
level of EGFR was compared in both parent cells (5637 and T24) and
their resistant counterparts (5637R and T24R). It was found that
the mRNA expression of EGFR was lower in 5637R cells than that in
5637 cells, 1.0±0.01 vs. 0.5±0.01 (5637 vs. 5637R, P<0.001).
However, compared with T24 cells, EGFR expression was notably
increased in T24R cells, 1.0±0.02 vs. 1.48±0.01 (T24 vs. T24R,
P<0.001) (Fig. S1). On the
other hand, there was no significant difference in EGFR protein
expression and phosphorylation between parent cells and resistant
cells (Fig. S1).
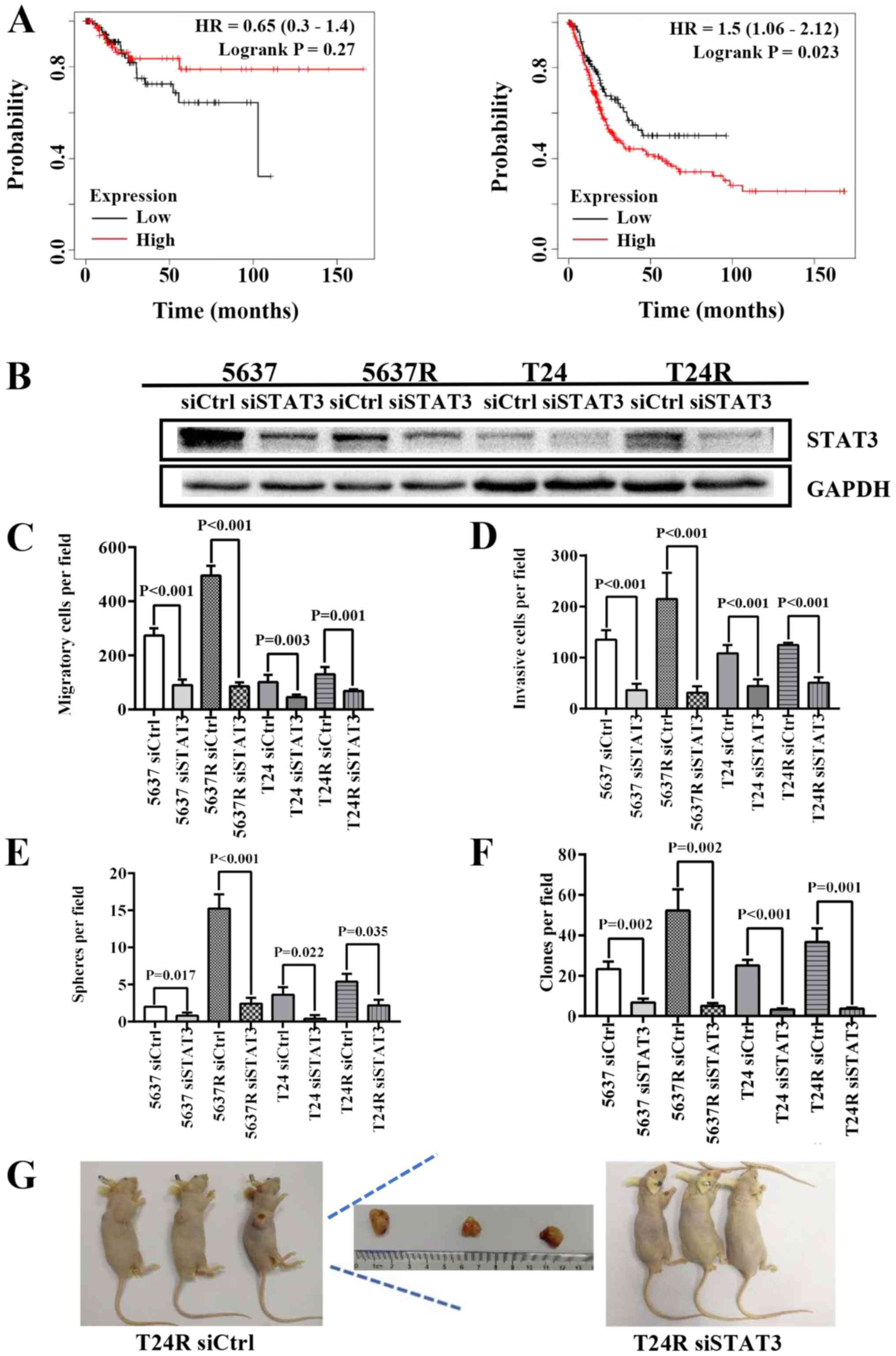 | Figure 4.Depletion of STAT3 abrogates the
self-renewal and tumorigenicity of human BCa cells. (A)
Kaplan-Meier curves comparing the RFS and OS between patients with
BCa expressing high or low levels of STAT3, a log-rank test was
performed (left, RFS; right, OS). (B) Western blot analysis of
STAT3, GAPDH served as a loading control. (C) Quantification of
migration analysis of siCtrl and siSTAT3 BCa cells. (D)
Quantification of invasion analysis of siCtrl and siSTAT3 BCa
cells. (E) Quantification of tumorspheres formed by siCtrl and
siSTAT3 BCa cells. (F) Quantification of soft agar colony formation
analysis of siCtrl and siSTAT3 BCa cells. The data are presented as
the mean ± SEM of triplicate experiments. (G) Results of the tumor
formation assays of siCtrl and siSTAT3 BCa cells (n=3). STAT3,
signal transduction and transcription activator 3; BCa, bladder
cancer; RFS, relapse-free survival; OS, overall survival; si-,
small interfering RNA; Ctrl, control; HR, hazard ratio; R,
radio-resistant cells. |
Discussion
Unlike individuals with other types of cancer, the
survival rate of patients with BCa has not improved over the past
three decades (29). Due to its
ability to preserve and promote the recovery of normal tissues, FI
is widely used to treat BCa. However, the redistribution of
surviving tumor cells during the long-term FI period limits the
efficacy of RT. In fact, redistributing tumors usually acquire ARR,
which results in treatment failure (30). CSCs are unique subpopulations of
cells within tumors. They are capable of self-renewal and
differentiation, and possess high DNA damage repair abilities,
reduced levels of reactive oxygen species production and low
proliferative capacity (31). These
functions impart resistance to a variety of therapeutic methods,
including RT (32). Therefore,
CSC-targeted drug screening presents a promising option for
overcoming ARR.
Our previous study reported that FI increases STAT3
phosphorylation in BCa cells (18).
STAT3 phosphorylation also suppressed the RR of glioblastoma stem
cells, and was associated with improved patient prognosis (33). JAK2 is preferentially upregulated in
colorectal CSC subpopulations, which is accompanied by the
phosphorylation of STAT3. JAK2/STAT3 signaling plays a vital role
in promoting tumor initiation and RR by limiting apoptosis and
enhancing clonogenic potential (34). In 2017, the KMT1A-GATA3-STAT3
pathway was confirmed to be a novel signaling pathway for the
self-renewal of human BCa stem cells (21). Consistent with the results of these
reports, the results of the present study demonstrated that STAT3
is a key molecule linking RR, tumor invasiveness and cancer
stem-like properties in BCa. BCa cells that survived FI were
confirmed to be RR, possess increased migratory and invasive
abilities, as well as enhanced CSC characteristics (Figs. 1 and 2), accompanied by increased
phosphorylation levels of STAT3 (Fig.
3). Furthermore, knockdown of STAT3 expression and inhibiting
its activation in BCa cells significantly inhibited the motility,
and reduced the anchorage and proliferative abilities of tumor
cells in low-adsorption media (Figs.
3 and 4). Notably,
downregulating STAT3 expression also resulted in a loss of tumor
formation ability in immunodeficient mice (Fig. 4G). Similarly, low STAT3 mRNA levels
in patients with BCa was associated with improved OS, compared with
STAT3high patients (Fig.
4A). Collectively, these data indicated that STAT3 plays an
important role in the malignant progression, metastasis and
recurrence of BCa.
Compared with 5637 and T24 cells, a notable
elevation in STAT3 phosphorylation at Tyr705 was observed in their
RR counterparts (5637R and T24R cells). Possible upstream molecular
mechanisms involving enhanced activation of STAT3 were also
investigated; two stem cell-related signaling pathways (namely
KMT1A-GATA3-STAT3 and JAK2-STAT3) were determined, and elevated
expression of KMT1A and JAK2 were detected in RR cells, accompanied
by the increased phosphorylation of STAT3 (Fig. 3B). In addition, the expression of
MMP2 and MMP9, downstream effectors of STAT3 and key molecules in
the regulation of tumor cell migration and invasiveness (35), were upregulated in both RR cell
lines. When STAT3 phosphorylation was inhibited with S3I-201, the
elevated expression of KMT1A, JAK2, MMP2 and MMP9 in RR cells was
significantly suppressed, and the radiosensitivity of these cells
was markedly enhanced (data not shown). Therefore, we hypothesized
that the KMT1A-GATA3/JAK2-STAT3-MMP2/MMP9 signaling cascade is the
key molecular mechanism involved in FI-induced tolerance to IR,
enhancing the motility and CSC-like properties of BCa cells.
However, the high cytotoxicity of S3I-201 limited its use in
further animal experiments. The identification of novel inhibitors
and appropriate methods of administration is still in progress.
In the current study, only T24R cells formed tumors
in nude mice. Although T24 cells (grade III) have a higher
malignant capacity than 5637 cells (grade II), the latter were not
able to form tumors under the current experimental conditions
(1×106 cells/per mouse). However, compared with T24
cells, T24R cells exhibited an increased tumorsphere formation rate
and formed more soft agar anchored clones, indicating that T24R
cells possessed more potent self-renewal and proliferative
abilities than T24 cells (Fig. 2).
In addition, compared with T24 cells, T24R cells exhibited greater
invasive capacity. It was therefore suggested that irradiation may
enrich stem-like BCa cells and promote their ability to form tumors
in vitro. When endogenous STAT3 expression was knocked down,
T24R cells lost the ability to form tumors in vitro, further
confirming that STAT3 plays an important role in maintaining the
CSC-like phenotype of BCa cells.
At present, it is still unclear how IR induces STAT3
phosphorylation in BCa cells. Although EGFR activation in response
to radiation has been demonstrated to be an upstream event of STAT3
phosphorylation (28), the present
study found that only T24R cells showed increased mRNA expression
of EGFR compared with T24 cells (Fig.
S1). These results suggested that EGFR is not likely to be the
key molecule linking IR and STAT3 phosphorylation. On the other
hand, it was observed that JAK2/STAT3 activation was elevated in RR
cells (Fig. 3), therefore it is
speculated that IR-treated cells can release excessive inflammatory
factors, such as IL-6, to activate the JAK-STAT signaling pathway.
Our future studies will focus on elucidating the relationship among
IR, cytokines and STAT3 activation following KMT1A and JAK2
overexpression.
In conclusion, the results of the present study
demonstrated that FI induced RR, increased migration and invasion
of cells, and enhanced the CSC-like characteristics of BCa cells,
which were associated with the aberrant activation of STAT3. These
findings suggested that STAT3 may be an effective therapeutic
target to prevent the progression, metastasis and recurrence of BCa
in patients receiving radiotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant no. 31870846), the
Natural Science Foundation of Shanghai (grant no. 18ZR1403600).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZK designed the experiments. FW, XM and GM performed
the experiments. FW, XM and XZ analyzed the data. ZK, FW and XM
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Committee for
Ethical Use of Experimental Animals at Fudan University (approval
no. 201802144S; Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaufman DS, Shipley WU and Feldman ASJL:
Bladder Cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar
|
|
2
|
Dong F, Xu T, Shen Y, Zhong S, Chen S,
Ding Q and Shen Z: Dysregulation of miRNAs in bladder cancer:
Altered expression with aberrant biogenesis procedure. Oncotarget.
8:27547–27568. 2017. View Article : Google Scholar
|
|
3
|
Park JC, Citrin DE, Agarwal PK and Apolo
AB: Multimodal management of muscle-invasive bladder cancer. Curr
Probl Cancer. 38:80–108. 2014. View Article : Google Scholar
|
|
4
|
Madersbacher S, Hochreiter W, Burkhard F,
Thalmann GN, Danuser H, Markwalder R and Studer UE: Radical
cystectomy for bladder cancer today - a homogeneous series without
neoadjuvant therapy. J Clin Oncol. 21:690–696. 2003. View Article : Google Scholar
|
|
5
|
Smith AB, Jaeger B, Pinheiro LC, Edwards
LJ, Tan HJ, Nielsen ME and Reeve BB: Impact of bladder cancer on
health-related quality of life. BJU Int. 121:549–557. 2018.
View Article : Google Scholar
|
|
6
|
Bajaj A, Martin B, Bhasin R, Hentz C,
Block AM, Harkenrider MM and Solanki AA: The impact of academic
facility type and case volume on survival in patients undergoing
curative radiation therapy for muscle-invasive bladder cancer. Int
J Radiat Oncol Biol Phys. 100:851–857. 2018. View Article : Google Scholar
|
|
7
|
Mitin T, Dengina N, Chernykh M, Usychkin
S, Gladkov O, Degnin C, Chen Y, Nosov D, Tsimafeyeu I, Thomas CR
Jr, et al: Management of muscle invasive bladder cancer with
bladder preservation in Russia: A survey-based analysis of current
practice and the impact of an educational workshop on clinical
expertise. J Cancer Educ. Mar 4–2020.(Epub ahead of print). doi:
10.1007/s13187-020-01728-y. View Article : Google Scholar
|
|
8
|
Shimura T: Acquired radioresistance of
cancer and the AKT/GSK3β/cyclin D1 overexpression cycle. J Radiat
Res (Tokyo). 52:539–544. 2011. View Article : Google Scholar
|
|
9
|
Sharda A, Rashid M, Shah SG, Sharma AK,
Singh SR, Gera P, Chilkapati MK and Gupta S: Elevated HDAC activity
and altered histone phospho-acetylation confer acquired
radio-resistant phenotype to breast cancer cells. Clin Epigenetics.
12:42020. View Article : Google Scholar
|
|
10
|
Anuja K, Kar M, Chowdhury AR, Shankar G,
Padhi S, Roy S, Akhter Y, Rath AK and Banerjee B: Role of telomeric
RAP1 in radiation sensitivity modulation and its interaction with
CSC marker KLF4 in colorectal cancer. Int J Radiat Biol.
96:790–802. 2020. View Article : Google Scholar
|
|
11
|
Pajonk F, Vlashi E and McBride WH:
Radiation resistance of cancer stem cells: The 4 R's of
radiobiology revisited. Stem Cells. 28:639–648. 2010. View Article : Google Scholar
|
|
12
|
Vlashi E and Pajonk F: Cancer stem cells,
cancer cell plasticity and radiation therapy. Semin Cancer Biol.
31:28–35. 2015. View Article : Google Scholar
|
|
13
|
Bighetti-Trevisan RL, Sousa LO, Castilho
RM and Almeida LO: Cancer stem cells: Powerful targets to improve
current anticancer therapeutics. Stem Cells Int. 2019:96180652019.
View Article : Google Scholar
|
|
14
|
Ishiguro T, Ohata H, Sato A, Yamawaki K,
Enomoto T and Okamoto K: Tumor-derived spheroids: Relevance to
cancer stem cells and clinical applications. Cancer Sci.
108:283–289. 2017. View Article : Google Scholar
|
|
15
|
Galoczova M, Coates P and Vojtesek B:
STAT3, stem cells, cancer stem cells and p63. Cell Mol Biol Lett.
23:122018. View Article : Google Scholar
|
|
16
|
Pattabiraman DR and Weinberg RA: Tackling
the cancer stem cells - what challenges do they pose? Nat Rev Drug
Discov. 13:497–512. 2014. View
Article : Google Scholar
|
|
17
|
Lin L, Fuchs J, Li C, Olson V, Bekaii-Saab
T and Lin J: STAT3 signaling pathway is necessary for cell survival
and tumorsphere forming capacity in
ALDH+/CD133+ stem cell-like human colon
cancer cells. Biochem Biophys Res Commun. 416:246–251. 2011.
View Article : Google Scholar
|
|
18
|
Mao G, Yao Y and Kong Z: Long term
exposure to γ rays induces radioresistance and enhances the
migration ability of bladder cancer cells. Mol Med Rep.
18:5834–5840. 2018.
|
|
19
|
Chang R, He H, Mao G and Kong Z:
Upregulating DAB2IP expression via EGR-1 inhibition, a new approach
for overcoming fractionated-irradiation-induced cross-tolerance to
ionizing radiation and mitomycin C in tumor cells. Int J Radiat
Biol. 93:386–393. 2017. View Article : Google Scholar
|
|
20
|
Sen N, Che X, Rajamani J, Zerboni L, Sung
P, Ptacek J and Arvin AM: Signal transducer and activator of
transcription 3 (STAT3) and survivin induction by varicella-zoster
virus promote replication and skin pathogenesis. Proc Natl Acad Sci
USA. 109:600–605. 2012. View Article : Google Scholar
|
|
21
|
Yang Z, He L, Lin K, Zhang Y, Deng A, Yong
Liang Y, Li C and Wen T: The KMT1A-GATA3-STAT3 circuit is a novel
self-renewal signaling of human bladder cancer stem cells. Clin
Cancer Res. 23:6673–6685. 2017. View Article : Google Scholar
|
|
22
|
Nagy Á, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:92272018.
View Article : Google Scholar
|
|
23
|
Livak and Schmittgen, . Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-DDct method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
24
|
Eun K, Ham SW and Kim H: Cancer stem cell
heterogeneity: origin and new perspectives on CSC targeting. BMB
Rep. 50:117–125. 2017. View Article : Google Scholar
|
|
25
|
Ahmad G and Amiji MM: Cancer stem
cell-targeted therapeutics and delivery strategies. Expert Opin
Drug Deliv. 14:997–1008. 2017. View Article : Google Scholar
|
|
26
|
Tran MN, Goodwin Jinesh G, McConkey DJ and
Kamat AM: Bladder cancer stem cells. Curr Stem Cell Res Ther.
5:387–395. 2010. View Article : Google Scholar
|
|
27
|
Abubaker K, Luwor RB, Zhu H, McNally O,
Quinn MA, Burns CJ, Thompson EW, Findlay JK and Ahmed N: Inhibition
of the JAK2/STAT3 pathway in ovarian cancer results in the loss of
cancer stem cell-like characteristics and a reduced tumor burden.
BMC Cancer. 14:3172014. View Article : Google Scholar
|
|
28
|
Gao L, Li FS, Chen XH, Liu QW, Feng J B,
Liu QJ and Su X: Radiation induces phosphorylation of STAT3 in a
dose- and time-dependent manner. Asian Pac J Cancer Prev.
15:6161–6164, 201. View Article : Google Scholar
|
|
29
|
Berdik C: Unlocking bladder cancer.
Nature. 551:S34–S35. 2017. View
Article : Google Scholar
|
|
30
|
Li JY, Li YY, Jin W, Yang Q, Shao ZM and
Tian XS: ABT-737 reverses the acquired radioresistance of breast
cancer cells by targeting Bcl-2 and Bcl-xL. J Exp Clin Cancer Res.
31:1022012. View Article : Google Scholar
|
|
31
|
Najafi M, Farhood B, Mortezaee K,
Kharazinejad E, Majidpoor J and Ahadi R: Hypoxia in solid tumors: A
key promoter of cancer stem cell (CSC) resistance. J Cancer Res
Clin Oncol. 146:19–31. 2020. View Article : Google Scholar
|
|
32
|
Ohishi T, Koga F and Migita T: Bladder
cancer stem-like cells: Their origin and therapeutic perspectives.
Int J Mol Sci. 17:172015. View Article : Google Scholar
|
|
33
|
Masliantsev K, Pinel B, Balbous A, Guichet
PO, Tachon G, Milin S, Godet J, Duchesne M, Berger A, Petropoulos
C, et al: Impact of STAT3 phosphorylation in glioblastoma stem
cells radiosensitization and patient outcome. Oncotarget.
9:3968–3979. 2017. View Article : Google Scholar
|
|
34
|
Park SY, Lee CJ, Choi JH, Kim JH, Kim JW,
Kim JY and Nam JS: The JAK2/STAT3/CCND2 Axis promotes colorectal
cancer stem cell persistence and radioresistance. J Exp Clin Cancer
Res. 38:3992019. View Article : Google Scholar
|
|
35
|
Huang S: Regulation of metastases by
signal transducer and activator of transcription 3 signaling
pathway: clinical implications. Clin Cancer Res. 13:1362–1366.
2007. View Article : Google Scholar
|