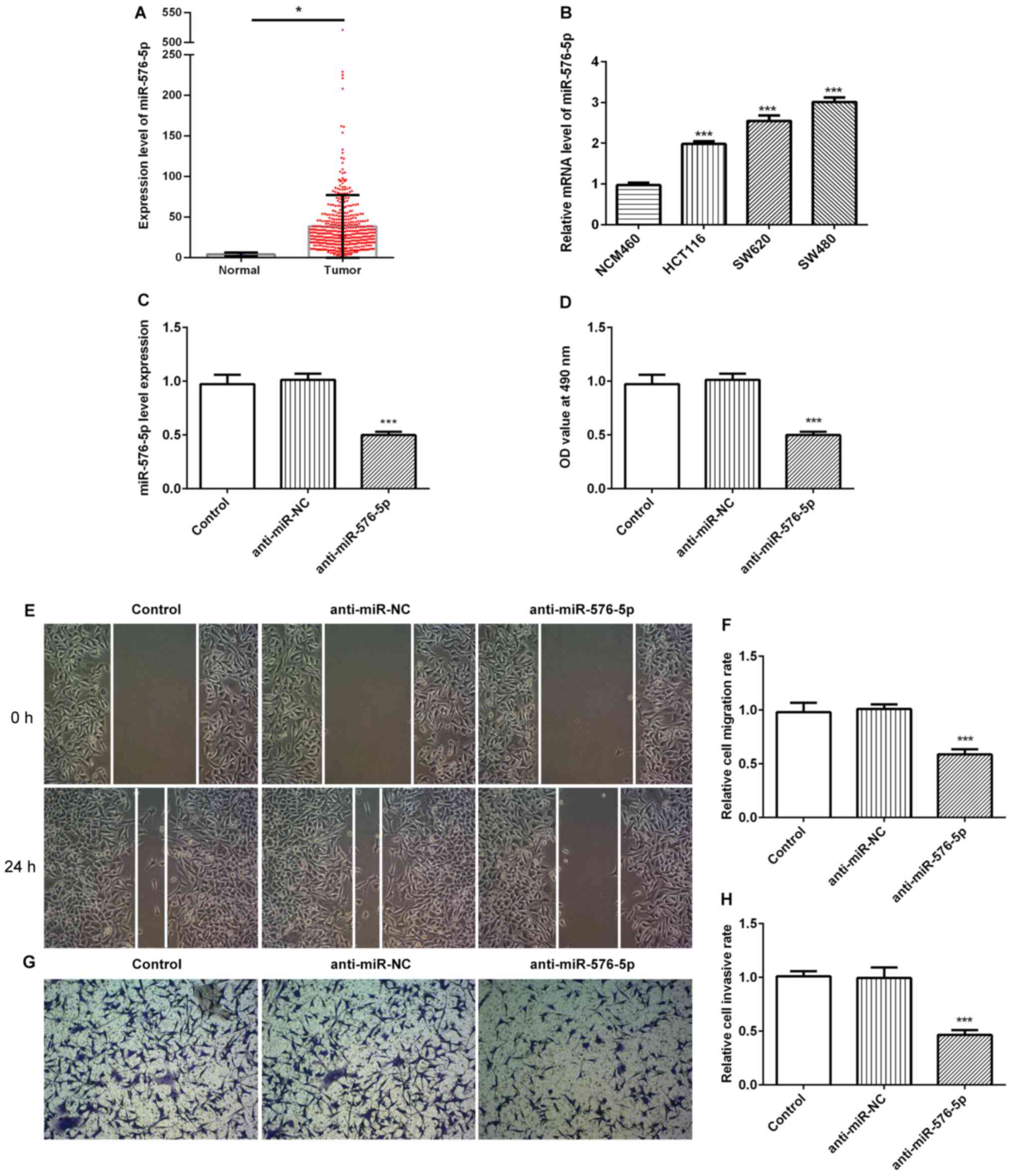
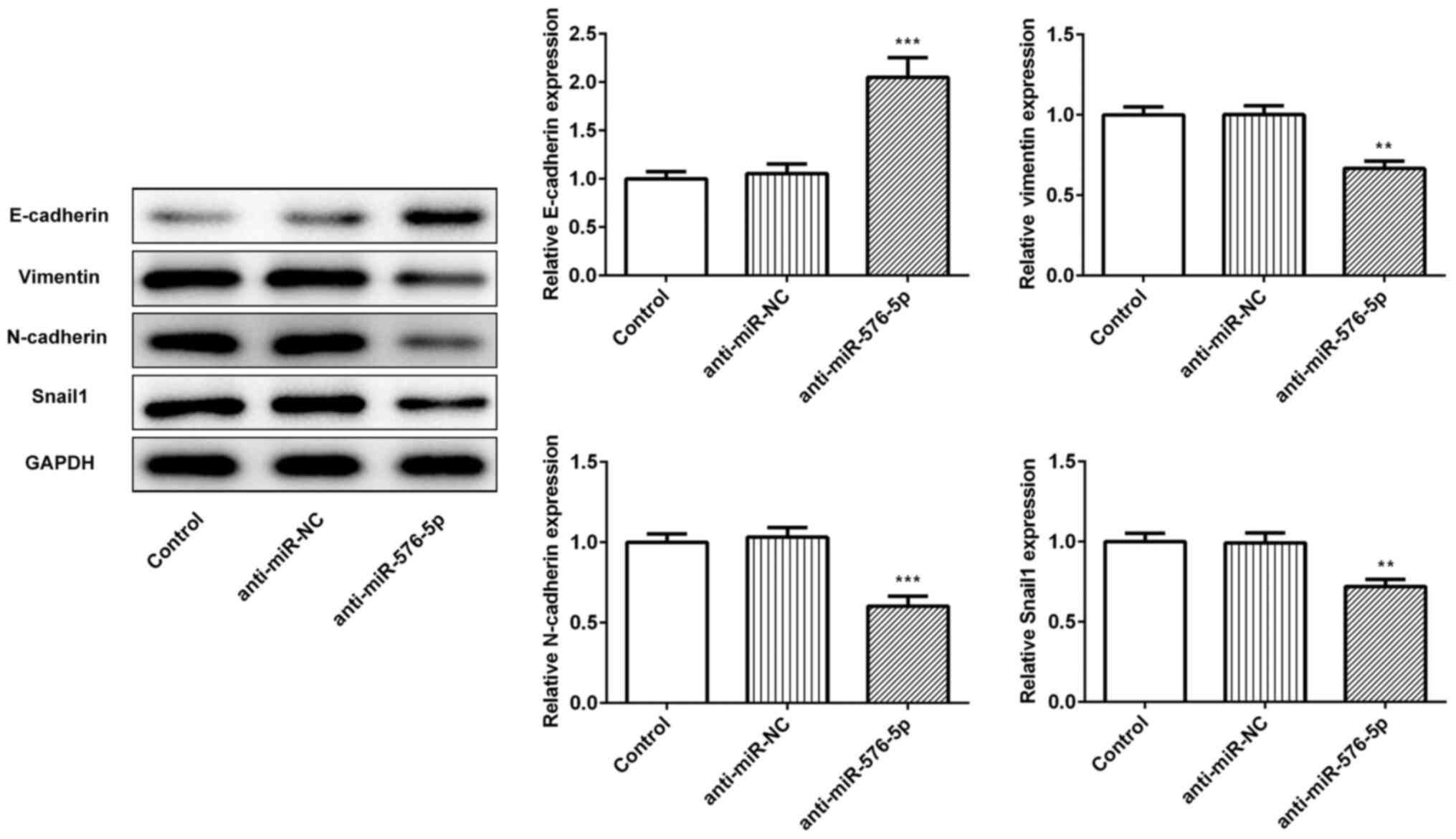
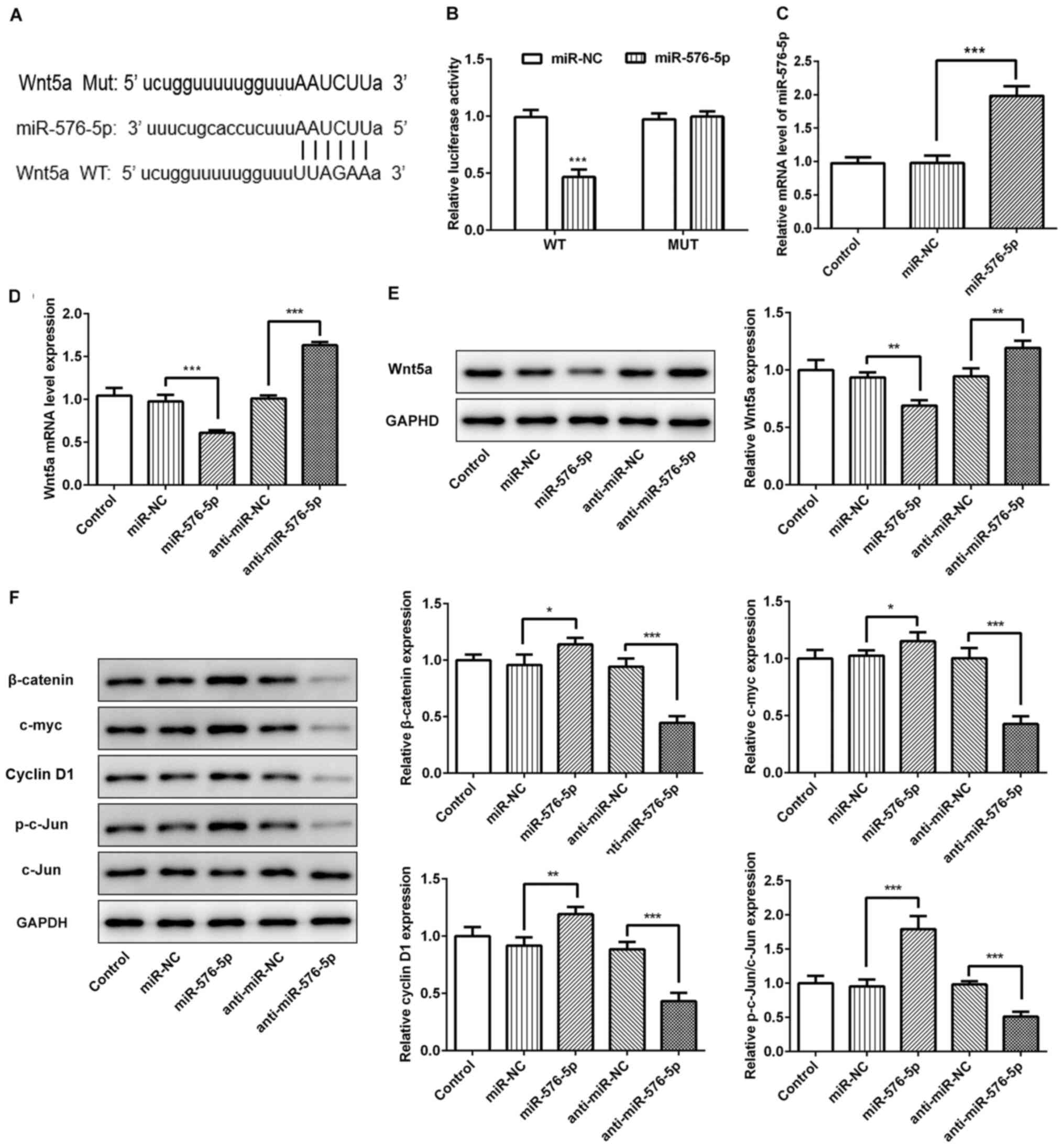
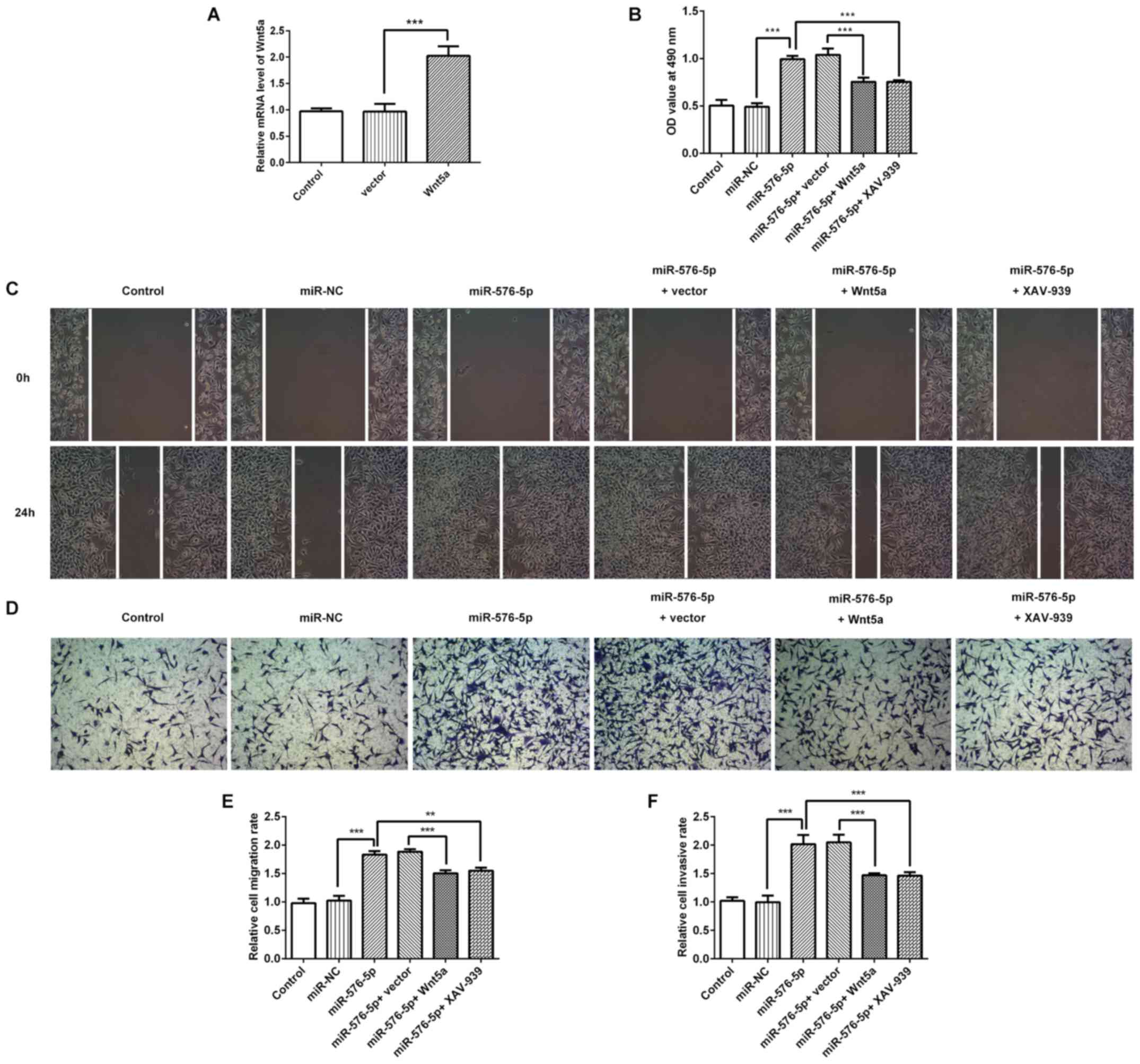
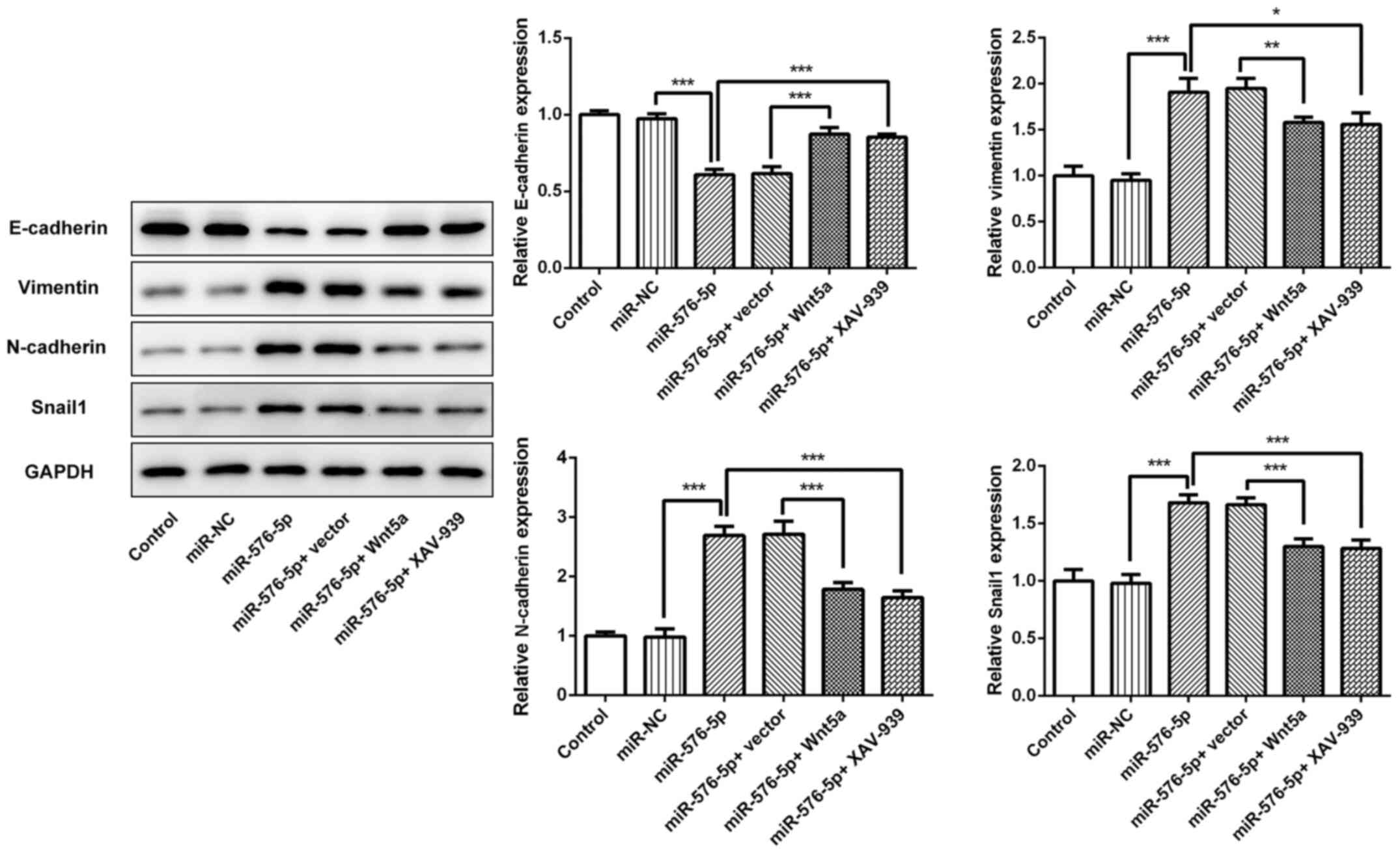
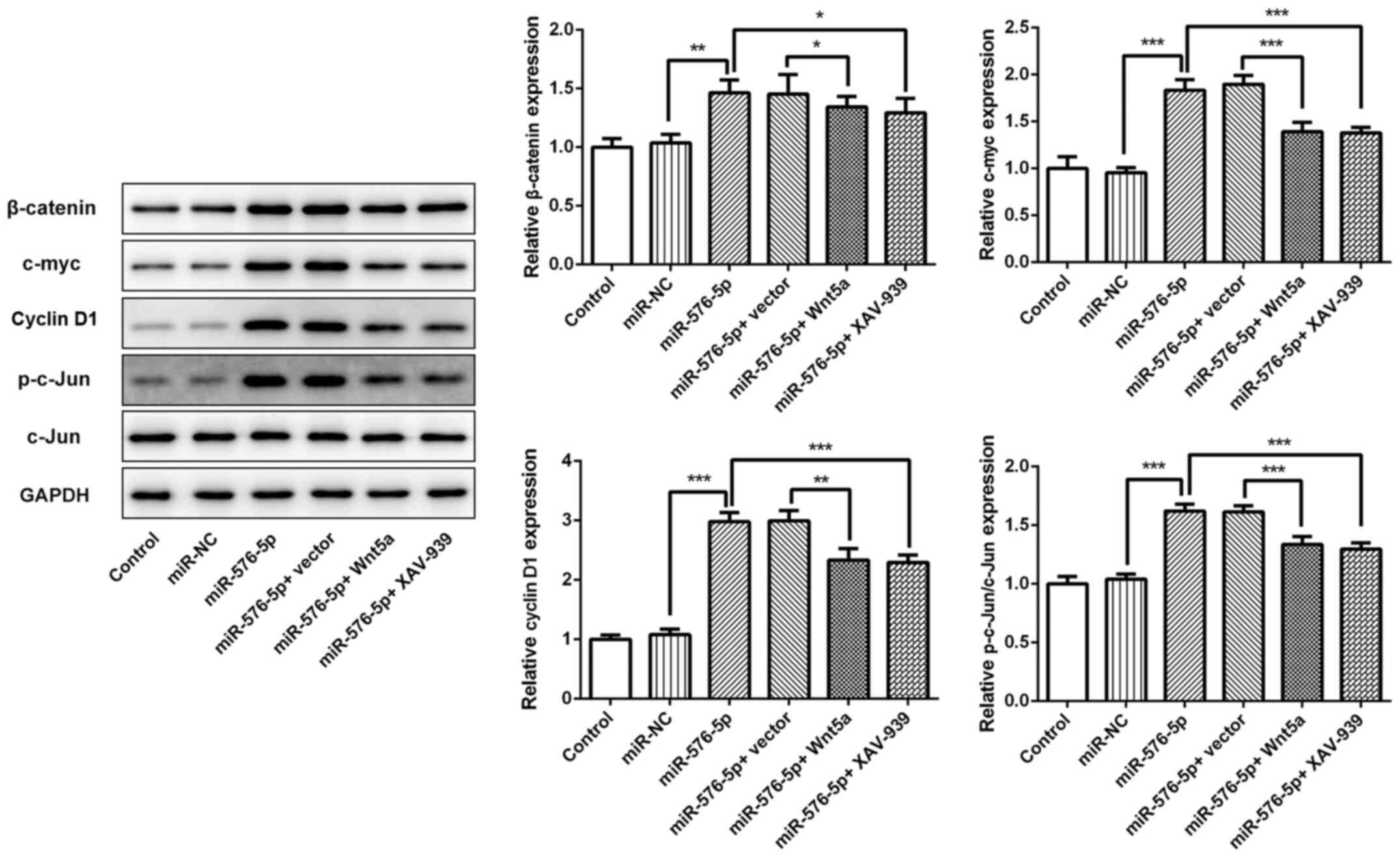
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu CW, ZhouDDXieTHaoJ L, Pant OP, Lu CB
and Liu XF: HOXAll antisense long noncoding RNA (HOXAll-AS): A
promising lncRNA in human cancers. Cancer Med. 7:3792–3799. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McArdle CS and Hole DJ: Outcome following
surgery for colorectal cancer: Analysis by hospital after
adjustment for case-mix and deprivation. Br J Cancer. 86:331–335.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holm M, Saraswat M, Joenväärä S, Ristimäki
A, Haglund C and Renkonen R: Colorectal cancer patients with
different C-reactive protein levels and 5-year survival times can
be differentiated with quantitative serum proteomics. PLoS One.
13:e01953542018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsuchiya S, Okuno Y and Tsujimoto G:
MicroRNA: Biogenetic and functional mechanisms and involvements in
cell differentiation and cancer. J Pharmacol Sci. 101:267–270.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartels CL and Tsongalis GJ: MicroRNAs:
Novel biomarkers for human cancer. Clin Chem. 55:623–631. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun GL, Li Z, Wang WZ, Chen Z, Zhang L, Li
Q, Wei S, Li BW, Xu JH, Chen L, et al: miR-324-3p promotes gastric
cancer development by activating Smad4-mediated Wnt/beta-catenin
signaling pathway. J Gastroenterol. 53:725–739. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan Z, Cui H, Xu X, Lin Z, Zhang X, Kang
L, Han B, Meng J, Yan Z, Yan X and Jiao S: MiR-125a suppresses
tumor growth, invasion and metastasis in cervical cancer by
targeting STAT3. Oncotarget. 6:25266–25280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang C, Hu X, Alattar M and Zhao H: miRNA
expression profiles associated with diagnosis and prognosis in lung
cancer. Expert Rev Anticancer Ther. 14:453–461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kordass T, Weber CEM, Eisel D, Pane AA,
Osen W and Eichmüller SB: miR-193b and miR-30c-1* inhibit, whereas
miR-576-5p enhances melanoma cell invasion in vitro. Oncotarget.
9:32507–32522. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ni XF, Zhao LH, Li G, Hou M, Su M, Zou CL
and Deng X: MicroRNA-548-3p and MicroRNA-576-5p enhance the
migration and invasion of esophageal squamous cell carcinoma cells
via NRIP1 down-regulation. Neoplasma. 65:881–887. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Chen J, Wang L, Chen L, Du Z, Zhu
L, Cui M, Zhang M and Song L: Linc-PINT acted as a tumor suppressor
by sponging miR-543 and miR-576-5p in esophageal cancer. J Cell
Biochem. 120:19345–19357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang YN, Chen ZH and Chen WC: Novel
circulating microRNAs expression profile in colon cancer: A pilot
study. Eur J Med Res. 22:512017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghahhari NM and Babashah S: Interplay
between microRNAs and WNT/β-catenin signalling pathway regulates
epithelial-mesenchymal transition in cancer. Eur J Cancer.
51:1638–1649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumawat K and Gosens R: WNT-5A: Signaling
and functions in health and disease. Cell Mol Life Sci. 73:567–587.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calin GA and Croce CM: MicroRNA-cancer
connection: The beginning of a new tale. Cancer Res. 66:7390–7394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu W, Luo X, Fu H, Liu L, Sun P and Wang
Z: MiR-3653 inhibits the metastasis and epithelial-mesenchymal
transition of colon cancer by targeting Zeb2. Pathol Res Pract.
215:1525772019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen E, Li Q, Wang H, Yang F, Min L and
Yang J: MiR-92a promotes tumorigenesis of colorectal cancer, a
transcriptomic and functional based study. Biomed Pharmacother.
106:1370–1377. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang L, Zhang Y, Li Z, Zhao X, Xi Z, Chen
H, Shi H, Xin T, Shen R and Wang T: MiR-4319 suppresses colorectal
cancer progression by targeting ABTB1. United European
Gastroenterol J. 7:517–528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rodrigo JP, Martínez P, Allonca E,
Alonso-Durán L, Suárez C, Astudillo A and García-Pedrero J:
Immunohistochemical markers of distant metastasis in laryngeal and
hypopharyngeal squamous cell carcinomas. Clin Exp Metastasis.
31:317–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hugo H, Ackland ML, Blick T, Lawrence MG,
Clements JA, Williams ED and Thompson EW: Epithelial-mesenchymal
and mesenchymal-epithelial transitions in carcinoma progression. J
Cell Physiol. 213:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li D, Tian B and Jin X: miR-630 inhibits
epithelial-to-mesenchymal transition (EMT) by regulating the
Wnt/β-catenin pathway in gastric cancer cells. Oncol Res. 27:9–17.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Montorsi L, Guizzetti F, Alecci C,
Caporali A, Martello A, Atene CG, Parenti S, Pizzini S, Zanovello
P, Bortoluzzi S, et al: Loss of ZFP36 expression in colorectal
cancer correlates to wnt/β-catenin activity and enhances
epithelial-to-mesenchymal transition through upregulation of ZEB1,
SOX9 and MACC1. Oncotarget. 7:59144–59157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kikuchi A, Yamamoto H, Sato A and
Matsumoto S: Wnt5a: Its signalling, functions and implication in
diseases. Acta Physiol (Oxf). 204:17–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Asem MS, Buechler S, Wates RB, Miller DL
and Stack MS: Wnt5a Signaling in Cancer. Cancers (Basel). 8:792016.
View Article : Google Scholar
|
|
28
|
Qin L, Yin YT, Zheng FJ, Peng LX, Yang CF,
Bao YN, Liang YY, Li XJ, Xiang YQ, Sun R, et al: WNT5A promotes
stemness characteristics in nasopharyngeal carcinoma cells leading
to metastasis and tumorigenesis. Oncotarget. 6:10239–10252. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Da Forno PD, Pringle JH, Hutchinson P,
Osborn J, Huang Q, Potter L, Hancox RA, Fletcher A and Saldanha GS:
WNT5A expression increases during melanoma progression and
correlates with outcome. Clin Cancer Res. 14:5825–5832. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang T, Liu X and Wang J: Up-regulation of
Wnt5a inhibits proliferation and migration of hepatocellular
carcinoma cells. J Cancer Res Ther. 15:904–908. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bi L, Liu X, Wang C, Cao Y, Mao R, Li P
and Geng M: Wnt5a involved in regulation of the biological behavior
of hepatocellular carcinoma. Int J Clin Exp Pathol. 7:987–995.
2014.PubMed/NCBI
|
|
32
|
Prasad CP, Manchanda M, Mohapatra P and
Andersson T: WNT5A as a therapeutic target in breast cancer. Cancer
Metastasis Rev. 37:767–778. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Q and Chen H: Silencing of Wnt5a during
colon cancer metastasis involves histone modifications.
Epigenetics. 7:551–558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng R, Sun B, Liu Z, Zhao X, Qi L, Li Y
and Gu Q: Wnt5a suppresses colon cancer by inhibiting cell
proliferation and epithelial-mesenchymal transition. J Cell
Physiol. 229:1908–1917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Amerongen R, Mikels A and Nusse R:
Alternative wnt signaling is initiated by distinct receptors. Sci
Signal. 1:re92008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Howard S, Deroo T, Fujita Y and Itasaki N:
A positive role of cadherin in Wnt/β-catenin signalling during
epithelial-mesenchymal transition. PLoS One. 6:e238992011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wijnhoven BP, Dinjens WN and Pignatelli M:
E-cadherin-catenin cell-cell adhesion complex and human cancer. Br
J Surg. 87:992–1005. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi L, Wu YX, Yu JH, Chen X, Luo XJ and
Yin YR: Research of the relationship between β-catenin and
c-myc-mediated Wnt pathway and laterally spreading tumors
occurrence. Eur Rev Med Pharmacol Sci. 21:252–257. 2017.PubMed/NCBI
|
|
40
|
Valenta T, Hausmann G and Basler K: The
many faces and functions of β-catenin. EMBO J. 31:2714–2736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fang Y, Shen ZY, Zhan YZ, Feng XC, Chen
KL, Li YS, Deng HJ, Pan SM, Wu DH and Ding Y: CD36 inhibits
β-catenin/c-myc-mediated glycolysis through ubiquitination of GPC4
to repress colorectal tumorigenesis. Nat Commun. 10:39812019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xie C, Pan Y, Hao F, Gao Y, Liu Z, Zhang
X, Xie L, Jiang G, Li Q and Wang E: C-Myc participates in
β-catenin-mediated drug resistance in A549/DDP lung adenocarcinoma
cells. APMIS. 122:1251–1258. 2014. View Article : Google Scholar : PubMed/NCBI
|