Introduction
Premature rupture of membranes (PROM) refers to the
spontaneous rupture of membranes surrounding the amniotic cavity in
pregnancies before labor (1). It is
reported that the incidence rate of PROM worldwide is 5–15%
(2), it can be observed at any
gestational age (3) and occurs in
~10% of pregnancies and in ~40% of preterm deliveries (4). Preterm PROM (PPROM) is the rupture of
fetal membranes in pregnancies between 20 and 37 weeks of gestation
and the incidence rate is 2.0–3.5% worldwide (1). Mature PROM (MPROM) occurs after 37
weeks of gestation with a 10% incidence rate (1). PROM without proper treatment can
result in endometritis, chorioamnionitis, placental abruption,
premature labor, fetal infection and fetal distress, which threaten
maternal and fetal health and life. Recent studies have
demonstrated that reproductive system infection is one of the
important causes of PROM, however, the specific mechanism is
unknown (5–7).
Inflammasomes have been recognized for their crucial
effects in host defense against pathogens; dysfunction of
inflammasome activation is associated with the development of
tumorigenesis and metabolic, neurodegenerative, autoimmune and
inflammatory diseases (8). To date,
four receptor proteins have been determined to assemble
inflammasomes, including the NLR family pyrin domain containing
(NLRP)1, NLRP3, NLR family CARD-domain containing 4 (NLRC4) and
absent in melanoma 2 (AIM2) (5).
Recognition of the inflammatory ligand by these receptors results
in sensor activation, oligomerization and the recruitment of an
adaptor protein known as apoptosis-associated speck-like protein
containing a caspase recruitment domain (ASC). ASC serves as a
bridge, connecting the sensor to the downstream effector caspase1
(Casp1) (9). Proximity-induced
autoprocessing results in the formation of the catalytical active
protease Casp1 p20 (10), which
initiates downstream responses, including the release and
conversion of IL-18 and IL-1β from proIL-18 and proIL-1β (11) and induces pyroptosis, which is a
lytic form of cell death. Indeed, the expression levels of Casp1
p20 is considered an indicator of inflammasome activity (12,13).
Although the activation of inflammasomes is linked to inflammatory
response to some extent, there is no report that it is related to
PROM progress, to the best of the authors' knowledge.
A disintegrin and metalloproteinase with
thrombospondin motifs 4 (ADAMTS4) is a member of ADAMTS protease
family that serves an important role in many processes such as
organogenesis, angiogenesis, ovulation, cervical dilation and
parturition (14). ADAMTS4 is
upregulated in PROM and affects the development of PROM through
Ras-mediated activation of NADPH oxidase (15,16).
However, the ADAMTS4 expression in placenta and fetal membrane
tissues of PPROM and MPROM patients and the relationship between
ADAMTS4 and inflammasome components remain to be elucidated.
The aims of the present study were to explore the
activation levels of several classical inflammasomes, NLRP1, NLRP3,
AIM2 and NLRC4, in PPROM, MPROM and control patients. Furthermore,
ADAMTS4 expression and the correlation analysis between ADAMTS4 and
inflammasome components in PROM patients were also
investigated.
Materials and methods
Study design
The present study was conducted at Xuzhou Maternity
and Child Health Care Hospital Affiliated to Xuzhou Medical
University between April 2017 and December 2019. The protocol of
the present study was approved by the Medical Ethics Committee of
Xuzhou Maternity and Child Health Care Hospital Affiliated to
Xuzhou Medical University (approval no. 201502) and all the
patients included in the study provided signed informed consent. A
total of 60 patients aged 23–41 years with a singleton pregnancy
who were admitted to the Department of Obstetrics were recruited,
including 20 cases of PPROM patients (gestational week <37
weeks), 20 cases of MPROM patients (gestational week ≥37 weeks) and
20 cases of healthy pregnancies as control (gestational week ≥37
weeks) groups. Gestational age was determined based on the first
trimester ultrasound scan for all pregnancies.
The diagnosis of PROM was performed using a speculum
examination by visualizing the characteristic pooling of amniotic
fluid in the vagina, together with a positive test for the presence
of insulin-like growth factor-binding protein (ACTIM PROM test; Oy
Medix Biochemica Ab) in vaginal fluid. The exclusion criteria of
all pregnancies in the present study were: Structural or
chromosomal abnormalities of the fetus, signs of fetal hypoxia,
signs of intrauterine fetal growth restriction, vaginal bleeding,
or any medical complication such as diabetes mellitus hypertension,
preeclampsia, or thyroid disease.
Samples
Placenta specimens were obtained from the chorion
frondosum of the maternal surface of placenta during the cesarean
section. Fetal membrane specimens were acquired near the rupture of
fetal membrane. A part of the specimens was stored at −80°C for
total RNA and protein extraction, the other parts were immersed
into 4% paraformaldehyde at room temperature for 24 h for
immunohistochemical staining.
Immunohistochemistry
Placenta and fetal membrane specimen sections (4 µm)
were deparaffinized, rehydrated and incubated with 3% hydrogen
peroxide at room temperature for 30 min for endogenous peroxidase
activity blockage. Following blocking with 5% bovine serum albumin
(cat. no. A3858; Sigma-Aldrich; Merck KGaA) for 1 h at room
temperature, sections were incubated with primary antibodies
against human NLRP1 (cat. no. ab3683; 1:100 dilution; Abcam), human
NLRRP3 (cat. no. ab214185; 1:100 dilution; Abcam), human AIM2 (cat.
no. 93015; 1:100 dilution; Abcam) and human NLRC4 (cat. no. 99860;
1:100 dilution; Abcam) at 4°C overnight. Tissue sections were then
rinsed with phosphate-buffered saline and treated with
streptavidin-peroxidase conjugated IgG secondary antibody (cat. no.
SP-9001; OriGene Technologies, Inc.) at 37°C for 30 min. The
sections were then stained a brown color under a light microscope
with diamino-3,3′-benzidine tetrahydrochloride (DAB; OriGene
Technologies, Inc.) incubated at room temperature for 5 min. Slides
were counterstained with hematoxylin at room temperature for 5 min,
dehydrated and mounted. Protein levels were analyzed by Image-Pro
Plus 6.0 software (Media Cybernetics, Inc.).
ELISA
The protein concentration of ADAMTS4 was determined
using human ADAMTS4 ELISA kit (cat. no. CSB-E11848h; Cusabio
Technology LLC) according to the manufacturer's instructions. The
absorbance was determined using a microplate reader and all samples
were tested in duplicate. The standard curves were drawn by
plotting absorbance values against the gradient standard
concentrations and the linear regression was then analyzed. The
ADAMTS4 concentration in cell supernatants of placenta and fetal
membrane was calculated according to the curve linear equation.
Protein preparation and western blot
analysis
Placenta and fetal membranes were homogenized and
the cells were lysed. In brief, ~100 mg placenta or fetal membrane
tissue was homogenized at 4°C with a homogenizer. Total protein was
extracted using Tissue or Cell Total Protein Extraction kit (cat.
no. C510003; Sangon Biotech Co., Ltd.) according to the
manufacturer's instructions. The concentration of proteins was
established with the bicinchoninic acid (BCA) method with BCA
Protein Concentration Assay kit (cat. no. P0012S; Beyotime
Institute of Biotechnology). Tissue homogenates were analyzed
through standard immunoblotting analysis. Briefly, samples were
boiled at 100°C for 5 min and 40 µg protein with equal volumes (20
µl) of each sample was loaded onto 12% sodium dodecyl
sulfate-acrylamide gels. Protein was separated at a constant
voltage of 120 V and transferred onto nitrocellulose filter
membrane. Following blocking with 5% skimmed milk at room
temperature for 2 h, membranes were incubated with antibodies
against human NLRP1 (cat. no. ab3683; 1:1,000 dilution; Abcam),
NLRP3 (cat. no. ab214185; 1:1,000 dilution; Abcam), AIM2 (cat. no.
ab93015; 1:1,000 dilution; Abcam), NLRC4 (cat. no. ab99860; 1:1,000
dilution; Abcam), ASC (cat. no. ab155970; 1:1,000 dilution; Abcam),
Casp1 (cat. no. 3866; 1:1,000 dilution; Cell Signaling Technology,
Inc.), Casp1 p20 (cat. no. 4199; 1:1,000 dilution; Cell Signaling
Technology, Inc.), proIL-18 (cat. no. 10663-1-AP; 1:1,000 dilution;
ProteinTech Group, Inc.), IL-18 (cat. no. sc-7954; 1:200 dilution;
Santa Cruz Biotechnology, Inc.), proIL-1β (cat. no. ab156791;
1:1,000 dilution; Abcam), IL-1β (cat. no. 83186; 1:1,000 dilution;
Cell Signaling Technology, Inc.) and ADAMTS4 (cat. no. ab185722;
1:1,000 dilution; Abcam) at 4°C overnight. Anti-GAPDH (cat. no.
ab9485; 1:1,000 dilution; Abcam) was applied as loading control.
Membranes were then washed with Tris-buffered saline (TBS)
containing 0.1% Tween-20 at room temperature for 5 min three times
and incubated with horseradish peroxidase (HRP)-conjugated
anti-mouse IgG (cat. no. 7076; 1:10,000 dilution; Cell Signaling
Technology, Inc.) or HRP-conjugated anti-rabbit IgG (cat. no. 7074;
1:10,000 dilution; Cell Signaling Technology, Inc.) at room
temperature for 2 h. The blots were visualized with an enhanced
chemiluminescence solution (cat. no. P0018; Beyotime Institute of
Biotechnology), then exposed to film and quantified with ImageJ
v2.0 software (National Institutes of Health). The expression level
of target protein was calculated by dividing the intensity of the
target protein by the intensity of GAPDH protein.
Reverse transcription-quantitative
(RT-q) PCR
The frozen placenta and fetal membrane tissues were
thawed and RNA was extracted using RNAiso Plus Total RNA extraction
reagent (cat. no. 9109; Takara Bio, Inc.), followed by DNase I
treatment. RNA concentration was analyzed using NanoDrop (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.) and RNA quality was
quantified by the Agilent 2100 Bioanalyzer (Agilent Technologies,
Inc.) to produce an electrophoresis trace from which the RNA
integrity number and DV200 index were calculated using the 2100
Expert Software (version no. B.02.10; Agilent Technologies, Inc.).
RNA was then reverse-transcribed to cDNA on a Veriti 96-well
Thermal Cycler (Applied Biosystems) with PrimeScript RT reagent kit
(Takara Bio, Inc.) with thermocycling conditions of 42°C for 15 min
and 85°C for 5 min. Primers were synthesized by Takara Bio, Inc.
(sequences in Table I). PCR
amplifications were performed on a LightCycler 480 Real-Time PCR
System (Roche Diagnostics GmbH) with SYBR-Green PCR Master mix
(Applied Biosystems) with thermocycling conditions of 95°C for 30
sec and 40 cycles at 95°C for 5 sec and 60°C for 30 sec. All
procedures including RNA extraction, cDNA synthesis and qPCR were
performed according to the manufacturer's protocols. Relative mRNA
expression was calculated using the 2−ΔΔCq method
(17) following normalization to
GAPDH expression.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Primer sequences |
|---|
| NLRP1 | F:
5′-CCACAACCCTCTGTCTACATTAC-3′ |
|
| R:
5′-GCCCCATCTAACCCATGCTTC-3′ |
| NLRP3 | F:
5′-GATCTTCGCTGCGATCAACA-3′ |
|
| R:
5′-GGGATTCGAAACACGTGCATTA-3′ |
| AIM2 | F:
5′-CTGCAGTGATGAAGACCATTCGTA-3′ |
|
| R:
5′-GGTGCAGCACGTTGCTTTG-3′ |
| NLRC4 | F:
5′-CCAGTCCCCTCACCATAGAAG-3′ |
|
| R:
5′-ACCCAAGCTGTCAGTCAGACC-3′ |
| ASC | F:
5′-AACCCAAGCAAGATGCGGAAG-3′ |
|
| R:
5′-TTAGGGCCTGGAGGAGCAAG-3′ |
| Caspase-1 | F:
5′-GCCTGTTCCTGTGATGTGGAG-3′ |
|
| R:
5′-TGCCCACAGACATTCATACAGTTTC-3′ |
| IL-18 | F:
5′-CTGCCACCTGCTGCAGTCTA-3′ |
|
| R:
5′-TCTACTGGTTCAGCAGCCATCTTTA-3′ |
| IL-1β | F:
5′-CCAGGGACAGGATATGGAGCA-3′ |
|
| R:
5′-TTCAACACGCAGGACAGGTACAG-3′ |
| ADAMTS4 | F:
5′-GAGGGAGGCACCCCTAACT-3′ |
|
| R:
5′-CCTTGACGTTGCACATGGGA-3 |
| GAPDH | F:
5′-GCACCGTCAAGGCTGAGAAC-3′ |
|
| R:
5′-TGGTGAAGACGCCAGTGGA-3′ |
Statistical analysis
Data are expressed as mean ± SEM and statistical
analyses were performed using SPSS 23.0 software (IBM Corp.).
Comparison between groups was carried out by unpaired Student's
t-test, chi-square test, or one-way ANOVA followed by Bonferroni's
post hoc test. Bivariate correlation analysis was performed using
Pearson or Spearman rank correlation. All calculated P-values were
two-sided and P<0.05 was considered to indicate a statistically
significant difference.
Results
Elevated expression of
inflammasome-related receptor proteins in placenta of PROM
pregnancies
The mRNA and protein expression of
inflammasome-related receptors in placenta tissues were identified.
As shown in Fig. 1A, the mRNA
expression of NLRP1, NLRP3, AIM2 and NLRC4 in PPROM groups were all
significantly increased compared with controls. In addition, mRNA
expression of these receptors was further upregulated in MPROM
groups compared with controls. As common components of several
inflammasomes, the expression of ASC and Casp1 mRNA were also
promoted in PPROM and MPROM groups when compared with control
group. The protein levels of these inflammasome components in PPROM
and MPROM groups changed in accordance with the changes in mRNA
levels (Fig. 1B-D). These results
suggested that the mRNA and protein expression of all inflammasome
components, NLRP1, NLRP3, AIM2, NLRC4, ASC and Casp1, were
upregulated in placenta tissue of PPROM patients. Notably, these
trends of increasing inflammasome components expression were more
pronounced in MPROM groups.
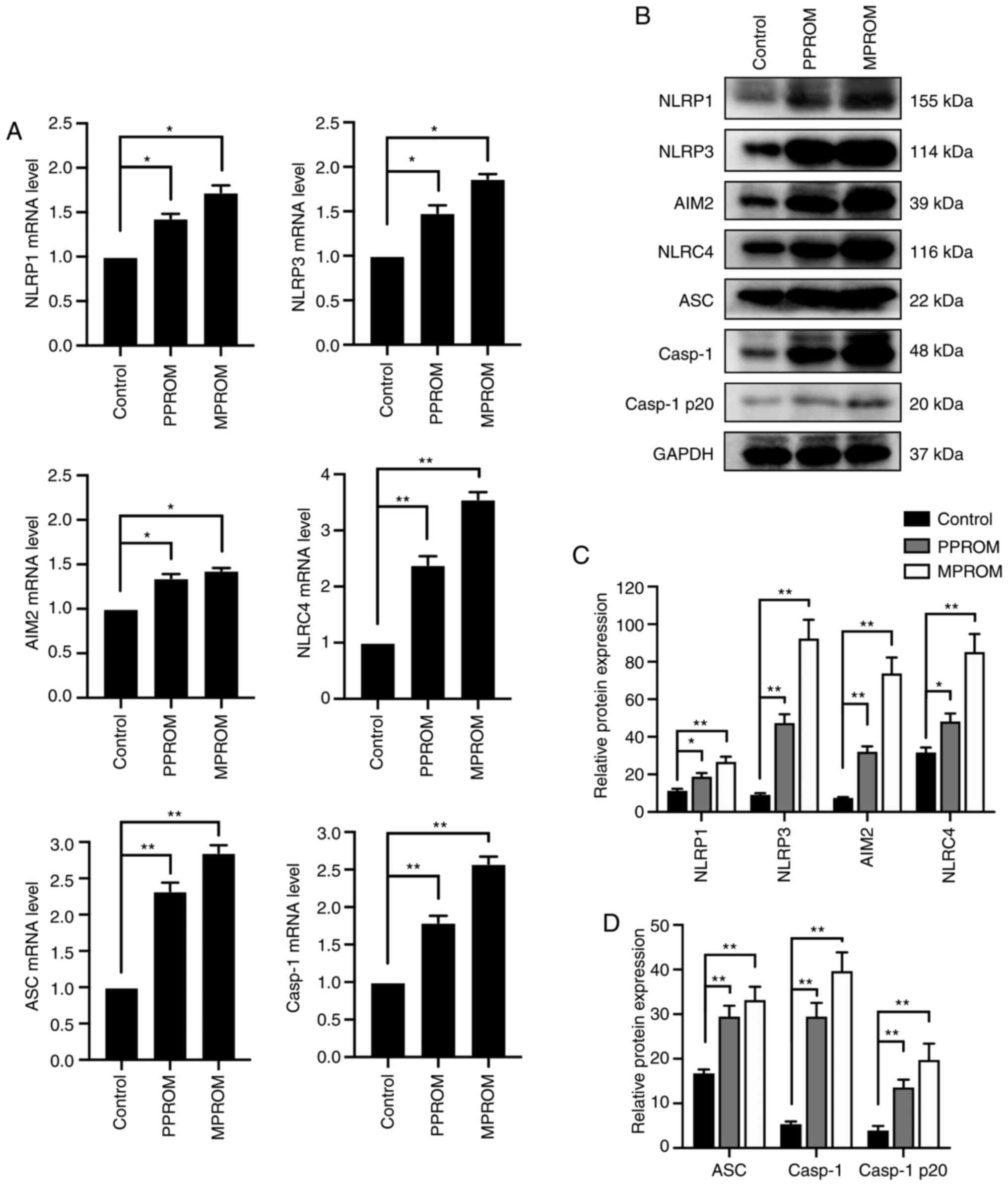 | Figure 1.Enhanced mRNA and protein levels of
inflammasome components in placenta tissues from PROM patients. (A)
The mRNA expression of NLRP1, NLRP3, AIM2, NLRC4, ASC and caspase-1
in control, PPROM and MPROM placenta tissues was determined by
reverse transcription-quantitative PCR (n=20 in each group). (B)
Representative blots of NLRP1, NLRP3, AIM2, NLRC4, ASC, Casp1 and
Casp1 p20 in control, PPROM and MPROM placenta tissues.
Corresponding quantitative analysis of (C) NLRP1, NLRP3, AIM2 and
NLRC4 and (D) ASC, Casp1 and Casp1 p20 (n=20 in each group).
*P<0.05, **P<0.01 vs. indicated groups. PROM, premature
rupture of membranes; NLRP, NLR family pyrin domain containing;
AIM2, absent in melanoma 2; NLRC4, CARD-domain containing 4; ASC,
the apoptosis-associated speck-like protein that contains a caspase
recruitment domain; Casp1, caspase-1; PPROM, preterm premature
rupture of membranes; MPROM, mature premature rupture of
membranes. |
Increased expression of inflammasomes
in fetal membrane of PROM patients
The mRNA levels of inflammasomes in fetal membrane
of PPROM were notably enhanced compared with controls except for
AIM2 expression (Fig. 2A). In
addition to the mRNA levels, protein expression of these
inflammasomes in PPROM groups was also increased compared with the
control group except for the Casp1 expression (Fig. 2B-D). Consistently, mRNA and protein
levels of inflammasomes in fetal membrane tissues of MPROM groups
were further elevated compared with controls.
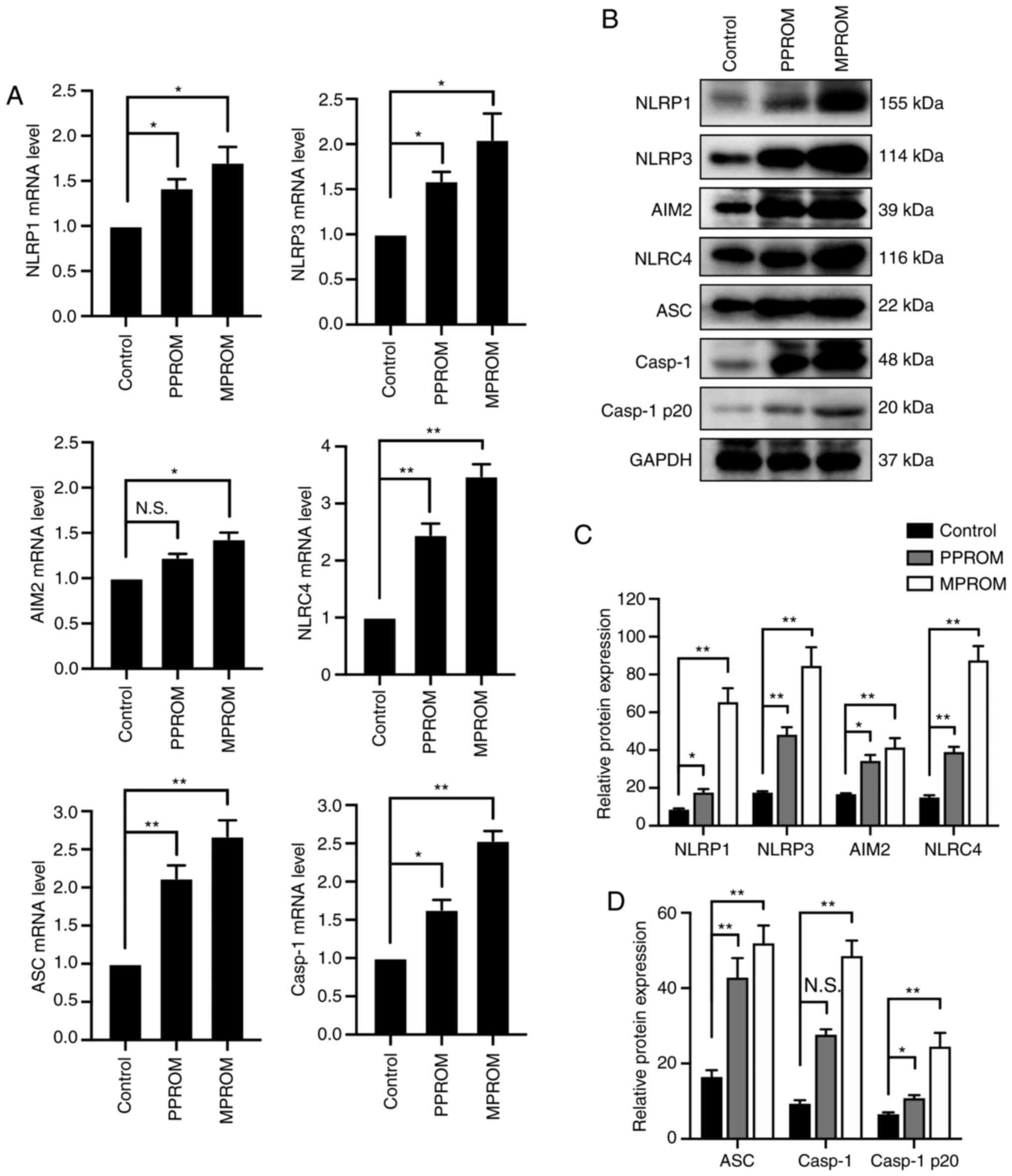 | Figure 2.Elevation of inflammasome expression
was observed in fetal membrane tissues of PROM pregnancies. (A) The
mRNA levels of NLRP1, NLRP3, AIM2, NLRC4, ASC and Casp1 in control,
PPROM and MPROM fetal membrane tissues (n=20 in each group). (B)
Representative blots of NLRP1, NLRP3, AIM2, NLRC4, ASC, Casp1 and
Casp1 p20 in control, PPROM and MPROM fetal membrane tissues.
Corresponding quantitative analysis of (C) NLRP1, NLRP3, AIM2 and
NLRC4 and (D) ASC, Casp1 and Casp1 p20 (n=20 in each group).
*P<0.05, **P<0.01 vs. indicated groups. PROM, premature
rupture of membranes; NLRP, NLR family pyrin domain containing;
AIM2, absent in melanoma 2; NLRC4, CARD-domain containing 4; ASC,
the apoptosis-associated speck-like protein that contains a caspase
recruitment domain; Casp1, caspase-1; PPROM, preterm premature
rupture of membranes; MPROM, mature premature rupture of membranes;
N.S., no significance. |
Increased maturation and expression of
inflammasome downstream effectors in PROM patients
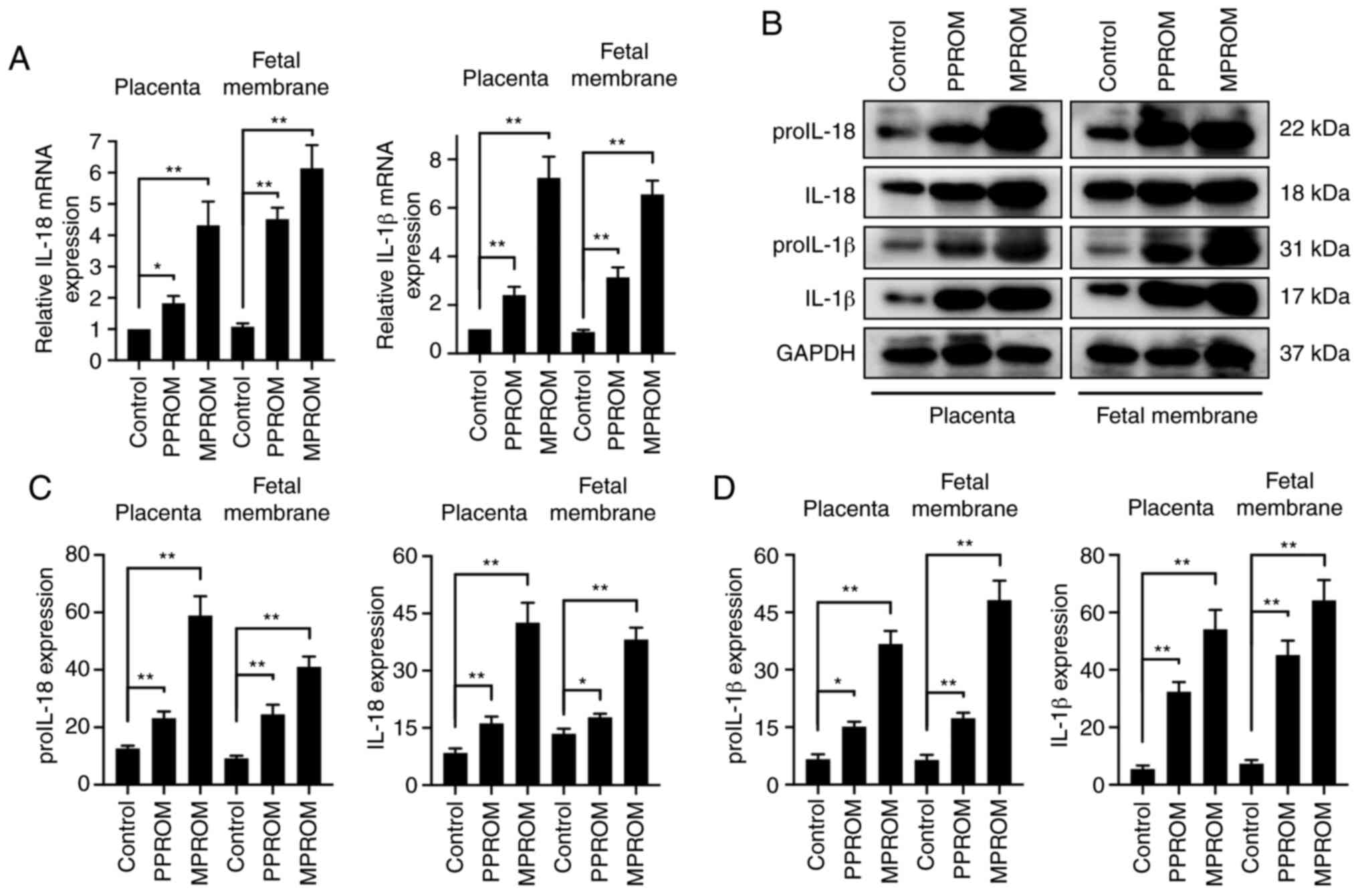
The mRNA expression of IL-18 and IL-1β in placenta
and fetal membrane of PROM pregnancies was also investigated. As
shown in Fig. 3A, IL-18 and IL-1β
mRNA levels in PPROM groups in both placenta and fetal membrane
were increased significantly compared with controls. A further
increase of IL-18 and IL-1β mRNA levels in MPROM groups was
observed compared with controls. Furthermore, the protein
expression of proIL-18, IL-18, proIL-1β and IL-1β in both placenta
and fetal membrane of PPROM patients were markedly elevated
compared with controls (Fig. 3B-D).
A consistent trend of an increase in protein expression of
proIL-18, IL-18, proIL-1β and IL-1β was observed in MPROM group, as
well as increased mRNA expression.
Analysis of NLRP1, NLRP3, AIM2 and
NLRC4 protein levels in placenta and fetal membrane by
immunohistochemistry
The expression of inflammasome components was then
determined by immunohistochemical staining in vivo. As shown
in Fig. 4A-C and E, NLRP1, NLRP3
and NLRC4 expression were all increased in placenta of PPROM groups
compared with controls, whereas no significant difference in AIM2
expression levels was observed between PPROMs and controls
(Fig. 4D). The expression of these
inflammasome components were all markedly upregulated in the
placenta of MPROMs compared with controls. Unexpectedly, no
significant difference was observed in their expression levels in
the fetal membrane of PPROMs compared with controls (Fig. 5A-E) and a promotion of inflammasome
component expression was identified in the fetal membrane of MPROMs
compared with controls. These results suggested that all four
inflammasomes may be responsible for PROM progress.
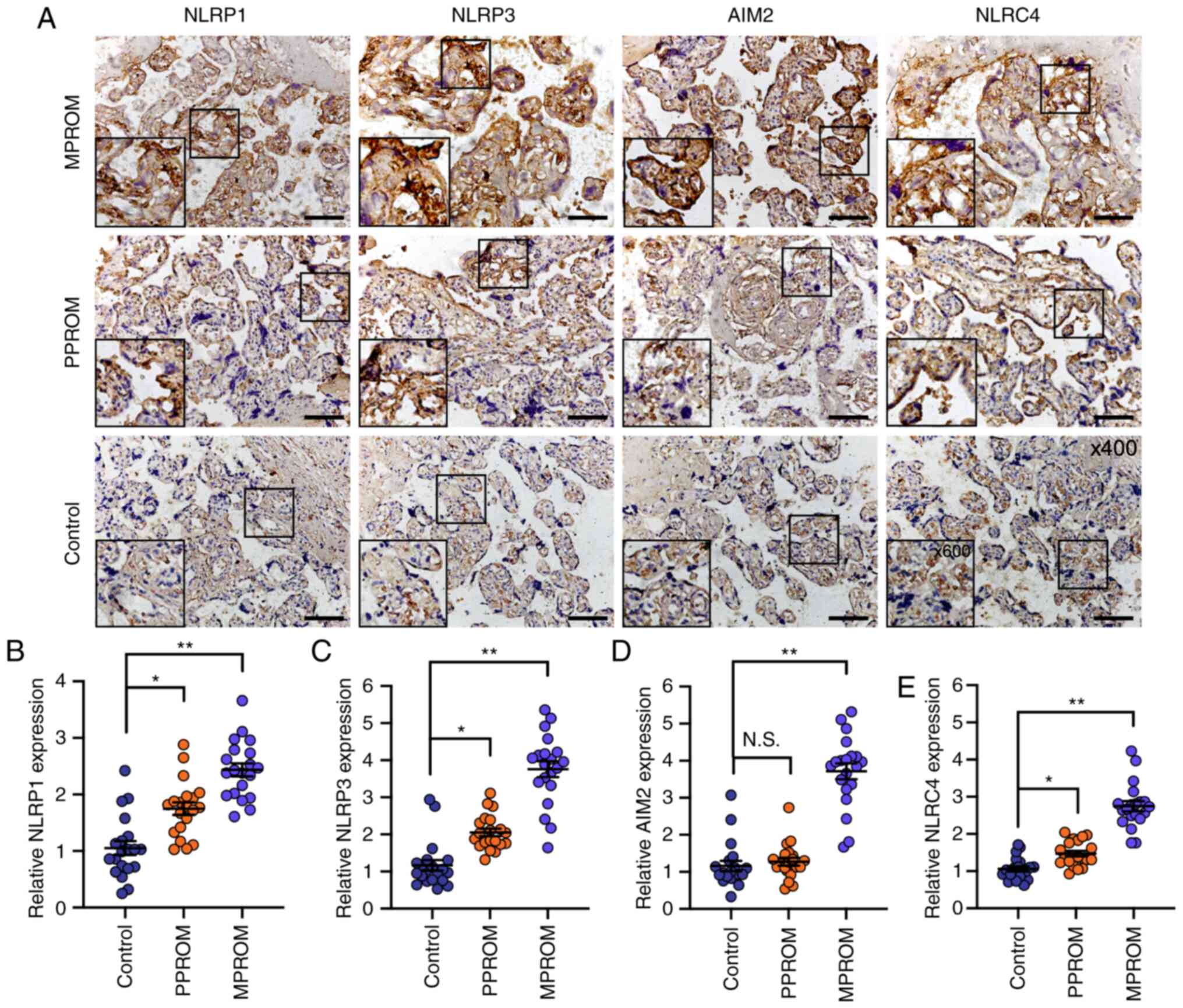 | Figure 4.Evaluation of NLRP1, NLRP3, AIM2 and
NLRC4 expression in placenta tissues. (A) Representative
immunohistochemical staining images of NLRP1, NLRP3, AIM2 and NLRC4
in placenta tissue sections from control, PPROM and MPROM patients
(magnification of main image, ×400; Scale bar, 250 µm; box
indicates area enlarged on lower left). Quantified expression
levels of (B) NLRP1, (C) NLRP3, (D) AIM2 and (E) NLRC4 in control,
PPROM and MPROM tissues (n=20 in each group). *P<0.05,
**P<0.01 vs. indicated groups. N.S. indicates no significance.
NLRP, NLR family pyrin domain containing; AIM2, absent in melanoma
2; NLRC4, CARD-domain containing 4; PPROM, preterm premature
rupture of membranes; MPROM, mature premature rupture of
membranes. |
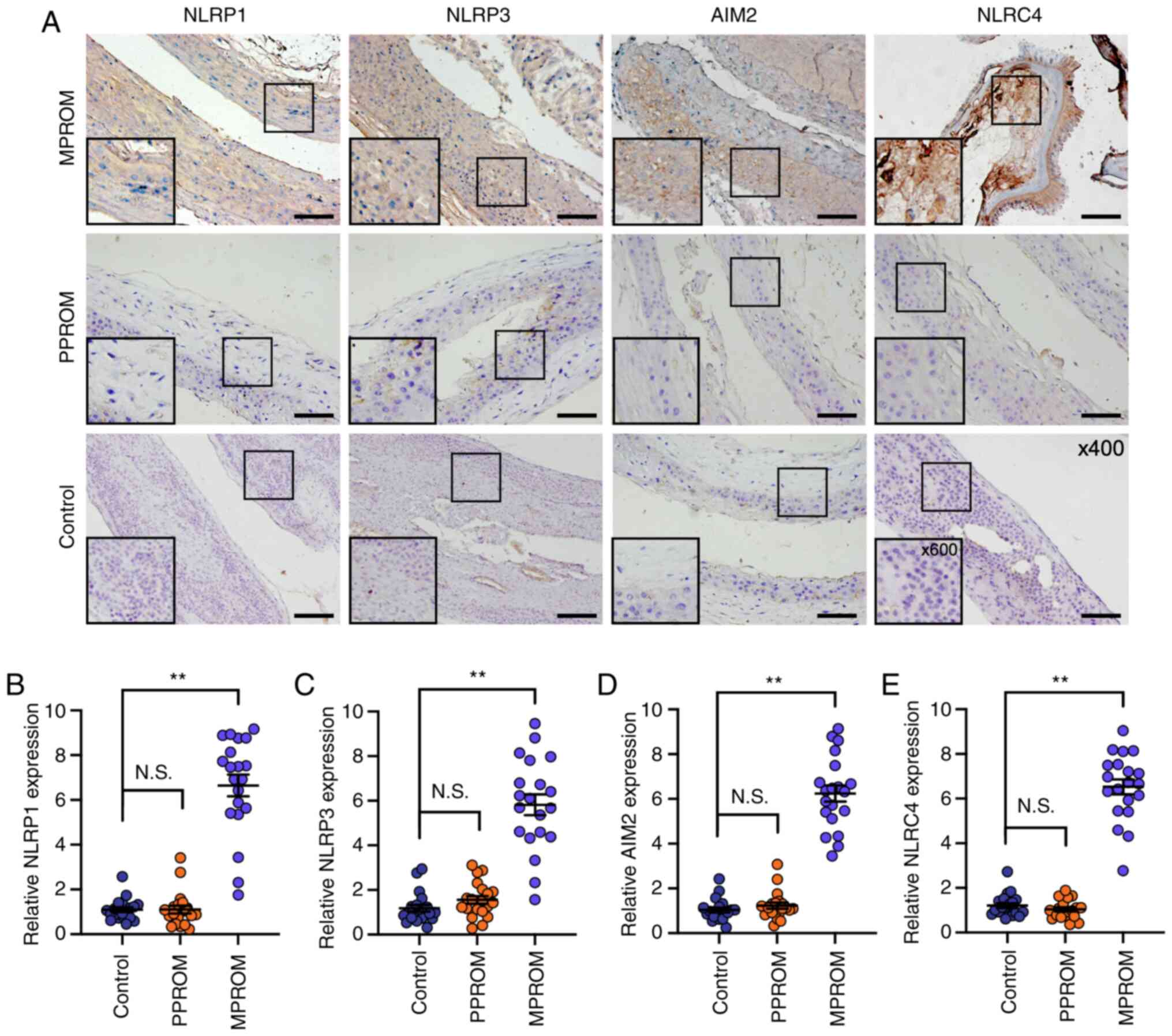 | Figure 5.Increased inflammasome components
levels in fetal membrane of PROM patients. (A) Representative
immunohistochemical staining images of NLRP1, NLRP3, AIM2 and NLRC4
in fetal membrane sections from control, PPROM and MPROM groups
(magnification of main image, ×400; Scale bar, 250 µm; box
indicates area enlarged on lower left). Quantified expression
levels of (B) NLRP1, (C) NLRP3, (D) AIM2 and (E) NLRC4 in control,
PPROM and MPROM tissues (n=20 in each group). **P<0.01 vs.
indicated groups. PROM, premature rupture of membranes; NLRP, NLR
family pyrin domain containing; AIM2, absent in melanoma 2; NLRC4,
CARD-domain containing 4; PPROM, preterm premature rupture of
membranes; MPROM, mature premature rupture of membranes. |
ADAMTS4 expression and its correlation
analysis with inflammasome components in placenta and fetal
membrane of PROM pregnancies
The expression of ADAMTS4, one of the risk factors
of PROM, was detected. As shown in Fig.
6A and B, ADAMTS4 protein levels in placenta and fetal membrane
of PPROM and MPROM groups were all significantly increased compared
with control groups. In addition to protein expression, upregulated
mRNA expression of ADAMTS4 was observed in placenta and fetal
membrane of PPROMs and MPROMs compared with controls (Fig. 6C). Furthermore, a general positive
correlation between ADAMTS4 and all four inflammasome components in
PPROMs (Fig. 6D) and MPROMs
(Fig. 6E) was observed.
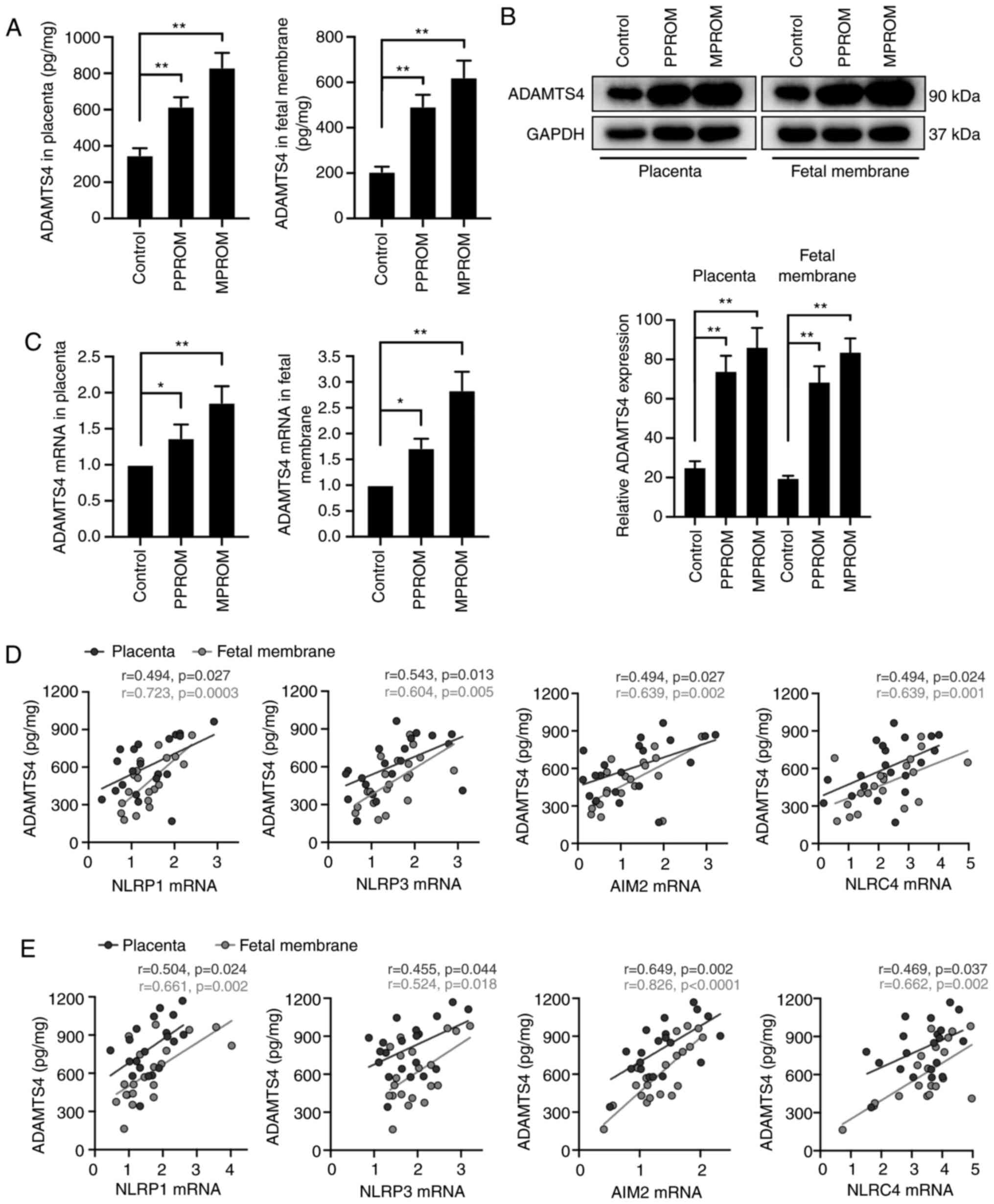 | Figure 6.Correlation between inflammasome mRNA
expression and ADAMTS4 concentrations in placenta and fetal
membrane tissues of PROM patients. (A) ADAMTS4 expression levels
were investigated in placenta and fetal membrane tissues by ELISA
analysis (n=20 in each group). (B) Western blot and quantified
expression levels of ADAMTS4 in placenta and fetal membrane of
control, PPROM and MPROM patients (n=20 in each group). (C) The
mRNA levels of ADAMTS4 in placenta and fetal membrane (n=20 in each
group). *P<0.05, **P<0.01 vs. indicated groups. Correlation
between ADAMTS4 protein levels and mRNA expression of inflammasome
components (NLRP1, NLRP3, AIM2 and NLRC4) in placenta and fetal
membrane of (D) PPROM or (E) MPROM patients (n=20 in each group). A
bivariate correlation analysis was performed using the Spearman
rank test. R indicates the Spearman correlation coefficient.
ADAMTS4, a disintegrin and metalloproteinase with thrombospondin
motifs 4; PROM, premature rupture of membranes; PPROM, preterm
premature rupture of membranes; MPROM, mature premature rupture of
membranes; NLRP, NLR family pyrin domain containing; AIM2, absent
in melanoma 2; NLRC4, CARD-domain containing 4. |
Discussion
PROM is defined as rupture of the fetal membrane
before (PPROM) or after (MPROM) 37 weeks of completed gestation. At
present, PROM is often ignored and regarded as an adverse outcome
of pregnancy (18). Despite
advanced progress in prenatal care over the past three decades,
rates of PROM and subsequent miscarriage remain high (1). The etiology of PROM is diverse and
complicated, including cervix relaxation, reproductive system
infection, abnormal amniotic cavity pressure and lack of trace
elements, and infection is considered as a crucial cause of PROM
(3,19,20).
The inflammasome was named by Martinon et al
(21) in 2002 to describe a
high-molecular-weight complex in the cytoplasm of stimulated immune
cells which modulates the activation of inflammatory caspases.
Since then, this research field has expanded substantially and
multiple major inflammasomes have been identified, with the
assembly of them being directed by a unique pattern-recognition
receptor in response to pathogen-associated molecular patterns or
endogenous danger signals in the cytoplasm of the host cells
(22). The four classic
inflammasome receptors NLRP1, NLRP3, AIM2 and NLRC4 are crucial for
inflammasome activation and host defense against pathogens
(8). However, the relationship
between inflammasomes and PROM remains to be elucidated.
ADAMTS4 is also known as aggrecanase-1 and possesses
a role in aggrecan cleavage and collagen degradation, which result
in extracellular matrix (ECM) remodeling (23), the vital pathogenesis of PROM.
ADAMTS4 is abundantly expressed in brain, lungs and myocardium
tissues and a small amount is expressed in placenta and skeletal
muscle cells (23). In addition,
its aggrecanase activity and mRNA expression are linked with IL-1
and TNF-α accumulation (24). The
combination of oxidative mediators with inflammation, infection and
proteases affect the structural and functional changes of ECM and
form a vicious cycle in ECM degradation (15). Inflammation and oxidative stress
also lead to proteoglycan degradation in ECM, collagen collapse and
degradation of cell connections via released proteases such as
ADAMTS4 (25). Decreased tensile
forces and thinning of the fetal membrane result in rupture of
membranes in the early weeks of pregnancy ultimately (15). As one of the risk factors of PROM,
whether ADAMTS4 is associated with inflammasome expression need to
be determined.
The present study demonstrated that the mRNA and
protein expression of all four inflammasomes plus ASC and Casp1
were upregulated in placenta and fetal membrane tissues of PPROM
patients compared with control groups (except for the AIM2
expression in fetal membrane; Fig.
2A). The expression of these inflammasomes was further elevated
in MPROM groups compared with controls. Additionally, the
expression levels of downstream proIL-18, IL-18, proIL-1β and IL-1β
demonstrated a consistent trend with the inflammasome expression in
PPROM and MPROM groups. These results indicated that the activation
of inflammasomes and their downstream effectors are more pronounced
in MPROM groups compared with the PPROM groups. Notably, results
from immunohistochemistry suggested that no significant difference
of inflammasome receptor expression was observed in fetal membrane
of PPROMs compared with controls (Fig.
5B-E). These results, which were inconsistent with the western
blotting results, suggested that the inflammasomes may not be
significantly activated in fetal membrane tissue of PPROM patients.
Finally, the mRNA and protein levels of ADAMTS4 were investigated.
Increased mRNA and protein expression of ADAMTS4 were observed in
placenta and fetal membrane of PPROM pregnancies. As expected,
ADAMTS4 expression was further promoted in MPROMs compared with
controls. A general positive correlation between ADAMTS4 and all
four inflammasome receptors in placenta and fetal membrane of
PPROMs and MPROMs was observed, indicating that the activation of
these inflammasomes may be potential factors influencing the
expression of ADAMTS4 which triggers the development of PROM
subsequently.
However, there are several limitations in the
present study. Since it is difficult to establish a PROM model in
small animals, how to determine whether deleting the expression of
inflammasomes can improve PROM at the animal level is worthy of
further study. In addition, although the inflammasome expression
has a significant correlation with ADAMTS4 expression, whether
inflammasome regulates the expression of ADAMTS4 remains to be
confirmed.
In conclusion, the present study suggested that
NLRP1, NLRP3, AIM2 and NLRC4 inflammasome activation and its
effector expression in PROM were upregulated. Increased expression
of ADAMTS4 was also observed in PROM group and was significantly
correlated with the expression of inflammasomes. Whether
downregulation of inflammasome elements contribute to modulating
ADAMTS4 expression and alleviating PROM progress requires further
evidence. The present study on the expression and correlation of
inflammatory bodies and ADAMTS4 may be instrumental in the
expansion of PROM etiology and may provide a potential therapeutic
target for clinical PROM treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the project of
Xuzhou Science and Technology (grant no. KC20121).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ, CM and XL conceived and designed the research.
JZ, CM, JL and FP were responsible for subject recruitment and
collection of specimens. JZ, CM and LH performed the experiments.
XL, JL, FP and LH contributed reagents, materials, instruments and
analysis tools. CM and JL analyzed the data. JZ and FP were
responsible for data analysis and writing of the manuscript. All
authors reviewed and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of Xuzhou Maternity and Child Health Care Hospital
Affiliated to Xuzhou Medical University (approval no. 201502) and
all the patients included in the study signed informed
consents.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goldenberg RL, Culhane JF, Iams JD and
Romero R: Epidemiology and causes of preterm birth. Lancet.
371:75–84. 2008. View Article : Google Scholar
|
|
2
|
Lee T and Silver H: Etiology and
epidemiology of preterm premature rupture of the membranes. Clin
Perinatol. 28:721–734. 2001. View Article : Google Scholar
|
|
3
|
Parry S and Strauss JF III: Premature
rupture of the fetal membranes. N Engl J Med. 338:663–670. 1998.
View Article : Google Scholar
|
|
4
|
Kumar D, Moore RM, Mercer BM, Mansour JM,
Redline RW and Moore JJ: The physiology of fetal membrane weakening
and rupture: Insights gained from the determination of physical
properties revisited. Placenta. 42:59–73. 2016. View Article : Google Scholar
|
|
5
|
Kacerovsky M, Pliskova L, Bolehovska R,
Musilova I, Hornychova H, Tambor V and Jacobsson B: The microbial
load with genital mycoplasmas correlates with the degree of
histologic chorioamnionitis in preterm PROM. Am J Obstet Gynecol.
205:213.e1–e7. 2011. View Article : Google Scholar
|
|
6
|
Hornychova H, Kacerovsky M, Musilova I,
Pliskova L, Zemlickova H, Matejkova A, Vosmikova H, Rozkosova K,
Cermakova P, Bolehovska R, et al: Cervical human papillomavirus
infection in women with preterm prelabor rupture of membranes. PLoS
One. 13:e02078962018. View Article : Google Scholar
|
|
7
|
Suzuki Y, Horie K, Yada Y, Kono Y,
Hirashima C, Usui R, Matsubara S and Ohkuchi A: Vaginal ureaplasma
species increase chorioamnionitis in very preterm infants with
preterm premature rupture of the membranes at <28 weeks of
gestation. Eur J Clin Microbiol Infect Dis. 37:2371–2380. 2018.
View Article : Google Scholar
|
|
8
|
von Moltke J, Ayres JS, Kofoed EM,
Chavarria-Smith J and Vance RE: Recognition of bacteria by
inflammasomes. Annu Rev Immunol. 31:73–106. 2013. View Article : Google Scholar
|
|
9
|
Liu D, Zeng X, Li X, Cui C, Hou R, Guo Z,
Mehta JL and Wang X: Advances in the molecular mechanisms of NLRP3
inflammasome activators and inactivators. Biochem Pharmacol.
175:1138632020. View Article : Google Scholar
|
|
10
|
Schroder K, Zhou R and Tschopp J: The
NLRP3 inflammasome: A sensor for metabolic danger? Science.
327:296–300. 2010. View Article : Google Scholar
|
|
11
|
Slaats J, Ten Oever J, van de Veerdonk FL
and Netea MG: IL-1beta/IL-6/CRP and IL-18/ferritin: Distinct
inflammatory programs in infections. PLoS Pathog. 12:e10059732016.
View Article : Google Scholar
|
|
12
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar
|
|
13
|
Lamkanfi M and Dixit VM: Mechanisms and
functions of inflammasomes. Cell. 157:1013–1022. 2014. View Article : Google Scholar
|
|
14
|
Porter S, Clark IM, Kevorkian L and
Edwards DR: The ADAMTS metalloproteinases. Biochem J. 386:15–27.
2005. View Article : Google Scholar
|
|
15
|
Ozler S, Oztas E, Guler BG, Ergin M, Uygur
D, Yucel A, Erel O and Danisman N: ADAMTS4 and
oxidative/antioxidative status in preterm premature rupture of
membranes. Fetal Pediatr Pathol. 35:239–250. 2016. View Article : Google Scholar
|
|
16
|
Ahmad R, Sylvester J, Ahmad M and
Zafarullah M: Adaptor proteins and Ras synergistically regulate
IL-1-induced ADAMTS-4 expression in human chondrocytes. J Immunol.
182:5081–5087. 2009. View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Menon R and Richardson LS: Preterm
prelabor rupture of the membranes: A disease of the fetal
membranes. Semin Perinatol. 41:409–419. 2017. View Article : Google Scholar
|
|
19
|
Murtha AP and Menon R: Regulation of fetal
membrane inflammation: A critical step in reducing adverse
pregnancy outcome. Am J Obstet Gynecol. 213:447–448. 2015.
View Article : Google Scholar
|
|
20
|
Gomez-Lopez N, Romero R, Xu Y, Garci
z-Flores V, Leng Y, Panaitescu B, Miller D, Abrahams VM and Hassan
SS: Inflammasome assembly in the chorioamniotic membranes during
spontaneous labor at term. Am J Reprod Immunol.
77:10.1111/aji.12648. 2017. View Article : Google Scholar
|
|
21
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar
|
|
22
|
Broz P and Dixit VM: Inflammasomes:
Mechanism of assembly, regulation and signalling. Nat Rev Immunol.
16:407–420. 2016. View Article : Google Scholar
|
|
23
|
Naito S, Shiomi T, Okada A, Kimura T,
Chijiiwa M, Fujita Y, Yatabe T, Komiya K, Enomoto H, Fujikawa K and
Okada Y: Expression of ADAMTS4 (aggrecanase-1) in human
osteoarthritic cartilage. Pathol Int. 57:703–711. 2007. View Article : Google Scholar
|
|
24
|
Pratta MA, Scherle PA, Yang G, Liu RQ and
Newton RC: Induction of aggrecanase 1 (ADAM-TS4) by interleukin-1
occurs through activation of constitutively produced protein.
Arthritis Rheum. 48:119–133. 2003. View Article : Google Scholar
|
|
25
|
Lockwood CJ and Kuczynski E: Risk
stratification and pathological mechanisms in preterm delivery.
Paediatr Perinat Epidemiol. 15 (Suppl 2):S78–S89. 2001. View Article : Google Scholar
|