Introduction
Osteoporosis is a systemic metabolic disease
associated with age, which is accompanied by a significant economic
and psychological burden to patients and their families (1). At present, the drugs used to treat
osteoporosis have several side effects and other deficiencies
(2); therefore, further determining
the pathogenesis of osteoporosis and identifying novel treatment
targets is of great economic and social significance. Under normal
physiological conditions, osteoblasts serve an important role in
bone formation. Osteoblasts are the main functional cells in bone
formation, and are responsible for the synthesis, secretion and
mineralization of bone matrix; the proliferation and
differentiation of osteoblasts are regulated by a variety of growth
factors (3,4). Therefore, understanding the mechanism
underlying osteoblast differentiation has a vital clinical
significance for the treatment of osteoporosis.
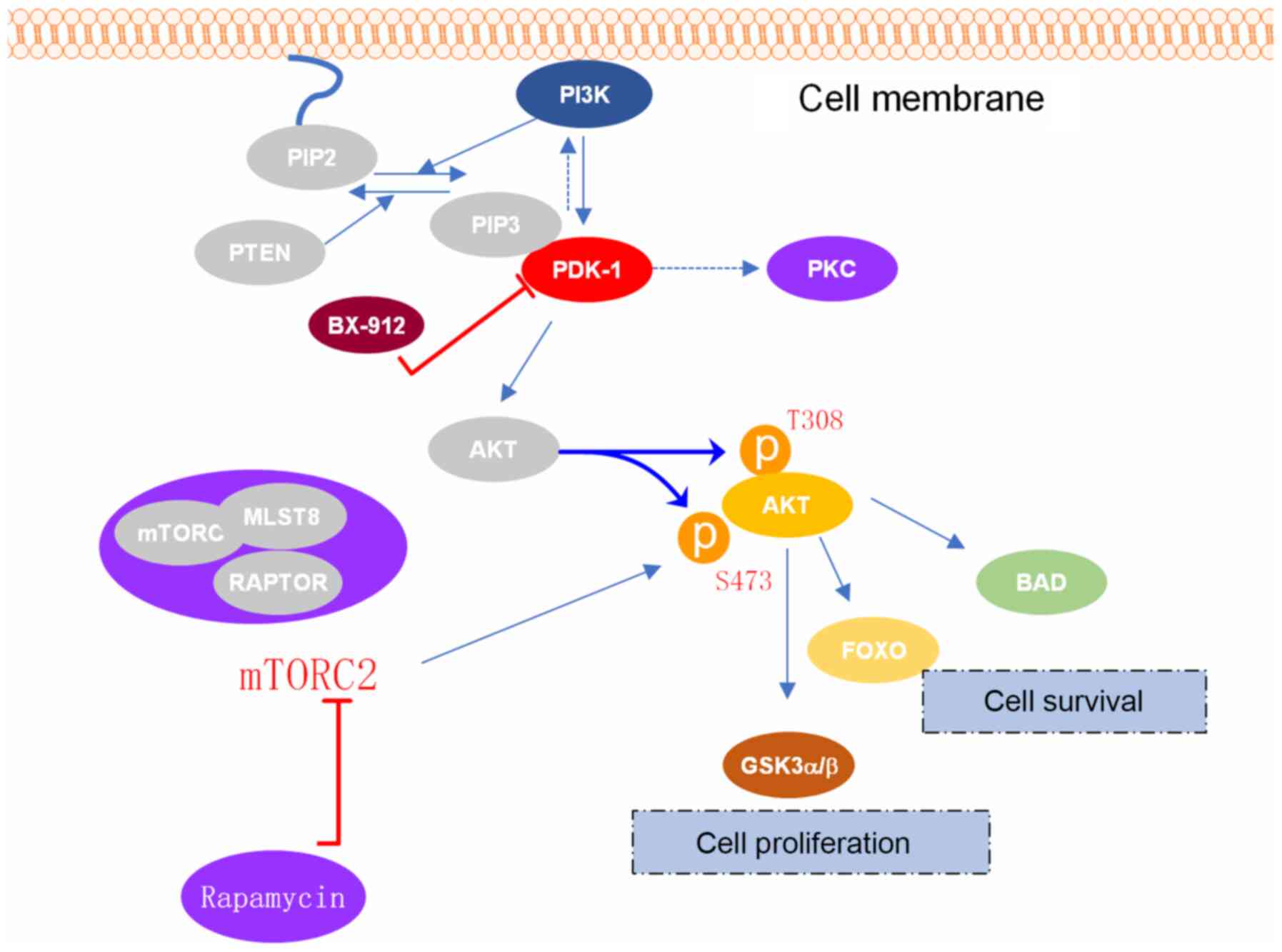
The PI3K/AKT signaling pathway is involved in the
regulation of cell proliferation, differentiation, apoptosis and
glucose transport, particularly in apoptosis and cell survival
(5). PI3K is a key control point in
several apoptotic pathways. When PI3K is activated, second
messengers PIP2 and PIP3 can be induced. AKT is an important
downstream target of PI3K and PIP3, which can interact with PIP3
recruited on the plasma membrane, and then be partially
phosphorylated and activated by 3-phosphoinositide-dependent
protein kinase-1 (PDK-1) to regulate downstream pathways and
promote cell survival (6). An
increasing body of evidence has reported that the PI3K/AKT
signaling pathway regulates the proliferation and differentiation
of osteoblasts (7). Notably, the
PDK-1 protein has attracted increasing attention in recent years;
however, to the best of our knowledge, the role of PDK-1 in
osteoblast differentiation has not been systematically studied.
Bones are affected by the balance in
osteoblast-mediated bone formation and osteoclast-mediated bone
resorption. Our previous study demonstrated that inhibiting the
expression of PDK-1 significantly inhibited the differentiation and
maturation of osteoclasts (8).
Therefore, the aim of present study was to determine the role of
PDK-1 in osteoblast differentiation.
Materials and methods
Ethics statement
The present study was approved by the Ethics
Committee of the Guangxi Medical University (Nanning, China;
approval no. 201910012).
Materials and reagents
Fetal bovine serum (FBS) and Dulbecco's modified
Eagle medium were purchased from Gibco (Thermo Fisher Scientific,
Inc.). Osteoblast induction medium (OBM; cat. no. MUBMX-90021) was
purchased from Cyagen Biosciences, Inc. Primary antibodies specific
to t-PDK1(rabbit; cat. no. 5662; 1:1,000), p-PDK1 (rabbit; cat. no.
3438; 1:1,000), t-Akt (rabbit; cat. no. 4685; 1:1,000), p-Akt
(rabbit; cat. no. 13038; 1:1,000), GAPDH (rabbit; cat. no. 5174;
1:1,000) and horseradish peroxidase-conjugated IgG secondary
antibody (anti-rabbit; cat. no. 5127; 1:2,000) were purchased from
Cell Signaling Technology, Inc. The E.Z.N.A. Total RNA kit I (cat.
no. R6834-02) was purchased from Omega Bio-Tek, Inc. The Alkaline
Phosphatase (ALP) Staining kit, ALP Activity Assay kit and Alizarin
Red S Staining kit were purchased from Beyotime Institute of
Biotechnology. The PrimeScript™ RT Reagent kit was obtained from
Takara Bio, Inc. Adenoviral vectors encoding Cre recombinase
(PHBAd-cre-GFP) were purchased from Hanbio Biotechnology Co., Ltd.
BX-912 (PDK-1 specific inhibitor) was purchased from Selleck
Chemicals. The commercial Cell Counting Kit-8 (CCK-8) assay kit was
purchased from Beyotime Institute of Biotechnology.
Animals
A total of three male C57BL/6 mice (age, 4–6 weeks;
weight, 10–16 g; homozygote PDK-1flox/flox) were
designed and provided by GemPharmatech Co., Ltd. The upstream and
downstream sequences of the PDK-1 gene were inserted into the loxP
site. The results of the identification were provided by
GemPharmatech Co., Ltd. and validated in the present study (shown
in Fig. S1). A total of three male
C57BL/6 mice (age, 4–6 weeks old; weight, 10–16 g; wild-type,
PDK-1+/+) were also purchased from GemPharmatech Co.,
Ltd. All animals had the same genetic background. All mice were
raised and maintained in the laboratory animal center of Guangxi
Medical University (Nanning, China). Mice were individually housed
at a controlled temperature (22–26°C) and humidity (50-60%) in
ventilated cages with a 12-h light/dark cycle and free access to
standard chow and fresh water. Experiments on all animals were
conducted according to the 2013 ARRIVE and AVMA guidelines for
euthanasia. Briefly, mice were anesthetized via inhalation of 2%
sevoflurane and were placed in a container; CO2 was then
injected into the container at a rate that replaced 25% of
container volume per minute. When it was confirmed that the
heartbeats of the mice had stopped and they were no longer
breathing, the CO2 was turned off. Finally, the mice
were observed for 2 min to confirm death. The mice were sacrificed
in November 2019.
Treatment groups
The control group was treated with OBM. The BX-912
group was treated with OBM containing 0.3 µM BX-912 at 37°C for 1
week. Subsequently, 1×104 cells/well of BMSCPDK-1
flox/flox in the empty vector and pHBAd-cre-EGFP virus groups
were infected with an empty vector or pHBAd-cre-EGFP with a MOI of
100 at 37°C, respectively. A total of 72 h post-infection, the
medium was replaced with OBM.
Isolation and culture of mouse bone
marrow mesenchymal stem cells (BMSCs)
After euthanasia, the femurs and tibiae of the mice
were isolated under aseptic conditions. Subsequently, a 1-ml
injection syringe was used to isolate mice bone marrow from the
femurs and tibiae of the PDK1flox/flox and
PDK1+/+ mice, according to a previously published
protocol (9). The bone marrow was
collected by a sterile Pasteur pipette, and filtered through a cell
filter and transferred to a centrifuge. Then, 1:4 volume
erythrocyte lysis buffer (Beijing Solarbio Science & Technology
Co., Ltd.) was added to lyse the red blood cells. Centrifugation
was performed at 100 × g for 5 min at 4°C. Resuspended cells were
transferred into a T75 cell bottle in DMEM containing 15% FBS, as
described by Klein et al (10).
Induction of BMSC differentiation into
osteoblasts
Primary BMSCs were isolated and cultured in an
incubator containing 5% CO2 at 37°C; the medium was
changed every 2–3 days. Third-generation BMSCs were collected and
seeded in 6-well plates previously coated with gelatin at a density
of 5×103 cells/cm2, and upon the BMSCs
reaching 70–80% confluence, 2 ml OBM was added to each well to
initiate induction, followed by culture in an incubator containing
5% CO2 at 37°C. OBM was replaced with fresh OBM every 3
days. The main components of OBM include 50 µg/ml ascorbic acid, 10
mM sodium β-glycerophosphate and 10−7 M dexamethasone.
After 1 week of induction, to avoid osteoblast shedding, 1 ml of
medium was changed every 2 days. The morphology of the osteoblasts
was observed under a light microscope (magnification, ×100; Leica
DMI8; Leica Microsystems GmbH).
Infection with adenoviral vectors
BMSCs from PDK1flox/flox mice
(BMSCPDK-1flox/flox) were infected with empty adenovirus
vectors or pHBAd-cre-EGFP adenovirus vectors (Hanbio Biotechnology
Co., Ltd.), as previously described (11). Preliminary investigations to
determine the optimal MOI value were performed in 96-well plates.
Then, BMSCs were cultured in 96-well plates at a density of
1×104 cells/well. Different MOI virus particle solutions
were premixed with medium and added to each culture well. The MOI
gradient was 25, 50, 100, 200, 300 and 400 (12). After 7 h, the medium was replaced
with ordinary culture medium. After 72 h, the MOI with the highest
fluorescence efficiency was selected as the optimal MOI.
Transfection efficiency (%)=(number of cells emitting green
fluorescence in the fluorescence microscope field/number of cells
in the light microscope field) ×100. A total of 72 h
post-adenovirus infection into BMSCs, the number of fluorescent
cells in three visual fields was counted in the empty vector and
pHBAd-cre-EGFP virus groups, and the transfection efficiency was
calculated. If the transfection efficiency reached ~80%, the next
experiment was conducted.
CCK-8 assay
CCK-8 was used to observe the effects of the
PDK1-specific inhibitor BX-912 on BMSC proliferation, and to
identify its maximum safe concentration. BMSCs were plated onto
96-well plates in triplicate at a density of 1×104
cells/well in complete medium. After 1 day, the BMSCs were treated
with different concentrations of BX-912 (0, 0.1, 0.3, 0.5, 0.7, 0.9
and 1.1 µM) for 24, 48, 72, 96 and 120 h at 37°C. Subsequently, 10
µl CCK-8 buffer was added to each well for 2 h at 37°C. The optical
density (OD) values were measured at a wavelength of 450 nm using a
microplate reader.
Staining and quantification of
extracellular matrix
For ALP staining and ALP activity assay, the cells
were washed five times with PBS, fixed with 4% paraformaldehyde for
30 min at 20°C, washed 3–5 times with PBS and further incubated
with freshly prepared 5-bromo-4-chloro-3-indolyl-phosphate/nitro
blue tetrazolium (Beyotime Institute of Biotechnology) working
solution at 37°C for 1 h. The cells were then washed with distilled
water 3–5 times, and observed under an inverted light microscope
(magnification, ×100) after being sealed in resin. The ALP activity
was assessed using an ALP assay kit (cat. no. P0321S; Beyotime
Institute of Biotechnology). The absorbance/optical density (OD) of
each sample was measured at 492 nm using a microplate reader.
Cellular ALP activity was quantified as previously described
(13).
For Alizarin Red staining and quantification of
mineralized extracellular matrix, the cells were maintained in
different groups for 21 days, washed with PBS and then fixed with
4% paraformaldehyde for 30 min at 20°C. After washing with PBS 3–5
times, cells were stained with Alizarin Red S solution (40 mmol/l;
pH 4.2; Beyotime Institute of Biotechnology) for 20 min at 20°C.
Cells were then washed with PBS and images were captured using an
inverted light microscope (magnification, ×100). The sample
solution was measured using a microplate reader at an OD of 402 nm.
The quantification of mineralized extracellular matrix was
performed as previously described (14).
Western blot analysis
Total protein was extracted using RIPA lysis buffer
(Shanghai Biyuntian Biotechnology Co., Ltd.), according to the
manufacturer's protocol. Briefly, protein lysis buffer (1 ml) was
added to each well, cells were lysed for 30 min at 4°C, and cell
lysates were collected at 4°C. Subsequently, the supernatants were
collected, and the protein concentration was determined using the
bicinchoninic acid assay method. A total of 20 µg protein was
separated via 10% SDS-PAGE. Proteins were transferred to
polyvinylidene fluoride membranes and incubated in 5% milk for 1 h
at 37°C. Subsequently, membranes were incubated with the following
primary antibodies overnight at 4°C: Anti-p-PDK1 (1:1,000),
anti-PDK1 (1:1,000), anti-β-actin (1:1,500), anti-AKT (1:1,000) and
anti-p-AKT (1:1,000). Subsequently, secondary antibodies
(anti-rabbit horseradish peroxidase-conjugated IgG) were added at a
1:1,000 dilution, and samples were incubated at 37°C for 1 h. Then,
0.5 ml chemiluminescent horseradish peroxidase substrate (EMD
Millipore) was then added to the membrane and incubated for 4 min
at 20°C. The membrane was carefully placed in a cassette and left
for 30 sec; during development, the film was completely immersed in
the developing solution. When a black band was observed on the
film, it was rinsed with clean water and placed in the fixing
solution. The blot was analyzed using ImageJ 5.0 software (National
Institutes of Health).
RNA preparation and reverse
transcription-quantitative PCR (qPCR)
Total RNA was extracted from cell lines using the
E.Z.N.A. Total RNA kit I (Omega Bio-Tek, Inc.; cat. no. R6834-02).
cDNA was reverse transcribed from 1–2 µg extracted RNA using the
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) under the following conditions: 37°C for 5 min,
42°C for 60 min and 70°C for 10 min. The PCR reaction was performed
using the PowerUp™ SYBR™ Green Master mix (cat. no. A25742; Applied
Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 95°C for 5 min; followed by 40 cycles at 95°C for 5
sec, 60°C for 34 sec and 72°C for 15 sec; and a final extension at
72°C for 1 min. The primers used were as follows: PDK-1, forward,
5′-TGTGCTTGGTGGATATTGAT-3′ and reverse, 5′-AAGGAGGAGAGGAGGAATGT-3′;
RUNX2, forward, 5′-TTCTCCAACCCACGAATGCAC-3′, and reverse,
5′-CAGGTACGTGTGGTAGTGAGT-3′; osteocalcin, forward,
5′-GAGGGCAATAAGGTAGTGAACAGA-3′ and reverse,
5′-AAGCCATACTGGTTTGATAGCTCG-3′; collagen I, forward,
5′-AATGGTGCTCCTGGTATTGC-3′ and reverse, 5′-GGCACCAGTGTCTCCTTTGT-3′;
and GAPDH, forward, 5′-GCATCTCCCTCACAATTTCCA-3′ and reverse,
5′-TGCAGCGAACTTTATTGATGGT-3′. The mRNA expression levels of RUNX2,
osteocalcin, collagen I and PDK-1 were normalized to those of
GAPDH. The 2−ΔΔCq method was used to determine the
relative expression levels of each target gene (8,15).
Statistical analysis
Experimental data are presented as the mean ±
standard deviation of ≥3 independent biological repeats and were
analyzed using SPSS 24.0 statistical software (IBM Corp.). One-way
analysis of variance followed by Tukey's test was used to compare
differences among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
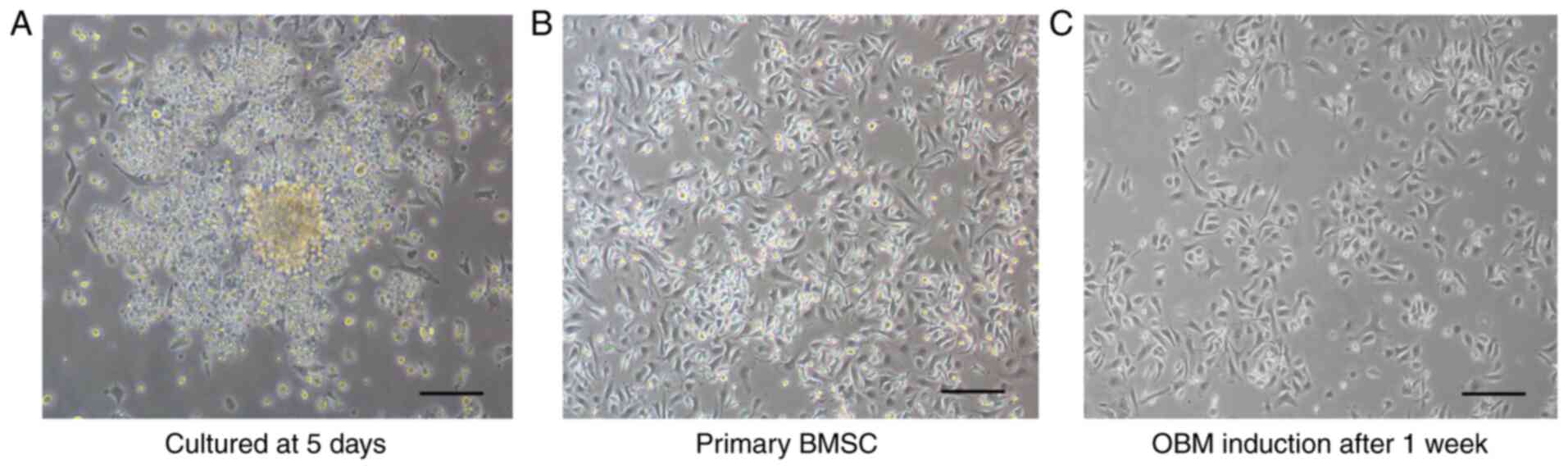
Observation of BMSCs under an inverted
microscope
After a 24-h cell culture, the majority of cells had
attached to the bottom of the flask. After 5 days, most cells had
gathered into clusters, and cell colonies were observed in the
culture flask (Fig. 1A). In the
primary generation, the cells were slender and fusiform with a
fence-like arrangement, and cell growth had clearly accelerated
(Fig. 1B). Following the addition
of OBM, the rate of cell growth was markedly decreased. After a
1-week culture, cell formation resembled that of osteoblasts
(triangular or polygonal) (Fig.
1C).
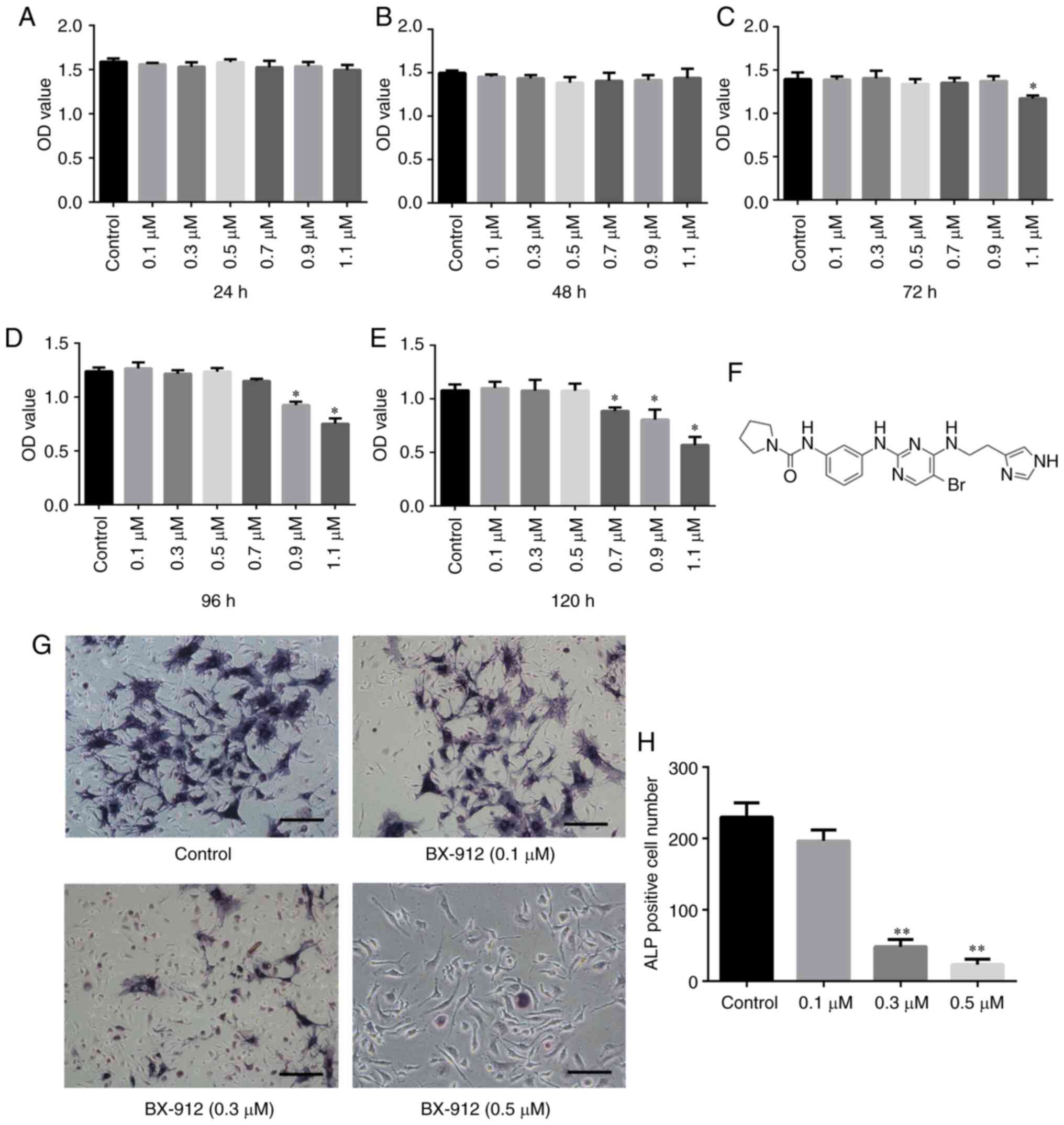
BX-912 inhibits BMSC differentiation
into osteoblasts in vitro
The survival rate of the primary BMSCs at 24, 48,
72, 96 and 120 h was measured using the CCK-8 assay. The results
revealed that BMSC proliferation was not inhibited by ≤0.5 µM
BX-912 (Fig. 2A-E). Therefore, for
subsequent experiments, 0.1–0.5 µM BX-912 was added to the OBM. ALP
staining was performed on day 7. The results revealed that ALP
formation in cells was significantly inhibited with the increase in
BX-912 concentration. When cells were treated with 0.3 and 0.5 µM
BX-912, the decrease in ALP was statistically significant
(P<0.01); the number of ALP-positive cells decreased from
230±20/well (0 µM) to 25±10/well (0.5 µM; Fig. 2G and H). These findings indicated
that BX-912 could significantly inhibit the differentiation of
BMSCs into osteoblasts in a dose-dependent manner in vitro.
Notably, 0.3 µM BX-912 was selected for follow-up experiments.
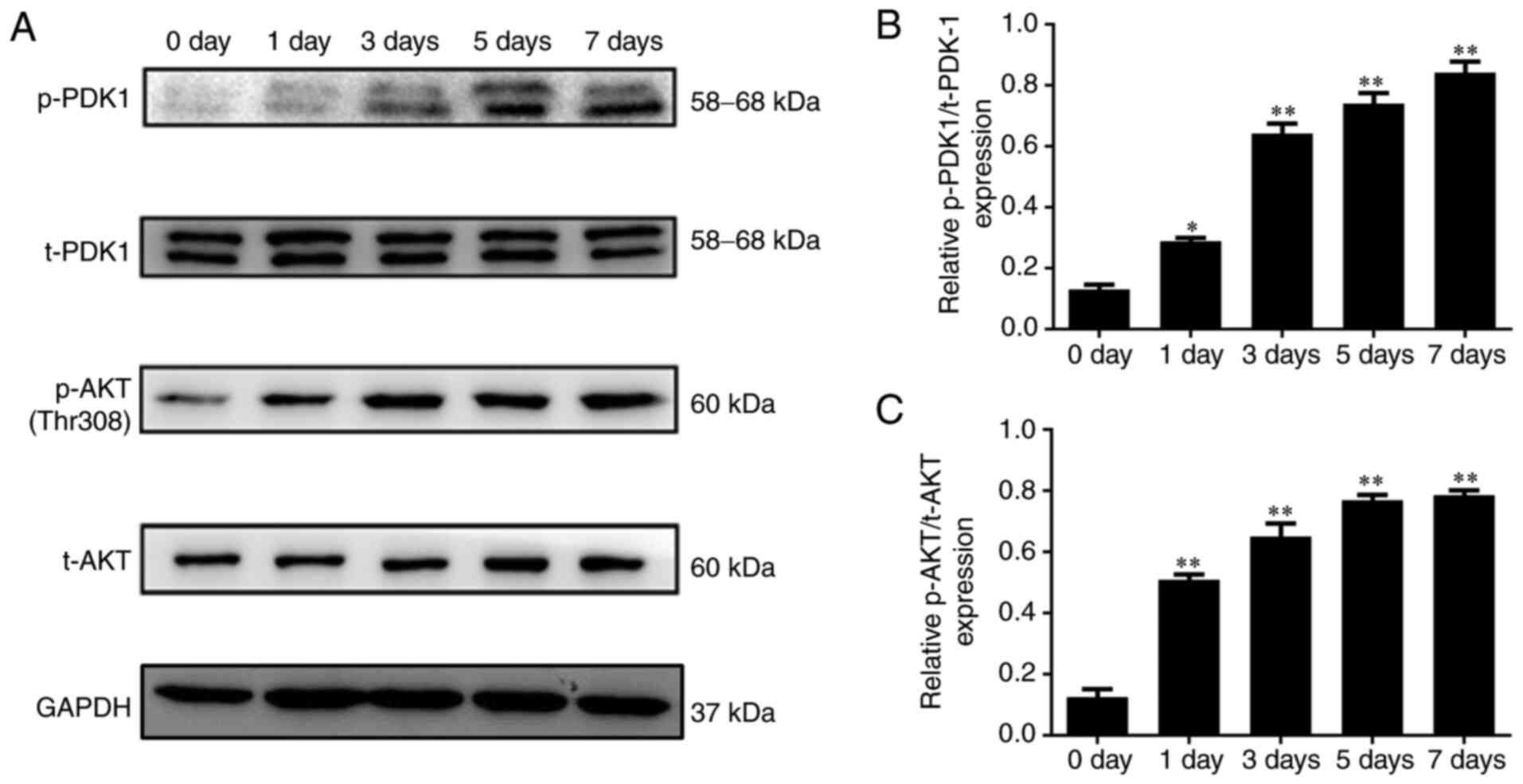
p-PDK-1 and p-AKT protein expression
is gradually increased during osteoblast differentiation
The protein expression levels of t-PDK1, p-PDK1,
t-AKT and p-AKT were detected by western blot analysis on days 0,
1, 3, 5 and 7 of the osteoblast differentiation process. The
results showed that the protein expression levels of p-PDK1 and
p-AKT were gradually increased in the osteoblasts (P<0.05;
Fig. 3).
pHBAd-cre-EGFP viral infection
decreases PDK-1 expression in BMSCsPDK-1flox/flox
An adenovirus vector was used to introduce the Cre
recombinase enzyme into BMSCsPDK-1flox/flox to disrupt
the PDK-1 gene. Based on the MOI gradient, it was observed that
infection efficiency was at its highest at a MOI of 100;
transfection efficiency was 85% when cells were infected with a MOI
of 100 (Fig. 4A). The BMSCs were
obtained from homozygote PDK-1flox/flox mice, in which
the upstream and downstream sequences of the PDK-1 gene were
inserted into the loxP site, so that the Cre recombinase enzyme
could knock out PDK-1 gene expression by recognizing the loxP site
(Fig. 4B). The RT-qPCR results
demonstrated that the mRNA expression levels of PDK-1 were higher
in the empty vector virus group on day 7 (12.69±0.61) compared with
those in the pHBAd-cre-EGFP virus group (3.6±0.2); mRNA expression
of PDK-1 was decreased by 72% at day 7 (P<0.01; Fig. 4C). Western blot analysis revealed
that p-PDK1, t-PDK1 and p-AKT protein expression gradually
decreased with time during the differentiation process of BMSCs
into osteoblasts (P<0.05; Fig.
4D). In conclusion, disrupting the floxed PDK-1 gene segment by
Cre recombinase downregulated PDK-1 expression in
BMSCsPDK-1flox/flox, as compared with the non-specific
effects that viral infection had on PDK-1 in BMSC
PDK-1flox/flox in the empty vector group.
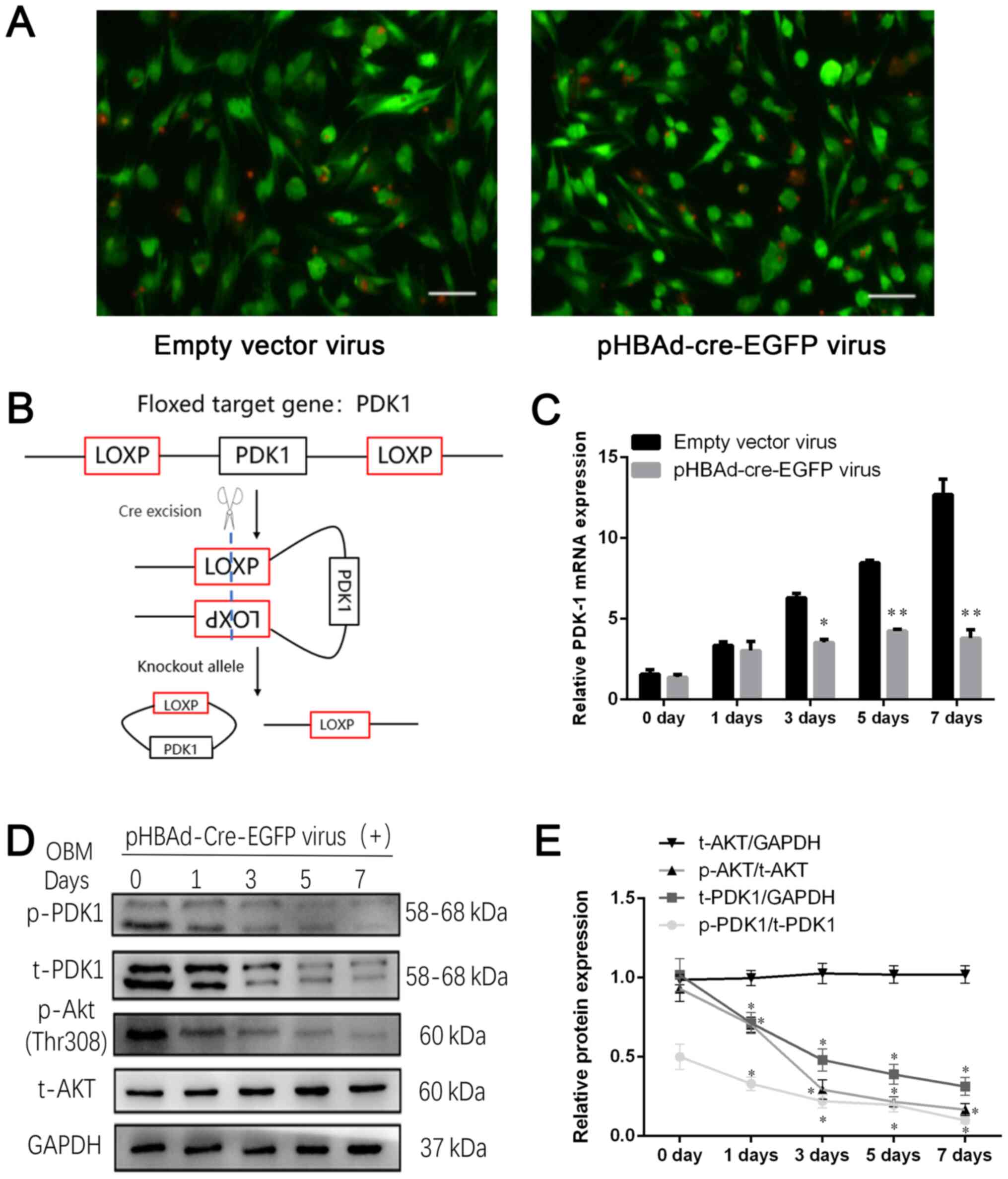 | Figure 4.pHBAd-cre-EGFP viral infection
decreases PDK-1 expression in BMSCPDK-1flox/flox. (A)
Green fluorescence of viral infection at a multiplicity of
infection of 100. Scale bar, 100 µm. (B) Schematic diagram of the
mechanism of action of Cre enzyme. (C) Reverse
transcription-quantitative PCR revealed that the relative mRNA
expression levels of PDK-1 were gradually inhibited following
pHBAd-cre-EGFP viral infection compared with those in the empty
vector virus group. (D) Western blot analysis of t-PDK-1, p-PDK-1,
t-AKT and p-AKT protein expression during the differentiation
process, following pHBAd-cre-EGFP viral infection. (E) Relative
protein expression levels of t-PDK1, t-AKT, p-PDK1 and p-AKT were
semi-quantified. Data are presented as the mean ± standard
deviation. *P<0.05, **P<0.01 vs. 0 day. PDK-1,
3-phosphoinositide-dependent protein kinase-1; t, total; p-,
phosphorylated. |
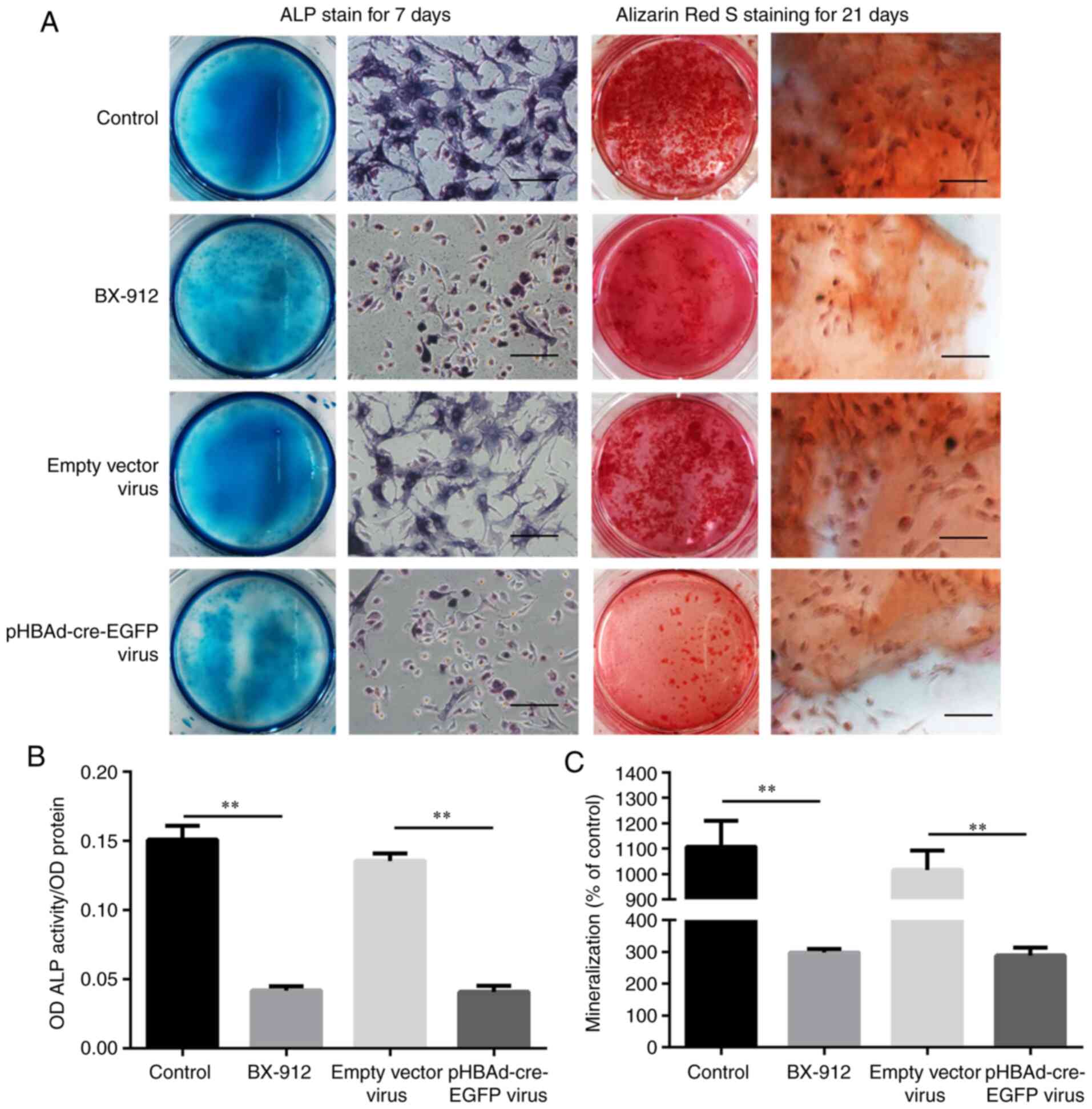
ALP activity and mineralization are
inhibited by BX-912 and pHBAd-cre-EGFP viral transfection
ALP expression is generally detected in the early
stage of osteoblast differentiation, whereas the mineralization
process generally occurs in the middle and late stages of
osteoblast differentiation (16).
Therefore, ALP staining and OD value determination were performed
on day 7, whereas Alizarin Red staining and quantitative detection
of mineralization (% control) were performed on day 21. The
findings revealed that a higher number of ALP-positive cells and
calcified nodules were observed in the control and empty vector
groups on days 7 and 21. However, the number of ALP-positive cells
and calcified nodules were markedly decreased in the BX-912 and
pHBAd-cre-EGFP virus groups on days 7 and 21 (Fig. 5A). The OD values of ALP activity
demonstrated that it was significantly lower in the BX-912 and
pHBAd-cre-EGFP virus groups compared with those in the control and
empty vector virus groups on day 7 (P<0.01; Fig. 5B). Mineralization (% control) was
also shown to be significantly lower in the BX-912 and
pHBAd-cre-EGFP virus groups compared with those in the control and
empty vector virus groups on day 21 (P<0.01; Fig. 5C). In conclusion, ALP activity and
mineralization (% control) formation were significantly inhibited
by BX-912 or pHBAd-cre-EGFP viral infection.
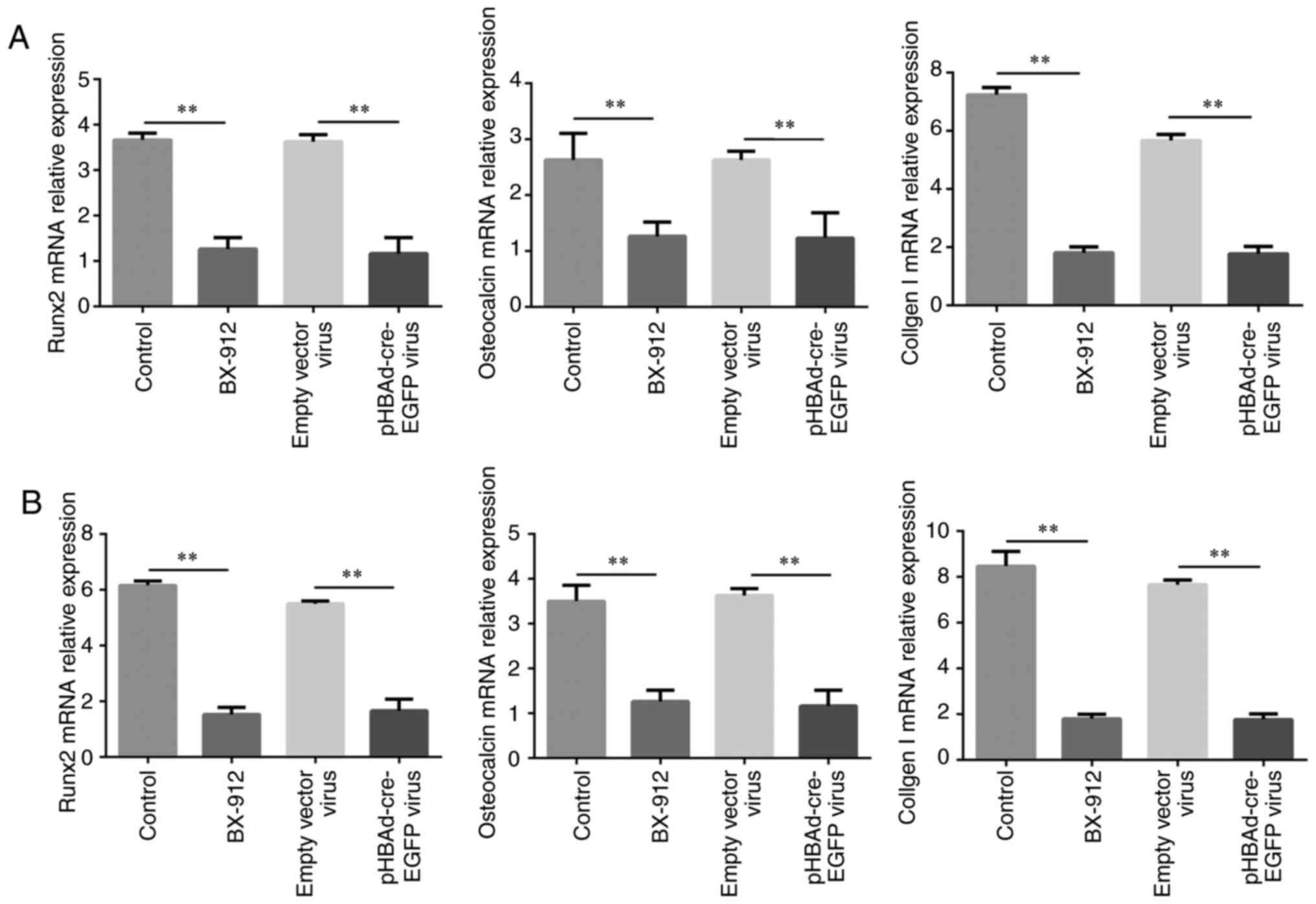
Osteoblast-related gene expression is
decreased by BX-912 and pHBAd-cre-EGFP viral infection
The mRNA expression levels of the osteoblast-related
genes RUNX2, osteocalcin, collagen I were detected by RT-qPCR on
days 7 and 21. The results demonstrated that the mRNA expression
levels of RUNX2, osteocalcin and collagen I were significantly
downregulated in the BX-912 and pHBAd-cre-EGFP virus groups,
compared with those in the control and empty vector virus groups
following treatment for 7 and 21 days (P<0.01; Fig. 6A and B).
Discussion
BMSCs are a commonly used seed cell in tissue
engineering. Under specific conditions, BMSCs can be induced to
differentiate into osteoblasts, adipocytes, chondrocytes, neurons,
etc., thus participating in tissue repair and regeneration. BMSCs
are also the main source of osteoblasts (17). Bone formation refers to the process
through which BMSCs form osteoblasts through migration,
proliferation and differentiation, and guide the formation of new
bone tissue (18). However, the
understanding of the principle and molecular mechanism underlying
the osteogenic differentiation of BMSCs is still limited.
Certain studies have reported that activation of the
PI3K/AKT signaling pathway may cause changes in numerous signaling
molecules associated with bone tissue, including bone morphogenetic
protein-2 and ALP bone formation markers, and promote osteoblast
proliferation and differentiation (5). During osteoblast differentiation, the
inhibition of this signaling pathway may lead to osteoblast damage
(19). AKT is a downstream effector
of PI3K. Mice with AKT gene knockout showed decreased bone mass
synthesis compared with in mice of the same age during childhood
and adulthood, indicating that this signaling pathway has the
function of regulating osteoblasts (6,20).
Based on the results of these studies, it may be hypothesized that
the PI3K/AKT signaling pathway serves an important role in
osteogenic differentiation.
Research on PDK-1 has mainly focused on tumor
metastasis, energy metabolism and cell growth (21), with PDK-1 rarely reported in the
field of bone metabolism. PDK-1 has been identified as a protein
kinase, which directly participates in the signal transduction
pathway downstream of AKT by promoting the phosphorylation of AKT
(22), subsequently affecting
carbohydrate metabolism, protein transcription and translation and
cell proliferation (23). AKT
protein can activate the downstream mTORC1 protein, which has been
shown to have a pivotal role in apoptosis, autophagy, proliferation
and differentiation (24). In
addition, AKT is one of the important signaling molecules
recognized and phosphorylated by PDK-1. When PDK-1 is activated,
the Thr308 site of the AKT protein is rapidly phosphorylated, and
downstream target proteins, such as Bcl-2-associated death
promoter, glycogen synthase kinases α and β, and mTORC1 are
activated or inhibited to regulate cell proliferation,
differentiation, apoptosis and migration (25). The translocation of AKT can
facilitate the phosphorylation of Thr308, which is accomplished
with the help of membrane-located PDK-1 (26). PDK-1 has been demonstrated to serve
a crucial role in PI3K/AKT signal transduction (Fig. 7). Therefore, it was hypothesized
that exploring the effect of the PDK-1 gene on osteoblast
differentiation and maturation may be of great significance to
skeletal metabolic diseases.
In the present study, in order to investigate the
physiological role of PDK-1 in bone metabolism, BX-912, a specific
inhibitor of PDK-1, was added into the OBM, and it was shown to
significantly inhibit the differentiation and maturation of
osteoblasts. BX-912 is an effective, complete PDK-1 inhibitor, and
its high specificity and selectivity when it comes to the PDK-1
gene has been widely recognized (27,28).
These characteristics were also verified in the present study. A
previous study also demonstrated that mice cannot survive if the
PDK1 gene is knocked out during the embryonic stage (29). Therefore, in order to understand the
effect of PDK-1 on osteoblasts, BMSCs were isolated from mice
(PDK-1flox/flox) carrying the PDK-1 gene and the
upstream and downstream sequence of the PDK-1 gene were inserted
into the loxP site respectively. After
BMSCsPDK-1flox/flox were infected with a virus
containing the Cre recombinase enzyme (pHBAd-cre-EGFP virus), the
mRNA expression levels of PDK-1 were significantly suppressed
compared with those in the empty vector virus group on days 3–7;
the p-AKT protein was also gradually downregulated.
It has also been reported that PDK-1 may act on
downstream proteins through the PI3K-independent pathway (12). Therefore, the present study focused
on changes in the expression of downstream AKT proteins. It is well
known that ALP is expressed at an early stage of osteoblast
differentiation, whereas mineralization occurs at a late stage.
Therefore, ALP staining and OD value determination were performed
on day 7, and Alizarin Red staining and quantitative detection of
mineralization (% control) were performed on day 21. In the present
study, the same effect that PDK-1 had on osteoblast differentiation
was also observed by BX-912 and PDK-1 gene disruption in
vitro. To the best of our knowledge, the present study was the
first to confirm that PDK-1 has an important role in osteoblast
differentiation.
Isomoto et al (30) reported that rapamycin, a specific
inhibitor of mTORC, inhibited osteoblast differentiation.
Phornphutkul et al (31) and
Singha et al (32) reported
the same results. Pan et al (33) revealed that inhibition of PI3K
kinase could block ALP activity in fetal rat skull cells. The
findings of those investigations indicated that the
PI3K/PDK-1/Akt/mTORC signaling pathway has an effect on osteoblast
differentiation, which were similar to the findings of the present
study. Owen and Pan (34) suggested
that there were three stages of osteoblast differentiation.
Initially, actively proliferating cells produce extracellular
matrix components, such as laminin and express cell growth
regulatory genes (35). After the
cells have entered the maturation stage of the extracellular
matrix, collagen I protein, which provides bone mechanical strength
and constitutes the structure of bone tissue, is deposited and the
expression of ALP, a marker gene of the osteoblast phenotype, is
increased. In the final stage of mineralization, the expression of
bone sialoprotein and osteocalcin increases. Osteocalcin, a
secreted protein, is synthesized by osteoblasts in the
non-proliferative stage (18). It
participates in bone development by producing carboxylated
osteocalcin, and is considered to be a sign of bone formation and
transformation. Furthermore, RUNX2, as a specific transcription
factor for osteoblasts, mainly regulates the differentiation of
mesenchymal stem cells into osteogenic and cartilage precursor
cells, and serves an important role in the formation and
reconstruction of bone tissue (7).
Therefore, the present study detected the expression of these three
genes. It was revealed that the mRNA expression levels of RUNX2,
osteocalcin and collagen I were significantly downregulated in the
BX-912 and pHBAd-cre-EGFP virus groups compared with those in the
other groups.
The main components of OBM include ascorbic acid,
sodium β-glycerophosphate and dexamethasone. It has been widely
accepted that this medium can promote the differentiation of BMSCs
into osteoblasts (36).
Dexamethasone has been reported to promote the differentiation and
maturation of osteoblasts, increase ALP, regulate the secretion of
insulin-like growth factor by osteoblasts and promote the synthesis
of extracellular matrix collagen (31). Ascorbic acid may promote the
synthesis of collagen I in cultured cells, and regulate ATP and ALP
activity and the synthesis of non-collagen matrix proteins
(37). β-glycerophosphate has been
shown to provide phosphate ions to osteoblasts, and promote the
deposition and calcification of physiological calcium salts, which
is a necessary condition for mineralized nodules in BMSCs (38). When medium contains these
components, BMSCs undergo a series of changes, resulting in them
obtaining the morphology and growth characteristics of osteoblasts
(39).
Notably, inhibition of osteoblasts was only observed
at the cellular level in the present study; a more in-depth study
on its specific biological mechanism is required. It should also be
noted that the downstream proteins of the PI3K/AKT signaling
pathway were not completely explored, which is another limitation
of the present study. Our future studies aim to focus on changes in
bone morphology after knocking out the PDK-1 gene in osteoblasts
in vivo and on the downstream proteins of the PI3K/AKT
signaling pathway, in order to obtain novel targets for the
treatment of osteoporosis or osteosclerosis.
In conclusion, the results of the present study
found that PDK-1 gene disruption and BX-912 can significantly
inhibit the maturation of BMSC into osteoblasts, indicating that
PDK-1 plays an important role in the process of osteoblast
differentiation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81860402), the
Guangxi Natural Science Foundation of China (grant no.
2017GXNSFAA198073), the Guangxi Key Research and Development
Project (grant no. GuikeAB17195001), and the High Level Innovation
Team and Excellence Scholars Program of Guangxi High Education
Institutions and Guangxi Medical High-level Key Talents Training
‘139’ Program Training Project.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YB, QiZ and QuZ performed the experiments and wrote
the manuscript. YZ and HN analyzed the data. ML and ZS performed
the experiments, analyzed the data, and prepared and drafted the
manuscript. SZ and GZ conceived and designed the study, and
reviewed and edited the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Animals
Ethics Committee of Guangxi Medical University and conducted
according to the Guide for the Care and Use of Laboratory Animals
(approval no. 201910012).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu P, Lee S, Knoll J, Rauch A, Ostermay
S, Luther J, Malkusch N, Lerner UH, Zaiss MM, Neven M, et al: Loss
of menin in osteoblast lineage affects osteocyte-osteoclast
crosstalk causing osteoporosis. Cell Death Differ. 24:672–682.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Föger-Samwald U, Vekszler G, Hörz-Schuch
E, Salem S, Wipperich M, Ritschl P, Mousavi M and Pietschmann P:
Molecular mechanisms of osteoporotic hip fractures in elderly
women. Exp Gerontol. 73:49–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Indran IR, Liang RL, Min TE and Yong EL:
Preclinical studies and clinical evaluation of compounds from the
genus Epimedium for osteoporosis and bone health. Pharmacol Ther.
162:188–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoon JY, Baek CW, Kim HJ, Kim EJ, Byeon GJ
and Yoon JU: Remifentanil negatively regulates RANKL-induced
osteoclast differentiation and bone resorption by inhibiting
c-Fos/NFATc1 expression. Tissue Eng Regen Med. 15:333–340. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gu YX, Du J, Si MS, Mo JJ, Qiao SC and Lai
HC: The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1
preosteoblast proliferation and differentiation on SLA and SLActive
titanium surfaces. J Biomed Mater Res A. 101:748–754. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ulici V, Hoenselaar KD, Agoston H,
McErlain DD, Umoh J, Chakrabarti S, Holdsworth DW and Beier F: The
role of Akt1 in terminal stages of endochondral bone formation:
Angiogenesis and ossification. Bone. 45:1133–1145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agas D, Sabbieti MG, Marchetti L, Xiao L
and Hurley MM: FGF-2 enhances Runx-2/Smads nuclear localization in
BMP-2 canonical signaling in osteoblasts. J Cell Physiol.
228:2149–2158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao D, Zhou Q, Gao Y, Cao B, Zhang Q,
Zeng G and Zong S: PDK1 is important lipid kinase for RANKL-induced
osteoclast formation and function via the regulation of the
Akt-GSK3β-NFATc1 signaling cascade. J Cell Biochem. 121:4542–4557.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng X, He J, Zhao J, Wu Y, Shi X, Du L,
Nong M, Zong S and Zeng G: Polygonatum sibiricum polysaccharide
promotes osteoblastic differentiation through the
ERK/GSK-3β/β-catenin signaling pathway in vitro. Rejuvenation Res.
21:44–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klein J, Fasshauer M, Ito M, Lowell BB,
Benito M and Kahn CR: beta(3)-adrenergic stimulation differentially
inhibits insulin signaling and decreases insulin-induced glucose
uptake in brown adipocytes. J Biol Chem. 274:34795–34802. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shibata H, Toyama K, Shioya H, Ito M,
Hirota M, Hasegawa S, Matsumoto H, Takano H, Akiyama T, Toyoshima
K, et al: Rapid colorectal adenoma formation initiated by
conditional targeting of the Apc gene. Science. 278:120–123. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakaue H, Nishizawa A, Ogawa W,
Teshigawara K, Mori T, Takashima Y, Noda T and Kasuga M:
Requirement for 3-phosphoinositide-kependent dinase-1 (PDK-1) in
insulin-induced glucose uptake in immortalized brown adipocytes. J
Biol Chem. 278:38870–38874. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Son KM, Park HC, Kim NR, Lee IS and Yang
HC: Enhancement of the ALP activity of C3H10T1/2 cells by the
combination of an oxysterol and apatite. Biomed Mater.
5:0441072010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai Y, Zheng C and Li H: Inhibition of
miR-23a-3p promotes osteoblast proliferation and differentiation. J
Cell Biochem. Nov 6–2019.(Epub ahead of print). doi:
10.1002/jcb.29497. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeon HL, Oh IS, Baek YH, Yang H, Park J,
Hong S and Shin JY: Zoledronic acid and skeletal-related events in
patients with bone metastatic cancer or multiple myeloma. J Bone
Miner Metab. 38:254–263. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu J, Zhang W, Ran Q, Xiang Y, Zhong JF,
Li SC and Li Z: The differentiation balance of bone marrow
mesenchymal stem cells is crucial to hematopoiesis. Stem Cells Int.
2018:15401482018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berendsen AD and Olsen BR: Bone
development. Bone. 80:14–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Ma XY, Feng YF, Ma ZS, Ma TC,
Zhang Y, Li X, Wang L and Lei W: Magnesium ions promote the
biological behaviour of rat calvarial osteoblasts by activating the
PI3K/Akt signalling pathway. Biol Trace Elem Res. 179:284–293.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng XD, Xu PZ, Chen ML, Hahn-Windgassen
A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman
KG and Hay N: Dwarfism, impaired skin development, skeletal muscle
atrophy, delayed bone development, and impeded adipogenesis in mice
lacking Akt1 and Akt2. Genes Dev. 17:1352–1365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu YR, Min H, Fang JF, Zhou F, Deng XW
and Zhang YQ: Activity of the novel dual phosphatidylinositol
3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 against
osteosarcoma. Cancer Biol Ther. 16:602–609. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alessi DR, James SR, Downes CP, Holmes AB,
Gaffney PR, Reese CB and Cohen P: Characterization of a
3-phosphoinositide-dependent protein kinase which phosphorylates
and activates protein kinase Balpha. Curr Biol. 7:261–269. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frödin M, Jensen CJ, Merienne K and
Gammeltoft S: A phosphoserine-regulated docking site in the protein
kinase RSK2 that recruits and activates PDK1. EMBO J. 19:2924–2934.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abeyrathna P and Su Y: The critical role
of Akt in cardiovascular function. Vascul Pharmacol. 74:38–48.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang F, Shan S, Huo Y, Xie Z, Fang Y, Qi
Z, Chen F, Li Y and Sun B: MiR-155-5p inhibits PDK1 and promotes
autophagy via the mTOR pathway in cervical cancer. Int J Biochem
Cell Biol. 99:91–99. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williams MR, Arthur JS, Balendran A, van
der Kaay J, Poli V, Cohen P and Alessi DR: The role of
3-phosphoinositide-dependent protein kinase 1 in activating AGC
kinases defined in embryonic stem cells. Curr Biol. 10:439–448.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiu Z, Li H, Zhang Z, Zhu Z, He S, Wang X,
Wang P, Qin J, Zhuang L, Wang W, et al: A pharmacogenomic landscape
in human liver cancers. Cancer Cell. 36:179–193.e111. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Coppé JP, Mori M, Pan B, Yau C, Wolf DM,
Ruiz-Saenz A, Brunen D, Prahallad A, Cornelissen-Steijger P, Kemper
K, et al: Mapping phospho-catalytic dependencies of
therapy-resistant tumours reveals actionable vulnerabilities. Nat
Cell Biol. 21:778–790. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qian XJ, Li XL, Xu X, Wang X, Feng QT and
Yang CJ: α-SMA-Cre-mediated excision of PDK1 reveals an essential
role of PDK1 in regulating morphology of cardiomyocyte and tumor
progression in tissue microenvironment. Pathol Biol (Paris).
63:91–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Isomoto S, Hattori K, Ohgushi H, Nakajima
H, Tanaka Y and Takakura Y: Rapamycin as an inhibitor of osteogenic
differentiation in bone marrow-derived mesenchymal stem cells. J
Orthop Sci. 12:83–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Phornphutkul C, Lee M, Voigt C, Wu KY,
Ehrlich MG, Gruppuso PA and Chen Q: The effect of rapamycin on bone
growth in rabbits. J Orthop Res. 27:1157–1161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Singha UK, Jiang Y, Yu S, Luo M, Lu Y,
Zhang J and Xiao G: Rapamycin inhibits osteoblast proliferation and
differentiation in MC3T3-E1 cells and primary mouse bone marrow
stromal cells. J Cell Biochem. 103:434–446. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pan JM, Wu LG, Cai JW, Wu LT and Liang M:
Dexamethasone suppresses osteogenesis of osteoblast via the
PI3K/Akt signaling pathway in vitro and in vivo. J Recept Signal
Transduct Res. 39:80–86. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Owen TA and Pan LC: Isolation and culture
of rodent osteoprogenitor cells. Methods Mol Biol. 455:3–18. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Selim AA, Castaneda JL, Owen TA, Popoff SN
and Safadi FF: The role of osteoactivin-derived peptides in
osteoblast differentiation. Med Sci Monit. 13:BR259–BR270.
2007.PubMed/NCBI
|
|
36
|
Xi Y, Huang H, Zhao Z, Ma J and Chen Y:
Corrigendum to ‘Tissue inhibitor of metalloproteinase 1 suppresses
growth and differentiation of osteoblasts and osteoclasts by
targeting the AKT pathway’ [Exp. Cell Res. 389 (2020)
111930–11940]. Exp Cell Res. 394:1121892020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hadzir SN, Ibrahim SN, Abdul Wahab RM,
Zainol Abidin IZ, Senafi S, Ariffin ZZ, Abdul Razak M and Zainal
Ariffin SH: Ascorbic acid induces osteoblast differentiation of
human suspension mononuclear cells. Cytotherapy. 16:674–682. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang RX and Tao J: Nicotinamide
mononucleotide attenuates glucocorticoid-induced osteogenic
inhibition by regulating the SIRT1/PGC-1α signaling pathway. Mol
Med Rep. 22:145–154. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu X, Yang H, Zhang H, Wang G, Liu K, Gu
Q, Tao Y, Chen G, Jiang X, Li G, et al: Improved osteogenesis and
upregulated immunogenicity in human placenta-derived mesenchymal
stem cells primed with osteogenic induction medium. Stem Cell Res
Ther. 7:1382016. View Article : Google Scholar : PubMed/NCBI
|