Introduction
Repeated stress events have a substantial role in
the modulation of immunoglobulin (Ig)A, a component of mucosal
immunity with a key anti-inflammatory role that is regarded as a
biomarker of animal wellness (1,2).
Experimental assays in animal models have shown, in most cases, the
downregulatory effects of chronic stress on parameters associated
with the IgA response, which include total and secretory IgA
production and the number of IgA+ plasma cells (3–9). In
other studies, the IgA response was found to be either
significantly or non-significantly increased under chronic stress
conditions (8,10,11).
The modulatory role of chronic stress on the intestinal IgA
response seems to entail crosstalk between the humoral and cellular
components of intestinal immunity and stress hormones, such as
corticosterone release by the adrenal glands (5,8,9). The
elevation of corticosterone levels is the endpoint of the
hypothalamic pituitary adrenal (HPA) axis activation via the
corticotropin-releasing hormone (CRH) pathway (12). The underlying molecular mechanisms
that control intestinal immunity are not fully known; however, in
the brain, corticosterone positively or negatively modulates
genomic and nongenomic expression through cytoplasmic and membrane
glucocorticoid receptors, respectively (12).
Bovine lactoferrin (bLf) is a multifunctional
iron-binding glycoprotein that enhances the IgA response and even
IgG expression levels, as previously shown in the distal or
full-length small intestine of healthy mice (13–15).
Similar to IgA, locally synthetized IgG contributes to the
protection of the intestinal mucosa (16). The effects of lactoferrin on the IgA
response result from the role of lactoferrin as a modulatory factor
in antigen presenting cells (17)
and CD3+ T cells (18)
as well as its action as a class-switch factor for IgA via the
transforming growth factor (TGF)-β receptor III signaling pathway
(19). In experimental models of
chronic immobilization stress, bLf treatment increases the number
of splenic antibody-secreting cells (20). The underlying mechanism seems to
entail both the upregulatory and downregulatory effects of bLf on
stress hormones such as corticosterone (21,22).
Chronic stress has an earlier proinflammatory effect in the ileum
compared with that in the duodenum, as previously documented in
mice that underwent crowding stress, a model that is used to
address the role of psychosocial stress in human disorders such as
irritable bowel syndrome (IBS) (23). The harmful effect of chronic stress
is associated in part with perturbations in the luminal microbiota
that, in turn, exhibits a gradient that increases from the proximal
to the distal small intestine (24,25).
Although previous studies have focused on separately
examining the effects of stress or bLf on intestinal immunity, the
present study assessed the effects of chronic stress on parameters
associated with the IgA response in the distal small intestine
during bLf treatment. As the modulatory properties of chronic
stress on the IgA response-associated parameters in the distal
intestine under conditions of bLf treatment have not yet been
documented, this approach may provide insights into the potential
use of bLf in pharmacological and neuroimmunological interventions
to maintain intestinal homeostasis.
Materials and methods
Animal handling
A total of 48 male BALB/c mice (age, 6 weeks; body
weight, 21–23 g) were purchased from Unidad de Producción y
Experimentación Animal, Universidad Autónoma Metropolitana Unidad
Xochimilco (Mexico City, Mexico). Mice individually housed, were
provided with water and fed Laboratory Rodent Diet 5001 (LabDiet)
ad libitum, and kept on a 12-h light/dark cycle in a
noiseless room, at 20°C and a relative humidity of 55%. The mice
were left for 2 weeks to adapt to the environmental conditions, and
the experimental interventions were started when the mice were
8-weeks-old. The animals were handled in accordance with Mexican
Federal Regulations for Animal Experimentation and Care
(NOM-062-ZOO-1999, Ministry of Agriculture, Mexico City, Mexico) in
concordance with ARRIVE guidelines for reporting animal research
(26). The protocol was approved by
the Institutional Animal Care and Use Committee of the Instituto
Politécnico Nacional. All handling and assays were carried out from
8:00-11:00 a.m. to normalize the influence of the circadian cycle
on adrenocorticotropic hormone and corticosterone fluctuations.
For immobilization, each mouse was placed in a prone
position, and the four limbs were gently stretched and attached
with adhesive tape to an expanded polystyrene board enveloped with
plastic film for easy cleaning. Limb immobilization occurred in the
following order: Forepaws, hindpaws and tail. This process was
accomplished by first placing very low-adhesive tape and then
covering that with high-adhesive tape to minimize pain after tape
removal. The adhesive tape was placed at the dorsum of the
forepaws, on the footpads of the hindpaws and in the middle of the
tail. Curve strips produced from paperboard-adhesive tape reels
were placed upon the adhesive tape as chewers for mice to prevent
self-inflicted injuries on the foreleg skin. In this experiment,
the head of each mouse was allowed to move freely, and the twisting
of limbs and tearing of whiskers were avoided by gentle and careful
handling of mice. After 1 h of immobilization, the adhesive tape
was removed carefully to reduce pain in the following order: Tail,
followed by the hindpaws and then the forepaws. This protocol was
repeated daily for 7 days. Food and water were removed from the
control group during the period of immobilization applied to the
stressed mice group.
Bovine lactoferrin
Iron-saturated (3%) bLf was purchased from
NutriScience Innovations, LLC, and the purity was confirmed.
Briefly, total protein of bLf was quantified using Bradford reagent
(cat. no. 500 0006; Bio-Rad Laboratories, Inc.) and 2 µg protein
mixed with NuPAGE™ LDS Sample Buffer (4X; cat. no. NP0007;
Invitrogen; Thermo Fisher Scientific, Inc.) was boiled at 85°C for
5 min and loaded onto a 10% SDS-PAGE gel alongside a molecular
weight marker (cat. no. MPSTD3; MilliporeSigma). Electrophoresis
was performed at 88 V for 2 h. After, the gels were stained with
Coomassie blue R solution (cat. no. 1610436; Bio-Rad Laboratories,
Inc.) for 1 h at room temperature with gentle agitation and the bLf
purity was confirmed by the presence of a single 80 kDa band
(Fig. S1). A volume of 100 µl
saline solution 0.9% w/v NaCl (Solución CS-PiSA®; PiSA
Pharmaceutical) containing 50, 500 or 5,000 µg bLf for the
bLf-treated groups or vehicle only (saline solution) for the
bLf-untreated groups were delivered by oral deposition with a
200-µl pipette tip.
Experimental protocol
A total of eight groups of six mice were used in
this study. Three groups were treated with either 50, 500 or 5,000
µg of bLf and underwent chronic stress for 1 h a day for 7 days by
board immobilization. bLf administration occurred 1 h before the
start of the stress session. Three groups of unstressed mice were
used as the controls, which were treated with corresponding bLf
doses. Two groups were untreated basal groups, one of which
underwent stress and one that was not stressed. On day 7, the mice
were sacrificed by exposing them with an isoflurane overdose
(Sofloran™; PiSA Pharmaceutical). Euthanasia procedure was
conducted by placing each mouse within a 500 ml clean chamber glass
jar (wide mouth) with a tight-fitting lid containing 300 µl of 100%
isoflurane. Following 3 min of isoflurane exposure, cardiac arrest
(no palpable heartbeat) and sphincter distention (urine and feces
release) were detected to confirm death, and the mice were
immediately exsanguinated by cardiac puncture with a heparinized
syringe. Plasma samples were collected and stored at −70°C to
analyze the corticosterone level by ELISA. A small 5-cm distal
segment proximal to the ileocecal valve was collected and dissected
for intestinal washings to evaluate antibody levels by ELISA.
Epithelial cells purified from the distal intestinal segment were
used to analyze the protein expression levels of α-chain and
polymeric immunoglobulin receptor (pIgR) by a chemiluminescent
western blot assay. The intestinal strip was scraped with a
coverslip to collect whole mucosa samples to analyze the mRNA
expression levels of α-chain, J-chain, pIgR, IL-2, IL-4, IL-5 and
IL-6 by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR).
ELISAs for antibody levels
The collection of intestinal washings was
accomplished by flushing the intestinal segment with 3 ml of
sterile PBS (pH 7.2) containing 0.02% sodium azide and the Complete
Mini protease inhibitor cocktail (cat. no. 11 836 153 001; Roche
Diagnostics GmbH). The intestinal washings were centrifuged at
10,000 × g for 20 min at 4°C, and the supernatants were removed and
stored at −70°C until used. The levels of antibodies in the
intestinal washings were tested in 96-well microtiter ELISA plates
using 100 µl of all reactants in each well in successive steps. For
this study, rabbit anti-mouse IgA myeloma serum to detect total IgA
was prepared as follows: Two male New Zealand rabbits (age, 12
weeks; body weight, 2.5 kg) were purchased from Unidad de
Producción y Experimentación Animal, Universidad Autónoma
Metropolitana Unidad Xochimilco (Mexico City, Mexico). Rabbits were
individually housed, provided with water and fed Laboratory Rabbit
Diet 5321 (LabDiet) ad libitum and kept on a 12-h light/dark
cycle in a noiseless room, at 20°C and a relative humidity of 55%.
Rabbits were immunized intramuscularly with IgA κ-chains from
murine myeloma (cat. no. M1421; Sigma-Aldrich; Merck KGaA). An
immunogen volume of 500 µl containing 0.3 µg was mixed with 500 µl
of either complete (cat. no. F5881; Sigma-Aldrich; Merck KGaA) or
incomplete Freund's adjuvant (cat. no. F5506; Sigma-Aldrich; Merck
KGaA). Three immunizations were applied for 7 days each, the first
with the immunogen plus complete Freund's adjuvant and the last two
with the immunogen plus with incomplete Freund's adjuvant. A total
of 1 ml blood was collected once from the marginal vein for
monitoring the immune response of rabbit. One week after the last
immunization, 10 ml blood was collected from the central ear artery
using a sterile syringe and applying 70% ethanol as a disinfectant
and 1 ml (1% w/v) lidocaine (Pisacaina®1%; PiSA
Pharmaceutical) as a local anesthetic subcutaneously. Blood was
centrifuged at 515 × g for 10 min at room temperature and serum
collected was aliquoted and stored at −70°C. Thereafter, euthanasia
was conducted by an intraperitoneal injection of a sodium
pentobarbital overdose (100 mg/kg body weight), death was confirmed
by detecting lack of pulse, breathing, corneal reflect, cardiac
arrest and sphincter distention.
For sandwich-type ELISA, coating was performed by
overnight incubation at 4°C with 0.1 µg/ml rabbit polyclonal
anti-myeloma mouse IgA antibody (prepared as described above) for
total IgA, 0.4 µg/ml goat polyclonal anti-SC (N-16) antibody (cat.
no. sc-20485; Santa Cruz Biotechnology, Inc.) for secretory IgA or
0.1 µg/ml rabbit polyclonal anti-mouse IgG secondary antibody (cat.
no. 61-6000; Invitrogen; Thermo Fisher Scientific, Inc.) for total
IgG in 0.1 M carbonate-bicarbonate buffer (pH 9.6). A total of five
rounds of washes with PBS containing 0.05% Tween-20 (PBST) were
performed before and after each incubation step at 37°C. Blocking
was performed with 3% skimmed milk in 0.1 M carbonate-bicarbonate
buffer (pH 9.6), and incubation for 2 h at 37°C. For the samples,
two-fold sample dilutions in a saline solution were tested in
duplicate and incubated for 1 h at 37°C. The conjugation step was
performed by 1 h incubation at 37°C with horseradish peroxidase
(HRP)-conjugated goat anti-mouse IgA (1:5,000; cat. no. 626720;
Invitrogen; Thermo Fisher Scientific, Inc.) for total and secretory
IgA or HRP-conjugated rabbit anti-mouse IgG (1:5,000; cat. no.
816720; Invitrogen; Thermo Fisher Scientific, Inc.) for total IgG
antibodies. A volume of 10 µl 30% hydrogen peroxide (cat. no.
H1009; Sigma-Aldrich; Merck KGaA) was mixed with 10 mg of
O-phenylenediamine (cat. no. P9029; Sigma-Aldrich; Merck KGaA) in
50 ml phosphate-citric acid citrate buffer (0.05 M, pH 5.0) was
prepared as the substrate and incubated at room temperature in the
dark for 20 min. The enzymatic reaction was stopped with 2.5 M
sulfuric acid (cat. no. 320501; Sigma-Aldrich; Merck KGaA). For
normalization purposes, the absorbance value (λ=490 nm) of each
sample was divided by the absorbance of an internal control (pool
of intestinal liquid samples from mice treated with bLf) and
multiplied by the corresponding dilution factor. The data from six
mice per group are reported in relative units as the mean ± SD.
For the evaluation of specific IgA and IgG levels,
an anti-bLf coating was performed with 0.2 µg/ml of bLf using
carbonate-bicarbonate buffer (pH 9.6) and the same incubation
conditions. Thereafter, the successive steps followed those
described for the total antibody ELISAs, but using HRP-conjugated
anti-mouse IgA (1:5,000; cat. no. 626720; Invitrogen; Thermo Fisher
Scientific, Inc.) or HRP-conjugated anti-mouse IgG (1:5,000; cat.
no. 816720; Invitrogen; Thermo Fisher Scientific, Inc.) antibodies.
The absorbance of the samples was divided by the absorbance of the
internal standard and multiplied by the corresponding dilution
factor. Data are expressed in relative units as the mean and
SD.
Western blot analysis
Epithelial cell protein extracts were examined by
western blot analysis to determine the expression of the α-chain
component of IgA and pIgR, which is involved in IgA-mediated
transcytosis (27). The expression
of the 78-kDa and 120-kDa forms of the pIgR protein were measured
(28). Epithelial cells were
purified as following: Intestinal segments were everted to expose
the luminal surface and mixed with 1.5 mM EDTA (pH 7.3) plus 1%
antibiotic-antimycotic (cat. no. 15240062; Gibco; Thermo Fisher
Scientific, Inc.) and 1% FBS (cat. no. 10437; Gibco; Thermo Fisher
Scientific, Inc.) in RPMI-1640 medium (cat. no. 11875085; Gibco;
Thermo Fisher Scientific, Inc.). Thereafter, the samples were
incubated for 30 min at 37°C in a water bath at 50 × g, and then a
gentle disaggregation was applied using a syringe rubber plunger
before passing the cell suspension through a 100-µm cell strainer
(cat. no. Z742101; Sigma-Aldrich; Merck KGaA) to remove the larger
debris. The cell suspension was centrifuged at 515 × g for 15 min
at 4°C, the supernatant was removed, and the cell pellet was
resuspended in RPMI-1640 medium, passed through a gauze and then
centrifuged as before. The cell pellet was subsequently suspended
in 20% Percoll (HyClone; Cytiva) and this mixture was overlaid upon
40% Percoll and then centrifuged at 515 × g for 30 min at 4°C. The
epithelial cells were recovered at the interphase between 20 and
40% and then washed with PBS and then centrifuged as indicated
above. From each group (n=6), epithelial samples of two mice were
mixed and three pools were obtained and analyzed in duplicate. The
epithelial cells were treated with protease inhibitor cocktail
(cat. no. 11836153001; Roche Diagnostics GmbH) and disrupted by
sonication with one 10 sec pulse at 100 W amplitude (Fisher Sonic
Dismembrator Model 3000). The samples were then centrifuged at
10,000 × g for 10 min at 4°C and the supernatants were collected
and stored at −70°C.
Total protein concentration was quantified using
Bradford reagent and 20 µg protein/lane was separated via 10%
SDS-PAGE at 88 V for 2 h. The separated proteins were transferred
onto PVDF membranes and blocked with 5% milk/1X TBST at room
temperature for 1 h. The membranes were then incubated at room
temperature for 1 h with primary antibodies [rabbit HRP-conjugated
anti-mouse IgA (1:10,000; cat. no. 626720; Invitrogen; Thermo
Fisher Scientific, Inc.), goat polyclonal anti-human SC (N-16)
antibody (1:400; cat. no. sc-20485; Santa Cruz Biotechnology, Inc.)
to detect pIgR or goat polyclonal anti-actin C-11 antibody (1:500;
cat. no. SC-1615; Santa Cruz Biotechnology, Inc.)]. Following the
primary antibody incubation, the membranes were washed with TBST
(thrice) and incubated with a secondary antibody just for SC and
actin [1:5,000; HRP-conjugated rabbit anti-goat polyclonal IgG
(H+L; cat. no. 61-1620; Invitrogen; Thermo Fisher Scientific, Inc.)
for 1 h at room temperature. The membranes were washed with TBST
(thrice) and protein bands were detected by chemiluminescence,
which was carried out using a streptavidin-HRP conjugate (cat. no.
18-152; Millipore; Merck KGaA) and the luminol-based SuperSignal
West Femto substrate (cat. no. 3409; Thermo Fisher Scientific,
Inc.). The protein bands were detected using an ImageQuant LAS 4000
instrument (GE Healthcare), and the bands were semi-quantified
using the ImageQuant TL v8.1 software (Amersham; Cytiva). The data
are reported in relative units and were computed by dividing the
signal of each band (α-chain, 60 kDa; pIgR, 78 and 120 kDa) by the
signal of the actin band (42 kDa). The data were normalized with
the control basal group and reported as the mean ± SD.
RT-qPCR
RT-qPCR was performed to assess the expression of
regulatory ILs involved in the generation of IgA-associated
proteins (α- and J-chains) and pIgR (27,29).
RNA extraction, cDNA synthesis and qPCR for α-chain, J-chain, pIgR
and IL-2, −4 and −6 (30) and IL-5
were performed. Briefly, total RNA from whole mucosa samples was
extracted using TRIzol® reagent (50 mg/ml; Invitrogen;
Thermo Fisher Scientific, Inc.), according to manufacturer's
protocol. Reverse transcription was performed using M-MLV reverse
transcriptase (cat. no. 28025-021; Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol at 37°C
for 50 min. qPCR was subsequently performed using a LightCycler
TaqMan Master reaction mix (cat. no. 04 535 286 001; Roche
Diagnostics GmbH) in a LightCycler instrument (Roche Diagnostics
GmbH). The following thermocycling conditions were used: Initial
denaturation at 95°C for 10 min; followed by 45 cycles for
amplification at 95°C for 10 sec, 60°C for 35 sec and 72°C for 1
sec; and 1 cycle for cooling at 40°C for 30 sec.
Original specific oligonucleotide primers were
generated by using the online assay design software ProbeFinder
(https://lifescience.roche.com/en_mx/brands/universal-probe-library.html)
as previously reported (30). The
nucleotide sequence of primers used for RT-qPCR are shown in
Table I. Universal ProbeLibrary
probes are all from Roche Diagnostics GmbH (α-chain, probe #27,
cat. no. 04687582001; J-chain, probe #11, cat. no. 04685105001;
pIgR, probe #80, cat. no. 04689038001; IL-2, probe #15, cat. no.
04685148001; IL-4, probe #2, cat. no. 04684982001; IL-5, probe #91,
cat. no. 04692080001; IL-6, probe #6, cat. no. 04685032001; 18S
ribosomal RNA, probe #48, cat. no. 04688082001. The samples were
analyzed in duplicate and the mRNA expression levels were
calculated by using the comparative parameter quantification cycle
method and normalized to the level of the 18S ribosomal RNA subunit
(31).
 | Table I.Nucleotide sequences of primers used
for reverse transcription-quantitative PCR. |
Table I.
Nucleotide sequences of primers used
for reverse transcription-quantitative PCR.
| Primer name | Sequence
(5′→3′) | Sequence
(3′→5′) |
|---|
| α-chain |
acctcagtcaccgtctcctc |
cggaagggaagtaatcgtga |
| J-chain |
gaactttgtataccatttgtcagacg |
ctgggtggcagtaacaacct |
| pIgR |
ctgtgcccgaaactggat |
tcaggttggcttcttgtatgag |
| IL-2 |
gctgttgatggacctacagga |
ttcaattctgtggcctgctt |
| IL-4 |
catcggcattttgaacgag |
cgagctcactctctgtggtg |
| IL-5 |
acattgaccgccaaaaagag |
atccaggaactgcctcgtc |
| IL-6 |
gctaccaaactggatataatcagga |
ccaggtagctatggtactccagaa |
| 18S ribosomal
RNA |
gcaattattccccatgaacg |
gggacttaatcaacgcaagc |
Corticosterone assay
Plasma corticosterone expression levels were
determined using a commercially available Corticosterone ELISA kit
(cat. no. 901-097; Enzo Life Sciences, Inc.). Two-fold sample
dilutions were analyzed in duplicate, and corticosterone
concentrations were calculated from a standard curve and are
reported in ng/ml as the mean ± SD of 6 mice per group.
Statistical analysis
Three independent assays were performed, and the
results are expressed as the mean ± SD from one representative
assay of 8 groups with 6 mice each. Data comparisons were analyzed
by one-way ANOVA followed by a Holm-Šidák pos hoc test. All
analyses were performed using the statistical program SigmaPlot
version 11 (Systat Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
bLf counteracts the effect of stress
on the increase of total IgA and IgG responses
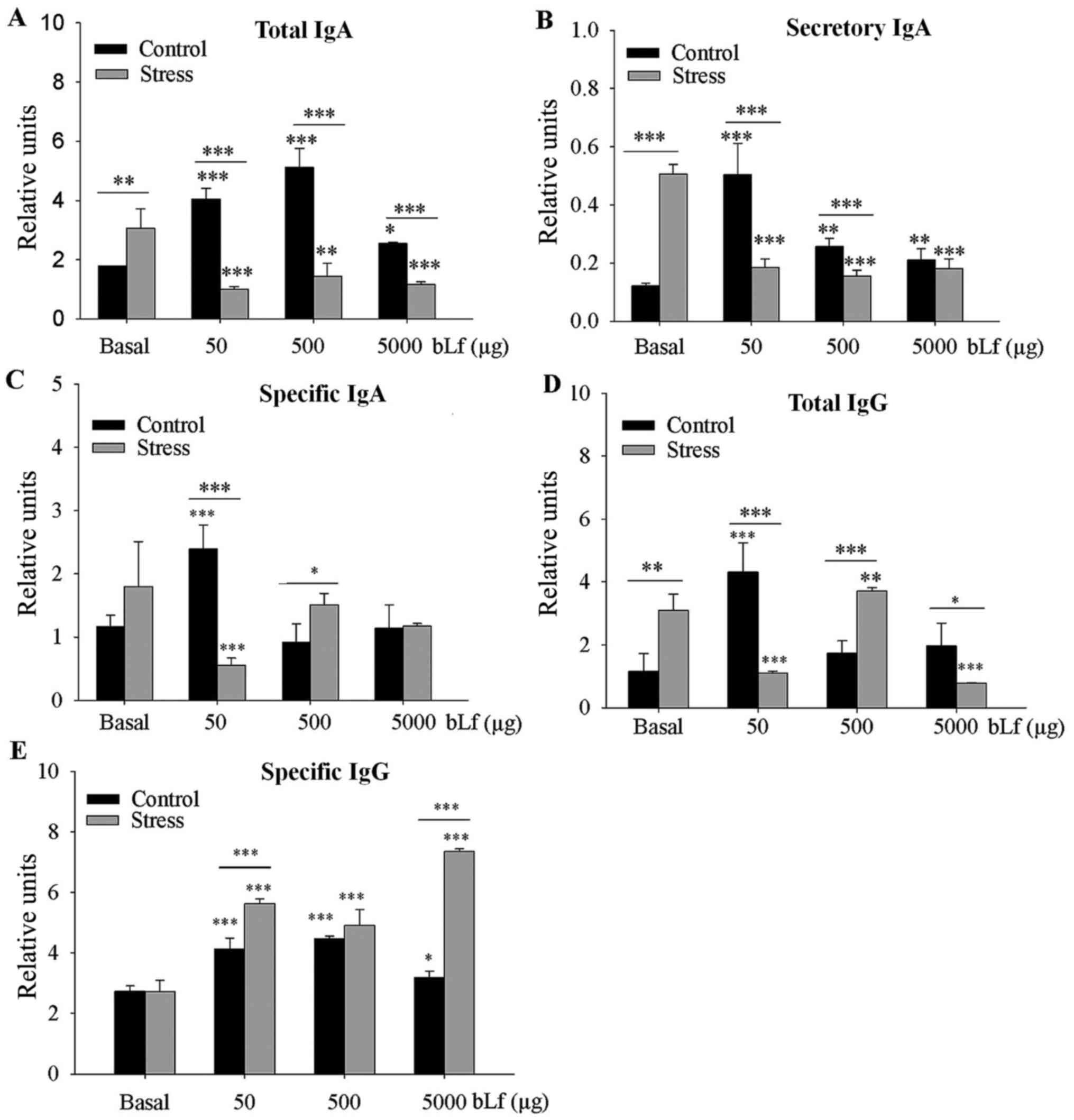
Analysis between the basal groups indicated that the
levels of total IgA (P<0.01) and SIgA (P<0.001) were
significantly greater in the stressed group compared with levels in
the control group. Compared with the corresponding basal group, the
expression levels of total IgA, SIgA (at all bLf dosages) and
specific IgA (50 µg bLf) were significantly lower in the bLf and
stress treated groups (P<0.001, except total IgA 500 µg bLf,
P<0.01). In comparison with their corresponding basal group,
expression levels of all bLf dosages of total IgA (Fig. 1A), SIgA (Fig. 1B) and specific IgA at 50 µg bLf
(Fig. 1C) were significantly higher
in control bLf-treated mice (P<0.001); total IgA treated with
5,000 µg bLf (P<0.05) and SIgA treated with 500 or 5,000 µg bLf
(P<0.01) were also significantly higher. Within bLf-treated
groups expression levels of total IgA at all bLf treatment doses
and SIgA treated with 50 or 500 µg bLf were significantly lower
(P<0.001) in stressed mice compared with control mice; the
specific-IgA expression levels were significantly lower
(P<0.001) when treated with 50 µg bLf, or significantly higher
(P<0.05) when treated with 500 µg bLf, in the stressed compared
with the unstressed mice.
Between the basal groups, the total-IgG expression
levels were significantly higher (P<0.05) in the stressed mice
compared with the control mice (Fig.
1D). In comparison with their corresponding basal group, total
IgG expression levels were significantly greater in the control
mice treated with 50 µg bLf (P<0.001) and stressed mice treated
with 500 µg bLf (P<0.05), whereas total IgG expression was
significantly lower in stressed mice treated with 50 or 5,000 µg
bLf (P<0.001). All bLf-treated mice had significantly higher
expression levels of specific-IgG compared with their corresponding
basal groups (all P<0.001, except P<0.05 for control mice
treated with 5,000 µg of bLf; Fig.
1E). Within bLf-treated groups anti-bLf IgG expression levels
were significantly higher in stressed mice treated with 50 or 5,000
µg bLf compared with the control mice with the same bLf treatment
(P<0.001).
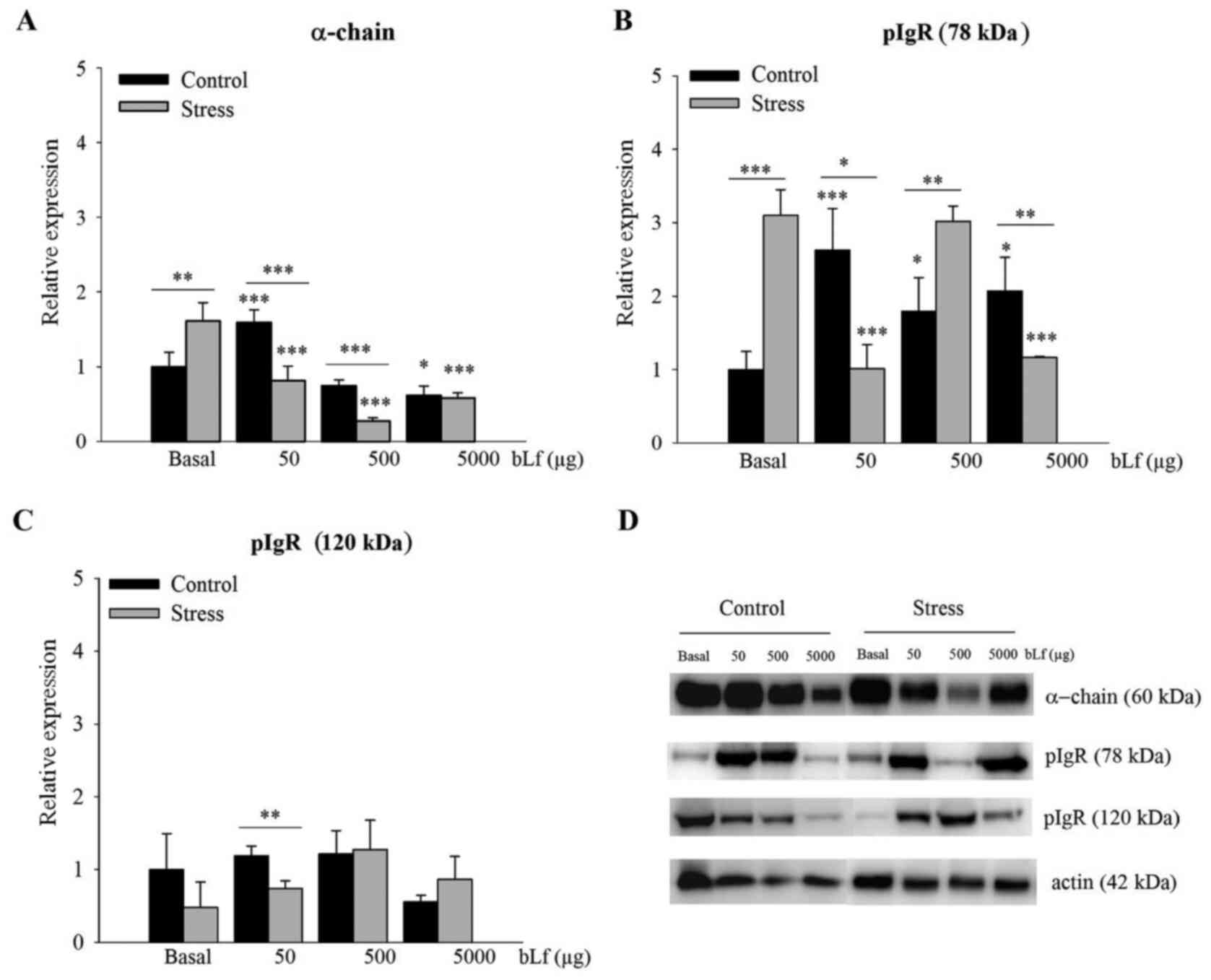
bLf counteracts the effects of stress
on increase protein expression levels of α-chain and 78-kDa
pIgR
Within the basal groups, α-chain (P<0.01;
Fig. 2A) and 78-kDa pIgR
(P<0.001; Fig. 2B) protein
expression levels were significantly higher in the stressed
compared with the control mice. In comparison with the basal group
of the stressed mice, expression levels of the α-chain treated at
any dose (Fig. 2A) and 78-kDa pIgR
treated with 50 or 5,000 µg of bLf (Fig. 2B) were significantly lower in
bLf-treated mice (P<0.001); no significant difference was
detected in the expression of 78-kDa pIgR of mice treated with 500
µg bLf (Fig. 2B). In the control
group, α-chain protein expression in mice treated with 50 µg bLf
(Fig. 2A) and 78-kDa pIgR
expression in mice treated with any bLf dose (Fig. 2B) were significantly greater in the
treatment groups compared with the basal group (P<0.001 for both
at 50 µg bLf; P<0.05 for 78-kDa pIgR at 500 or 5,000 µg bLf).
Notably, the expression of the α-chain was significantly lower when
treated with 5,000 µg bLf, compared with the basal control
(P<0.05; Fig. 2A) in bLf-treated
control mice.
Comparisons within the bLf-treated groups indicated
that in stressed mice, the relative protein expression levels of
α-chain following treatment with 50 µg bLf (P<0.01) or 500 µg
bLf (P<0.001) (Fig. 2A), 78-kDa
pIgR following treatment with 50 µg bLf (P<0.05) or 5,000 µg
bLf, (P<0.01) (Fig. 2B), and
120-kDa pIgR in mice treated with 50 µg bLf (P<0.01) (Fig. 2C) were significantly lower, whereas
78-kDa pIgR protein expression was significantly higher following
treatment with 500 µg bLf (P<0.01; Fig. 2B). Representative western blotting
images are presented in Fig.
2D.
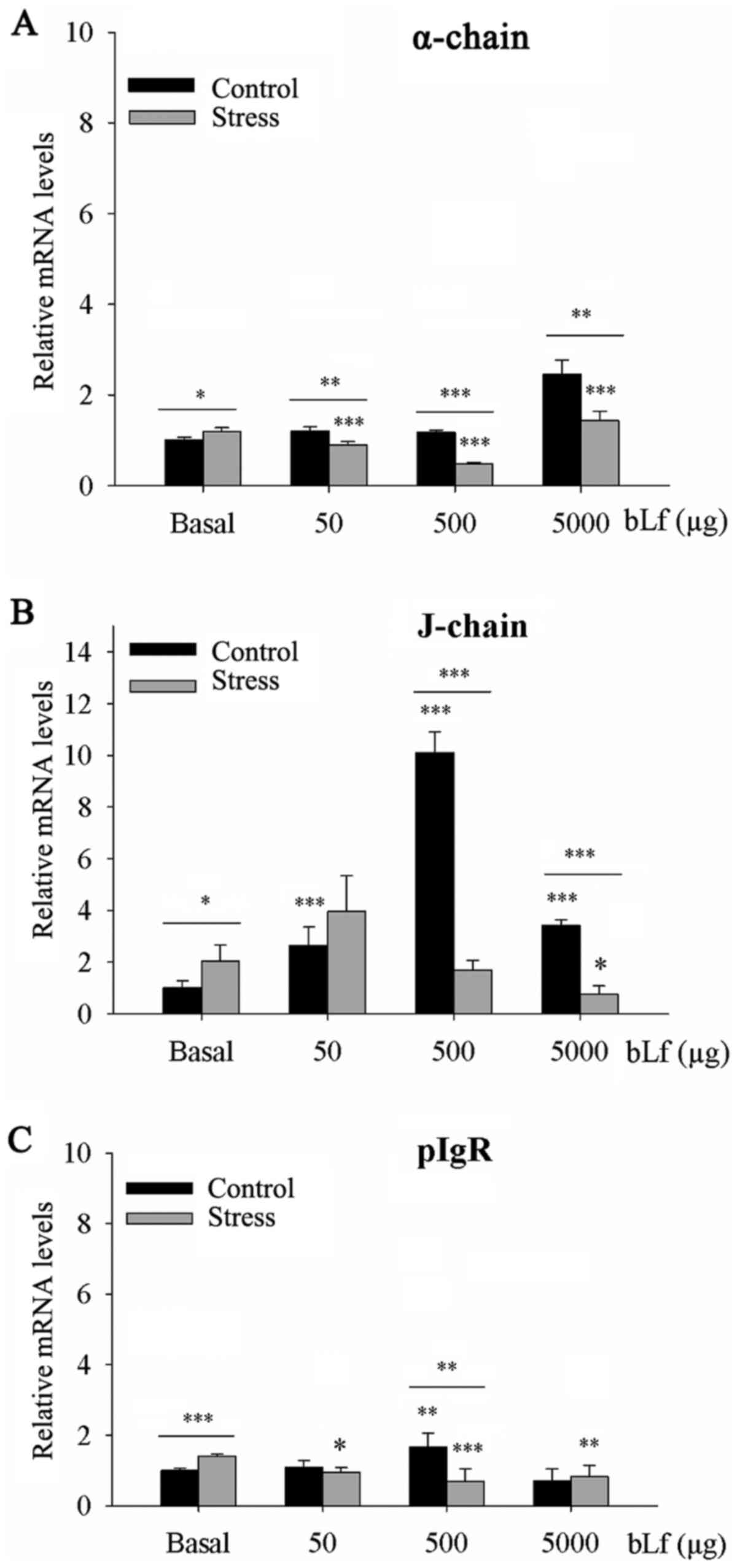
bLf counteracts the effects of stress
on increased α-chain and 78-kDa pIgR mRNA expression levels
Within the basal groups, α-chain (P<0.05),
J-chain (P<0.05) and pIgR (P<0.001) mRNA expression levels
were significantly higher in the stressed compared with the control
mice (Fig. 3). In comparison with
their corresponding basal group, α-chain mRNA expression levels
were significantly elevated following treatment with 5,000 µg bLf
in both the control (P<0.001) and stressed (P<0.01) groups
(Fig. 3). Notably, following
treatment with 50 or 500 µg bLf, stressed mice experienced a
significant reduction in α-chain mRNA expression (P<0.001;
Fig. 3A). The mRNA expression
levels of J-chain following treatment at all bLf doses (P<0.001;
Fig. 3B) and pIgR treated with 500
µg bLf (P<0.01; Fig. 3C) in the
bLf-treated control mice were significantly elevated. By contrast,
J-chain mRNA expression following treatment with 5,000 µg bLf
(P<0.05; Fig. 3B) and pIgR mRNA
expression levels treated with 50 µg bLf (P<0.05), 500 µg bLf
(P<0.001) or 5,000 µg bLf (P<0.01) were significantly lower
in the stressed bLf-treated mice compared with the basal control
(Fig. 3C). Analysis within the
bLf-treated groups showed that mRNA expression levels of α-chain
following treatment with 50 or 5,000 µg bLf (P<0.01) or 500 µg
bLf (P<0.001), the J-chain expression following treatment with
500 or 5,000 µg bLf (P<0.001), and pIgR in mice treated with 500
µg bLf (P<0.01) were lower in the stressed mice compared with
the unstressed mice (Fig.
3A-C).
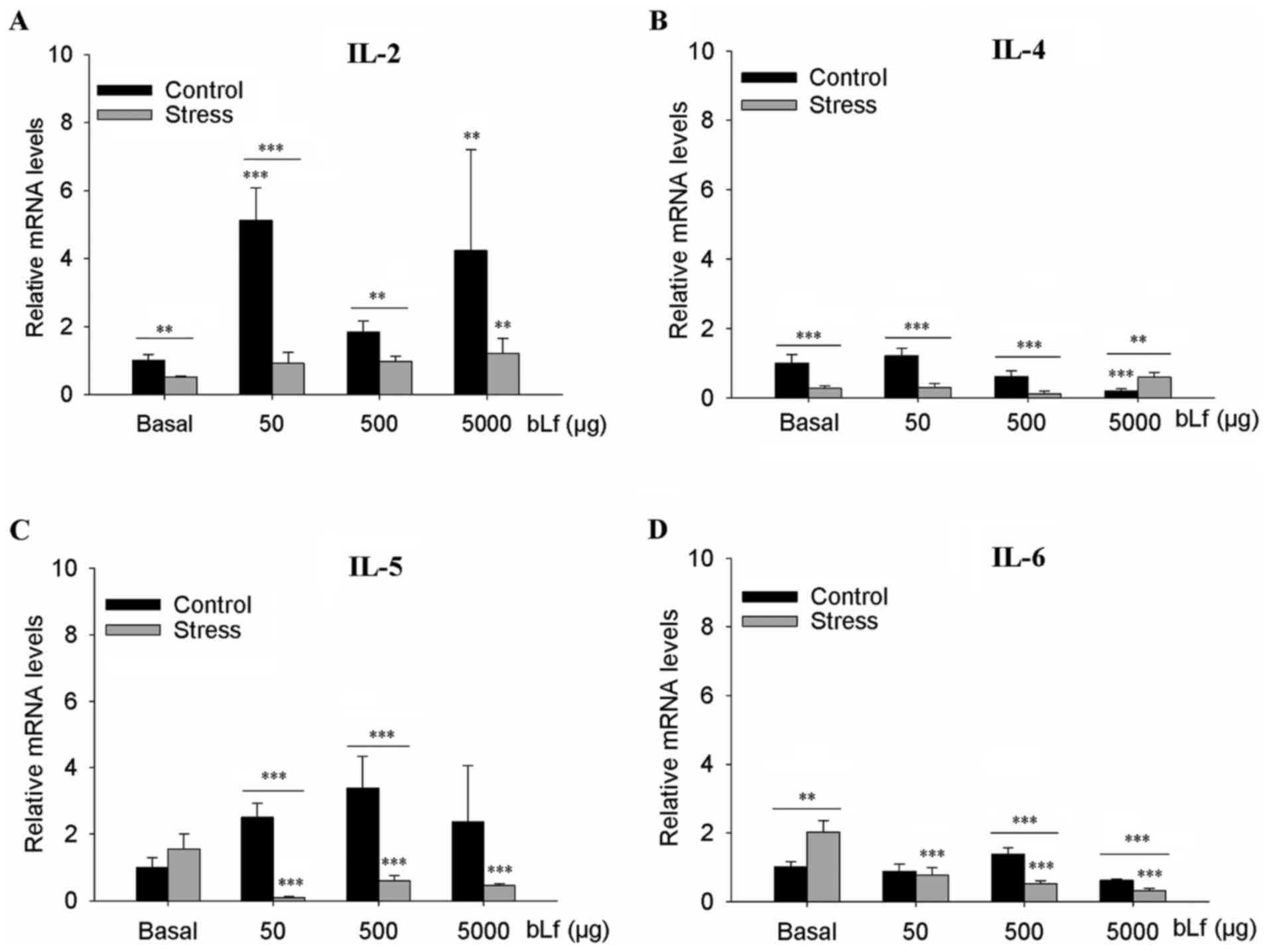
bLf does not counteract the effects of
stress on the mRNA expression of IgA-associated ILs
Within the basal groups, stressed mice showed
significantly elevated IL-6 mRNA expression levels (P<0.01;
Fig. 4D) and lower IL-2 (P<0.01;
Fig. 4A) and IL-4 (P<0.001;
Fig. 4B) mRNA expression levels. In
comparison with the corresponding basal group, stressed mice had
elevated IL-2 mRNA when treated with 5,000 µg bLf (P<0.01;
Fig. 4A) and significantly lower
IL-5 and IL-6 mRNA expression at all bLf dosages (P<0.001;
Fig. 4C and D). In comparison with
the basal group, the control mice had significantly elevated IL-2
mRNA expression levels at 50 µg bLf (P<0.001) and 5,000 µg bLf
(P<0.01) (Fig. 4A) and
significantly lower IL-4 mRNA expression levels when treated with
5,000 µg bLf (P<0.001; Fig. 4B).
There were no statistically significant differences in the mRNA
expression levels of IL-5 or IL-6 of the control mice at any
concentration of bLf treatment (Fig. 4C
and D). Within the bLf-treated mice, stressed mice had lower
expression levels of IL-2, IL-4 and IL-5 when treated with 50 or
500 µg bLf (all P<0.001, except P<0.01 IL-2 500 µg bLf;
Fig. 4A-C) and significantly lower
expression levels of IL-6 mRNA when treated with 500 or 5,000 µg
bLf (P<0.001; Fig. 4D).
Moreover, stressed mice had greater IL-4 mRNA expression levels
when treated with 5,000 µg bLf (P<0.001; Fig. 4B).
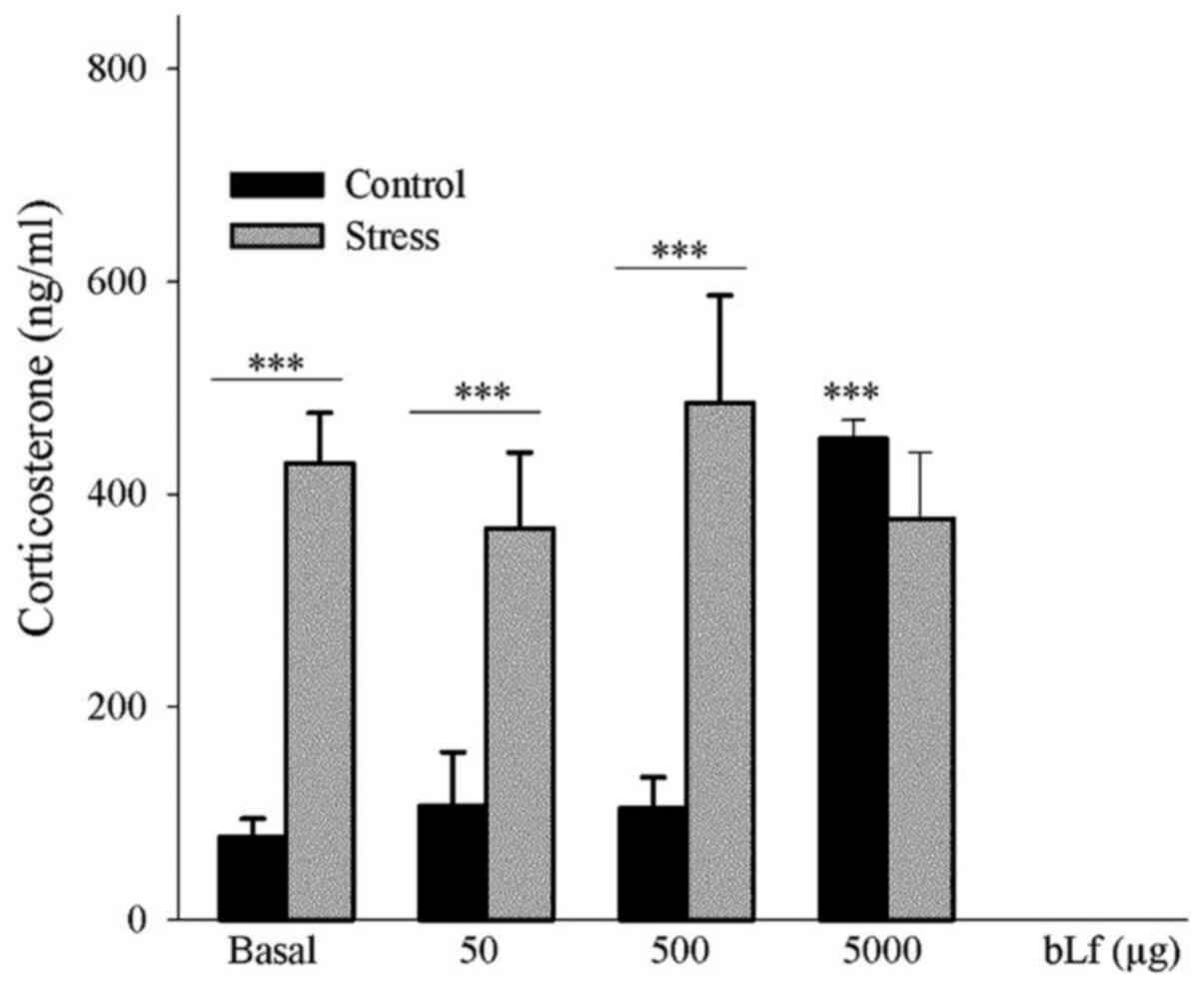
bLf does not counteract the effect of
stress on the elicitation of corticosterone levels
Analyses of the expression levels of corticosterone,
which is used as a stress biomarker (5), indicated that within the basal groups
the concentration of corticosterone were found to be significantly
greater in the stressed mice compared with the control mice
(P<0.001). Furthermore, in comparison with the corresponding
basal groups, the unstressed control mice treated with 5,000 µg bLf
had a significantly higher corticosterone expression level
(P<0.001). Within the bLf-treated groups, the stressed mice
treated with 50 or 500 µg bLf had a significantly elevated
corticosterone concentration compared with the control mice
receiving the same treatment (P<0.001) (Fig. 5).
Discussion
The effects of chronic stress on parameters
associated with the IgA antibody response have mostly been analyzed
in the colon (3,7,9,10,32)
and, to a lesser extent, in the full-length small intestine
(5), jejunum (6), duodenum or ileum (11). In addition, the modulatory
properties of bLf on the antibody response have been documented in
the distal and full-length small intestine of healthy mice
(13–15). Moreover, the effects of chronic
stress in mice treated with bLf have been demonstrated by an
increased response of splenic antibody-secreting cells (20). The present study aimed to address
the impact of chronic stress on some parameters associated with the
IgA response in the distal small intestine under conditions of bLf
treatment.
Chronic stress has a substantial upregulatory effect
on the generation of IgA, as previously found in the distal small
intestine and mesenteric lymph nodes (8,11). In
the present study, overall data analysis within the bLf treated
groups indicated that, in general terms, the upregulatory effects
of chronic stress on the concentration of total antibodies (IgA,
SIgA and IgG), α-chain protein and the mRNA expression of α-chain
and J-chain were reduced by bLf. These findings may reflect a
protective role of bLf against increased gut permeability, causing
an increase in luminal IgA, as previously found in the ileal
content of mice that underwent massive bowel resection and as
supported by in vitro assays (33,34).
No significant effects of chronic stress on specific IgA and IgG
antibody expression levels were observed; however, specific IgG
expression levels were triggered by bLf with and without chronic
stress. The biological meaning is currently unknown, but the
luminal increase of serum-derived IgG may provide a strategy of
protection like that described in aged mice (35).
In the present study, the effect of chronic stress
on the induction of 78-kDa pIgR protein and pIgR mRNA expression
was also decreased by bLf. Similar to the IgA response, the
downregulation of pIgR expression may result from the control
mechanism of bLf when gut permeability is compromised under
conditions of chronic stress. bLf induces an increased number of
positive IL-4-TCD3+CD4+ cells localized at
the distal region of the small intestine (13) and is also known to induce the IL-4
response in the full-length small intestine of healthy mice
(15). Chronic stress, by contrast,
is known to downregulate the expression level of IL-4 mRNA, as
previously described in the jejunum of heat-stressed rats (6). IL-4 activates signaling pathways that
upregulate pIgR (28). Moreover,
stress hormones such as corticosterone elicit an increase in pIgR
mRNA expression levels, as previously found in the proximal
intestine of suckling rats (36).
In the present study, bLf did not counteract the impact of chronic
stress on the decrease of IL-4 mRNA expression levels and on
corticosterone triggering; it appears that the downregulation of
pIgR mRNA expression levels by bLf under conditions of chronic
stress involves pathways independent of IL-4 and
corticosterone.
IL-4, IL-2 and IL-5 are known to be involved in
signaling pathways for the generation of IgA (31). IL-2 has a key role in the
transcription of the J-chain of dimeric IgA (28). Both IL-4 and IL-5 enhance the IgA
class-switching of B cell precursors, elicit autocrine TGF-β
release by B cells primed by CD40 ligand and promote the
proliferation and plasma cell differentiation of IgA+ B
cells (31). bLf induces the
generation of not only IL-4 but also IL-2, as previously found in
the full-length small intestine of healthy mice (15), whereas chronic stress has been shown
to decrease IL-2 transcription in the jejunum of heat-stressed rats
(6). In the present study, bLf did
not counteract the downregulatory impact of chronic stress on IL-2
mRNA as was found for IL-4 mRNA. Under stress conditions, the
downregulatory properties of bLf on the transcription of IL-2 and
IL-4 mRNA may underlie the decreased antibody response.
Chronic stress is known to increase circulatory
levels of IL-6 (37). IL-6 is a
pleiotropic factor for IgA generation but is also a potent
stimulator of corticosterone through a CRH-independent pathway
(38). The elicitation of
corticosterone by chronic stress has previously been associated
with a decrease in the IgA response (3,5,8,9).
In the present study, IL-6 mRNA expression levels elicited by
chronic stress were reduced by treatment with bLf, and the
elevation of corticosterone levels induced by chronic stress was
not significantly affected by any dose of bLf. These findings may
reflect a regulatory role of bLf on IL-6 and corticosterone
responses to decrease the luminal IgA response and thus maintain
gut homeostasis under stress conditions. In the present study,
unstressed control mice treated with 5,000 µg of bLf was found to
elevate serum corticosterone levels. These findings are in line
with an upregulatory effect of bLf on endogenous corticosterone
that relies on, in part, the propensity of bLf to elicit a
Th2-associated IL profile (22). As
suggested, bLf acts as a HPA axis stimulator to cause
corticosterone release in healthy mice, potentially by interacting
with receptors on cells in the central nervous system (22).
In the present assay, dose-dependent effects of bLf
were not detected, resulting in part from the extent of the luminal
elimination of bLf complexes that accumulate through the uptake of
bLf and/or the removal of bLf-complexes generated by the chronic
ingestion of bLf; the clearance of bLf complexes by immune
exclusion may prevent their entry into systemic compartments with
potentially harmful outcomes (39).
Apparent inconsistencies were found between the production and mRNA
expression of parameters of the IgA response evoked by chronic
stress in the presence of bLf. These findings may result from the
potential ability of bLf to elicit the local response of
corticosterone. Indeed, corticosterone is known to be released by
the epithelial monolayer of the full-length small intestine in mice
(40). Moreover, bLf interacts with
toll-like receptor (TLR)4, which is expressed in immune competent
cells (41). In the intestinal
epithelium, TLR4 expression is higher in the distal than in the
proximal small intestine (42).
Thus, the inconsistent data found in the present study may reflect
the impact of chronic stress on the modulation of IgA and pIgR by
bLf and their interplay with microbiota and TLR4 (43,44).
Although an obvious limitation involves the
assessment of ILs only following transcription, the current study
may be an experimental representation of human disorders such IBS
that are associated with disturbances in the luminal microbiota,
which exhibits a gradient that increases from the proximal to the
distal small intestine (24,25).
In conclusion, results from the present study indicated that bLf
may counteract the upregulatory impact of chronic stress on the
elevation of most parameters associated with the IgA response but
at a dose of 5,000 µg, elevates the expression of corticosterone
and the specific IgG antibody response at 50, 500 and 5,000 µg.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Consejo Nacional
de Ciencia y Tecnología (CONACyT; grant no. 167345); CONACyT also
provided a graduate scholarship (grant no. 281258).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TRCH performed the ELISAs. DCGJ performed the
reverse transcription-quantitative PCR assays. RCR made substantial
contributions to the conception and design of the study. MGV
conducted the western blot assays, and the acquisition, analysis
and interpretation of data. MEDS participated in the design of the
experiments, and performed the analysis and interpretation of the
data, was involved in drafting the manuscript and revised it
critically for important intellectual content. Each author
participated sufficiently in the work to take public responsibility
for appropriate portions of the content and agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Animals were handled in accordance with Mexican
federal regulations for animal experimentation and care
(NOM-062-ZOO-1999, Ministry of Agriculture, Mexico City, Mexico).
The protocol was approved by the Institutional Animal Care and Use
Committee of the Instituto Politécnico Nacional (Mexico City,
Mexico).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
bLf
|
bovine lactoferrin
|
|
SIgA
|
secretory IgA
|
|
pIgR
|
polymeric immunoglobulin receptor
|
References
|
1
|
Campos-Rodríguez R, Godínez-Victoria M,
Abarca-Rojano E, Pacheco-Yépez J, Reyna-Garfias H, Barbosa-Cabrera
RE and Drago-Serrano ME: Stress modulates intestinal secretory
immunoglobulin A. Front Integr Nuerosci. 7:862013. View Article : Google Scholar
|
|
2
|
Staley M, Conners MG, Hall K and Miller
LJ: Linking stress and immunity: Immunoglobulin A as a non-invasive
physiological biomarker in animal welfare studies. Horm Behav.
102:55–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caso JR, Hurtado O, Pereira MP,
García-Bueno B, Menchén L, Alou L, Gómez-Lus ML, Moro MA, Lizasoain
I and Leza JC: Colonic bacterial translocation as a possible factor
in stress-worsening experimental stroke outcome. Am J Physiol Regul
Integr Comp Physiol. 296:R979–R985. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eriksson E, Royo F, Lyberg K, Carlsson HE
and Hau J: Effect of metabolic cage housing on immunoglobulin A and
corticosterone excretion in faeces and urine of young male rats.
Exp Physiol. 89:427–433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jarillo-Luna A, Rivera-Aguilar V, Garfias
HR, Lara-Padilla E, Kormanovsky A and Campos-Rodríguez R: Effect of
repeated restraint stress on the levels of intestinal IgA in mice.
Psychoneuroendocrinology. 32:681–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu X, Li H, Lu A, Zhong Y, Hou X, Wang N,
Jia D, Zan J, Zhao H, Xu J and Liu F: Reduction of intestinal
mucosal immune function in heat-stressed rats and bacterial
translocation. Int J Hyperthermia. 28:756–765. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ponferrada A, Caso JR, Alou L, Colón A,
Sevillano D, Moro MA, Lizasoain I, Menchén P, Gómez-Lus ML, Lorenzo
P, et al: The role of PPARgamma on restoration of colonic
homeostasis after experimental stress-induced inflammation and
dysfunction. Gastroenterology. 132:1791–1803. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto S, Motomura A, Akahoshi A,
Takahashi K and Minami H: Immunoglobulin secretions in the
mesenteric lymph node in stressed rats. J Nutr Sci Vitaminol
(Tokyo). 55:191–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zoppi S, Madrigal JL, Pérez-Nievas BG,
Marín-Jiménez I, Caso JR, Alou L, García-Bueno B, Colón A,
Manzanares J, Gómez-Lus ML, et al: Endogenous cannabinoid system
regulates intestinal barrier function in vivo through cannabinoid
type 1 receptor activation. Am J Physiol Gastrointest Liver
Physiol. 302:G565–G571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aguilera M, Vergara P and Martínez V:
Stress and antibiotics alter luminal and wall-adhered microbiota
and enhance the local expression of visceral sensory-related
systems in mice. Neurogastroenterol Motil. 25:e515–e529. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reyna-Garfias H, Miliar A, Jarillo-Luna A,
Rivera-Aguilar V, Pacheco-Yepez J, Baeza I and Campos-Rodríguez R:
Repeated restraint stress increases IgA concentration in rat small
intestine. Brain Behav Immun. 24:110–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Joëls M, Sarabdjitsingh RA and Karst H:
Unraveling the time domains of corticosteroid hormone influences on
brain activity: Rapid, slow, and chronic modes. Pharmacol Rev.
64:901–938. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arciniega-Martínez IM, Campos-Rodríguez R,
Drago-Serrano ME, Sánchez-Torres LE, Cruz-Hernández TR and
Reséndiz-Albor AA: Modulatory effects of oral bovine lactoferrin on
the IgA response at inductor and effector sites of distal small
intestine from BALB/c mice. Arch Immunol Ther Exp (Warsz).
64:57–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Debbabi H, Dubarry M, Rautureau M and Tomé
D: Bovine lactoferrin induces both mucosal and systemic immune
response in mice. J Dairy Res. 65:283–293. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sfeir RM, Dubarry M, Boyaka PN, Rautureau
M and Tomé D: The mode of oral bovine lactoferrin administration
influences mucosal and systemic immune responses in mice. J Nutr.
134:403–409. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bouvet JP and Fischetti VA: Diversity of
antibody-mediated immunity at the mucosal barrier. Infect Immun.
67:2687–2691. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Puddu P, Valenti P and Gessani S:
Immunomodulatory effects of lactoferrin on antigen presenting
cells. Biochimie. 91:11–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nielsen SM, Hansen GH and Danielsen EM:
Lactoferrin targets T cells in the small intestine. J
Gastroenterol. 45:1121–1128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jang YS, Seo GY, Lee JM, Seo HY, Han HJ,
Kim SJ, Jin BR, Kim HJ, Park SR, Rhee KJ, et al: Lactoferrin causes
IgA and IgG2b isotype switching through betaglycan binding and
activation of canonical TGF-β signaling. Mucosal Immunol.
8:906–917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zimecki M, Artym J, Chodaczek G, Kocieba M
and Kruzel M: Effects of lactoferrin on the immune response
modified by the immobilization stress. Pharmacol Rep. 57:811–817.
2005.PubMed/NCBI
|
|
21
|
Maekawa Y, Sugiyama A and Takeuchi T:
Lactoferrin ameliorates corticosterone-related acute stress and
hyperglycemia in rats. J Vet Med Sci. 79:412–417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zimecki M, Artym J and Kocieba M:
Endogenous steroids are responsible for lactoferrin-induced
myelopoiesis in mice. Pharmacol Rep. 61:705–710. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vicario M, Guilarte M, Alonso C, Yang P,
Martínez C, Ramos L, Lobo B, González A, Guilà M, Pigrau M, et al:
Chronological assessment of mast cell-mediated gut dysfunction and
mucosal inflammation in a rat model of chronic psychosocial stress.
Brain Behav Immun. 24:1166–1175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mowat AM and Agace WW: Regional
specialization within the intestinal immune system. Nat Rev
Immunol. 14:667–685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Malley D: Immunomodulation of enteric
neural function in irritable bowel syndrome. World J Gastroenterol.
21:7362–7366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. PLoS Biol.
8:e10004122010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johansen FE and Kaetzel CS: Regulation of
the polymeric immunoglobulin receptor and IgA transport: New
advances in environmental factors that stimulate pIgR expression
and its role in mucosal immunity. Mucosal Immunol. 4:598–602. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimada S, Kawaguchi-Miyashita M, Kushiro
A, Sato T, Nanno M, Sako T, Matsuoka Y, Sudo K, Tagawa Y, Iwakura Y
and Ohwaki M: Generation of polymeric immunoglobulin
receptor-deficient mouse with marked reduction of secretory IgA. J
Immunol. 163:5367–5373. 1999.PubMed/NCBI
|
|
29
|
Cerutti A: The regulation of IgA class
switching. Nat Rev Immunol. 8:421–434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Viloria M, Lara-Padilla E,
Campos-Rodríguez R, Jarillo-Luna A, Reyna-Garfias H, López-Sánchez
P, Rivera-Aguilar V, Salas-Casas A, Berral de la Rosa FJ and
García-Latorre E: Effect of moderate exercise on IgA levels and
lymphocyte count in mouse intestine. Immunol Invest. 40:640–656.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aoki-Yoshida A, Aoki R, Moriya N, Goto T,
Kubota Y, Toyoda A, Takayama Y and Suzuki C: Omics studies of the
murine intestinal ecosystem exposed to subchronic and mild social
defeat stress. J Proteome Res. 15:3126–3138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu J, Chen J, Wu W, Shi J, Zhong Y, van
Tol EA, Tang Q and Cai W: Enteral supplementation of bovine
lactoferrin improves gut barrier function in rats after massive
bowel resection. Br J Nutr. 112:486–492. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao X, Xu XX, Liu Y, Xi EZ, An JJ, Tabys
D and Liu N: The in vitro protective role of bovine lactoferrin on
intestinal epithelial barrier. Molecules. 24:1482019. View Article : Google Scholar
|
|
35
|
Senda S, Cheng E and Kawanishi H: IgG in
murine intestinal secretions. Aging effect and possible
physiological role. Scand J Immunol. 29:41–47. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li TW, Wang J, Lam JT, Gutierrez EM,
Solorzano-Vargus RS, Tsai HV and Martín MG: Transcriptional control
of the murine polymeric IgA receptor promoter by glucocorticoids.
Am J Physiol. 276:G1425–G1434. 1999.PubMed/NCBI
|
|
37
|
Voorhees JL, Tarr AJ, Wohleb ES, Godbout
JP, Mo X, Sheridan JF, Eubank TD and Marsh CB: Prolonged restraint
stress increases IL-6, reduces IL-10, and causes persistent
depressive-like behavior that is reversed by recombinant IL-10.
PLoS One. 8:e584882013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bethin KE, Vogt SK and Muglia LJ:
Interleukin-6 is an essential, corticotropin-releasing
hormone-independent stimulator of the adrenal axis during immune
system activation. Proc Natl Acad Sci USA. 97:9317–9322. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fischer R, Debbabi H, Blais A, Dubarry M,
Rautureau M, Boyaka PN and Tome D: Uptake of ingested bovine
lactoferrin and its accumulation in adult mouse tissues. Int
Immunopharmacol. 7:1387–1393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cima I, Corazza N, Dick B, Fuhrer A,
Herren S, Jakob S, Ayuni E, Mueller C and Brunner T: Intestinal
epithelial cells synthesize glucocorticoids and regulate T cell
activation. J Exp Med. 200:1635–1646. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Puddu P, Latorre D, Carollo M, Catizone A,
Ricci G, Valenti P and Gessani S: Bovine lactoferrin counteracts
Toll-like receptor mediated activation signals in antigen
presenting cells. PLoS One. 6:e225042011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ortega-Cava CF, Ishihara S, Rumi MA,
Kawashima K, Ishimura N, Kazumori H, Udagawa J, Kadowaki Y and
Kinoshita Y: Strategic compartmentalization of Toll-like receptor 4
in the mouse gut. J Immunol. 170:3977–3985. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Diebel LN and Liberati DM: Disparate
effects of bacteria and Toll-like receptor-dependant bacterial
ligand stimulation on immunoglobulin A transcytosis. J Trauma.
70:691–700. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Salerno-Goncalves R, Safavie F, Fasano A
and Sztein MB: Free and complexed-secretory immunoglobulin A
triggers distinct intestinal epithelial cell responses. Clin Exp
Immunol. 185:338–347. 2016. View Article : Google Scholar : PubMed/NCBI
|