Introduction
Type 2 diabetes mellitus (T2DM) is characterized by
insulin resistance and dysfunction of pancreatic β-cells. Enhanced
pancreatic β-cells apoptosis causes β-cells loss in T2DM (1,2).
Therefore, the regulation of pancreatic β-cells mass and its
function serve a critical role in the pathogenesis of T2DM.
Currently, there are a limited number of drugs, such as liraglutide
and metformin, approved for the prevention of islet β-cells from
injury (1,3–5).
Autophagy is characterized by the lysosomal
degradation of cellular material, and is a process of subcellular
membrane rearrangement sequestering cytoplasm, proteins and
organelles, forming the autophagosome (6). Previous studies have revealed that
autophagy can prevent β-cells injury and death by protecting
against endoplasmic reticulum stress (ERs), inflammation and
oxidative stress (7–11). In our previous studies, autophagy
was found to exert a protective effect by mitigating inflammation
in β-cells (1,8,10). It
has also been reported that Atg7-KO ob/ob mice develop severe
diabetes and have increased levels of apoptotic β-cells and
decreased β-cell viability by imposing ERs (12). These studies revealed that autophagy
serves a protective role in pancreatic β-cells.
Forkhead box O1 (FoxO1) belongs to the forkhead box
protein family and is involved in a series of intracellular
functions, including apoptosis, oxidative stress and mitochondrial
dysfunction (13–16). FoxO1 is highly expressed in islet
β-cells and serves a protective role in T2DM (14,17).
For instance, FoxO1 protects against β-cells failure, contributes
to localize in the nucleus of β-cells and induces neuronal
differentiation 1 and MAF bZIP transcription factor A, which
directly bind to the insulin 2 gene promoter and promotes its
transcription (17). Furthermore,
FoxO1 protects lipopolysaccharide-induced INS-1 cells from
oxidative stress damage and mitochondrial dysfunction (16). In db/db mouse model, FoxO1 knockout
decreases glucose-responsive insulin secretion (18). Moreover, FoxO1 regulates autophagic
flux in various cell types, such as vascular endothelial cells
(19), human cholangiocarcinoma
QBC939 cells (15) and rat
mesangial cells (20). However, the
effect of FoxO1 on autophagy in pancreatic β-cell has not been
fully elucidated.
Liraglutide is a glucagon-like peptide-1 (GLP-1)
analog, which is known to regulate the synthesis of insulin,
inhibits the apoptosis of pancreatic β-cells and is widely used for
the treatment of T2DM. Liraglutide prevents high glucose or free
fatty acids (FFA)-induced INS-1 cell apoptosis by targeting
autophagy (1,3). Fan et al (4) reported that liraglutide can enhance
autophagy and promote islet β-cells proliferation in a high-fat-fed
and streptozotocin-induced mouse model of T2DM. Moreover,
liraglutide markedly improves islet β-cells function under lipid
stress and blocks the inhibitory effect of palmitate (PA) on FoxO1
(21). It has also been shown that
GLP-1 increases the levels of phosphorylated FoxO1 in β-cells
(22). However, the molecular
mechanism via which liraglutide exerts its action on autophagy and
FoxO1 remains unknown.
Based on the autophagy functions and the roles of
FoxO1 in the regulation of islet β-cells, it was hypothesized that
the protective effect and corresponding mechanisms of liraglutide
on pancreatic β-cells may be associated with induced autophagy and
exerted by activating FoxO1. In the present study, INS-1 cells
(induced by PA) were used to validate this hypothesis.
Materials and methods
Reagents and chemicals
INS-1 rat insulinoma cells were purchased from the
American Type Culture Collection. RPMI-1640 medium was purchased
from Thermo Fisher Scientific, Inc. FBS was purchased from Hangzhou
Sijiqing Biological Engineering Materials Co. Ltd. Liraglutide was
purchased from Novo Nordisk Hellas Ltd. PA and chloroquine (CQ)
were obtained from Sigma-Aldrich (Merck KGaA). FoxO1 small
interfering RNA (siRNA) was obtained from Shanghai GenePharma Co.,
Ltd. The following antibodies were used at a 1:1,000 dilution:
Microtubule-associated protein 1 light chain3 (LC3; cat. no. 2775;
Cell Signaling Technology, Inc.), phosphorylated (p)-FoxO1 (cat.
no. 9461; Cell Signaling Technology, Inc.), FoxO1 (cat. no. 2880;
Cell Signaling Technology, Inc.), cleaved caspase-3 (cat. no.
AF1150; Beyotime Institute of Biotechnology), β-actin (cat. no.
8457; Cell Signaling Technology, Inc.) and GAPDH (cat. no.
sc-32233; Santa Cruz Biotechnology, Inc.). Horseradish
peroxidase-conjugated secondary antibodies were used at a 1:5,000
dilution, which included goat anti-rabbit and goat anti-mouse
secondary antibodies from Jackson ImmunoResearch Laboratories, Inc.
(cat. nos. 111-545-003 and 115-005-003). Cell Counting Kit-8
(CCK-8) was obtained from Dojindo Molecular Technologies, Inc.
SDS-PAGE and an ECL detection kit were obtained from Cytiva.
Cell culturing
INS-1 cells were cultured in RPMI-1640 medium
supplemented with 10% (v/v) FBS, in a humidified atmosphere
containing 95% air and 5% CO2 (1). All experiments were performed at 80%
cell confluence. A PA-induced lipid toxicity model of INS-1 cells
was established to investigate whether GLP-1 protects against
PA-induced β-cells injury via FoxO1. CQ, a lysosome inhibitor, was
used to block the autophagic flux (23). The concentration of CQ was selected
according to a previous study (23). The concentration of liraglutide was
chosen based on a previous study (1,23).
First, purified INS-1 cells were incubated at 37°C
and in various concentrations of PA (0, 0.1, 0.3 and 0.5 mmol/l)
for 24 h. Then, INS-1 cells were divided into eight groups: i)
Control (CON) group; ii) PA group (0.5 mmol/l); iii) CQ group (10
µmol/l) (22); iv) PA + CQ group
(PA 0.5 mmol/l + CQ 10 µmol/l); v) LIRA group (liraglutide 100
nmol/l) (1,20); vi) PA + LIRA group (PA 0.5 mmol/l +
liraglutide 100 nmol/l); vii) LIRA + CQ group (liraglutide 100
nmol/l + CQ 10 µmol/l); and viii) PA + LIRA + CQ group (PA 0.5
mmol/l + liraglutide 100 nmol/l + CQ 10 µmol/l).
siRNA interference technology was used to inhibit
FoxO1 gene expression in INS-1 cells. After the transfection, cells
were randomly divided into four groups: PA + siCON group (PA 0.5
mmol/l + negative controls siRNA), PA + siFoxO1 group (PA 0.5
mmol/l + siFoxO1), LIRA + siCON group (liraglutide 100 nmol/l +
negative controls siRNA) and LIRA + siFoxO1 group (liraglutide 100
nmol/l + siFoxO1).
Cell viability assay
Cell viability was assessed with a CCK-8 according
to the manufacturer's protocol. INS-1 cells were treated with 10 µl
CCK-8 for 1 h at 37°C. Absorbance was measured at 450 nm using a
microplate reader (Tecan Group, Ltd.).
siRNA transfection
INS-1 cells were seeded in 6-well plates at a
density of 2×105 per well with RPMI-1640 supplemented
with 10% FBS at 37°C for 24 h. INS-1 cells were transfected with 80
nM siRNA against FoxO1 or their negative controls duplexes using
PepMute siRNA. The sequences of the designed siFoxO1 were as
follows: FoxO1 siRNA, 5′-GGACAGCAAAUCAAGUUAUtt-3′. The PepMute™
siRNA transfection reagent (Signa Gen, SL100566) was used according
to the manufacturer's instructions. INS-1 cells were transfected at
37°C for 24 h, then treated with 0.5 mmol/l PA or 100 nmol/l
liraglutide at 37°C. After 24 h of treatment, protein extracts from
cells were collected and used for western blot analysis.
Western blotting
INS-1 cells were harvested for 24 h as
aforementioned. RIPA lysis buffer (cat. no. P0013K; Beyotime
Institute of Biotechnology) was used to extract protein. Lysates
were centrifuged at 4°C and 12,000 × g for 5 min and supernatant
was collected. After using BCA protein assay kit (cat. no. P0012S;
Beyotime Institute of Biotechnology) to determine the protein
concentration, 45 µg protein was boiled for 5 min and separated on
8 or 12% SDS-PAGE. The proteins were transferred to PVDF membrane,
then 5% non-fat milk was used for blocking for 1 h at room
temperature. After washing, the membranes were incubated with the
primary antibody at 4°C overnight. After washing, the membranes
were incubated with the second antibody for 1 h at room
temperature. Western blot analyses were performed using previously
described methods (1,16). The relative expression level of a
specific protein was normalized to GAPDH. Immunoblots were
semi-quantified via densitometric analysis using Image Lab software
v2.0.1 (Bio-Rad Laboratories, Inc.).
Immunofluorescence assay
Following the aforementioned treatments, INS-1 cells
were fixed at room temperature for 20 min in fresh 4%
paraformaldehyde solution (Sigma-Aldrich; Merck KGaA) and washed
three times with PBS. Then, the cells were permeabilized using PBS
containing 0.5% Triton-X 100 (Sigma-Aldrich; Merck KGaA) for 1 h
and then blocked with PBS supplemented with 4% BSA and 0.3%
Triton-X 100 for another 1 h at room temperature. Cells were
incubated with anti-LC3 (1:100; Cell Signaling Technology)
overnight at 4°C. Cells were then washed three times with PBS and
incubated with goat anti-rabbit and anti-mouse secondary antibodies
for 1 h at room temperature. Digital images were obtained using an
Olympus CKX41SF inverted microscope (magnification, ×100; Olympus
Corporation)
Transmission electron microscopy
(TEM)
INS-1 cells treated with 0.5 mmol/l PA and 10 µmol/l
CQ for 24 h at 37°C were harvested using a method described in our
previous report (10). INS-1 cells
were fixed with Karnovsky's fixative solution [1% (v/v)
paraformaldehyde, 2% (v/v) glutaraldehyde, 2 mmol/l calcium
chloride and 100 mmol/l cacodylatebuffer (pH 7.4)] for 2 h at room
temperature and washed with cacodylate buffer. After post-fixing
with a fixative solution containing 1% (v/v) osmium tetroxide and
1.5% (v/v) potassium ferrocyanide for 1 h at room temperature, the
cells were dehydrated with 50–100% (v/v) alcohol and stained with
en bloc in 0.5% (v/v) uranyl acetate at 4°C overnight. The cells
were then embedded in Poly/Bed 812 resin (Pelco Scientific) and
polymerized, after which they were sliced into 70 nm sections using
a Reichert-Jung Ultracut E Ultramicrotome (Leica Microsystems GmbH)
and stained with uranyl acetate and lead citrate at room
temperature for 30 min. The cells were observed and imaged under a
TEM (EM902A; Carl Zeiss MicroImaging GmbH).
Statistical analysis
Data are presented as the mean ± SD of ≥3
independent experiments. Differences between the various groups
were determined with by paired and unpaired Student's t-test using
SPSS 19.0 software (IBM Corp.) or one-way ANOVA followed by Tukey's
multiple comparison tests using GraphPad Prism Version 7 (GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of PA in INS-1 cells injury,
autophagy and FoxO1
INS-1 cells were incubated with various
concentrations of PA (0, 0.1, 0.3 and 0.5 mmol/l) for 24 h to study
the role of PA in the process of pancreatic β-cell injury,
autophagy and FoxO1.
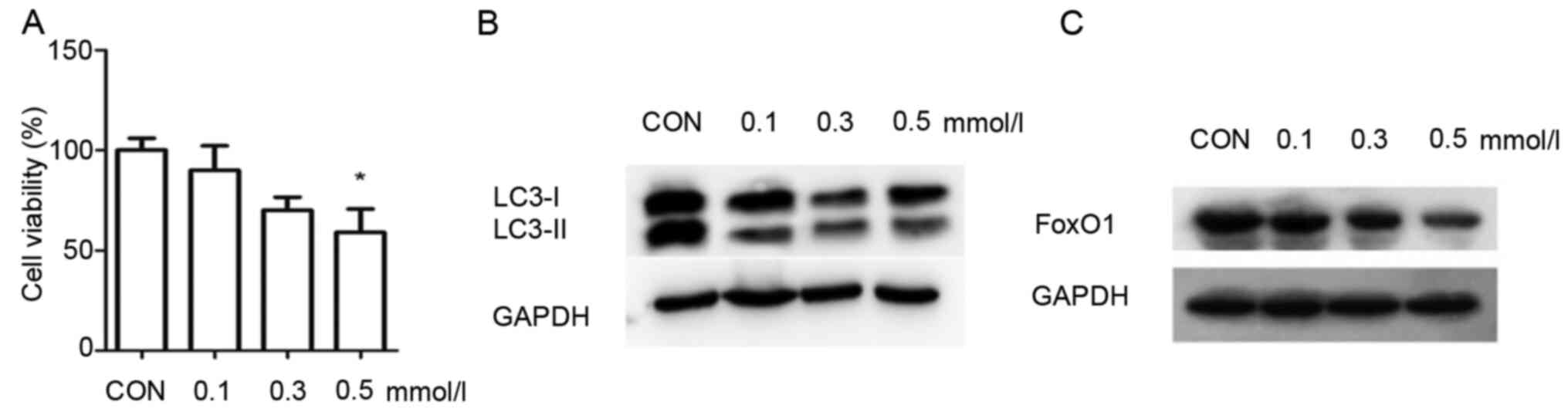
The viability of INS-1 cells was significantly
decreased to 61% in the 0.5 mmol/l PA group compared with the
control group (Fig. 1A). These
findings demonstrated that PA induces injury in INS-1 cells. PA at
a concentration of 0.5 mmol/l significantly decreased the INS-1
cell viability, and thus this was selected as the suitable
concentration for the PA group.
As LC3 is a landmark protein associated with
autophagy, western blotting was used to measure the expression of
LC3 in the INS-1 cells. The results demonstrated that the
expression of LC3II/I was enhanced with the increasing
concentrations of PA, compared with the control group (Fig. 1B). These results suggested that
autophagy was activated in INS-1 cells in response to PA. The
expression of FoxO1 was also analyzed via western blotting. The
expression of FoxO1 was markedly decreased when INS-1 cells were
cultured in 0.5 mmol/l PA compared with the control group (Fig. 1C). These results indicated that PA
decreased the expression of FoxO1 in INS-1 cells.
Effect of autophagy on PA-induced
INS-1 cell injury and FoxO1
INS-1 cells were treated with 0.5 mmol/l PA and 10
µmol/l CQ, an autophagy inhibitor, for 24 h to study the role of
autophagy in PA-induced INS-1 cell injury and FoxO1.
Compared with the control group, the viability of
INS-1 cell treated with CQ alone was significantly decreased
(Fig. 2A). Moreover, the survival
of INS-1 cells was significantly decreased in the PA + CQ group
compared with the PA group (Fig.
2A). These results suggested that CQ accelerated PA-induce
cytotoxicity, and that autophagy was essential for the survival of
INS-1 cells, whether in a lipotoxic or a normal environment.
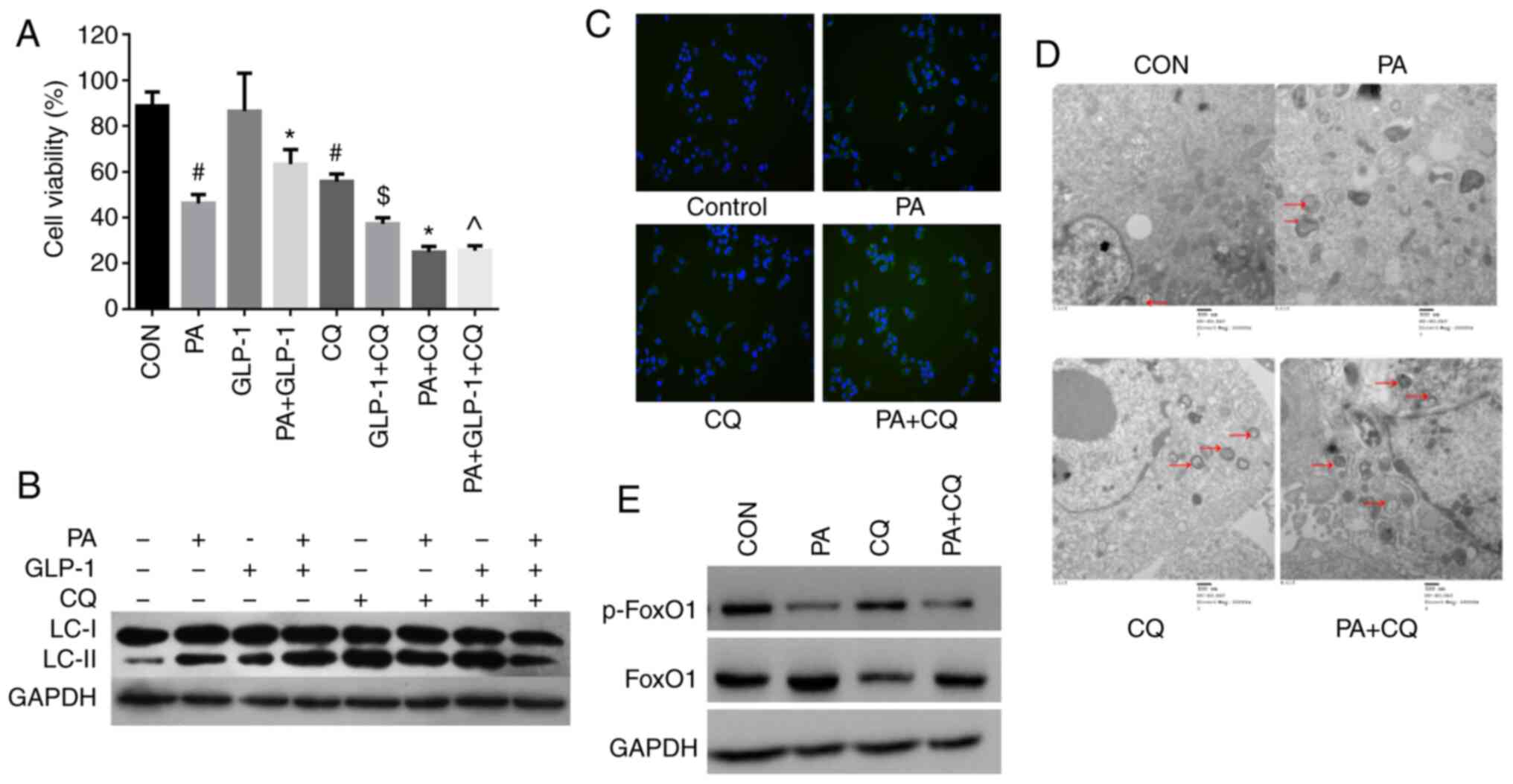 | Figure 2.Effect of autophagy in PA-induced
INS-1 cells injury and FoxO1. (A) Cell Counting Kit-8 analysis of
the viability of INS-1 cells in each group. Data are presented as
the mean ± SD (n=3). #P<0.05 vs. CON group;
*P<0.05 vs. PA group; $P<0.05 vs. LIRA group;
^P<0.05 vs. PA + LIRA group. Data were analyzed by
unpaired t-test. (B) Western blotting was used to detect the
expression of LC3 in each group. (C) Immunofluorescence analysis of
LC3 puncta in each group (magnification, ×100). (D)
Micromorphological changes in cellular organelles were examined via
transmission electron microscopy. The red arrow points to
autophagic vesicles (magnification, ×20,000; n=3). The red circles
indicate the presence of autophagic vacuole structures. (E) Western
blotting, using GAPDH as a control, was conducted to determine
p-FoxO1 and FoxO1 protein expression levels in each group. FoxO1,
Forkhead box O1; LC3, microtubule-associated protein 1 light
chain3; CON, control; PA, palmitate; CQ, chloroquine; GLP-1,
glucagon-like peptide-1; p-, phosphorylated. |
The expression of LC3II/I was increased in the PA +
CQ group compared with the PA group (Fig. 2B). Immunofluorescence was also used
to detect LC3. The results identified that LC3 puncta were
significantly increased in the PA group compared with in the
control group (Fig. 2C). Moreover,
the LC3 puncta numbers per viable cell was increased in the PA + CQ
group compared with the PA group (Fig.
2C). TEM was used to examine the typical autophagic structures
in INS-1 cells and provided evidence of autophagy activation. The
double membrane autophagic vesicles containing cell organelles in
the cytoplasm of INS-1 cells are integrated autophagosomes, shown
via ultrastructural image analysis. It was found that the number of
autophagic vacuoles was increased after PA treatment (Fig. 2D). Compared with the PA group, there
were numerous different forms of autophagosomes, which were
enhanced when pre-treated with CQ (Fig.
2D). These results further suggested that autophagy was
activated in INS-1 cells in response to PA. Furthermore, CQ
aggravated the impairment of autophagy induced by PA in INS-1
cells. It was also identified that moderate autophagy had a
protective effect in INS-1 cells and was necessary to maintain the
normal architecture and function of INS-1 cells.
Next, it was investigated whether autophagy could
regulate FoxO1 protein expression in INS-1 cells. As
phosphorylation is critical for the FoxO1 function (24,25),
the levels of phosphorylated FoxO1 and total FoxO1 were detected in
each group. There was no difference in the expression of p-FoxO1
between the PA group and the PA + CQ group (Fig. 2E). The results suggested that
autophagy does not regulate the expression of FoxO1 in INS-1
cells.
Effects of liraglutide on PA-induced
INS-1 cells injury, autophagy and FoxO1
To study the effects of liraglutide in PA-induced
INS-1 cells injury, autophagy and FoxO1, INS-1 cells were cultured
with 0.5 mmol/l PA and 100 nmol/l liraglutide for 24 h.
Treatment of INS-1 cells with PA decreased cell
viability, which was partially prevented by co-treatment with
liraglutide (Fig. 2A). To further
identify the effects of the liraglutide in PA-induced INS-1 cells
injury. Western blotting was used to detect the expression of
cleaved caspase-3 in INS-1 cells. Treatment of INS-1 cells with PA
increased the expression of cleaved caspase-3, which was partially
prevented by co-treatment with liraglutide (Fig. 3A). However, there was no significant
difference between the control and liraglutide group in terms of
cell viability and apoptosis. These results suggested that
liraglutide has a protective effect on PA-induced INS-1 cells
rather than on normal conditions. Moreover, INS-1 cells viability
was significantly decreased in PA + LIRA + CQ group compared with
the PA + LIRA group (Fig. 2A).
These results indicated that the activation of autophagy was a
crucial component of liraglutide mediated PA-induced INS-1 cell
survival, although other pathways affecting cell survival cannot be
eliminated.
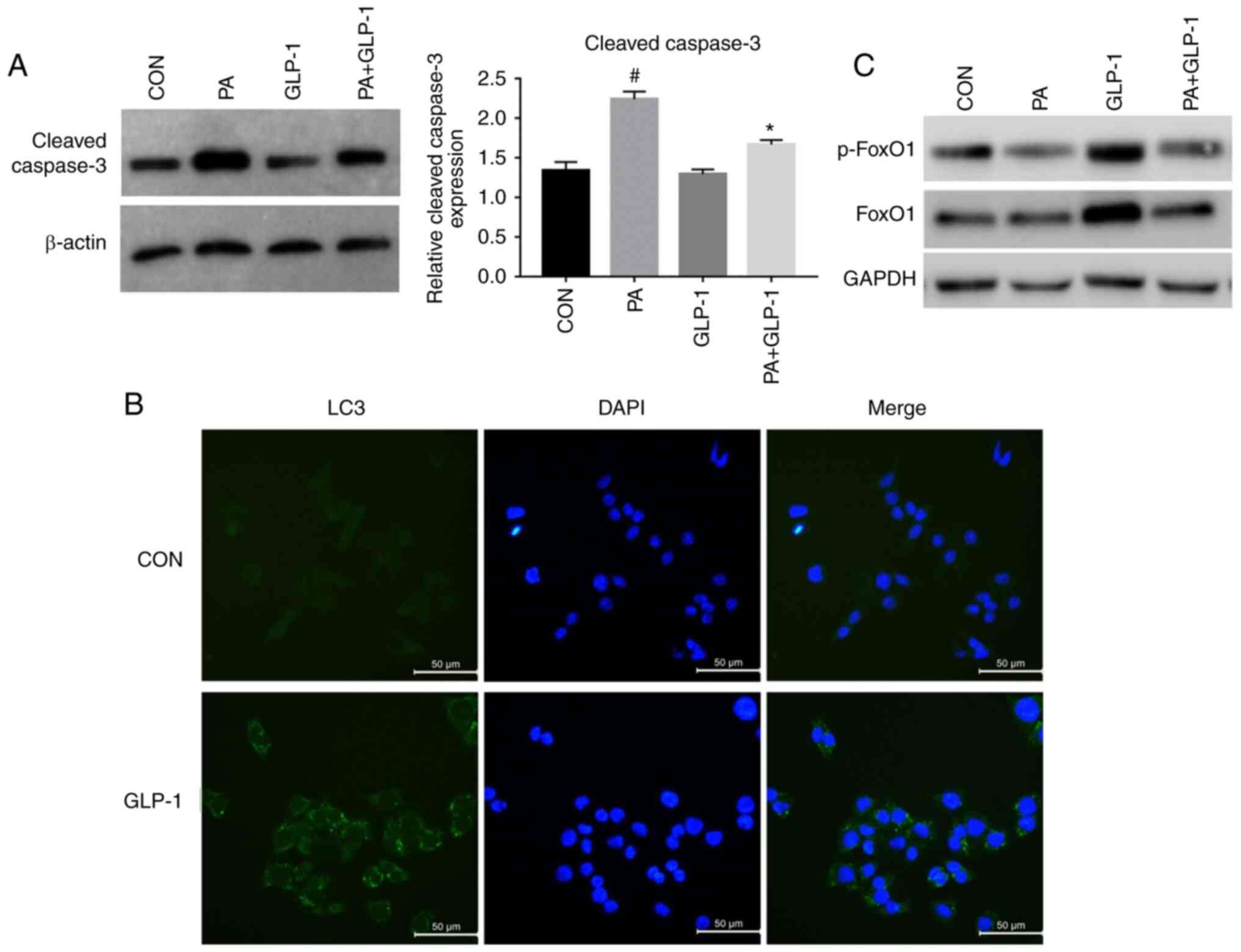 | Figure 3.Effect of liraglutide in PA-induced
INS-1 cells injury, autophagy and FoxO1. (A) Western blotting,
using β-actin as a control, for determining cleaved caspase-3
protein expression. Data were analyzed by paired t-test and are
presented as the mean ± SD (n=3). #P<0.05 vs. CON
group; *P<0.05 vs. PA group. (B) Immunofluorescence analysis of
LC3 puncta in control and GLP-1 group. Scale bar, 50 µm. (C).
Western blotting was used to detect the expression levels of
p-FoxO1 and FoxO1 in each group. FoxO1, Forkhead box O1; LC3,
microtubule-associated protein 1 light chain3; CON, control; PA,
palmitate; GLP-1, glucagon-like peptide-1; p-, phosphorylated. |
Western blotting was performed to investigate the
effect of liraglutide on the expression of LC3 protein in INS-1
cells of each group. The expression of LC3II/I was increased in the
LIRA group compared with the control group. Additionally, compared
with the PA group, the expression of LC3II/I was increased in the
PA + LIRA group, but was decreased when pretreated with CQ
(Fig. 2B). These findings indicated
that liraglutide partly reverses the effect of autophagy between CQ
and PA-induced INS-1 cells. Immunofluorescence analysis of LC3
puncta was used to examine the effect of liraglutide in INS-1
cells. The green fluorescence intensity of LC3 was increased in the
liraglutide group compared with the control group (Fig. 3B). These data demonstrated that
liraglutide prevented INS-1 cells from PA-induced damage by
enhancing the level of autophagy.
When compared with the control group, the
phosphorylation of FoxO1 was decreased in the PA group (Fig. 3C). Pretreatment with liraglutide
notably increased the PA-induced phosphorylation of FoxO1 in INS-1
cells (Fig. 3C). These results also
indicated that liraglutide pretreatment could alleviate the
reduction of p-FoxO1 protein expression induced by PA in INS-1
cells.
Liraglutide, via FoxO1, improved the
viability and autophagy induced by PA
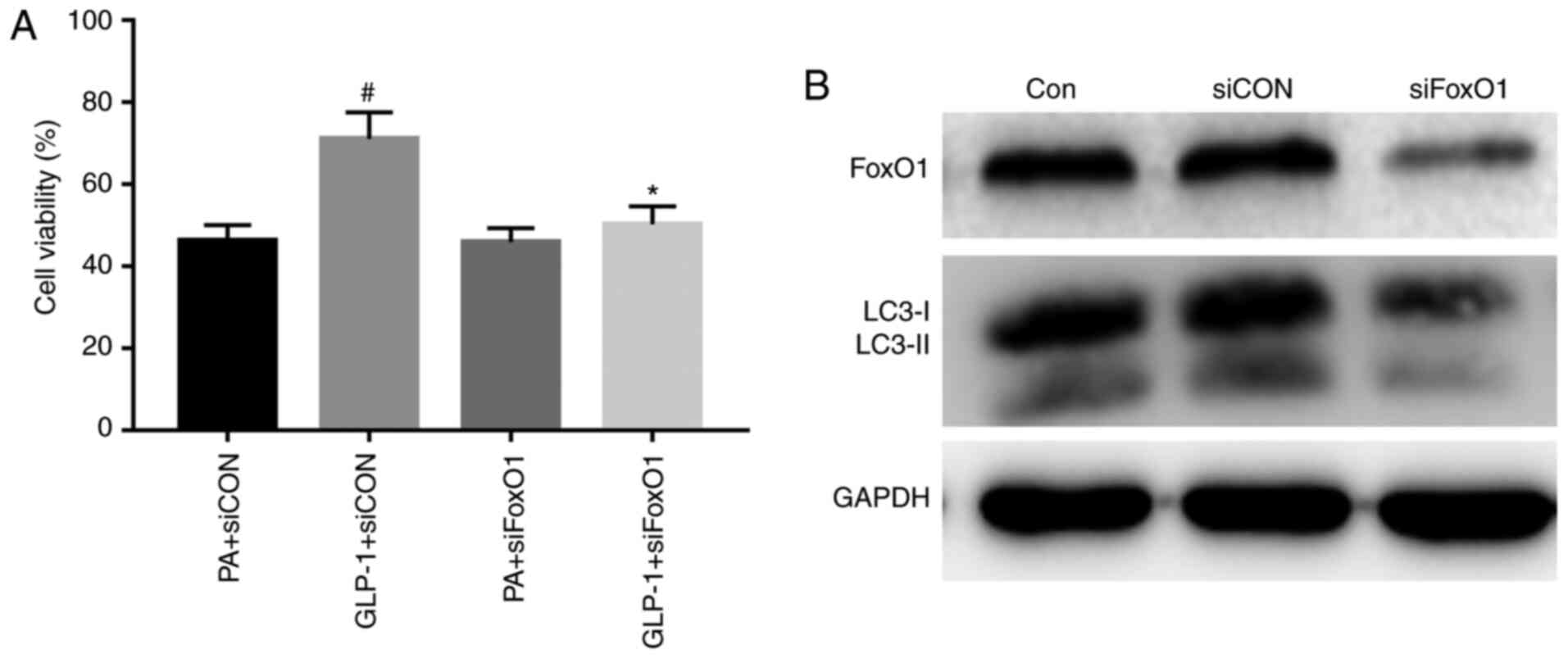
To study the effects of FoxO1 in INS-1 cell injury,
the present study interfered with the expression of FoxO1, and
found that INS-1 cell viability was not different between the PA +
siCON group and the PA + siFoxO1 group (Fig. 4A). The results demonstrated that
interfering with the expression of FoxO1 did not regulate INS-1
cell viability in a high-fat environment. The viability of INS-1
cells was significantly increased in the LIRA + siCON group,
compared with the PA + siCON group (Fig. 4A). However, INS-1 cell viability was
substantially decreased in the LIRA + siFoxO1 group, compared with
the LIRA + siCON group (Fig. 4A).
These results indicated that silencing FoxO1 could attenuate the
protection of liraglutide on the viability of INS-1 cells.
To confirm the role of FoxO1 in the effect of
autophagy in INS-1 cells, the expression of FoxO1 was knocked down.
FoxO1 expression was successfully suppressed in INS-1 cells that
were transfected with siRNA targeting FoxO1 (Fig. 4B). The expression of LC3-II was
decreased in the siFoxO1 group compared with the siCON group. The
results demonstrated that silencing FoxO1 downregulated the level
of autophagy-associated proteins in INS-1 cells (Fig. 4B). Collectively, the results
suggested that FoxO1 restored autophagy.
Discussion
T2DM is characterized by insulin resistance and
dysfunction of islet β-cells (2,10).
Substantial evidence indicates that oxidative stress, inflammatory
response and ERs can lead to dysfunction of β-cells (8,26,27).
Functional damage and quantity reduction of islet β-cells serve a
vital role in the occurrence and development of T2DM (2,13).
Moreover, hyperlipidemia is part of the main risk factors of
diabetes (3). The toxic effect of
FFA on β-cells is called lipotoxicity. Islet β-cell apoptosis
induced by FFA is currently considered to be one of the main
pathogenesis of T2DM, but its exact mechanism remains unknown. PA
is a FFA that is recognized as the most widely used molecule to
damage islet β-cells via lipotoxicity (21). Therefore, the present study used PA
to treat INS-1 cells to establish a pancreatic β-cells injury
model, which caused in vitro β-cell dysfunction in T2DM.
In the present experiment, INS-1 cells were treated
with different concentrations of PA. The results demonstrate that
INS-1 cell viability was decreased with the increasing
concentrations of PA. Our previous study reported similar results
that PA significantly reduced islet β-cell viability in a
dose-dependent manner (28). The
present study incubated INS-1 cells with 0.5 mmol/l PA for 24 h and
found that the expression of cleaved caspase-3 was increased.
Moreover, it was identified that chronic exposure to PA increased
INS-1 cells injury. These results are in line with the previous
findings and provide a possible mechanism to link lipotoxicity to
T2DM (29).
Autophagy regulates the degradation of organelles
and proteins in eukaryotic cells, which helps to maintain cellular
homeostasis (6). As autophagy
serves a critical role in maintaining cell homeostasis, autophagy
is indispensable in β-cells physiology (7). A lack of autophagy promotes the
impairment of islet β-cells function and mass, eventually lead to
the onset of diabetes (1,10,12).
The present findings demonstrated that the autophagy inhibitor CQ
further decreased the survival rate of INS-1 cells incubated in PA.
The results also indicated that autophagy promoted pancreatic
β-cells survival, which was consistent with our previous studies
(1,28). It was found that autophagy protected
β-cells from PA-induced injury and exerted a protective role in the
occurrence of T2DM. Autophagy also serves a major role in
maintaining islet β-cells function and survival. To further
investigate the interaction between autophagy and lipotoxicity in
INS-1 cells, the present study cultured INS-1 cells in a lipotoxic
environment and found that the autophagy hallmark protein LC3-II
was enhanced with increasing PA concentrations. These results were
further confirmed by immunofluorescence results, which identified
that LC3 puncta were significantly increased with 0.5 mmol/l PA.
The current immunofluorescence results were consistent with the
results of TEM analysis. The present data are consistent with the
prior studies showing that elevated serum levels of FFA mediated
induction of β-cell autophagy (28,30).
The increase of autophagosome formation and the
decrease of lysosomal fusion and degradation can result in the
accumulation of LC3-II (6,7). To further verify whether LC3-II
accumulation induced by PA resulted from impaired clearance due to
defective fusion with lysosomes or from the true autophagic flux,
the present study measured LC3-II in the presence of CQ and found
that the level of LC3-II protein was increased. The results were
also confirmed via immunofluorescence, in which the number of LC3
puncta increased. In addition, TEM identified that the number of
autophagic vacuoles was increased in the CQ group and PA + CQ
group. The present data are in agreement with the previous studies
(5,7,28),
which reported that CQ increased PA-induced defective autophagic
turnover in INS-1 cells.
Liraglutide, a GLP-1 analog, serves a pivotal role
in pancreatic β-cell proliferation, anti-apoptotic function and
autophagy (1,3,4,21).
Liraglutide protects islet β-cells from FFA and affects glucolipid
metabolism by activating autophagy (3). While it is known that GLP-1 regulates
β-cell autophagy by defending against oxidative stress and ERs
(31), further studies are required
to elucidate the mechanisms between liraglutide and autophagy in
pancreatic β-cells. The present study investigated the protective
effect of liraglutide on PA-induced INS-1 cells injury. Liraglutide
significantly inhibited the expression of cleaved caspase-3 protein
in INS-1 cells induced by PA acid, as well as increased the
survival and autophagy of INS-1 cells induced by PA. This was
verified by an increase in LC3-II formation and cell viability.
However, this effect can be reversed by the autophagy inhibitor CQ.
These data demonstrated that liraglutide exerted its cytoprotective
effects on PA-induced INS-1 cells injury by regulating autophagy,
and these are similar to a previous study (28).
It has been revealed that the GLP-1 receptor agonist
exendin-4 improves the β-cell survival induced by glucolipotoxicity
and the effect was dependent on the restoration of autophagic
signaling (31). GLP-1 can change
the subcellular distribution of FoxO1 and promote its translocation
in pancreatic β-cells (32). The
GLP-1 receptor inhibitor exendin9-39 downregulates the expression
of p-FoxO1 in NIT-1 insulinoma cells (33). Moreover, the GLP-1 receptor agonist
exendin-4 upregulates adiponectin levels in adipocytes via FoxO1,
both in vitro and in vivo (34). Shao et al (21) reported that liraglutide markedly
improved β-cells function under FFA, and the protective action of
liraglutide was mediated by the PI3K/AKT/FoxO1 pathway. Exendin can
also increase the levels of p-FoxO1 via protein kinase A in β-cells
(22). Geniposide enhances the
phosphorylation of Foxo1 and inhibits apoptosis in PA-treated INS-1
cells by activating the GLP-1 receptor (35). Furthermore, GLP-1 enhances FoxO1
acetylation and stimulate β-cells mass expansion (36). Therefore, the interactions between
liraglutide and FoxO1 autophagy are worth investigating in
pancreatic β-cells. However, whether liraglutide mediates β-cell
autophagy via FoxO1 remains unknown. The present study demonstrated
that liraglutide increased the expression of p-FoxO1 protein in
PA-induced INS-1 cells. Furthermore, FoxO1 silencing attenuated the
protective effect of liraglutide on INS-1 cells. Collectively, the
results indicated that liraglutide protected INS-1 cells against PA
by upregulating the expression of FoxO1.
To determine the effect of FoxO1-mediated cell
viability and autophagy in INS-1 cells, the present study blocked
the expression of FoxO1 in INS-1 cells. FoxO1 is a downstream
effector of insulin/insulin-like growth factor-I signaling and can
be regulated by Akt via phosphorylation, following nuclear
exclusion (13,24). Furthermore, FoxO1 is a key
regulatory factor governing a variety of physiological functions,
including the pancreatic β-cells proliferation, apoptosis and
autophagy (14,15,18,37).
FoxO1 also protects pancreatic β-cells by inhibiting
thioredoxin-interacting protein transcription (38). In the present study, the expression
of FoxO1 was downregulated with the increase in concentrations of
PA. FoxO1 silencing suppressed the viability of
liraglutide-pretreated INS-1 cells. These data suggested that FoxO1
silencing attenuated the protective effect of liraglutide on INS-1
cells. The relationship between FoxO1 and autophagy was examined,
and the present results suggested that blocking the FoxO1
expression suppressed the autophagy-related protein LC3-II in INS-1
cells. Liu et al (39)
revealed that FoxO1 antagonist treatment induces similar responses
in adipocytes. However, in the present study, the autophagy
inhibitor CQ did not affect the expression of p-FoxO1 in INS-1
cells. The current results were consistent with previously reported
findings that the effect of autophagy can be regulated by FoxO1 but
the autophagy inhibitor 3-methyadenine had no effect on FoxO1 in
vascular endothelial cells (19).
These results indicated that FoxO1 improved the viability of INS-1
cells against PA by upregulating the expression of
autophagy-associated proteins. However, the mechanism of FoxO1
regulating autophagy requires further investigation.
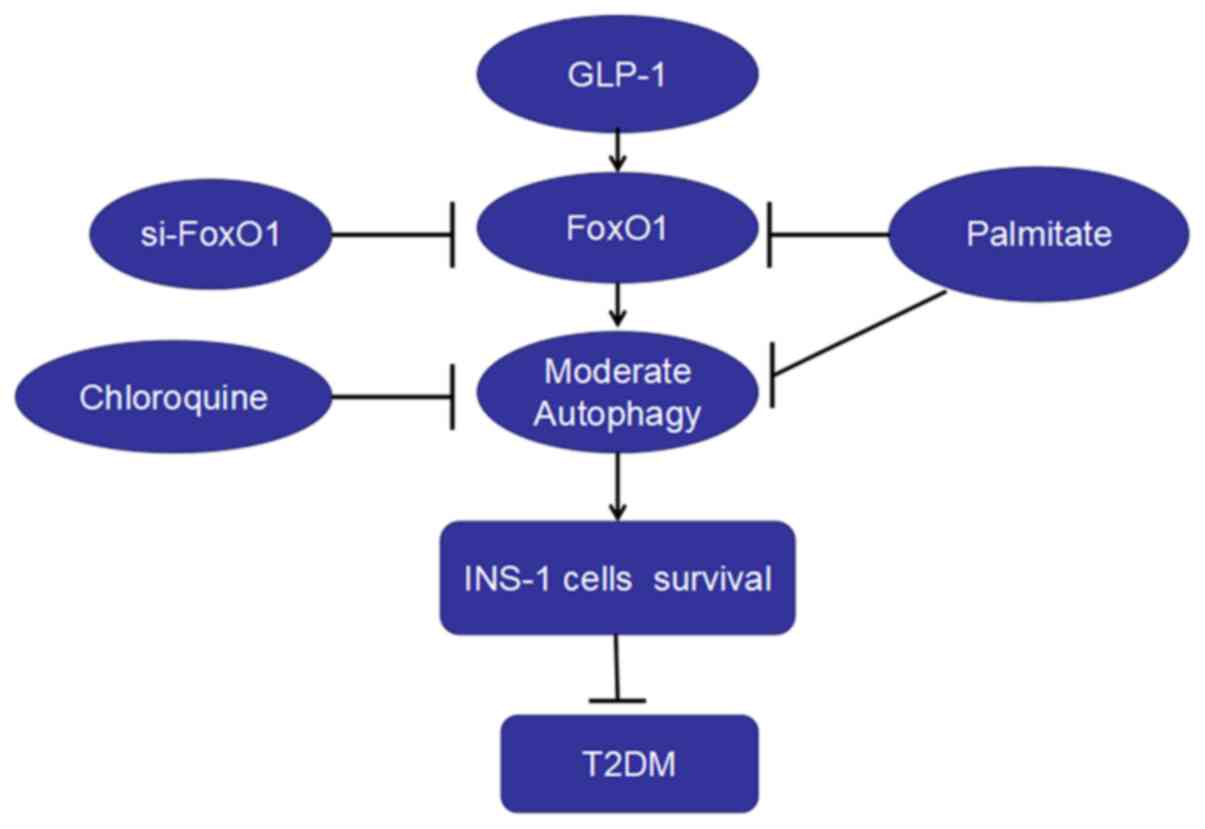
In the present study, it was found that: i) PA
decreased the viability, increased apoptosis and autophagy and
depressed the expression of FoxO1 in INS-1 cells; ii) inhibiting
the effect of autophagy decreased the survival and the expression
of FoxO1 in INS-1 cells; iii) liraglutide increased INS-1 cell
survival, autophagy and the expressions of FoxO1 induced by PA; and
iv) FoxO1 silencing decreased the level of autophagy and the
protection of liraglutide on the viability of INS-1 cells.
Therefore, liraglutide protected INS-1 cells against PA-induced
injury, which may occur via the upregulation of autophagy mediated
by FoxO1 (Fig. 5).
There are certain limitations to the present study.
First, PA was used to test the cell viability, apoptosis and
autophagy of pancreatic β-cells, based on its lipotoxicity
(40,41). Previous studies also proposed that
PA could participate in apoptosis via reactive oxygen species and
ERs generation in isolated rat islets of Langerhans (28,29,37,42).
Therefore, further studies are required obtain an in-deep insight
into the role of PA in apoptosis. Second, the main purpose of the
present study was to examine the mechanism of liraglutide on the
autophagy of islet β-cells; however, the improvement of insulin
secretion function in β-cells due to the autophagy effect of
liraglutide was not been studied. Third, GLP-1 regulates autophagy
in multiple signal pathways, including PI3K/Akt and 5′
AMP-activated protein kinase (43).
Therefore, further research to investigate the pathway between
GLP-1 and autophagy is required. Moreover, there are some
limitations with regards to the model. For instance, CQ does not
simply prevent autophagy, but blocks the pathway leading to a
build-up of autophagosomes and lysosomes. Therefore, dysfunctional
autophagy may have a much larger impact on cell survival and
function than simply preventing autophagy. In addition, due to the
limited funds, intracellular phosphorylation of FoxO1/total FoxO1
was not examined in the present study. However, a literature search
was conducted and it was found that the effects of FoxO1 depend on
its activation, which can be influenced by its abundance,
post-transcriptional modification, nuclear-cytoplasmic shuttling
and subcellular localization (13,17,18).
Among these activating factors, phosphorylation is critical for the
FoxO1 function (24,25). Finally, the present study lacked
in vivo experiments, which will further validate the use of
liraglutide for diabetes treatment.
In conclusion, to the best of our knowledge, this is
the first study that demonstrated that the GLP-1 agonist
liraglutide protected INS-1 cells against PA-induced injury via the
upregulation of autophagy mediated by FoxO1. This may provide a
novel theoretical basis and experimental support for liraglutide in
the treatment of diabetes.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81770820).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
XDL performed the cell experiments. XDL and SSH
performed CCK8 and immunofluorescence assays and western blot
analysis, XDL and TTW performed transmission electron microscopy.
XDL and YBL contributed to the study design and writing of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CCK-8
|
Cell Counting Kit-8
|
|
ERs
|
endoplasmic reticulum stress
|
|
FFA
|
free fatty acids
|
|
FoxO1
|
Forkhead box O1
|
|
GLP-1
|
glucagon-like peptide-1
|
|
LC3
|
microtubule-associated protein 1 light
chain3
|
|
p-FoxO1
|
phosphorylated FoxO1
|
|
siRNA
|
small interfering RNA
|
|
TEM
|
transmission electron microscopy
|
|
T2DM
|
type 2 diabetes mellitus
|
References
|
1
|
Chen ZF, Li YB, Han JY, Yin JJ, Wang Y,
Zhu LB and Xie GY: Liraglutide prevents high glucose level induced
insulinoma cells apoptosis by targeting autophagy. Chin Med J
(Engl). 126:937–941. 2013.PubMed/NCBI
|
|
2
|
Chen ZF, Li YB, Han JY, Wang J, Yin JJ, Li
JB and Tian H: The double-edged effect of autophagy in pancreatic
beta cells and diabetes. Autophagy. 7:12–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J, Wu J, Wu H, Liu X, Chen Y, Wu J,
Hu C and Zou D: Liraglutide protects pancreatic β-cells against
free fatty acids in vitro and affects glucolipid metabolism in
apolipoprotein E-/- mice by activating autophagy. Mol Med Rep.
12:4210–4218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan M, Jiang H, Zhang Y, Ma Y, Li L and Wu
J: Liraglutide enhances autophagy and promotes pancreatic β cell
proliferation to ameliorate type 2 diabetes in high-fat-fed and
streptozotocin-treated mice. Med Sci Monit. 24:2310–2316. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Q, Jia S, Xu L, Li B and Chen N:
Metformin-induced autophagy and irisin improves INS-1 cell function
and survival in high-glucose environment via AMPK/SIRT1/PGC-1α
signal pathway. Food Sci Nutr. 7:1695–1703. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee YH, Kim J, Park K and Lee MS: β-cell
autophagy: Mechanism and role in β-cell dysfunction. Mol Metab.
27S:S92–S103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toledo M and Singh R: Complement C3 and
autophagy keep the β cell alive. Cell Metab. 29:4–6. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu H, Yin JJ, Cao MM, Liu GD, Su Y and Li
YB: Endoplasmic reticulum stress induced by lipopolysaccharide is
involved in the association between inflammation and autophagy in
INS-1 cells. Mol Med Rep. 16:5787–5792. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
King BC, Kulak K, Krus U, Rosberg R, Golec
E, Wozniak K, Gomez MF, Zhang E, O'Connell DJ, Renström E, et al:
Complement component C3 is highly expressed in human pancreatic
islets and prevents β cell death via ATG16L1 interaction and
autophagy regulation. Cell Metab. 29:202–210.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu L-B, Cao M-M, Wang J, Su Y, Jiang W,
Liu GD and Li YB: Role of autophagy in LPS-induced inflammation in
INS-1 cells. Mol Med Rep. 19:5211–5218. 2019.PubMed/NCBI
|
|
11
|
Zhou X-T, Pu Z-J, Liu L-X, Li GP, Feng JL,
Zhu HC and Wu LF: Inhibition of autophagy enhances
adenosine-induced apoptosis in human hepatoblastoma HepG2 cells.
Oncol Rep. 41:829–838. 2019.PubMed/NCBI
|
|
12
|
Quan W, Hur KY, Lim Y, Oh SH, Lee JC, Kim
KH, Kim GH, Kim SW, Kim HL, Lee MK, et al: Autophagy deficiency in
beta cells leads to compromised unfolded protein response and
progression from obesity to diabetes in mice. Diabetologia.
55:392–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kitamura T: The role of FOXO1 in β-cell
failure and type 2 diabetes mellitus. Nat Rev Endocrinol.
9:615–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang T, Kim DH, Xiao X, Lee S, Gong Z,
Muzumdar R, Calabuig-Navarro V, Yamauchi J, Harashima H, Wang R, et
al: FoxO1 Plays an Important Role in Regulating β-Cell Compensation
for Insulin Resistance in Male Mice. Endocrinology. 157:1055–1070.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He W, Zhang A, Qi L, Na C, Jiang R, Fan Z
and Chen J: FOXO1, a potential therapeutic target, regulates
Autophagic flux, oxidative stress, mitochondrial dysfunction, and
apoptosis in human cholangiocarcinoma QBC939 cells. Cell Physiol
Biochem. 45:1506–1514. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mo X, Wang X, Ge Q and Bian F: The effects
of SIRT1/FoxO1 on LPS induced INS-1 cells dysfunction. Stress.
22:70–82. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kitamura YI, Kitamura T, Kruse JP, Raum
JC, Stein R, Gu W and Accili D: FoxO1 protects against pancreatic
beta cell failure through NeuroD and MafA induction. Cell Metab.
2:153–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kobayashi M, Kikuchi O, Sasaki T, Kim HJ,
Yokota-Hashimoto H, Lee YS, Amano K, Kitazumi T, Susanti VY,
Kitamura YI, et al: FoxO1 as a double-edged sword in the pancreas:
Analysis of pancreas- and β-cell-specific FoxO1 knockout mice. Am J
Physiol Endocrinol Metab. 302:E603–E613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Q, Hu Y, Jiang M, Wang F and Gong G:
Effect of autophagy regulated by Sirt1/FoxO1 pathway on the release
of factors promoting thrombosis from vascular endothelial cells.
Int J Mol Sci. 20:41322019. View Article : Google Scholar
|
|
20
|
Ren H, Shao Y, Wu C, Ma X, Lv C and Wang
Q: Metformin alleviates oxidative stress and enhances autophagy in
diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol Cell
Endocrinol. 500:1106282020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shao S, Nie M, Chen C, Chen X, Zhang M,
Yuan G, Yu X and Yang Y: Protective action of liraglutide in beta
cells under lipotoxic stress via PI3K/Akt/FoxO1 pathway. J Cell
Biochem. 115:1166–1175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muhammad AB, Xing B, Liu C, Naji A, Ma X,
Simmons RA and Hua X: Menin and PRMT5 suppress GLP1 receptor
transcript and PKA-mediated phosphorylation of FOXO1 and CREB. Am J
Physiol Endocrinol Metab. 313:E148–E166. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin J, Wang Y, Gu L, Fan N, Ma Y and Peng
Y: Palmitate induces endoplasmic reticulum stress and autophagy in
mature adipocytes: Implications for apoptosis and inflammation. Int
J Mol Med. 35:932–940. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xing YQ, Li A, Yang Y, Li XX, Zhang LN and
Guo HC: The regulation of FOXO1 and its role in disease
progression. Life Sci. 193:124–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen B, Zhou W, Zhao W, Yuan P, Tang C,
Wang G, Leng J, Ma J, Wang X, Hui Y, et al: Oxaliplatin reverses
the GLP-1R-mediated promotion of intrahepatic cholangiocarcinoma by
altering FoxO1 signaling. Oncol Lett. 18:1989–1998. 2019.PubMed/NCBI
|
|
26
|
Wang F, Yin J, Ma Y, Jiang H and Li Y:
Vitexin alleviates lipopolysaccharide-induced islet cell injury by
inhibiting HMGB1 release. Mol Med Rep. 15:1079–1086. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao MM, Lu X, Liu GD, Su Y, Li YB and Zhou
J: Resveratrol attenuates type 2 diabetes mellitus by mediating
mitochondrial biogenesis and lipid metabolism via Sirtuin type 1.
Exp Ther Med. 15:576–584. 2018.PubMed/NCBI
|
|
28
|
Fu J, Nchambi KM, Wu H, Luo X, An X and
Liu D: Liraglutide protects pancreatic β cells from endoplasmic
reticulum stress by upregulating MANF to promote autophagy
turnover. Life Sci. 252:1176482020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bugliani M, Mossuto S, Grano F, Suleiman
M, Marselli L, Boggi U, De Simone P, Eizirik DL, Cnop M, Marchetti
P, et al: Modulation of autophagy influences the function and
survival of human pancreatic beta cells under endoplasmic reticulum
stress conditions and in type 2 diabetes. Front Endocrinol
(Lausanne). 10:522019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chu KY, O'Reilly L, Mellet N, Meikle PJ,
Bartley C and Biden TJ: Oleate disrupts cAMP signaling,
contributing to potent stimulation of pancreatic β-cell autophagy.
J Biol Chem. 294:1218–1229. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zummo FP, Cullen KS, Honkanen-Scott M,
Shaw JAM, Lovat PE and Arden C: Glucagon-Like peptide 1 protects
pancreatic β-cells from death by increasing autophagic flux and
restoring lysosomal function. Diabetes. 66:1272–1285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Tokui Y, Yamagata K, Kozawa J,
Sayama K, Iwahashi H, Okita K, Miuchi M, Konya H, Hamaguchi T, et
al: Continuous stimulation of human glucagon-like peptide-1 (7–36)
amide in a mouse model (NOD) delays onset of autoimmune type 1
diabetes. Diabetologia. 50:1900–1909. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Y-J, Wu Y-B, Fang Z-H, Chen MQ, Wang
YF, Wu CY and Lv MA: Danzhi Jiangtang capsule mediates NIT-1
insulinoma cell proliferation and apoptosis by GLP-1/Akt signaling
pathway. Evid Based Complement Alternat Med. 2019:53568252019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang A, Li T, An P, Yan W, Zheng H, Wang B
and Mu Y: Exendin-4 upregulates adiponectin level in adipocytes via
Sirt1/Foxo-1 signaling pathway. PLoS One. 12:e01694692017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu J, Yin F, Xiao H, Guo L and Gao X:
Glucagon-like peptide 1 receptor plays an essential role in
geniposide attenuating lipotoxicity-induced β-cell apoptosis.
Toxicol In Vitro. 26:1093–1097. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bastien-Dionne PO, Valenti L, Kon N, Gu W
and Buteau J: Glucagon-like peptide 1 inhibits the sirtuin
deacetylase SirT1 to stimulate pancreatic β-cell mass expansion.
Diabetes. 60:3217–3222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shaklai S, Grafi-Cohen M, Sharon O, Sagiv
N, Shefer G, Somjen D and Stern N: Pancreatic beta-cell
proliferation induced by estradiol-17β is Foxo1 dependent. Horm
Metab Res. 50:485–490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kibbe C, Chen J, Xu G, Jing G and Shalev
A: FOXO1 competes with carbohydrate response element-binding
protein (ChREBP) and inhibits thioredoxin-interacting protein
(TXNIP) transcription in pancreatic beta cells. J Biol Chem.
288:23194–23202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu L, Zheng LD, Zou P, Brooke J, Smith C,
Long YC, Almeida FA, Liu D and Cheng Z: FoxO1 antagonist suppresses
autophagy and lipid droplet growth in adipocytes. Cell Cycle.
15:2033–2041. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Min S, Zhu T, Tong J, Caidan R, Wang K,
Kai G, Zhang W, Ru L, Pengcuo J and Tong L: Screening active
components from Rubus amabilis for pancreatic β-cells
protection. Pharm Biol. 58:674–685. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu X, Zeng X, Chen X, Luo R, Li L, Wang
C, Liu J, Cheng J, Lu Y and Chen Y: Oleic acid protects
insulin-secreting INS-1E cells against palmitic acid-induced
lipotoxicity along with an amelioration of ER stress. Endocrine.
64:512–524. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu C, Fu Y, Li CE, Chen T and Li X:
Phycocyanin-functionalized selenium nanoparticles reverse palmitic
acid-induced pancreatic β cell apoptosis by enhancing cellular
uptake and blocking reactive oxygen species (ROS)-mediated
mitochondria dysfunction. J Agric Food Chem. 65:4405–4413. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang Y, Fang H, Xu G, Zhen Y, Zhang Y,
Tian J, Zhang D, Zhang G and Xu J: Liraglutide improves cognitive
impairment via the AMPK and PI3K/Akt signaling pathways in type 2
diabetic rats. Mol Med Rep. 18:2449–2457. 2018.PubMed/NCBI
|