Introduction
Atherosclerosis (AS), which is characterized by
hyperlipidemia, lipid plaque formation and an accompanying complex
vascular inflammatory response, is considered as the major
contributor for the development of cardiovascular disease worldwide
(1–3). Accumulating evidence has revealed the
role of autoimmunity in AS (4).
Clinical studies have identified that patients with autoimmune
diseases, such as antiphospholipid syndrome (APS) or systemic lupus
erythematous, experience significant morbidity and mortality due to
AS (5,6).
Endothelial inflammation was discovered to
significantly contribute to the initiation and progression of AS by
damaging the vascular wall, promoting monocyte adhesion and
infiltration, and accelerating lipid accumulation in the
subendothelial space (7,8). IL-1β, IL-6 and TNF-α, which are
secreted by endothelial cells, are reported to be closely
associated with arterial damage and vascular inflammation during
the early stages of AS (9). In
addition, adhesion molecules released from endothelial cells, such
as intercellular adhesion molecule (ICAM)-1 and vascular adhesion
molecule (VCAM)-1, are observed to serve important roles in the
recruitment of circulating monocytes (10).
Oxidized low-density lipoprotein (oxLDL) is known to
be an important lipid component in AS, and exerts roles throughout
almost every stage of the disease (11). OxLDL accelerates AS progression by
triggering vascular inflammation and lipid accumulation, which are
the important pathological factors involved in the initiation and
development of AS (12). However,
previous clinical evidence has indicated that the
oxLDL/β2-glycoprotein I (β2GPI) complex may be a more substantial
indicator of cardiovascular complications compared with oxLDL alone
in patients with APS (13,14). Previous studies have also reported
that the increased risk of AS in patients with APS was mainly due
to β2GPI and its autoantibodies (4–6). For
example, our previous in vivo study confirmed that the
presence of the anti-β2GPI antibody (Ab) accelerated plaque
formation in ApoE-/- mice (15). In
addition, an in vitro study found that the
oxLDL/β2GPI/anti-β2GPI Ab complex induced the proatherogenic
activation of vascular smooth muscle cells (VSMCs) by enhancing
their migratory abilities and the secretion of active molecules
(16).
Similar to the majority of the members of the
pattern recognition receptor family, Toll-like receptor (TLR) 4 is
capable of recognizing a wide range of danger-associated molecules,
including microbial components, such as lipopolysaccharide (LPS)
and modified endogenous molecules, including oxLDL (17). Upon recognition, the invaded
pathogens are eliminated by triggering the inflammatory response
(18). Several studies have
revealed that TLR4 is involved in antiphospholipid Ab-mediated
thrombosis and the activation of endothelial cells, which could
activate p38 MAPK and NF-κB, leading to the subsequent upregulation
of various genes and the production of proinflammatory cytokines in
patients with APS (19–21). Moreover, the activation of the
TLR4/NF-κB signaling pathway was discovered to promote the
secretion of inflammatory cytokines and the lipid accumulation of
macrophages during the pathogenesis of AS (22,23).
Our previous studies have demonstrated that the
oxLDL/β2GPI/anti-β2GPI Ab complex induced the differentiation of
macrophages to foam cells and altered the proliferation and
apoptosis of VSMCs via a biphasic effect (24,25).
Considering the crucial role of inflammatory activation of
endothelial cells in AS development, the effect of the
oxLDL/β2GPI/anti-β2GPI Ab complex on endothelial cells requires
further investigation. Therefore, the present study aimed to
evaluate the expression levels of inflammatory cytokines, adhesive
molecules and chemokines in human umbilical vein endothelial cells
(HUVECs) in the presence of the oxLDL/β2GPI/anti-β2GPI Ab complex,
in addition to determining the ability of HUVECs to recruit
monocytes and identifying the role of the TLR4/NF-κB signaling
pathway in these processes.
Materials and methods
Cell culture
HUVECs were obtained from the Shanghai Institutes
for Biological Sciences. Cells were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% heat-inactivated FBS
(Biological Industries), 4.5 g/l glucose, 1% glutamine and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
The cells were maintained at 37°C and 5% CO2 in a
humidified incubator until 90% confluence.
THP-1, a human monocytes cell-line, was purchased
from Shanghai Institutes for Biological Sciences. THP-1 monocytes
were cultured at 37°C in a 5% CO2/95% humidified air
incubator (Thermo Fisher Scientific, Inc.) in RPMI-1640 (Gibco;
Thermo Fisher Scientific, Inc.) with 10% FBS (Biological
Industries) and 1% penicillin-streptomycin antibiotics (Gibco;
Thermo Fisher Scientific, Inc.).
Preparation and identification of
stimuli
Following 12 h of serum deprivation, HUVECs were
stimulated with DMEM, oxLDL (50 µg/ml; cat. no. YB-002; http://www.yiyuanbiotech.com/PRO.asp?id=562#section2;
Guangzhou Yiyuan Biotech. Co. Ltd.), oxLDL(50 µg/ml)/β2GPI (100
µg/ml; cat. no. G9173; Sigma-Aldrich; Merck KGaA) complex, oxLDL
(50 µg/ml)/anti-β2GPI Ab (100 µg/ml; cat. no. HPA001654;
Sigma-Aldrich; Merck KGaA) complex, oxLDL (50 µg/ml)/β2GPI (100
µg/ml)/anti-β2GPI Ab (100 µg/ml) complex (24–26) or
LPS (500 ng/ml; Sigma-Aldrich; Merck KGaA) at 37°C for 2 or 24
h.
For preparation of the complex of oxLDL/β2GPI, 50 µg
oxLDL and 100 µg β2GPI were added to 1 ml DMEM (with 10% FBS and 1%
antibiotics), and then incubated at 37°C and pH 7.4 for 16 h, as
previously described (24,25). The complex of oxLDL/anti-β2GPI Ab
and oxLDL/β2GPI/anti-β2GPI Ab were prepared by incubating oxLDL (50
µg/ml) or oxLDL (50 µg/ml)/β2GPI (100 µg/ml) complex with
anti-β2GPI Ab (100 µg/ml) at 37°C for 30 min (26). The concentrations and incubation
times of the aforementioned reagents were selected according to
established protocols and previous studies (24–27).
After performing the aforementioned incubations, the
binding of complex was confirmed via ELISA according to previous
studies (26,27). Firstly, anti-human apoB-100 Ab (cat.
no. SAB2500080; Sigma-Aldrich; Merck KGaA) was adsorbed onto
microtiter plates by incubating at 8 mg/ml (dissolved in Carbonate
buffered saline, 50 µl/well) at 4°C overnight. After blocking with
PBS containing 10% Newborn Calf Serum (NBCS; cat. no. 80230-6412;
Zhejiang Tianhang Biotechnology Co., Ltd.) at 37°C for 1 h, the
samples (oxLDL/β2GPI, oxLDL/anti-β2GPI Ab or oxLDL/β2GPI/anti-β2GPI
Ab) diluted (1:100) with PBS containing 10% NBCS were added to the
wells (100 µl/well) to be incubated at 37°C for 2 h. The blank
control well was added with PBS containing 10% NBCS. The wells were
then incubated with anti-β2GPI Ab at 37°C for 30 min and then
incubated with horseradish peroxidase (HRP)-labeled anti-IgG Ab
[cat. no. 05-4030-05; Hangzhou Multi Sciences (Lianke) Biotech Co.,
Ltd.] at room temperature (RT) for 2 h (for Blank control and
oxLDL/β2GPI) or HRP-labeled anti-IgG Ab (for oxLDL/anti-β2GPI Ab
and oxLDL/β2GPI/anti-β2GPI Ab) at RT for 2 h. Extensive washing
between steps was performed with PBS containing 0.05% Tween-20.
Color was developed with tetramethylbenzidine (TMB) and
H2O2 at RT for 15 min. The reaction was
terminated, and optical density at 450 nm was measured.
For the inhibition of TLR4 and NF-κB, the cells were
pretreated with 5 µM TAK-242 (Invitrogen; Thermo Fisher Scientific,
Inc.) or 20 µM 2-phosphonobutane-1,2,4, -tricarboxylic acid (PDTC;
Sigma-Aldrich; Merck KGaA) at 37°C for 6 or 1.5 h, respectively,
according to the manufacturer's protocols.
Reverse transcription-quantitative PCR
(RT-qPCR)
The cells were seeded at a density of
2×106 cells/well into a 6-well plate and starved in
serum-free media for 12 h prior to stimulation. Total RNA was
extracted from HUVECs using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Total RNA was reverse transcribed
into cDNA using the HiScript™ First-strand cDNA Synthesis kit
(Vazyme Biotech Co., Ltd.) at 50°C for 15 min and followed by 85°C
for 2 min. oligo dT-primers (Sangon Biotech, Co., Ltd.) were used
for the reverse transcription alongside 1 µg total RNA in a 10 µl
reaction volume. The expression levels of target mRNAs in the
HUVECs were subsequently analyzed via qPCR using SYBR-Green I dye
(Vazyme Biotech Co., Ltd.). The primer pairs used for the qPCR are
listed in Table SI. The following
thermocycling conditions were used for the amplification run:
Initial denaturation at 95°C for 30 sec; followed by 39 cycles at
56°C (VCAM-1)/58°C (ICAM-1, IL-1β and IL-6)/60°C [TNF-α, monocyte
chemotactic protein (MCP-1), TLR4 and β-actin] for 30 sec and
extension at 72°C for 30 sec. The expression levels of the target
genes were quantified using the 2−Δ∆Cq method (28) and normalized to the control gene,
β-actin.
Western blotting
The cells were stimulated with different stimuli as
aforementioned and then total protein was extracted using RIPA
lysis buffer (cat. no. P0013K; Beyotime Institute of Biotechnology)
for 1 h in an ice bath. Cellular protein concentrations were
determined using a BCA Protein assay kit (cat. no. P0011; Beyotime
Institute of Biotechnology), according to the manufacturer's
protocol. Protein samples (100 µg) were separated by
electrophoresis on 8–10% polyacrylamide gels and the separated
proteins were subsequently transferred onto PVDF membranes (Bio-Rad
Laboratories, Inc.). The membranes were blocked in fresh 5% dry
non-fat milk diluted in TBS-0.05% Tween-20 (TBS/T) for 1 h at room
temperature. After being washed with TBS/T for 15 min three times,
the membranes were then incubated with the following primary Abs at
4°C overnight: Monoclonal rabbit anti-TLR4 (1:500; cat. no.
sc-76B357.1; Santa Cruz Biotechnology, Inc.), monoclonal rabbit
anti-phosphorylated (p)-NF-κB-p65 Ser536 (1:1,000; cat. no. 3033;
Cell Signaling Technology, Inc.), monoclonal rabbit anti-NF-κB p65
(1:1,000; cat. no. 8242; Cell Signaling Technology, Inc.),
monoclonal rabbit anti-p-AKT (1:1,000; cat. no. 4060; Cell
Signaling Technology, Inc.), monoclonal rabbit anti-AKT (1:1,000;
cat. no. 4685; Cell Signaling Technology, Inc.) and polyclonal
anti-β-actin (1:5,000; cat. no. AP0060; Bioworld Technology).
Following the primary Ab incubation, the membranes were incubated
with a horseradish peroxidase-conjugated goat anti-rabbit secondary
Ab (1:5,000; cat. no. BS13278; Bioworld Technology) at RT for 1 h
after washing three times with TBS/T for 15 min. Protein bands were
visualized using enhanced ECL western blotting detection reagent
(Cytiva) on an Image Quant LAS 4000 imager, and the densitometric
analysis was performed using LANE 1D (version 4.0; Beijing Sage
Creation Science Co., Ltd.).
ELISAs
The cells were seeded at a density of
1.0×105 cells/well into 24-well plates and treated with
different stimuli as previously described following serum
starvation for 12 h. For TLR4 or NF-κB inhibition, cells in
specific wells were pretreated with TAK-242 (5 µM) for 6 h or PDTC
(20 µM) at 37°C for 1.5 h prior to the other treatments. Following
stimulation, the cell culture supernatants were centrifuged for 10
min at 300 × g at 4°C. The concentrations of TNF-α, IL-1β, IL-6 and
MCP-1 in the cell culture supernatants were analyzed using TNF-α
[cat. no. 70-EK182-96; Hangzhou Multi Sciences (Lianke) Biotech
Co., Ltd.], IL-1β [cat. no. 70-EK101B-96; Hangzhou Multi Sciences
(Lianke) Biotech Co., Ltd.], IL-6 [cat. no. 70-EK106/2-96; Hangzhou
Multi Sciences (Lianke) Biotech Co., Ltd.] and MCP-1 [cat. no.
70-EK187-96; Hangzhou Multi Sciences (Lianke) Biotech Co., Ltd.]
according to the manufacturer's protocols. The concentrations of
the cytokines were expressed as pg/ml.
Immunofluorescence staining
HUVECs were seeded at a density of
1.0×105 cells/well into 24-well plates. Following 24 h
of incubation with the stimuli, the cells were fixed in 4%
paraformaldehyde at 4°C for 30 min and then washed gently with PBS
three times. The cells were subsequently blocked with 5% BSA [cat.
no. A600332; Sangon Biotech (Shanghai) Co., Ltd.] at RT for 1 h and
then incubated overnight at 4°C with the following primary Ab:
Anti-ICAM-1 (1:500; cat. no. ab2213; Abcam) and anti-VCAM-1 (1:500;
cat. no. ab134047; Abcam). Subsequently, the cells were incubated
with an AF488-conjugated secondary Ab (1:500; cat. no.
K0034G-AF488; Beijing Solarbio Science & Technology Co., Ltd.).
The nuclei were stained with DAPI (cat. no. C0065, Beijing
Solarbio, Science & Technology Co., Ltd.) for 15 min at 37°C.
The expression levels of the proteins were observed and imaged
using the fluorescent microscopes on the BioTek Cytation 5 Cell
Imaging Multi-Mode reader (BioTek Instruments, Inc.).
Monocyte adhesion assay
HUVECs were stimulated with different stimuli as
aforementioned for 24 h and then rinsed with PBS. Subsequently,
1×105 THP-1 cells/well, which were seeded into 24-well
plates and pre-stained with 5 µM Dil (cat. no. D9780; Beijing
Solarbio Science & Technology Co., Ltd.) at 37°C for 30 min,
were added to the HUVEC cultures and incubated at 37°C and 5%
CO2 for 30 min in a humidified incubator. After being
washed with PBS, THP-1 cells that were adhered to HUVECs were
visualized and imaged under a fluorescence microscope at a
magnification of ×100 (Leica Microsystems GmbH). The Dil (red
fluorescence) intensity in each field of view was calculated using
ImageJ 2× 2.1 software (National Institute of Health), which
reflected the number of adhered THP-1 cells.
Statistical analysis
All the experiments were repeated ≥3 times for each
experimental condition and the representative results are
presented. Normally distributed data are expressed as the mean ±
SEM. Differences between control and experimental conditions were
assessed using the one-way ANOVA with Tukey's multiple group
comparison test. The two-factor treatment results were analyzed
using a two-way ANOVA with Bonferroni's test. All the statistical
analyses were performed using SPSS 24.0 software (IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of the oxLDL/β2GPI/anti-β2GPI
Ab complex on the expression levels of inflammatory cytokines
(TNF-α, IL-1β and IL-6) in HUVECs
To investigate the role of the
oxLDL/β2GPI/anti-β2GPI Ab complex in endothelial inflammation,
HUVECs were treated with DMEM, oxLDL, oxLDL/β2GPI, oxLDL/anti-β2GPI
Ab, oxLDL/β2GPI/anti-β2GPI Ab complex or LPS for 2 or 24 h. The
oxLDL/β2GPI, oxLDL/anti-β2GPI Ab and oxLDL/β2GPI/anti-β2GPI Ab
complex were prepared and validated prior to experiment (P<0.01;
Fig. S1). Following incubation
with the stimuli, the mRNA and protein expression levels of TNF-α,
IL-1β and IL-6 were analyzed using RT-qPCR and ELISAs,
respectively. Treatment of cells with oxLDL and the
oxLDL/β2GPI/anti-β2GPI Ab complex significantly upregulated the
mRNA and protein expression levels of TNF-α (Fig. 1A and D), IL-1β (Fig. 1B and E) and IL-6 (Fig. 1C and F) compared with the DMEM
control group (P<0.01; Fig. 1).
The expression levels of the inflammatory cytokines in the
oxLDL/β2GPI and oxLDL/anti-β2GPI Ab groups were also upregulated,
although not all the changes were statistically significant. The
effects of the oxLDL/β2GPI/anti-β2GPI Ab complex treatment were
more notable compared with the oxLDL, oxLDL/β2GPI and
oxLDL/anti-β2GPI Ab groups, although similar results were observed
using LPS.
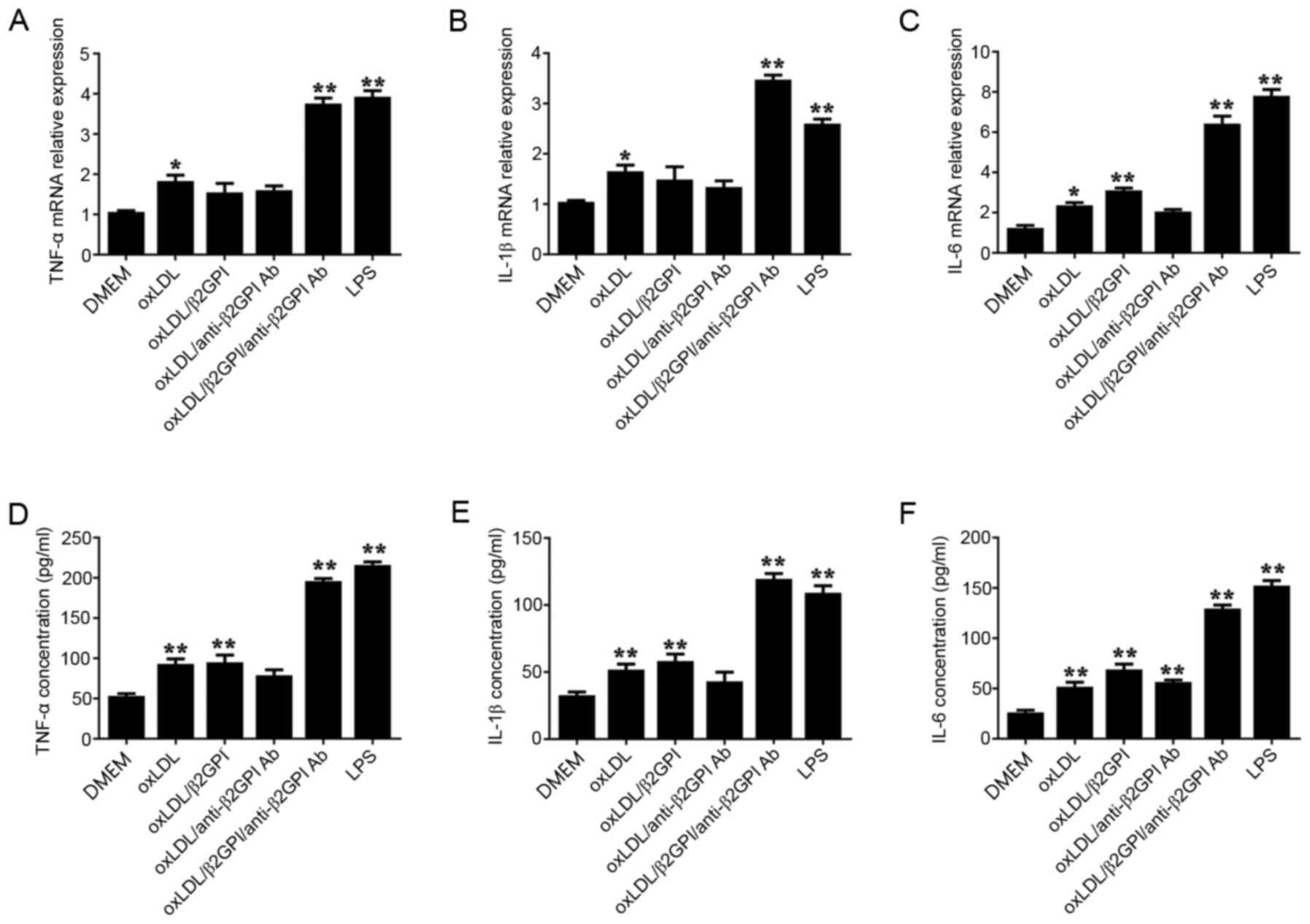 | Figure 1.Effects of the oxLDL/β2GPI/anti-β2GPI
Ab complex on the expression levels of the inflammatory cytokines
TNF-α, IL-1β and IL-6. HUVECs were treated with DMEM, oxLDL,
oxLDL/β2GPI complex, oxLDL/anti-β2GPI Ab complex,
oxLDL/β2GPI/anti-β2GPI Ab complex or LPS for 2 h (for qPCR) or for
24 h (for ELISA). Total RNA was extracted from HUVECs and the mRNA
expression levels of (A) TNF-α, (B) IL-1β and (C) IL-6 were
analyzed using RT-qPCR. The cell culture supernatants of the HUVECs
were collected for the detection of (D) TNF-α, (E) IL-1β and (F)
IL-6 concentrations using ELISAs. *P<0.05, **P<0.01 vs. DMEM
group. OxLDL, oxidized low-density lipoprotein; β2GPI, β2
glycoprotein I; LPS, lipopolysaccharide; RT-qPCR, reverse
transcription-quantitative PCR; HUVECs, human umbilical vein
endothelial cells; Ab, antibody. |
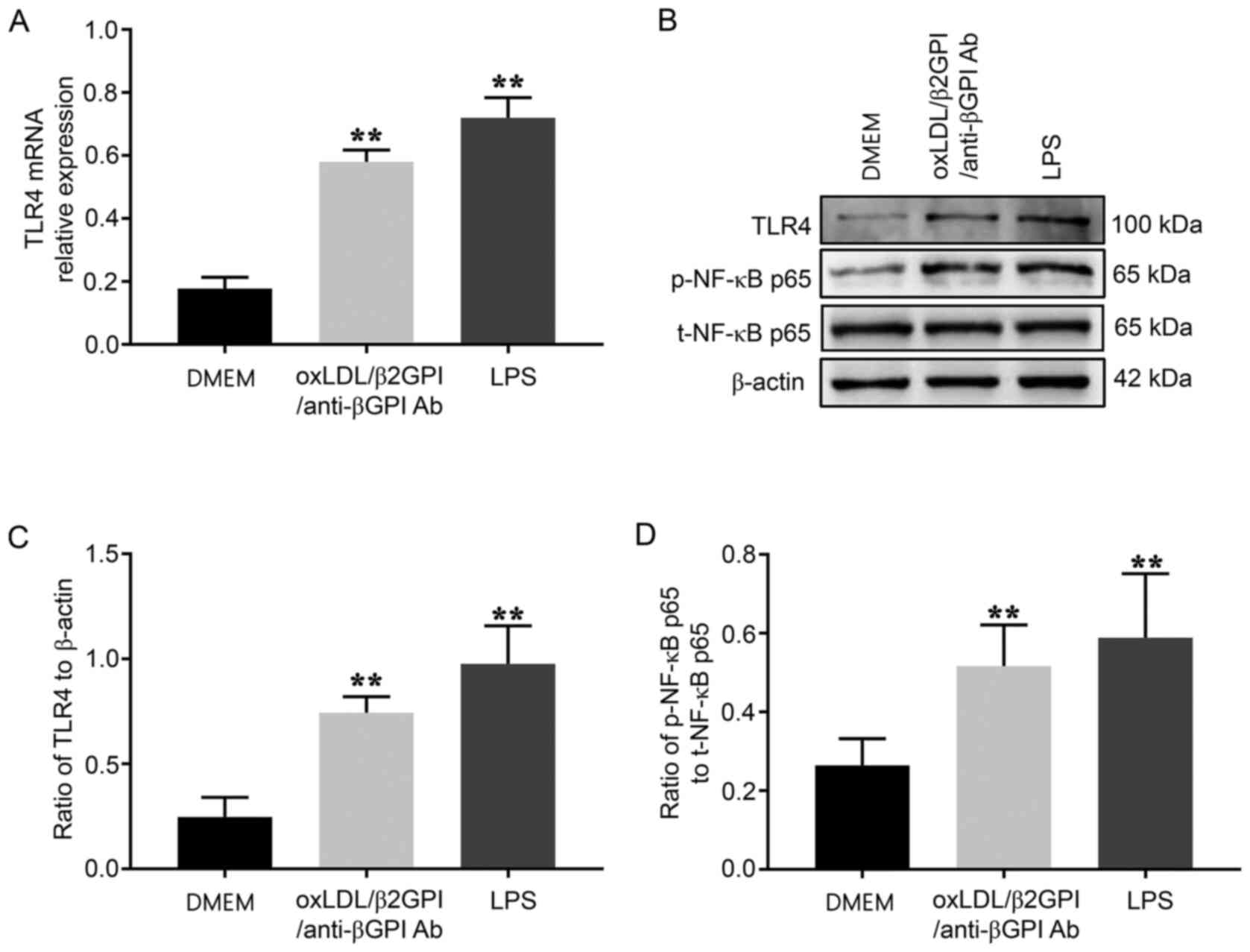
OxLDL/β2GPI/anti-β2GPI Ab complex
upregulates the expression of TLR4 and the phosphorylation of NF-κB
in HUVECs
TLR4 and NF-κB signaling pathways have been widely
confirmed to serve critical roles in the pathological process of AS
(22,29). In the present study, TLR4 expression
levels and the phosphorylation of NF-κB were analyzed during the
endothelial inflammation induced by the oxLDL/β2GPI/anti-β2GPI Ab
complex. The oxLDL/β2GPI/anti-β2GPI Ab complex significantly
upregulated the mRNA and protein expression levels of TLR4 in
HUVECs compared with the DMEM group, and a similar effect was
observed following LPS treatment (P<0.01; Fig. 2A and C). Moreover, the
phosphorylation levels of NF-κB p65 at Ser536 were significantly
increased following the incubation with the oxLDL/β2GPI/anti-β2GPI
Ab complex or LPS compared with the DMEM group (P<0.01; Fig. 2D). As the other down-stream members
of TLR4 signaling, AKT was also found to be phosphorylated in
oxLDL/β2GPI/anti-β2GPI Ab complex or LPS-treated HUVECs compared
with the DMEM group (P<0.05; Fig.
S2).
Involvement of the TLR4/NF-κB
signaling pathway in the oxLDL/β2GPI/anti-β2GPI Ab complex-induced
expression of inflammatory cytokines in HUVECs
To further determine the involvement of TLR4 and
NF-κB in the oxLDL/β2GPI/anti-β2GPI Ab complex-induced expression
of inflammatory cytokines in HUVECs, TAK-242 or PDTC were used to
inhibit their signal transduction, respectively. The results
demonstrated that the upregulated mRNA expression levels TNF-α,
IL-1β and IL-6 induced by the oxLDL/β2GPI/anti-β2GPI Ab complex
were blocked by pretreating the cells with TAK-242 or PDTC
(P<0.01; Fig. 3A-C). Identical
results were observed in the protein expression (P<0.01;
Fig. 3D-F). The inhibition of NF-κB
imposed weaker effects on abrogating the induction of IL-1β and
IL-6, as well as the protein secretion of TNF-α compared with the
inhibition of TLR4 (P<0.01; Fig. 3B,
D, E and F; P<0.05; Fig.
3C). Therefore, these results indicated that the blockade of
the TLR4/NF-κB signaling pathway may effectively inhibit the
expression levels of TNF-α, IL-1β and IL-6 induced by the
oxLDL/β2GPI/anti-β2GPI Ab complex.
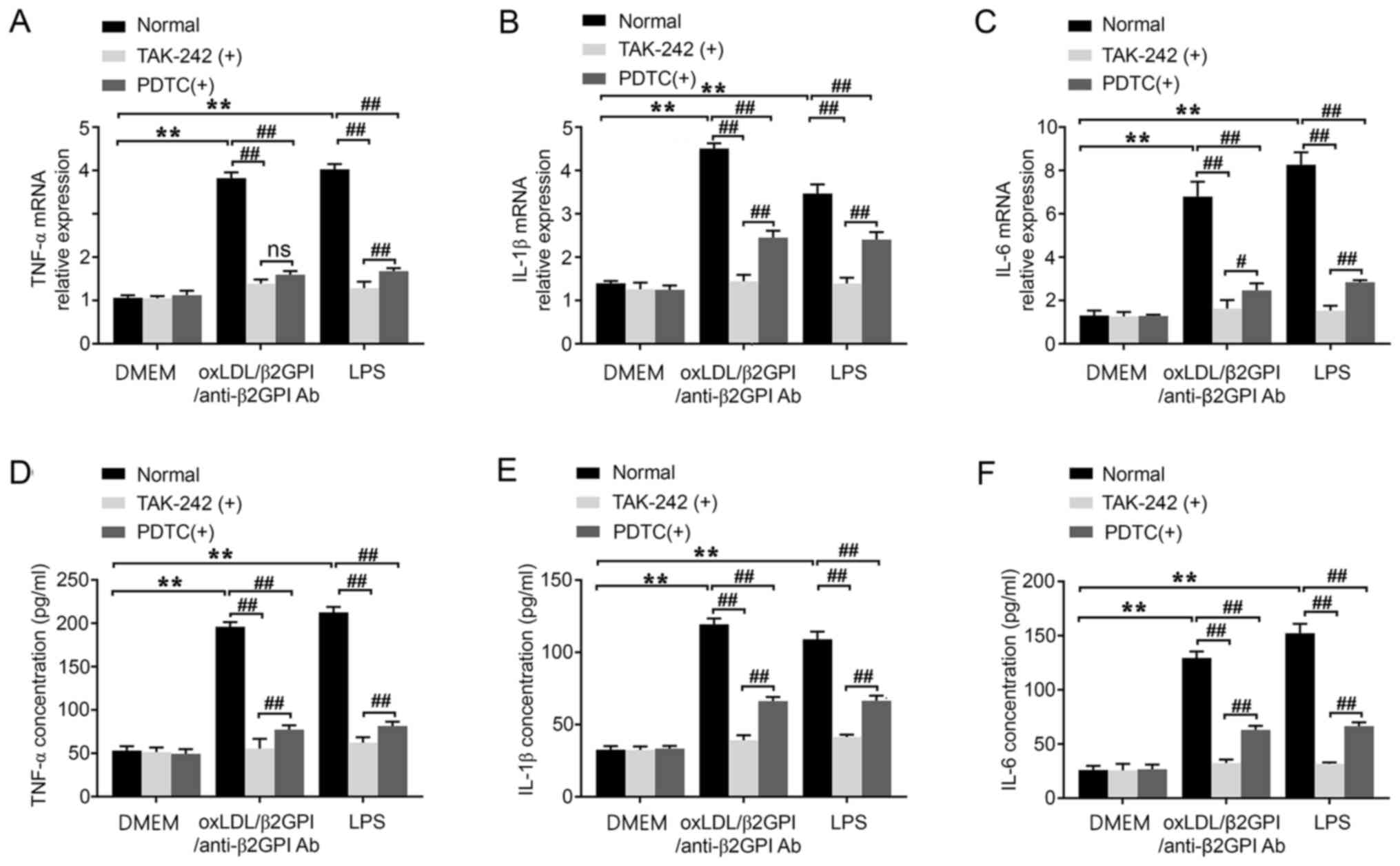 | Figure 3.Effects of the oxLDL/β2GPI/anti-β2GPI
Ab complex on the expression levels of the inflammatory cytokines,
TNF-α, IL-1β and IL-6, and the involvement of the Toll-like
receptor 4/NF-κB signaling pathway. HUVECs were treated with DMEM,
oxLDL/β2GPI/anti-β2GPI Ab complex and LPS with or without TAK-242
or PDTC pretreatment. Total RNA was extracted from HUVECs to
determine the mRNA expression levels of (A) TNF-α, (B) IL-1β and
(C) IL-6 using reverse transcription-quantitative PCR. The cell
culture supernatants of HUVECs were collected for analyzing the
secretion of (D) TNF-α, (E) IL-1β and (F) IL-6 using commercial
ELISA kits. **P<0.01; #P<0.05;
##P<0.01. OxLDL, oxidized low-density lipoprotein;
β2GPI, β2 glycoprotein I; LPS, lipopolysaccharide; HUVECs, human
umbilical vein endothelial cells; ns, not significant; Ab,
antibody; PDTC, 2-phosphonobutane-1,2,4,-tricarboxylic acid. |
OxLDL/β2GPI/anti-β2GPI Ab complex
induces the expression of adhesion molecules and the involvement of
the TLR4/NF-κB signaling pathway
Endothelial activation includes the expression of
adhesion molecules (ICAM-1 and VCAM-1) on cell surface (8). Adhesion molecules are essential in
endothelial inflammatory responses due to their ability to affect
leukocyte adherence and to activate the vascular endothelium, which
perpetuates a chronic proinflammatory state and fosters the
progression of atherosclerotic lesion (10,30).
Both oxLDL/β2GPI/anti-β2GPI Ab complex and LPS stimulation were
able to significantly upregulate the mRNA expression levels of
ICAM-1 and VCAM-1 compared with the DMEM group (P<0.01; Fig. 4A and B). Similarly, the protein
expression levels of ICAM-1 and VCAM-1 in presence of the
oxLDL/β2GPI/anti-β2GPI Ab complex and LPS were notably upregulated
compared with that in DMEM group (Fig.
4C and D).
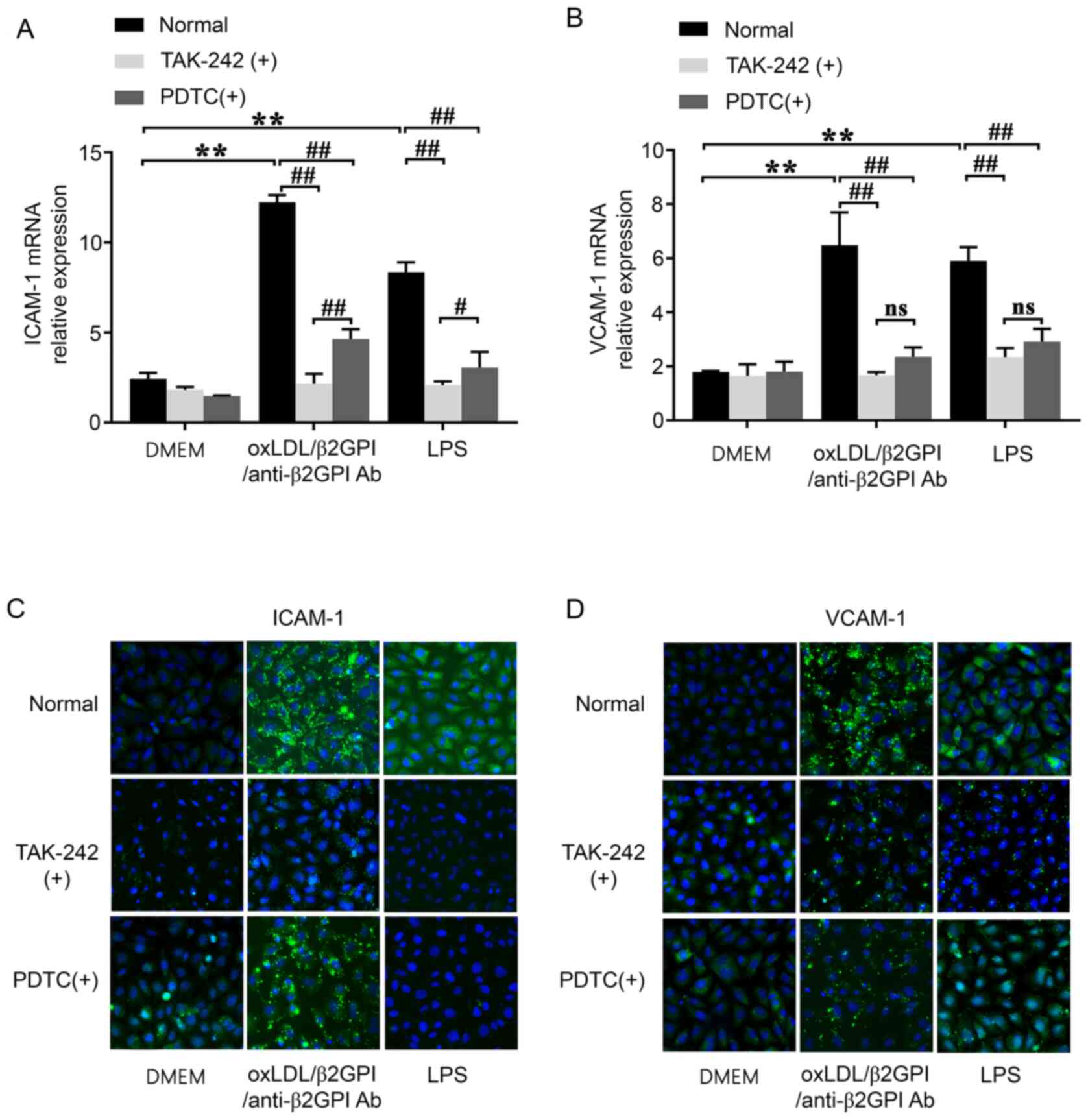 | Figure 4.Effects of the oxLDL/β2GPI/anti-β2GPI
Ab complex on the expression levels of the adhesion molecules,
ICAM-1 and VCAM-1, and the involvement of the Toll-like receptor
4/NF-κB signaling pathway. HUVECs were treated with DMEM,
oxLDL/β2GPI/anti-β2GPI Ab complex and LPS with or without TAK-242
or PDTC pretreatment. Total RNA was extracted from the cells to
analyze the mRNA expression levels of (A) ICAM-1 and (B) VCAM-1
using reverse transcription-quantitative PCR. Representative
immunofluorescence (green; magnification, ×200) was used to analyze
the protein expression levels of (C) ICAM-1 and (D) VCAM-1.
**P<0.01; #P<0.05; ##P<0.01. OxLDL,
oxidized low-density lipoprotein; β2GPI, β2 glycoprotein I; LPS,
lipopolysaccharide; ICAM-1, intercellular adhesion molecule-1;
VCAM-1, vascular adhesion molecule-1; HUVECs, human umbilical vein
endothelial cells; ns, not significant; Ab, antibody; PDTC,
2-phosphonobutane-1,2,4,-tricarboxylic acid. |
Subsequently, cells were pretreated with TAK-242 or
PDTC prior to the other treatments. The results demonstrated that
TAK-242 and PDTC pretreatment blocked the upregulation in the
expression levels of ICAM-1 and VCAM-1 induced by the
oxLDL/β2GPI/anti-β2GPI Ab complex or LPS compared with the groups
without inhibitors (P<0.01; Fig.
4). The effects produced by TAK-242 on the induction of ICAM-1
were found to be more significant compared with those induced by
PDTC (P<0.05; Fig. 4A), and
there was no difference between these two inhibitors pretreatment
on the induction of VCAM-1(P>0.05; Fig. 4B).
Involvement of the TLR4/NF-κB
signaling pathway in the oxLDL/β2GPI/anti-β2GPI Ab complex-induced
upregulation of MCP-1 expression and the adhesion of THP-1 to
HUVECs
MCP-1 has been discovered to trigger monocytes,
which circulate in the blood to aggravate vascular inflammation and
induce thrombosis in the plaque (31). To investigate whether the induction
of the oxLDL/β2GPI/anti-β2GPI Ab complex could increase monocyte
adhesion, the adhesion of Dil-stained THP-1 cells to HUVECs was
analyzed using an improved monocyte-EC adhesion assay, in addition
to evaluating the expression of MCP-1. The results indicated that
the oxLDL/β2GPI/anti-β2GPI Ab complex and LPS were able to
upregulate the mRNA and protein expression levels of MCP-1 compared
with the DMEM control group (P<0.01; Fig. 5A and B). Similarly, the adhesion of
THP-1 to HUVECs in the presence of the oxLDL/β2GPI/anti-β2GPI Ab
complex or LPS was significantly increased compared with the
DMEM-treated group (P<0.01; Fig. 5C
and D). Moreover, these effects were significantly attenuated
by the pretreatment with either TAK-242 or PDTC compared with the
groups without inhibitors (P<0.01; Fig. 5). However, the attenuation effects
of TAK-242 were found to be more significant than PDTC on the mRNA
expression of MCP-1 induced by oxLDL/β2GPI/anti-β2GPI Ab complex or
LPS, as well as the protein secretion of MCP-1 induced by LPS
(P<0.01; Fig. 5A and B).
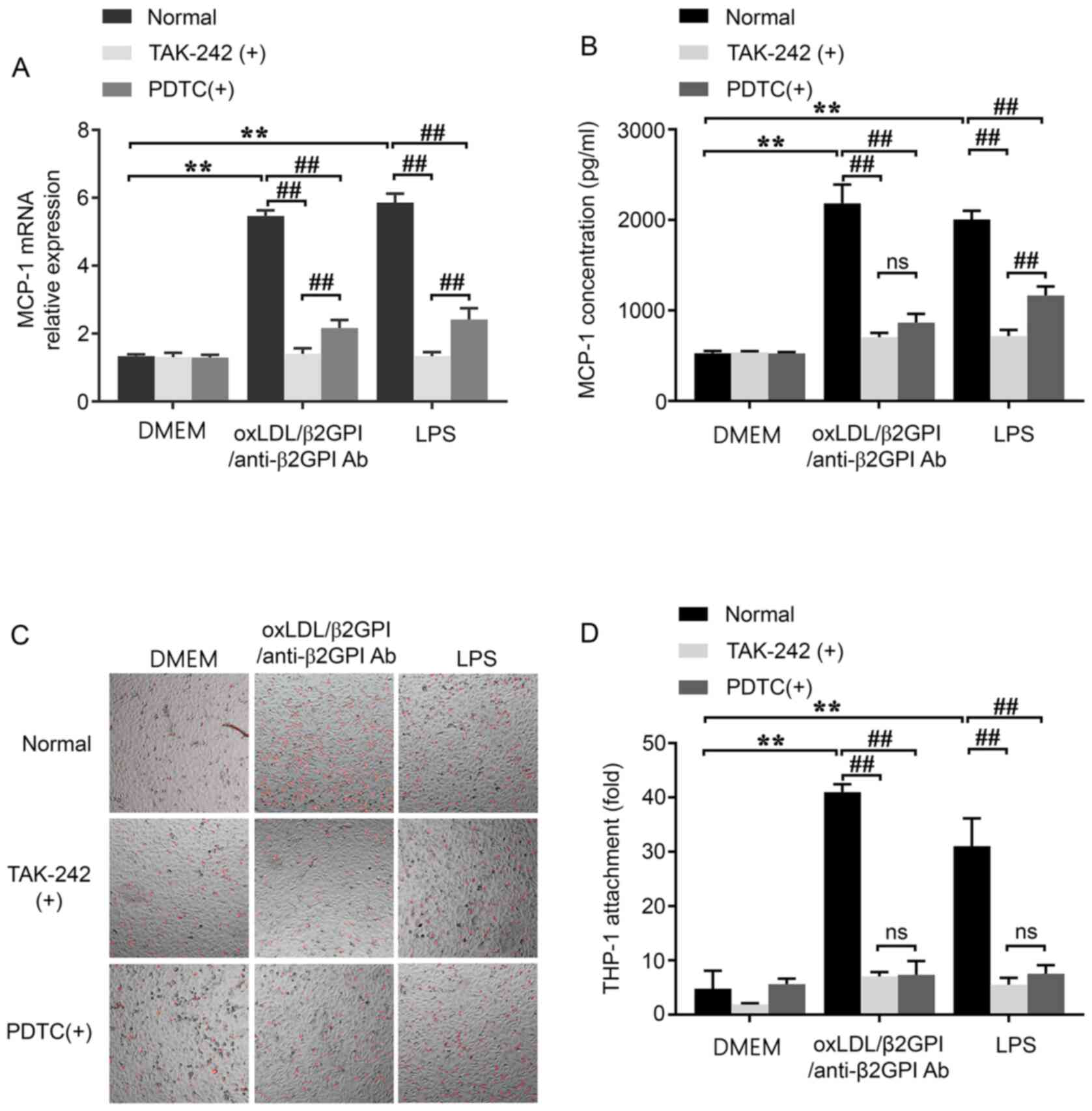 | Figure 5.Effects of the oxLDL/β2GPI/anti-β2GPI
Ab complex on the expression of MCP-1 and the adhesion of THP-1 to
HUVECs, and the involvement of the Toll-like receptor 4/NF-κB
signaling pathway. HUVECs were stimulated with DMEM,
oxLDL/β2GPI/anti-β2GPI Ab complex or LPS pretreated with or without
TAK-242 or PDTC. (A) mRNA expression levels of MCP-1 were analyzed
using reverse transcription-quantitative PCR. (B) Protein
expression levels of MCP-1 were detected using commercial ELISAs.
(C) Monocyte recruitment ability of HUVECs were detected using a
Dil-stained monocyte-EC adhesion assay (magnification, ×100) and
the (D) results were quantified. **P<0.01;
##P<0.01. OxLDL, oxidized low-density lipoprotein;
β2GPI, β2 glycoprotein I; Ab, antibody; LPS, lipopolysaccharide;
MCP-1, monocyte chemoattractant protein 1; ns, not significant;
HUVECs, human umbilical vein endothelial cells; PDTC,
2-phosphonobutane-1,2,4,-tricarboxylic acid. |
Discussion
According to the increasing basic and clinical
research conducted on AS, autoimmunity has been confirmed as an
important factor in its pathogenesis (5,32). An
accumulating number of studies have suggested that APS,
characterized by the presence of a group of heterogeneous
autoantibodies and by the occurrence of thromboembolic
complications in the arterial and/or venous vasculature, may be a
potential cause of the accelerated progression and eventual
mortality in patients with AS (33,34).
Furthermore, the oxLDL/β2GPI/anti-oxLDL/β2GPI complex was
identified as the circulating immune complex that exerts a
proatherogenic effect in patients with APS (35). Our previous studies also discovered
that the oxLDL/β2GPI/anti-β2GPI Ab complex increased the conversion
of macrophages into foam cells and induced the phenotypic changes,
proliferation and apoptosis of VSMCs (24,26).
However, to the best of our knowledge, the roles of the
oxLDL/β2GPI/anti-β2GPI Ab complex in the activation of endothelial
cells have not been well-defined.
Abnormal inflammatory activation and dysfunction in
the endothelium are recognized as early steps that contribute to
the development and exacerbation of AS (36). The inflammatory process involves the
increased expression of inflammatory cytokines, cell adhesion
molecules and chemokines (37).
TNF-α, which is predominantly released by endothelial cells,
monocytes and macrophages during the early stages of the
inflammatory response, was revealed to activate endothelial cells
and macrophages and lead to the release of other inflammatory
cytokines (38). In addition, IL-6
and IL-1β were found to regulate the expression of adhesion
molecules on endothelial cells and enhance the recruitment of
inflammatory cells to lesion sites in the early stages of the
development of AS (39,40).
In the present study, HUVECs were treated with DMEM,
oxLDL, oxLDL/β2GPI complex, oxLDL/anti-β2GPI Ab complex,
oxLDL/β2GPI/anti-β2GPI Ab complex or LPS. LPS was confirmed to
activate the vascular endothelium by affecting leukocyte adherence,
and it an established natural TLR4 agonist (17). The concentrations of inflammatory
cytokines (IL-1β, IL-6 and TNF-α) induced by the
oxLDL/β2GPI/anti-β2GPI Ab complex and LPS were increased compared
with the DMEM group, whereas the effects of the oxLDL/β2GPI complex
and oxLDL/anti-β2GPI Ab complex were relatively weaker. These
findings provided evidence to support the hypothesis that the
oxLDL/β2GPI/anti-β2GPI Ab complex may be able to promote the
inflammatory response in endothelial cells, and that it may share a
common signaling pathway with LPS. The present study also
identified that the upregulated expression levels of inflammatory
cytokines induced by the oxLDL/β2GPI/anti-β2GPI Ab complex could be
attenuated by the inhibition of either TLR4 or NF-κB. Based on
these results, it was suggested that the oxLDL/β2GPI/anti-β2GPI Ab
complex may promote the endothelial inflammatory response via the
TLR4-mediated intracellular signaling transduction pathway in the
pathological process of AS.
TLR4-mediated intracellular signaling transduction
is critical for inflammation and immune regulation, and it has been
indicted to regulate the expression of genes encoding numerous
important molecules involved in AS (41–43).
TLR4 is expressed in different cell types present in the
atherosclerotic plaque, among which it is expressed at low levels
in healthy endothelial cells, but its expression is upregulated in
human atherosclerotic lesions (44). The activation of the transcription
factor NF-κB is known to be involved in endothelial dysfunction and
the synthesis of inflammatory cytokines (45). In the present study, the activation
of the TLR4/NF-κB signaling pathway during the inflammatory process
was induced by the oxLDL/β2GPI/anti-β2GPI Ab complex in HUVECs. The
results demonstrated that the expression of TLR4 at both the mRNA
and protein levels, as well as the phosphorylation levels of NF-κB
were largely increased in the presence of the
oxLDL/β2GPI/anti-β2GPI Ab complex, and that the activation of NF-κB
was dependent on LPS-mediated TLR4 activation. However, the effects
of the oxLDL/β2GPI/anti-β2GPI complex and LPS were not completely
consistent. For instance, the oxLDL/β2GPI/anti-β2GPI complex
demonstrated weaker effects compared with LPS in inducing the
expression levels of TLR4 and IL-6 and the phosphorylation of
NF-κB, suggesting that TLR4-mediated NF-κB activation may be the
major signaling pathway of the oxLDL/β2GPI/anti-β2GPI complex,
thereby inducing the production of IL-6. By contrast, the effects
of the oxLDL/β2GPI/anti-β2GPI complex in inducing the production of
IL-1β, ICAM-1 and VCAM-1 were stronger compared with LPS,
indicating that other AS-related cell surface receptors, such as
TLR2, may also serve a role during the inflammatory activation of
endothelial cells induced by the oxLDL/β2GPI/anti-β2GPI complex, in
addition to TLR4.
As aforementioned, AS is characterized by vascular
endothelial inflammation, and the first stage involves circulating
monocytes adhering to the dysfunctional endothelium and migrating
across into the sub-endothelial space, where they differentiate
into macrophages (8). The concerted
actions of the activated endothelial cells, monocytes and
macrophages result in the production of a complex paracrine milieu
of cytokines, which perpetuates a chronic proinflammatory state and
fosters the progression of atherosclerotic lesion (8,46). The
cell adhesion molecules, such as ICAM-1 and VCAM-1, have been
identified to mediate the binding of leukocytes to vascular
endothelial cells via very late activation antigen-4 (VLA-4) and
lymphocyte function associated antigen-1 (LFA-1) (30,47).
The present study demonstrated that the upregulated expression
levels of ICAM-1 and VCAM-1 appeared to be closely associated with
the oxLDL/β2GPI/anti-β2GPI Ab complex, indicating a potential
central regulatory role of the oxLDL/β2GPI/anti-β2GPI Ab complex in
the release of these cell adhesion molecules.
The involvement of TLR4 and NF-κB in the
oxLDL/β2GPI/anti-β2GPI Ab complex-induced release of adhesion
molecules was investigated in the current study using of TAK-242
and PDTC pretreatment. TAK-242 is a novel cyclohexene that blocks
the signaling mediated by the intracellular domain of TLR4
(48), while PDTC inhibits the
activation of NF-κB by suppressing both NF-κB DNA binding and
NF-κB-dependent transcriptional activity (49). TAK-242 and PDTC could largely
attenuate the effects of the oxLDL/β2GPI/anti-β2GPI Ab complex on
the expression levels of ICAM-1 and VCAM-1, suggesting that TLR4
and NF-κB may contributes to the signaling pathways that mediate
endothelial inflammation induced by the oxLDL/β2GPI/anti-β2GPI Ab
complex. Furthermore, the effects produced by TAK-242 on the
induction of IL-1β and IL-6 and the protein secretion of TNF-α were
found to be more significant compared with those induced by PDTC.
Thus, it should be examined whether, in addition to NF-κB, other
signaling pathways regulated by TLR4 serve a role in this process.
The oxLDL/β2GPI/anti-β2GPI Ab complex induced the phosphorylation
of AKT by HUVECs, which indicated that AKT, or other TLR4-related
signaling pathways, such as p38 MAPK and ERK1/2 (50), may also be involved in the
activation of endothelial cells induced by the
oxLDL/β2GPI/anti-β2GPI Ab complex. These results may explain why
the effects produced by TAK-242 are more significant compared with
those produced by PDTC; however, the relevant mechanisms required
further investigation.
Monocytes adhere and migrate into the
sub-endothelial space in response to chemotactic signals, where
they differentiate into macrophages and then transform into
lipid-loaded foam cells (30,31).
MCP-1 is a well-known chemotactic cytokine that regulates the
recruitment of mononuclear inflammatory cells during the process of
AS (31). As identified in the
current study, both the oxLDL/β2GPI/anti-β2GPI Ab complex and LPS
treatment upregulated the mRNA and protein expression levels of
MCP-1. Similarly, the number of THP-1 cells bound to the HUVECs
monolayer was increased by the oxLDL/β2GPI/anti-β2GPI Ab complex
and LPS treatment. Notably, the effects of the
oxLDL/β2GPI/anti-β2GPI Ab complex were stronger compared with LPS
treatment. These results are consistent with the effects of the
oxLDL/β2GPI/anti-β2GPI Ab complex on the expression of IL-1β, ICAM1
and VCAM-1. Furthermore, the effects of the oxLDL/β2GPI/anti-β2GPI
Ab complex were found to be impaired by the inhibition of TLR4 or
NF-κB. These results suggested that the oxLDL/β2GPI/anti-β2GPI Ab
complex may promote the recruitment of monocytes to endothelial
cells via the TLR4/NF-κB signaling pathway.
In conclusion, the present study demonstrated that
the oxLDL/β2GPI/anti-β2GPI Ab complex significantly upregulated the
expression levels of proinflammatory cytokines and pro-adhesive
molecules in HUVECs, as well as enhancing their ability to recruit
monocytes. Moreover, the TLR4/NF-κB signaling pathway was indicated
to be involved in these processes. However, the present study has
limitations. In addition to TLR4, the other endothelial cell
surface receptors, including TLR2 and Fcγ, may also be involved in
the oxLDL/β2GPI/anti-β2GPI complex-induced endothelial activation,
should be further determined to clarify the results of the present
study. Overall, the available results suggested that autoimmune
factors may be potential molecular targets for the therapeutic
intervention at the early vascular inflammatory stage of patients
with AS with an autoimmune background. However, further studies are
required to clarify the underlying mechanisms.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science
Foundation of China (grant no. 81370614), the Youth Fund of Jiangsu
Province (grant no. BK20150532) and the Project of Natural Science
in Colleges and Universities of Jiangsu Province (grant no.
15KJB310002).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
HZ and JY conceived and designed the study. GZ and
QC performed the experiments. CH and YC collected the experimental
results. PZ, LX and TW analyzed and interpreted the experimental
data. GZ drafted and revised the article. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
APS
|
antiphospholipid syndrome
|
|
AS
|
atherosclerosis
|
|
oxLDL
|
oxidized low-density lipoprotein
|
|
β2GPI
|
β2 glycoprotein I
|
|
anti-β2GPI Ab
|
anti-β2 glycoprotein I antibody
|
|
VSMCs
|
vascular smooth muscle cells
|
|
TLR4
|
Toll-like receptor 4
|
|
HUVECs
|
human umbilical vein cells
|
|
ICAM-1
|
intercellular adhesion molecule-1
|
|
VCAM-1
|
vascular adhesion molecule-1
|
|
MCP-1
|
monocyte chemoattractant protein 1
|
|
LPS
|
lipopolysaccharide
|
References
|
1
|
Glass CK and Witztum JL: Atherosclerosis.
The road ahead. Cell. 104:503–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mozaffarian D, Benjamin EJ, Go AS, Arnett
DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ,
Howard VJ, et al: Heart disease and stroke statistics-2015 update:
A report from the American Heart Association. Circulation.
131:e29–e322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hansson GK, Libby P and Tabas I:
Inflammation and plaque vulnerability. J Intern Med. 278:483–493.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsuura E, Kobayashi K, Koike T and
Shoenfeld Y: Autoantibody-mediated atherosclerosis. Autoimmun Rev.
1:348–353. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shoenfeld Y, Gerli R, Doria A, Matsuura E,
Cerinic MM, Ronda N, Jara LJ, Abu-Shakra M, Meroni PL and Sherer Y:
Accelerated atherosclerosis in autoimmune rheumatic diseases.
Circulation. 112:3337–3347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benagiano M, Gerosa M, Romagnoli J, Mahler
M, Borghi MO, Grassi A, Della Bella C, Emmi G, Amedei A, Silvestri
E, et al: β2 Glycoprotein I recognition drives Th1 inflammation in
atherosclerotic plaques of patients with primary antiphospholipid
syndrome. J Immunol. 198:2640–2648. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Onat D, Brillon D, Colombo PC and Schmidt
AM: Human vascular endothelial cells: A model system for studying
vascular inflammation in diabetes and atherosclerosis. Curr Diab
Rep. 11:193–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gimbrone MA Jr and García-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berg AH and Scherer PE: Adipose tissue,
inflammation, and cardiovascular disease. Circ Res. 96:939–949.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lawson C and Wolf S: ICAM-1 signaling in
endothelial cells. Pharmacol Rep. 61:22–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen B, Meng L, Shen T, Gong H, Qi R, Zhao
Y, Sun J, Bao L and Zhao G: Thioredoxin attenuates oxidized
low-density lipoprotein induced oxidative stress in human umbilical
vein endothelial cells by reducing NADPH oxidase activity. Biochem
Biophys Res Commun. 490:1326–1333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Profumo E, Buttari B, Alessandri C, Conti
F, Capoano R, Valesini G, Salvati B and Riganò R:
Beta2-glycoprotein I is a target of T cell reactivity in patients
with advanced carotid atherosclerotic plaques. Int J Immunopathol
Pharmacol. 23:73–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kasahara J, Kobayashi K, Maeshima Y,
Yamasaki Y, Yasuda T, Matsuura E and Makino H: Clinical
significance of serum oxidized low-density
lipoprotein/beta2-glycoprotein I complexes in patients with chronic
renal diseases. Nephron Clin Pract. 98:c15–c24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu R, Yuan Y, Niu D, Song J, Liu T, Wu J
and Wang J: Elevated beta2-glycoprotein I-low-density lipoprotein
levels are associated with the presence of diabetic microvascular
complications. J Diabetes Complications. 29:59–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Zhu X, Zhou H, Xia L and Wang T,
Wang Z, Li Y, Yan J and Wang T: Anti-β2GPI antibodies enhance
atherosclerosis in ApoE-deficient mice. Biochem Biophys Res Commun.
512:72–78. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang T, Ouyang H, Zhou H, Xia L, Wang X
and Wang T: Pro-atherogenic activation of A7r5 cells induced by the
oxLDL/β2GPI/anti-β2GPI complex. Int J Mol Med. 42:1955–1966.
2018.PubMed/NCBI
|
|
17
|
Bierhaus A, Chen J, Liliensiek B and
Nawroth PP: LPS and cytokine-activated endothelium. Semin Thromb
Hemost. 26:571–587. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lundberg AM and Hansson GK: Innate immune
signals in atherosclerosis. Clin Immunol. 134:5–24. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pierangeli SS, Vega-Ostertag ME, Raschi E,
Liu X, Romay-Penabad Z, De Micheli V, Galli M, Moia M, Tincani A,
Borghi MO, et al: Toll-like receptor and antiphospholipid mediated
thrombosis: In vivo studies. Ann Rheum Dis. 66:1327–1333. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brandt KJ, Kruithof EK and de Moerloose P:
Receptors involved in cell activation by antiphospholipid
antibodies. Thromb Res. 132:408–413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie H, Sheng L, Zhou H and Yan J: The role
of TLR4 in pathophysiology of antiphospholipid syndrome-associated
thrombosis and pregnancy morbidity. Br J Haematol. 164:165–176.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roshan MH, Tambo A and Pace NP: The Role
of TLR2, TLR4, and TLR9 in the Pathogenesis of Atherosclerosis. Int
J Inflamm. 2016:15328322016.
|
|
23
|
Ostuni R, Zanoni I and Granucci F:
Deciphering the complexity of Toll-like receptor signaling. Cell
Mol Life Sci. 67:4109–4134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang T, Zhou H, Chen Y, Zhang P and Wang
T: The biphasic effects of the oxLDL/β2 GPI/anti-β2 GPI complex on
VSMC proliferation and apoptosis. Cell Signal. 57:29–44. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Y, Kong X, Zhou H, Zhang X, Liu J, Yan
J, Xie H and Xie Y: oxLDL/β2GPI/anti-β2GPI complex induced
macrophage differentiation to foam cell involving TLR4/NF-kappa B
signal transduction pathway. Thromb Res. 134:384–392. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang R, Zhou SJ, Li CJ, Wang XN, Tang YZ,
Chen R, Lv L, Zhao Q, Xing QL, Yu DM and Yu P: C-reactive
protein/oxidised low-density lipoprotein/beta2-glycoprotein I
complex promotes atherosclerosis in diabetic BALB/c mice via
p38mitogen-activated protein kinase signal pathway. Lipids Health
Dis. 12:422013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lopez LR, Kobayashi K, Matsunami Y and
Matsuura E: Immunogenic oxidized low-density
lipoprotein/beta2-glycoprotein I complexes in the diagnostic
management of atherosclerosis. Clin Rev Allergy Immunol. 37:12–19.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Galkina E and Ley K: Vascular adhesion
molecules in atherosclerosis. Arterioscler Thromb Vasc Biol.
27:2292–2301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin J, Kakkar V and Lu X: Impact of MCP-1
in atherosclerosis. Curr Pharm Des. 20:4580–4588. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsuura E, Atzeni F, Sarzi-Puttini P,
Turiel M, Lopez LR and Nurmohamed MT: Is atherosclerosis an
autoimmune disease? BMC Med. 12:472014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Denas G, Jose SP, Bracco A, Zoppellaro G
and Pengo V: Antiphospholipid syndrome and the heart: A case series
and literature review. Autoimmun Rev. 14:214–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hughes GR, Harris NN and Gharavi AE: The
anticardiolipin syndrome. J Rheumatol. 13:486–489. 1986.PubMed/NCBI
|
|
35
|
Bassi N, Ghirardello A, Iaccarino L,
Zampieri S, Rampudda ME, Atzeni F, Sarzi-Puttini P, Shoenfeld Y and
Doria A: OxLDL/beta2GPI-anti-oxLDL/beta2GPI complex and
atherosclerosis in SLE patients. Autoimmun Rev. 7:52–58. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tuttolomondo A, Di Raimondo D, Pecoraro R,
Arnao V, Pinto A and Licata G: Atherosclerosis as an inflammatory
disease. Curr Pharm Des. 18:4266–4288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zernecke A and Weber C: Inflammatory
mediators in atherosclerotic vascular disease. Basic Res Cardiol.
100:93–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ohta H, Wada H, Niwa T, Kirii H, Iwamoto
N, Fujii H, Saito K, Sekikawa K and Seishima M: Disruption of tumor
necrosis factor-alpha gene diminishes the development of
atherosclerosis in ApoE-deficient mice. Atherosclerosis. 180:11–17.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schuett H, Luchtefeld M, Grothusen C,
Grote K and Schieffer B: How much is too much? Interleukin-6 and
its signalling in atherosclerosis. Thromb Haemost. 102:215–222.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Grebe A, Hoss F and Latz E: NLRP3
Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ Res.
122:1722–1740. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rocha DM, Caldas AP, Oliveira LL, Bressan
J and Hermsdorff HH: Saturated fatty acids trigger TLR4-mediated
inflammatory response. Atherosclerosis. 244:211–215. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Higashimori M, Tatro JB, Moore KJ,
Mendelsohn ME, Galper JB and Beasley D: Role of toll-like receptor
4 in intimal foam cell accumulation in apolipoprotein E-deficient
mice. Arterioscler Thromb Vasc Biol. 31:50–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Płóciennikowska A, Hromada-Judycka A,
Borzęcka K and Kwiatkowska K: Co-operation of TLR4 and raft
proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life
Sci. 72:557–581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Edfeldt K, Swedenborg J, Hansson GK and
Yan ZQ: Expression of toll-like receptors in human atherosclerotic
lesions: A possible pathway for plaque activation. Circulation.
105:1158–1161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kempe S, Kestler H, Lasar A and Wirth T:
NF-kappaB controls the global pro-inflammatory response in
endothelial cells: Evidence for the regulation of a pro-atherogenic
program. Nucleic Acids Res. 33:5308–5319. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tousoulis D, Kampoli AM, Papageorgiou N,
Androulakis E, Antoniades C, Toutouzas K and Stefanadis C:
Pathophysiology of atherosclerosis: The role of inflammation. Curr
Pharm Des. 17:4089–4110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Scott DW, Dunn TS, Ballestas ME, Litovsky
SH and Patel RP: Identification of a high-mannose ICAM-1 glycoform:
Effects of ICAM-1 hypoglycosylation on monocyte adhesion and
outside in signaling. Am J Physiol Cell Physiol. 305:C228–C237.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kawamoto T, Ii M, Kitazaki T, Iizawa Y and
Kimura H: TAK-242 selectively suppresses Toll-like receptor
4-signaling mediated by the intracellular domain. Eur J Pharmacol.
584:40–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Németh ZH, Deitch EA, Szabó C and Haskó G:
Pyrrolidinedithiocarbamate inhibits NF-kappaB activation and IL-8
production in intestinal epithelial cells. Immunol Lett. 85:41–46.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tsai CS, Huang CY, Chen CH, Lin YW, Shih
CM, Tsao NW, Chiang KH, Lee CY, Jeng H and Lin FY: Eotaxin-2
increased toll-like receptor 4 expression in endothelial cells in
vitro and exacerbates high-cholesterol diet-induced atherogenesis
in vivo. Am J Transl Res. 8:5338–5353. 2016.PubMed/NCBI
|