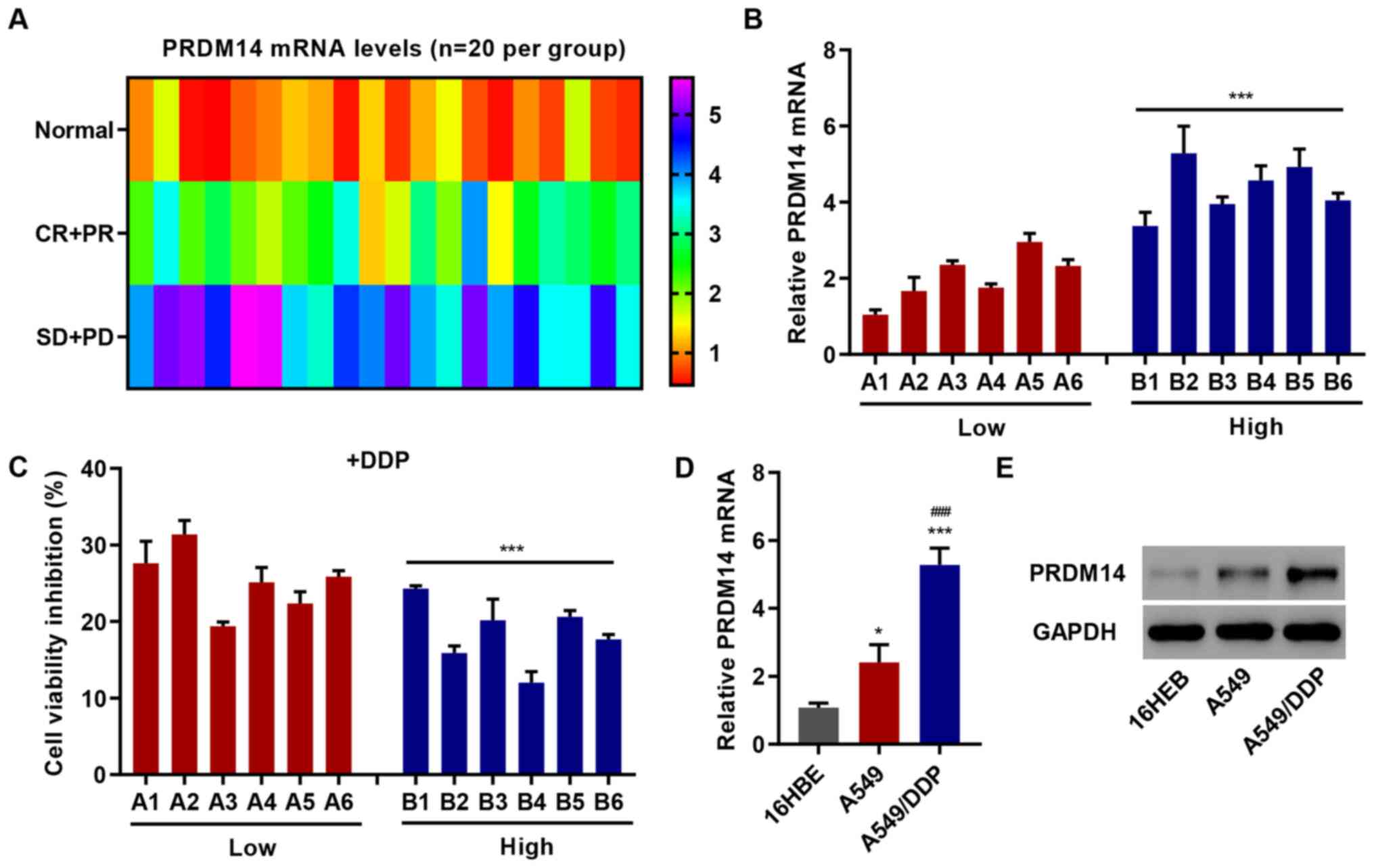
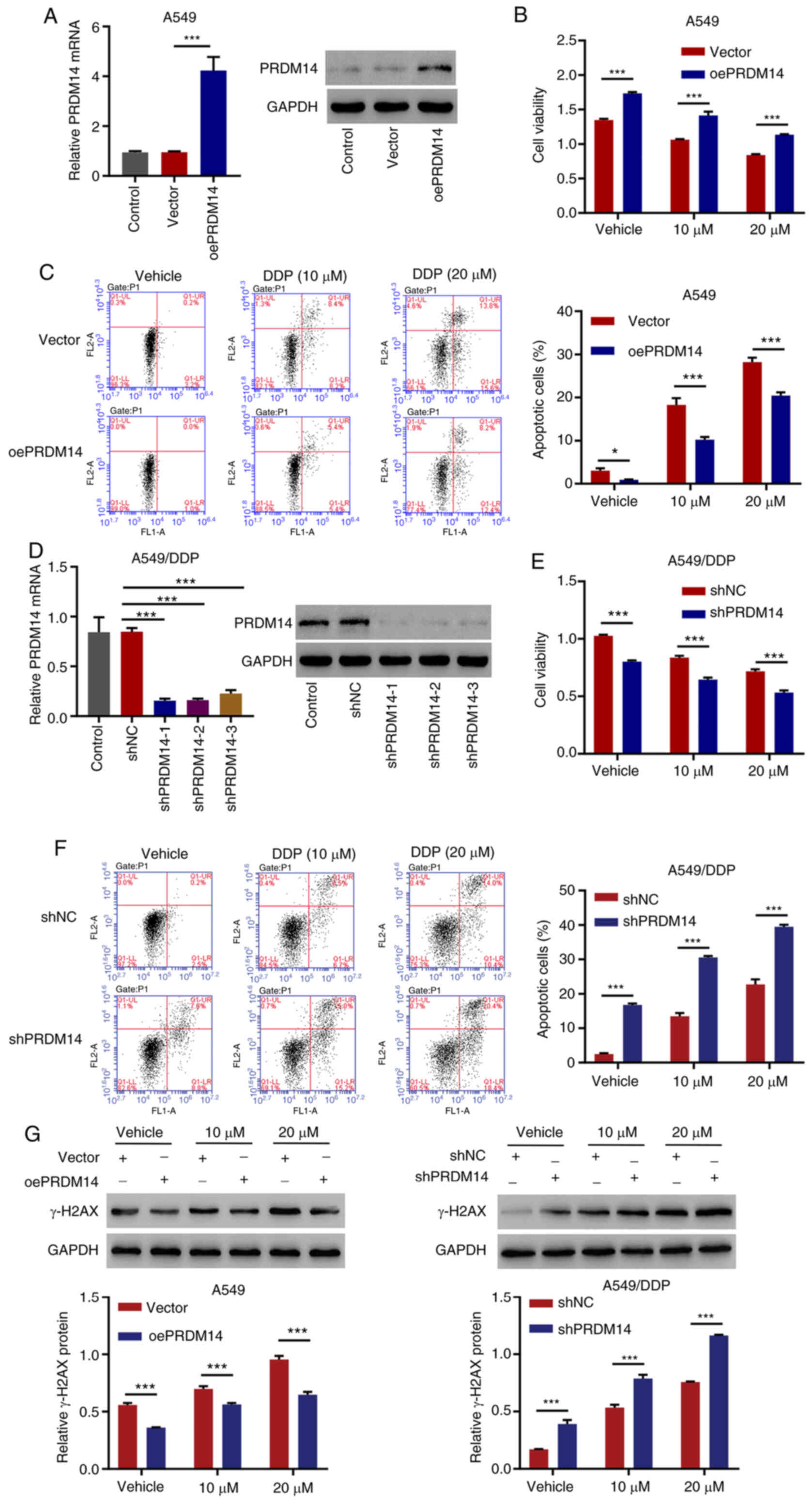
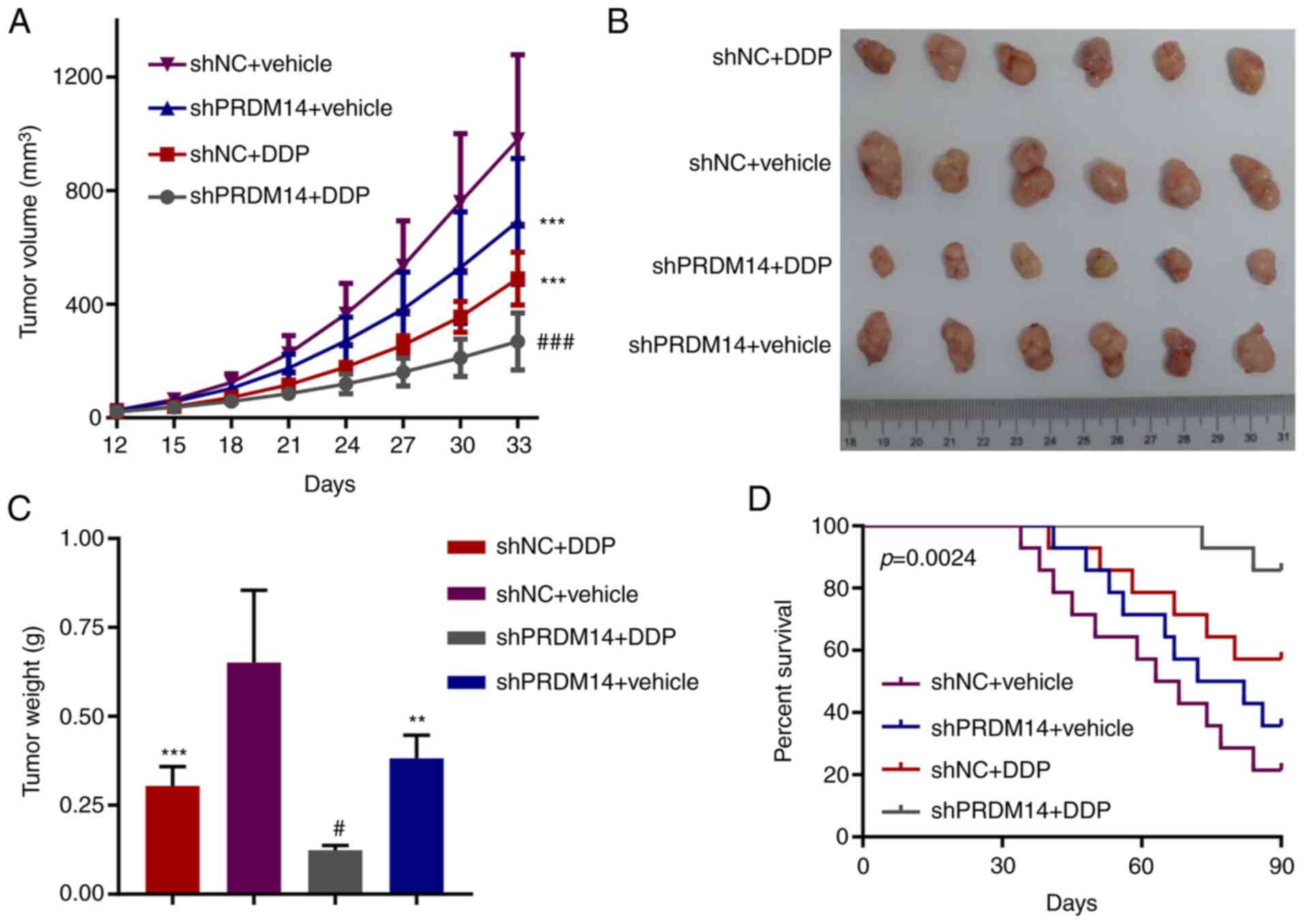
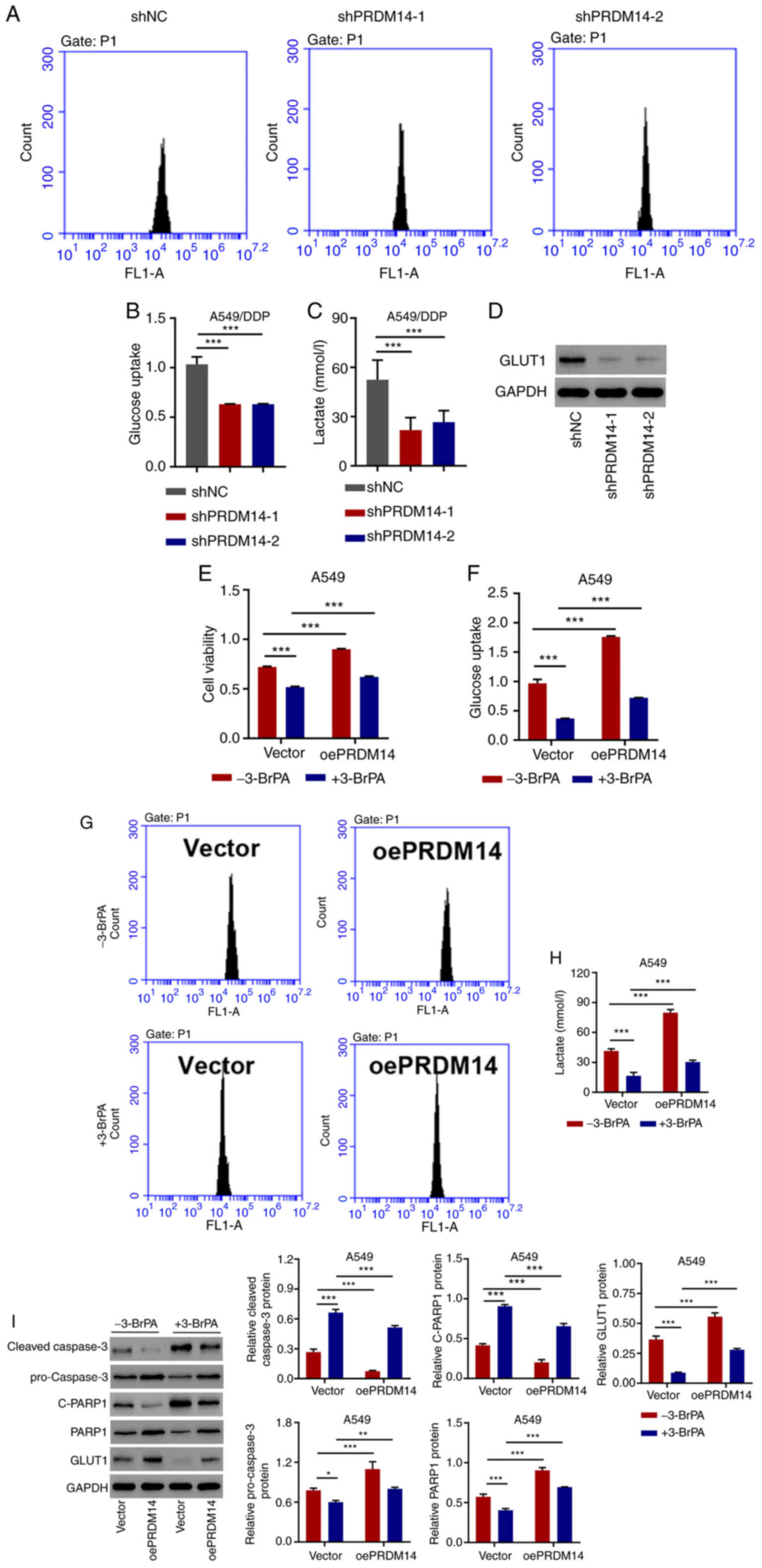
|
1
|
Pan X, Chen Y, Shen Y and Tantai J:
Knockdown of TRIM65 inhibits autophagy and cisplatin resistance in
A549/DDP cells by regulating miR-138-5p/ATG7. Cell Death Dis.
10:4292019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stankovic B, Bjørhovde HAK, Skarshaug R,
Aamodt H, Frafjord A, Müller E, Hammarström C, Beraki K, Bækkevold
ES, Woldbæk PR, et al: Immune cell composition in human non-small
cell lung cancer. Front Immunol. 9:31012018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rossi A and Di Maio M: Platinum-based
chemotherapy in advanced non-small-cell lung cancer: Optimal number
of treatment cycles. Expert Rev Anticancer Ther. 16:653–660. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li FL, Liu JP, Bao RX, Yan GQ, Feng X, Xu
YP, Sun YP, Yan W, Ling ZQ, Xiong Y, et al: Acetylation accumulates
PFKFB3 in cytoplasm to promote glycolysis and protects cells from
cisplatin-induced apoptosis. Nat Commun. 9:5082018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bose S and Le A: Glucose metabolism in
cancer. Adv Exp Med Biol. 1063:3–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JH, Nam B, Choi YJ, Kim SY, Lee JE,
Sung KJ, Kim WS, Choi CM, Chang EJ, Koh JS, et al: Enhanced
glycolysis supports cell survival in EGFR-Mutant lung
adenocarcinoma by inhibiting autophagy-mediated EGFR degradation.
Cancer Res. 78:4482–4496. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang W, Bouchard G, Yu A, Shafiq M,
Jamali M, Shrager JB, Ayers K, Bakr S, Gentles AJ, Diehn M, et al:
GFPT2-expressing cancer-associated fibroblasts mediate metabolic
reprogramming in human lung adenocarcinoma. Cancer Res.
78:3445–3457. 2018.PubMed/NCBI
|
|
10
|
Higashi K, Yamagishi T, Ueda Y, Ishigaki
Y, Shimasaki M, Nakamura Y, Oguchi M, Takegami T, Sagawa M and
Tonami H: Correlation of HIF-1α/HIF-2α expression with FDG uptake
in lung adenocarcinoma. Ann Nucl Med. 30:708–715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huber SM, Misovic M, Mayer C, Rodemann HP
and Dittmann K: EGFR-mediated stimulation of sodium/glucose
cotransport promotes survival of irradiated human A549 lung
adenocarcinoma cells. Radiother Oncol. 103:373–379. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ciribilli Y, Singh P, Inga A and Borlak J:
c-Myc targeted regulators of cell metabolism in a transgenic mouse
model of papillary lung adenocarcinoma. Oncotarget. 7:65514–65539.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gong T, Cui L, Wang H, Wang H and Han N:
Knockdown of KLF5 suppresses hypoxia-induced resistance to
cisplatin in NSCLC cells by regulating HIF-1α-dependent glycolysis
through inactivation of the PI3K/Akt/mTOR pathway. J Transl Med.
16:1642018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Lu F, Gong Y, Zhao C, Pan Q,
Ballantyne S, Zhao X, Tian S and Chen H: High expression of
synthesis of cytochrome c oxidase 2 and TP53-induced glycolysis and
apoptosis regulator can predict poor prognosis in human lung
adenocarcinoma. Hum Pathol. 77:54–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Zhang Z and Yu Z: Identification
of a novel glycolysis-related gene signature for predicting
metastasis and survival in patients with lung adenocarcinoma. J
Transl Med. 17:4232019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan SX, Hu RC, Xia Q, Tan YL, Liu JJ, Gan
GX and Wang LL: The methylation profiles of PRDM promoters in
non-small cell lung cancer. Onco Targets Ther. 11:2991–3002. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fog CK, Galli GG and Lund AH: PRDM
proteins: Important players in differentiation and disease.
Bioessays. 34:50–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hohenauer T and Moore AW: The prdm family:
Expanding roles in stem cells and development. Development.
139:2267–2282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taniguchi H and Imai K: PRDM14, a zinc
finger protein, regulates cancer stemness. Methods Mol Biol.
1867:3–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bi HX, Shi HB, Zhang T and Cui G: PRDM14
promotes the migration of human non-small cell lung cancer through
extracellular matrix degradation in vitro. Chin Med J (Engl).
128:373–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taniguchi H and Imai K: Silencing PRDM14
via oligonucleotide therapeutics suppresses tumorigenicity and
metastasis of breast cancer. Methods Mol Biol. 1974:233–243. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tuthill MH, Montinaro A, Zinngrebe J,
Prieske K, Draber P, Prieske S, Newsom-Davis T, von Karstedt S,
Graves J and Walczak H: TRAIL-R2-specific antibodies and
recombinant TRAIL can synergise to kill cancer cells. Oncogene.
34:2138–2144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiong S, Zheng Y, Jiang P, Liu R, Liu X
and Chu Y: MicroRNA-7 inhibits the growth of human non-small cell
lung cancer A549 cells through targeting BCL-2. Int J Biol Sci.
7:805–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakurai Y, Ichinoe M, Yoshida K, Nakazato
Y, Saito S, Satoh M, Nakada N, Sanoyama I, Umezawa A, Numata Y, et
al: Inactivation of REV7 enhances chemosensitivity and overcomes
acquired chemoresistance in testicular germ cell tumors. Cancer
Lett. 489:100–110. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki S, Okada M, Takeda H, Kuramoto K,
Sanomachi T, Togashi K, Seino S, Yamamoto M, Yoshioka T and
Kitanaka C: Involvement of GLUT1-mediated glucose transport and
metabolism in gefitinib resistance of non-small-cell lung cancer
cells. Oncotarget. 9:32667–32679. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan T, Sun G, Sun X, Zhao L, Zhong R and
Peng Y: Tumor energy metabolism and potential of 3-bromopyruvate as
an inhibitor of aerobic glycolysis: Implications in tumor
treatment. Cancers (Basel). 11:3172019. View Article : Google Scholar
|
|
28
|
Zhang Q, Pan J, North PE, Yang S, Lubet
RA, Wang Y and You M: Aerosolized 3-bromopyruvate inhibits lung
tumorigenesis without causing liver toxicity. Cancer Prev Res
(Phila). 5:717–725. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Igarashi H, Taniguchi H, Nosho K, Ishigami
K, Koide H, Mitsuhashi K, Okita K, Takemasa I, Imai K and Nakase H:
PRDM14 promotes malignant phenotype and correlates with poor
prognosis in colorectal cancer. Clin Transl Oncol. 22:1126–1137.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moriya C, Taniguchi H, Miyata K, Nishiyama
N, Kataoka K and Imai K: Inhibition of PRDM14 expression in
pancreatic cancer suppresses cancer stem-like properties and liver
metastasis in mice. Carcinogenesis. 38:638–648. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang T, Ye X, Yan D, Deng C, Li Z and
Tian B: FAM46B promotes apoptosis and inhibits glycolysis of
prostate cancer through inhibition of the MYC-LDHA axis. Onco
Targets Ther. 13:8771–8782. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heydarzadeh S, Moshtaghie AA, Daneshpoor M
and Hedayati M: Regulators of glucose uptake in thyroid cancer cell
lines. Cell Commun Signal. 18:832020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chakraborty PK, Mustafi SB, Xiong X,
Dwivedi SKD, Nesin V, Saha S, Zhang M, Dhanasekaran D, Jayaraman M,
Mannel R, et al: MICU1 drives glycolysis and chemoresistance in
ovarian cancer. Nat Commun. 8:146342017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ganapathy-Kanniappan S and Geschwind JF:
Tumor glycolysis as a target for cancer therapy: Progress and
prospects. Mol Cancer. 12:1522013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kikuchi R, Iwai Y, Tsuji T, Watanabe Y,
Koyama N, Yamaguchi K, Nakamura H and Aoshiba K: Hypercapnic tumor
microenvironment confers chemoresistance to lung cancer cells by
reprogramming mitochondrial metabolism in vitro. Free Radic Biol
Med. 134:200–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu Y, Wan Z, Zhang X, Zhong X, Rui L and
Li Z: PRDM14 inhibits 293T cell proliferation by influencing the
G1/S phase transition. Gene. 595:180–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Snellenberg S, Cillessen SA, Van Criekinge
W, Bosch L, Meijer CJLM, Snijders PJF and Steenbergen RDM:
Methylation-mediated repression of PRDM14 contributes to apoptosis
evasion in HPV-positive cancers. Carcinogenesis. 35:2611–2618.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moriya C, Taniguchi H, Nagatoishi S,
Igarashi H, Tsumoto K and Imai K: PRDM14 directly interacts with
heat shock proteins HSP90α and glucose-regulated protein 78. Cancer
Sci. 109:373–383. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feng Y, Hu J, Xie D, Qin J, Zhong Y, Li X,
Xiao W, Wu J, Tao D, Zhang M, et al: Subcellular localization of
caspase-3 activation correlates with changes in apoptotic
morphology in MOLT-4 leukemia cells exposed to X-ray irradiation.
Int J Oncol. 27:699–704. 2005.PubMed/NCBI
|