Introduction
Androgens and androgen receptors (ARs) have a
crucial role in maintaining spermatogenesis (1). Increasing evidence has demonstrated
that low testosterone levels and mutations in the AR gene could
result in spermatogenesis failure and male infertility (2–4). A
previous study using a Sertoli cell-specific AR knockout (SCARKO)
mouse model revealed spermatogenesis arrest at the diplotene stage
(5), indicating that the function
of androgens in Sertoli cells is critical for spermatogenesis.
Free testosterone binds to cytoplasmic AR in Sertoli
cells, inducing a conformational change in AR and the nuclear
translocation of the testosterone/AR complex. Subsequently, the
complex binds to the androgen response element (ARE) in the
chromatin, to induce target gene transcriptional activation or
repression (1). At present,
reproductive homeobox 5 is the only target gene that has been
identified (6). Therefore,
additional AR target genes, that may be physiologically relevant to
spermatogenesis, need to be identified.
Our previous study (7) showed that ATPase Ca++
transporting plasma membrane 4 (ATP2B4, also known as PMCA4) was
one of the 2,276 downregulated genes in AR knockout (ARKO) mice
compared with WT mice. PMCA4 belongs to the family of P-type
primary ion transport ATPases, which have key roles in
intracellular Ca2+ homeostasis (8). Schuh et al (9) demonstrated that PMCA4 is required for
sperm motility and male fertility in mice, while Patel et al
(10) showed that PMCA4 is critical
for murine sperm maturation. Olli et al (11) found that deletion of PMCA4 gene
resulted in decreased sperm motility and infertility and suggested
the potential involvement of PMCA4 mutations in human
asthenospermia. However, the underlying mechanism responsible for
the decreased sperm motility resulting from the absence or
knockdown of PMCA4 has not been fully elucidated (11). The present study aimed to explore
the expression pattern and function of PMCA4 during mouse
spermatogenesis.
Materials and methods
Animals
A total of 30 C57BL/6 male mice were purchased from
Guangdong Experimental Animal Center (Foshan, China). SCARKO (n=3)
and ARKO (n=3) mice were provided by Dr Yaoting Gui, at the Peking
University Shenzhen Hospital (Shenzhen, China). Experimental
protocols involving animals were reviewed and approved by the
ethics committee of The People's Hospital of Longhua (Shenzhen,
China; approval no. LHRY-1907014). ARKO mouse testes were collected
from 8–9-week-old mice. WT postnatal testes were collected from
C57BL/6 mice aged 1–8 weeks (3 mice in each group). Samples from
other organs mentioned in the present study were from C57BL/6 adult
mice (age, 8–9 weeks; n=6). Mice were maintained for at least 5
days under standard conditions with water and chow ad libitum and a
16-h light/8-h dark cycle at 22–25°C and 50–60% relative humidity.
Euthanasia was performed by cervical dislocation after
CO2 sedation.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from mouse testes using
TRIzol (Thermo Fisher Scientific, Inc.) and cDNA synthesis was
performed using the PrimeScript RT Master Mix kit (Takara Bio,
Inc.) according to the manufacturer's protocol. RT-qPCR reactions
were performed with the SYBR® Premix EX TaqTMII PCR kit
(Takara Bio, Inc.), with primers specific for mouse PMCA4 (forward,
5′-CTGAGGGAATGGACGAGAT-3′ and reverse, 5′-CAACTGCTGCGGAATAGGA-3′;
product size, 204 bp), with GAPDH (forward,
5′-AGTGGCAAAGTGGAGATT-3′; and reverse, 5′-GTGGAGTCATACTCCAACA-3′;
product size, 116 bp) used as the endogenous control. The annealing
temperature was 60°C, with 40 cycles. Data were calculated using
2−ΔΔCq method (12).
Western blot analysis
Protein samples were extracted from tissue and cells
using RIPA lysis buffer (Beyotime Institute of Biotechnology), then
20 µg protein samples (determined by bicinchoninic acid) were
loaded and run on 10% SDS-PAGE, and transferred onto a
polyvinylidene fluoride (PVDF) membrane. The membranes were blocked
with 10% (w/v) non-fat milk in TBS buffer (Dalian Meilun Biology
Technology Co., Ltd.) with 0.05% Tween-20 (Sigma-Aldrich; Merck
KGaA) at room temperature for 1 h. After incubation overnight at
4°C with primary antibodies targeting PMCA4 (140 kDa, Abcam; cat.
no. ab2783; 1:1,000 dilution), GAPDH (37 kDa, Cell Signaling
Technology, Inc.; cat. no. 5174; 1:1,000 dilution), or AR (110 kDa,
Santa Cruz Biotechnology, Inc., cat. no. sc-816; 1:1,000). The
membranes were treated with horseradish peroxidase-labeled
secondary antibody (anti-rabbit and anti-mouse IgG; both 1:1000;
cat nos. 7074 and 7076, respectively; both Cell Signaling
Technology, Inc.) for 1 h at room temperature. Positive bands were
detected using an enhanced chemiluminescence kit (Thermo Fisher
Scientific, Inc.). The densitometry was determined by ImageJ 1.48
software (imagej.net/).
Sperm collection
Mouse epididymides for sperm preparation were
collected from C57BL/6 mice as described previously (13). Briefly, cauda epididymis was
dissected and placed in Quinn's Advantage Fertilization (HTF)
Medium (pH 7.4; SAGE®Media; cat. no. ART-1020; Cooper
Surgical, Inc.) at 37°C for 10 min following shearing, to allow the
sperm to be dispersed. After a wash with fresh medium, the sperm
was centrifuged at 800 × g for 10 min at room temperature, and then
resuspended in fresh Quinn's medium (1×107 cells/ml) for
the next step of experiments.
Immunofluorescence
Mouse testes were dissected, fixed with 4%
paraformaldehyde for 24 h at 4°C, then embedded in paraffin. The
paraffin-embedded testicular tissue was cut in 2-µm sections. After
dewaxing and rehydrating, the slides were subjected to antigen
retrieval, by immersing in 10 mM sodium citrate (pH 6.0) and
microwaving at 1,000 W for 30 min. The sections were blocked in 10%
bovine serum albumin at 37°C for 30 min, followed by cooling to
room temperature (RT). An anti-PMCA4 antibody (1:100; cat. no.
ab2783; Abcam) was added on to the slides and kept at 4°C
overnight. The following day, after three washes with PBS, the
sections were incubated with an anti-mouse Alexa Fluor 594 antibody
(1:500; cat. no. A-11005; Thermo Fisher Scientific, Inc.) for 1 h
at RT. The sections were counterstained with Hoechst 33342
(1:2,000; Invitrogen; Thermo Fisher Scientific, Inc.) for 5 min at
RT. Following two additional washes, the sections were mounted with
SlowFade (Invitrogen; Thermo Fisher Scientific, Inc.) and observed
using a fluorescent microscope (magnification, ×200; Zeiss
GmbH).
Cell transfection
Murine Sertoli cell line TM4 was purchased from the
American Type Culture Collection. Cells were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum (v/v) and 100 µg/ml each penicillin and streptomycin at 37°C
with atmospheric conditions of 95% air and 5% CO2.
Plasmids pcDNA3.1-AR and pcDNA3.1 were constructed by IGE
Biotechnology Ltd. Cells were plated in 10-cm culture dishes; upon
reaching 50% confluence, cells were transfected (37°C; 24–48 h)
with 2.5 µg plasmid using Lipofectamine® 3000 (Thermo
Fisher Scientific, Inc.). Cells were harvested 24–48 h after
transfection.
Dual-luciferase assay
At 24 h post-transfection, TM4 cells were cultured
in serum-free medium [0.1% BSA in DMEM (cat. no. C11995500BT;
Gibco; Thermo Fisher Scientific, Inc.)] for 6 h before addition of
10 nM testosterone (Dalian Meilun Biology Technology Co., Ltd.) or
ethanol. Following 6 h incubation at 37°C and removal of the
medium, cells were lysed by addition of 100 µl passive lysis buffer
(Promega Corporation). Luciferase activity was measured following
the manufacturer's instructions (cat. no. E1910; Promega
Corporation). The activity of luciferase reporter was normalized to
that of Renilla luciferase.
Statistical analysis
All experiments in the present study were repeated
at least three times. Statistical analysis was performed using
Sigmaplot 16.0 (Systat Software, Inc.). Data were expressed as the
mean ± standard error of mean. Statistical significance was
evaluated by one -way ANOVA followed by Fisher's protected
least-significant difference post hoc test, unless otherwise
specified. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of PMCA4 during
mouse testes development
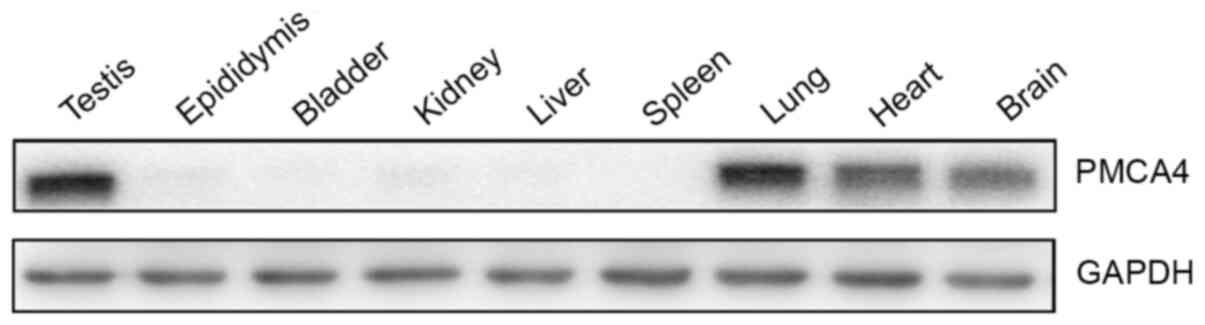
First, the protein expression levels of PMCA4 were
examined in various tissues from adult mice. Western blotting
results revealed that the PMCA4 protein was expressed in the
testes, as well as in the lung, heart and brain (Fig. 1). No protein expression of PMCA4 was
observed in epididymis, bladder, liver, kidney and spleen tissue
(Fig. 1).
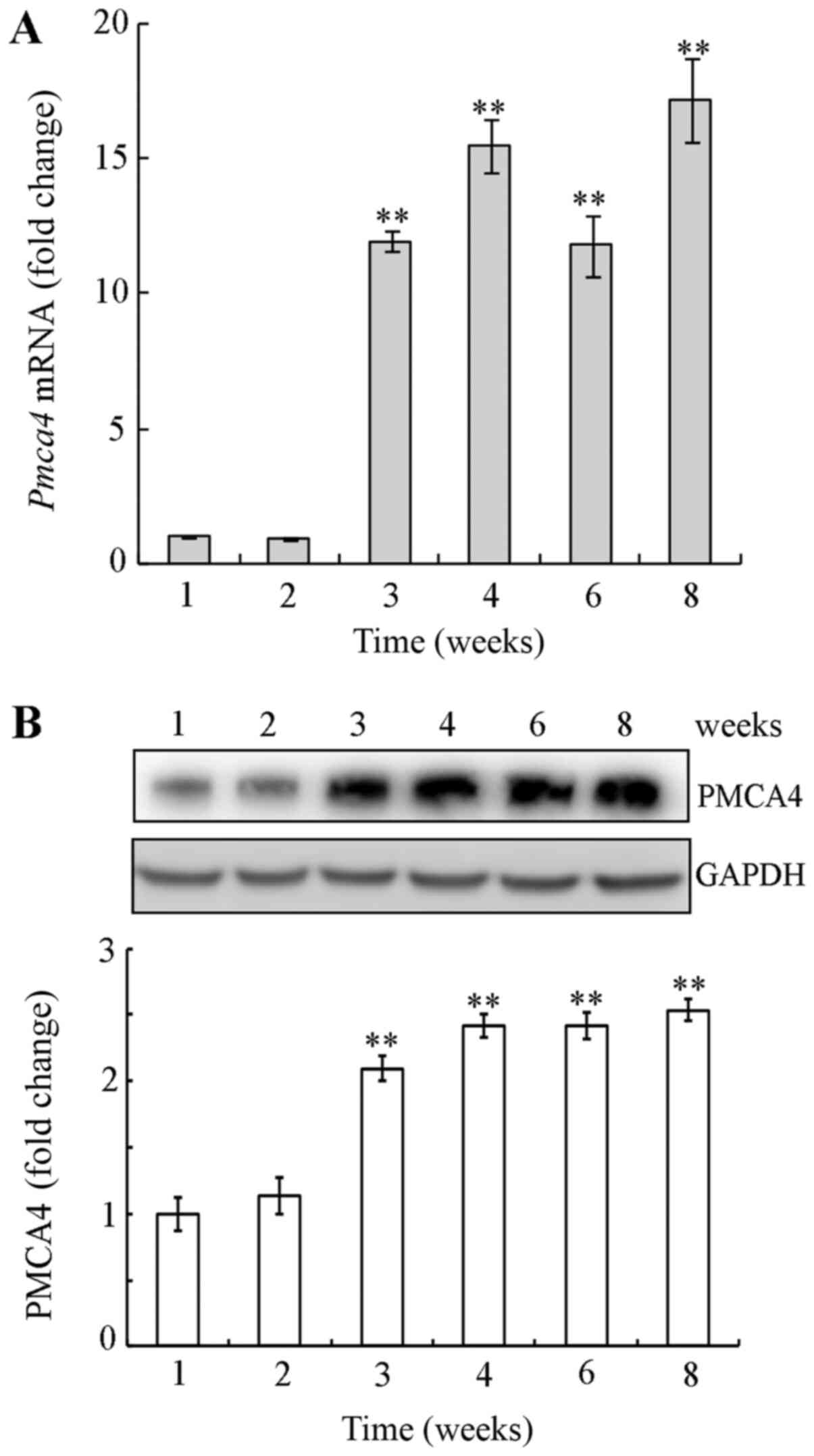
Next, the mRNA and protein expression levels of
PMCA4 were examined by RT-qPCR and western blotting, respectively,
in mouse testicular tissues during postnatal development. PMCA4
expression levels were significantly increased at week 3–8
postnatal compared with week 1, both at the mRNA and the protein
level (Fig. 2). These findings
suggest that PMCA4 was upregulated during mouse testes postnatal
development.
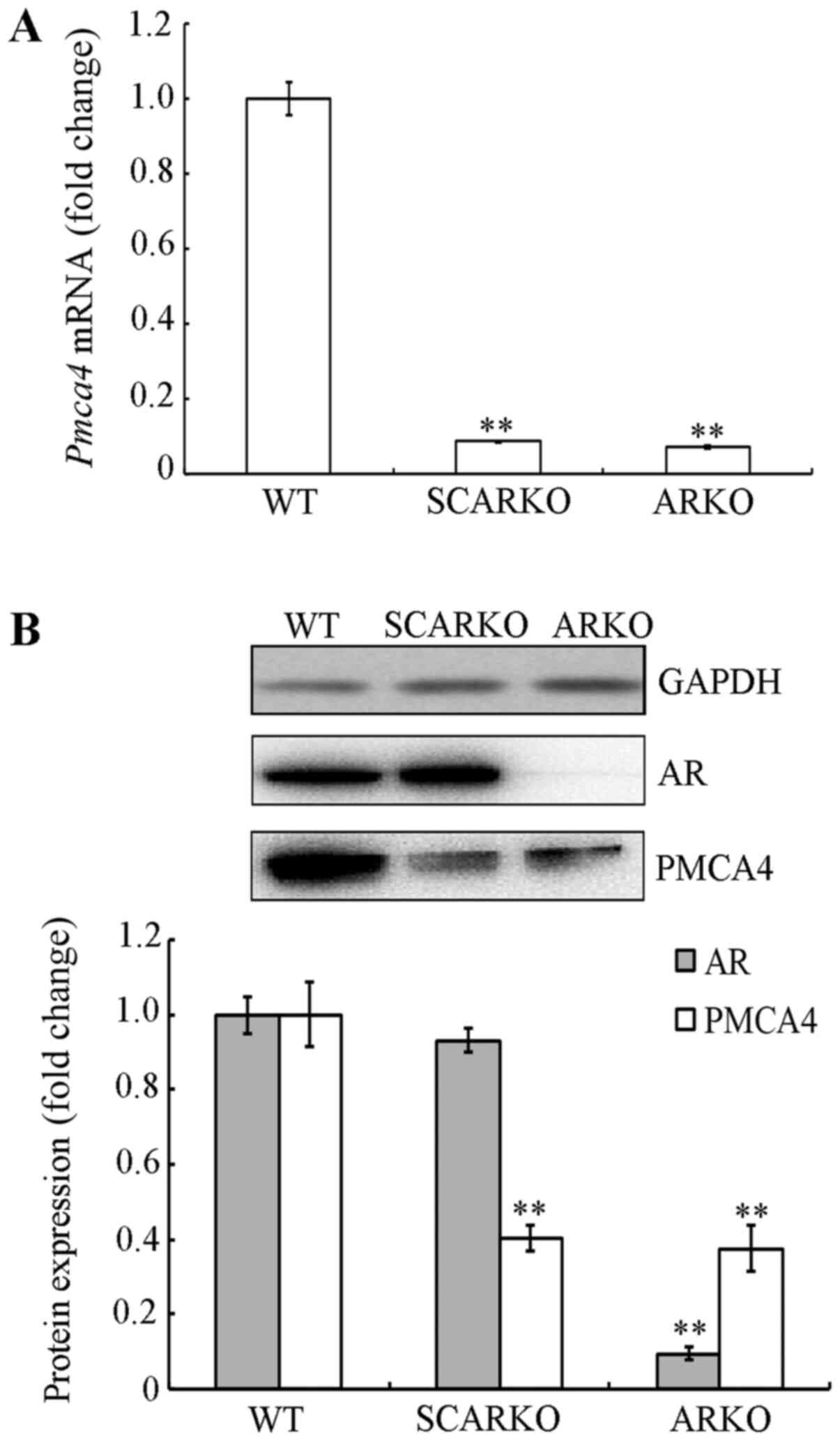
PMCA4 expression is decreased in the
testes of AR-knockout mice
To confirm the results from our previous gene
expression analysis (8), PMCA4 mRNA
and protein expression levels were examined in the testes of
SCARKO, ARKO and WT mice. Compared with WT mice, PMCA4 mRNA levels
were decreased in testicular tissues from SCARKO (0.087±0.002;
P<0.001) and ARKO (0.075±0.004; P<0.001) mice (Fig. 3A). Similar results were obtained for
the protein expression levels, as shown in Fig. 3B. These findings indicated that
PMCA4 expression was decreased in AR knockout mice, thus, it was
hypothesized that PMCA4 may be regulated by AR in mouse testes.
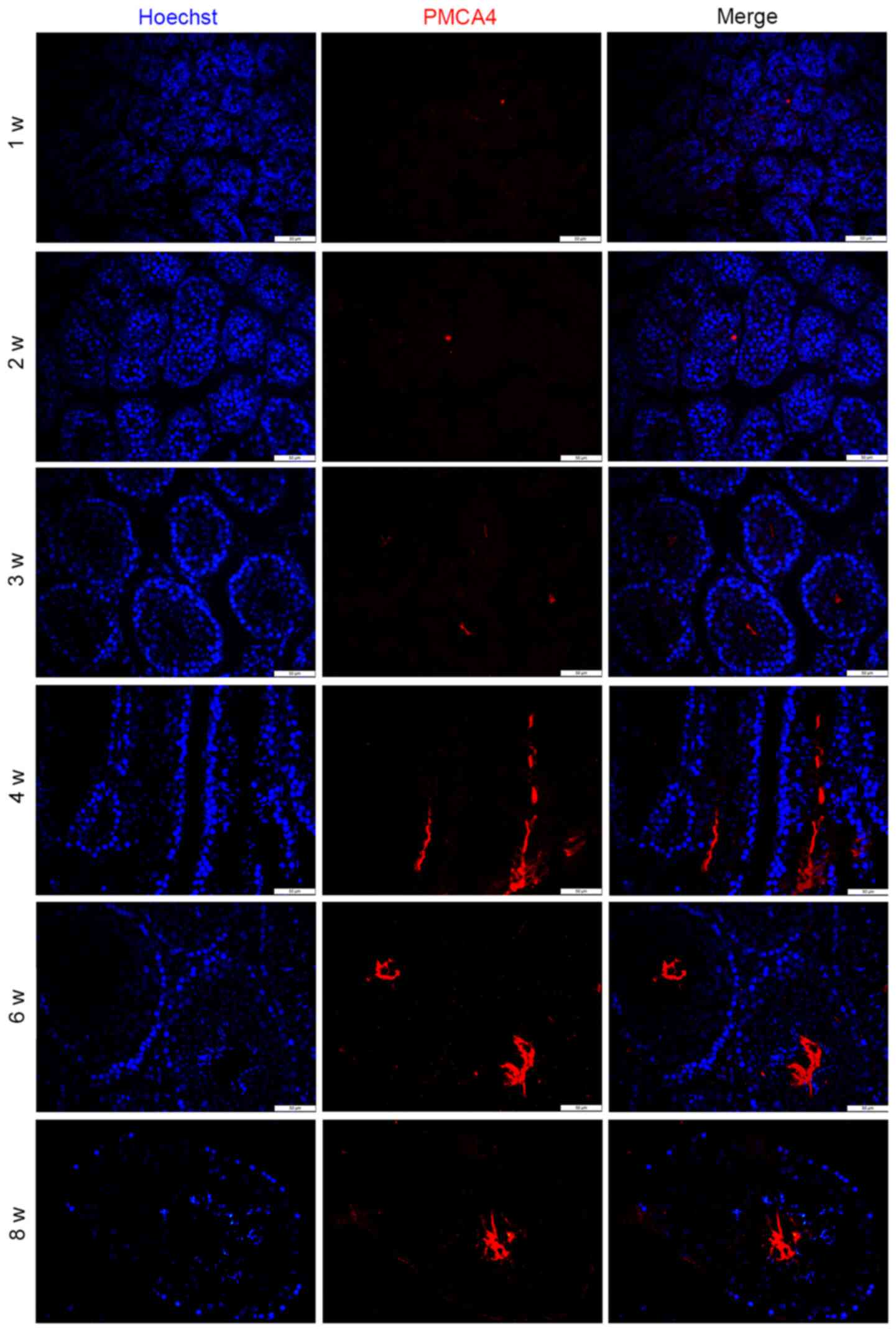
PMCA4 expression during
spermatogenesis
To further explore the potential role of PMCA4 in
spermatogenesis, the subcellular localization of PMCA4 during
testes development was investigated by immunofluorescence staining.
As presented in Fig. 4, PMCA4
immunostaining was absent before 3 weeks postnatal. In addition,
the staining pattern in the microscopy images revealed that PMCA4
expression was located in the elongated spermatids (Fig. 4).
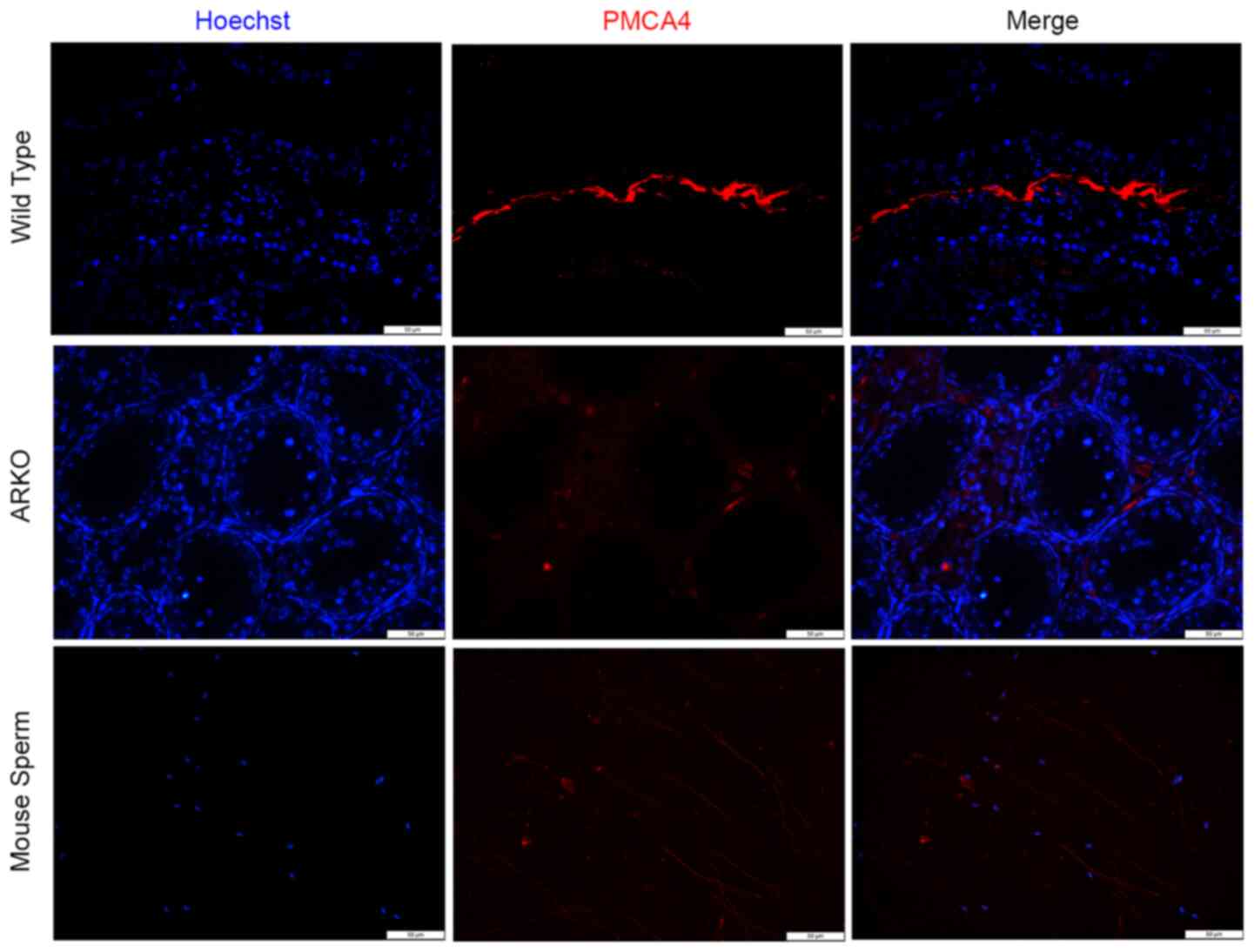
Next, immunofluorescence staining for PMCA4 was
performed in testicular tissues from WT and ARKO mice. In the
testis from WT mice, high intensity fluorescence (red) was observed
in the lumen of the testicular tubule and was co-localized with the
mature sperm (Fig. 5). Consistent
with the western blotting data in Fig.
3B, minimal florescence signal was observed in the testis from
ARKO mice (Fig. 5). Immunostaining
of PMCA4 in healthy mouse sperm revealed that the protein was
located at the sperm tail. The present data suggested that PMCA4
may be involved in the movement and motility of the sperm.
PMCA4 promoter is activated by
testosterone in vitro
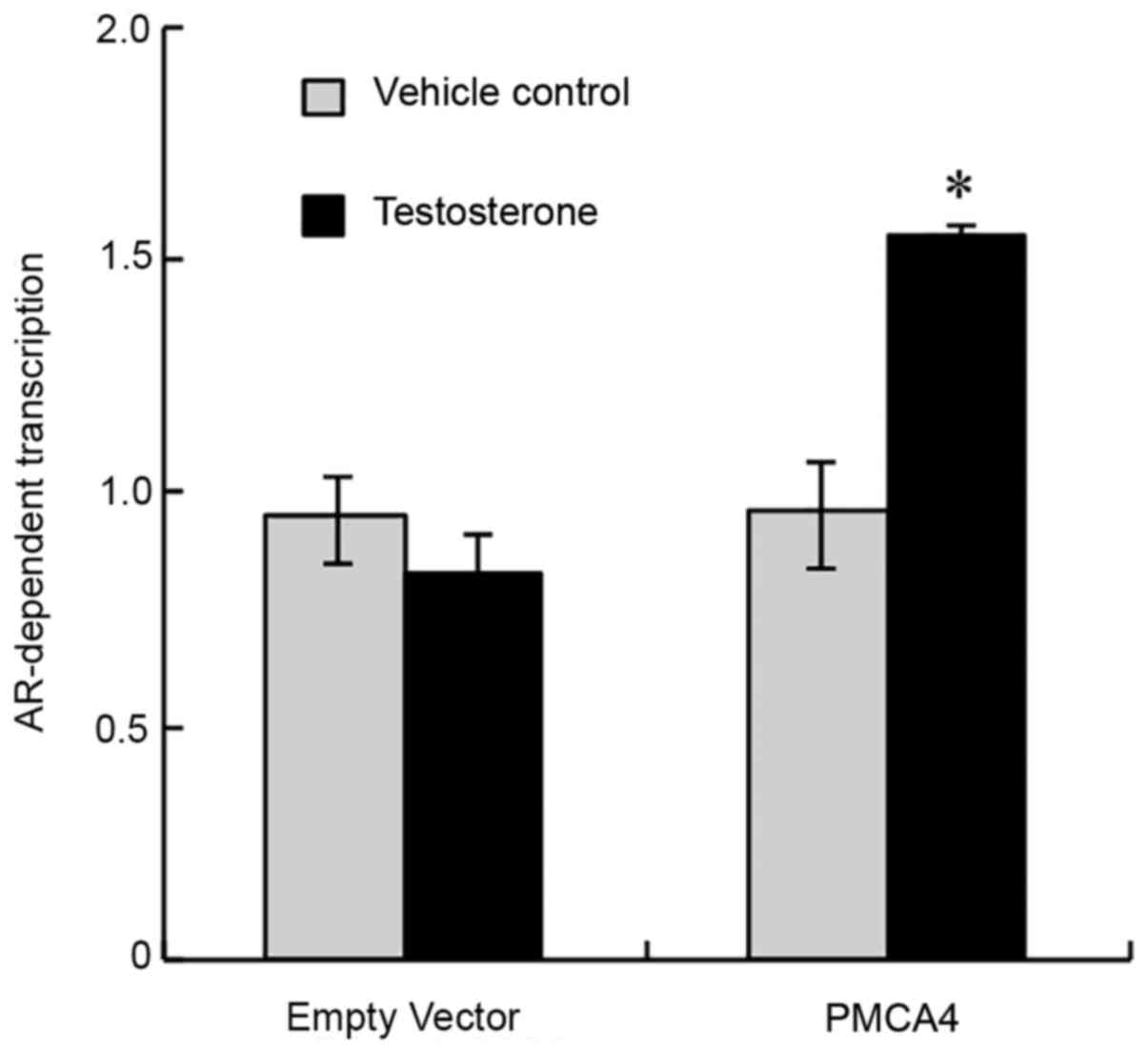
To evaluate whether testosterone and AR affect the
transcription of PMCA4, a dual-luciferase assay was used to
determine the PMCA4 promoter-driven luciferase activity. As shown
in Fig. 6, following treatment of
TM4 cells with testosterone or ethanol (vehicle control), cells
transfected with the control empty vector exhibited no difference
in luciferase activity following exposure to testosterone. By
contrast, cells transfected with the PMCA4 promoter-driven vector
exhibited significantly increased luciferase activity following
testosterone treatment (Fig. 6).
The present data indicated that testosterone and AR regulated the
promoter activity of PMCA4.
PMCA4 protein expression is regulated
by AR in vitro
To further confirm that AR regulates PMCA4 protein
expression, TM4 cells were transfected with pcDNA3.1-AR or pcDNA3.1
(empty vector). A significant increase in PMCA4 and AR protein
expression levels was observed by western blotting in the
AR-overexpressing cells compared with the control-transfected cells
(Fig. 7A). In Fig. 7B, cells were pre-incubated for 6 h
with 30 µM flutamide, an AR antagonist (14), and then were exposed to 10 nM
testosterone for 24 h. As expected, testosterone induced an
increase in PMCA4 and AR protein expression levels in the negative
control group, which were treated with vehicle for flutamide
(Fig. 7B). Flutamide pre-treatment,
however, significantly decreased the testosterone-mediated
induction in PMCA4 and AR protein expression levels (Fig. 7B).
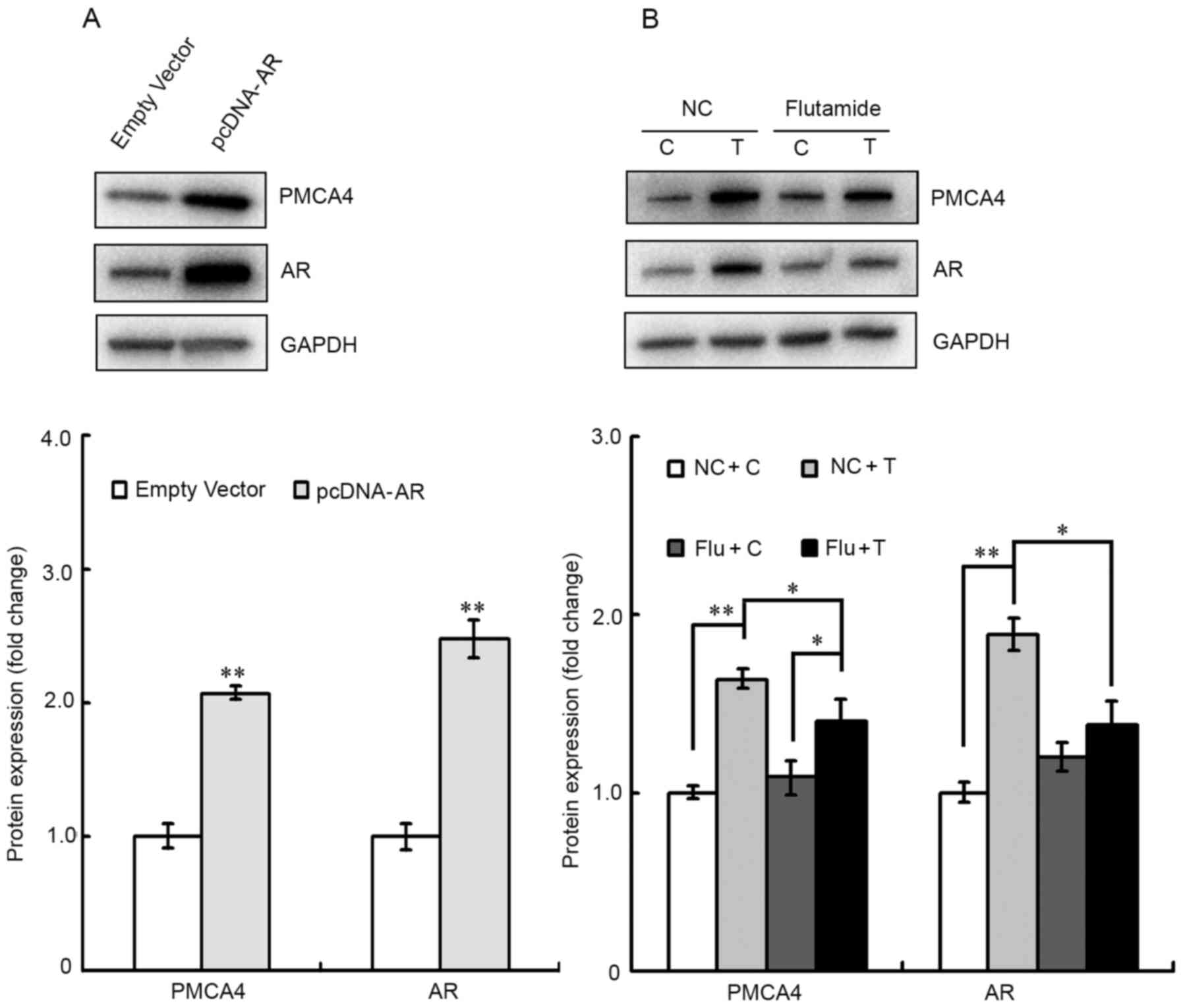 | Figure 7.PMCA4 expression is upregulated by
androgens and AR in TM4 cells. (A) TM4 cells were transfected with
either empty vector or pcDNA-AR, and subsequently the AR and PMCA4
protein expression levels were evaluated via western blotting.
GAPDH was used as an internal control. Data are presented as the
mean ± standard error of the mean (n=3). **P<0.01 vs. empty
vector group. (B) Cells were pre-treated with flutamide for 6 h,
prior to 10 nM testosterone administration for 24 h. AR and PMCA4
protein expression levels were then evaluated by western blotting.
Testosterone increased PMCA4 and AR protein expression, and this
increase was blocked by the AR antagonist flutamide. Data are
presented as the mean ± standard error of the mean (n=3). The
statistical significance of the differences between groups was
determined by one-way ANOVA followed by all pairwise Holm-Sidak
test. *P<0.05 and **P<0.01, with comparisons shown in
brackets. PMCA4, ATPase Ca++ transporting plasma
membrane 4; AR, androgen receptor; NC, negative control for Flu; C,
control for T and ethanol; T, testosterone; Flu, flutamide. |
Discussion
Ionic homeostasis has a key role in sperm
maturation, capacitation and gamete communication (15); the balance of ion transport systems
is central to sperm motility (16).
Thus, there is little doubt as to the importance of calcium
homeostasis in sperm motility and fertilization (17,18).
AR is capable of transmitting testosterone signals
by at least two known mechanisms, the classical and non-classical
pathways. In the non-classical Ca2+ influx pathway,
androgen interacts with a Gq coupled G-protein coupled receptor
(GPCR) in the plasma membrane. Phospholipase C (PLC) is then
activated to cleave phosphatidylinositol 4,5-bisphosphate (PIP2)
into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG)
(19). Decreased PIP2 concentration
inhibits ATP-sensitive potassium channels causing membrane
depolarization and Ca2+ entry via L-type Ca2+
channels (20). Voltage dependent
L-type Ca2+ channels open allowing the influx of
Ca2+, which can regulate multiple cellular processes
(20). However, the physiological
functions regulated by the testosterone-mediated influx of
Ca2+ have not been identified (19).
PMCA represents a family of enzymes that extrude
Ca2+ from the cytoplasm across the plasma membrane of
eukaryotic cells. PMCA4 is the most common PMCA isoform in sperm
(21), and has two major splice
variants 4a and 4b. The latter one is thought to be a key regulator
in calcium clearance in murine sperm (22,23),
where PMCA4 variant 4b deletion disrupts calcium homeostasis and
results in the loss of both progressive and hyperactivated sperm
motility, ultimately leading to male infertility (9,24). A
study conducted by Chen et al (25) reported that PMCA4 was downregulated
in seminoma, the most common testicular malignant germ cell tumor,
suggesting that PMCA4 has an important role in the spermatogenesis
and male fertility; however, the molecular mechanism remains
unclear. Therefore, the present study aimed to explore the
expression pattern and function of PMCA4 during mouse
spermatogenesis.
Results from western blot analysis in different
murine tissues revealed that PMCA4 was expressed in the testes,
lungs, heart and brain. PMCA4 mRNA and protein expression levels
were markedly increased in mice testis at 3 weeks postnatal, which
is the time point when the first wave of spermatogenesis occurs
(26), suggesting that PMCA4
expression may be primarily restricted to post-meiotic germ cells.
This was further confirmed by immunofluorescence results showing
that PMCA4 was located at the lumen and inner layers of
seminiferous tubules. Previous reports using rats (22), as well as bovines (27), have demonstrated that PMCA4 is
expressed in the epididymis epithelium. The present findings are in
agreement with a previous study published by Patel et al
(10), which reported that PMCA4
protein is expressed in the testis and throughout the epididymis.
However, the molecular mechanism by which PMCA4 influences
spermatogenesis has not been reported to date. In the present
study, PMCA4 mRNA levels in testis tissues from SCARKO and ARKO
mice were significantly decreased compared with WT controls, and
PMCA4 protein expression levels were decreased in ARKO mice
compared with WT controls. These findings suggested that AR may
regulate PMCA4 expression in mouse testes. By using a luciferase
activity assay in vitro, the present study confirmed that
activation of AR by testosterone administration increased the
activity of the PMCA4 promoter. Cells overexpressing AR in vitro
also had higher expression levels of the PMCA4 protein, and the
increase in the PMCA4 protein expression induced by testosterone
was prevented by pre-treatment with the AR antagonist
flutamide.
It was previously reported that PMCA4 and the nitric
oxide synthases (NOSs) are interacting partners that have been
identified in a quaternary complex including Caveolin-1 (11). A previous study also found that in
mouse Sertoli cells AR localizes in the plasma membrane by
association to Caveolin-1 (28).
The aforementioned evidence supports the hypothesis that AR
regulates PMCA4 expression. In general, androgens and AR affect the
development of spermatogenic cells in Sertoli cells (5,29). The
present results demonstrated that PMCA4 was almost exclusively
localized in the sperm tail. A study by Lestari et al
(30) reported that PMCA4
expression in the normozoospermia group was much higher than in the
asthenozoospermia group due to impaired sperm structure and
function, suggesting that PMCA4 defects may be a factor in sperm
dysfunction and infertility.
In conclusion, the present study demonstrated that
AR regulates PMCA4 expression and that the expression levels of
PMCA4 mRNA and protein are downregulated in testes form AR knockout
mice compared with WT mice. The present data indicated that PMCA4
may serve a potentially important role in spermatogenesis, and
likely in male reproduction. Further studies are needed to better
understand the molecular mechanisms by which AR regulates the PMCA4
expression and function in spermatogenesis.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Natural Science Foundation (grant no. 31800984), the Science and
Technology Planning Project of Guangdong province (grant nos.
2018A0303130337 and A2019565), the Science and Technology Planning
Project of Shenzhen municipality (grant nos. JCYJ20180228164047023
and JCYJ20190808095407464) and the Longhua Science and Technology
Planning Project (grant nos. 2017029, 1150A20190513BA7B6B0 and
2020006).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RS, QD, HG and ZW performed the experiments. HL and
QD analyzed the data and wrote the manuscript. QD obtained funding
and revised the manuscript. All the authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All experimental protocols involving animals were
reviewed and approved by the Ethics Committee of The People's
Hospital of Longhua (Shenzhen, China; approval no.
LHRY-1907014).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Walker WH: Testosterone signaling and the
regulation of spermatogenesis. Spermatogenesis. 1:116–120. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yong EL, Lim LS, Wang Q, Mifsud A, Lim J,
Ong YC and Sim KS: Androgen receptor polymorphisms and mutations in
male infertility. J Endocrinol Invest. 23:573–577. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tahmasbpour E, Balasubramanian D and
Agarwal A: A multi-faceted approach to understanding male
infertility: Gene mutations, molecular defects and assisted
reproductive techniques (ART). J Assist Reprod Genet. 31:1115–1137.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Hara L and Smith LB: Androgen receptor
roles in spermatogenesis and infertility. Best Pract Res Clin
Endocrinol Metab. 29:595–605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Gendt K, Swinnen JV, Saunders PT,
Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens
F, Lécureuil C, et al: A Sertoli cell-selective knockout of the
androgen receptor causes spermatogenic arrest in meiosis. Proc Natl
Acad Sci USA. 101:1327–1332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
MacLean JA II and Wilkinson MF: The Rhox
genes. Reproduction. 140:195–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang QX, Zhang XY, Zhang ZM, Lu W, Liu L,
Li G, Cai ZM, Gui YT and Chang C: Identification of
testosterone-/androgen receptor-regulated genes in mouse Sertoli
cells. Asian J Androl. 14:294–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calì T, Brini M and Carafoli E: Regulation
of cell calcium and role of plasma membrane calcium ATPases. Int
Rev Cell Mol Biol. 332:259–296. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schuh K, Cartwright EJ, Jankevics E,
Bundschu K, Liebermann J, Williams JC, Armesilla AL, Emerson M,
Oceandy D, Knobeloch KP, et al: Plasma membrane Ca2+
ATPase 4 is required for sperm motility and male fertility. J Biol
Chem. 279:28220–28226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patel R, Al-Dossary AA, Stabley DL, Barone
C, Galileo DS, Strehler EE and Martin-DeLeon PA: Plasma membrane
Ca2+-ATPase 4 in murine epididymis: Secretion of splice
variants in the luminal fluid and a role in sperm maturation. Biol
Reprod. 89:62013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olli KE, Li K, Galileo DS and
Martin-DeLeon PA: Plasma membrane calcium ATPase 4 (PMCA4)
co-ordinates calcium and nitric oxide signaling in regulating
murine sperm functional activity. J Cell Physiol. 233:11–22. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma Q, Li Y, Luo M, Guo H, Lin S, Chen J,
Du Y, Jiang Z and Gui Y: The expression characteristics of FAM71D
and its association with sperm motility. Hum Reprod. 32:2178–2187.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng Q, Zhang Z, Wu Y, Yu WY, Zhang J,
Jiang ZM, Zhang Y, Liang H and Gui YT: Non-genomic action of
androgens is mediated by rapid phosphorylation and regulation of
androgen receptor trafficking. Cell Physiol Biochem. 43:223–236.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vignini A, Buldreghini E, Nanetti L,
Amoroso S, Boscaro M, Ricciardo-Lamonica G, Mazzanti L and Balercia
G: Free thiols in human spermatozoa: Are
Na+/K+-ATPase, Ca2+-ATPase
activities involved in sperm motility through peroxynitrite
formation? Reprod Biomed Online. 18:132–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jimenez T, Sánchez G and Blanco G:
Activity of the Na,K-ATPase α4 isoform is regulated during sperm
capacitation to support sperm motility. J Androl. 33:1047–1057.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wood CD, Darszon A and Whitaker M: Speract
induces calcium oscillations in the sperm tail. J Cell Biol.
161:89–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukami K, Yoshida M, Inoue T, Kurokawa M,
Fissore RA, Yoshida N, Mikoshiba K and Takenawa T: Phospholipase
Cdelta4 is required for Ca2+ mobilization essential for
acrosome reaction in sperm. J Cell Biol. 161:79–88. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rahman F and Christian HC: Non-classical
actions of testosterone: An update. Trends Endocrinol Metab.
18:371–378. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smith LB and Walker WH: The regulation of
spermatogenesis by androgens. Semin Cell Dev Biol. 30:2–13. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calamera J, Buffone M, Ollero M, Alvarez J
and Doncel GF: Superoxide dismutase content and fatty acid
composition in subsets of human spermatozoa from normozoospermic,
asthenozoospermic, and polyzoospermic semen samples. Mol Reprod
Dev. 66:422–430. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilhelm B, Brandenburger T, Post H and
Aumüller G: Expression and localization of PMCA4 in rat testis and
epididymis. Histochem Cell Biol. 129:331–343. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Di Leva F, Domi T, Fedrizzi L, Lim D and
Carafoli E: The plasma membrane Ca2+ ATPase of animal cells:
Structure, function and regulation. Arch Biochem Biophys.
476:65–74. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okunade GW, Miller ML, Pyne GJ, Sutliff
RL, O'Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V,
Doetschman T, et al: Targeted ablation of plasma membrane
Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function
for PMCA1 and a critical role in hyperactivated sperm motility and
male fertility for PMCA4. J Biol Chem. 279:33742–33750. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Qi C, Xia L and Li G:
Identification of novel genetic etiology and key molecular pathways
for seminoma via network-based studies. Int J Oncol. 51:1280–1290.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Margolin G, Khil PP, Kim J, Bellani MA and
Camerini-Otero RD: Integrated transcriptome analysis of mouse
spermatogenesis. BMC Genomics. 15:392014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brandenburger T, Strehler EE, Filoteo AG,
Caride AJ, Aumüller G, Post H, Schwarz A and Wilhelm B: Switch of
PMCA4 splice variants in bovine epididymis results in altered
isoform expression during functional sperm maturation. J Biol Chem.
286:7938–7946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deng Q, Wu Y, Zhang Z, Wang Y, Li M, Liang
H and Gui Y: Androgen receptor localizes to plasma membrane by
binding to caveolin-1 in mouse sertoli cells. Int J Endocrinol.
2017:39859162017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang RS, Yeh S, Tzeng CR and Chang C:
Androgen receptor roles in spermatogenesis and fertility: Lessons
from testicular cell-specific androgen receptor knockout mice.
Endocr Rev. 30:119–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lestari SW, Miati DN, Seoharso P,
Sugiyanto R and Pujianto DA: Sperm Na+,
K+-ATPase α4 and plasma membrane Ca2+-ATPase
(PMCA) 4 regulation in asthenozoospermia. Syst Biol Reprod Med.
63:294–302. 2017. View Article : Google Scholar : PubMed/NCBI
|