Introduction
Sepsis is a severe systemic inflammatory response
syndrome caused by a dysregulated host response to infection
(1), and is the leading cause of
morbidity and mortality in critically ill patients worldwide
(2). Multiple complications occur
during sepsis, such as acute lung injury (3), kidney lesions (4), cardiac dysfunction (5), liver disorders (6) and brain damage (7). Among these manifestations,
sepsis-associated encephalopathy (SAE) appears earlier and more
frequently than other complications (8). Despite advancements in the treatment
of patients in the intensive care unit (ICU), individuals with SAE
exhibit longer ICU stays and higher 28-day mortality rates
(9), with survivors experiencing a
substantial cognitive deficit (10). Learning and memory impairments have
also been observed in septic mammalian models (11,12).
Thus, identifying the pathogenic mechanisms of SAE and developing
more effective therapeutic strategies are critical.
Inflammatory lesions, oxidative damage and cell
death are well associated with SAE (13,14).
Inflammatory triggers, such as lipopolysaccharide (LPS), can bind
to Toll-like receptor 4 (TLR4) and subsequent activate downstream
signals of TLR4, such as mitogen-activated protein kinases (MAPKs)
and nuclear factor-κB (15,16), and untimely induce the production of
adhesion molecules and proinflammatory factors, including tumor
necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 in vascular
endothelial cells (17), which can
result in endothelial abnormalities, leakage of the blood-brain
barrier (BBB), immune cell migration, neuronal death, and
ultimately brain damage (18,19).
In addition, intracellular calcium is involved in LPS-stimulated
cytokine production; calcium influx due to calcium store depletion
is referred to as store-operated calcium entry (20). Calcium signaling involves activation
of tyrosine kinases and leads to activation of MAPKs in endothelial
cells (21). Reportedly, oxidative
stress triggers neuronal injury and cerebral dysfunction during
sepsis (22,23). Therefore, the alleviation of
inflammation, oxidative stress and apoptosis would be an
efficacious remedy for SAE. Heme oxygenases (HOs) are the
rate-limiting enzymes that convert heme into biliverdin, ferrous
iron and carbon monoxide. Heme oxygenase-1 (HO-1), the inducible
isoform of HO, confers cytoprotection by exerting
anti-inflammatory, antioxidative and anti-apoptotic functions
(24–26). HO-1 upregulation and activation
ameliorates sepsis-induced multiorgan injury, including lung,
kidney, liver and brain damage (27–30).
Therefore, HO-1-targeted treatments are a promising avenue for
neuroprotection during sepsis.
Isoflurane (ISO) is a widely used inhalational
anesthetic during surgery. Mounting evidence suggests that besides
its use as an anesthetic, ISO possesses non-anesthetic physiologic
properties against inflammation, oxidation and apoptosis (31,32).
ISO affords neuroprotection against hypoxic ischemia-induced
cerebral damage (33,34), and ISO post-treatment improves
pulmonary vascular permeability in septic rats by upregulating HO-1
(27). ISO elicits its
anti-inflammatory activity by activating the HO-1 pathway in
LPS-stimulated macrophages (35).
ISO also protects against oxygen glucose deprivation-induced neuron
injury, which relies on the upregulation of HO-1 (36). However, 1.2–2.5% ISO has adverse
effects for patients in the ICU who cannot tolerate its hemodynamic
effects. ISO at <1% has a weak effect on hemodynamics, which is
more beneficial for ICU patients (37). The previous literature revealed that
0.7% ISO inhalation for 1 h at 1 and 6 h post-zymosan insult
reduced zymosan-induced inflammation and apoptosis in vitro
and in vivo (38–40). Nevertheless, the roles of HO-1 in
the neuroprotective effects of 0.7% ISO during sepsis remain
largely unknown.
In the present study, the protective effects of 0.7%
ISO were evaluated using an SAE mouse model created by cecal
ligation and puncture (CLP), and the beneficial effects of 0.7% ISO
were investigated for their potential association with HO-1
augmentation and/or activation.
Materials and methods
Animals and ethical guidelines
A total of 150 male C57BL/6 mice (age, 8 weeks;
weight, 20–25 g) were purchased from the Animal Center of Xi'an
Jiaotong University (Xi'an, China). The mice were maintained in
specific pathogen-free conditions at 22±2°C with 60% relative
humidity, 12-h dark/light cycles, and free access to food and
water. This study was conducted in accordance with the ARRIVE
guidelines for the Care and Use of Laboratory Animals (41), and the experimental procedures were
approved by the Ethics Committee of Xi'an Jiaotong University
(approval no. XAJU.No20180730A10120). All efforts were made to
minimize suffering. The mice were anaesthetized with 50 mg/kg of
pentobarbital sodium though intraperitoneal injection. At the end
of the experiments, the mice were administrated with 30% volume
displacement rate of CO2 for euthanasia. No heartbeat
and mydriasis were used to confirm animal death.
CLP-induced SAE mouse model
A CLP-induced SAE mouse model was established as
previously described with slight modifications (42). After anesthesia with pentobarbital
sodium (50 mg/kg), the mice were immobilized on an aseptic
operating table and received a 1-cm abdominal midline incision to
expose the cecum. The distal 20% of the cecum (below the ileocecal
valve and 1 cm from the tip) was ligated with a 6-0 suture and
punctured twice with a 20-gauge needle. The cecum was gently
squeezed to extrude a small amount of feces from the perforation
sites, and then returned to the abdomen. The incision was
subsequently sutured with sterile 6-0 silk. In the sham group, the
mice underwent a similar procedure without ligation and puncture of
the cecum. All animals received fluid resuscitation by subcutaneous
injection of 1 ml pre-warmed 0.9% saline solution using a 25-gauge
needle.
ISO administration
As previously reported, 0.7% ISO inhalation for 1 h
was conducted at 1 and 6 h post-challenge in mice (40,43).
The mice were placed in an air-tight plastic chamber
(Billups-Rothenberg, Inc.) with an inflow and an outflow. ISO
(Sigma-Aldrich; Merck KGaA) was delivered into the chamber by air
at 2 l/min through a tube, and Baralyme (Allied Healthcare
Products, Inc.) was used to remove CO2 from the chamber
gases. The concentration (0.7%) of ISO in the outflow hose was
continuously monitored using an anesthetic gas analyzer (Smart
Anesthesia Multi-gas Module; Cytiva), and the temperature of the
room and chamber was maintained at 20–24°C.
Experimental groups
A total of 150 mice were randomly assigned to the
following five groups (n=30 per group): i) Control group, mice
received sham surgery and no drug treatments; ii) ISO group, mice
received sham surgery and ISO exposure; iii) CLP group, mice
received CLP surgery and no drug treatments; iv) CLP + ISO group,
mice received CLP surgery and ISO exposure; and v) CLP + ISO + zinc
protoporphyrin IX (ZnPP; Sigma-Aldrich; Merck KGaA) group, mice
received CLP surgery and both ISO and ZnPP treatments. 0.7% ISO
inhalation for 1 h was conducted at 1 and 6 h post-CLP surgery, and
ZnPP (10 mg/kg) was peritoneally injected into the mice 1 h before
CLP surgery.
Evaluation of survival rate
Following CLP or sham treatment, 10 mice from each
group were returned to their cages and the survival rate was
calculated up to 7 days post-surgery.
Morris water maze (MWM) test
To evaluate the spatial learning and memory
abilities of the mice, the MWM test was performed as previously
described (44). The apparatus
consisted of a circular pool (diameter, 150 cm; height, 60 cm)
filled with water (22±2°C) containing food-grade titanium dioxide
(Shanghai Jianghu Industrial Co., Ltd.). A removable platform
(diameter, 10 cm) was placed in one quadrant of the pool and
submerged 1 cm below the surface of the water. The pool was divided
into four quadrants and the swimming paths of the mice were
captured using a video camera above the center of the pool. From
4–7 days post-treatment, CLP or sham-treated mice (n=10) were
individually placed into the pool at each of the four quadrants
(facing the sidewalls) and allowed to circumnavigate in search of
the platform for four trials per day (60 sec per trial). If a mouse
failed to find the platform within 60 sec, it was guided by the
investigator and permitted to search for an extra 10 sec. The
escape latency, swimming distance and time spent in the target
quadrant were recorded at each trial. The probe test comprised a
single trial with the platform removed. The mice were released into
the water and allowed to locate the original platform for 90 sec,
and the time spent in the target quadrant was recorded. On the 7th
day, the mice were subjected to the probe trial with the platform
removed, 2 h after the last training trial. Each mouse was
monitored for 60 sec to observe the swimming path, time spent in
the target quadrant of the platform, and the number of times that
the platform was crossed.
Brain water content
Brain water content was measured using the standard
wet-dry method as previously reported (45). The mice were anesthetized and
sacrificed 48 h post-CLP or sham surgery, and the brains were
immediately removed and weighed to obtain the wet weight.
Subsequently, the brain samples were dried in an oven at 100°C for
48 h, and the dry weight was acquired. Brain water content was
calculated as follows: (Wet weight-dry weight/wet weight)
×100%.
BBB permeability assay
BBB permeability was evaluated by extravasation of
Evans blue (EB) as previously reported (30). The mice were injected with 2% EB (3
ml/kg; Sigma-Aldrich; Merck KGaA) via the tail vein at 48 h
post-CLP or sham treatment. After 2 h, the mice were anesthetized
and transcardially perfused with normal saline. The brain samples
were harvested, weighted, homogenized in formamide (10 ml/g), and
incubated at 37°C for 48 h. The supernatants were collected after
centrifugation at 10,000 × g for 30 min at 4°C, and the optical
density (OD) at 625 nm was determined using a microplate reader
(Molecular Devices, LLC). Standard formamide was used as the blank
control, and the results were expressed as the relative amount of
EB (µg/g wet weight).
Blood collection and hippocampus
tissue preparation
At 48 h after CLP or sham surgery, the mice were
anesthetized and blood samples were collected from the cervical
artery, which were centrifuged at 1,000 × g (4°C) for 30 min. The
mice were then euthanized, and the hippocampus tissues were
immediately harvested. The tissues were homogenized and centrifuged
at 10,000 × g and 4°C for 10 min. The sera and supernatants were
stored at −80°C for the measurement of inflammatory mediators and
oxidative parameters.
Enzyme-linked immunosorbent assay
(ELISA)
The levels of TNF-α (cat. no. RTA00), IL-1β (cat.
no. RLB00), IL-6 (cat. no. R6000B) and IL-10 (cat. no. R1000) in
the serum and hippocampus supernatants were determined using
commercially available ELISA kits (R&D Systems, Inc.),
according to the manufacturer's instructions. The serum and
hippocampus supernatants were collected and the OD at 450 nm was
measured on an ELISA plate reader (Molecular Devices, LLC) and the
results are expressed as pg/ml serum or pg/mg protein.
Reactive oxygen species (ROS)
assay
ROS production was assessed using a
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) kit (Nanjing
Jiancheng Bioengineering Institute) as per the manufacturer's
protocol. Briefly, 50 µl serum or hippocampal supernatant were
added to a 96-well plate and incubated with 100 µM DCFH-DA solution
(200 µl) for 30 min (37°C) in the dark. DCFH-DA can be hydrolyzed
by cellular esterases to form DCFH, which is oxidized by ROS to
produce the fluorescent compound dichlorofluorescein (DCF). DCF
oxidation was fluorometrically measured using a fluorescence
microplate reader (Molecular Devices, LLC) at 485 nm excitation and
525 nm emission wavelengths. The data are represented as nmol/ml
serum or nmol/mg protein.
Malondialdehyde (MDA), superoxide
dismutase (SOD) and catalase (CAT) production
The levels of MDA (cat. no. A003-1-2), SOD (cat. no.
A001-3-2) and CAT (cat. no. A007-1-1) in the serum and hippocampal
supernatants were analyzed using commercial kits (Nanjing Jiancheng
Bioengineering Institute) following the manufacturer's protocols.
Briefly, the serum and supernatants were collected and the
absorbance at 530, 450 and 405 nm was measured using a microplate
reader (Bio-Rad Laboratories, Inc.) to determine MDA content and
SOD and CAT levels, respectively. The MDA content is displayed as
nmol/ml serum or nmol/mg protein, and the SOD and CAT activities
were calculated as U/ml serum or U/mg protein.
Terminal deoxynucleotidyl transferase
(TDT) dUTP nick end labeling (TUNEL) assay
TUNEL assay was carried out to detect neuronal
apoptosis using the Cell Death Detection kit (Roche Diagnostics) as
per the manufacturer's protocol. Hippocampal tissues were
homogenized and centrifuged at 500 × g (4°C) for 5 min. The
supernatants were discarded and the cell pellets were resuspended
in PBS; 1×105 cells were seeded into 6-well plates.
Cells were fixed with 4% paraformaldehyde for 1 h at 25°C. The TDT
Enzyme (EMD Millipore) was added to the wells, which were then
incubated for 1 h at 37°C in a dark humidified chamber. Stop/Wash
buffer (EMD Millipore) was added for 10 min at room temperature,
and the wells were rinsed with PBS before anti-digoxigenin
fluorescein (EMD Millipore) was added. The cells were then
incubated for 30 min at room temperature in a dark humidified
chamber. In the negative control group, TDT was omitted. The cells
were then incubated with DAPI for 15 min at 37°C in the dark. The
slides were mounted with neutral balsam (Sangon Biotech Co., Ltd.).
Stained cells were observed in five randomly selected fields of
view using a fluorescence microscope (Olympus Corporation). The
apoptotic index is expressed as the ratio of TUNEL+
(green) to DAPI+ cells (blue) ×100%.
Flow cytometric assessment of
apoptosis
Hippocampal neuron apoptosis was detected via flow
cytometry using the propidium iodide (PI)-Annexin V-FITC Apoptosis
Detection kit (BD Biosciences). Hippocampal tissues were
homogenized and centrifuged at 500 × g (4°C) for 5 min; the
supernatants were discarded and the cell pellets were resuspended
in 500 µl 1X Annexin V Binding Buffer. Subsequently, 5 µl Annexin
V-FITC and 5 µl PI were added and the cells were incubated for 10
min in the dark at room temperature. The cells were immediately
analyzed using a FACSCalibur™ flow cytometer (BD Biosciences) and
the percentage of apoptotic cells (early + late apoptosis) was
calculated by FlowJo software (version 7.6.2; FlowJo LLC).
Caspase-3 activity assay
Caspase-3 activity was measured using the
Caspase-3/CPP32 Colorimetric Assay kit (BioVision, Inc.).
Hippocampal tissues were homogenized and centrifuged at 500 × g
(4°C) for 5 min. The supernatants were discarded and the cell
pellets were resuspended in PBS; 1×105 cells were
incubated on ice for 10 min with 50 µl chilled lysis buffer. The
supernatant was then centrifuged at 12,000 × g for 5 min at 4°C,
and 100 µg protein (in a total volume of 40 µl) was added to 40 µl
2X reaction buffer containing 5 µl N-Acetyl-Asp-Glu-Val-Asp-pNA
substrate (150 µM final concentration). After incubation at 37°C
for 2 h, N-Acetyl-Asp-Glu-Val-Asp-pNA cleavage was monitored by
detecting the enzyme-catalyzed release of pNA using a microplate
reader (BioTek Instruments, Inc.) at 405 nm.
Reverse transcription-quantitative
(RT-q) PCR analysis
Total RNA was isolated from the hippocampal tissues
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) as per the manufacturer's instructions. cDNA was
synthesized using the PrimeScript™ Reverse Transcription Reagent
kit (Takara Biotechnology Co., Ltd.) with the following conditions:
37°C for 15 min, 85°C for 5 sec and maintained at 4°C. The qPCR
assay was performed using the SYBR®
Advantage® qPCR Premix (Takara Biotechnology Co., Ltd.)
and conducted on a 7500 Fast Real-Time System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The following thermocycling
conditions were used for qPCR: 95°C for 10 min; followed by 40
cycles of 95°C for 10 sec and 60°C for 60 sec. Relative mRNA levels
were calculated using the 2−ΔΔCq method (46). The primers sequences were as
follows: HO-1 forward, 5′-TTCAGAAGGGTCAGGTCC-3′ and reverse,
5′-CAGTGAGGCCCATACCAGAA-3′; and GAPDH forward,
5′-ACCCAGAAGACTGTGGATGG-3′ and reverse,
5′-CACATTGGGGGTAGGAACAC-3′.
Western blot analysis
Hippocampal tissues were homogenized in RIPA lysis
buffer (Beyotime Institute of Biotechnology) containing a protease
inhibitor cocktail (Sigma-Aldrich; Merck KGaA). Total protein was
quantified using a BCA protein assay kit (Beyotime Institute of
Biotechnology). Equal amounts of protein sample (30 µg/lane) were
separated via 10% SDS-PAGE, and then transferred onto
polyvinylidene fluoride membranes (BD Biosciences). After blocking
with 10% non-fat milk in Tris-buffered saline containing Tween-20
(50 mM Tris, pH 7.4, 250 mM NaCl and 0.1% Tween-20) at room
temperature for 1 h, the membranes were incubated with primary
antibodies against HO-1 (1:1,000; cat. no. ab189491; Abcam), Bax
(1:1,000; cat. no. 5023s; Cell Signaling Technology, Inc.), Bcl-2
(1:1,000; cat. no. 3498s; Cell Signaling Technology, Inc.),
caspase-3 (1:1,000; cat. no. 14220s; Cell Signaling Technology,
Inc.), cleaved (cl)-caspase-3 (1:1,000; cat. no. 9664s; Cell
Signaling Technology, Inc.) and β-actin (1:1,000; cat. no. 4967s;
Cell Signaling Technology, Inc.) overnight at 4°C. Following
treatment with the appropriate horseradish peroxidase-conjugated
anti-rabbit IgG (1:2,000; cat. no. 7074s; Cell Signaling
Technology, Inc.), the protein bands were visualized using an
enhanced chemiluminescence detection kit (Amersham; Cytiva) and
Kodak radiography film (FUJIFILM Wako Pure Chemical Corporation).
The Western blot results were normalized to those of the internal
control β-actin. The proteins were visualized using Image Quant™
LAS 4000 (GE Healthcare) and semi-quantified using ImageJ Software
(version 1.46; National Institutes of Health).
Statistical analysis
All data are expressed as the mean ± standard
deviation (SD). Survival rates were assessed using the Kaplan-Meier
method and compared using the log-rank test. The latency, distance
and time during water maze training were analyzed using two-way
ANOVA followed by Bonferroni's post hoc test. Other comparisons
among multiple groups were made using one-way ANOVA followed by
Tukey's multiple comparisons test. The statistical analyses were
performed using SPSS 18.0 software (SPSS, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
0.7% ISO increases the expression and
activity of HO-1 in the hippocampus of CLP-induced septic mice
RT-qPCR and western blot assays were performed to
investigate the expression of HO-1 in the hippocampus of
CLP-challenged mice. As shown in Fig.
1A-C, CLP resulted in an increase in HO-1 mRNA and protein
expression in the hippocampus, which was further enhanced by 0.7%
ISO. However, the elevation of HO-1 was attenuated by treatment
with the HO-1 inhibitor ZnPP. Similarly, HO-1 activity increased
substantially in the hippocampus of mice subjected to CLP, compared
with those in the control group. 0.7% ISO exposure resulted in
further elevation of HO-1 activity compared with the CLP group,
while ZnPP treatment reduced this augmented HO-1 activity (Fig. 1D). These results suggested that 0.7%
ISO increases the expression and activity of HO-1 in the
hippocampus of CLP-induced septic mice.
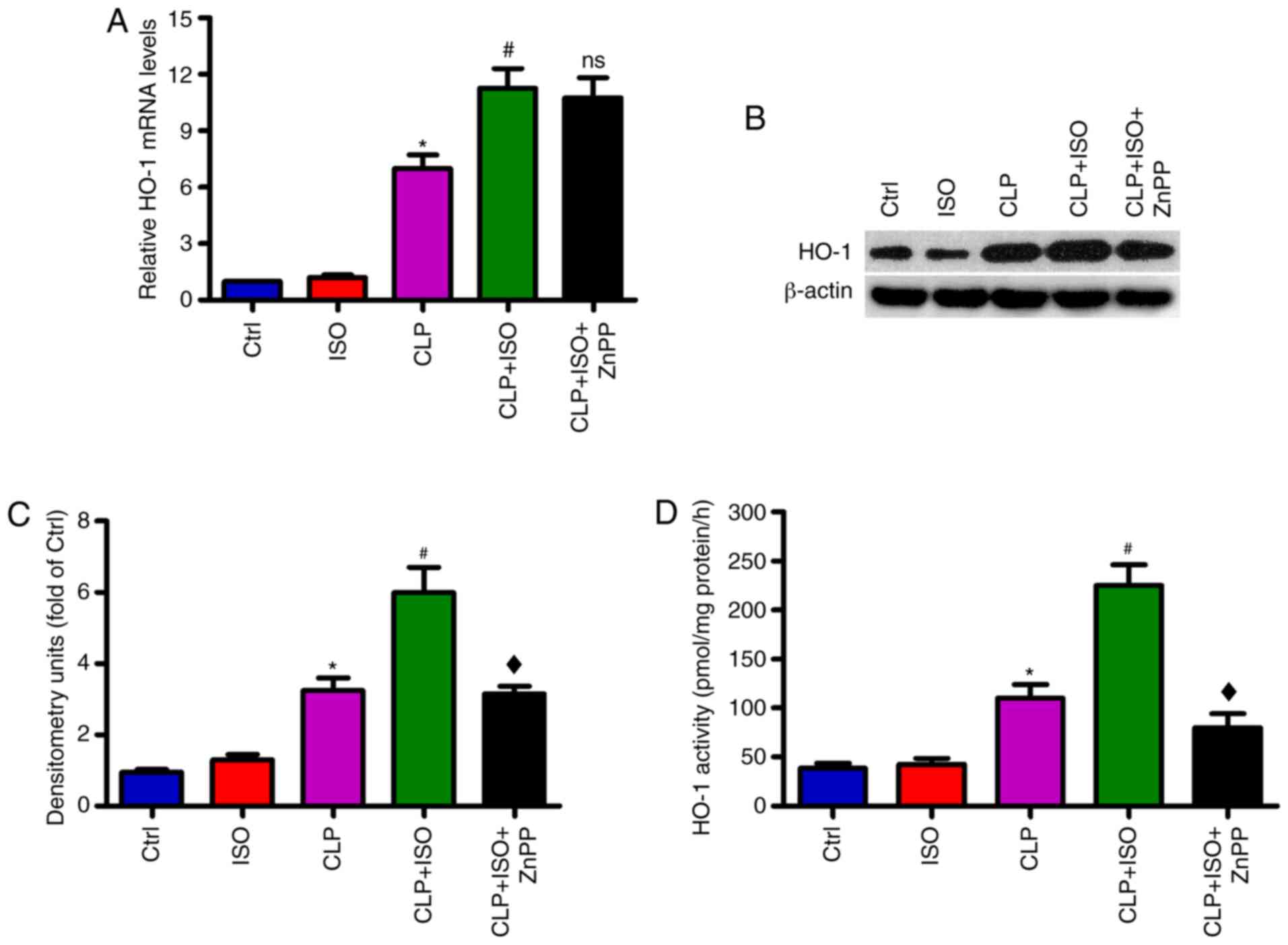 | Figure 1.0.7% ISO enhances HO-1 expression and
activity in the hippocampus of the CLP-induced mice. Mice were
treated with or without 0.7% ISO by inhalation for 1 h, starting at
1 and 6 h after sham or CLP surgery, respectively, in the presence
or absence of ZnPP (10 mg/kg), peritoneally administered 1 h before
sham or CLP surgery. At 48 h post-surgery, the mice were
anesthetized and the hippocampi were isolated. (A) Reverse
transcription-quantitative PCR and (B) western blotting assays were
performed to determine the mRNA and protein levels of HO-1. GAPDH
and β-actin were used as the endogenous controls. (C) HO-1 protein
levels were calculated and (D) HO-1 activity was detected.
Representative data are expressed as the mean ± SD from three
independent experiments. *P<0.05 vs. Ctrl or ISO group;
#P<0.05 vs. CLP group; ♦P<0.05 vs. CLP
+ ISO group. HO-1, heme oxygenase-1; ISO, isoflurane; CLP, cecal
ligation and puncture; ZnPP, Zinc protoporphyrin IX; Ctrl, control;
ns, not significant. |
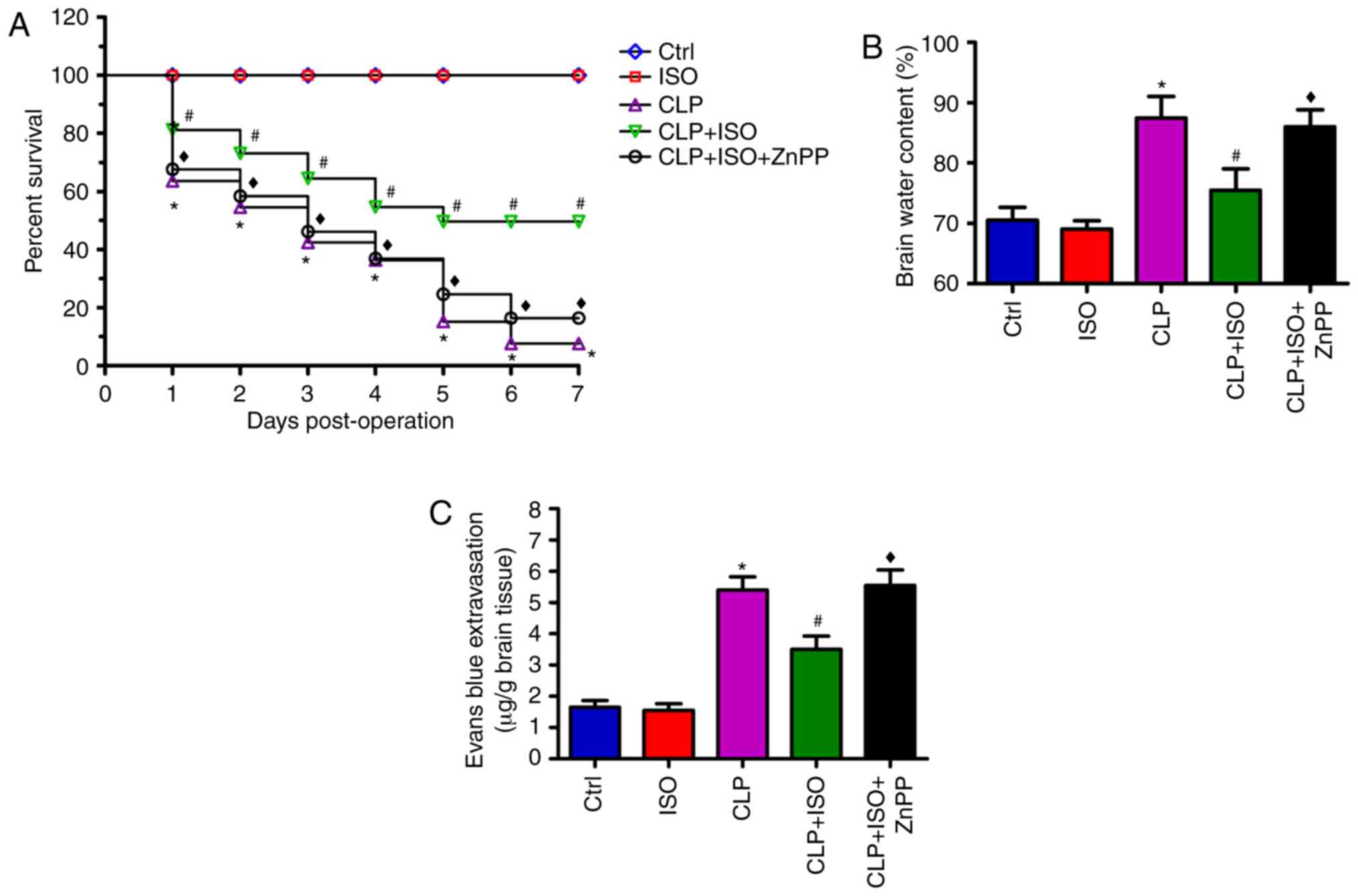
0.7% ISO reduces the mortality, brain
water content and BBB disruption in CLP-induced septic mice
Next, the effects of 0.7% ISO on the survival rate
of CLP-treated mice were investigated. During the 7-day observation
period, no mortalities were observed in the control mice or those
treated with ISO alone. However, the survival rate significantly
decreased following CLP surgery, compared with that of the control
group. 0.7% ISO significantly prolonged the survival time of
CLP-challenged mice, which was subsequently abolished by ZnPP
administration (Fig. 2A). As
depicted in Fig. 2B and C, mice in
the CLP group exhibited an increase in brain water content and a
decrease in BBB integrity. 0.7% ISO exposure attenuated these
effects in the CLP + ISO group compared with the CLP group.
However, these beneficial effects were reversed by ZnPP treatment.
These findings indicated that 0.7% ISO could decrease mortality
rates and brain edema, and increase BBB integrity in CLP-induced
mice, which partly depends on HO-1 activation.
0.7% ISO improves the memory and
learning capacity of CLP-induced septic mice
A MWM training assay was conducted to investigate
the effects of 0.7% ISO on the learning abilities of CLP-induced
mice. Compared with the control group, CLP-treated mice showed
markedly prolonged escape latency from the 5th day post-surgery.
CLP-challenged mice exposed to 0.7% ISO displayed considerably
shorter escape latency compared with mice that underwent CLP
surgery only. Furthermore, ZnPP administration attenuated the
effects of 0.7% ISO on escape latency (Fig. 3A). Of note, there were no
significant differences in the distance and time spent in the
target quadrant among all five groups (Fig. 3B and C). Following water maze
training, the platform was removed to analyze the role of 0.7% ISO
on the memory of CLP-challenged mice, including the distance, time
spent in the target quadrant, and the frequency at which the
platform was crossed. As depicted in Fig. 3D, the travelled distance was similar
among the five groups. However, the CLP-treated mice spent a
significantly shorter time in the target quadrant than the control
mice, which was significantly prolonged by 0.7% ISO; the time spent
at the quadrant was subsequently decreased by ZnPP treatment
(Fig. 3E). Similarly, the frequency
of platform crossing was significantly reduced in the CLP group
compared with the control mice, which was significantly increased
by 0.7% ISO, and decreased by ZnPP administration (Fig. 3F). These data demonstrated that 0.7%
ISO ameliorates the learning and memory functions of CLP-induced
septic mice, which is indicated to involve activated HO-1.
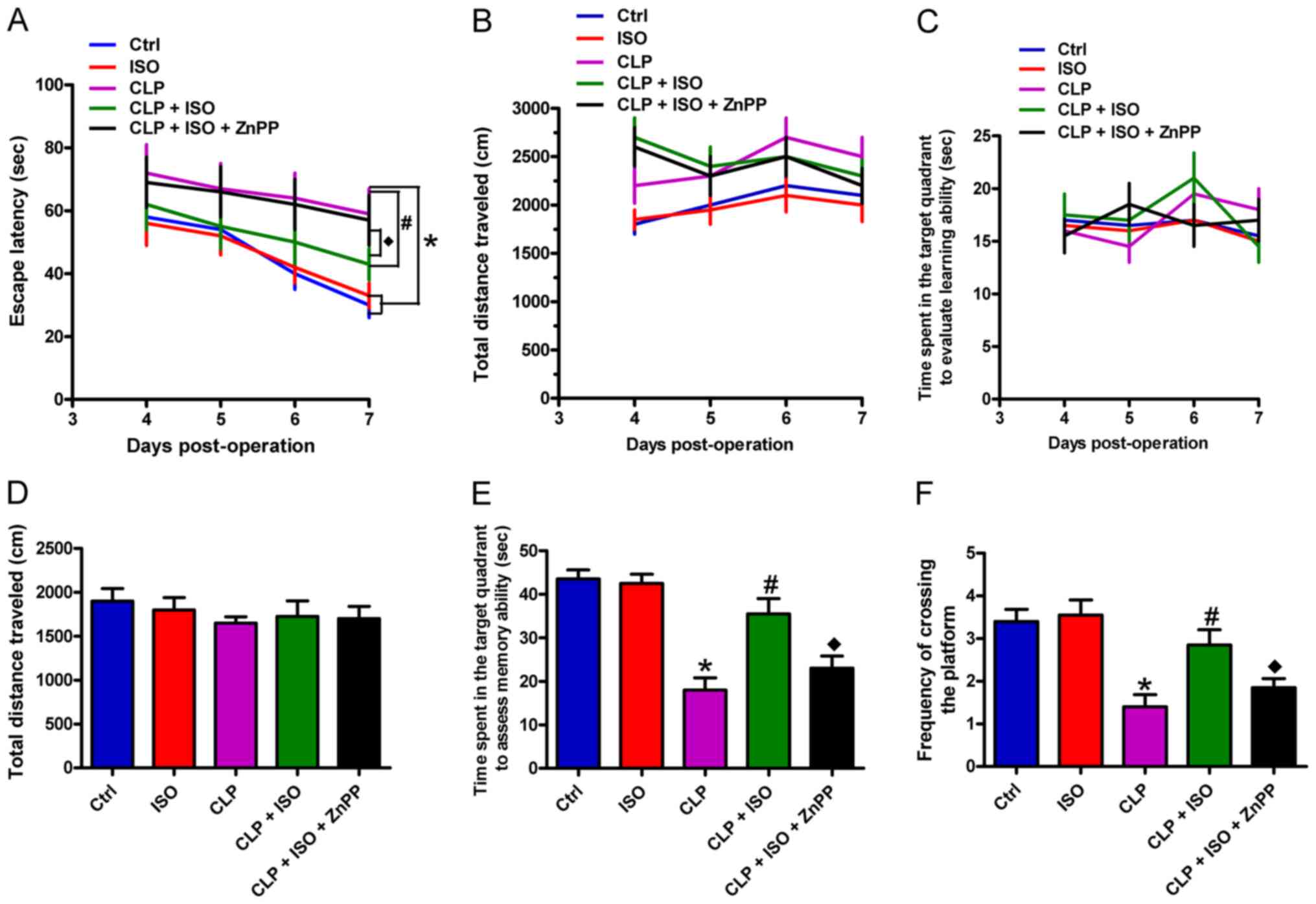 | Figure 3.0.7% ISO ameliorates the learning and
memory abilities of CLP-induced mice. Mice were treated with or
without 0.7% ISO by inhalation for 1 h, starting at 1 and 6 h after
sham or CLP treatment, respectively, in the presence or absence of
ZnPP (10 mg/kg), peritoneally administered 1 h before sham or CLP
surgery. Morris water maze training and hidden platform assays were
conducted from day 4 to 7 post-surgery. (A) Escape latency, (B)
total distance travelled and (C) time spent in the target quadrant
were recorded to evaluate the learning ability. (D) Total distance
travelled, (E) time spent in the target quadrant and (F) the
frequency of platform crossing were calculated to assess memory
ability. Representative data are expressed as the mean ± SD from
three independent experiments. *P<0.05 vs. Ctrl group;
#P<0.05 vs. CLP or ISO group; ♦P<0.05
vs. CLP + ISO group. ISO, isoflurane; CLP, cecal ligation and
puncture; ZnPP, zinc protoporphyrin IX; Ctrl, control. |
0.7% ISO inhibits CLP-induced
inflammation in the serum and hippocampus of mice
To identify the effects of 0.7% ISO on inflammatory
responses in CLP-induced septic mice, ELISA assays were conducted
to evaluate the levels of pro- and anti-inflammatory factors in the
serum and hippocampus. CLP was found to significantly increase the
levels of pro-inflammatory cytokines, including TNF-α (Fig. 4A), IL-1β (Fig. 4B) and IL-6 (Fig. 4C), and the anti-inflammatory
cytokine IL-10 (Fig. 4D) in the
serum. 0.7% ISO resulted in a significant reduction of TNF-α, IL-1β
and IL-6, and a further elevation of IL-10 in the sera of
CLP-treated mice. Whereas, ZnPP reversed the changes in TNF-α,
IL-1β, IL-6 and IL-10 expression (Fig.
4A-D). Consistently, 0.7% ISO exposure significantly reduced
the CLP-induced increase in the levels of TNF-α, IL-1β and IL-6,
and further enhanced the production of IL-10 in the hippocampus,
which were significantly counteracted by ZnPP (Fig. 4E-H). These results indicated that
HO-1 is highly important to the anti-inflammatory effects of 0.7%
ISO in CLP-induced septic mice.
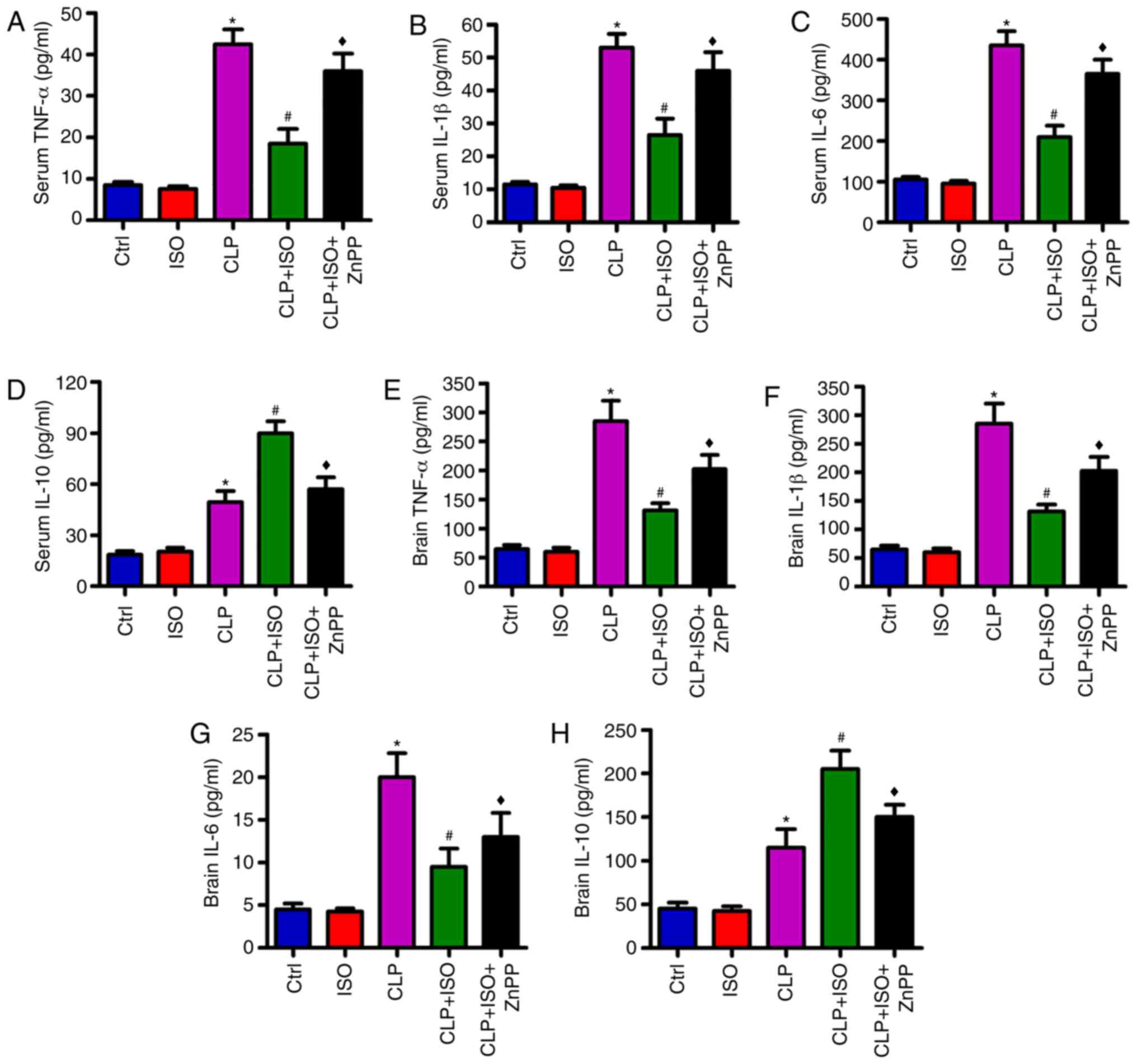 | Figure 4.0.7% ISO reduces the levels of
pro-inflammatory cytokines in the serum and hippocampal tissues of
CLP-treated mice. Mice were treated with or without 0.7% ISO by
inhalation for 1 h, starting at 1 and 6 h after sham or CLP
treatment, respectively, in the presence or absence of ZnPP (10
mg/kg), peritoneally administered 1 h before sham or CLP surgery.
At 48 h post-surgery, the mice were anesthetized and the serum and
hippocampi were collected. The levels of (A) TNF-α, (B) IL-1β, (C)
IL-6 and (D) IL-10 in the serum, and the production of (E) TNF-α,
(F) IL-1β, (G) IL-6 and (H) IL-10 in hippocampus were determined by
an enzyme-linked immunosorbent assay. Representative data are
expressed as the mean ± SD from three independent experiments.
*P<0.05 vs. Ctrl group; #P<0.05 vs. CLP or ISO
group; ♦P<0.05 vs. CLP + ISO group. ISO, isoflurane;
CLP, cecal ligation and puncture; ZnPP, zinc protoporphyrin IX;
Ctrl, control; TNF-α, tumor necrosis factor-α; IL-,
interleukin. |
0.7% ISO represses CLP-induced
oxidative stress in the serum and hippocampus of mice
To further elucidate the effects of 0.7% ISO on
sepsis-induced oxidative stress, the ROS and MDA contents, and
anti-oxidative SOD and CAT activities were determined in the serum
and hippocampal tissues of the mice. Compared with the control
group, the production of ROS (Fig.
5A) and MDA (Fig. 5B)
significantly increased, and the SOD (Fig. 5C) and CAT (Fig. 5D) activities significantly decreased
in the sera of CLP-challenged mice. 0.7% ISO exposure significantly
reduced the levels of ROS and MDA, and enhanced the activities of
SOD and CAT, which were reversed by ZnPP administration (Fig. 5A-D). Similarly, 0.7% ISO reduced
CLP-induced ROS and MDA, and enhanced CLP-reduced SOD and CAT
activity in the hippocampus, and ZnPP reversed these alterations
(Fig. 5E-H). These findings
illustrated that the antioxidative effects of 0.7% ISO on
CLP-induced septic mice are reliant on the activation of HO-1.
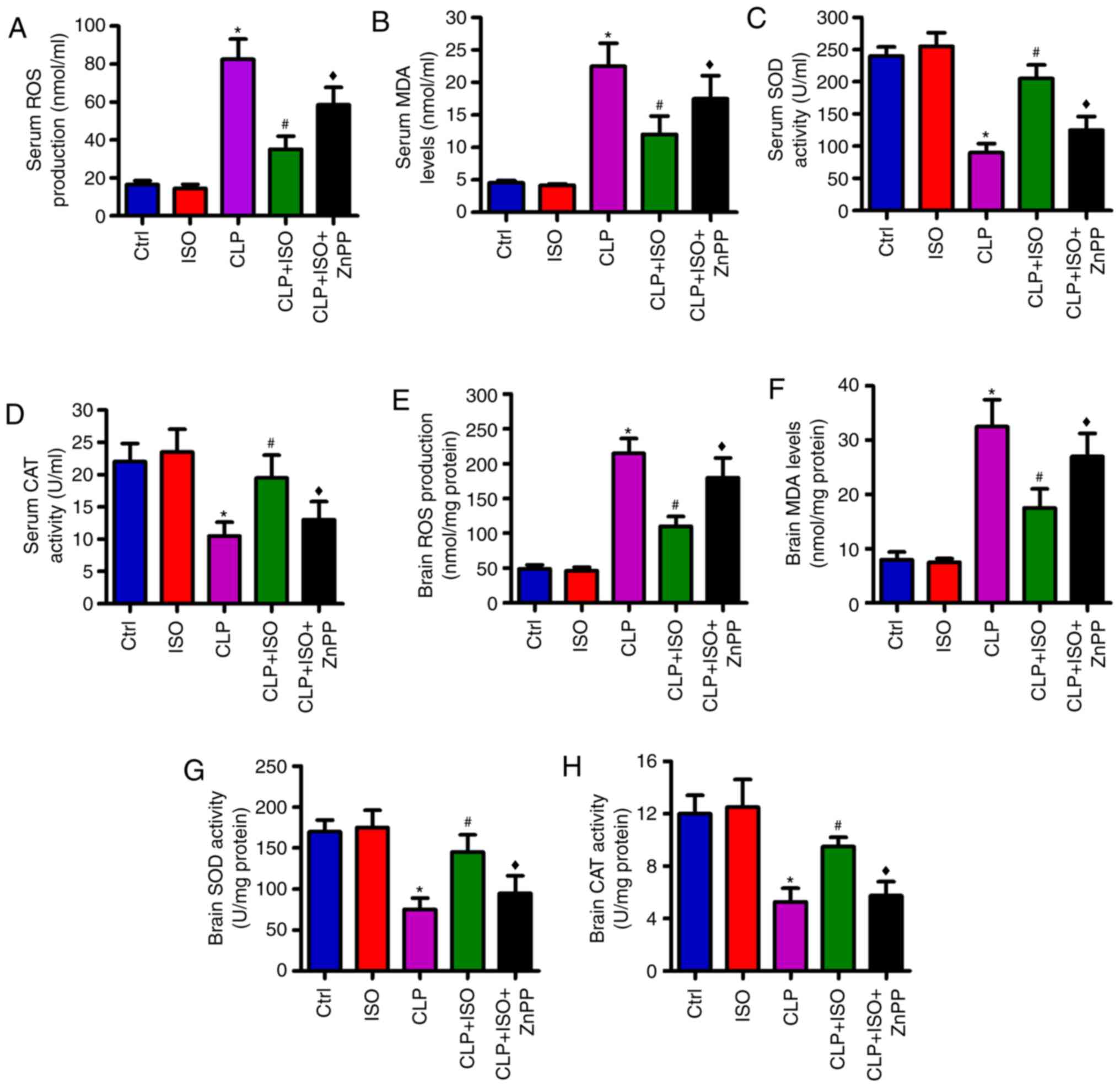 | Figure 5.0.7% ISO inhibits oxidation and
increases antioxidative processes in the serum and hippocampi of
CLP-injured mice. Mice were treated with or without 0.7% ISO by
inhalation for 1 h, starting at 1 and 6 h after sham or CLP
treatment, respectively, in the presence or absence of ZnPP (10
mg/kg), peritoneally administered 1 h before sham or CLP surgery.
At 48 h post-surgery, the mice were anesthetized and the serum and
hippocampus were collected. (A) ROS and (B) MDA content, as well as
(C) SOD and (D) CAT activity in the serum, the production of (E)
ROS and (F) MDA, and the activities of (G) SOD and (H) CAT in the
hippocampus were determined using commercial kits. Representative
data are expressed as mean ± SD from three independent experiments.
*P<0.05 vs. Ctrl group; #P<0.05 vs. CLP or ISO
group; ♦P<0.05 vs. CLP + ISO group. ISO, isoflurane;
CLP, cecal ligation and puncture; ZnPP, zinc protoporphyrin IX;
Ctrl, control; ROS, reactive oxygen species; MDA, malondialdehyde;
SOD, superoxide dismutase; CAT, catalase. |
0.7% ISO suppresses CLP-induced
neuronal apoptosis in the mouse hippocampus
Next, a TUNEL assay was performed to assess the
impacts of 0.7% ISO on CLP-induced neuronal apoptosis. As shown in
Fig. 6A and B, an increased number
of apoptotic neurons were observed in the hippocampus of
CLP-challenged mice than those of the control group. 0.7% ISO
significantly decreased the number of TUNEL+ cells in
the hippocampus, while ZnPP abrogated these protective effects. The
flow cytometry results also demonstrated that 0.7% ISO decreased
CLP-induced neuronal apoptosis (Fig. 6C
and D) in the hippocampus, which was reversed by ZnPP
administration. Furthermore, 0.7% ISO decreased CLP-induced
caspase-3 activation (Fig. 6E) in
the hippocampus, whereas ZnPP administration significantly reversed
this effect. On a molecular level, 0.7% ISO resulted in a
significant upregulation in anti-apoptotic Bcl-2 and downregulation
in pro-apoptotic Bax and cl-caspase-3 in the hippocampus of
CLP-treated mice. ZnPP reversed the expression changes in these
proteins (Fig. 6F-I). There were no
significance differences in the expression of caspase-3 among the
five groups (Fig. 6F and J). These
data indicated that HO-1 is implicated in the anti-apoptotic
functions of 0.7% ISO in CLP-induced septic mice.
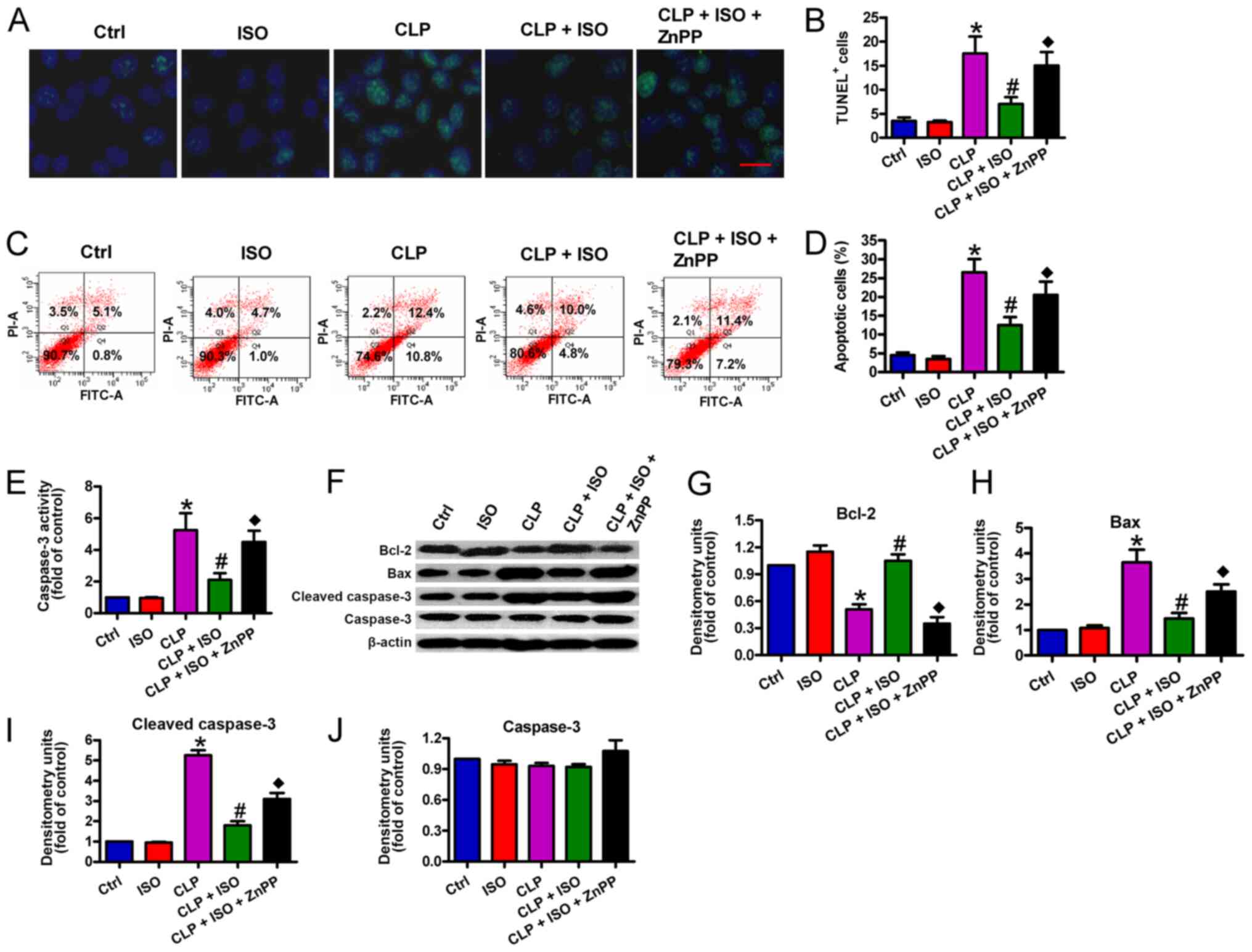 | Figure 6.0.7% ISO suppresses neuronal
apoptosis in the hippocampi of CLP-treated mice. Mice were treated
with or without 0.7% ISO by inhalation for 1 h, starting at 1 and 6
h after sham or CLP surgery respectively, in the presence or
absence of peritoneal ZnPP administration (10 mg/kg) at 1 h before
sham or CLP surgery. At 48 h post-surgery, the mice were
anesthetized and the hippocampi were collected. (A and B) TUNEL
assays were conducted and the number of TUNEL+ cells was
calculated (scale bar, 10 µm). (C and D) Neuronal apoptosis was
analyzed by flow cytometry and the percentage of apoptotic neurons
was determined. (E) Neuronal caspase-3 activity was detected. (F)
Representative western blotting results for Bcl-2, Bax,
cl-caspase-3 and caspase-3 detection; β-actin was used as the
endogenous control. Protein levels of (G) Bcl-2, (H) Bax, (I)
cl-caspase-3 and (J) caspase-3 were calculated. Representative data
are expressed as the mean ± SD from three independent experiments.
*P<0.05 vs. Ctrl group; #P<0.05 vs. CLP or ISO
group; ♦P<0.05 vs. CLP + ISO group. ISO, isoflurane;
CLP, cecal ligation and puncture; ZnPP, zinc protoporphyrin IX;
cl-, cleaved; Ctrl, control. |
Discussion
The present study demonstrated that 0.7% ISO exerted
neuroprotective effects on CLP-induced septic mice, which involved
HO-1 signaling. The following key findings were observed in septic
mice: i) ISO enhanced the expression and activity of HO-1 in the
hippocampus; ii) ISO improved the survival rate and reduced brain
water content and BBB disruption; iii) ISO ameliorated the memory
and learning ability; iv) ISO reduced the production of
pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) and increased
the release of the anti-inflammatory cytokine IL-10 in the serum
and hippocampus; v) ISO resulted in decreased oxidative products
(ROS and MDA) and a simultaneous increase in antioxidant enzyme
(SOD and CAT) activity in the serum and hippocampus; vi) ISO
inhibited neuronal apoptosis by upregulating Bcl-2 and
downregulating Bax and cl-caspase-3 in the hippocampus; and vii)
the neuroprotective effects of ISO were mediated by HO-1
activation. Overall, 0.7% ISO prevented CLP-associated neuronal
injury by reducing inflammation, oxidative stress and apoptosis,
which was dependent on HO-1 activation.
A previous study reported that the use of ISO during
surgery is considered optimal for neuroprotection (47). ISO post-conditioning notably
decreases the infarct volume and improves the neurobehavioral
performance of rats subjected to middle cerebral artery occlusion,
thereby providing increased ischemic tolerance in the brain
(48). ISO increases the weight
ratio and neuronal density of neonatal rats, and ameliorates the
memory and learning ability of adolescent rats following brain
hypoxia and ischemia (49). ISO
exerts a protective effect on hypoxia-induced neuronal injury in
the hippocampus by suppressing apoptosis (50). The present study highlighted that
ISO exerted neuroprotection against CLP-induced SAE in mice by
increasing overall survival, and reducing brain water content and
BBB disruption, as well as increasing the memory capacity and
learning ability.
Systemic and neuroinflammation can induce
endothelial cell damage, microglial activation and neuronal death
(51). As a critical structure of
learning and memory, the hippocampus is vulnerable to systemic
inflammatory factors (52,53), and pro-inflammatory cytokines, such
as TNF-α, IL-1β and IL-6, can readily promote endothelial
dysfunction, BBB disruption and brain edema (54,55).
IL-10 is a potent anti-inflammatory cytokine that alleviates such
injuries (56). Reportedly, ISO
represses neuroinflammation, coinciding with a decrease in TNF-α
and IL-1β in the mouse brain following subarachnoid hemorrhage
(57). In the current study, 0.7%
ISO decreased the production of TNF-α, IL-1β and IL-6, and
increased IL-10 generation in the serum and hippocampi of
CLP-induced septic mice. Previous studies have suggested that ISO
induces HO-1 expression in Hep3B cells (58) and hippocampal neurons (36). HO-1 signaling mediates the
anti-inflammatory effects of ISO in CLP-induced lung injury
(27) and
ischemia/reperfusion-induced liver injury (59), as well as in LPS-challenged
macrophages (35). Consistently,
the present study revealed that 0.7% ISO induced the expression and
activity of HO-1 in the hippocampi of CLP-treated mice, and that
ZnPP reversed the anti-inflammatory abilities of 0.7% ISO,
suggesting that the protective effects of 0.7% ISO against
CLP-induced sepsis partially rely on activated HO-1 signaling.
Oxidative stress is one of the primary causes of SAE
during sepsis (60). Excessive ROS
generation induces lipid peroxidation, disrupts cellular and
mitochondrial membranes, and ultimately results in the apoptosis
and necrosis of cells (61). MDA is
a direct product of lipid peroxidation and is recognized as an
indicator of ROS-mediated injury (62). Antioxidant enzymes, such as SOD and
CAT, are key ROS scavengers that specifically eliminate superoxide
radicals and prevent ROS-induced brain damage during sepsis
(24). A published review
summarizes the anti-oxidative aspects of ISO in acute brain injury
(63). HO-1 is a key participant in
sepsis (27–29), and has been reported to hamper
oxidative stress during sepsis-induced acute lung injury (64). ISO enhanced HO-1 expression in the
lungs of CLP-treated rats (27).
Herein, the present findings demonstrated that 0.7% ISO decreased
the ROS and MDA content, and enhanced SOD and CAT activity in the
serum and hippocampi of CLP-induced septic mice. Moreover, ZnPP
administration reversed these effects, indicating that HO-1
signaling is implicated in the anti-oxidative effects of 0.7% ISO
in septic mice.
Due to its close association with cognitive
dysfunction, neuronal apoptosis in the hippocampus is a major
causative factor of SAE (65). The
balance between anti- and pro-apoptotic Bcl-2 family proteins
dominates the progression of neuron apoptosis (66). Pro-apoptotic Bax translocates to the
outer mitochondrial membrane, disrupting mitochondrial
transmembrane potential and ultimately promoting cell death. On the
contrary, anti-apoptotic Bcl-2 inhibits Bax activation (67). Caspase-3, the final apoptotic
executor, plays a crucial role in neuronal apoptosis (14). ISO has been confirmed to increase
Bcl-2 expression and reduce cytochrome c release from mitochondria
in the ischemic penumbra of the rat brain (68). Bedirli et al (69) reported that ISO upregulates Bcl-2
and downregulates Bax in the rat brain following focal cerebral
ischemia. ISO reduces oxygen and glucose deprivation-induced
hippocampal neuron apoptosis by inactivating caspase-3 (50). HO-1 signaling mediates the
inhibitory effects of 0.7% ISO on zymosan-induced pulmonary cell
apoptosis (70). In line with
previous findings, it was confirmed in the present study that 0.7%
ISO repressed neuronal apoptosis in the hippocampus of CLP-induced
septic mice, which was accompanied by reduced expression of Bax and
cl-caspase-3, and increased Bcl-2 expression. Treatment with ZnPP,
a HO-1 inhibitor, neutralized the anti-apoptotic effects of 0.7%
ISO, implying that HO-1 signaling is involved in the 0.7%
ISO-reduced neuronal apoptosis in CLP-induced septic mice.
In conclusion, the present study demonstrated that
0.7% ISO plays crucial neuroprotective roles in a CLP-established
SAE mouse model, largely involving the activation of HO-1. The
upregulation and activation of HO-1 mediates the anti-inflammatory,
antioxidative and anti-apoptotic effects of 0.7% ISO on CLP-induced
neuronal damage in the brains of septic mice. These findings
provided strong evidence for the potential application of 0.7% ISO
for the treatment of patients with SAE.
Acknowledgements
Not applicable.
Funding
This study was supported in part by the National
Natural Science Foundation of China (grant no. 81401138).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW contributed to the conception and design of the
present study. LZ and XZ conducted all the experiments. ZW and LZ
drafted the manuscript and revised it critically for important
intellectual content. TW and XP interpreted and analyzed the data.
ZW provided final approval of the version to be published. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experimental protocols were approved by
the Animal Care and Use Committee of Xi'an Jiaotong University
(approval no. XAJU.No20180730A10120) and were conducted in
accordance with the National Institutes of Health guidelines for
the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Delano MJ and Ward PA: Sepsis-induced
immune dysfunction: Can immune therapies reduce mortality? J Clin
Invest. 126:23–31. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dellinger RP, Levy MM, Rhodes A, Annane D,
Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke
R, et al: Surviving sepsis campaign: International guidelines for
management of severe sepsis and septic shock: 2012. Crit Care Med.
41:580–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gong Y, Lan H, Yu Z, Wang M, Wang S, Chen
Y, Rao H, Li J, Sheng Z and Shao J: Blockage of glycolysis by
targeting PFKFB3 alleviates sepsis-related acute lung injury via
suppressing inflammation and apoptosis of alveolar epithelial
cells. Biochem Biophys Res Commun. 491:522–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alobaidi R, Basu RK, Goldstein SL and
Bagshaw SM: Sepsis-associated acute kidney injury. Semin Nephrol.
35:2–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martin L, Derwall M, Al Zoubi S,
Zechendorf E, A Reuter D, Thiemermann C and Schuerholz T: The
septic heart: Current understanding of molecular mechanisms and
clinical implications. Chest. 155:427–437. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan J and Li S and Li S: The role of the
liver in sepsis. Int Rev Immunol. 33:498–510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Y, Wang K, Ma Z, Liu D, Yang Y, Sun M,
Wen A, Hao Y, Ma S, Ren F, et al: SIRT1 activation by butein
attenuates sepsis-induced brain injury in mice subjected to cecal
ligation and puncture via alleviating inflammatory and oxidative
stress. Toxicol Appl Pharmacol. 363:34–46. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gofton TE and Young GB: Sepsis-associated
encephalopathy. Nat Rev Neurol. 8:557–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang LN, Wang XT, Ai YH, Guo QL, Huang L,
Liu ZY and Yao B: Epidemiological features and risk factors of
sepsis-associated encephalopathy in intensive care unit patients:
2008–2011. Chin Med J (Engl). 125:828–831. 2012.PubMed/NCBI
|
|
10
|
Iwashyna TJ, Ely EW, Smith DM and Langa
KM: Long-term cognitive impairment and functional disability among
survivors of severe sepsis. JAMA. 304:1787–1794. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barichello T, Martins MR, Reinke A, Feier
G, Ritter C, Quevedo J and Dal-Pizzol F: Cognitive impairment in
sepsis survivors from cecal ligation and perforation. Crit Care
Med. 33:221–223, 262-223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Semmler A, Frisch C, Debeir T, Ramanathan
M, Okulla T, Klockgether T and Heneka MT: Long-term cognitive
impairment, neuronal loss and reduced cortical cholinergic
innervation after recovery from sepsis in a rodent model. Exp
Neurol. 204:733–740. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwalm MT, Pasquali M, Miguel SP, JPAD
Santos, Vuolo F, Comim CM, Petronilho F, Quevedo J, Gelain DP,
Moreira JCF, et al: Acute brain inflammation and oxidative damage
are related to long-term cognitive deficits and markers of
neurodegeneration in sepsis-survivor rats. Mol Neurobiol.
49:380–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Comim CM, Barichello T, Grandgirard D,
Dal-Pizzol F, Quevedo J and Leib SL: Caspase-3 mediates in part
hippocampal apoptosis in sepsis. Mol Neurobiol. 47:394–398. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heine H, Rietschel ET and Ulmer AJ: The
biology of endotoxin. Mol Biotechnol. 19:279–296. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vaure C and Liu Y: A comparative review of
toll-like receptor 4 expression and functionality in different
animal species. Front Immunol. 5:3162014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shukla P, Rao GM, Pandey G, Sharma S,
Mittapelly N, Shegokar R and Mishra PR: Therapeutic interventions
in sepsis: Current and anticipated pharmacological agents. Br J
Pharmacol. 171:5011–5031. 2014.PubMed/NCBI
|
|
18
|
Sonneville R, Verdonk F, Rauturier C,
Klein IF, Wolff M, Annane D, Chretien F and Sharshar T:
Understanding brain dysfunction in sepsis. Ann Intensive Care.
3:152013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Danielski LG, Giustina AD, Badawy M,
Barichello T, Quevedo J, Dal-Pizzol F and Petronilho F: Brain
barrier breakdown as a cause and consequence of neuroinflammation
in sepsis. Mol Neurobiol. 55:1045–1053. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abramowitz J and Birnbaumer L: Physiology
and pathophysiology of canonical transient receptor potential
channels. FASEB J. 23:297–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fleming I, Fisslthaler B and Busse R:
Calcium signaling in endothelial cells involves activation of
tyrosine kinases and leads to activation of mitogen-activated
protein kinases. Circ Res. 76:522–529. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barichello T, Fortunato JJ, Vitali AM,
Feier G, Reinke A, Moreira JCF, Quevedo J and Dal-Pizzol F:
Oxidative variables in the rat brain after sepsis induced by cecal
ligation and perforation. Crit Care Med. 34:886–889. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dal-Pizzol F, Ritter C, Cassol OJ Jr,
Rezin GT, Petronilho F, Zugno AI, Quevedo J and Streck EL:
Oxidative mechanisms of brain dysfunction during sepsis. Neurochem
Res. 35:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoetzel A and Schmidt R: Regulatory role
of anesthetics on heme oxygenase-1. Curr Drug Targets.
11:1495–1503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alcaraz MJ, Fernandez P and Guillen MI:
Anti-inflammatory actions of the heme oxygenase-1 pathway. Curr
Pharm Des. 9:2541–2551. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen K, Gunter K and Maines MD: Neurons
overexpressing heme oxygenase-1 resist oxidative stress-mediated
cell death. J Neurochem. 75:304–313. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong X, Hu R, Sun Y, Li Q and Jiang H:
Isoflurane post-treatment improves pulmonary vascular permeability
via upregulation of heme oxygenase-1. Exp Lung Res. 39:295–303.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu JB, Zhou F, Yao SL, Tang ZH, Wang M and
Chen HR: Effect of heme oxygenase-1 on the kidney during septic
shock in rats. Transl Res. 153:283–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park JS, Choi HS, Yim SY and Lee SM: Heme
oxygenase-1 protects the liver from septic injury by modulating
TLR4-mediated mitochondrial quality control in mice. Shock.
50:209–218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu L, Xie K, Chen H, Dong X, Li Y and Yu
Y, Wang G and Yu Y: Inhalation of hydrogen gas attenuates brain
injury in mice with cecal ligation and puncture via inhibiting
neuroinflammation, oxidative stress and neuronal apoptosis. Brain
Res. 1589:78–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Flondor M, Hofstetter C, Boost KA, Betz C,
Homann M and Zwissler B: Isoflurane inhalation after induction of
endotoxemia in rats attenuates the systemic cytokine response. Eur
Surg Res. 40:1–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jamnicki-Abegg M, Weihrauch D, Pagel PS,
Kersten JR, Bosnjak ZJ, Warltier DC and Bienengraeber MW:
Isoflurane inhibits cardiac myocyte apoptosis during oxidative and
inflammatory stress by activating Akt and enhancing Bcl-2
expression. Anesthesiology. 103:1006–1014. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Head BP and Patel P: Anesthetics and brain
protection. Curr Opin Anaesthesiol. 20:395–399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sakai H, Sheng H, Yates RB, Ishida K,
Pearlstein RD and Warner DS: Isoflurane provides long-term
protection against focal cerebral ischemia in the rat.
Anesthesiology. 106:92–99; discussion 98–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li QF, Zhu YS, Jiang H, Xu H and Sun Y:
Heme oxygenase-1 mediates the anti-inflammatory effect of
isoflurane preconditioning in LPS-stimulated macrophages. Acta
Pharmacol Sin. 30:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Q, Zhu Y, Jiang H, Xu H and Liu H:
Up-regulation of heme oxygenase-1 by isoflurane preconditioning
during tolerance against neuronal injury induced by oxygen glucose
deprivation. Acta Biochim Biophys Sin (Shanghai). 40:803–810. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sackey PV, Martling CR, Carlswärd C,
Sundin O and Radell PJ: Short- and long-term follow-up of intensive
care unit patients after sedation with isoflurane and midazolam-a
pilot study. Crit Care Med. 36:801–806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li JT, Wang H, Li W, Wang LF, Hou LC, Mu
JL, Liu X, Chen HJ, Xie KL, Li NL and Gao CF: Anesthetic isoflurane
posttreatment attenuates experimental lung injury by inhibiting
inflammation and apoptosis. Mediators Inflamm. 2013:1089282013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang H, Fan J, Li NL, Li JT, Yuan SF, Yi
J, Wang L, Chen JH, Lv YG, Yao Q, et al: A subanesthetic dose of
isoflurane during postconditioning ameliorates zymosan-induced
neutrophil inflammation lung injury and mortality in mice.
Mediators Inflamm. 2013:4796282013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang H, Wang L, Li NL, Li JT, Yu F, Zhao
YL, Wang L, Yi J, Wang L, Bian JF, et al: Subanesthetic isoflurane
reduces zymosan-induced inflammation in murine Kupffer cells by
inhibiting ROS-activated p38 MAPK/NF-κB signaling. Oxid Med Cell
Longev. 2014:8516922014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kilkenny C, Browne W, Cuthill IC, Emerson
M and Altman DG; National Centre for the Replacement, Refinement
and Reduction of Amimals in Research, : Animal research: Reporting
in vivo experiments-the ARRIVE guidelines. J Cereb Blood Flow
Metab. 31:991–993. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Toscano MG, Ganea D and Gamero AM: Cecal
ligation puncture procedure. J Vis Exp. 7:28602011.
|
|
43
|
Mu J, Xie K, Hou L, Peng D, Shang L, Ji G,
Li J, Lu Y and Xiong L: Subanesthetic dose of isoflurane protects
against zymosan-induced generalized inflammation and its associated
acute lung injury in mice. Shock. 34:183–189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sui DM, Xie Q, Yi WJ, Gupta S, Yu XY, Li
JB, Wang J, Wang JF and Deng XM: Resveratrol protects against
sepsis-associated encephalopathy and inhibits the NLRP3/IL-1β axis
in microglia. Mediators Inflamm. 2016:10456572016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hatashita S, Hoff JT and Salamat SM:
Ischemic brain edema and the osmotic gradient between blood and
brain. J Cereb Blood Flow Metab. 8:552–559. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jiang M, Sun L, Feng DX, Yu ZQ, Gao R, Sun
YZ and Chen G: Neuroprotection provided by isoflurane
pre-conditioning and post-conditioning. Med Gas Res. 7:48–55. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang S, Yin J, Ge M, Dai Z, Li Y, Si J, Ma
K, Li L and Yao S: Transforming growth-beta 1 contributes to
isoflurane postconditioning against cerebral ischemia-reperfusion
injury by regulating the c-Jun N-terminal kinase signaling pathway.
Biomed Pharmacother. 78:280–290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu Y, Xue H, Zhao P, Yang Y, Ji G, Yu W,
Han G, Ding M and Wang F: Isoflurane postconditioning induces
concentration- and timing-dependent neuroprotection partly mediated
by the GluR2 AMPA receptor in neonatal rats after brain
hypoxia-ischemia. J Anesth. 30:427–436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhao DA, Bi LY, Huang Q, Zhang FM and Han
ZM: Isoflurane provides neuroprotection in neonatal hypoxic
ischemic brain injury by suppressing apoptosis. Braz J Anesthesiol.
66:613–621. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Matsuda A, Jacob A, Wu R, Aziz M, Yang WL,
Matsutani T, Suzuki H, Furukawa K, Uchida E and Wang P: Novel
therapeutic targets for sepsis: Regulation of exaggerated
inflammatory responses. J Nippon Med Sch. 79:4–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Calabresi P, Castrioto A, Di Filippo M and
Picconi B: New experimental and clinical links between the
hippocampus and the dopaminergic system in Parkinson's disease.
Lancet Neurol. 12:811–821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Alexander JJ, Jacob A, Cunningham P,
Hensley L and Quigg RJ: TNF is a key mediator of septic
encephalopathy acting through its receptor, TNF receptor-1.
Neurochem Int. 52:447–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rothwell NJ and Hopkins SJ: Cytokines and
the nervous system II: Actions and mechanisms of action. Trends
Neurosci. 18:130–136. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sharief MK and Thompson EJ: In vivo
relationship of tumor necrosis factor-alpha to blood-brain barrier
damage in patients with active multiple sclerosis. J Neuroimmunol.
38:27–33. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Williams LM, Ricchetti G, Sarma U, Smallie
T and Foxwell BM: Interleukin-10 suppression of myeloid cell
activation-a continuing puzzle. Immunology. 113:281–292. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Altay O, Suzuki H, Hasegawa Y, Ostrowski
RP, Tang J and Zhang JH: Isoflurane on brain inflammation.
Neurobiol Dis. 62:365–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li QF, Wang XR, Yang YW and Su DS:
Up-regulation of hypoxia inducible factor 1alpha by isoflurane in
Hep3B cells. Anesthesiology. 105:1211–1219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Schmidt R, Tritschler E, Hoetzel A, Loop
T, Humar M, Halverscheid L, Geiger KK and Pannen BH: Heme
oxygenase-1 induction by the clinically used anesthetic isoflurane
protects rat livers from ischemia/reperfusion injury. Ann Surg.
245:931–942. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hopkins RO: Sepsis, oxidative stress, and
brain injury. Crit Care Med. 35:2233–2234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chandra J, Samali A and Orrenius S:
Triggering and modulation of apoptosis by oxidative stress. Free
Radic Biol Med. 29:323–333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Requena JR, Fu MX, Ahmed MU, Jenkins AJ,
Lyons TJ and Thorpe SR: Lipoxidation products as biomarkers of
oxidative damage to proteins during lipid peroxidation reactions.
Nephrol Dial Transplant. 11 (Suppl 5):48–53. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang T, Sun Y and Zhang F: Anti-oxidative
aspect of inhaled anesthetic gases against acute brain injury. Med
Gas Res. 6:223–226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yu J, Wang Y, Li Z, Dong S, Wang D, Gong
L, Shi J, Zhang Y, Liu D and Mu R: Effect of heme oxygenase-1 on
mitofusin-1 protein in LPS-induced ALI/ARDS in rats. Sci Rep.
6:365302016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Semmler A, Okulla T, Sastre M,
Dumitrescu-Ozimek L and Heneka MT: Systemic inflammation induces
apoptosis with variable vulnerability of different brain regions. J
Chem Neuroanat. 30:144–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yao XL, Liu J, Lee E, Ling GS and McCabe
JT: Progesterone differentially regulates pro- and anti-apoptotic
gene expression in cerebral cortex following traumatic brain injury
in rats. J Neurotrauma. 22:656–668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Renault TT, Teijido O, Antonsson B, Dejean
LM and Manon S: Regulation of Bax mitochondrial localization by
Bcl-2 and Bcl-x(L): Keep your friends close but your enemies
closer. Int J Biochem Cell Biol. 45:64–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li L, Peng L and Zuo Z: Isoflurane
preconditioning increases B-cell lymphoma-2 expression and reduces
cytochrome c release from the mitochondria in the ischemic penumbra
of rat brain. Eur J Pharmacol. 586:106–113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bedirli N, Bagriacik EU, Emmez H, Yilmaz
G, Unal Y and Ozkose Z: Sevoflurane and isoflurane preconditioning
provides neuroprotection by inhibition of apoptosis-related mRNA
expression in a rat model of focal cerebral ischemia. J Neurosurg
Anesthesiol. 24:336–344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang L, Zhao YL, Liu NN, Zhu XS, Liu QQ,
Mei HY, Wang LF, Yang AG, Gao CF and Li JT: Epithelial HO-1/STAT3
affords the protection of subanesthetic isoflurane against
zymosan-induced lung injury in mice. Oncotarget. 8:54889–54903.
2017. View Article : Google Scholar : PubMed/NCBI
|