Introduction
Stem cell therapy has been broadly used to treat a
variety of diseases, including acute/chronic inflammatory and
vascular diseases (1–3). The primary sources of stem cells are
the bone marrow (BM), dental tissue, adipose tissue, and umbilical
cord blood. BM is considered the primary source of multipotent stem
cells and thus has been used as an important source of stem cells
for clinical cellular therapy (4–8).
However, BM stem cell culture requires BM aspiration via femoral
puncture, an invasive procedure that may be both a risk and a
burden to patients. Additionally, BM stem cell viability ex
vivo is influenced by the patient's unique background (9). Thus, BM stem cells from patients with
critical illnesses may not be suitable for ex vivo culture
for transplantation purposes.
Studies have been carried out to investigate adipose
tissue as an alternative to BM, and adipose-derived stem cells
(ADSCs) obtained through minimally invasive procedures have been
found to express stem cell-specific markers. Additionally, these
ADSCs have also been found to possess the ability for self-renewal,
differentiation into multiple cell lineages, and paracrine action,
which are similar characteristics to those observed in BM
mesenchymal stem cells (BMSCs). Notably, ADSCs have a high rate of
repopulation in vitro, thereby providing sufficient cell
numbers for patient use; moreover, as compared to that of BMSCs,
the cellular activity of ADSC has been reported to be rarely
altered by the underlying disease condition of the donor. The
efficacy of ADSCs has been confirmed in the treatment of many
disorders and injuries, including muscle injury, cartilage damage,
neurodegenerative disorders, and rheumatoid arthritis (10–13).
Thus, ADSCs have emerged as a crucial alternative source of stem
cells.
ADSC transplantation is typically performed using
autologous stem cells from host adipose tissue; the main
beneficiary group requiring stem cell therapy is the elderly,
rather than the young. With aging, the number of inflammatory
markers present in the human body also tends to increase (14,15)
and thus, the elderly are more susceptible to illnesses than the
young. Alterations in the immune responsiveness of the elderly can
affect their stem cell activity, given that stem cells are
profoundly injured by changes in physiological conditions, such as
aging or systemic inflammation. BMSCs from the elderly exhibit a
reduced differentiation capacity with a low level of cytokine
production (16–20). The same has been observed for ADSCs,
and the reduced cell activity of ADSCs can undeniably lead to
compromised therapeutic effects (21).
Therefore, donor age is a critical factor in
estimating the efficacy of stem cell therapy. However, studies on
the effect of age on ADSC activity remain relatively inconclusive;
therefore, this study aimed to address this gap, which is crucial
for stem cell therapy using ADSCs.
We hypothesized that age affects the cellular
activity of ADSCs. To verify our hypothesis, we comparatively
analyzed the essential functions of ADSCs obtained from young and
elderly donors, by evaluating the cell proliferation rate,
differentiation potential, and cytokine profile.
Materials and methods
Materials
Healthy adipose tissues were provided by the Kyung
Hee University Medical Center [Total of eight donors (6 male, 2
female); Seoul, Korea; (IRB# 2016-12-022)] with donors' written
consent. Adipose tissue (2×2 cm) was suspended in saline in a 50 ml
tube at 4°C and transferred to the cell culture room. ADSC
isolation was performed immediately upon arrival to the culture
room. ADSCs from healthy young individuals were purchased from
ScienCell Research Laboratories (individuals <30 years old).
Alizarin Red S powder, Oil Red O solution, formaldehyde solution,
and cetylpyridinium chloride were purchased from Sigma-Aldrich;
Merck KGaA. Stempro osteogenesis and adipogenesis kits, α-MEM
medium, and FBS were purchased from Gibco; Thermo Fisher
Scientific, Inc. Phosphate-buffered saline,
penicillin/streptomycin, and 0.25% trypsin-EDTA solution were
provided by Welgene. Vascular endothelial growth factor (VEGF),
hepatocyte grow factor (HGF), stromal cell-derived factor-α
(SDF-1α), transforming growth factor-β1 (TGF-β1), and the bone
morphogenetic protein (BMP)-2 ELISA kits were obtained from R&D
Systems.
Cell culture
ADSCs were isolated as previously reported (21) and cultured in an α-MEM medium
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin. The culture medium was replaced with fresh
medium every other day. The ADSCs between 3rd and 5th passages were
used for all the experiments. Endothelial progenitor cells (EPCs)
were cultured from BM mononuclear cells (STEMCELL, 70001.4) in an
EGM-2 medium (Lonza) on fibronectin-coated dishes. The medium was
changed every other day and cells in passage 2 were used for this
study. ADSC was analyzed for specific marker expression by
FACSCalibur using Cell Quest software (version 3.0, BD
Biosciences).
WST-1 assay
The ADSCs (2×104 cells/well) were seeded
in a 96-well plate and incubated for 24 h. Ten microliters of WST-1
solution was added at 10% of the total medium volume, and the plate
was incubated for 90 min at 37°C with 5% CO2. Optical
density values for each well were measured at 450 nm using a Versa
Max Microplate Reader (Molecular Devices).
Cytokine measurement
The ADSCs (5×105 cells/well) were seeded
in a 6-well plate in 2.5 ml of α-MEM medium. The concentrations of
VEGF, HGF, SDF-1α, TGF-β1 and BMP-2 in the conditioned medium were
analyzed using ELISA kits according to the manufacturer's
instructions.
Matrigel tube formation assay
Matrigel (BD Biosciences, 100 µl) was applied on 48
well-plates and incubated for 30 min at 37°C. The EPCs
(2×104 cells at passage 2) in conditioned medium from
ADSCs (young or elderly) were added to the Matrigel matrix and
incubated for 3 h at 37°C with 5% CO2. The effect of
ADSC paracrine factors on tube structure was observed under a
stereomicroscope.
Sample preparation and western blot
analysis
Protein lysates were quantified and then denatured
and electrophoresed using sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to a nitrocellulose membrane. After
blocking with 5% skim milk, the membranes were incubated with
primary antibodies for bone morphogenetic protein receptor 1A
(BMPR1A) and GAPDH, followed by anti-immunoglobulin G horseradish
peroxidase-conjugated secondary antibody. The blots were developed
and visualized with ECL (Dogen Bio) and analyzed under
chemiluminescence using Amersham imager 600 (GE Healthcare).
Protein expression levels were quantified using the ImageJ
software.
Osteogenic induction assay
The ADSCs were maintained in α-MEM medium.
Osteogenic medium was replaced with the fresh medium every three
days. At day 20, the cells were fixed using 3.7% formaldehyde and
stained with Alizarin Red S solution (2%, w/w) for 10 min prior to
visualization of calcium deposition. Alizarin Red S was eluted
using cetylpyridinium chloride (10%, w/v), and calcium deposition
was quantified as the absorbance value at 560 nm (Molecular
Devices).
Adipogenic induction assay
Adipogenesis was induced using the Stempro
adipogenesis kit following the manufacturer's instructions. At day
15, the cells were fixed using 3.7% formaldehyde and stained with
Oil Red O solution to evaluate lipid droplet formation. Oil Red O
was eluted using isopropyl alcohol (Daejung, Siheung, Korea), and
the absorbance value was measured at 490 nm (Molecular
Devices).
Statistical analysis
All data are presented as mean ± standard deviation
(SD) of three independent experiments. Statistical analyses of all
data were carried out using an unpaired, two-tailed Student's
t-test. P-values <0.05 were interpreted to indicate
statistically significant differences (P<0.05, P<0.01,
P<0.001).
Results
Age reduces the viability and
proliferation rate of ADSCs
The ADSCs harvested from the young (<30 years)
and elderly (>70 years) groups were cultured in vitro and
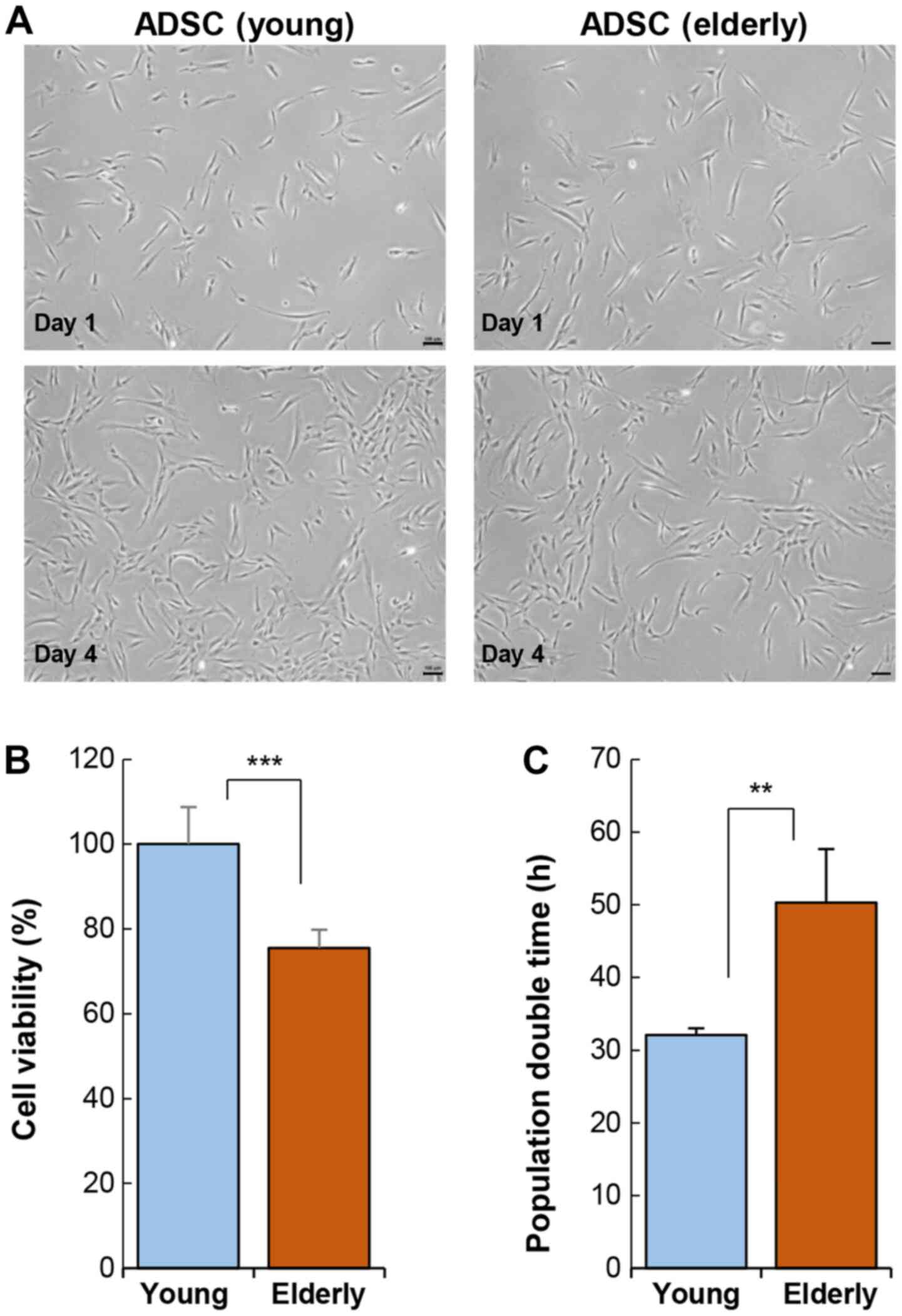
analyzed. Cellular morphology was not affected by age (Fig. 1A). ADSCs exhibited a typical shape
of fibroblastic cells that sustained over a long period in
vitro. Specific marker gene expression was also not affected by
age (Table SI). However, ADSC
activity varied considerably depending on age. The viability of
ADSCs was significantly reduced in the elderly group as compared to
that in the young group (Fig. 1B).
This reduction in cell activity also led to an increase in
population doubling time (Fig.
1C).
Stem cell transplantation requires a large number of
cells, approximately 1×108−1×109, indicating
that the ADSCs from the elderly require more time to reach the
desired cell number for transplantation, as compared to those from
the young. An increase in the time required for cellular expansion
is a key indicator of cellular senescence or dysfunction.
Specifically, ADSC viability can be affected by the physiological
condition of the elderly and may rapidly undergo cellular
senescence during ex vivo culture. Therefore, ADSCs from the
elderly may lose their therapeutic efficacy during ex vivo
culture.
Paracrine action of ADSCs is altered
by age
Paracrine actions of stem cells account for the
hallmark function of stem cell therapy in vivo. Stem cells
tend to lose their ability to secret cytokines or growth factors
due to senescence. Given that transplanted stem cells primarily act
through paracrine factors, preservation of their ability to produce
cytokines can be critical in predicting their treatment efficacy
following transplantation (14).
For screening secreted cytokines, conditioned medium
from ADSCs was analyzed with cytokine array (Fig. S1). Compared to the ADSCs from the
young group, those from the old group tended to produce higher
levels of inflammatory factors and lower levels of angiogenic
factors, including HGF and VEGF.
Among the identified secretory factors in ADSCs, we
quantitatively determined the secretion levels of angiogenic
factors using ELISA. VEGF, HGF, SDF-1α and TGF-β are involved in
vascularization or bone formation, as well as cell survival. These
represent important factors to consider in stem cell
transplantation for the treatment of vascular or skeletal diseases
(22–25).
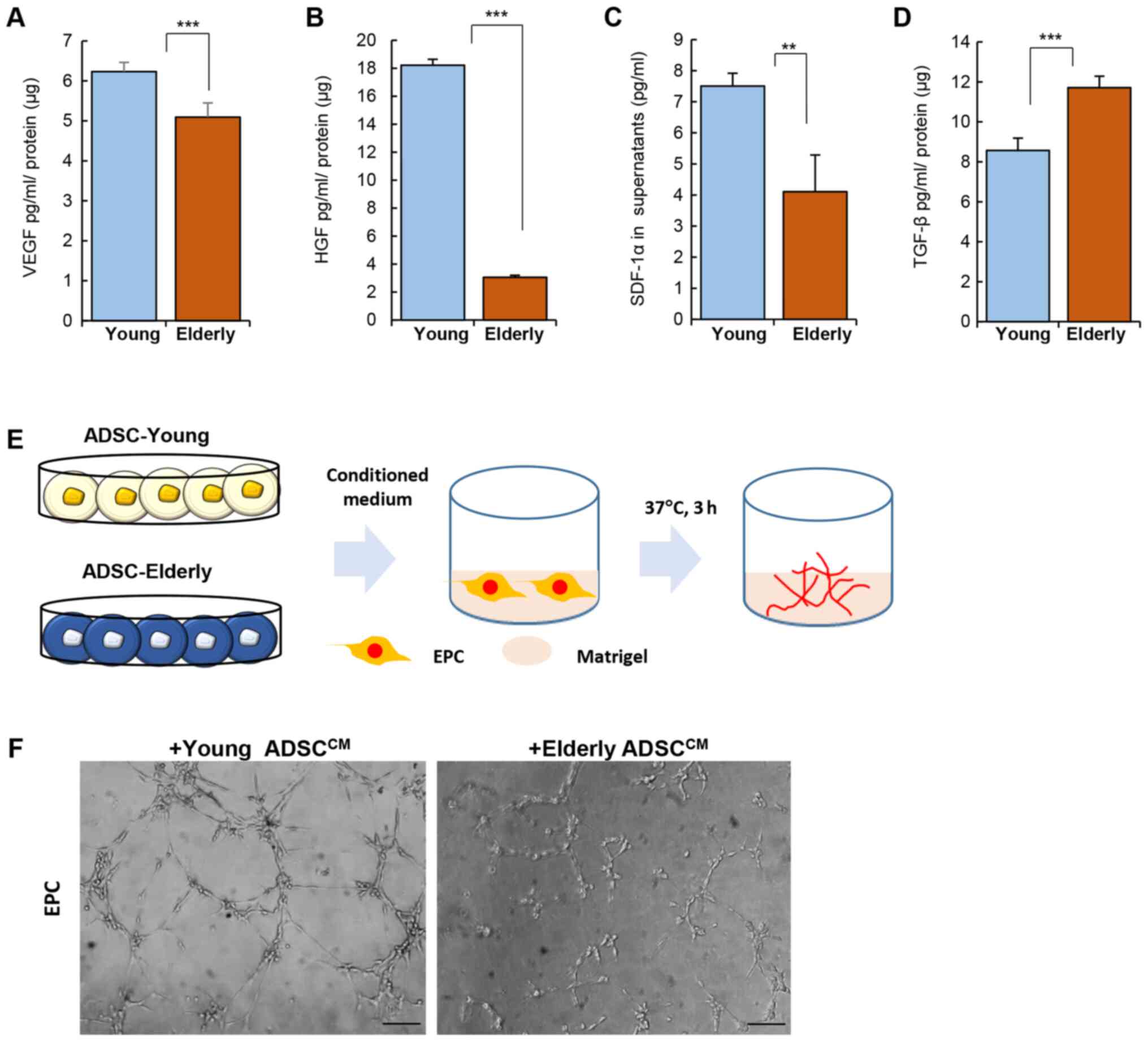
Levels of VEGF, HGF, and SDF-1α were clearly reduced
in the ADSCs from the elderly group when compared to those from the
young group (Fig. 2A-C; VEGF,
Young: 6.23±0.23, Elderly: 5.09±0.36 pg/ml/µg protein, P<0.001;
HGF Young: 18.22±0.41, Elderly: 3.04±0.15 pg/ml/µg protein,
P<0.001; SDF-1α Young: 7.5±0.41, Elderly: 4.1±1.18 pg/ml/µg
protein, P<0.01). However, TGF-β production was elevated in the
ADSCs from the elderly group (Fig.
2D; TGF-β, Young: 8.57±0.61, Elderly: 11.71±0.58 pg/ml/µg
protein, P<0.001).
The difference in the levels of angiogenic factors
produced by ADSCs of the young and elderly groups was expected to
affect angiogenesis in vivo. To determine the effect of
soluble factors from ADSCs on vessel formation, conditioned medium
from ADSCs was collected and then applied to EPCs on matrigel in
vitro (Fig. 2E). As shown in
Fig. 2F, the conditioned medium
from ADSCs from the young group could promote tube formation by
EPCs much more than that from the ADSCs from the elderly group.
These data suggest that age can alter the cytokine
secretion profile of ADSCs and this may be related to treatment
efficacy in vivo.
Age weakens the differentiation
potential of ADSCs
We have proven that age can influence cell
repopulation rate and cytokine secretion. This suggests that age
may disrupt the differentiation potential of ADSCs. To test this,
we induced adipogenesis and osteogenesis in ADSCs from the young
and elderly groups and compared their differentiation
potential.
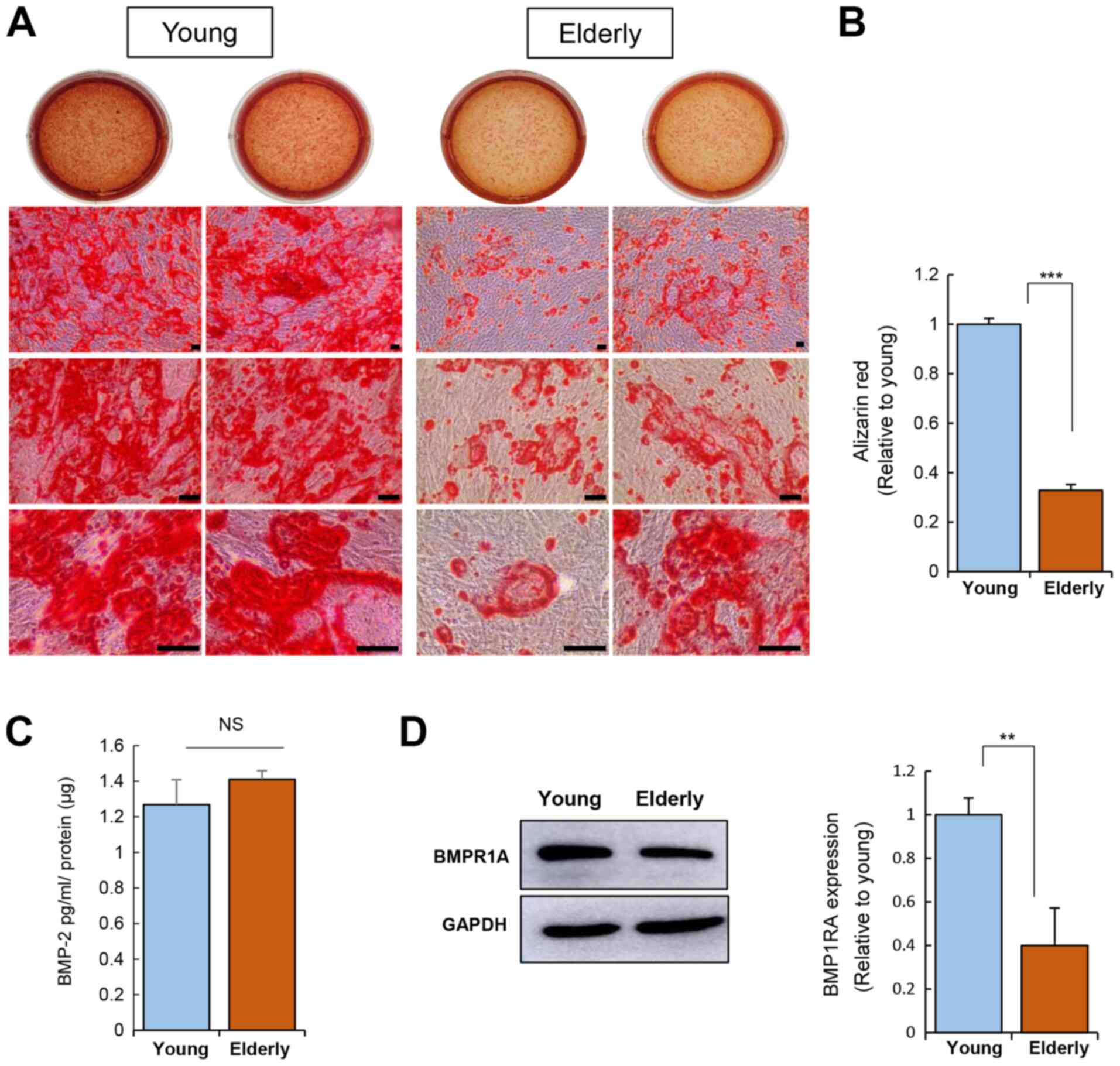
Osteogenic induction of ADSCs was carried out for 20
days and calcium deposition was then assessed using Alizarin Red S
staining (Fig. 3A and B). This
corroborated the impairment of the osteogenic potential of ADSCs
from the elderly group.
Osteogenesis is driven by BMP-2 signaling,
emphasizing the role of BMP signaling in osteogenesis (26). The level of BMP-2 secreted from
ADSCs was not influenced by age (Fig.
3C). Next, the expression of BMP receptor 1A (BMPR1A; receptor
for BMP-2) was determined, revealing that the ADSCs from the
elderly group have reduced BMPR1A expression as compared to that of
the ADSCs from the young group (Fig.
3C). This suggests that ADSCs from the elderly might have a
weak response to exogenous BMP-2 due to poor expression of its
receptor, which can contribute to poor osteogenesis.
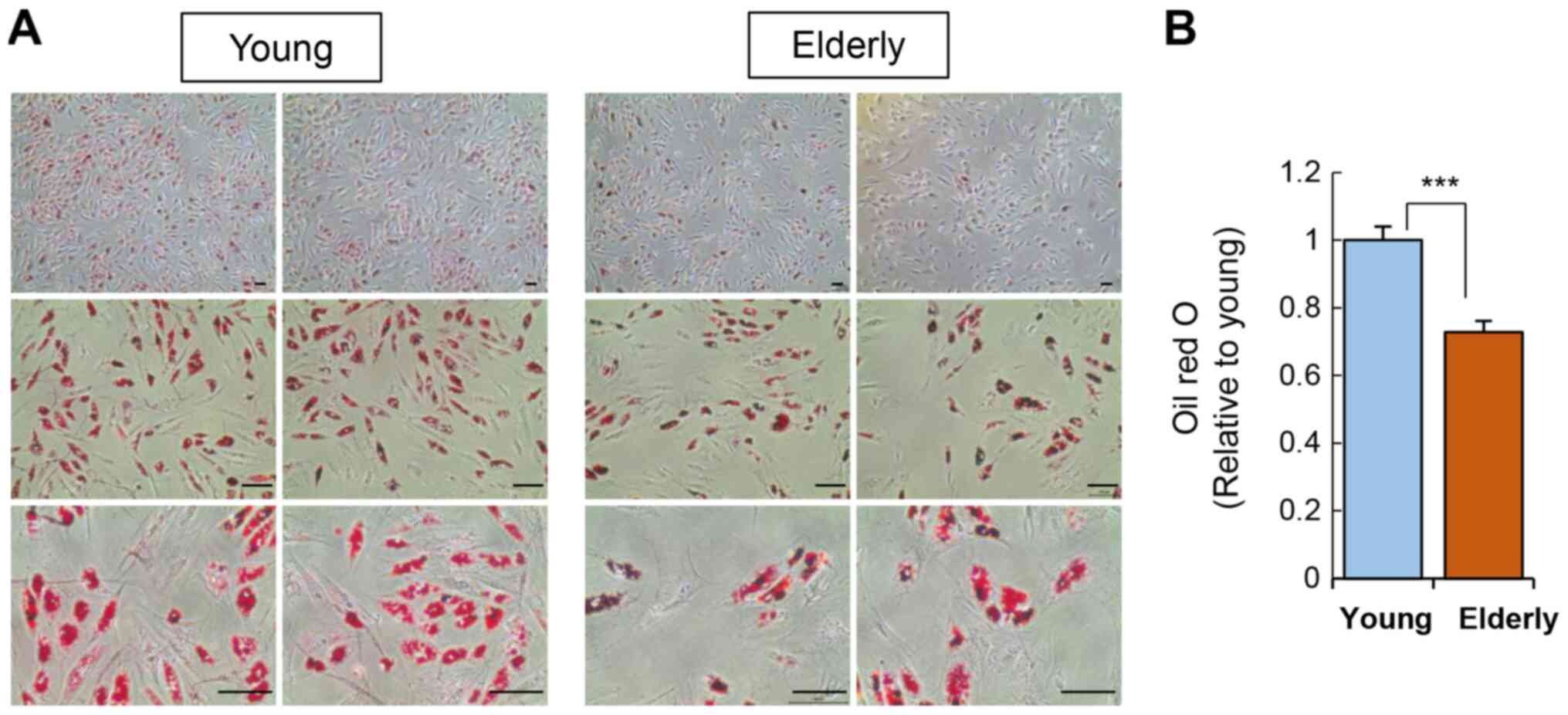
In addition to osteogenesis, we also verified the
adipogenic potential of ADSCs by culturing the ADSCs in adipogenic
media for two weeks and then staining cellular lipid droplets using
Oil Red O (Fig. 4). ADSCs from the
elderly group showed a significant reduction in adipogenic
potential compared to that of the young group.
Collectively, the differentiation potential of ADSCs
from the elderly group was clearly impaired, regardless of the
specific cell type polarization. This overall depletion in the
differentiation potential of ADSCs in the elderly group may be
caused by alterations in paracrine action or receptor expression,
given that cell differentiation is induced by specific growth
factors.
Discussion
Stem cell therapy remains a revolutionary and novel
treatment modality with considerable potential to become one of the
most important breakthroughs of medical science (19,27).
The global stem cell therapy market is highly competitive, and
growth in this market is further fueled by the increasing number of
scientific research activities utilizing the therapeutic potency of
stem cells for medical treatment.
BMSCs are the most actively utilized cell type for
transplantation, and their therapeutic effect on various chronic
diseases is evidenced by treatment outcomes, such as suppression of
inflammation or enhancement of tissue growth to promote
regeneration. However, depending on the underlying disease
condition of a given patient, BMSCs may not be viable due to the
risk of access or poor cell viability. Adipose tissue has been
actively investigated as a promising treatment for incurable
disorders (10–13). ADSC therapy exhibits therapeutic
effects on neurodegenerative disorders, cardiac injuries, and
cutaneous damage. Thus, ADSCs are undoubtedly a novel clinical
therapeutic agent. To validate the efficacy of ADSC therapy, the
cellular function of ADSCs should be analyzed prior to
transplantation, and this standard should be considered as a
quality control.
This study explored the effect of age on the
cellular activity of ADSCs. Although ADSCs have inherent
self-renewal capacity and exhibit paracrine action in vivo,
these functions were expected to be altered by the donor's age.
Previous reports have explored the relationship between aging and
ADSC activity (28–31). However, these studies mainly focused
on the effect of aging on the efficacy and differentiation of stem
cells in non-clinical settings and determined the expression of
paracrine factors at the transcriptional level. Although this work
provided important insights, it was limited.
In this study, ADSCs from the elderly group showed
low activity and repopulation rate compared to those from the young
group. The levels of cytokine secretion were also quite different
in the young group compared to those in the elderly. Notably, the
production of angiogenic factors, including VEGF, SDF-1α, and HGF,
was reduced in the ADSCs from the elderly group. These differences
indicate that stem cell therapy using ADSCs from the elderly may
not provide the expected paracrine action and cannot survive in
vivo.
This low repopulation rate and weakened paracrine
potential may lead to the impairment of the differentiation
potential of stem cells. We found that ADSCs from the elderly group
had a poor differentiation potential when polarized into
osteoblasts or adipocytes, whereas ADSCs from the young group
showed a prompt response to differentiation stimuli. This
difference might be attributed to the altered expression of
ligands/receptors in ADSCs due to aging.
Aging is a complex physiological process in the
human body that encompasses a wide spectrum of biological
processes, including oxidative stress, chronic inflammation, and
cellular senescence. This complex physiological environment can
affect stem cell viability. From a clinical perspective, ADSC
therapy is often used in older patients. Many previous trials have
relentlessly aimed to improve the stem cell proliferation rate to
achieve the desired cell number for transplantation purposes, but
have overlooked the aspects of paracrine action or differentiation
capacity. ADSCs from the elderly with chronic inflammation were
found to secrete higher levels of pro-inflammatory cytokines
compared to those from the young. Particularly, the ADSCs with
altered cytokine profiles may predispose the recipients to certain
risks and thus ADSC activity should be examined in the elderly,
prior to transplantation.
Taken together, with the above-mentioned information
on the relationship between age and the cellular activity of ADSCs
prior to transplantation, we can prepare additional procedures to
augment the function of ADSCs derived from elderly patients.
Aging is accompanied by chronic diseases and stem
cell therapy is chiefly applied in aged patients; therefore, we
could not ignore the effect of diseases in age-mediated stem cell
impairment. As such, the study of the complex response from elderly
patients may be practically meaningful in clinical settings.
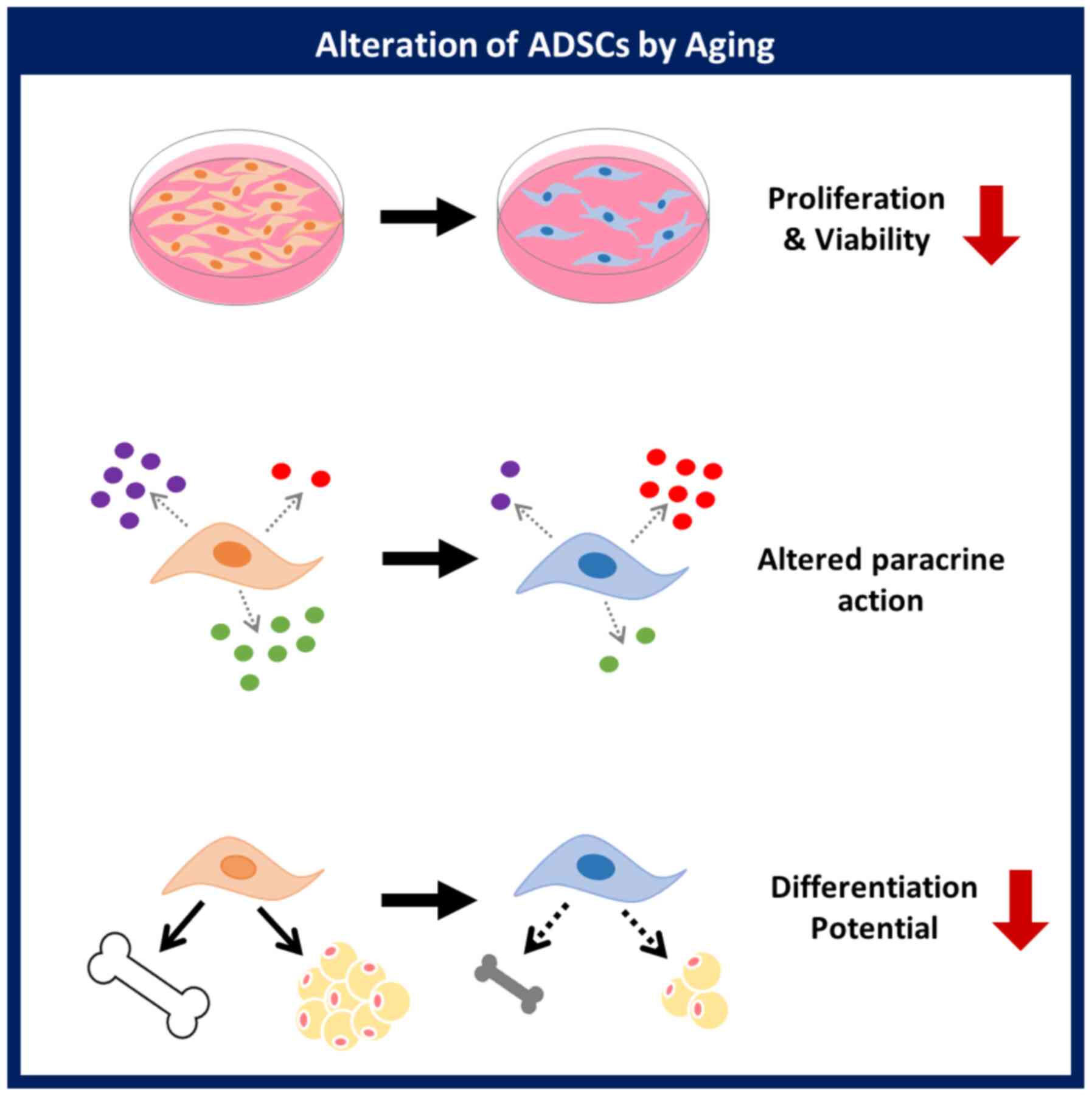
This study demonstrated that a donor's age affects
the proliferative activity, paracrine action, and differentiation
potential of ADSCs. Evaluation of the cellular activity of ADSCs
based on age will be helpful for the development of ADSCs as a
cellular therapeutic agent in stem cell therapy (Fig. 5). The relationship between specific
background diseases and stem cell activity will be explored in
future studies.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by a Korean Health
Technology R&D Project grant (grant no. HI18C1492) from the
Ministry of Health and Welfare (Sejong, South Korea) and Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education (grant no.
2018R1D1A1B0704104813).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HSH conceived and designed the study. JSP, HSH and
GP analyzed and interpreted the data. JSP and HSH wrote the
article. JSP, HSH and GP gave final approval of the article. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed in
accordance with the ethical standards as recommended by Kyung Hee
University Hospital. Donor samples were obtained with written
consent after personal explanation (IRB# 2016-12-022; Institutional
Review Board of Kyung Hee University Hospital, Seoul, Republic of
Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADSCs
|
adipose-derived stem cells
|
|
BMP-2
|
bone morphogenetic protein 2
|
|
BMPR1A
|
bone morphogenetic protein receptor
1
|
|
HGF
|
hepatocyte growth factor
|
|
SDF-1
|
stromal cell-derived factor-1
|
|
TGF-β
|
transforming growth factor-β
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Baraniak PR and McDevitt TC: Stem cell
paracrine actions and tissue regeneration. Regen Med. 5:121–143.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biehl JK and Russell B: Introduction to
stem cell therapy. J Cardiovasc Nurs. 24:98–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goradel NH, Hour FG, Negahdari B,
Malekshahi ZV, Hashemzehi M, Masoudifar A and Mirzaei H: Stem cell
therapy: A new therapeutic option for cardiovascular diseases. J
Cell Biochem. 119:95–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L, Tredget EE, Wu PY and Wu Y:
Paracrine factors of mesenchymal stem cells recruit macrophages and
endothelial lineage cells and enhance wound healing. PLoS One.
2:e18862008. View Article : Google Scholar
|
|
5
|
Uemura R, Xu M, Ahmad N and Ashraf M: Bone
marrow stem cells prevent left ventricular remodeling of ischemic
heart through paracrine signaling. Circ Res. 9:1414–1421. 2006.
View Article : Google Scholar
|
|
6
|
Miao C, Lei M, Hu W, Han S and Wang Q: A
brief review: The therapeutic potential of bone marrow mesenchymal
stem cells in myocardial infarction. Stem Cell Res Ther. 8:2422017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu S, Zhou Z, Li H, Liu Z, Pan X, Wang F,
Huang Y, Li X, Xiao Y, Pan J, et al: BMSCs ameliorate septic
coagulopathy by suppressing inflammation in cecal ligation and
puncture-induced sepsis. J Cell Sci. 131:jcs2111512018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Watanabe S, Uchida K, Nakajima H, Matsuo
H, Sugita D, Yoshida A, Honjoh K, Johnson WE and Baba H: Early
transplantation of mesenchymal stem cells after spinal cord injury
relieves pain hypersensitivity through suppression of pain-related
signaling cascades and reduced inflammatory cell recruitment. Stem
Cells. 33:1902–1914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Q, Zhao B, Li C, Rong JS, Tao SQ and
Tao TZ: Decreased proliferation ability and differentiation
potential of mesenchymal stem cells of osteoporosis rat. Asian Pac
J Trop Med. 7:358–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kesireddy V: Evaluation of adipose-derived
stem cells for tissue-engineered muscle repair construct-mediated
repair of a murine model of volumetric muscle loss injury. Int J
Nanomedicine. 11:1461–1473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kasir R, Vernekar VN and Laurencin CT:
Regenerative engineering of cartilage using adipose-derived stem
cells. Regen Eng Transl Med. 1:42–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chi K, Fu RH, Huang YC, Chen SY, Hsu CJ,
Lin SZ, Tu CT, Chang LH, Wu PA and Liu SP: Adipose-derived stem
cells stimulated with n-butylidenephthalide exhibit therapeutic
effects in a mouse model of Parkinson's disease. Cell Transplant.
27:456–470. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ueyama H, Okano T, Orita K, Mamoto K,
Sobajima S, Iwaguro H and Nakamura H: Local transplantation of
adipose-derived stem cells has a significant therapeutic effect in
a mouse model of rheumatoid arthritis. Sci Rep. 10:30762020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bektas A, Schurman SH, Sen R and Ferrucci
L: Aging, inflammation and the environment. Exp Gerontol.
105:10–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferrucci L and Fabbri E: Inflammageing:
Chronic inflammation in ageing, cardiovascular disease, and
frailty. Nat Rev Cardiol. 15:505–522. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Asumda FZ and Chase PB: Age-related
changes in rat bone-marrow mesenchymal stem cell plasticity. BMC
Cell Biol. 12:442011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baek SM, Son Y and Hong HS: Substance P
blocks the impairment of paracrine potential of MSC due to long
term culture. Mol Cell Toxicol. 14:283–290. 2018. View Article : Google Scholar
|
|
18
|
Stolzing A, Jones E, McGonagle D and Scutt
A: Age-related changes in human bone marrow-derived mesenchymal
stem cells: consequences for cell therapies. Mech Ageing Dev.
129:163–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun LY, Zhang HY, Feng XB, Hou YY, Lu LW
and Fan LM: Abnormality of bone marrow-derived mesenchymal stem
cells in patients with systemic lupus erythematosus. Lupus.
16:121–128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murphy JM, Dixon K, Beck S, Fabian D,
Feldman A and Barry F: Reduced chondrogenic and adipogenic activity
of mesenchymal stem cells from patients with advanced
osteoarthritis. Arthritis Rheum. 46:704–713. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim S, Piao J, Son Y and Hong HS:
Substance P enhances proliferation and paracrine potential of
adipose-derived stem cells in vitro. Biochem Biophys Res Commun.
485:131–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu K and Olsen BR: The roles of vascular
endothelial growth factor in bone repair and regeneration. Bone.
91:30–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhen R, Yang J, Wang Y, Li Y, Chen B, Song
Y, Ma G and Yang B: Hepatocyte growth factor improves bone
regeneration via the bone morphogenetic protein-2-mediated NF-κB
signaling pathway. Mol Med Rep. 17:6045–6053. 2018.PubMed/NCBI
|
|
24
|
Li Y, Chang S, Li W, Tang G, Ma Y, Liu Y,
Yuan F, Zhang Z, Yang GY and Wang Y: cxcl12-engineered endothelial
progenitor cells enhance neurogenesis and angiogenesis after
ischemic brain injury in mice. Stem Cell Res Ther. 9:1392018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen G, Deng C and Li YP: TGF-β and BMP
signaling in osteoblast differentiation and bone formation. Int J
Biol Sci. 8:272–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salazar VS, Gamer LW and Rosen V: BMP
signalling in skeletal development, disease and repair. Nat Rev
Endocrinol. 12:203–221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zakrzewski W, Dobrzyński M, Szymonowicz M
and Rybak Z: Stem cell: Past, present, and future. Stem Cell Res
Ther. 10:682019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dufrane D: Impact of age on human adipose
stem cells for bone tissue engineering. Cell Transplant.
26:1496–1504. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei W, Niklason L and Steinbacher DM: The
effect of age on human adipose-derived stem cells. Plast Reconstr
Surg. 131:27–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin Y, Yang L, Zhang Y, Gao W, Yao Z, Song
Y and Wang Y: Effects of age on biological and functional
characterization of adipose-derived stem cells from patients with
end-stage liver disease. Mol Med Rep. 16:3510–3518. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marędziak M, Marycz K, Tomaszewski K,
Kornicka K and Henry B: The influence of aging on the regenerative
potential of human adipose derived mesenchymal stem cells. Stem
Cells Int. 2016:21524352016. View Article : Google Scholar : PubMed/NCBI
|