Introduction
Ischemic heart diseases are amongst the leading
causes of mortality worldwide (1–3), with
a death rate of 46–324 deaths per 100,000 individuals/year
(3). Early reperfusion is key for
the effective recovery of the ischemic myocardium; however,
reperfusion may also result in ischemia/reperfusion (I/R) injury
(4). Under normal circumstances,
mitochondria provide ATP for myocardial survival and contraction;
however, they are also the site of myocardial oxidative stress and
calcium overload during an I/R situation (4,5).
Therefore, mitochondria are important factors (both preventative
and causative) during myocardial I/R injury. Studies have shown
that myocardial I/R injury and different types of conditioning can
affect mitochondrial function (6–9).
Furthermore, the expression levels of certain mitochondrial
proteins are altered following myocardial I/R injury and ischemic
preconditioning (10–12). Recently, it was reported that D-Post
may protect Langendorff I/R hearts via the mitochondrial
ATP-sensitive potassium channel (mitoKATP) and the
hypoxia-inducible factor-1/hypoxia response element pathway
(13).
Following the discovery in 1991 of
mitoKATP (14), which is
located at the inner mitochondrial membrane, mitoKATP
was demonstrated to be a trigger for the protective effects of
ischemic pre-conditioning (15,16).
Pharmacological conditioning is currently being evaluated as an
alternative method for the treatment of I/R injury (17). Similar myocardial protection can be
obtained from drugs such as mitoKATP openers, including
diazoxide (15,18). A previous study observed that
diazoxide post-conditioning (D-Post) is cardioprotective in I/R rat
cardiomyocytes (12), which has
been also reported by Penna et al (19). However, the mechanisms via which
D-Post protects the myocardium against I/R injury have not been
fully elucidated.
The aim of the present study was to analyze the
differential expression of mitochondrial proteins in normal, I/R
and D-Post rat hearts, which may aid the exploration of potential
targets for the treatment of myocardial I/R injury.
Materials and methods
Animals
A total of 65 male Sprague-Dawley rats (age, 16–20
weeks old, weight, 200–250 g) were purchased from the Center of
Laboratory Animals of The Third Military Medical University
(Chongqing, China). The rats were housed in cages with 12 h
light/dark cycles, ad libitum access to food and water at a
constant humidity (50–60%) and temperature (22±1°C). All animals
received humane care in compliance with the Guide for the Care and
Use of Laboratory Animals (20),
and all experimental protocols were approved by the Animal Care and
Use Committee of Zunyi Medical University.
Materials
SDS, ammonium persulfate, sucrose, acrylamide,
methylene bis-acrylamide, mannitol, glycerol and glycine were
purchased from Amresco, LLC. Diazoxide, Nycodenz®, urea,
thiourea, EDTA and tetramethylethylenediamine were obtained from
Sigma-Aldrich (Merck KGaA). Protein quantification kits,
immobilized pH gradient (IPG) strips, dithiothreitol (DTT),
BIO-Lyte, CHAPS, agarose, bromophenol blue, β-mercaptoethanol,
iodoacetamide and PVDF were acquired from Bio-Rad Laboratories,
Inc. Anti-cytochrome c oxidase subunit IV (COX IV; cat. no.
ab14744), anti-2-oxoglutarate dehydrogenase (OGDH; cat. no.
ab137773), anti-NADH dehydrogenase (ubiquinone) flavoprotein 1
(NDUFV1; cat. no. ab203208) and anti-NADH-ubiquinone oxidoreductase
75 kDa subunit (NDUFS1; cat. no. ab169540) antibodies were
purchased from Abcam. All reagents were of analytical grade.
Perfusion protocol
Rats were intraperitoneally anesthetized using
sodium pentobarbital (40 mg/kg) containing heparin (250 U/kg). When
rats were in deep anesthesia and had lost the pinch reflex, rat
hearts were rapidly excised and placed in cold K-H solution (2.50
mM CaCl2, 11.1 mM glucose, 4.75 mM KCl, 1.19 mM
KH2PO4, 118.00 mM NaCl, 1.19 mM
MgCl2•6H2O and 24.80 mM NaHCO3, pH
7.40). Then, hearts were quickly removed and connected to a
Langendorff perfusion system. Hearts were perfused with 37°C K-H
buffer bubbled for 10 min before perfusion with 5% CO2
and 95% O2 at 5.8 kPa for 20 min before equilibration.
Exsanguination following heart removal resulted in rat mortality;
death was confirmed from the loss of pinch reflexes and rigor
mortis after the removal of heart. The control, I/R and D-Post
hearts were perfused using the Langendorff apparatus as previously
reported (9,21,22),
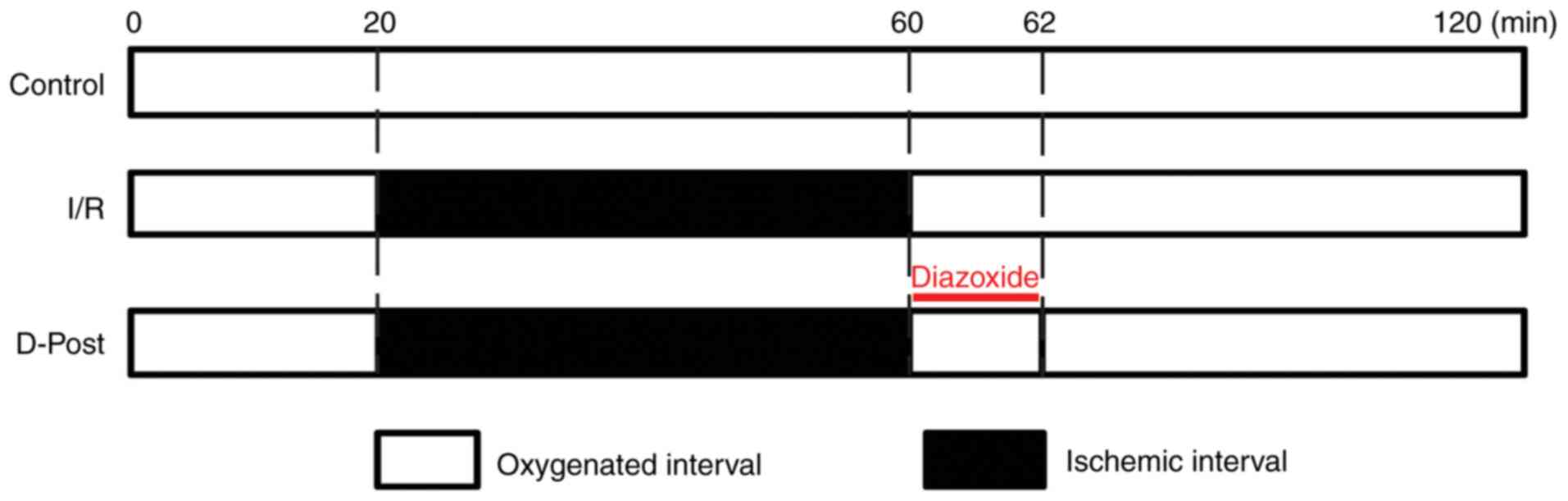
and the protocols are outlined in Fig.
1. I/R injury was induced by hypoxia in the ischemia session
and subsequent reperfusion with oxygenated K-H solution.
A total of 30 rat hearts were randomly allocated to
the Control, I/R and D-Post groups (n=10/group). For equilibration,
all hearts were perfused using the Langendorff apparatus with K-H
solution for 20 min. Hearts in the Control group were continuously
perfused with oxygenated K-H solution for 100 min. After
equilibration, the I/R and D-Post hearts were subjected to 40 min
of ischemia; the I/R hearts were then reperfused with K-H solution
for 60 min, while the D-Post hearts were reperfused with diazoxide
(50 µM in K-H solution) for 2 min, and then with K-H solution for
58 min. Cardiac functional parameters, including heart rate (HR),
the maximum rate of the rise in intraventricular pressure
(dp/dtmax), left ventricular developed pressure (LVDP)
and left ventricular end-diastolic pressure (LVEDP) were recorded
using the PowerLab system (ADInstruments) following equilibration
(T1) and reperfusion (T2). At the end of equilibration, if
premature systoles were <2/min, HR >250 bpm and LVDP >80
mmHg, the ventricular tissues were collected for further
experimentation at the end of the reperfusion period.
Transmission electron microscopy
(TEM)
The cardiac tissues were evaluated via electron
microscopy, which was conducted as previously reported (9,23).
Briefly, 1 mm3 of the left ventricle was fixed in 0.25%
glutaraldehyde and 3% paraformaldehyde at room temperature for 2 h.
The tissues were then mounted with 1% osmic acid, dehydrated with
acetone and embedded using ethoxyline 618 (35°C overnight, 45°C for
8 h, 60°C for 48 h). The myocardial sections were cut (50 nm
thickness), stained in uranyl acetate and lead citrate for 30 and
10 min at room temperature, respectively, and then photographed
with a transmission electron microscope (HITACHI-H7500; Hitachi,
Ltd.) and ultrastructural damage was evaluated using Flameng's
scoring method (24) as previously
reported (9).
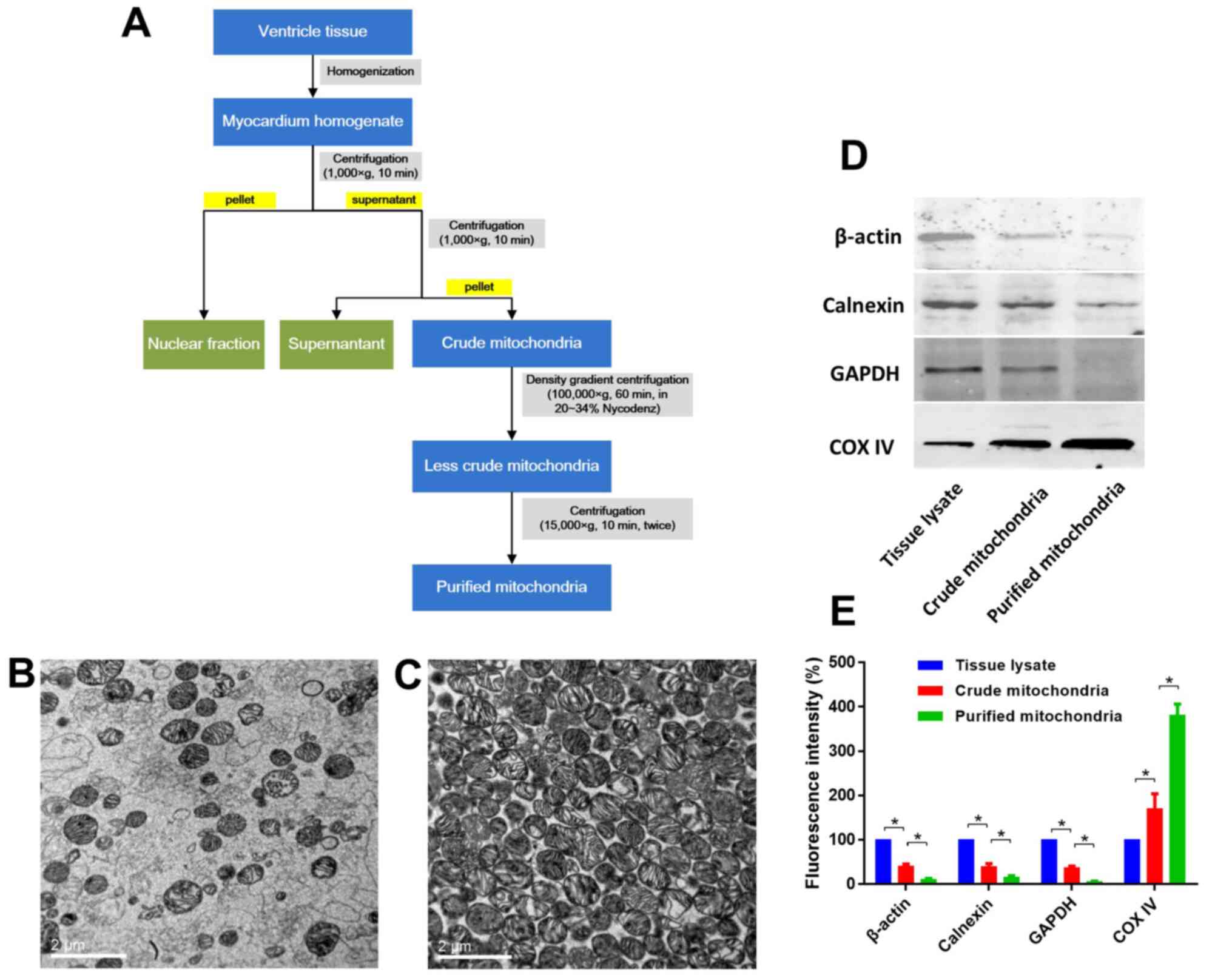
Mitochondria extraction
The purity of the extracted mitochondria is
important for the accuracy of mitochondrial proteomics analysis. As
previously described (9), the
ventricular tissues were cut and placed into ice-cold
mitochondrion-separating medium (700 mM sucrose, 210 mM mannitol, 1
mM EDTA and 10 mM Tris-HCl; pH 7.4), homogenized and centrifugated
at 1,500 × g for 10 min at 4°C. The supernatant was then
centrifuged at 12,000 × g for 10 min at 4°C to harvest the crude
mitochondria. Finally, Nycodenz density gradient medium was layered
into an ultracentrifuge tube (Beckman Coulter, Inc.) with layers of
the following concentrations: i) 34% 0.5 ml; ii) 30% 0.8 ml; iii)
25% 1.2 ml (containing the crude mitochondrial solution); and iv)
20% 0.3 ml. The samples were then centrifugated at 100,000 × g for
60 min at 4°C to obtain purified mitochondria. To confirm the
mitochondrial purity, TEM was performed to evaluate the status of
the mitochondria.
Two-dimensional electrophoresis (2-DE)
of mitochondrial proteins
To obtain the mitochondrial proteins, mitochondrial
pellets were dissolved in hydration loading buffer (7 M urea, 2 M
thiourea, 40 mM Tris base, 4% CHAPS and 1% DTT), sonicated for 10
sec, and centrifugated at 12,000 × g at room temperature for 20
min. 2-DE was performed as previously described (9,25)
according to the manufacturer's protocol (Bio-Rad Laboratories,
Inc.), but with minor modifications.
A 24-cm IPG strip (pH 5–8) was rehydrated for 14 h
with hydration buffer containing 500 µg mitochondrial protein.
Isoelectric focusing was carried out at 250 V for 1 h as follows:
1,000 V for 3 h, 4,000 V for 3 h, and then with incremental
increases of 10,000 V until reaching 80,000 V/h. IPG strips were
placed in 8 ml equilibration solution (375 mM Tris-HCl, 6 M urea,
20% glycerol, 2% SDS and 0.001% bromophenol blue) and protein
separation was performed using a Bio-Rad system (Bio-Rad
Laboratories, Inc.). The IPG strips were loaded onto a 12% SDS-PAGE
gel in running buffer (192 mM glycine, 25 mM Tris and 0.1% mM SDS;
pH 8.3), and a constant current was applied for 16 h.
Protein identification
Gels were stained with silver nitrate at room
temperature for 30 min and digital images of protein dots on the
gels were captured using a scanner (Seiko Epson Corporation).
PDQuest 8.0 software (Bio-Rad Laboratories, Inc.) was used to
identify differential spots between the Control, I/R and D-Post
groups as previously described (9,25), and
these differential protein spots were excised from the gels and
digested. The peptide mass fingerprint was obtained using
matrix-assisted laser desorption ionization-time of flight mass
spectrometry (MALDI-TOF MS) with a mass spectrometer (Ultraflex
III; Bruker Corporation) and compared with that from the NCBInr
protein database (http://www.matrixscience.com/help/seq_db_setup_nr.html)
as reported previously (9).
Peptides were extracted with 50 mM NH4HCO3:ACN (1:1, v/v). The
peptide solution (3 µl) was applied to a target disk to evaporate,
and mixed with 0.1 µl matrix solution (4 mg/ml in 70% ACN and 30%
0.1% TFA, v/v), spectra was obtained with MALDI TOF/TOF mass.
BioTools 3.0 (Bruker Corporation) and Mascot software (Matrix
Science, Inc.) were the databases used to identify proteins via
peptide mass fingerprinting. NCBInr was chosen as the sequence
database. The names of the differentially expressed proteins were
then confirmed.
Western blotting
Western blotting was conducted following standard
procedures (26). The expression
levels of β-actin, calnexin, GAPDH and COX IV in purified
mitochondria were detected to confirm the purity of mitochondria
used in the present study, and the expression levels of NDUFV1,
NDUFS1 and OGDH were detected to compare with 2-DE results. Equal
quantities of protein (60 µg) from isolated mitochondria from
Control, I/R and D-Post hearts were subjected to 12% SDS-PAGE, and
transferred to PVDF membranes for immunoblotting. The membranes
were blocked for 2 h at room temperature in TBS (20 mM Tris and 150
mM NaCl, pH 8.0), and then incubated at 4°C overnight with mouse
anti-β-actin (cat. no. ab8226), rabbit anti-calnexin (cat. no.
ab22595), mouse anti-GAPDH (cat. no. ab8245), mouse anti-COX IV
(cat. no. ab33985), rabbit anti-NDUFV1 (cat. no. ab221998), rabbit
anti-NDUFS1 (cat. no. ab169540) and rabbit anti-OGDH (cat. no.
ab137773) primary antibodies (all 1:500 and purchased from Abcam).
Then, membranes were subsequently incubated with HRP-conjugated
anti-rabbit (cat. no. ab6721) or anti-mouse (cat. no. ab6728)
secondary antibodies (1:2,000; Abcam). Protein expression was
visualized via enhanced chemiluminescence (Cytiva) with a ChemiDoc
MP system (Bio-Rad Laboratories, Inc. Image Lab software (version
5.2.1; Bio-Rad Laboratories, Inc.), NDUFV1, NDUFS1 and OGDH levels
were normalized to that of COX IV.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Comparisons of protein expression among groups were
conducted with one-way ANOVA followed by Sidak's post hoc test for
multiple comparisons. Comparisons at different time points in the
same group, and comparisons at the same time point in 3 groups were
conducted with two-way mixed ANOVA followed by Sidak's post hoc
test for multiple comparisons. Comparisons of Flameng's score among
different groups were conducted using Kruskal-Wallis test followed
by Dunn's post hoc test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
D-Post improves cardiac function
D-Post effectively reversed I/R-induced hemodynamic
dysfunction (Fig. 2). There was no
significant difference in HR (Fig.
2A), dp/dtmax (Fig.
2B), LVDP (Fig. 2C) or LVEDP
(Fig. 2D) among the three groups at
the end of T1. However, at the end of T2, the HR,
dp/dtmax and LVDP of the Control and D-Post groups were
significantly greater than those of the I/R group, whereas the
LVEDP was significantly decreased in these two groups compared with
I/R (P<0.05).
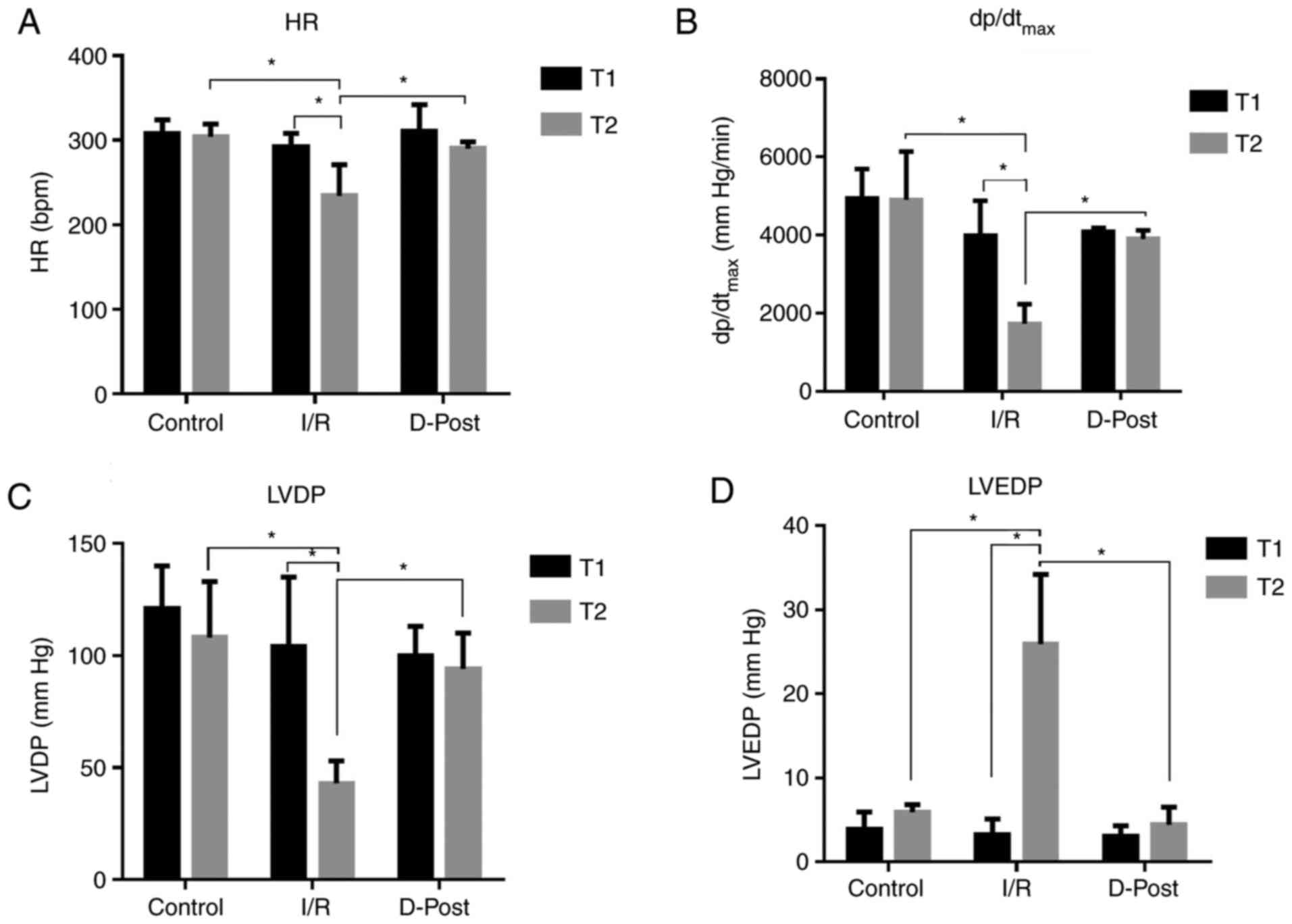 | Figure 2.Comparison of hemodynamic parameters.
D-Post effectively improves (A) HR, (B) dp/dtmax, (C)
LVDP and (D) LVEDP after I/R injury. n=10 in each group.
Comparisons at different time points in the same group, and
comparisons at the same time point in different groups were
conducted using two-way ANOVA followed Sidak's post hoc test.
*P<0.05. I/R, ischemia/reperfusion; D-Post, diazoxide
post-conditioning; HR, heart rate; dp/dtmax, maximum
rate of the rise in intraventricular pressure; LVDP, left
ventricular developed pressure; LVEDP, left ventricular
end-diastolic pressure; T1, time point following equilibration; T2,
time point following reperfusion. |
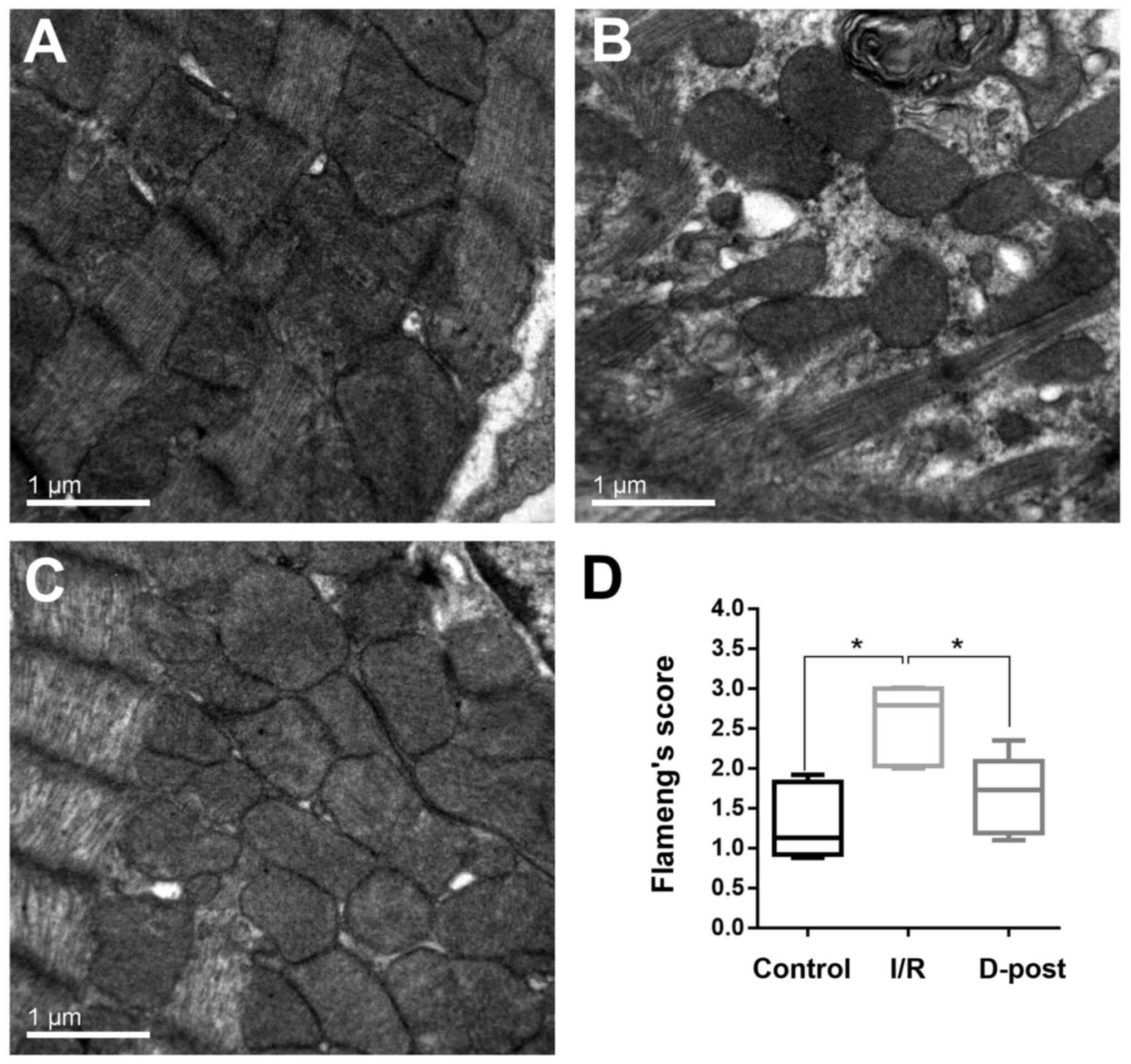
Electron microscopy
In the I/R group, the myocardial fibers were
arranged in a disordered manner, the mitochondria were swollen, and
the cristae were fractured and fuzzy (Fig. 3B). The myocardia of the D-Post group
exhibited a more normal morphology; the myocardial fibers were
arranged in an orderly manner, and fewer mitochondria were swollen
(and to a lesser degree than those in the I/R group), but with an
intact appearance (Fig. 3C).
Quantification of mitochondrial damage was determined using
Flameng's method (Fig. 3D).
Flameng's score was considerably higher in the I/R group compared
with the Control group (2.6±0.46 vs. 1.3±0.45), and subsequently
decreased to 1.7±0.48 in the D-Post group.
Evaluation of mitochondrial
purity
Mitochondria were extracted and purified as depicted
in Fig. 4A. Mitochondrial purity
was evaluated using TEM images and western blotting of β-actin,
calnexin, GAPDH and COX IV, which indicated that high purity
mitochondria were obtained (Fig.
4B-E). Representative TEM micrographs of the isolated
mitochondria are displayed in Fig.
4. The mitochondria were intact with no swelling or rupturing,
and the cristae were intact and organized (Fig. 4C). Few impurities were present
within these fields of view (Fig.
4C). Western blotting data also indicated that the purified
mitochondria exhibited high purity (high expression of COX IV, and
low expression of β-actin, calnexin and GAPDH), as shown in
Fig. 4D and E.
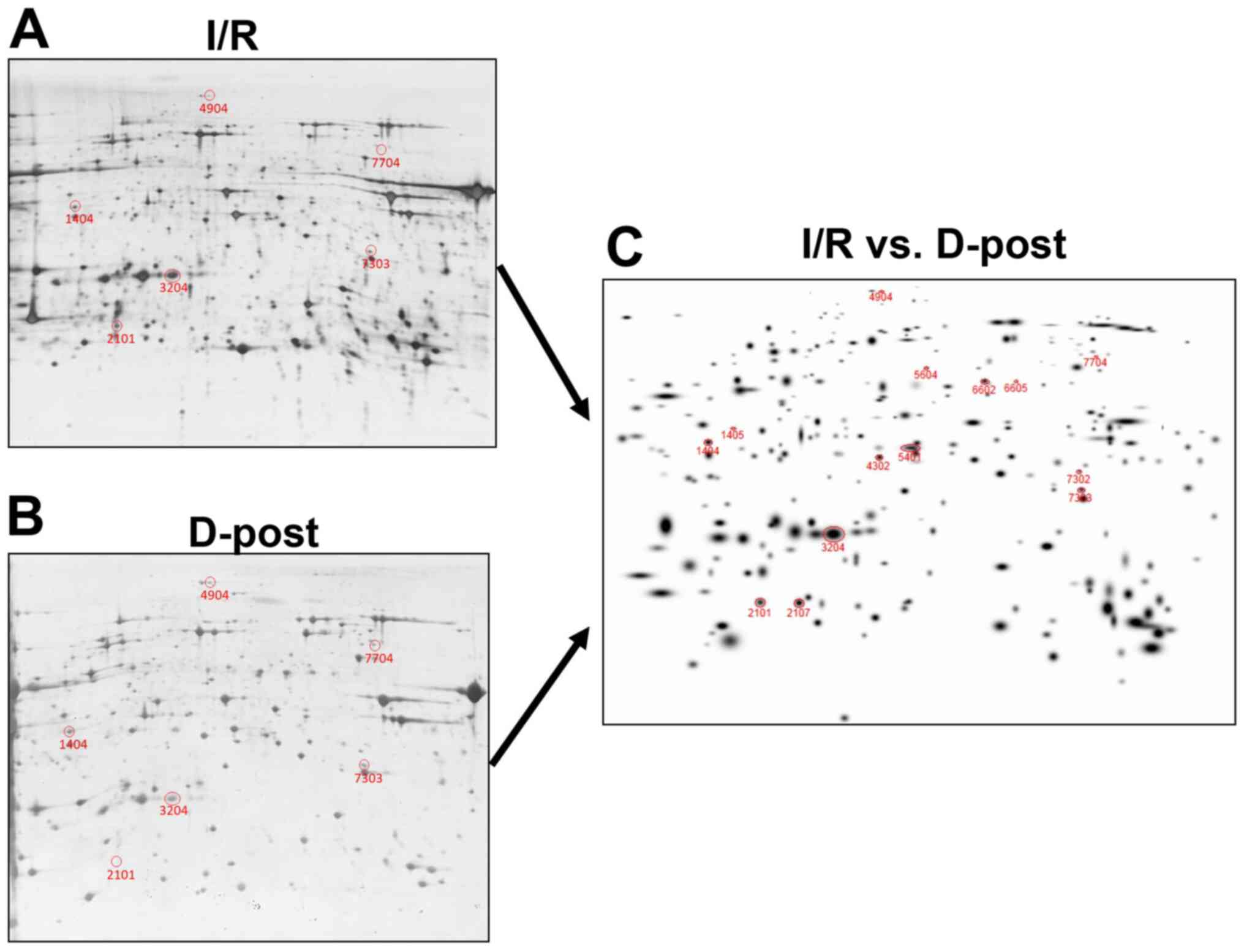
D-Post alters mitochondrial protein
expression
The expression levels of mitochondrial proteins in
the I/R and D-Post groups were compared following 2-DE. The gels
were stained with silver nitrate and scanned, and representative
images are presented in Fig. 5A
(I/R) and 5B (D-Post). In total, 14 spots were identified with
expression differences >50% between the two groups (Fig. 5C).
Protein identification with MALDI-TOF
MS
A total of 14 spots were isolated from the 2-DE gels
of the I/R group, and subjected to MALDI-TOF MS. The peptide mass
peaks were compared with those in the NCBInr protein database,
revealing five differentially expressed proteins [NDUFV1, NDUFS1,
OGDH, ATP synthase (isoform CRA_c, isoform CRA_b)]; descriptions of
these five proteins are listed in Table
I.
 | Table I.Information of differentially
expressed proteins. |
Table I.
Information of differentially
expressed proteins.
| Protein name | Gene name | Calculated PI | SSPa | NCBI GI no. | AA
coverageb, % |
Fold-changec |
P-valued |
|---|
| NADH dehydrogenase
(ubiquinone) flavoprotein 1 | NDUFV1 | 5.89 | 1405 | 149061921 | 24 | 3.6±0.2 | 0.002 |
| 2-oxoglutarate
dehydrogenase | OGDH | 6.30 | 4904 | 62945278 | 12 | 3.3±0.5 | 0.005 |
| NADH-ubiquinone
oxidoreductase 75 kDa subunit | NDUFS1 | 5.74 | 5604 | 149046009 | 24 | 2.6±0.5 | 0.008 |
| ATP synthase, H+
transporting, mitochondrial F1 complex, α subunit, isoform 1,
isoform CRA_c | ATP5F1A | 9.50 | 7704 | 149029482 | 25 | 2.5±0.8 | 0.003 |
| ATP synthase, H+
transporting, mitochondrial F1 complex, α subunit, isoform 1,
isoform CRA_b | ATP5F1A | 6.92 | 2107 | 149029480 | 38 | −2.4±0.7 | 0.001 |
PPC alters the mitochondrial
expression levels of NDUFV1, NDUFS1 and OGDH
To validate the data obtained from 2-DE and
MALDI-TOF MS, three of the five differentially expressed proteins
were selected and subjected to western blotting. The expression
levels of NDUFV1, NDUFS1 and OGDH in the D-Post group were
significantly upregulated compared with in the I/R group
(P<0.05, Fig. 6), consistent
with the 2-DE expression trends for these proteins.
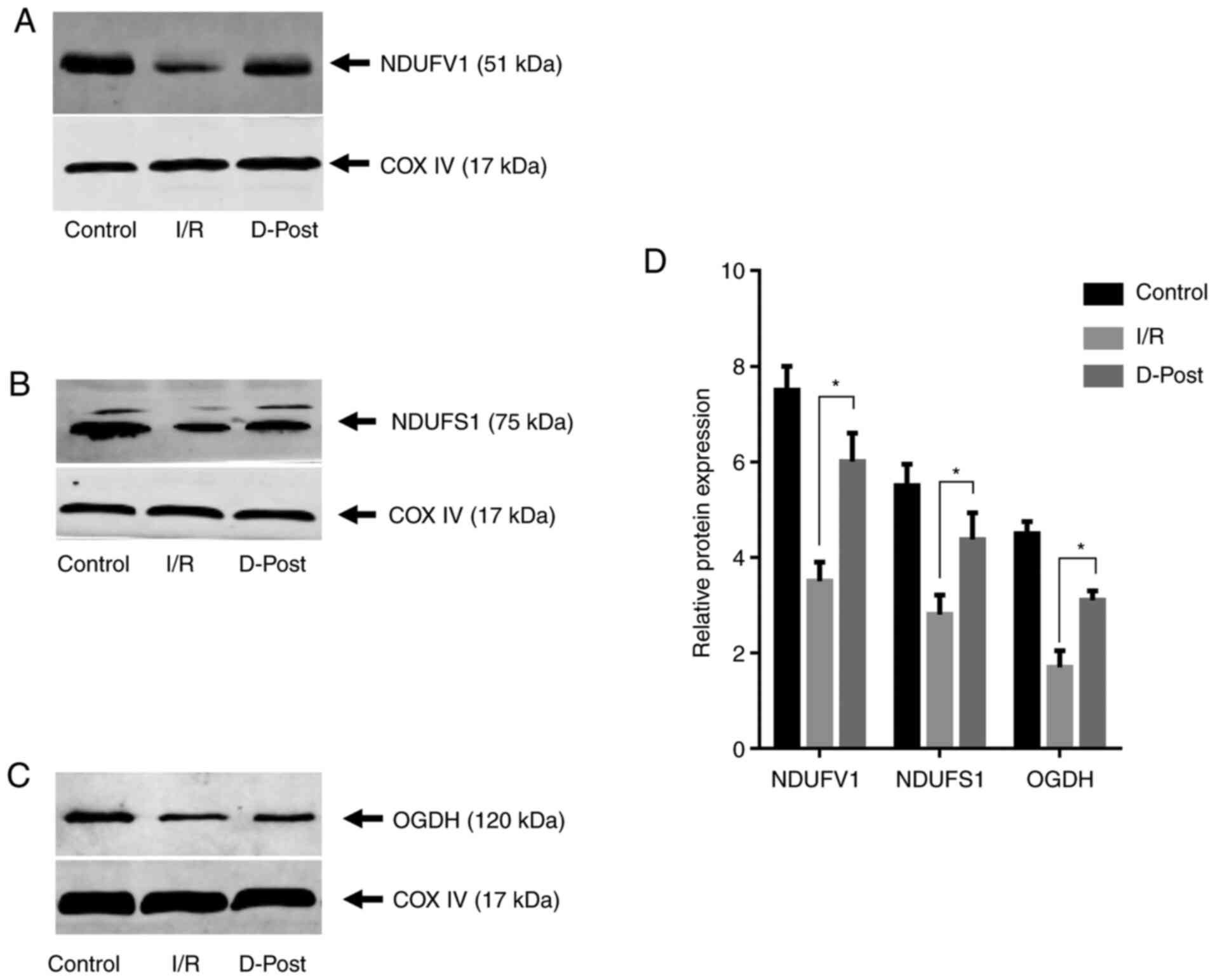 | Figure 6.Expression levels of NDUFV1, NDUFS1
and OGDH in normal, I/R and D-Post hearts. (A) NDUFV1, (B) NDUFS1
and (C) OGDH expression as determined via western blotting. (D)
Compared with the I/R group, expression of each protein was
upregulated in the D-Post group, which was consistent with the
expression trends observed with two-dimensional electrophoresis.
n=3 in each group. *P<0.05. I/R, ischemia/reperfusion; D-Post,
diazoxide post-conditioning; COX IV, cytochrome c oxidase
subunit IV; NDUFV1, NADH dehydrogenase (ubiquinone) flavoprotein 1;
NDUFS1, NADH-ubiquinone oxidoreductase 75 kDa subunit; OGDH,
2-oxoglutarate dehydrogenase. |
Discussion
D-Post was previously revealed to effectively
attenuate I/R injury in primary adult rat cardiomyocytes (12). In the present ex vivo study,
D-Post alleviated I/R injury in rat hearts. Studies have shown that
mitoKATP opening may be the trigger point and
end-effector of the myocardial protective effects of certain drugs
(27–29). Therefore, comparative mitochondrial
proteomics analyses were used to detect potential effectors
responsible for the protective effects of D-Post. As a result, five
differentially expressed proteins between I/R and D-Post hearts
were identified, all of which are associated with the mitochondrial
respiratory chain or energy metabolism, and may therefore be
potential myocardial protective effectors for I/R.
In the present study, the expression of NDUFV1 and
NDUFS1 was increased following D-Post in I/R hearts. These NADH
dehydrogenase subunits constitute the catalytic core of complex I
(30,31); the overexpression of NDUFV1 and
NDUFS1 may therefore enhance the function of complex I and restore
energy production in I/R, in which a lack of oxygen retards
oxidation reactions and energy generation.
OGDH is one of the components of the ketoglutarate
dehydrogenase complex, which is a key regulatory point in the
tricarboxylic acid cycle, and catalyzes the oxidative
decarboxylation of α-ketoglutarate to succinyl-CoA, NADH and
CO2 (32). In addition,
OGDH also regulates mitochondrial redox potential (NADH/NAD+), and
has been reported to be a significant source of reactive oxygen
species (ROS) during mitochondrial succinate metabolism in the
porcine heart (33). OGDH
expression is reportedly decreased in the I/R heart (10,34),
which may influence ROS and energy production in the myocardium. In
the present study, 2-DE and western blotting revealed an increase
in OGDH expression following D-Post, and this recovery in
expression may restore ROS and energy homeostasis in the I/R heart,
thus decreasing damage to the ischemic myocardium.
The expression of ATP synthase subunit α (ATPA) was
found to be altered in I/R hearts subjected to D-Post. Of note, two
isoforms of ATPA were revealed to be differentially expressed; the
CRA_c isoform was upregulated in D-Post hearts, while the CRA_b
isoform was downregulated. As there are no specific antibodies that
differentiate between these two isoforms, their protein expression
levels cannot be separately detected by western blotting. I/R has
been reported to promote the inhibition of ATP synthase, and
subsequently decrease ATP production (35,36).
In addition, ATP synthase contributes to the effects of
mitoKATP opening in I/R hearts (37,38).
D-Post increases ATP synthase activity, which may be a direct
reason for its cardioprotective effects (38). However, whether the effects of
D-Post are specifically associated with ATPA is yet to be
investigated.
The present proteomics analysis of mitochondrial
proteins between I/R and D-Post rat hearts is a practical way to
highlight potential D-Post effector proteins. A total of five
differentially expressed proteins were identified using 2-DE and
MALDI-TOF MS (and validated via western blotting), and each
warrants further investigation. Proteomic changes between normal
Langendorff and I/R hearts have been investigated previously and 4
differentially expressed proteins (ATPA, isoform 2 of cytochrome
c1, electron-transferring flavoprotein and NDUFS2) have been
identified with the same protocol (25). Therefore, the differentially
expressed proteins between the Control and I/R groups were not
investigated in the present study.
There were limitations to the present study: i) The
function of these differentially expressed proteins in I/R and
D-Post tissues were not studied further (for example, it is not
clear whether interventions in the expression of these proteins may
affect I/R injury in the myocardium); ii) the use of narrow-range
pH IPG strips (pH 5–8) may result in other potential proteins not
being detected, which may be the reason that only five
differentially expressed proteins were identified between the I/R
and D-Post hearts; iii) for the evaluation of the protective
effects of D-Post, triphenyltetrazolium chloride staining of
Langendorff hearts would be a more convincing indicator and should
be used to measure myocardial infarct area; and iv) as mentioned
above, the lack of a normal control group for the 2-DE experiments
is a limitation of the present study.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The
Special Scientific Research Fund for Public Welfare, Ministry of
Health of China (grant no. 200802173) and the National Natural
Science Foundation of China (grant no. 8176020318).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YP and YW confirm the authenticity of all the raw
data. YP, YW, WS and SC performed the experiments, wrote the
manuscript and prepared the figures. SC, YL and TY conceived the
study, and provided the reagents and materials. All authors
reviewed the data and drafts of the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All animals received humane care in compliance with
the Guide for the Care and Use of Laboratory Animals in China, and
all experimental protocols were approved by the Animal Care and Use
Committee of Zunyi Medical University (Zunyi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Paiva S and Agbulut O: MiRroring the
multiple potentials of MicroRNAs in acute myocardial infarction.
Front Cardiovasc Med. 4:732017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaski JC, Crea F, Gersh BJ and Camici PG:
Reappraisal of ischemic heart disease. Circulation. 138:1463–1480.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nowbar AN, Gitto M, Howard JP, Francis DP
and Al-Lamee R: Mortality from ischemic heart disease. Circ
Cardiovasc Qual Outcomes. 12:e0053752019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuznetsov AV, Javadov S, Margreiter R,
Grimm M, Hagenbuchner J and Ausserlechner MJ: The role of
mitochondria in the mechanisms of cardiac ischemia-reperfusion
injury. Antioxidants (Basel). 8:4542019. View Article : Google Scholar
|
|
5
|
Lesnefsky EJ, Chen Q, Tandler B and Hoppel
CL: Mitochondrial dysfunction and myocardial ischemia-reperfusion:
Implications for novel therapies. Annu Rev Pharmacol Toxicol.
57:535–565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meng W, Xu Y, Li D, Zhu E, Deng L, Liu Z,
Zhang G and Liu H: Ozone protects rat heart against
ischemia-reperfusion injury: A role for oxidative preconditioning
in attenuating mitochondrial injury. Biomed Pharmacother.
88:1090–1097. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang L, Xie P, Wu J, Yu J, Yu T, Wang H,
Wang J, Xia Z and Zheng H: Sevoflurane postconditioning improves
myocardial mitochondrial respiratory function and reduces
myocardial ischemia-reperfusion injury by up-regulating HIF-1. Am J
Transl Res. 8:4415–4424. 2016.PubMed/NCBI
|
|
8
|
Pagliaro P, Femmino S, Popara J and Penna
C: Mitochondria in cardiac postconditioning. Front Physiol.
9:2872018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao S, Liu Y, Wang H, Mao X, Chen J, Liu
J, Xia Z, Zhang L, Liu X and Yu T: Ischemic postconditioning
influences electron transport chain protein turnover in
Langendorff-perfused rat hearts. PeerJ. 4:e17062016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim N, Lee Y, Kim H, Joo H, Youm JB, Park
WS, Warda M, Cuong DV and Han J: Potential biomarkers for ischemic
heart damage identified in mitochondrial proteins by comparative
proteomics. Proteomics. 6:1237–1249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Q, Younus MS, Thompson J, Hu Y,
Hollander JM and Lesnefsky EJ: Intermediary metabolism and fatty
acid oxidation: Novel targets of electron transport chain driven
injury during ischemia and reperfusion. Am J Physiol Heart Circ
Physiol. 314:H787–H795. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao S, Liu Y, Sun W, Zhao L, Zhang L, Liu
X and Yu T: Genome-wide expression profiling of
anoxia/reoxygenation in rat cardiomyocytes uncovers the role of
MitoKATP in energy homeostasis. Oxid Med Cell Longev.
2015:7565762015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Zhou W, Chen W, Wang H, Zhang Y and
Yu T: Mechanism of the hypoxia inducible factor 1/hypoxic response
element pathway in rat myocardial ischemia/diazoxide
post-conditioning. Mol Med Rep. 21:1527–1536. 2020.PubMed/NCBI
|
|
14
|
Inoue I, Nagase H, Kishi K and Higuti T:
ATP-sensitive K+ channel in the mitochondrial inner
membrane. Nature. 352:244–247. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garlid KD, Paucek P, Yarov-Yarovoy V,
Murray HN, Darbenzio RB, D'Alonzo AJ, Lodge NJ, Smith MA and Grover
GJ: Cardioprotective effect of diazoxide and its interaction with
mitochondrial ATP-sensitive K+ channels Possible
mechanism of cardioprotection. Circ Res. 81:1072–1082. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Sato T, O'Rourke B and Marban E:
Mitochondrial ATP-dependent potassium channels: Novel effectors of
cardioprotection? Circulation. 97:2463–2469. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caricati-Neto A, Errante PR and
Menezes-Rodrigues FS: Recent advances in pharmacological and
Non-pharmacological strategies of cardioprotection. Int J Mol Sci.
20:40022019. View Article : Google Scholar
|
|
18
|
Garlid KD, Paucek P, Yarov-Yarovoy V, Sun
X and Schindler PA: The mitochondrial KATP channel as a receptor
for potassium channel openers. J Biol Chem. 271:8796–8799. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Penna C, Perrelli MG, Tullio F, Angotti C,
Camporeale A, Poli V and Pagliaro P: Diazoxide postconditioning
induces mitochondrial protein S-nitrosylation and a redox-sensitive
mitochondrial phosphorylation/translocation of RISK elements: No
role for SAFE. Basic Res Cardiol. 108:3712013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press; Washington (DC): 2011, The
National Academies Collection: Reports funded by National.
|
|
21
|
Yu T, Fu XY, Liu XK and Yu ZH: Protective
effects of pinacidil hyperpolarizing cardioplegia on myocardial
ischemia reperfusion injury by mitochondrial KATP channels. Chin
Med J (Engl). 124:4205–4210. 2011.PubMed/NCBI
|
|
22
|
Yu T, Yu Z, Liu X, Yang S and Ye Y:
Myocardial protection with pinacidil induced hyperpolarized arrest
during cardiopulmonary bypass. Chin Med J (Engl). 114:1245–1248.
2001.PubMed/NCBI
|
|
23
|
Yang L and Yu T: Prolonged donor heart
preservation with pinacidil: The role of mitochondria and the
mitochondrial adenosine triphosphate-sensitive potassium channel. J
Thorac Cardiovasc Surg. 139:1057–1063. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Flameng W, Borgers M, Daenen W and
Stalpaert G: Ultrastructural and cytochemical correlates of
myocardial protection by cardiac hypothermia in man. J Thorac
Cardiovasc Surg. 79:413–424. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei Y, Li K, Wang H, et al: Potential
biomarkers for myocardial ischemia-reperfusion injury and pinacidil
post-conditioning identified with mitochondrial proteomics in rats.
Int J Clin Exp Med. 12:5060–5068. 2019.
|
|
26
|
Cao S, Xiao Z, Sun M and Li Y: D-serine in
the midbrain periaqueductal gray contributes to morphine tolerance
in rats. Mol Pain. 12:17448069166467862016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Testai L, Rapposelli S, Martelli A,
Breschi MC and Calderone V: Mitochondrial potassium channels as
pharmacological target for cardioprotective drugs. Med Res Rev.
35:520–553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mironova GD, Rozova EV, Belosludtseva NV
and Man'kovskaya IN: Dynamic restructuring of the myocardial
mitochondria in response to uridine modulation of the activity of
mitochondrial ATP-Dependent potassium channel under conditions of
acute hypoxic hypoxia. Bull Exp Biol Med. 166:806–810. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Henn MC, Janjua MB, Kanter EM, Makepeace
CM, Schuessler RB, Nichols CG and Lawton JS: Adenosine
triphosphate-sensitive potassium channel kir subunits implicated in
cardioprotection by diazoxide. J Am Heart Assoc. 4:e0020162015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bjorkman K, Sofou K, Darin N, Holme E,
Kollberg G, Asin-Cayuela J, Holmberg Dahle KM, Oldfors A, Moslemi
AR and Tulinius M: Broad phenotypic variability in patients with
complex I deficiency due to mutations in NDUFS1 and NDUFV1.
Mitochondrion. 21:33–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Benit P, Chretien D, Kadhom N, de
Lonlay-Debeney P, Cormier-Daire V, Cabral A, Peudenier S, Rustin P,
Munnich A and Rötig A: Large-scale deletion and point mutations of
the nuclear NDUFV1 and NDUFS1 genes in mitochondrial complex I
deficiency. Am J Hum Genet. 68:1344–1352. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Veys K, Alvarado-Diaz A and De Bock K:
Measuring glycolytic and mitochondrial fluxes in endothelial cells
using radioactive tracers. Methods Mol Biol. 1862:121–136. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mailloux RJ, Gardiner D and O'Brien M:
2-Oxoglutarate dehydrogenase is a more significant source of
O2.−/H2O2 than pyruvate
dehydrogenase in cardiac and liver tissue. Free Radic Biol Med.
97:501–512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kumar V, Kleffmann T, Hampton MB, Cannell
MB and Winterbourn CC: Redox proteomics of thiol proteins in mouse
heart during ischemia/reperfusion using ICAT reagents and mass
spectrometry. Free Radic Biol Med. 58:109–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Granger DN and Kvietys PR: Reperfusion
injury and reactive oxygen species: The evolution of a concept.
Redox Biol. 6:524–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bagheri F, Khori V, Alizadeh AM,
Khalighfard S, Khodayari S and Khodayari H: Reactive oxygen
species-mediated cardiac-reperfusion injury: Mechanisms and
therapies. Life Sci. 165:43–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ala-Rami A, Ylitalo KV and Hassinen IE:
Ischaemic preconditioning and a mitochondrial KATP channel opener
both produce cardioprotection accompanied by F1F0-ATPase inhibition
in early ischaemia. Basic Res Cardiol. 98:250–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jasova M, Kancirova I, Murarikova M,
Farkašová V, Waczulíková I, Ravingerová T, Ziegelhöffer A and Ferko
M: Stimulation of mitochondrial ATP synthase activity-a new
diazoxide-mediated mechanism of cardioprotection. Physiol Res. 65
(Suppl 1):S119–S127. 2016. View Article : Google Scholar : PubMed/NCBI
|