Introduction
Intervertebral disc degeneration (IVDD) is a leading
cause of back pain and occurs frequently in adults (1). IVDD is often accompanied by various
neurological symptoms and has high social and economic costs.
Surgical choices for discogenic back pain are finite and frequently
invasive. One of the most commonly performed procedures is
discectomy, with or without fusion (2). Nevertheless, long-term clinical trials
have revealed that the procedure results in relapse and adjacent
segment disease (3,4). Therefore, minimally invasive
regenerative therapies are being investigated.
With the degeneration of the intervertebral disc,
nucleus pulposus cells (NPCs) are unable to maintain healthy
nucleus pulposus (NP) tissue; therefore, the proteoglycan content
decreases and collagen type II is slowly replaced by collagen type
I (5). It has therefore been
suggested that the transplantation of resident cells into the disc
serves an important role in maintaining NP homeostasis by
sustaining cell numbers and synthesizing new matrix (6). Certain researchers have examined the
transplantation of mature autologous disc cells, chondrocytes, or
stem cells (SCs) to the intervertebral disc (7,8). Among
these, mesenchymal stem cells (MSCs) have received widespread
attention. The main advantages of MSCs include easy isolation from
various tissues (e.g., bone marrow and adipose tissue) and their
multilineage potential and immunomodulatory properties, which leads
to the reduction of local inflammation and the secretion of growth
factors that support regeneration (9–12).
The regenerative effects of MSCs on IVD diseases
have been reported in vitro in preclinical studies and in
certain clinical trials. The results can be summarized into two
primary findings: Firstly, MSCs may acquire an NP-like phenotype
under appropriate culture conditions (13–15);
and MSCs exhibit anti-inflammatory, anti-apoptotic and anabolic
properties, and release trophic factors (16,17).
Although the use of MSCs has been proven successful in certain
respects, MSCs are limited by their inferior ability to produce
native-like NP tissue, which has a limited proliferative ability
and poor regenerative potential, as well as their inability to
survive in the challenging microenvironment of the discs.
Therefore, a more appropriate cell source is required.
In the field of IVD regeneration, notochord cells
(NCs) have become the focus of research due to their potential
properties, and as they are native cells that constitute the NP
tissue in early life. Degenerative changes are observed following
the loss of NCs, which has been confirmed by a number of studies.
For example, NCs were not found in chondrodystrophic dogs or
humans, and signs of degeneration were observed in young dogs of
chondrodystrophic breeds (18). By
contrast, NCs were well preserved in the IVDs of adult
non-chondrodystrophic dogs, with degeneration occurring relatively
infrequently, only at selected spinal levels, and mostly in old
ages. Although older animals exhibit moderate histopathological
changes, the extracellular matrix is healthy (19). Therefore, NCs serve an important
role in maintaining the NP and may have regenerative potential for
IVDD.
Although NCs are the ideal cell source for the
regenerative purposes, the limitation of isolating and passaging
NCs has prevented their application. In the present study, a
porcine NP matrix was applied to induce MSC differentiation towards
NC-like cells, which express typical notochordal marker genes,
including brachyury (T), keratin-8 (KRT-8) and keratin-18 (KRT-18),
generating NP-like extracellular matrix. The findings of the
present study may provide a potential ideal source for the cellular
regenerative therapy of IVDD.
Materials and methods
Generation of porcine NP matrix
NP tissue was collected by a surgical blade from all
lumbar, thoracic and cervical IVDs of two male pigs (Miaodi
Biotechnology Co., Ltd.), aged 2 weeks and weighing 3.6 kg, they
were euthanized immediately after their delivery. All animal
experiments were approved by the Ethics Committee on Animal
Experiments of Fudan University (Shanghai, China). All experiments
were performed in accordance with relevant guidelines and
regulations. Porcine spines were separated under aseptic
conditions, and the surrounding soft tissues were completely
removed to ensure the identification of IVDs. NP tissues were
removed followed by incision of the disc. They were then frozen in
liquid nitrogen. The frozen NP tissues became brittle and were
easily ground into powder using a sterile mortar. The powder was
collected and stored in at −80°C until further use.
Isolation and culture of primary bone
marrow-derived MSCs (BM-MSCs)
BM-MSCs were harvested and isolated from the
aforementioned pigs. In brief, bone marrow was obtained from the
femurs and tibiae under sterile conditions. Bone marrow was further
diluted in phosphate-buffered saline (PBS; Beyotime Institute of
Biotechnology) and filtered through a 200-µm mesh (Beyotime
Institute of Biotechnology). The filtered suspension was washed
with PBS and centrifuged at 800 × g for 5 min at 25°C three times.
Cell pellets were cultured in MSC expansion medium, consisting of
low-glucose Dulbecco's modified Eagle's medium (the glucose
concentration was 1.0 g/l; Nanjing Keygen Biotech Co., Ltd.), 10%
fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.), and 100
U/ml penicillin and 100 µg/ml streptomycin (Beyotime Institute of
Biotechnology) in 25 cm2 cell culture flasks, at 37°C in
a 5% CO2 atmosphere. After 24 h, the suspended cells and
medium were removed, and the adherent cells were cultured
continuously in 25 cm2 cell culture flasks at 37°C and
expanded by replacing the medium every 2–3 days.
Evaluation of multi-differentiation
potential
To verify the multi-differentiation potential of the
acquired cells, the cells were induced to differentiate towards
osteogenic and adipogenic lineages in vitro. In brief,
third-generation BM-MSCs were seeded into 6-well plates at
1×105 cells per well. Once the cells reached 90%
confluence, morphological characteristics were observed using a
phase contrast microscope (magnification, ×40; Olympus IX50;
Olympus Corporation). The cells were then incubated with complete
culture medium to induce osteogenic differentiation (Chem
Biotechnology), which was comprised of 10% FBS, 0.5 mmol/l ascorbic
acid, 10 mmol/l sodium β-glycerophosphate, and 10 mmol/l
dexamethasone. Induction was terminated at ~3 weeks, and Alizarin
red dye was utilized to identify mineral deposits. The medium was
removed, and the cells were washed with PBS three times, followed
by fixing at 37°C with 4% paraformaldehyde for 20 min.
Subsequently, 0.5% Alizarin red dye was added (1 ml/well) and the
cells were incubated at 37°C for 15 min. Adipogenic differentiation
was induced using an induction kit (Chem Biotechnology) with basic
medium A and B, 10% FBS, 10 mg/l insulin, 0.5 mmol/l
1-methyl-3-isobutylxanthine, 200 µmol/l rosiglitazone, and 10
mmol/l dexamethasone. According to the manufacturer's protocol, the
aforementioned cells were cultured in induction medium A for 3
days, in induction medium B for one day, and this cycle was then
repeated five times. Next, cells were fixed at 37°C with 4%
paraformaldehyde for 20 min and incubated at 37°C in 0.5% Oil Red O
solution for 15 min. The residual stain was removed by washing with
PBS several times and the cells were observed under the phase
contrast microscope.
Immunofluorescence microscopy
Immunofluorescence was used to detect the expression
of the membrane surface markers, CD90, CD105, and CD45, according
to the manufacturer's protocol. In brief, the cells were fixed at
25°C with pre-chilled 4% paraformaldehyde for 20 min, and 0.1%
Triton X-100 in PBS was used to increase cell membrane
permeability. Following the application of blocking buffer (Cell
Signaling Technology, Inc.) for 1 h at room temperature, the cells
were incubated with primary antibodies (Cell Signaling Technology,
Inc.) against CD90 (1:200; cat. no. 13801), CD105 (1:200; cat. no.
4335), and CD45 (1:200; cat. no. 13917) for 1 h at 37°C. The cells
were then incubated with Alexa Fluor® 555-conjugated
goat-derived IgG secondary antibody (1:5,000; cat. no. 95235; Cell
Signaling Technology, Inc.) for 1 h at 37°C in the dark. Nuclear
staining with DAPI (Beyotime Institute of Biotechnology) was
conducted at 37°C for 3 min, and results were observed under a
fluorescence microscope (magnification, ×200; Olympus IX50; Olympus
Corporation).
Induction of NC-like cells
To induce NC-like cell differentiation, the
collected ground porcine NP tissue was frozen at −80°C and
freeze-dried overnight in order to remove alive cells; the powder
prepared from one disc was ~equally added into two wells of 6-well
culture plates. Third passage BM-MSCs (~2×105) suspended
in 2 ml medium (Nanjing Keygen Biotech Co., Ltd.) were seeded into
6-well culture plates. The samples were divided into two groups as
follows: The control group was NP-free, and the experimental group
was NP-treated with the pulverized porcine NP matrix. After 48 h,
the medium was changed carefully, taking care not to remove the NP
tissue powder. The medium was replenished every 2–3 days. Induction
persisted for ~3 weeks, and cell morphology was observed under a
microscope (magnification, ×200; Olympus IX50; Olympus Corporation)
and detections were conducted.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
RT-qPCR was performed to determine whether the
aforementioned cells exhibited an NC phenotype. After 3 weeks in
differentiation culture, total RNA was extracted using TRIzol
reagent (Sangon Biotech Co., Ltd.). A spectrophotometer (ND-20000c;
Thermo Fisher Scientific, Inc.) was used to examine the quantity of
isolated RNA, and the RNA was subsequently reverse transcribed (at
37°C for 15 min) into cDNA using a Synthesis kit (RR718; Takara
Bio, Inc.). RT-qPCR was performed to identify the genes that define
the notochordal phenotype: T, KRT-8, and KRT-18. A reaction mixture
containing cDNA, PrimeScript™ RT Master mix (Takara Bio, Inc.), and
primers (Takara Bio, Inc.) was subjected to RT-qPCR (7300 Real-Time
System; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The primers sequences were as follows: T
forward, GGCAAGGGATGGGAATAAGG and reverse, TGAGGATGGACAAAGGTGGTG;
KRT-8 forward, GCTGTCACAGTGAACCAGAGC and reverse,
AAGGAGGCAAACTTATTGTTGAGA; KRT-18 forward, AATGCCCGTCTTGCTGCT and
reverse, CAATCTGGTTTTGTAGACCCTTT; and β-actin (reference gene)
forward, TTCTGGCTCTTCTGCTCTCACTC and reverse,
TGCTCTTCCCTTCTTCTCATTACC. The thermocycling conditions were as
follows: Initial denaturation at 95°C for 2 min, followed by 40
cycles of denaturation at 95°C for 15 sec, annealing at 59°C for 20
sec and elongation at 72°C for 20 sec, and a final extension at
72°C for 10 min. The data were normalized to β-actin and quantified
using the 2−∆∆Cq method (20).
Western blot analysis
The expression of aggrecan and collagen II was
detected by western blot analysis according to the manufacturer's
protocol, and β-actin was used as an internal control for protein
loading. NP-treated and NP-free cells were washed with ice-cold PBS
and lysed in RIPA buffer (Beyotime Institute of Biotechnology) on
ice for 15 min. Protein concentrations were measured using a BCA
protein assay kit (Beyotime Institute of Biotechnology). Samples
(20 µg) were loaded and separated by 12% SDS-PAGE, and then
transferred onto polyvinylidene difluoride membranes (EMD
Millipore; Merck KGaA). Following blocking with 5% non-fat milk in
Tris buffered saline for 1 h at 37°C, the membranes were incubated
overnight with primary antibodies (all from Beyotime Institute of
Biotechnology) directed against aggrecan (1:1,000; cat. no.
AF6126), and collagen II (1:1,000; cat. no. AF6528), followed by
further incubation with a horseradish peroxidase-conjugated goat
anti-rabbit IgG secondary antibody (1:5,000; cat. no. A0208;
Beyotime Institute of Biotechnology) for 1 h at room temperature.
Specific proteins were visualized using an enhanced
chemiluminescence system (Amersham; GE Healthcare). Densitometry
was calculated using ImageJ software (1.8.0; National Institutes of
Health).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using Stata 10.0 software
(StataCorp LP). Data were analyzed by one-way analysis of variance,
followed by the Bonferroni post-hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Isolation and culture of BM-MSCs
BM-MSCs isolated from total bone marrow culture
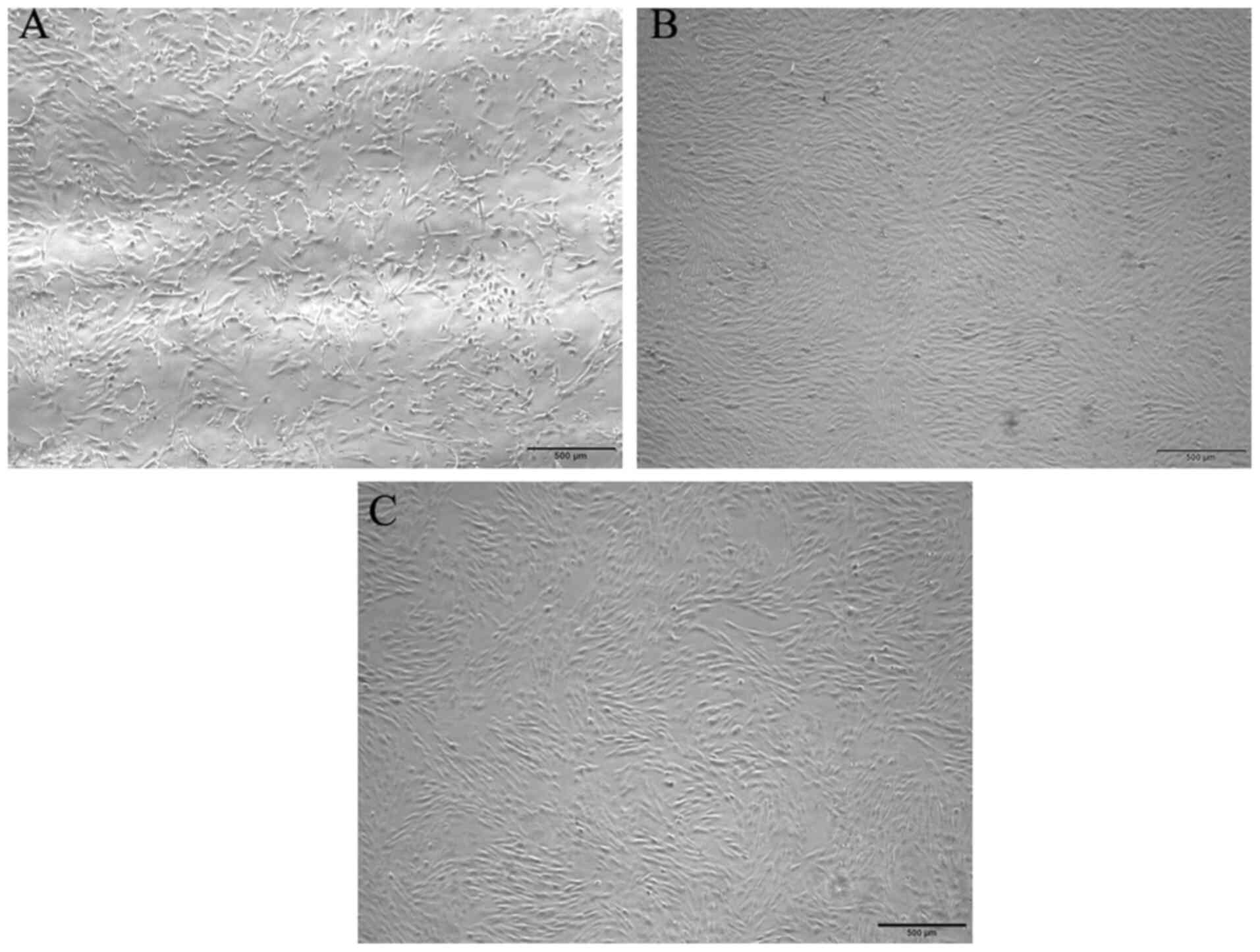
adhered to the bottom of the plate following primary culture and
acquired a polygonal or fibriform appearance (Fig. 1A). At ~12 days, the cells reached
~90% confluence and displayed a fibriform or spindle-shaped
morphology in a monolayer culture, indicating plastic adhesion
ability (Fig. 1B and C). The cell
monolayer was tightly arranged and exhibited a vortex-like
shape.
Multi-differentiation potential of
BM-MSCs
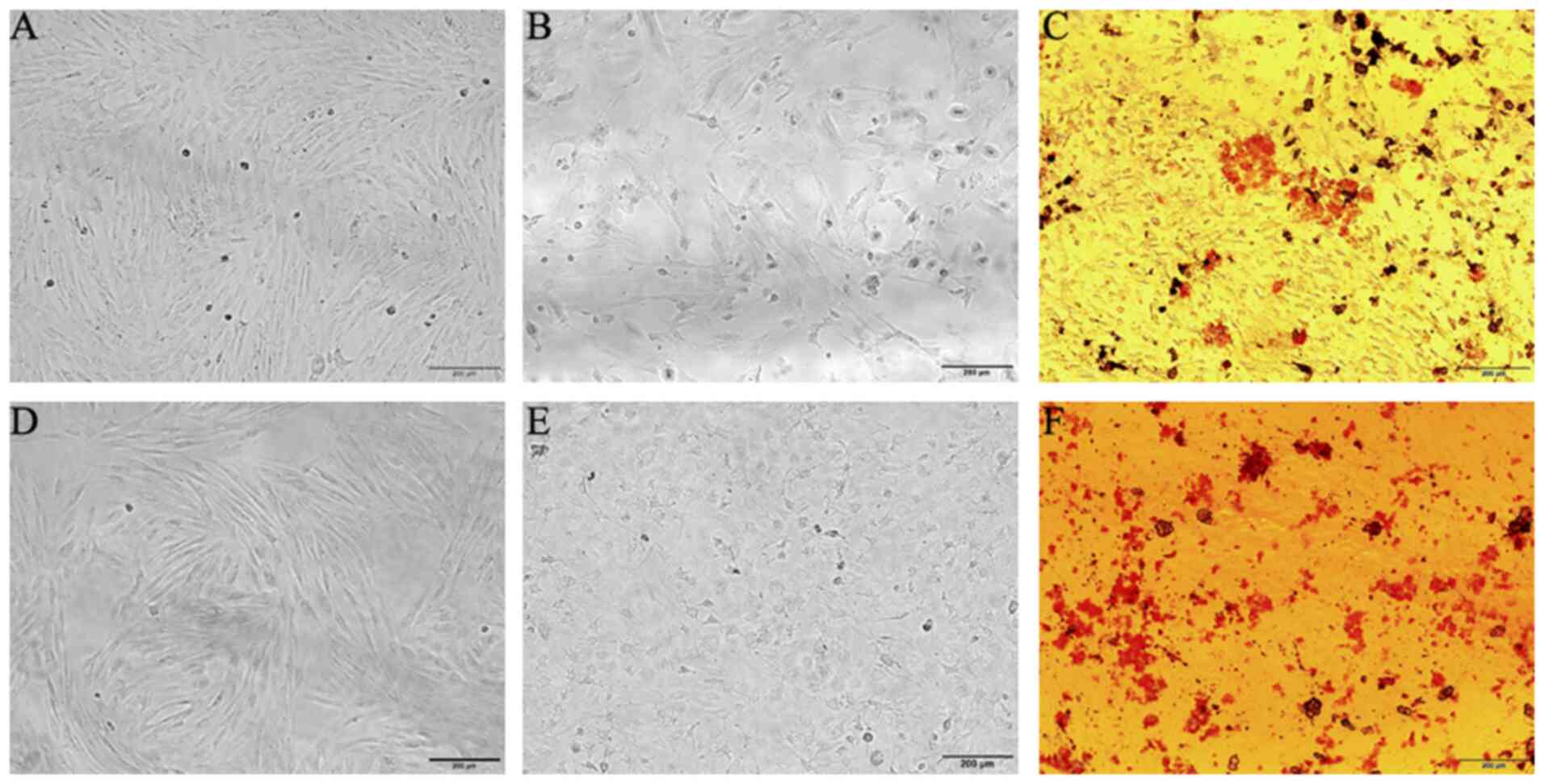
In the osteogenic differentiation experiment, the
cell phenotype changed from a spindle-shaped to a polygonal one,
and particles were observed in the cytoplasm. As time elapsed, the
cells came together with calcified nodules appearing, and a clear
sense of frosting was observed. Alizarin Red staining revealed that
the BM-MSCs had the potential for osteogenic differentiation
(Fig. 2A-C). The cells subjected to
adipogenic differentiation acquired a short fibrous and elliptical
appearance, and fat droplets were observed in the cytoplasm,
identified by Oil Red O staining (Fig.
2D-F). These results revealed that these cells were capable of
osteogenic and adipogenic differentiation under the proper
induction conditions.
Identification of BM-MSCs surface
markers
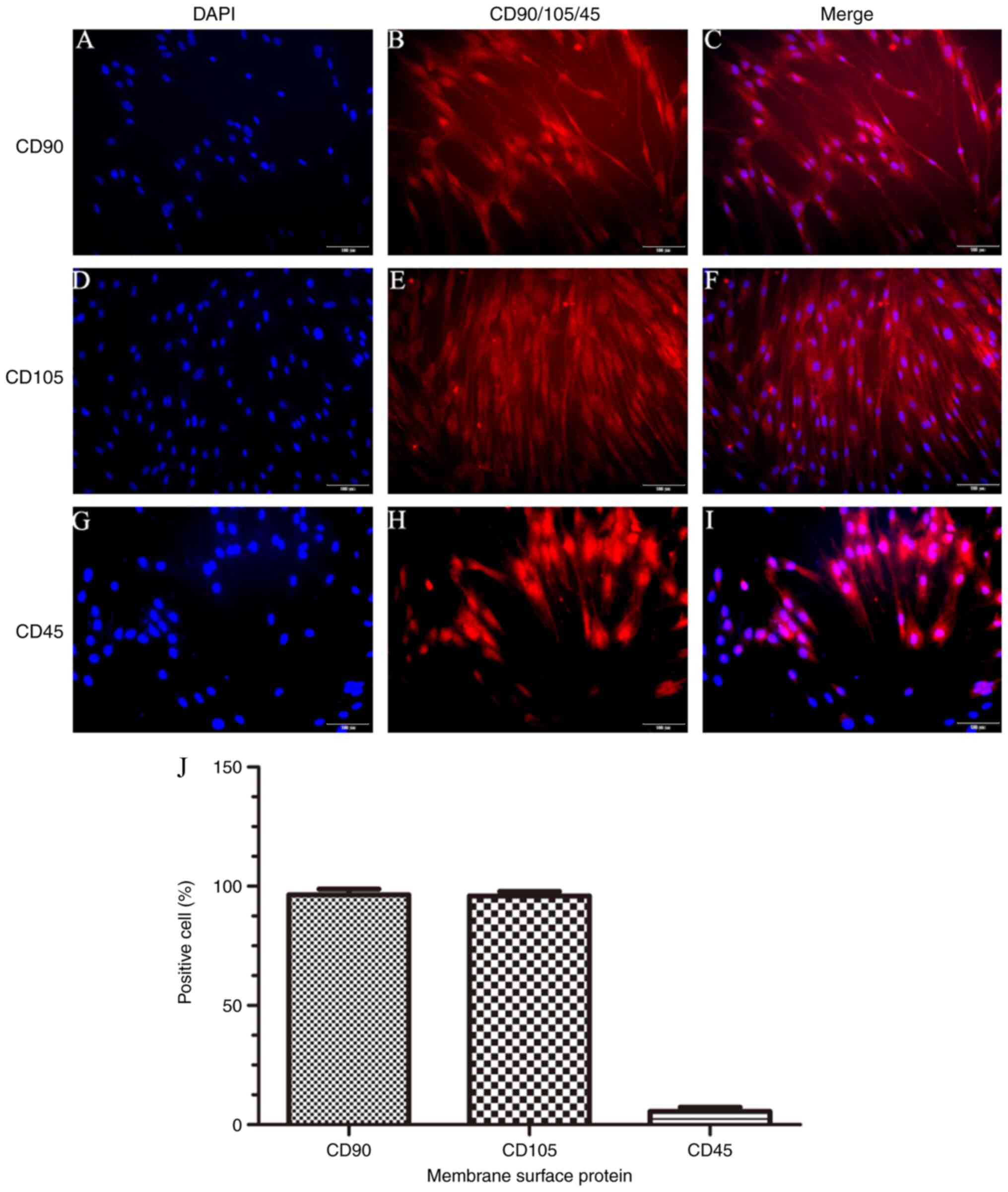
To investigate the expression of surface markers of
BM-MSCs, a fluorescence microscope assay was conducted. The results
revealed that there was a large number of cells expressing CD90
(98.6±0.8%) and CD105 (96.0±0.7%), and a very small number
expressing CD45 (5.8±0.4%) (Fig.
3). This indicated that the BM-MSCs fulfilled the features for
the phenotype of MSCs.
Expression of differentiation-related
genes
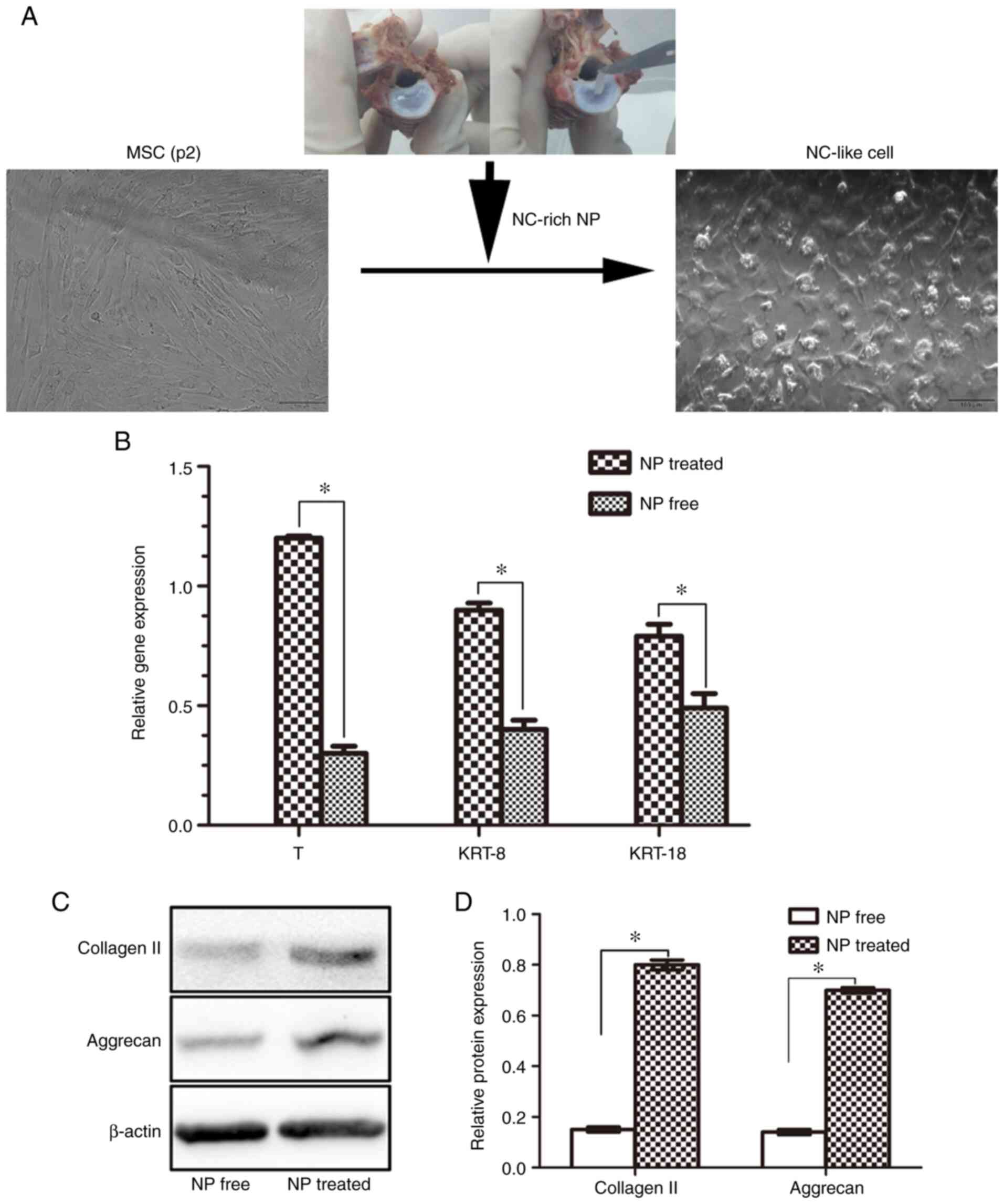
To determine whether identified NC markers were
expressed in the induced NC-like cells, the expression levels of
marker genes were detected by RT-qPCR. The results revealed that
all associated genes were expressed at higher levels in the
NP-treated group compared with the NP free group (all genes;
P<0.05; Fig. 4B). These results
demonstrated that porcine NP matrix markedly affects gene
expression, and the examined NC marker genes were expressed in
NC-like cells.
Effects of NC-rich matrix on
ECM-related protein expression
The morphology of the NC-like cells differed from
that of the BM-MSCs, in that they exhibited a fibroblast-like or
round appearance (Fig. 4A).
Furthermore, it was found that the NC-like cells exhibited an
enhanced synthesis of the ECM proteins, collagen II and aggrecan.
Compared with the NP-free group, the levels of collagen II and
aggrecan in the NP-treated group co-cultured with NC-rich matrix
(NC-like cells) were significantly higher (P<0.05; Fig. 4C and D).
Discussion
Since there are limitations in terms of current
treatment strategies for IVDD diseases, minimally invasive and
regenerative therapies, including cell or growth factors alone or
in combination, are being investigated. Among these approaches,
MSCs possess regenerative potential for the treatment of IDD.
However, with the increasing amount of research into using MSCs,
certain defects have emerged. To begin with, the harsh
microenvironment within the degenerative IVD may compromise the
viability and activity of externally delivered cell populations
(21). Furthermore, an MSC
injection may cause potential side-effects, including cell leakage
inducing osteophyte formation (22). Additionally, MSCs do not exert
evident regenerative effects in dogs with spontaneous degenerative
IVD disease (10). By contrast, NCs
are remnants of the chorda of embryonic tissue that are generally
lost (at the latest) by the tenth year of human life and may
function as stem cells in affected IVD tissue (23). Based on this hypothesis, the present
study attempted to restore NP cellularity, and therefore to restore
the biochemistry and biomechanical functionality of NP tissue by
the transplantation of NC-like cells.
In the present study, NC-like cells were generated
using MSCs due to their well-known pluripotency and abundance.
These cells were isolated from porcine tibiae and femurs by primary
culture, and the cells were identified according to the
International Society for Cellular Therapy standards (24). Primary cells exhibited plastic
adhesion ability and displayed a vortex-like proliferation. They
expressed CD90 and CD105, but did not express CD45. In addition,
they possessed the ability to differentiate into osteoblasts and
adipocytes. These characteristics fulfilled the criteria for the
consideration of these cells as MSCs. To the best of our knowledge,
no such growth factor or molecular inductive agent has been
successfully used to effectively direct notochordal
differentiation. In the present study, porcine NP matrix was used
to induce the notochordal differentiation. The porcine NP matrix
consists of a substantial population of NCs and may contain
regulatory factors that may induce the notochordal differentiation
(25).
In the present study, to confirm phenotypic
alterations in MSCs, the expression of NC-related cell markers,
including T, KRT-8 and KRT-18 was examined. The results revealed
that porcine NC-rich NP matrix induced the expression of T, KRT-8
and KRT-18. The protein encoded by the T gene is an embryonic
nuclear transcription factor, which localizes to
notochordal-derived cells (26).
Members of the cytokeratin family are typically expressed by
epithelial cells, but are found in a wide range of tissues,
including the developing NC (27,28).
The positive transcription of a panel of these genes in porcine
NC-rich NP matrix induced NC-like cells with an NC phenotype. In
the present study, NC-like cells generated NP-like extracellular
matrix with collagen II and aggrecan. The production of these two
important NP matrix components indicates that these NC-like cells
possessed biological functions similar to those of NP tissue. In
addition, NCs are more resistant to acute mechanical stresses than
are NP cells (29). Therefore,
NC-like cells may be an ideal cell source for regenerative
therapies for IVDD.
The mechanisms responsible for the promotion of the
differentiation and regeneration of porcine MSCs by porcine NP
matrix have come to light. The NP matrix may serve as an
‘instructive matrix’, locally increasing growth factor
concentrations and promoting their biological activity. A previous
study by Bach et al (30)
demonstrated that NC-conditioned medium (NCCM) exerted its anabolic
effects, mainly through soluble factors. It was hypothesized that
these characteristics are also applicable to NP matrix as they
later possess more sufficient active factors than NCCM. Among the
soluble factors, potentially noteworthy factors, including
transforming growth factor β-1 (TGFβ-1) and connective tissue
growth factor have been identified (31).
Considering the broad potential of NCs in the field
of cell therapy for the treatment of disc degeneration, certain
studies have focused on generating NC-like cells from various stem
cells. Liu et al (32,33)
demonstrated a proof of concept for using native porcine NP matrix
to direct the notochordal differentiation of human induced
pluripotent stem cells (hiPSCs). Although successful in certain
respects, patient-specific hiPSCs should be generated by
reprogramming or somatic cell nuclear transfer techniques (34). The complex production process would
hamper the development of an NC-based treatment for IVDD. By
contrast, MSCs are known for their pluripotency, abundance, and
easy access for collection, representing an ideal source. MSC
differentiation is induction factor-dependent, and multiple cell
types may be induced in response to different triggering stimuli.
In the field of disc regeneration, MSCs may differentiate toward
IVD cells or NP-like cells when induced by TGFβ and NCCM,
respectively (35,36). In the present study, it was
demonstrated that porcine NC-rich matrix induced porcine BM-MSCs to
differentiate into NC-like cells.
Despite these significant results, the present study
has certain limitations. Although the positive effects of porcine
NC-rich NP matrix on notochordal differentiation from BM-MSCs were
demonstrated, the exact underlying mechanisms remain unclear and
require further investigation. Furthermore, these conclusions are
based on in vitro evidence; no precise animal experiments
were performed to examine the therapeutic effects on disc
degeneration in vivo.
In conclusion, by co-culturing the obtained BM-MSCs
with porcine NP matrix, NC-like cells were generated, which
expressed typical notochordal marker genes, including T, KRT-8 and
KRT-18, exhibiting excellent functional ability to generate NP
extracellular matrix. This method may provide an ideal cell source
for regenerative therapies for disc degeneration.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Program for
Talent Cultivation of Jinshan Hospital (grant no. 2018-ISYYQH-07),
the Jinshan Science and Technology Committee (grant no. 2015-3-6)
and the Jinshan District Health Committee (grant no.
JSKJ-KTMS-2019-02).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL, QZ and ZJ performed the experiments, analyzed
the data and interpreted the results. DL, WL and LD revised the
manuscript and interpreted results. DL and MB analyzed the data. DL
and QZ prepared the manuscript. JW designed the study. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee on Animal Experiments of Fudan University (Shanghai,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hoy D, March L, Woolf A, Blyth F, Brooks
P, Smith E, Vos T, Barendregt J, Blore J, Murray C, et al: The
global burden of low back pain: Estimates from the global burden of
disease 2010 study. Ann Rheum Dis. 73:968–974. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee EH and Hui JH: The potential of stem
cells in orthopaedic surgery. J Bone Joint Surg Br. 88:841–851.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luo J, Wang H, Peng J, Deng Z, Zhang Z,
Liu S, Wang D, Gong M and Tang S: Rate of adjacent segment
degeneration of cervical disc arthroplasty versus fusion
meta-analysis of randomized controlled trials. World Neurosurg.
113:225–231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu TK, Meng Y, Wang BY, Hong Y, Rong X,
Ding C, Chen H and Liu H: Is the behavior of disc replacement
adjacent to fusion affected by the location of the fused level in
hybrid surgery? Spine J. 18:2171–2180. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johannessen W and Elliott DM: Effects of
degeneration on the biphasic material properties of human nucleus
pulposus in confined compression. Spine (Phila Pa 1976).
30:E724–E729. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li XC, Wu YH, Bai XD, Ji W, Guo ZM, Wang
CF, He Q and Ruan DK: BMP7-based functionalized self-assembling
peptides protect nucleus pulposus-derived stem cells from apoptosis
in vitro. Tissue Eng Part A. 22:1218–1228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crevensten G, Walsh AJ, Ananthakrishnan D,
Page P, Wahba GM, Lotz JC and Berven S: Intervertebral disc cell
therapy for regeneration: Mesenchymal stem cell implantation in rat
intervertebral discs. Ann Biomed Eng. 32:430–434. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berebichez-Fridman R, Gómez-García R,
Granados-Montiel J, Berebichez-Fastlicht E, Olivos-Meza A, Granados
J, Velasquillo C and Ibarra C: The Holy Grail of orthopedic
surgery: Mesenchymal stem cells-their current uses and potential
applications. Stem Cells Int. 2017:26383052017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vadalà G, Russo F, Ambrosio L, Loppini M
and Denaro V: Stem cells sources for intervertebral disc
regeneration. World J Stem Cells. 8:185–201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steffen F, Smolders LA, Roentgen AM,
Bertolo A and Stoyanov J: Bone marrow-derived mesenchymal stem
cells as autologous therapy in dogs with naturally occurring
intervertebral disc disease: Feasibility, safety, and preliminary
results. Tissue Eng Part C Methods. 23:643–651. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marcucio RS, Nauth A, Giannoudis PV,
Bahney C, Piuzzi NS, Muschler G and Miclau T III: Stem cell
therapies in orthopaedic trauma. J Orthop Trauma. 29 (Suppl
12):S24–S27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han XB, Zhang YL, Li HY, Chen B, Chang X,
Zhang W, Yang K, Zhou Y and Li CQ: Differentiation of human
ligamentum flavum stem cells toward nucleus pulposus-like cells
induced by coculture system and hypoxia. Spine (Phila Pa 1976).
40:E665–E674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng Y, Feng S, Liu W, Fu Q, Li Y, Li X,
Chen C, Huang C, Ge Z and Du Y: Preconditioning of mesenchymal
stromal cells toward nucleus pulposus-like cells by
microcryogels-based 3D cell culture and syringe-based pressure
loading system. J Biomed Mater Res B Appl Biomater. 105:507–520.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Colombier P, Clouet J, Boyer C, Ruel M,
Bonin G, Lesoeur J, Moreau A, Fellah BH, Weiss P, Lescaudron L, et
al: TGF-β1 and GDF5 act synergistically to drive the
differentiation of human adipose stromal cells toward nucleus
pulposus-like cells. Stem Cells. 34:653–667. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu J, Deng G, Tian Y, Pu Y, Cao P and Yuan
W: An in vitro investigation into the role of bone
marrowderived mesenchymal stem cells in the control of disc
degeneration. Mol Med Rep. 12:5701–5708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peroglio M, Douma LS, Caprez TS, Janki M,
Benneker LM, Alini M and Grad S: Intervertebral disc response to
stem cell treatment is conditioned by disc state and cell carrier:
An ex vivo study. J Orthop Translat. 9:43–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bergknut N, Rutges JP, Kranenburg HJ,
Smolders LA, Hagman R, Smidt HJ, Lagerstedt AS, Penning LC,
Voorhout G, Hazewinkel HA, et al: The dog as an animal model for
intervertebral disc degeneration? Spine (Phila Pa 1976).
37:351–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smolders LA, Bergknut N, Grinwis GC,
Hagman R, Lagerstedt AS, Hazewinkel HA, Tryfonidou MA and Meij BP:
Intervertebral disc degeneration in the dog. Part 2:
Chondrodystrophic and non-chondrodystrophic breeds. Vet J.
195:292–299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Acosta FJ, Lotz J and Ames CP: The
potential role of mesenchymal stem cell therapy for intervertebral
disc degeneration: A critical overview. Neurosurg Focus. 19:E42005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vadalà G, Sowa G, Hubert M, Gilbertson LG,
Denaro V and Kang JD: Mesenchymal stem cells injection in
degenerated intervertebral disc: Cell leakage may induce osteophyte
formation. J Tissue Eng Regen Med. 6:348–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hunter CJ, Matyas JR and Duncan NA: The
notochordal cell in the nucleus pulposus: A review in the context
of tissue engineering. Tissue Eng. 9:667–677. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salzig D, Schmiermund A, Gebauer E,
Fuchsbauer HL and Czermak P: Influence of porcine intervertebral
disc matrix on stem cell differentiation. J Funct Biomater.
2:155–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vujovic S, Henderson S, Presneau N, Odell
E, Jacques TS, Tirabosco R, Boshoff C and Flanagan AM: Brachyury, a
crucial regulator of notochordal development, is a novel biomarker
for chordomas. J Pathol. 209:157–165. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Götz W, Kasper M, Fischer G and Herken R:
Intermediate filament typing of the human embryonic and fetal
notochord. Cell Tissue Res. 280:455–462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Minogue BM, Richardson SM, Zeef LA,
Freemont AJ and Hoyland JA: Transcriptional profiling of bovine
intervertebral disc cells: Implications for identification of
normal and degenerate human intervertebral disc cell phenotypes.
Arthritis Res Ther. 12:R222010. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saggese T, Thambyah A, Wade K and
McGlashan SR: Differential response of bovine mature nucleus
pulposus and notochordal cells to hydrostatic pressure and glucose
restriction. Cartilage. 11:221–233. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bach FC, de Vries SA, Riemers FM, Boere J,
van Heel FW, van Doeselaar M, Goerdaya SS, Nikkels PG, Benz K,
Creemers LB, et al: Soluble and pelletable factors in porcine,
canine and human notochordal cell-conditioned medium: Implications
for IVD regeneration. Eur Cell Mater. 32:163–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gantenbein B, Calandriello E, Wuertz-Kozak
K, Benneker LM, Keel MJ and Chan SC: Activation of intervertebral
disc cells by co-culture with notochordal cells, conditioned medium
and hypoxia. BMC Musculoskelet Disord. 15:4222014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, Fu S, Rahaman MN, Mao JJ and Bal
BS: Native nucleus pulposus tissue matrix promotes notochordal
differentiation of human induced pluripotent stem cells with
potential for treating intervertebral disc degeneration. J Biomed
Mater Res A. 103:1053–1059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Rahaman MN and Bal BS: Modulating
notochordal differentiation of human induced pluripotent stem cells
using natural nucleus pulposus tissue matrix. PLoS One.
9:e1008852014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chung YG, Eum JH, Lee JE, Shim SH,
Sepilian V, Hong SW, Lee Y, Treff NR, Choi YH, Kimbrel EA, et al:
Human somatic cell nuclear transfer using adult cells. Cell Stem
Cell. 14:777–780. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Steck E, Bertram H, Abel R, Chen B, Winter
A and Richter W: Induction of intervertebral disc-like cells from
adult mesenchymal stem cells. Stem Cells. 23:403–411. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
de Vries SA, Potier E, van Doeselaar M,
Meij BP, Tryfonidou MA and Ito K: Conditioned medium derived from
notochordal cell-rich nucleus pulposus tissue stimulates matrix
production by canine nucleus pulposus cells and bone marrow-derived
stromal cells. Tissue Eng Part A. 21:1077–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|