Introduction
Periodontal tissues, mainly derived from the dental
follicle (DF), comprise the gingiva, cementum, alveolar bone, and
periodontal ligament (PDL) (1). The
periodontium functions as a unit to support and invest the tooth,
disperse occlusal force, and maintain the long-term stability of
dentognathic system. The periodontium is a highly dynamic
microenvironment that constantly undergoes turnover; under normal
conditions, the rates of tissue formation are in balance with the
rates of tissue degradation (2).
External stimuli, such as microorganisms, mechanical stress, or any
factors that may cause developmental defects or immune disorders,
can affect this dynamic balance (3–5).
Subsequently, the host can raise responses to these challenges
through various factors and molecules, in order to achieve a new
dynamic balance (6,7).
The Wnt signaling pathway has long been considered
to serve an essential role in the development and disease of
periodontal tissues. Canonical Wnt signaling (through β-catenin)
was recently confirmed to be associated with the development of
cementum and constitutive activation of β-catenin might cause
cementum hyperplasia (8). Wnt
family member 5a (Wnt5a), a prototypical β-catenin-independent Wnt
family member, has also been found to be highly involved in
periodontal tissue development and periodontitis.
Wnt5a may participate in the development of
periodontal tissues, maintaining a non-mineralized state of
periodontal ligament, and regulating bone homeostasis. In
periodontitis, excessive production of Wnt5a might function as a
proinflammatory factor amplifying local inflammation. However,
other studies have reported a positive role for Wnt5a in the
regeneration of integrated periodontal complex. The present review
article focused on the current understanding of Wnt5a and its
functions in periodontal tissue development, maintenance, and
periodontitis, as well as its potential effect on periodontal
regeneration.
Wnt5a signaling and functions
The Wnt5a cDNA was first isolated from fetal mice
(9) and was later sequenced in
humans (10). The Wnt5a gene, which
is located on chromosome 3p14-p21, has been identified to encode
two different protein isoforms, Wnt5a-long (Wnt5a-L) and
Wnt5a-short (Wnt5a-S), due to alternative transcriptional start
sites. The protein Wnt5a-L is 380 amino acids in size, and Wnt5a-S
comprises 365 amino acids (11).
Biochemical analysis has demonstrated that these two purified
proteins are similar in stability, hydrophobicity, and
Wnt/β-catenin signaling activity. However, they are differentially
expressed in tissues and might serve distinct roles in cancer. It
has been reported that Wnt5a-L inhibits, while Wnt5a-S promotes,
the growth of tumor cell lines (11).
The receptors of Wnt5a mainly include receptor
tyrosine kinase-like orphan receptors (RoRs), the frizzled family
of receptors (Fzd receptors), and co-receptors low-density
lipoprotein receptor-related protein (Lrp) 5 and 6. When binding to
these receptors, Wnt5a exerts its intracellular effects
predominantly by activating two non-canonical Wnt signaling
pathways (Fig. 1) (12). One branch is the planar cell
polarity pathway, which involves the activation of small GTPases
Rho and Rac, and the subsequent regulation of c-Jun N-terminal
kinase (Jnk) to control cell orientation (13). The other branch is the calcium
pathway, which when activated, increases the intracellular
Ca2+ levels, subsequently activating calcium/calmodulin
dependent protein kinase II (CaMKII) and protein kinase C. This
signaling pathway has been demonstrated to mediate cell
proliferation, migration, and adhesion (14). Notably, in addition to activating
the non-canonical Wnt pathway, Wnt5a can have regulatory effects on
the canonical Wnt/β-catenin signaling pathway to control cell
proliferation and differentiation (Fig.
1), depending on the receptor and cell type context (15). Secreted frizzled-related protein 5
(Sfrp5), a soluble protein, is one of the most common Wnt5a
antagonists. Sfrp5 has been demonstrated to inhibit Wnt5a signaling
in both physiological and pathological processes (16).
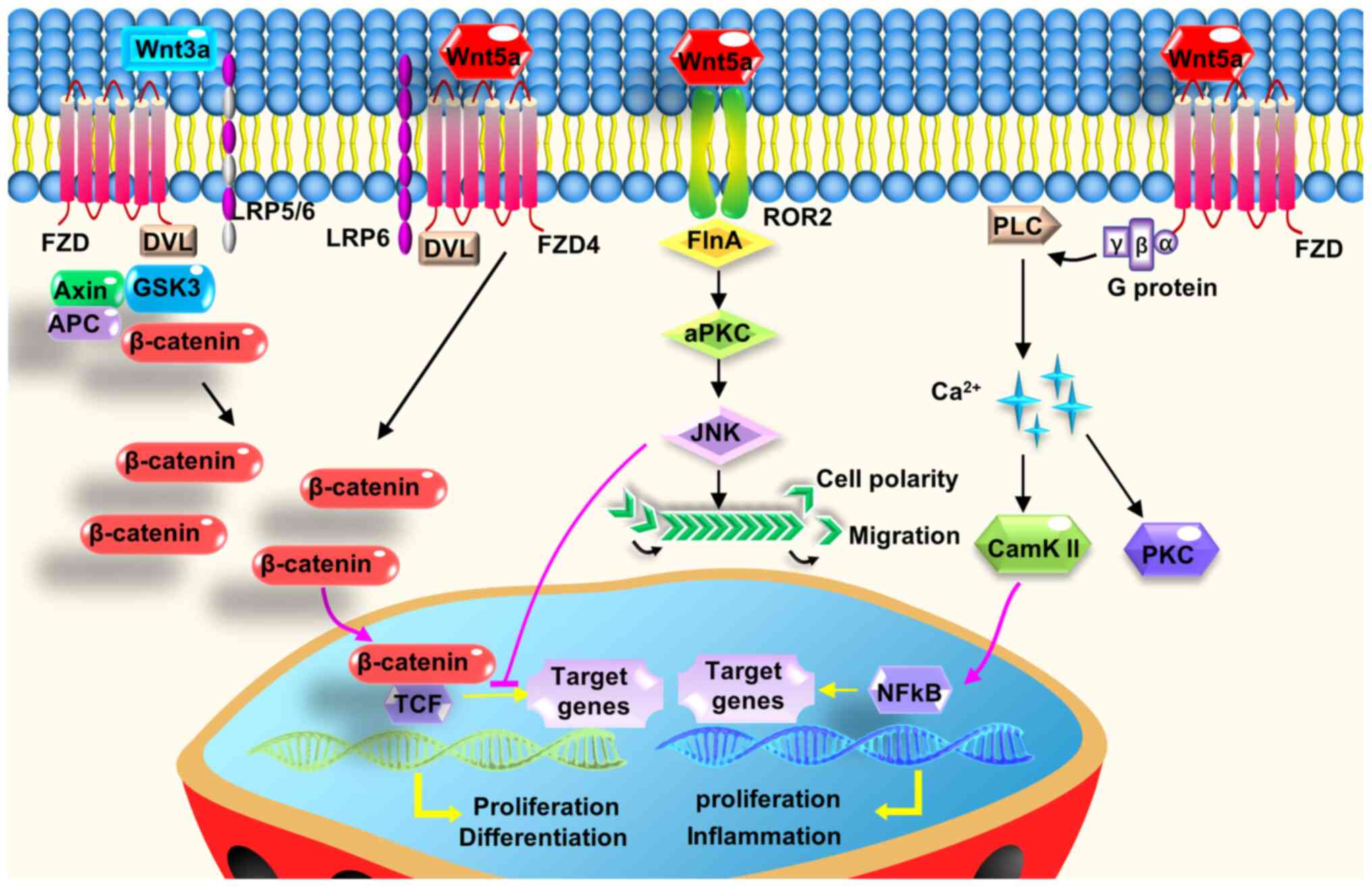 | Figure 1.Wnt5a exerts its intracellular
effects by activating two non-canonical Wnt signaling pathways, or
the interplay with the canonical Wnt signaling pathway. Wnt5a
regulates cell polarity, proliferation, migration, and adhesion,
predominantly through its downstream planar cell polarity and
calcium pathway. Wnt5a can antagonize canonical Wnt signaling
mostly by inhibiting the downstream part of β-catenin/Tcf-mediated
transcription or other mechanisms. In the presence of FZD4 and
LRP5, Wnt5a stabilizes β-catenin to activate the canonical Wnt
signaling pathway. APC, adenomatous polyposis coli; CaMKII,
calcium/calmodulin dependent protein kinase II; DVL, disheveled;
FlnA, filamin A; FZD, frizzled; GSK3, glycogen synthase kinase 3;
LRP, low-density lipoprotein receptor-related protein; PKC, protein
kinase C; PLC, phospholipase C; Tcf, T-cell factor; Wnt5a, Wnt
family member 5a. |
Wnt5a has been shown to be highly conserved among
species and associated with many processes of development and
disease. Homozygous Wnt5a-knockout mouse embryos are perinatally
lethal (17). In addition, Wnt5a
affects the morphogenesis of internal organs, such as the heart
(18) and the lungs (19). It is associated with proinflammatory
factor production and immune cell recruitment in inflammation
(20), and related to the
occurrence and prognosis of breast and colorectal cancers (21). Of note, previous studies have
reported that Wnt5 is involved in tooth growth, patterning,
odontoblast differentiation (22)
and determining tooth size (23).
Wnt5a in periodontal tissue development
Wnt5a is expressed at all stages of periodontal
tissue development. An early study demonstrated that Wnt5a mRNA was
mainly expressed in the dental follicle in developing murine tooth
germ (24), while later studies
reported that Wnt5a was strongly expressed in both the dental
epithelium and mesenchyme (23,25).
In postnatal periodontium, Wnt5a expression is robust in the
alveolar bone, as well as in the ameloblast and odontoblast layers
(26). Wnt5a can also be expressed
in mature periodontal tissues and is closely related to the
maintenance of periodontal soft and hard tissues under
physiological conditions (27,28).
Hasegawa et al (27) found
that Wnt5a was predominantly detected in mature PDL in rats.
Similarly, Wnt5a and its receptors (Ror2, Fzd2, Fzd4 and Fzd5) were
expressed in human periodontal ligament cells (HPDLCs) (27).
The expression of Wnt5a in the periodontium appears
to be mediated by physiological mechanical stress of occlusion and
mastication. Mechanical stress caused by stretch loading augmented
the expression levels of Wnt5a and its receptors in HPDLCs, while
the removal of occlusal pressure decreased Wnt5a expression levels
in rat PDL tissue (27). Wnt5a was
highly expressed at the tension sites of PDL and might further
contribute to osteogenesis through activation of canonical Wnt
signaling through its receptors Fzd4 and Lrp5 in an orthodontic
tooth movement model (28).
Wnt5a is hypothesized to participate in root
formation and tooth eruption, and further affect the development
and formation of cementum and alveolar bone (1). It was observed that the expression
levels of Wnt5a increased gradually from the center of the dental
follicle (beneath the apical foramen) to the lateral coronal
corner, where the DF differentiates into the periodontium during
tooth root development in swine (29). This spatial gradient expression
suggested the possible regulatory effect of Wnt5a on the
differentiation of DF. It is consistent with an earlier study
reporting that Wnt5a was expressed on
precementoblasts/cementoblasts during late tooth root development
(30). Furthermore, Wnt5a is
closely related to the growth and modeling of alveolar bone in the
process of tooth eruption. The expression of Wnt5a in rat alveolar
bone was increased during tooth eruption (31), especially during the
osteoclastogenesis burst (3–11 days postnatal) (26). Therefore, the spatial distribution
regulation of Wnt5a in alveolar bone may be crucial because tooth
eruption depends on the coordination of osteogenesis and
osteoclastogenesis in time and space.
Wnt5a in periodontal tissue maintenance
Despite containing stem cells that retain the
potential to differentiate into osteoblasts, cementoblasts and
fibroblasts (1), the mature
periodontal ligament never ossifies under physiological conditions,
a phenomenon known as the periodontal mineral homeostasis. The Wnt
pathway has been identified to be involved in the process, and
especially the Wnt antagonist Sfrp1. It has been demonstrated that
selective inhibition of Sfrp1 increased PDLC mineralization and
expression levels of mineralization-related genes, including
β-catenin, alkaline phosphatase, osteocalcin, collagen I, and
runt-related transcription factor 2 (32). Hasegawa et al (27) found that Wnt5a suppressed
mineralization but enhanced collagen production in HPDLCs. In
specific, Wnt5a upregulated the expression of periostin through
transforming growth factor-β1 (TGFβ1) (27), and periostin is essential for the
integrity and function of the periodontal ligament (33,34).
These findings suggest that Wnt5a may be a positive regulator in
fibrillogenesis to remodel PDL tissue and accelerate maturation of
PDL fibers, and to prevent non-physiological mineralization
(Fig. 2).
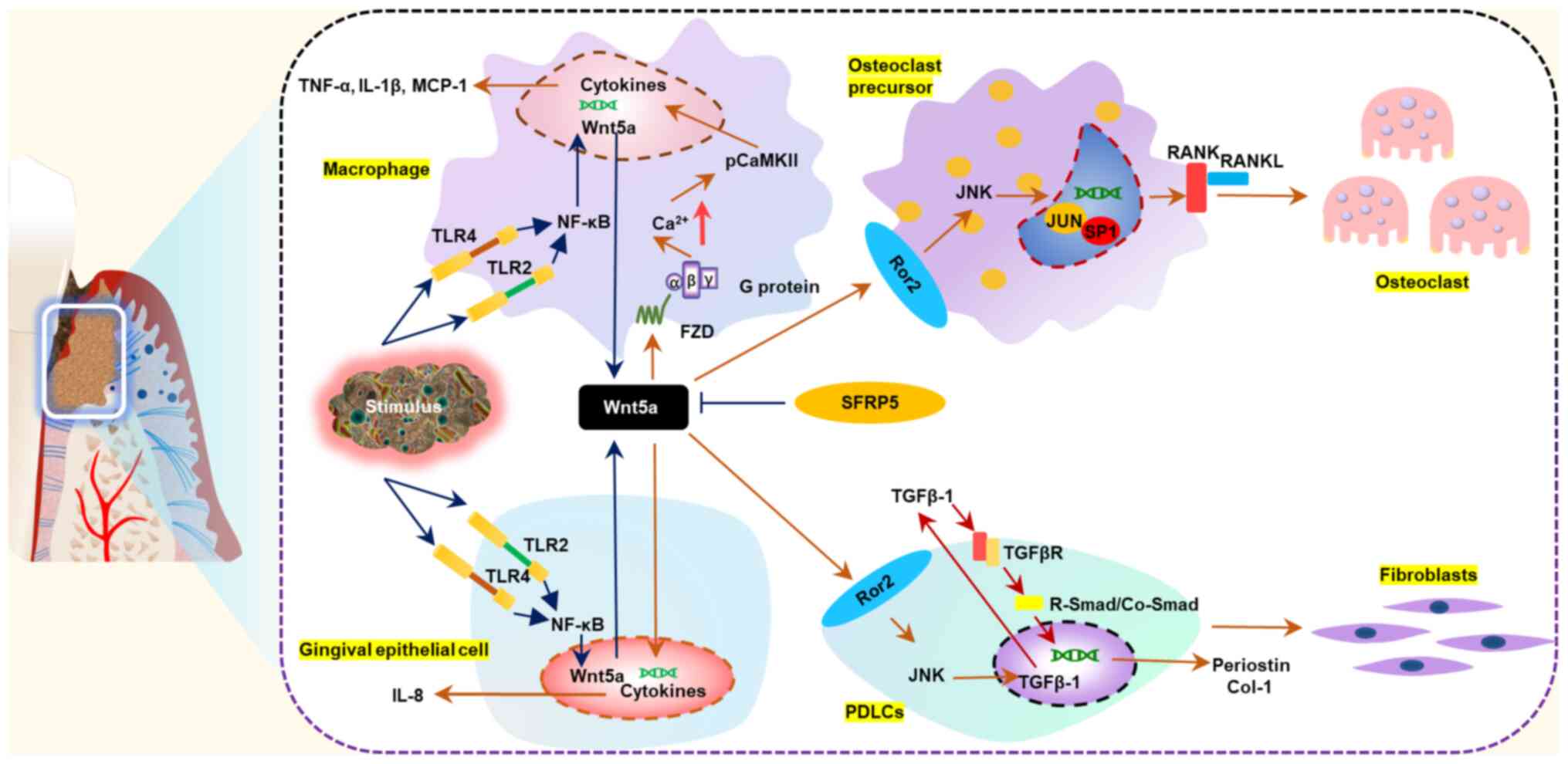 | Figure 2.Wnt5a participates in the
pathogenesis of periodontitis and regulates periodontal
non-mineralized homeostasis and bone homeostasis. Under
physiological conditions, Wnt5a contributes to maintaining a
non-mineralized state of periodontal ligament and regulating bone
homeostasis by promoting fibrillogenesis of periodontal ligament
cells and osteoclastogenesis of osteoclast precursors. During the
occurrence and development of periodontitis, Wnt5a expression
levels increase in response to pathogen stimulation. Excessive
production of Wnt5a amplifies the inflammatory response and
accelerates the destruction of periodontal tissues. Sfrp5
antagonizes the proinflammatory effects of Wnt5a. CaMKII,
calcium/calmodulin dependent protein kinase II; Col-1, collagen 1;
FZD, frizzled; MCP-1, monocyte chemotactic protein 1; PDLC,
periodontal ligament cell; RANK, receptor activator of NF-κB;
RANKL, receptor activator of NF-κB ligand; Ror2, receptor tyrosine
kinase-like orphan receptor 2; Sfrp5, secreted frizzled-related
protein 5; TLR, toll-like receptor; Wnt5a, Wnt family member
5a. |
In addition to the stability of soft tissues, the
maintenance of hard tissues in periodontium is equally important.
An early study showed that Wnt5a overexpression mediated dental
follicle cells (DFCs) to undergo moderate osteogenesis by
augmenting receptor activator of nuclear factor-κB (RANK) ligand
expression but not osteoprotegerin (26). This suggested that Wnt5a might
participate in bone homeostasis of periodontal tissues. Several
later studies confirmed the role of Wnt5a in regulating bone
homeostasis. Wnt5a has been reported to promote osteoclast fusion
in bone marrow-derived macrophages by antagonizing another
non-canonical Wnt signaling molecule, Wnt16 (35). Osteoblast-derived Wnt5a, when
binding to Ror2, increased RANK expression in osteoclast precursor
cells through the Jnk pathway, thereby promoting osteoclast
differentiation and bone resorption (36). Conversely, conditional deletion of
Wnt5a in mature osteoclasts resulted in reduced bone mass by
decreasing bone formation with unchanged osteoclast numbers
(37). It is generally hypothesized
that the nature of the response to Wnt5a is determined to a large
extent by the responding cells, and it might contribute to the bone
homeostasis rather than promoting osteogenesis or
osteoclastogenesis unilaterally.
Wnt5a in periodontitis
Wnt5a is associated with
periodontitis
A previous genome-wide association study has
demonstrated that the Wnt5a gene is correlated with severe
periodontitis (38). Wnt5a mRNA
expression levels in gingival tissues were upregulated in patients
with chronic periodontitis compared with healthy subjects (39–41).
In addition, the expression levels of Wnt5a mRNA increased in
gingival tissues from patients with aggressive periodontitis
compared those with chronic periodontitis (41). Furthermore, a positive association
was identified between the mRNA expression levels of Wnt5a and the
clinical parameters of periodontitis, including clinical attachment
loss (CAL) and periodontal pocket depth (PPD) (41). A cross-sectional study revealed that
severely diseased sites from patients with generalized forms of
moderate and severe chronic periodontitis (≥30% of the sites with
PPD ≥4 mm, CAL ≥3 mm and presence of bleeding on probing) exhibited
statistically significantly higher levels of Wnt5a in gingival
crevicular fluid (GCF) compared with healthy sites within the same
patient (42).
Wnt5a regulates the innate immune
response to periodontal pathogen invasion
It is generally accepted that the interplay between
subgingival plaque biofilm and host immune response has an
important role in the occurrence and development of periodontitis.
Previous studies have indicated that Wnt5a is highly involved in
the innate immune response of periodontitis, in which gingival
epithelial cells and monocyte/macrophage-lineage cells act as
barriers to prevent pathogen invasion. Maekawa et al
(40) found that Wnt5a was
predominantly localized in the epithelial layer, rather than in the
subjacent connective tissue, and that Wnt5a expression in human
gingival epithelial cells (HGECs) was significantly upregulated
following a 12-h stimulation with Porphyromonas
gingivalis-derived (Pg-) lipopolysaccharide (LPS) (0.1 and 1.0
µg/ml) (40). Another study
demonstrated that monocytes, but not human gingival fibroblasts
(HGFs), had an important role in upregulating Wnt5a expression at
inflamed gingiva sites (39). The
expression levels of Wnt5a mRNA were constant following treatment
with 1 µg/ml Pg-LPS in HGFs, while they were significantly
increased at 2 h and reached peak value at 4 h following Pg-LPS
stimulation in primary monocytes (39). Additionally, silencing of toll-like
receptor (Tlr)2 and Tlr4 by RNA interference downregulated the
Pg-LPS-induced Wnt5a expression (39). Luciferase reporter assay and
electrophoretic mobility analysis confirmed that this process was
dependent on the NF-κB signaling pathway, and the addition of NF-κB
inhibitors (wedelolactone or the inhibitor of κB kinase inhibitor
VII) could reduce the expression of Wnt5a in a dose-dependent
manner (39). Further studies
indicated that p65, one of the classical NF-κB transcription
factors, may upregulate Wnt5a via inflammatory stimulation
(43,44). Finally, Pg-LPS and interferon-γ
synergistical stimulation activated Wnt5a in monocytes, partly
through the glycoprotein130/Janus kinase/STAT1 signaling pathway
(39).
Wnt5a triggers proinflammatory
signaling cascades and increases secretion of proinflammatory
cytokines
Wnt5a has been demonstrated to regulate inflammatory
cytokines and to aggravate the destruction of periodontium.
Inhibition of Wnt5a by small interfering (si)RNA or a neutralizing
antibody significantly reduced the expression of interleukin
(IL)-1β, monocyte chemotactic protein 1, and matrix
metalloproteinase 2 in P. gingivalis-infected macrophages
(45). Local administration of
exogenous Wnt5a in the gingiva of mice with ligature-induced
periodontitis exacerbated alveolar bone loss (40). It has been reported that the calcium
pathway mediated by the Wnt5a receptor Fzd5 might be involved in
the process, since Wnt5a stimulation increased intracellular
calcium levels and activated CaMKII (46).
The effects of Wnt5a on periodontitis have also been
found to be linked to the interaction with its antagonist Sfrp5
(47). It was demonstrated that
Sfrp5 may have a protective role in periodontitis to prevent
LPS-induced inflammation, whereas Wnt5a was proinflammatory. The
expression of Sfrp5 in gingival tissues from patients with
periodontitis was significantly decreased, which was consistent
with in vitro results from HGECs following Pg-LPS
stimulation. Additionally, Sfrp5 inhibited the increase of IL-8
mRNA expression mediated by Pg-LPS and/or Wnt5a stimulation. Local
treatment of Sfrp5 in mice with ligature-induced periodontitis
significantly reduced the expression of Wnt5a as well as that of
proinflammatory factors (IL-1β, IL-6, and IL-17), and inhibited
alveolar bone loss (40). In
addition, the Sfrp5/Wnt5a axis might have a role in the association
between periodontitis and systemic inflammatory disorders
(including obesity, type 2 diabetes, and coronary artery disease).
A nested case-control study found that in overweight individuals
(body mass index, 24–38 kg/m2), Sfrp5 serum levels in
patients with periodontitis involving tooth loss were significantly
lower than in patients with periodontitis without tooth loss and
the matched control group, while Wnt5a had no significant
difference (48). Obese human
subjects had significantly lower serum Sfrp5 levels than lean
controls, while they had significantly higher serum Wnt5a levels
(49). Similarly, this inverse
pattern of expression for Sfrp5 and Wnt5a was detected in the serum
of patients with coronary artery disease (50) and the plasma of patients with type 2
diabetes mellitus (51). An in
vitro study confirmed that Sfrp5 alleviated the endothelial
dysfunction damage mediated by Wnt5a (52).
Taken together, Wnt5a may serve an important role in
periodontitis. It not only regulates the innate immune response to
periodontal pathogen invasion, but also triggers proinflammatory
signaling cascades and increases secretion of proinflammatory
cytokines, ultimately resulting to the destruction of periodontium
(Fig. 2). However, Wnt5a has been
reported to have an anti-inflammatory role under certain
conditions. It might be crucial to the functions of macrophages in
maintaining CD14 expression, an important immune response mediator,
and supporting macrophage survival (44). Wnt5a induced the expression of
anti-inflammatory mediator IL-10 in primary human monocytes
(53), and promoted macrophage M2
polarization in kidney fibrosis (54). In addition, Wnt5a was found to have
an important role in killing Mycobacterium tuberculosis by
enhancing autophagy of human monocyte-derived macrophages (55). Thus, the specific role of Wnt5a in
inflammation remains inconclusive, and it may depend on the
tissue-specific immune microenvironment or the stage of immune
response. Further studies are required to fully comprehend the
exact role of Wnt5a in periodontitis.
Wnt5a in periodontal regeneration
Untreated periodontitis leads to periodontal tissue
destruction and tooth loss. In recent years, periodontal tissue
engineering has been greatly developed, and stem cell therapy
remains a research hotspot (56).
Previous studies have revealed that the canonical Wnt signaling
pathway is essential in periodontal tissue regeneration, especially
in stem cell self-renewal and multi-directional differentiation
(57,58). The non-canonical Wnt signaling
pathways have also been observed to regulate the biological
behavior of periodontal stem cells.
Wnt5a may regulate the biological behavior of
periodontal stem cells. Wnt5a inhibited the induction of DFCs
cellular senescence, supported cell proliferation and prevented
cell death (59). Wnt5a
significantly enhanced the proliferation of HPDLCs by
phosphorylating two major mitogen-activated protein kinases,
extracellular signal-regulated kinase and Jnk (27), which are important signaling
molecules influencing cell proliferation (60). In addition, Wnt5a promoted the
migration of HPDLCs by phosphorylating Akt, which serves pivotal
roles in cell migration (61).
Similar to its function in bone homeostasis, Wnt5a has dual effects
on osteogenic differentiation of periodontal stem cells. Wnt5a
suppressed osteoblastic differentiation via Ror2/Jnk signaling in
HPDLCs (62). Additionally,
Sakisaka et al (30) found
that Wnt5a might function as a negative regulator in the
Wnt3a-mediated osteogenic differentiation of DFCs by inhibiting the
downstream part of β-catenin/T-cell factor-mediated transcription.
As a canonical Wnt family member, Wnt3a has been reported to have a
potential role in stimulating cementoblast/osteoblast
differentiation of dental follicle cells via the Wnt/β-catenin
signaling pathway (63). Several
studies have suggested that Wnt5a was a positive regulator in
osteogenic differentiation of stem/progenitor cells. Xiang et
al (26) found that in the
presence of 100 ng/ml bone morphogenetic protein (BMP)2, Wnt5a
strongly prompted mineralization of DFCs. Another study indicated
that Wnt5a signaling might be a substantial constituent in
BMP2-mediated osteoblastogenesis (64). In addition, Wnt5a-induced
non-canonical Wnt signaling has been shown to suppress
adipogenesis, which, in turn, promoted the differentiation of
mesenchymal stem cells into osteoblast lineage cells (65).
With the development of stem cell therapy, several
scholars have hypothesized that stem cells may obtain improved
regeneration ability in the inflammatory microenvironment (66). When they interact with the
inflammatory microenvironment, stem cells can repair themselves and
secret more regulatory factors to affect surrounding cells to
promote regeneration. In turn, surrounding cells, such as
tissue-resident immune cells, can influence the fate and behavior
of stem cells by releasing immune modulators, and further
contribute to tissue regeneration. Recently, it was observed that
Wnt5a was abundantly expressed in the regenerating gastric tissue
following Helicobacter pylori infection (67). Conditional deletion of Wnt5a or
depletion of innate lymphoid cells, the cells that act as the main
source of Wnt5a, reduced Mist1+ stem cell proliferation
and impaired healing and regeneration in experimental gastric ulcer
(67). This in vivo study
suggested that Wnt5a may contribute to the interaction between stem
cells and immune cells under inflammatory microenvironment. The
effect of Wnt5a on periodontal regeneration under the inflammatory
microenvironment is at the initial stages of being explored. An
in vitro study demonstrated that the Wnt5a-mediated
Ca2+ signaling pathway had an inhibitory role in
cementogenic/osteogenic differentiation of HPDLCs following
stimulation by pro-inflammatory cytokines IL-6 and TNF-α (68). However, another study suggested that
the inflammatory microenvironment may suppress the
Wnt/Ca2+ signaling pathway and lead to reduced
osteogenic differentiation of HPDLCs (69). This discrepancy may be due partly to
the pleiotropic effects of Wnt5a in periodontal regeneration in
inflammatory microenvironments. Wnt5a not only influences the
regeneration potential of stem cells, but also has an impact on
immune cells. Further studies in vivo will be required to
fully understand the effect of Wnt5a on periodontal regeneration in
a synthetic in vivo environment.
In addition, periodontal regeneration emphasizes the
reconstruction of integrated periodontal tissues, not just the
regeneration of hard tissues. Regeneration of periodontal ligament
guarantees functional recovery and long-term stability of
periodontal tissues. At present, and although further research is
warranted, Wnt5a is hypothesized to be a candidate molecule for
periodontal regeneration due to its ability to promote
fibrillogenesis and to moderate osteogenesis in periodontal
stem/progenitor cells.
Conclusion
As a prototypical non-canonical Wnt, Wnt5a is
involved in many processes of periodontal tissue development,
maintenance, and periodontitis. Based on its broad biological
functions, Wnt5a may also serve a role in periodontal regeneration.
However, current studies have primarily conducted in vitro
experiments, so there is a lack of in vivo studies to verify
the results. Moreover, the specific mechanism underlying Wnt5a
regulation of periodontal homeostasis is not completely understood.
Therefore, identifying how to regulate Wnt5a to maintain the
balance between osteogenesis and osteoclastosis in periodontal
tissues is important. Moreover, Wnt5a might display a diagnostic
value in periodontitis as Wnt5a can be detected in GCF, and its
expression level is correlated with the diseased site. Future
studies should investigate whether the protein expression levels of
Wnt5a in the GCF of patients with chronic periodontitis are related
to the active and stable phases of the diseased site, as well as
assessing the predictive value of Wnt5a.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural and Science Foundation of China (grant no. 81771077) and
the Key Research and Development Program of Sichuan Province (grant
no. 2020YFS0175).
Availability of data and materials
Not applicable.
Authors' contributions
XW and QL performed the literature search, wrote the
manuscript and designed the figures. SG and YW designed the
framework of this review and modified the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cho MI and Garant PR: Development and
general structure of the periodontium. Periodontology 2000.
24:9–27. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luan X, Zhou X, Trombetta-eSilva J,
Francis M, Gaharwar AK, Atsawasuwan P and Diekwisch TGH: MicroRNAs
and periodontal homeostasis. J Dent Res. 96:491–500. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mombelli A: Microbial colonization of the
periodontal pocket and its significance for periodontal therapy.
Periodontology 2000. 76:85–96. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Massoudi D, Ren Y, Muir AM, Harris
SE, Greenspan DS and Feng JQ: BMP1 and TLL1 are required for
maintaining periodontal homeostasis. J Dent Res. 96:578–585. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujita A, Morimatsu M, Nishiyama M, Naruse
K and Takashiba S: Mechanical stress modulates the homeostasis of
periodontal ligament. Mol Biol Cell. 29:12018.PubMed/NCBI
|
|
6
|
Li J, Ke X, Yan F, Lei L and Li H:
Necroptosis in the periodontal homeostasis: Signals emanating from
dying cells. Oral Dis. 24:900–907. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin Y, Liu DX and Lin XP: IL-35 may
maintain homeostasis of the immune microenvironment in
periodontitis. Exp Ther Med. 14:5605–5610. 2017.PubMed/NCBI
|
|
8
|
Xie X, Wang J, Wang K, Li C, Zhang S, Jing
D, Xu C, Wang X, Zhao H and Feng JQ: Axin2+-mesenchymal
PDL cells, instead of K14+ epithelial cells, play a key
role in rapid cementum growth. J Dent Res. 98:1262–1270. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gavin BJ, McMahon JA and McMahon AP:
Expression of multiple novel Wnt-1/int-1-related genes during fetal
and adult mouse development. Genes Dev. 4:2319–2332. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clark CC, Cohen I, Eichstetter I,
Cannizzaro LA, McPherson JD, Wasmuth JJ and Iozzo RV: Molecular
cloning of the human proto-oncogene Wnt-5A and mapping of the gene
(WNT5A) to chromosome 3p14-p21. Genomics. 18:249–260. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bauer M, Bénard J, Gaasterland T, Willert
K and Cappellen D: WNT5A encodes two isoforms with distinct
functions in cancers. PLoS One. 8:e805262013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumawat K and Gosens R: WNT-5A: Signaling
and functions in health and disease. Cell Mol Life Sci. 73:567–587.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Butler MT and Wallingford JB: Planar cell
polarity in development and disease. Nat Rev Mol Cell Biol.
18:375–388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De A: Wnt/Ca2+ signaling
pathway: A brief overview. Acta Biochim Biophys Sin (Shanghai).
43:745–756. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niehrs C and Acebron SP: Mitotic and
mitogenic Wnt signalling. EMBO J. 31:2705–2713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang D, Zhang Y and Shen C: Research
update on the association between SFRP5, an anti-inflammatory
adipokine, with obesity, type 2 diabetes mellitus and coronary
heart disease. J Cell Mol Med. 24:2730–2735. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamaguchi TP, Bradley A, McMahon AP and
Jones S: A Wnt5a pathway underlies outgrowth of multiple structures
in the vertebrate embryo. Development. 126:1211–1223.
1999.PubMed/NCBI
|
|
18
|
Bisson JA, Mills B, Paul Helt JC, Zwaka TP
and Cohen ED: Wnt5a and Wnt11 inhibit the canonical Wnt pathway and
promote cardiac progenitor development via the caspase-dependent
degradation of AKT. Dev Biol. 398:80–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li CG, Xiao J, Hormi K, Borok Z and Minoo
P: Wnt5a participates in distal lung morphogenesis. Dev Biol.
248:68–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pashirzad M, Shafiee M, Rahmani F,
Behnam-Rassouli R, Hoseinkhani F, Ryzhikov M, Moradi Binabaj M,
Parizadeh MR, Avan A and Hassanian SM: Role of Wnt5a in the
pathogenesis of inflammatory diseases. J Cell Physiol.
232:1611–1616. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asem MS, Buechler S, Wates RB, Miller DL
and Stack MS: Wnt5a signaling in cancer. Cancers (Basel). 8:792016.
View Article : Google Scholar
|
|
22
|
Lin M, Li L, Liu C, Liu H, He F, Yan F,
Zhang Y and Chen Y: Wnt5a regulates growth, patterning, and
odontoblast differentiation of developing mouse tooth. Dev Dyn.
240:432–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai J, Mutoh N, Shin JO, Tani-Ishii N,
Ohshima H, Cho SW and Jung HS: Wnt5a plays a crucial role in
determining tooth size during murine tooth development. Cell Tissue
Res. 345:367–377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sarkar L and Sharpe PT: Expression of Wnt
signalling pathway genes during tooth development. Mech Dev.
85:197–200. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng L, Ren LB, Dong G, Wang CL, Xu P, Ye
L and Zhou XD: Wnt5a promotes differentiation of human dental
papilla cells. Int Endod J. 43:404–412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiang L, Chen M, He L, Cai B, Du Y, Zhang
X, Zhou C, Wang C, Mao JJ and Ling J: Wnt5a regulates dental
follicle stem/progenitor cells of the periodontium. Stem Cell Res
Ther. 5:1352014. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hasegawa D, Wada N, Maeda H, Yoshida S,
Mitarai H, Tomokiyo A, Monnouchi S, Hamano S, Yuda A and Akamine A:
Wnt5a induces collagen production by human periodontal ligament
cells through TGFβ1-mediated upregulation of periostin expression.
J Cell Physiol. 230:2647–2660. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fu HD, Wang BK, Wan ZQ, Lin H, Chang ML
and Han GL: Wnt5a mediated canonical Wnt signaling pathway
activation in orthodontic tooth movement: Possible role in the
tension force-induced bone formation. J Mol Histol. 47:455–466.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu XS, Hu L, Li Y, Wang F, Ma P, Wang J,
Zhang C, Jiang C and Wang S: SCAPs regulate differentiation of
DFSCs during tooth root development in swine. Int J Med Sci.
15:291–299. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sakisaka Y, Tsuchiya M, Nakamura T, Tamura
M, Shimauchi H and Nemoto E: Wnt5a attenuates Wnt3a-induced
alkaline phosphatase expression in dental follicle cells. Exp Cell
Res. 336:85–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wise G: Cellular and molecular basis of
tooth eruption. Orthod Craniofac Res. 12:67–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gopinathan G, Foyle D, Luan X and
Diekwisch TGH: The Wnt antagonist SFRP1: A key regulator of
periodontal mineral homeostasis. Stem Cells Dev. 28:1004–1014.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rios HF, Ma D, Xie Y, Giannobile WV,
Bonewald LF, Conway SJ and Feng JQ: Periostin is essential for the
integrity and function of the periodontal ligament during occlusal
loading in mice. J Periodontol. 79:1480–1490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Panchamanon P, Pavasant P and Leethanakul
C: Periostin plays role in force-induced stem cell potential by
periodontal ligament stem cells. Cell Biol Int. 43:506–515. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kobayashi Y, Thirukonda GJ, Nakamura Y,
Koide M, Yamashita T, Uehara S, Kato H, Udagawa N and Takahashi N:
Wnt16 regulates osteoclast differentiation in conjunction with
Wnt5a. Biochem Biophys Res Commun. 463:1278–1283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maeda K, Kobayashi Y, Udagawa N, Uehara S,
Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, et
al: Wnt5a-Ror2 signaling between osteoblast-lineage cells and
osteoclast precursors enhances osteoclastogenesis. Nat Med.
18:405–412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Roberts JL, Liu G, Paglia DN, Kinter CW,
Fernandes LM, Lorenzo J, Hansen MF, Arif A and Drissi H: Deletion
of Wnt5a in osteoclasts results in bone loss through decreased bone
formation. Ann N Y Acad Sci. 1463:45–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Divaris K, Monda KL, North KE, Olshan AF,
Reynolds LM, Hsueh WC, Lange EM, Moss K, Barros SP, Weyant RJ, et
al: Exploring the genetic basis of chronic periodontitis: A
genome-wide association study. Hum Mol Genet. 22:2312–2324. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nanbara H, Wara-aswapati N, Nagasawa T,
Yoshida Y, Yashiro R, Bando Y, Kobayashi H, Khongcharoensuk J,
Hormdee D, Pitiphat W, et al: Modulation of Wnt5a expression by
periodontopathic bacteria. PLoS One. 7:e344342012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maekawa T, Kulwattanaporn P, Hosur K,
Domon H, Oda M, Terao Y, Maeda T and Hajishengallis G: Differential
expression and roles of secreted frizzled-related protein 5 and the
wingless homolog Wnt5a in periodontitis. J Dent Res. 96:571–577.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Haftcheshmeh SM, Mohammadi A, Soltani A,
Momtazi-Borojeni AA and Sattari M: Evaluation of STAT1 and Wnt5a
gene expression in gingival tissues of patients with periodontal
disease. J Cell Biochem. 120:1827–1834. 2019. View Article : Google Scholar
|
|
42
|
Chatzopoulos GS, Mansky KC, Lunos S,
Costalonga M and Wolff LF: Sclerostin and WNT-5a gingival protein
levels in chronic periodontitis and health. J Periodont Res.
54:555–565. 2019. View Article : Google Scholar
|
|
43
|
Ge XP, Can YH, Zhang CG, Zhou CY, Ma KT,
Meng JH and Ma XC: Requirement of the NF-κB pathway for induction
of Wnt-5A by interleukin-1β in condylar chondrocytes of the
temporomandibular joint: Functional crosstalk between the Wnt-5A
and NF-κB signaling pathways. Osteoarthritis Cartilage. 19:111–117.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Naskar D, Maiti G, Chakraborty A, Roy A,
Chattopadhyay D and Sen M: Wnt5a-Rac1-NF-κB homeostatic circuitry
sustains innate immune functions in macrophages. J Immunol.
192:4386–4397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Q, Liu J, Ma L, Bai N and Xu H:
Wnt5a is involved in LOX-1 and TLR4 induced host inflammatory
response in peri-implantitis. J Periodont Res. 55:199–208. 2020.
View Article : Google Scholar
|
|
46
|
Pereira C, Schaer DJ, Bachli EB, Kurrer MO
and Schoedon G: Wnt5A/CaMKII signaling contributes to the
inflammatory response of macrophages and is a target for the
antiinflammatory action of activated protein C and interleukin-10.
Arterioscler Thromb Vasc Biol. 28:504–510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ouchi N, Higuchi A, Ohashi K, Oshima Y,
Gokce N, Shibata R, Akasaki Y, Shimono A and Walsh K: Sfrp5 is an
anti-inflammatory adipokine that modulates metabolic dysfunction in
obesity. Science. 329:454–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schulz J, Knappe C, Graetz C, Mewes L,
Türk K, Black AK, Lieb W, Schäfer AS, Fawzy El-Sayed KM, Dörfer CE,
et al: Secreted frizzled-related protein 5 serum levels in human
periodontitis-A nested case-control study. J Clin Periodontol.
46:522–528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schulte DM, Müller N, Neumann K,
Oberhäuser F, Faust M, Güdelhöfer H, Brandt B, Krone W and Laudes
M: Pro-inflammatory wnt5a and anti-inflammatory sFRP5 are
differentially regulated by nutritional factors in obese human
subjects. PLoS One. 7:e324372012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tong S, Du Y, Ji Q, Dong R, Cao J, Wang Z,
Li W, Zeng M, Chen H, Zhu X and Zhou Y: Expression of Sfrp5/Wnt5a
in human epicardial adipose tissue and their relationship with
coronary artery disease. Life Sci. 245:1173382020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lu YC, Wang CP, Hsu CC, Chiu CA, Yu TH,
Hung WC, Lu LF, Chung FM, Tsai IT, Lin HC and Lee YJ: Circulating
secreted frizzled-related protein 5 (Sfrp5) and wingless-type MMTV
integration site family member 5a (Wnt5a) levels in patients with
type 2 diabetes mellitus. Diabetes Metab Res Rev. 29:551–556.
2013.PubMed/NCBI
|
|
52
|
Cho YK, Kang YM, Lee SE, Lee Y, Seol SM,
Lee WJ, Park JY and Jung CH: Effect of SFRP5 (secreted
frizzled-related protein 5) on the WNT5A (wingless-type family
member 5A)-induced endothelial dysfunction and its relevance with
arterial stiffness in human subjects. Arterioscler Thromb Vasc
Biol. 38:1358–1367. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mehmeti M, Bergenfelz C, Kallberg E,
Millrud CR, Björk P, Ivars F, Johansson-Lindbom B, Kjellström S,
André I and Leandersson K: Wnt5a is a TLR2/4-ligand that induces
tolerance in human myeloid cells. Commun Biol. 2:1762019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Feng Y, Liang Y, Zhu X, Wang M, Gui Y, Lu
Q, Gu M, Xue X, Sun X, He W, et al: The signaling protein Wnt5a
promotes TGFβ1-mediated macrophage polarization and kidney fibrosis
by inducing the transcriptional regulators Yap/Taz. J Biol Chem.
293:19290–19302. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gao YC, Wen Q, Hu SF, Zhou X, Xiong W, Du
X, Zhang L, Fu Y, Yang J, Zhou C, et al: IL-36γ promotes killing of
Mycobacterium tuberculosis by macrophages via WNT5A-induced
noncanonical WNT signaling. J Immunol. 203:922–935. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen FM and Jin Y: Periodontal tissue
engineering and regeneration: Current approaches and expanding
opportunities. Tissue Eng Part B Rev. 16:219–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu N, Gu B, Liu N, Nie X, Zhang B, Zhou X
and Deng M: Wnt/β-catenin pathway regulates cementogenic
differentiation of adipose tissue-deprived stem cells in dental
follicle cell-conditioned medium. PLoS One. 9:e933642014.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang F, Luo K, Rong Z, Wang Z, Luo F,
Zhang Z, Sun D, Dong S, Xu J and Dai F: Periostin upregulates
Wnt/β-catenin signaling to promote the osteogenesis of
CTLA4-modified human bone marrow-mesenchymal stem cells. Sci Rep.
7:416342017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Morsczeck C, Reck A and Reichert TE: WNT5A
supports viability of senescent human dental follicle cells. Mol
Cell Biochem. 455:21–28. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yang X, Zhang C, Jiang J and Li Y:
Baicalein retards proliferation and collagen deposition by
activating p38MAPK-JNK via microRNA-29. J Cell Biochem.
120:15625–15634. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhao Y, Wang H, Li X, Cao M, Lu H, Meng Q,
Pang H, Li H, Nadolny C, Dong X and Cai L: Ang II-AT1R increases
cell migration through PI3K/AKT and NF-κB pathways in breast
cancer. J Cell Physiol. 229:1855–1862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hasegawa D, Wada N, Yoshida S, Mitarai H,
Arima M, Tomokiyo A, Hamano S, Sugii H and Maeda H: Wnt5a
suppresses osteoblastic differentiation of human periodontal
ligament stem cell-like cells via Ror2/JNK signaling. J Cell
Physiol. 233:1752–1762. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nemoto E, Sakisaka Y, Tsuchiya M, Tamura
M, Nakamura T, Kanaya S, Shimonishi M and Shimauchi H: Wnt3a
signaling induces murine dental follicle cells to differentiate
into cementoblastic/osteoblastic cells via an osterix-dependent
pathway. J Periodont Res. 51:164–174. 2016. View Article : Google Scholar
|
|
64
|
Nemoto E, Ebe Y, Kanaya S, Tsuchiya M,
Nakamura T, Tamura M and Shimauchi H: Wnt5a signaling is a
substantial constituent in bone morphogenetic protein-2-mediated
osteoblastogenesis. Biochem Biophys Res Commun. 422:627–632. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hashimoto Y, Matsuzaki E, Higashi K,
Takahashi-Yanaga F, Takano A, Hirata M and Nishimura F:
Sphingosine-1-phosphate inhibits differentiation of C3H10T1/2 cells
into adipocyte. Mol Cell Biochem. 401:39–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fawzy El-Sayed KM, Elahmady M, Adawi Z,
Aboushadi N, Elnaggar A, Eid M, Hamdy N, Sanaa D and Dörfer CE: The
periodontal stem/progenitor cell inflammatory-regenerative cross
talk: A new perspective. J Periodont Res. 54:81–94. 2019.
View Article : Google Scholar
|
|
67
|
Nienhuser H, Kim W, Malagola E, Ruan T,
Valenti G, Middelhoff M, Bass A, Der CJ, Hayakawa Y and Wang TC:
Mist1+ gastric isthmus stem cells are regulated by Wnt5a
and expand in response to injury and inflammation in mice. Gut. Jul
24–2020.(Epub ahead of print). doi: 10.1136/gutjnl-2020-320742.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Han P, Lloyd T, Chen Z and Xiao Y:
Proinflammatory cytokines regulate cementogenic differentiation of
periodontal ligament cells by Wnt/Ca(2+) signaling pathway. J
Interferon Cytokine Res. 36:328–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu N, Shi S, Deng M, Tang L, Zhang G, Liu
N, Ding B, Liu W, Liu Y, Shi H, et al: High levels of β-catenin
signaling reduce osteogenic differentiation of stem cells in
inflammatory microenvironments through inhibition of the
noncanonical Wnt pathway. J Bone Miner Res. 26:2082–2095. 2011.
View Article : Google Scholar : PubMed/NCBI
|
















