Introduction
Radiation therapy, which is used for the treatment
of some cancer types, can cause delayed heart damage (1–3). In
the past two decades, it was found that radiation therapy increases
the risk of radiation related to cardiac damage in cancer survivors
(4,5). A significant increase in death rates
in the follow-up after 10 years was found in patients
post-radiation therapy in the US (6). A previous study also revealed that
radiation therapy increased cardiovascular mortalities in females
treated for the left breast compared with those who were treated
only for the right breast from earlier studies during the 1970s and
1980s (7). However, the underlying
causes and biomarkers of radiation-induced cardiotoxicity are
currently unknown, prompting the need for studies investigating the
differences in sensitivity and resistance to the development of
radiation-induced cardiotoxicity (8–10).
Circular RNAs (circRNAs) are a recently recognized
type of functional non-coding RNA that consist of a circular
configuration through a typical 3′ to 5′ phosphodiester bond. Since
circRNAs do not contain a free 5′ or 3′ terminus, they are much
more stable compared with linear RNAs in cells (11). The functional roles of circRNAs in
cardiovascular diseases, such as myocardial infarction,
ischemia-reperfusion injury, atherosclerosis, cardiomyopathy and
cardiac fibrosis have been increasingly reported (12–16).
circRNA_101237 has been demonstrated to mediate
anoxia/reoxygenation injury by targeting let-7a-5p/insulin-like
growth factor 2-binding protein 3 in cardiomyocytes (12). A previous study reported that
circFndc3b modulates cardiac repair after myocardial infarction via
the fused in sarcoma/VEGF-A axis (15). circRNA-Ttn105-110 was found to play
a protective role in doxorubicin-induced cardiotoxicity (16). Moreover, increasing evidence has
revealed that circRNAs may serve as endogenous competing RNAs and
affect gene expression by binding to microRNAs (miRs/miRNAs) as
sponges (17). circRNAs can also
interact with proteins as sponges, decoys or scaffolds (18). Additionally, circRNAs that are
retained in the nucleus can interfere with transcription and
promote alternative splicing (19).
circFOXO3 was first identified by a research group
in Toronto. Several findings have been reported regarding circFOXO3
function. Lower levels of circFOXO3 have been detected in breast
cancer, however the expression was revealed to increase when cancer
cells underwent apoptosis (20).
Subsequent studies found that circFOXO3 also played a role in the
progression of acute myeloid leukemia (21), glioblastoma (22), breast cancer (23) and cardiac senescence (24). However, the role of circFOXO3 in
radiation-induced cardiotoxicity remains to be elucidated.
The present study aimed to identify the functions of
circFOXO3 in radiation-induced cardiotoxicity. The current study
established circFOXO3-knockdown (KD) or -overexpression (OE) models
in cardiomyocytes and the effects of circFOXO3 on DNA damage and
cell apoptosis were observed. In addition, the effect of circFOXO3
on the apoptotic pathway was investigated. The data indicated that
circFOXO3 protected cardiomyocytes from radiation-induced
cardiotoxicity by reducing DNA damage and apoptosis. Thus,
circFOXO3 may be a potential therapeutic target against
radiation-induced cardiotoxicity.
Materials and methods
Cell culture
Human cardiomyocytes (AC16; American Type Culture
Collection) were cultured at 80% confluency in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
Cells were incubated at 37°C with 5% CO2.
RNA Fluorescence in Situ Hybridization
(FISH)
circFOXO3 probes were designed and synthesized by
Guangzhou RiboBio Co., Ltd. The probe signals were detected with a
FISH kit (Guangzhou RiboBio Co., Ltd.) according to the
manufacturer's instructions. Briefly, 5×104 AC16 cells
were fixed in 4% paraformaldehyde for 1 h at room temperature.
Pre-hybridization were conducted with 200 µl pre-hybridization
buffer at 37°C for 30 min. Then, 0.5 µM circFOXO3 FISH Probe Mix or
controls (U6 and 18S) were incubated overnight at 37°C. Fluorescent
microscopy (magnification, ×400) were used to detect the
signal.
Radiation treatment
Cells in culture were irradiated with an irradiator
(Gammacell 3000 Elan; Atomic Energy of Canada Ltd.) using a 137Cs
source at a dose rate of 6 Gy.
Construction of circFOXO3-KD or -OE
cell models
OE and KD of circFOXO3 were achieved by the
infection of circFOXO3-OE or circFOXO3-targeting short hairpin RNA
(shRNA) lentiviruses. circFOXO3 expression in AC16 cells was
assessed in circFOXO3-KD, circFOXO3-OE and negative control (NC)
groups. circFOXO3-KD or -OE cells were constructed as previously
described (22).
Reverse transcription-quantitative PCR
(RT-qPCR)
For RT-qPCR, RNA was exacted using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) and
was reverse transcribed using the Prime Script RT Master Mix at
37°C (15 min) (Takara Bio, Inc.). PCR was performed using a PCR
Master Mix (2X; Thermo Fisher Scientific, Inc.). To quantify the
levels of circRNA and mRNA, qPCR was performed using a SYBR Premix
Ex Taq kit (Takara Bio, Inc.) with GAPDH as the internal control.
The following primers were used: circFOXO3 forward,
5′-attgtccatggagacagcccgccg-3′ and reverse,
5′-gtggggaacttcactggtgctaag-3′; and GAPDH forward,
5′-GTCTCCTCTGACTTCAACAGCG-3′ and reverse,
5′-ACCACCCTGTTGCTGTAGCCAA-3′. The qPCR cycling conditions were as
follows: Initial denaturation for 3 min at 95°C, followed by 45
cycles at 95°C (10 sec) and 58°C (45 sec); data were acquired at
the end of the annealing/extension phase. Each sample was
replicated three times and the data were analyzed by comparing Cq
values (25).
Comet assay
Cells grown in 100-mm3 dishes were
radiated at dose rates 0 or 6 Gy. At the specified times, the cells
were suspended at a concentration of 1×105 cells/ml and
mixed with melted, low-melting point agarose (Trevigen, Inc.) at a
1:10 ratio and transferred onto a Comet slide (Trevigen, Inc.). The
slides were placed at 4°C for 30 min before being immersed in lysis
solution (Trevigen, Inc.) for 1 h at 4°C. Subsequently, the slides
were run on a horizontal electrophoresis apparatus for 10 min 20 V.
Propidium iodide (Becton-Dickinson and Company) was added to the
slides and stained at room temperature for 15 min. The slides were
visualized on a Leica microscope (Leica Microsystems, Inc.). The
olive tail moment (OTM) was recorded for each cell using ImageJ
1.52 (National Institutes of Health).
Flow cytometry (FCM) analysis
Cell apoptosis was measured using Annexin V-FITC
staining. Briefly, cells were radiated at dose rates of 0 or 6 Gy
and cultured for 24 h. The cells were then harvested, washed twice
with PBS, stained with Annexin V-FITC and PI in binding buffer and
detected by FCM (Beckman gallios, Beckman Coulter, Inc.; Flowjo
10.07, BD Biosciences) after 15 min incubation at room temperature
in the dark. Early apoptotic cells (Annexin
V+/PI−) and late apoptotic cells (Annexin
V+/PI+) were quantified.
Western blotting
Cell lysates were prepared using RIPA buffer
(Beyotime Institute of Biotechnology) containing protease
inhibitors. Protein concentration was determined using a
bicinchoninic acid protein assay kit. A total of 30 µg protein was
separated via 10% SDS-PAGE, followed by transfer to a
polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.) and
blocking with 5% non-fat milk in TBS-Tween buffer 7 (0.12 M
Tris-base, 1.5 M NaCl, 0.1% Tween-20) for 1 h at room temperature.
Immunoreactive bands were detected using an ECL kit (cat. no.
PI32209; Pierce; Thermo Fisher Scientific, Inc.). Primary
antibodies targeting the following proteins were used at 1:1,000:
Bax (cat. no. 2772; Cell Signaling Technology, Inc.), Bcl-2 (cat.
no. 4223; Cell Signaling Technology, Inc.), caspase 3 (cat. no.
9662; Cell Signaling Technology, Inc.), cleaved-caspase 3 (cat. no.
9602; Cell Signaling Technology, Inc.), cleaved-caspase 7 (cat. no.
8438; Cell Signaling Technology, Inc.) and actin (cat. no. AB0035;
Abways Technology). HRP-conjugated goat anti-mouse (cat. no.
SA00001-1) and goat anti-rabbit (cat. no. SA00001-2) antibodies
were used as secondary antibodies (1:10,000; ProteinTech Group,
Inc.). Images are representative of four independent experiments.
Image-Pro Plus 6.0 software (Media Cybernetics, Inc.) was used to
semi-quantify the relative band intensities from western blotting
images.
Statistical analysis
Data are presented as the mean ± SD from at least
three replicates. Student's two-tailed unpaired t-test was used to
determine differences between two groups. One-way ANOVA followed by
Tukey's post hoc test was applied to determine differences among at
least three groups. Statistical analyses were performed using SPSS
v17 software (SPSS, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
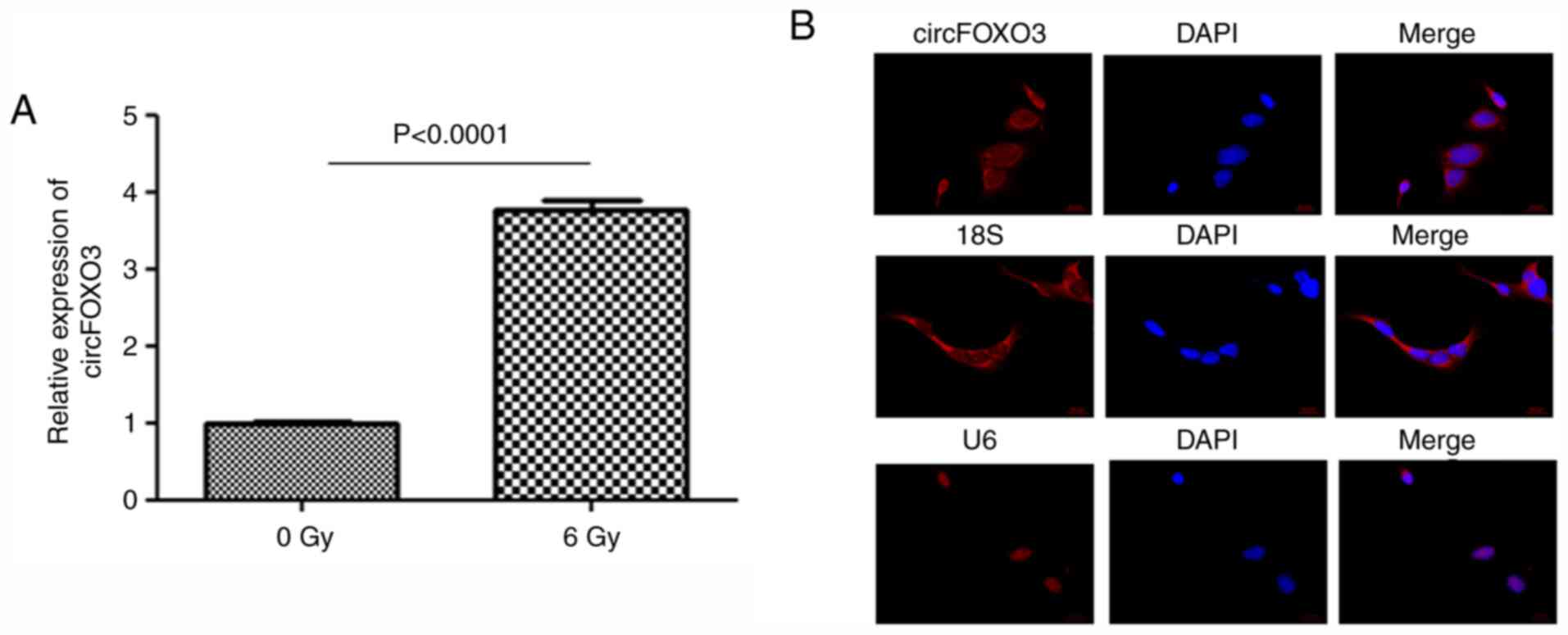
circFOXO3 is significantly upregulated
in cardiomyocytes after radiation
AC16 cells with or without radiation were collected.
qPCR results showed that circFOXO3 expression was significantly
upregulated after radiation (Fig.
1A). The subcellular localization of circFOXO3 in AC16 cells
after radiation was then examined. As shown in Fig. 1B, circFOXO3 was mainly localized in
the cytosol, consistent with 18S (cytosol localization) (Fig. 1B). U6 served as a nuclear
localization control (Fig. 1B).
These results indicated that circFOXO3 might be involved in
radiation-induced cardiotoxicity.
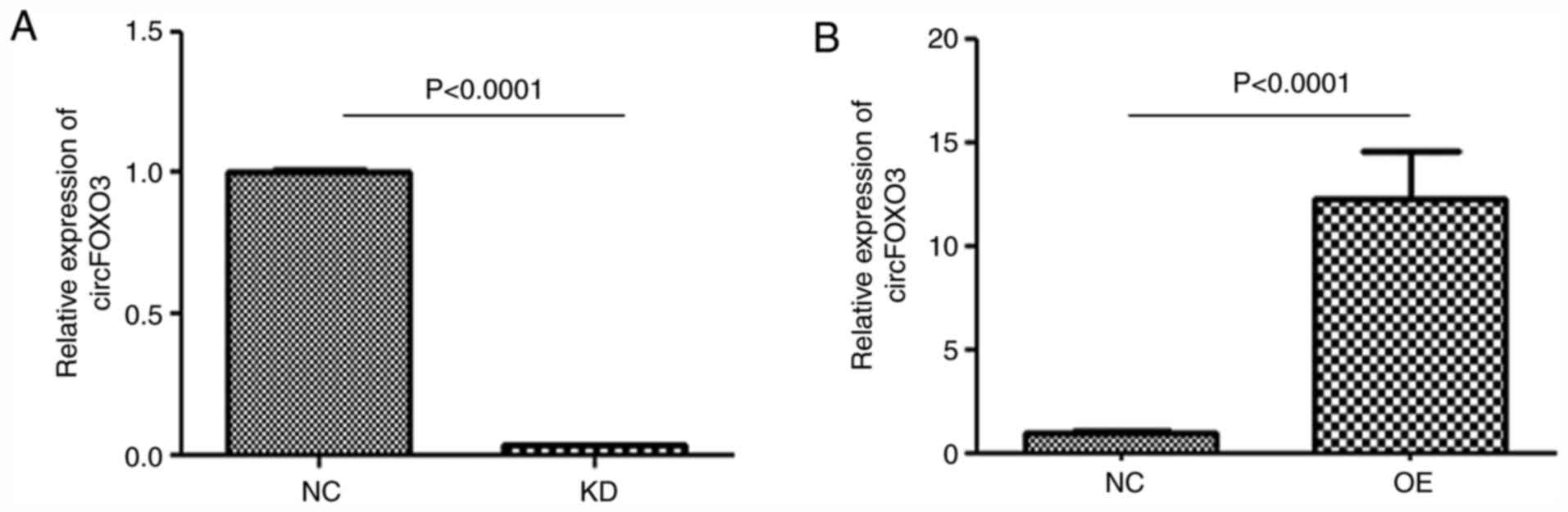
Successful establishment of
circFOXO3-KD or -OE cardiomyocytes
circFOXO3 expression was significantly downregulated
in the KD group (P<0.001 vs. NC) and upregulated in the OE group
(P<0.001 vs. NC) (Fig. 2),
indicating that circFOXO3-KD or -OE cardiomyocytes were
successfully established.
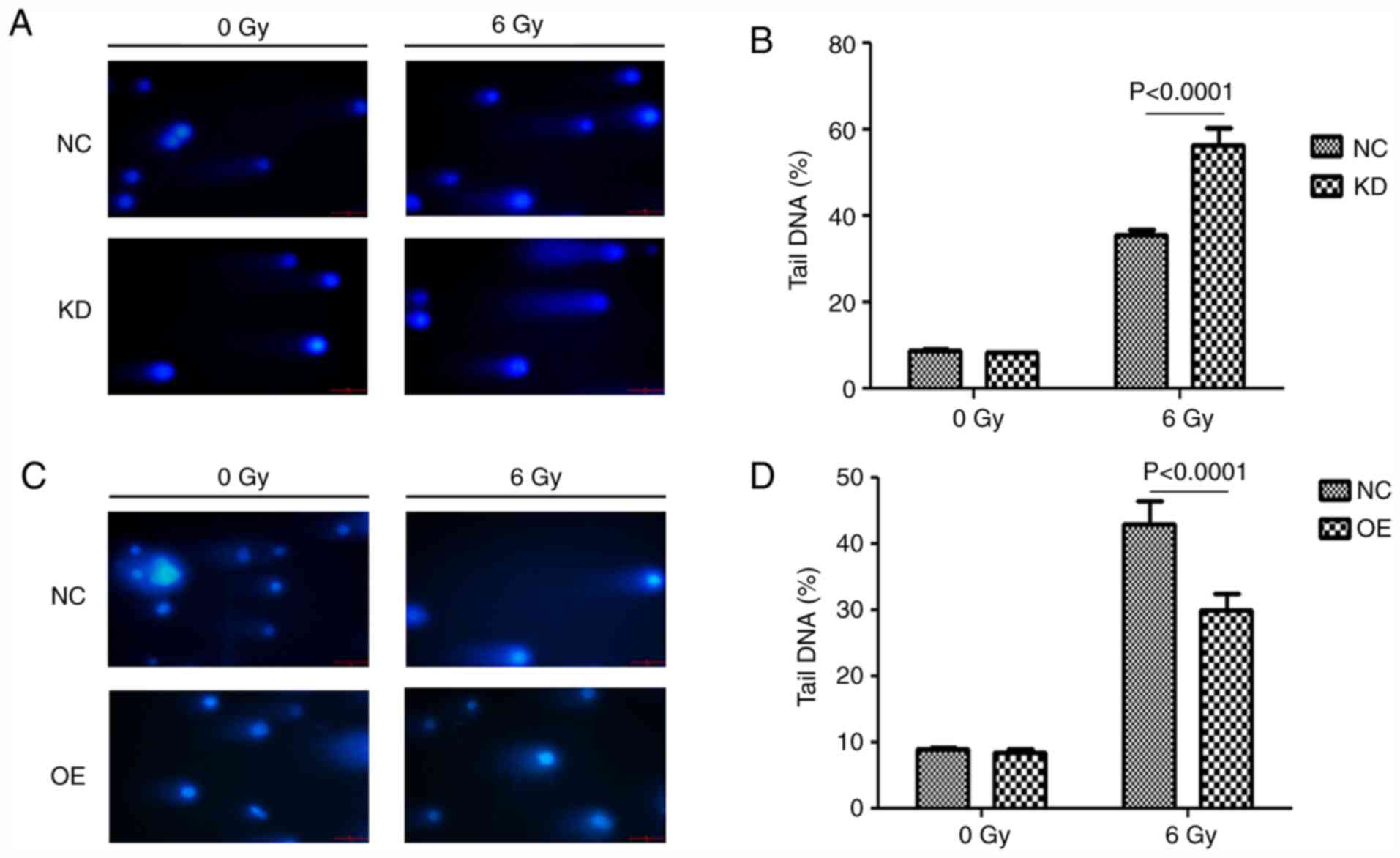
circFOXO3 elevates the DNA repair
processes after radiotherapy in cardiomyocytes
A comet assay was performed to determine the effects
of circFOXO3 on radiation-induced DNA damage. The results showed
that circFOXO3 KD significantly increased DNA damage (Fig. 3A and B). However, circFOXO3 OE
significantly reduced DNA damage (Fig.
3C and D).
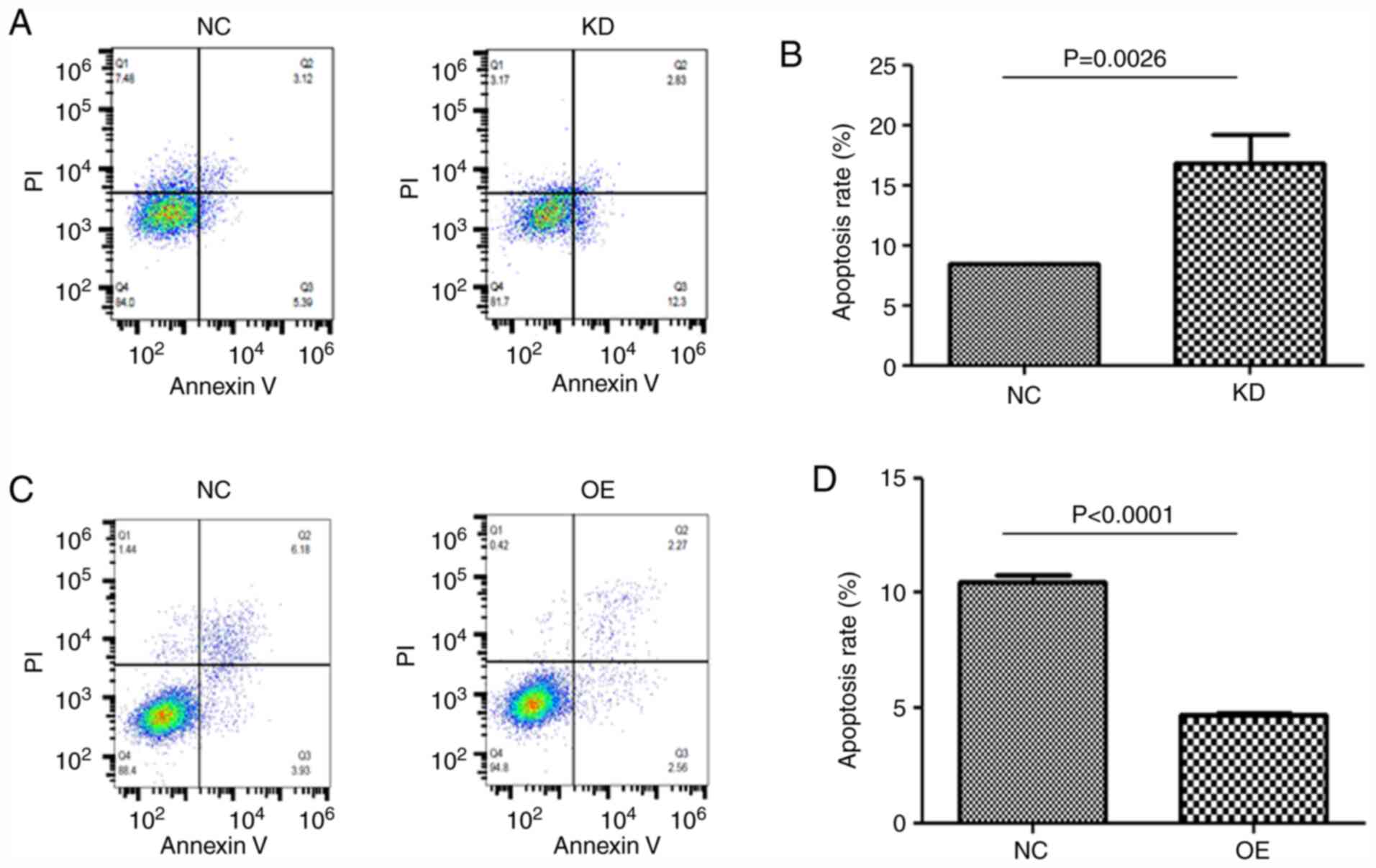
circFOXO3 decreases the percentage of
apoptotic cells after radiotherapy in cardiomyocytes
FCM was performed to test the effects of circFOXO3
on cell viability. Quantitative analysis indicated that apoptosis
was increased in circFOXO3-KD cells after radiation (P=0.0026 vs.
NC; Fig. 4A and B). Conversely, the
apoptosis rate was suppressed in circFOXO3-OE cells (P<0.001 vs.
NC; Fig. 4C and D).
circFOXO3 suppresses apoptotic protein
expression after radiotherapy in cardiomyocytes
The apoptotic signaling pathway serves a vital role
in radiation-induced cardiotoxicity. The expression levels of Bax,
Bcl-2, caspase 3 and caspase 7 were investigated using western
blotting. The results showed that circFOXO3 KD suppressed the
expression of Bcl-2 (P<0.001 vs. NC), whereas it increased the
expression of Bax, caspase 3 and caspase 7 (P<0.001 vs. NC)
(Fig. 5A and B). By contrast,
circFOXO3 OE elevated the expression of Bcl-2 (P<0.001 vs. NC),
whereas it decreased the expression of Bax, caspase 3 and caspase 7
(P<0.001 vs. NC) (Fig. 5C and
D).
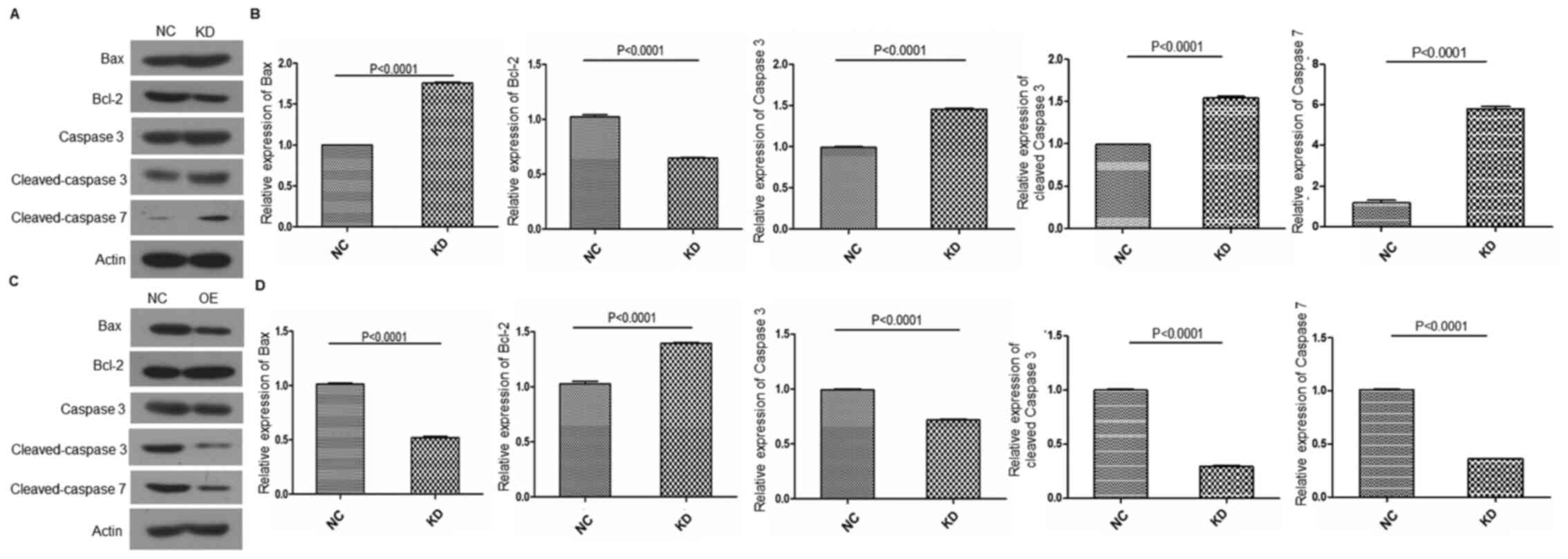 | Figure 5.circFOXO3 inhibits the activation of
the apoptotic pathway following radiation in cardiomyocytes. (A)
Protein expression levels of Bax, Bcl-2, pro- and cleaved-caspase 3
and cleaved-caspase 7 were evaluated by western blotting in AC16
cells with or without circFOXO3 KD after radiation. (B) Relative
expression of Bax, Bcl-2, pro- and cleaved-caspase 3 and
cleaved-caspase 7 was semi-quantified using Image-Pro Plus 6.0
software (Media Cybernetics, Inc.). (C) Protein expression levels
of Bax, Bcl-2, pro- and cleaved-caspase 3 and cleaved-caspase 7
were evaluated by western blotting in AC16 cells with or without
circFOXO3 OE following radiation. (D) Relative expression of Bax,
Bcl-2 and caspase 3 was semi-quantified using Image-Pro Plus 6.0
software. All experiments were repeated at least three times.
P<0.0001 vs. NC. circ, circular RNA; OE, overexpression; KD,
knockdown; NC, negative control. |
To further clarify the anti-apoptotic function of
circFOXO3 on AC16 cells in the absence of radiation, the levels of
Bax, caspase 3 and Bcl-2 in circFOXO3-KD, -OE and NC AC16 cells in
the absence of radiation were measured. As shown in Fig. S1, the protein levels of Bax,
caspase 3 and Bcl-2 were not notably altered in the absence of
radiation among these groups of cells, suggesting that the
anti-apoptotic effects of circFOXO3 is dependent on radiation
treatment.
Discussion
The present study constructed circFOXO3-KD or -OE
cardiomyocytes in order to assess the function of circFOXO3 on
radiation-induced cardiotoxicity. The results revealed that
downregulation of circFOXO3 significantly increased DNA damage and
apoptosis after radiation, whereas the upregulation of circFOXO3
showed the opposite results. Mechanistically, inhibition of
circFOXO3 increased the expression of pro-apoptotic proteins Bax
and caspase 3, while decreasing the expression of Bcl-2 in radiated
cardiomyocytes.
Several studies have focused on the function of
circRNAs in cancer biology. For instance, it was found that
circ-ABCA promoted proliferation and reduced apoptosis in ovarian
cancer by negatively regulating miR-1271, miR-1252 and miR-203
(26). Another study observed that
circ-ITCH suppressed cell proliferation and promoted apoptosis via
sponging miR-10a (27). Circulating
miRNAs have been proposed as biomarkers of radiation-induced
cardiac toxicity in non-small cell lung cancer (28). However, to the best of our
knowledge, the function of circFOXO3 in radiation-induced
cardiotoxicity has not yet been reported. The present study
observed that downregulation of circFOXO3 significantly suppressed
cell survival after radiation, whereas upregulation of circFOXO3
exerted the opposite results. These data suggested that circFOXO3
is involved in radiation-induced cardiotoxicity. However, Du et
al (20) reported that
circFOXO3 expression increased in breast cancer in cells undergoing
apoptosis by enhancing FOXO3 activity. A possible explanation for
this discrepancy might be related to the novel functions of FOXO3
and circFOXO3 in tumor progression. Recently, it was reported that
FOXO3A promoted glioblastoma multiforme (GBM) cell proliferation
and invasion (29). Moreover,
circFOXO3 enhanced GBM progression (22).
The apoptotic pathway is closely associated with the
progression of radiation-induced cardiotoxicity. It was previously
reported that irradiation can induce the upregulation of Bax and
downregulation of Bcl-2 in cardiomyocytes, leading to apoptosis and
subsequent development of fibrosis (30,31).
In the present study, while circFOXO3 KD promoted apoptosis, OE of
circFOXO3 decreased the expression of pro-apoptotic proteins Bax
and caspase 3 while increasing the expression of Bcl-2 in radiated
cardiomyocytes. Thus, the present results showed that circFOXO3
could effectively inactivate the apoptotic pathway.
In conclusion, the present study demonstrated that
circFOXO3 served a role in regulating cell apoptosis in
cardiomyocytes and may be a potential therapeutic target for
radiation-induced cardiotoxicity.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YQ was involved in the conceptualization, performed
the experiments, formal analysis, writing the original draft and
reviewing and editing the manuscript. XX and LL were involved in
the investigation, formal analysis and data validation. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Menezes KM, Wang H, Hada M and Saganti PB:
Radiation matters of the heart: A mini review. Front Cardiovasc
Med. 5:832018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spetz J, Moslehi J and Sarosiek K:
Radiation-Induced cardiovascular toxicity: Mechanisms, prevention,
and treatment. Curr Treat Options Cardiovasc Med. 20:312018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barjaktarovic Z, Kempf SJ, Sriharshan A,
Merl-Pham J, Atkinson MJ and Tapio S: Ionizing radiation induces
immediate protein acetylation changes in human cardiac
microvascular endothelial cells. J Radiat Res. 56:623–632. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wakeford R: Radiation in the workplace-a
review of studies of the risks of occupational exposure to ionising
radiation. J Radiol Prot. 29:A61–A79. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roychoudhuri R, Robinson D, Putcha V,
Cuzick J, Darby S and Moller H: Increased cardiovascular mortality
more than fifteen years after radiotherapy for breast cancer: A
population-based study. BMC Cancer. 7:92007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cohn KE, Stewart JR, Fajardo LF and
Hancock EW: Heart disease following radiation. Medicine
(Baltimore). 46:281–298. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cuzick J, Stewart H, Peto R, Baum M,
Fisher B, Host H, Lythgoe JP, Ribeiro G, Scheurlen H and Wallgren
A: Overview of randomized trials of postoperative adjuvant
radiotherapy in breast cancer. Cancer Treat Rep. 71:15–29.
1987.PubMed/NCBI
|
|
8
|
Darby SC, McGale P, Taylor CW and Peto R:
Long-term mortality from heart disease and lung cancer after
radiotherapy for early breast cancer: Prospective cohort study of
about 300,000 women in US SEER cancer registries. Lancet Oncol.
6:557–565. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Nimwegen FA, Ntentas G, Darby SC,
Schaapveld M, Hauptmann M, Lugtenburg PJ, Janus CPM, Daniels L, van
Leeuwen FE, Cutter DJ and Aleman BMP: Risk of heart failure in
survivors of Hodgkin lymphoma: Effects of cardiac exposure to
radiation and anthracyclines. Blood. 129:2257–2265. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Little MP, Tawn EJ, Tzoulaki I, Wakeford
R, Hildebrandt G, Paris F, Tapio S and Elliott P: A systematic
review of epidemiological associations between low and moderate
doses of ionizing radiation and late cardiovascular effects, and
their possible mechanisms. Radiat Res. 169:99–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsiao KY, Sun HS and Tsai SJ: Circular
RNA-New member of noncoding RNA with novel functions. Exp Biol Med
(Maywood). 242:1136–1141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gan J, Yuan J, Liu Y, Lu Z, Xue Y, Shi L
and Zeng H: Circular RNA_101237 mediates anoxia/reoxygenation
injury by targeting let-7a-5p/IGF2BP3 in cardiomyocytes. Int J Mol
Med. 45:451–460. 2020.PubMed/NCBI
|
|
13
|
Pan RY, Zhao CH, Yuan JX, Zhang YJ, Jin
JL, Gu MF, Mao ZY, Sun HJ, Jia QW, Ji MY, et al: Circular RNA
profile in coronary artery disease. Am J Transl Res. 11:7115–7125.
2019.PubMed/NCBI
|
|
14
|
Su Q and Lv X: Revealing new landscape of
cardiovascular disease through circular RNA-miRNA-mRNA axis.
Genomics. 112:1680–1685. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garikipati VN, Verma SK, Cheng Z, Liang D,
Truongcao MM, Cimini M, Yue Y, Huang G, Wang C, Benedict C, et al:
Circular RNA CircFndc3b modulates cardiac repair after myocardial
infarction via FUS/VEGF-A axis. Nat Commun. 10:43172019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aonuma T, Bayoumi AS, Tang Y and Kim IM: A
circular RNA regulator quaking: A novel gold mine to be unfolded in
doxorubicin-mediated cardiotoxicity. Noncoding RNA Investig.
2:192018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kulcheski FR, Christoff AP and Margis R:
Circular RNAs are miRNA sponges and can be used as a new class of
biomarker. J Biotechnol. 238:42–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen B and Huang S: Circular RNA: An
emerging non-coding RNA as a regulator and biomarker in cancer.
Cancer Lett. 418:41–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kristensen LS, Hansen TB, Veno MT and
Kjems J: Circular RNAs in cancer: Opportunities and challenges in
the field. Oncogene. 37:555–565. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du WW, Fang L, Yang W, Wu N, Awan FM, Yang
Z and Yang BB: Induction of tumor apoptosis through a circular RNA
enhancing Foxo3 activity. Cell Death Differ. 24:357–370. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou J, Zhou LY, Tang X, Zhang J, Zhai LL,
Yi YY, Yi J, Lin J, Qian J and Deng ZQ: Circ-Foxo3 is positively
associated with the Foxo3 gene and leads to better prognosis of
acute myeloid leukemia patients. BMC Cancer. 19:9302019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang S, Liao K, Miao Z, Wang Q, Miao Y,
Guo Z, Qiu Y, Chen B, Ren L, Wei Z, et al: CircFOXO3 promotes
glioblastoma progression by acting as a competing endogenous RNA
for NFAT5. Neuro Oncol. 21:1284–1296. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu WY: Roles of the circular RNA
circ-Foxo3 in breast cancer progression. Cell Cycle. 16:589–590.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du WW, Yang W, Chen Y, Wu ZK, Foster FS,
Yang Z, Li X and Yang BB: Foxo3 circular RNA promotes cardiac
senescence by modulating multiple factors associated with stress
and senescence responses. Eur Heart J. 38:1402–1412.
2017.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Y, Ye X, Xia X and Lin X: Circular
RNA ABCB10 correlates with advanced clinicopathological features
and unfavorable survival, and promotes cell proliferation while
reduces cell apoptosis in epithelial ovarian cancer. Cancer
Biomark. 26:151–161. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo L, Gao YQ and Sun XF: Circular RNA
ITCH suppresses proliferation and promotes apoptosis in human
epithelial ovarian cancer cells by sponging miR-10a-α. Eur Rev Med
Pharmacol Sci. 22:8119–8126. 2018.PubMed/NCBI
|
|
28
|
Ning L, Long B, Zhang W, Yu M, Wang S, Cao
D, Yang J, Shen K, Huang Y and Lang J: Circular RNA profiling
reveals circEXOC6B and circN4BP2L2 as novel prognostic biomarkers
in epithelial ovarian cancer. Int J Oncol. 53:2637–2646.
2018.PubMed/NCBI
|
|
29
|
Qian ZR, Ren L, Wu DC, Yang X, Zhou ZY,
Nie QM, Jiang G, Xue S, Weng W, Qiu Y and Lin Y: Overexpression of
FoxO3a is associated with glioblastoma progression and predicts
poor patient prognosis. Int J Cancer. 140:2792–2804. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salata C, Ferreira-Machado SC, De Andrade
CBV, Mencalha AL, Mandarim-De-Lacerda CA and de Almeida CE:
Apoptosis induction of cardiomyocytes and subsequent fibrosis after
irradiation and neoadjuvant chemotherapy. Int J Radiat Biol.
90:284–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sarosiek KA, Chi X, Bachman JA, Sims JJ,
Montero J, Patel L, Flanagan A, Andrews DW, Sorger P and Letai A:
BID Preferentially activates BAK while BIM preferentially activates
BAX, affecting chemotherapy response. Mol Cell. 51:751–765. 2013.
View Article : Google Scholar : PubMed/NCBI
|