Introduction
Polycystic ovary syndrome (PCOS) is a prevalent
endocrine disorder, which is characterized by hyperandrogenaemia,
chronic anovulation and polycystic ovary. PCOS is typically
accompanied by insulin resistance, central obesity, cardiovascular
diseases and/or early subclinical arteriosclerosis (1). It has been reported that 4–8% of women
worldwide are affected by PCOS (2).
Follicular dysplasia and degeneration, and thinning of the
granulosa cell layer are major pathological lesions of PCOS
(3), which may be attributed to
increased apoptosis of granulosa cells (4). Several candidate genes have thus far
been identified as pathogenic factors of PCOS, including
insulin-like growth factor (IGF), insulin receptor, luteinizing
hormone (LH)/human chorionic gonadotropin, sex hormone-binding
globulin and DENN domain containing 1A (5–8).
However, they have not been demonstrated to be the main reasons for
the pathogenesis of PCOS.
MicroRNAs (miRNAs) are small-chain noncoding RNAs,
18–22 nucleotides in length, which can regulate gene expression by
complementary base pairing to the 3 untranslated region (3UTR) of
target mRNAs and thus induce their degradation or inhibit
translation. miRNAs are vital regulators involved in the
progression of PCOS. Hossain et al (9) identified 89 miRNAs that are
significantly upregulated or downregulated in rats with
5α-dihydrotestosterone (DHT)-induced PCOS and high levels of
androgens. In blastospheres harvested from patients with PCOS,
hsa-let-7a, hsa-miR-19a, hsa-miR-19b, hsa-miR-24, hsa-miR-92 and
has-miR-93 were found to be significantly downregulated (10). Previous studies have shown that
miR-613 has an anticancer role (11–13),
but the potential function of miR-613 in the progression of PCOS
remains largely unknown. The expression of miR-613 in the ovarian
tissues of patients with PCOS was investigated in the present
study.
The abnormal upregulation of IGF-1 and granulosa
cell apoptosis are critical indicators of PCOS, whereas their
potential effects are unclear. In the present study, an in
vitro PCOS model was generated to elucidate the regulatory
effects of miR-613 and IGF-1 on KGN cell phenotypes.
Materials and methods
Subjects and samples
A total of 24 patients diagnosed with PCOS in the
First Affiliated Hospital of Nanchang University (Nanchang, China)
were recruited from June 2018 to June 2019. During this period, 24
healthy female volunteers with matched baseline characteristics,
including age, weight and waist measurement, were also recruited
(Table SI). PCOS was diagnosed in
accordance with the criteria provided in the Revised Rotterdam
European Society of Human Reproduction and Embryology/American
Society for Reproductive Medicine criteria (2003) (14). Ovarian tissues from the recruited
subjects were collected during laparoscopic inspection or the
diagnosis of pelvic pain. The study was approved by the Medical
Research Ethics Committee of the First Affiliated Hospital of
Nanchang University (approval no. 2018089), and written informed
consent was obtained from each subject.
Cell culture
Human KGN ovarian granulosa cells and IOSE80 ovarian
epithelial cells were purchased from the American Type Culture
Collection (ATCC). The cells were frozen at −80°C for storage.
After recovery, the cells were cultured in Dulbeccos modified
Eagles medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
containing 10% foetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin in a humidified
atmosphere of 5% CO2 at 37°C. Cell passage was conducted
using trypsin until adherent cells were grown to >80%
confluence.
293T cells were also purchased from the ATCC and
used for lentiviral packaging. Briefly, the cells were cultured in
DMEM containing 10% FBS and (both from Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin in a humidified
atmosphere of 5% CO2 at 37°C, when the cells were
>80% confluent, they were digested with trypsin and inoculated
into a new culture dish. When the cells were adherent, they were
incubated with serum-free Opti-MEM™ (Gibco; Thermo Fisher
Scientific, Inc.) for 4 h, after which transfection and lentiviral
packaging were carried out.
Cell transfection
miR-613 mimic, miR-613 inhibitor and the respective
negative controls, mimic-NC and inhibitor-NC (miR-613 mimic,
5′-AGGAAUGUUCCUUCUUUGCC-3′; mimic-NC, 5′-UUCUCCGAACGUGUCACGUTT-3′;
miR-613 inhibitor, 5′-GGCAAAGAAGGAACAUUCCU-3′; inhibitor-NC,
5′-UUCUCCGAACGUGUCACGUTT-3′) were synthesized by Guangzhou RiboBio
Co., Ltd. KGN cells were seeded at a density of 5×104
cells/well in a 12-well plate and cultured for 24 h. When 80% cell
confluence was reached, the medium was replaced with Opti-MEM and
the cells were cultured for 4 h. The miRNAs were gently mixed with
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), then incubated for 20 min at room temperature
and added to each well at a final concentration of 100 nM. The
cells were cultured at 37°C in a humidified atmosphere containing
5% CO2 for 40 min. Opti-MEM was then replaced with DMEM
containing 10% FBS and 1% penicillin-streptomycin for another 24 h
of cell culture. The FAM-labeled miRNAs were observed under a
fluorescence microscope to evaluate the transfection efficacy 24–48
h after transfection.
Target prediction and dual-luciferase
reporter assay
The site of IGF-1 targeted by miR-613 was predicted
using TargetScan 7.2 (http://www.targetscan.org/vert_72/). Based on this,
vectors containing wild-type and mutant (mut) IGF-1 3UTRs, namely
pmirGLO-IGF1-3UTR and pmirGLO-IGF1-mut 3UTR, were generated by
CoBioer Biosciences Co., Ltd. The DNAs were directly synthesized by
annealing, and the luciferase vectors were subsequently constructed
using pmirGLO Dual-Luciferase miRNA Target Expression Vector
(Promega Corporation). In addition, an miR-613 overexpression
plasmid was generated using pcDNA3.1(+) and named as
pcDNA3.1(+)-miR-613. The KGN cells were cultured to 80% density and
transfected with pmirGLO-IGF1-3UTR or pmirGLO-IGF1-mut 3UTR, alone
or in combination with pcDNA3.1(+) or pcDNA3.1(+)-miR-613, using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The miRNAs and plasmids were gently mixed with
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), then incubated for 20 min at room temperature.
The mRNA/plasmid/Lipofectamine 2000 mixture was then added to the
cells for 40 min at 37°C in a humidified atmosphere containing 5%
CO2. Cells were transfected at 37°C in a humidified
atmosphere containing 5% CO2 for 48 h, and then lysed
with 100 µl lysis buffer (Promega Corporation) per well and
centrifuged at 14,000 × g at 4°C for 5 min. A Dual- Luciferase
Reporter Assay system (Promega Corporation) was then used to detect
the luciferase activity (Promega Corporation). Relative luciferase
activity was expressed as the ratio of firefly luciferase activity
to Renilla luciferase activity.
Viability determination
Cell viability was assessed by MTT assay.
Transfected cells were collected and centrifuged at 2,500 × g and
4°C for 5 min. The precipitate was resuspended to prepare a cell
suspension, which was transferred to a 96-well plate at
5×103 cells/well for culture. At 24, 48, 72 and 96 h, 20
µl MTT was added to each well and incubated for 4 h at 37°C. The
cells were subsequently treated with 150 µl dimethyl sulfoxide for
10 min at room temperature to dissolve the formazan, and the
optical density at 570 nm was measured using an ultraviolet
spectrophotometer.
Colony formation assay
Transfected cells were seeded in 60-mm culture
dishes with 100 cells/dish and then cultivated in DMEM containing
10% FBS for 14 days. Visible colonies were washed with PBS, fixed
in 4% paraformaldehyde for 30 min, and stained with 1% crystal
violet for 15 min at room temperature. After air-drying, images of
the colonies were captured under a fluorescence microscope for
counting.
Determination of cell cycle
progression
Cells were transfected for 48 h, and cell cycle
progression was then determined. The cells were digested with
trypsin and resuspended in PBS. After fixing at 4°C for 30 min, the
cells were stained using propidium iodide (US Everbright, Inc.) at
room temperature for 30 min. Cells were analyzed on a FACScan flow
cytometer (BD Biosciences) and the results were analyzed using
FlowJo software (FlowJo, version 10, Ashland).
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was conducted according to the manufacturers
protocols for the PrimeScript™ RT Reagent Kit and SYBR
Premix Ex Taq™II with Tli RNaseH (Takara Bio, Inc.), using an ABI
Prism 7500 system (Thermo Fisher Scientific, Inc.). Using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), total RNA was first isolated from cells and ovarian tissues.
The RNA was treated with a gDNA eraser and reversely transcribed to
cDNAs. Subsequently, the cDNAs were subjected to qPCR for
denaturation at 95°C for 15 min, followed by 40 cycles of 95°C for
5 sec, 60°C for 30 sec and 72°C for 40 sec. Annealing was finally
conducted at 72°C for 10 min. GAPDH and U6 were used as internal
references. When analyzing changes in mRNA levels, the expression
values relative to those of a housekeeping control were compared.
The primer sequences and mRNA products are listed in Table I. The data were quantified using the
2−ΔΔCt method (15).
 | Table I.Primers used in the study. |
Table I.
Primers used in the study.
| Genes | Primers | Product size
(bp) |
|---|
| miR-613 | F:
GTGAGTGCGTTTCCAAGTGT | 84 |
|
| R:
TGAGTGGCAAAGAAGGAACAT |
|
| IGF-1 | F:
CATGTCCTCCTCGCATCTCT | 213 |
|
| R:
AGCAGCACTCATCCACGATA |
|
| Cyclin D1 | F:
CGATGCCAACCTCCTCAACGA | 153 |
|
| R:
TCGCAGACCTCCAGCATCCA |
|
| CDK1 | F:
CCTAGCATCCCATGTCAAAAACTTGG | 108 |
|
| R:
TGATTCAGTGCCATTTTGCCAGA |
|
| GAPDH | F:
AGCCACATCGCTCAGACAC | 66 |
|
| R:
GCCCAATACGACCAAATCC |
|
| U6 | F:
CTCGCTTCGGCAGCACA | 94 |
|
| R:
AACGCTTCACGAATTTGCGT |
|
Western blot analysis
Protein levels of IGF-1, cyclin D1 and CDK1 in cells
were determined by western blot analysis. The antibodies purchased
from ABclonal Biotech Co., Ltd. were as follows: Rabbit primary
antibodies IGF-1 (cat. no. A11985), cyclin D1 (cat. no. A11022),
CDK1 (cat. no. A0220) and GAPDH (cat. no. AC001), and HRP goat
anti-rabbit secondary antibody (cat. no. AS014). Total proteins
were first isolated by RIPA lysis buffer (Beyotime Institute of
Biotechnology), and their concentrations were evaluated using the
BCA method. Protein samples (50 µg/lane) were separated by SDS-PAGE
on 12% gels, then transferred to PVDF membranes. After incubation
in 5% skimmed milk for 1 h, the membranes were incubated with
primary antibodies (1:1,000) at 4°C overnight and then with
secondary antibody (1:1,000) at room temperature for 1 h. The bands
were visualized by Quantity One software (Bio-Rad Laboratories,
Inc.).
Lentivirus transfection
Vector GV287-IGF-1 was generated by cleaving the
GV287 plasmid (Shanghai GeneChem Co., Ltd.) using AgeI and
splicing in IGF-1. A 2nd generation system was used to the package
of lentivirus. The lentiviral plasmid, packaging vector and
envelope vector were mixed at a 4:3:2 ratio for a total DNA mass of
20 µg and incubated with 1 ml Lenti-Easy Packaging Mix (Shanghai
GeneChem Co., Ltd.) for 15 min. The mixture was then incubated for
another 20 min incubation with Lipofectamine® 2000, then
added into 293T cell culture medium for 6 h at 37°C. In brief, the
293T cells were seeded at a density of 2.5×105
cells/plate in a 10-cm plate and cultured to 80% confluence,
incubated in Opti-MEM for 4 h, and then in with the transfection
mixture as described in the Cell transfection section. The
supernatant of the transfected 293T cells was collected after three
days by filtering through a 0.45-µm filter, and the viral particles
were concentrated by ultracentrifugation at 70,000 × g for 2 h at
4°C. The structure of the GV287 plasmid is shown in Fig. S1. KGN cells were infected with the
lentivirus at a multiplicity of infection of 5 and with polybrene
(Sigma-Aldrich; Merck KGaA) at a final concentration of 8 µg/ml at
37°C with 5% CO2 for 24 h. Fresh culture medium was then
used to replace the old medium. Fluorescence was measured 72 h
post-infection when the achieved infection efficiency was 80%.
Screening of stable cell lines using green fluorescent protein.
Statistical analysis
Statistical analysis was performed using GraphPad
8.0 software (GraphPad Software, Inc.). Data are expressed as the
mean ± standard deviation. All data in the current study conform to
a normal distribution. One-way ANOVA was used to assess differences
among the groups, and Tukeys and Bonferronis tests were used for
post hoc testing following ANOVA. P<0.05 was considered to
indicate a statistically significant difference.
Results
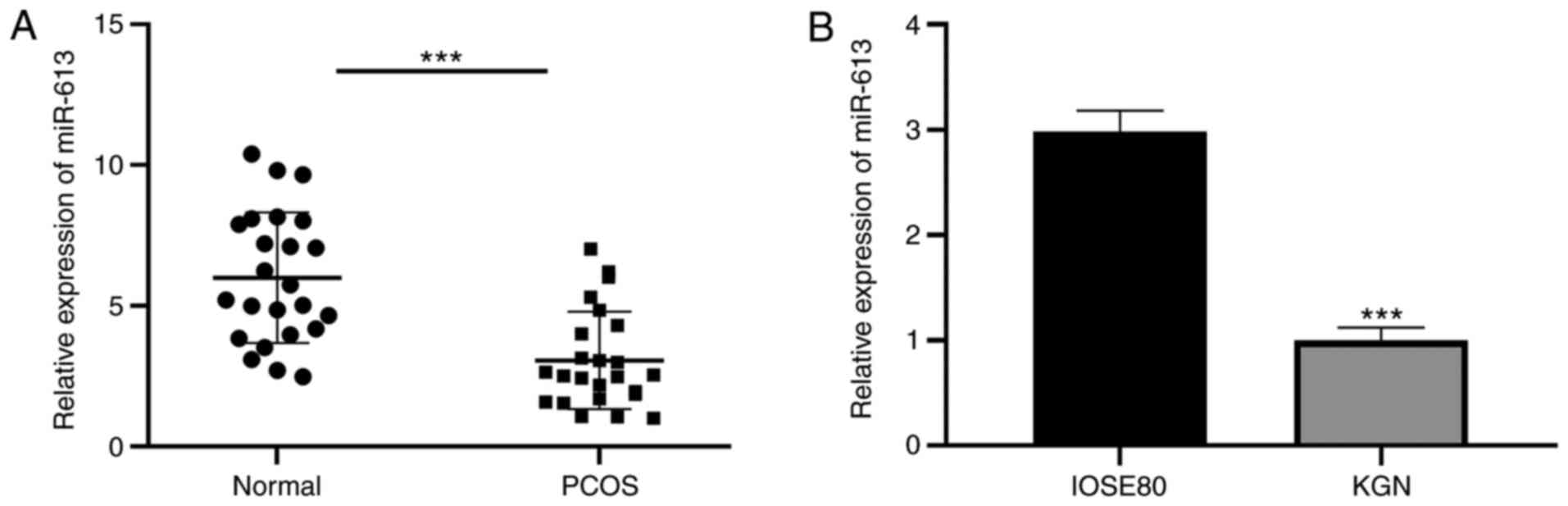
miR-613 is downregulated in patients
with PCOS
To explore the biological function of miR-613 in
PCOS, the levels of miR-613 in ovarian tissues and KGN cells were
first examined. The results revealed that miR-613 was significantly
downregulated in the ovarian tissues of patients with PCOS compared
with those in the healthy controls, and in KGN cells compared with
IOSE80 cells (P<0.001; Fig. 1A),
suggesting a significant involvement of miR-613 in PCOS.
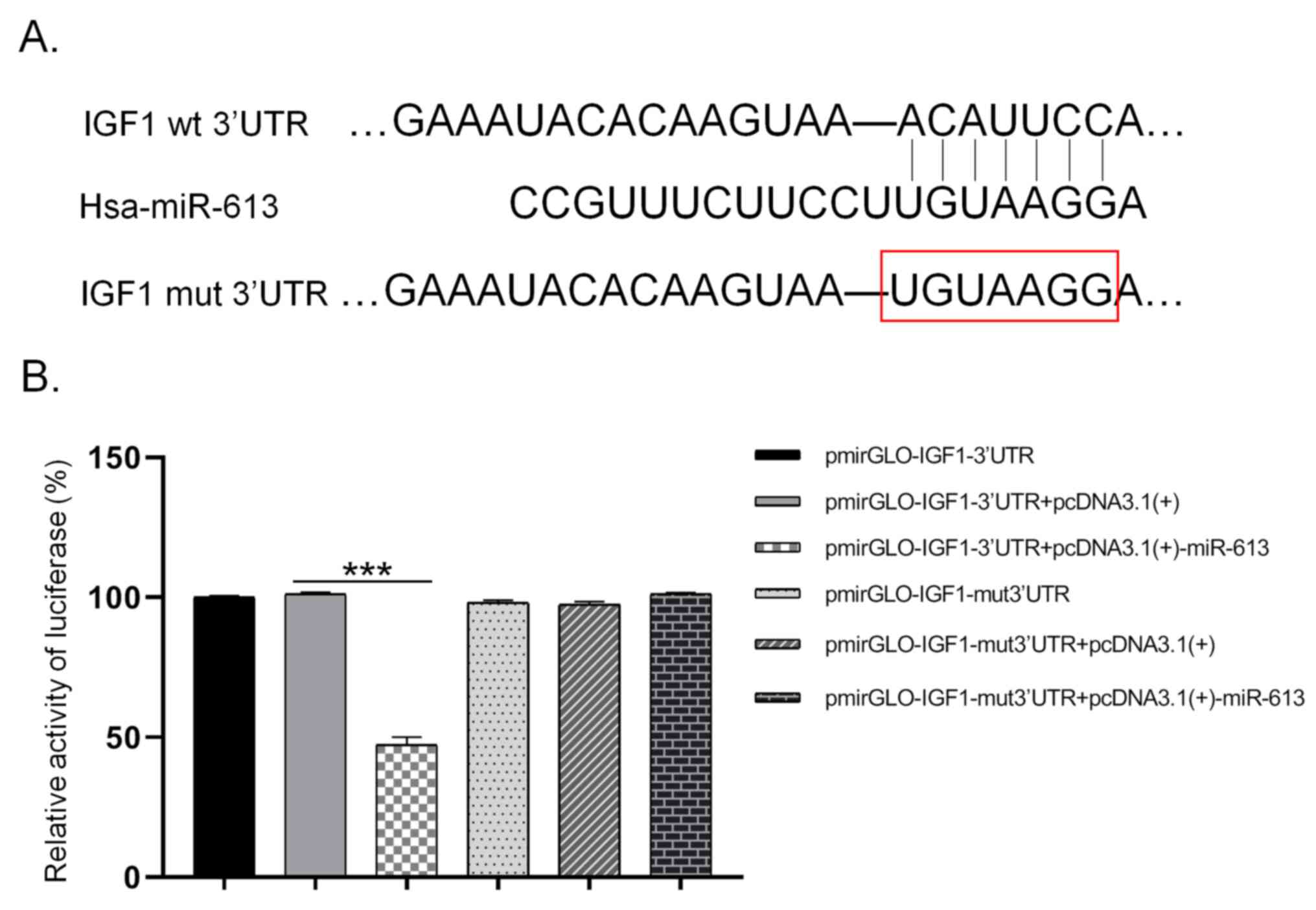
miR-613 inhibits IGF-1 expression by
directly binding to its 3UTR
Using TargetScan, binding sites were predicted for
miR-613 in the 3UTR of IGF-1 (Fig.
2A). A dual-luciferase reporter assay then revealed that the
luciferase intensity in the pmirGLO-IGF1-3UTR + pcDNA3.1(+)-miR-613
group was only about 47% of that in the pmirGLO-IGF1-3UTR +
pcDNA3.1(+) group, which was a significant difference (P<0.001).
Furthermore, no significant difference in luciferase intensity was
observed between the pmirGLO-IGF1-mut 3UTR + pcDNA3.1(+)-miR-613
group and the pmirGLO-IGF1-mut 3UTR + pcDNA3.1(+) group (Fig. 2B). These results demonstrate that
miR-613 targeted the 3UTR of IGF-1 and thus regulated its
post-transcriptional level.
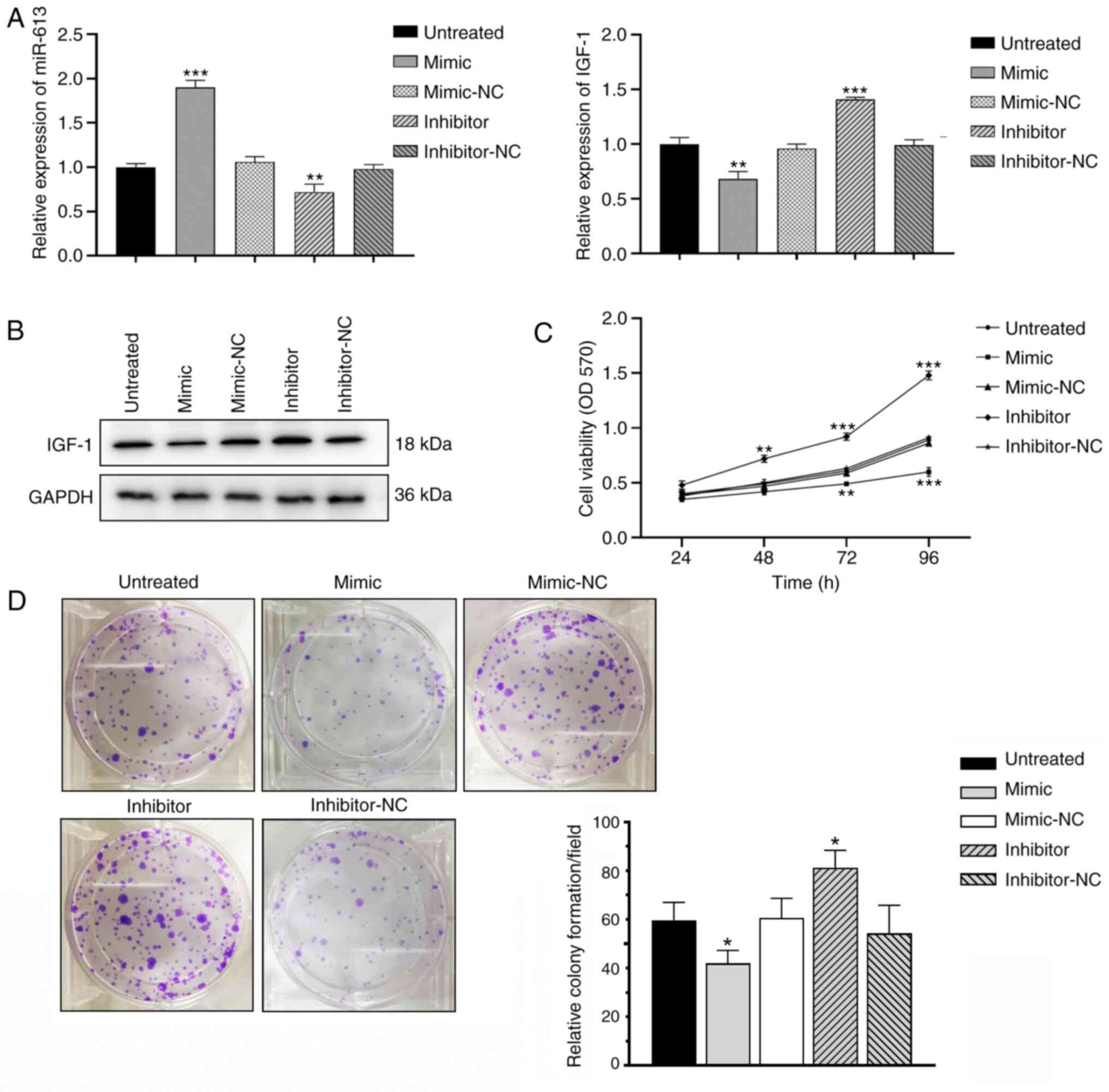
Overexpression of miR-613 inhibits KGN
cell proliferation
Total RNAs and proteins were extracted from KGN
cells transfected with miR-613 mimic, miR-613 inhibitor or the
respective negative controls and then subjected to RT-qPCR and
western blot analysis, respectively (Fig. 3A and B). Transfection of the KGN
cells with miR-613 mimic markedly reduced the translational level
of IGF-1. MTT assay revealed that transfection with miR-613 mimic
significantly decreased the viability of KGN cells, whereas the
knockdown of miR-613 significantly increased cell KGN cell
viability compared with the respective negative controls
(P<0.01; Fig. 3C). Colony
formation was reduced in KGN cells overexpressing miR-613, whereas
the knockdown of miR-613 increased the number of colonies formed,
compared with the respective negative controls (P<0.05; Fig. 3D). Collectively, these results
indicate that miR-613 inhibited KGN cell proliferation.
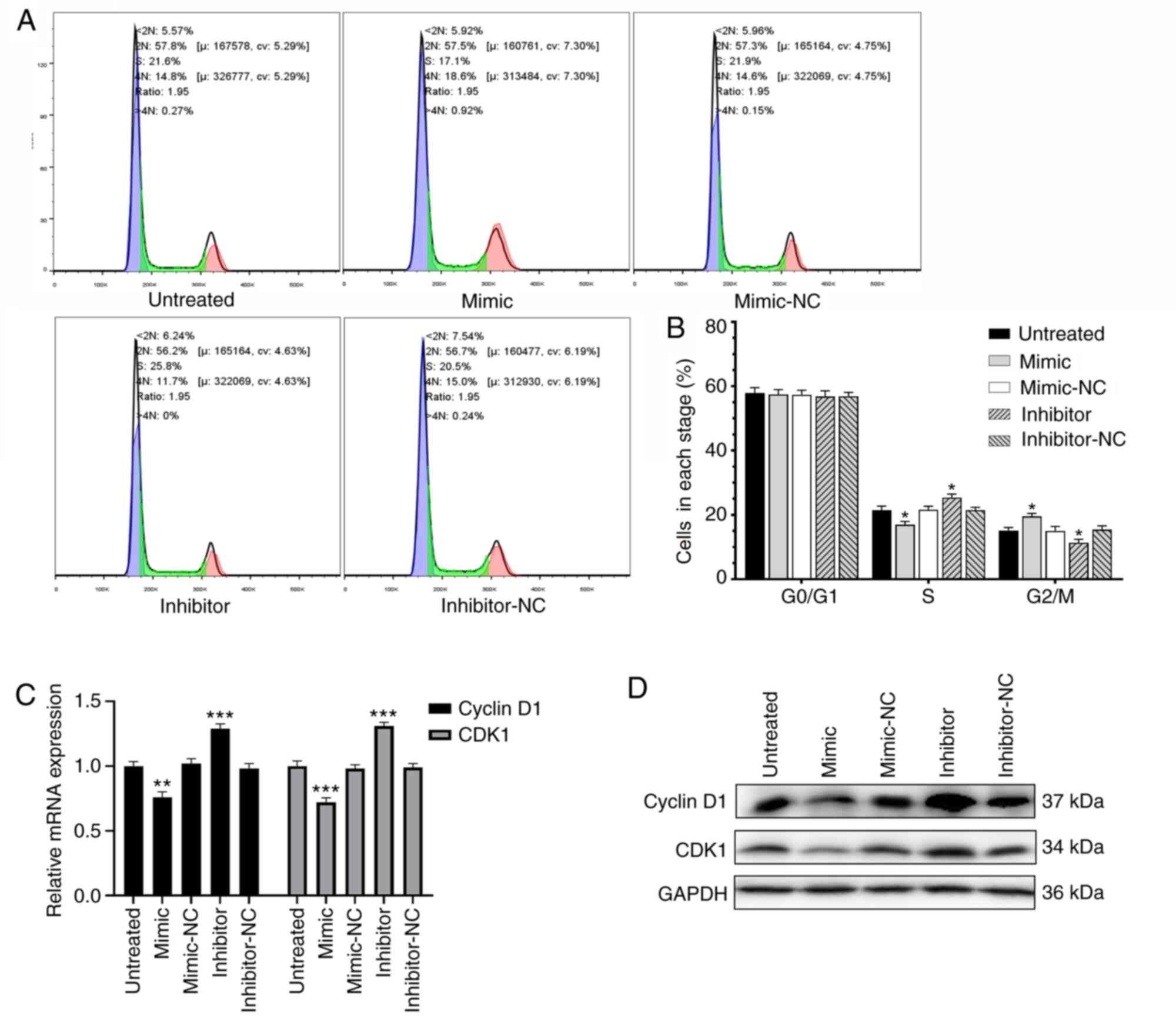
Overexpression of miR-613 inhibits KGN
cell proliferation by arresting cell cycle progression
Flow cytometry was used to assess the effect of
abnormal expression levels of miR-613 on cell cycle progression in
KGN cells. Compared with the respective control, the KGN cells
overexpressing miR-613 showed a reduced percentage of cells in the
S phase and an increased percentage of cells in the G2/M phase,
whereas the knockdown of miR-613 yielded the opposite results
(P<0.05; Fig. 4 and B).
Moreover, whether the expression of levels of cell cycle-associated
mRNAs and proteins were regulated by miR-613 was determined by
RT-qPCR and western blot analysis, respectively. The overexpression
of miR-613 downregulated the expression of cyclin D1 and CDK1,
whereas the knockdown of miR-613 upregulated them at the mRNA
(P<0.05) and protein levels (Fig. 4C
and D). These results indicate that the overexpression of
miR-613 arrested cell cycle progression in the G2/M phase by
downregulating cyclin D1 and CDK1, and inhibited KGN cell
proliferation.
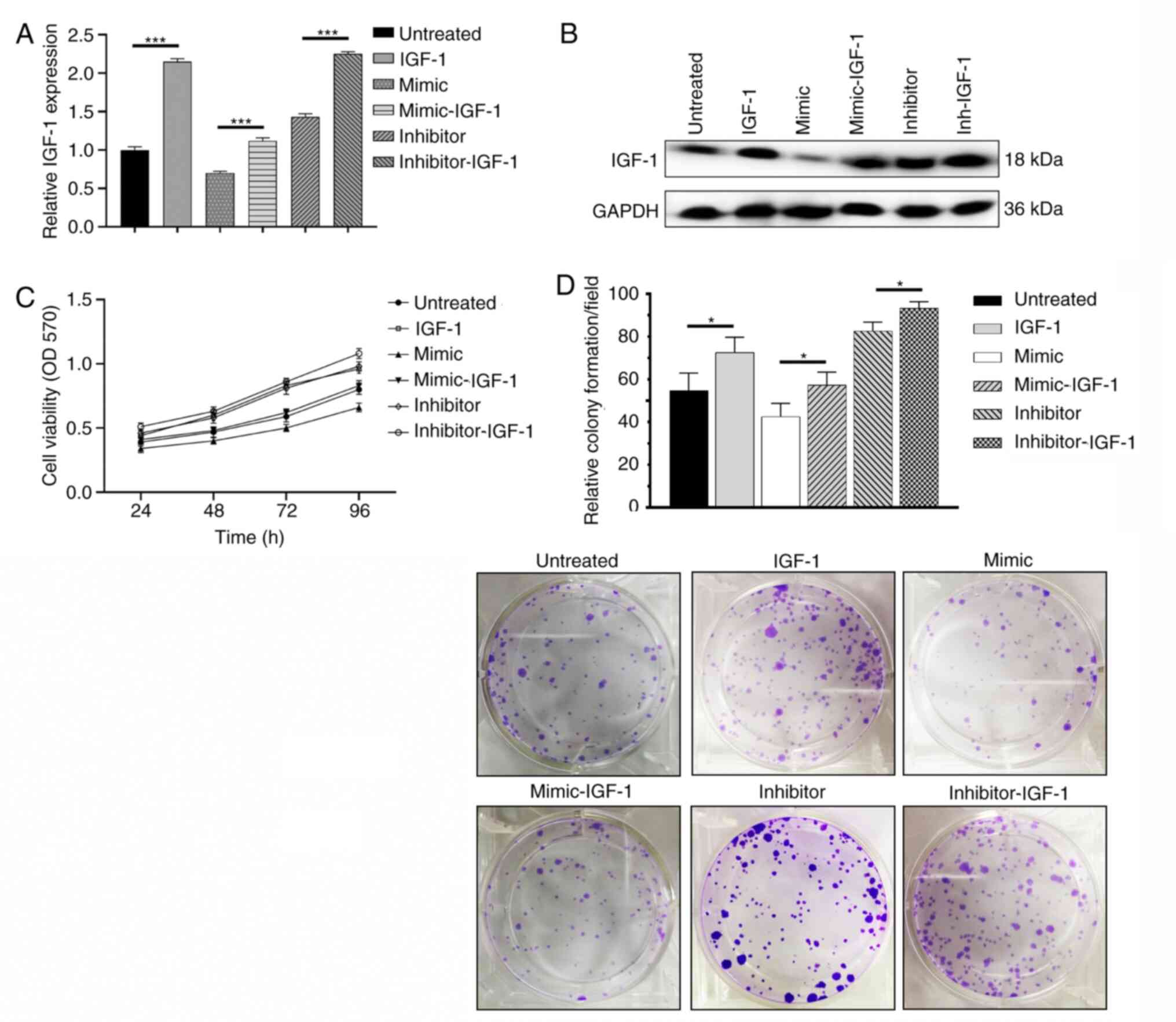
Overexpression of IGF-1 promotes KGN
cell proliferation
The KGN cell line with stably overexpressed IGF-1
was generated by lentiviral transfection. IGF-1 expression was
significanty increased following lentivirus infection, compared
with cells transduce with an empty vector (Fig. S2). Subsequently, miR-613 mimic,
miR-613 inhibitor, or their negative controls were transfected. The
mRNA and protein levels of IGF-1 were assessed. Overexpression of
IGF-1 significantly reversed the effects of miR-613 mimics and
increased the expression of IGF-1 (Fig.
5A and B). Using MTT and colony formation assays, the
overexpression of IGF-1 was shown to markedly increase the
viability and proliferation of KGN cells compared with that in the
respective control (Fig. 5C and
D).
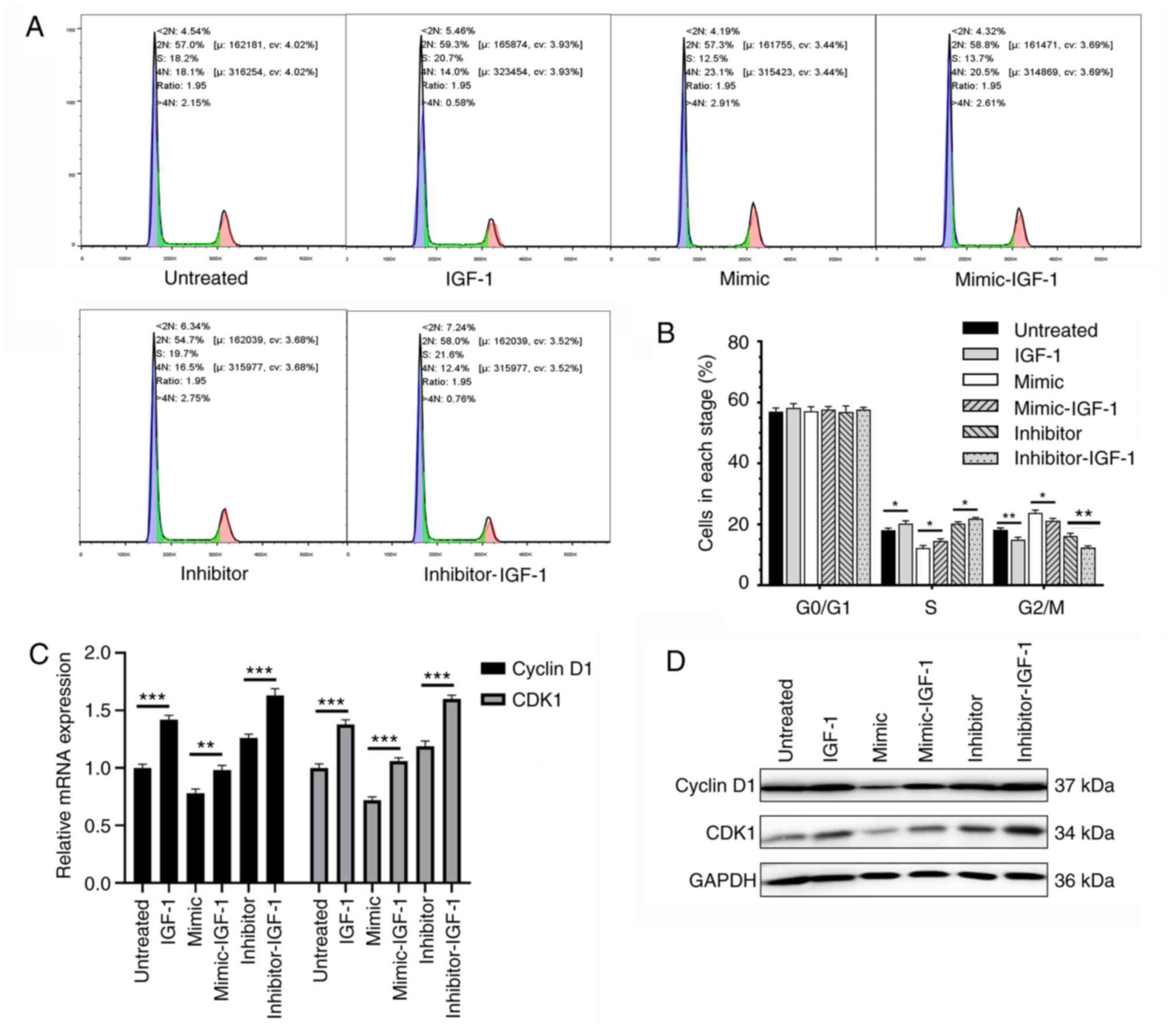
Overexpression of IGF-1 accelerates
cell cycle progression in KGN cells
The regulatory effect of IGF-1 overexpression on the
cell cycle progression of KGN cells was evaluated. Compared with
that in the respective control, the cell cycle arrest in KGN cells
induced by the overexpression of miR-613 was significantly
attenuated by the overexpression of IGF-1, and the overexpression
of IGF-1 further accelerated cell cycle progression in KGN cells
with miR-613 knockdown (P<0.05; Fig.
6A and B). Moreover, the overexpression of IGF-1 eliminated the
inhibitory effects of miR-613 on the expression of cyclin D1 and
CDK1 at the mRNA (P<0.01) and protein levels (Fig. 6C and D). These results indicate that
the inhibitory effects of miR-613 on the proliferation and cell
cycle progression of KGN cells may be attributable to IGF-1.
Discussion
PCOS is a common endocrine and metabolic disease in
women of reproductive age. The disease has a complex etiology, and
its pathogenesis remains largely unclear. Pathological changes that
occur in PCOS include the presence of multiple immature follicles
in the ovary, lack of dominant follicle selection, atresia of small
follicles, and degeneration and thinning of the granular cell layer
(3). Follicular growth and
development are delicate and complex processes in which granulosa
cells are of great significance. PCOS-induced elevated androgen
levels, insulin resistance and chronic inflammation have a close
association with granulosa cells (16). High androgen levels are the most
typical hormonal change observed in patients with PCOS, and are
associated with hair thinning, anovulation, infertility, hirsutism,
acne and seborrheic alopecia (16).
Yang et al (17) observed an
increase in the level of testosterone in follicular fluid and a
reduction in aromatase expression in the luteinized granulosa cells
of patients with PCOS, indicating an association between high
androgen levels in follicles and the progression of PCOS. Insulin
resistance and compensatory hyperinsulinemia are important
characteristics of PCOS, which may result in metabolic syndrome,
impaired glucose tolerance and reproductive disorders. Belani et
al (18) determined that high
levels of insulin stimulate apoptosis in the granulosa cells of
rats; however, the molecular mechanism underlying this has not yet
been identified. Moreover, patients with PCOS exist in a
pathological state of chronic and low-level inflammation. The
upregulation of inflammation-associated genes in the granulosa
cells of patients with PCOS may be attributed to abnormal ovulation
and ovarian hyperstimulation syndrome. A recent study proposed that
high levels of androgen activate ovarian chemerin and thereby
stimulate the chemokine-induced recruitment of monocytes to the
ovaries (19). Zhang et al
(20) detected high expression
levels of lysyl oxidase and interleukin-1β in the granulosa cells
and follicular fluid of patients with PCOS. They also found that
the inhibition of lysyl oxidase activity ameliorated anovulation,
suggesting that anti-inflammatory treatment is effective for PCOS.
Granulosa cells are generally regarded as being vital during
follicular development and the pathological process of PCOS.
miRNAs are endogenous, small-molecular, non-coding
RNAs that induce the degradation, inhibit translation or promote
the deadenylation of target mRNAs by recognizing and binding to the
3UTR of target genes. miRNAs are extensively involved in the
pathological process of PCOS. Sirotkin et al (21) reported that 36 of 80 different
miRNAs tested in human ovarian cells exerted regulatory effects on
progesterone secretion, among which, notably, miR-107 significantly
stimulated androgen secretion. Yao et al (22) demonstrated the involvement of
miR-224 in the regulation of the TGF-β1-induced proliferation of
mouse granulosa cells and the stimulation of estrogen secretion,
which proceeded via the targeting of Smad4. In addition, miR-224
has been reported to be highly expressed in the follicular fluid of
patients with PCOS, indicating a potential role of miR-224 in the
hormonal regulation of PCOS (23).
Furthermore, in a DTH-induced rat model of PCOS, 17 downregulated
miRNAs and 27 upregulated miRNAs were identified in rats with
hyperandrogenemia (9). It has been
suggested that the expression of miRNAs in patients with PCOS may
be affected by obesity and the serum level of free testosterone
(24).
The first study to feature miR-613 was reported in
2011 (25). miR-613 has been
identified to have anticancer effects. For example, Wang et
al (11) identified that
miR-613 is significantly downregulated in human
cytomegalovirus-positive glioblastoma, and plays a
tumor-suppressive role via the targeting of arginase 2. Another
study demonstrated that as gastric cancer progresses, miR-613
arrests cell cycle progression by targeting the cell cycle protein
CDK9 (26). Furthermore, miR-613
has been demonstrated to attenuate the proliferation and invasion
of glioma cells by targeting CDK14 (12). Gao et al (27) revealed that miR-613 prevents
angiogenesis in nasopharyngeal carcinoma by inactivation of the Akt
signaling pathway via FN1 downregulation, thereby attenuating tumor
progression. In addition, the progression and metastasis of
colorectal carcinoma have been shown to be inhibited by
miR-613-mediated cell cycle arrest in the G1 phase, with miR-613
directly targeting FMNL2 (13).
However, the role of miR-613 in the regulation of granulosa cells
and PCOS progression has rarely been reported. The findings of the
present study demonstrate that miR-613 was downregulated in the
ovarian tissues of PCOS patients and in KGN cells. In addition,
miR-613 regulated the transcription activity of IGF-1 via directly
targeting its 3UTR, thereby arresting cell cycle progression and
inhibiting the proliferation of KGN cells.
IGF-1 regulates cell growth and proliferation, which
serve critical roles in tumor development (28). Wang et al (29) demonstrated that blocking autocrine
IGF-1 via the application of a specific anti-IGF-1 antibody
inactivated the Akt/GSK-3β signaling pathway, which contributed to
the reduced activity of umbilical cord mesenchymal stem cells and
the arrest of cell cycle progression. IGF-1 is closely associated
with hyperinsulinemia and mammalian aging. Furthermore, in worms,
insects and yeast, the inhibition of insulin/IGF-like signal
transduction prolongs their lifespans (30–32).
Anisimov (33) and Bartke (34) reported that IGF-1 deficiency
significantly extends the lifespan of mice. Mice with IGF-1
deficiency exhibit decreased IGF-1 and insulin levels in the
circulation but markedly increased insulin resistance. Previous
studies have detected high expression levels of IGF-1 in the
ovarian tissues and granulosa cells of patients with PCOS. In one
of these studies, IGF-1 was found to be upregulated in the cumulus
cells collected from patients with PCOS, whereas miR-483-5p and
miR-486-5p were downregulated. Further analysis suggested that
miR-483-5p significantly regulates insulin resistance, and
miR-486-5p triggers cumulus cell proliferation by activating the
PI3K/Akt signaling pathway (35).
In another study, Homburg et al (36) collected serum samples from patients
with PCOS or hypopituitarism, and healthy subjects to detect the
levels of IGF-1, IGF binding protein-1, insulin and LH. Patients
with PCOS were found to express markedly high levels of IGF-1,
insulin and LH. In the current study, IGF-1 was upregulated in the
ovarian tissues of patients with PCOS and in KGN cells. The
translation of IGF-1 was inhibited by miR-613, which downregulated
the expression of IGF-1 in KGN cells. The reduced expression of
IGF-1 was associated with changes in the expression of cyclin D1
and CDK1 at the protein level, arrest at the G2/M phase of the cell
cycle and the inhibition of KGN cell proliferation.
In conclusion, miR-613 is significantly
downregulated in the ovarian tissues of patients with PCOS and in
KGN cells. It arrests cell cycle progression and attenuates the
proliferation of KGN cells via the targeting of IGF-1. Therefore,
miR-613 and IGF-1 may be potential diagnostic biomarkers and
therapeutic targets for PCOS.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key R&D
Program Projects in Jiangxi Province (grant no. 20171BBG70009).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
JW and SL made substantial contributions to
conception and design, acquisition, analysis and interpretation of
data, and given final approval of the version to be published
together. All authors agree to be accountable for all aspects of
the work in ensuring that questions related to the accuracy and
integrity of any part of the work are appropriately investigated
and resolved. SL drafted the manuscript and revised it critically
for important intellectual content. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Research
Ethics Committee of the First Affiliated Hospital of Nanchang
University (approval no. 2018089), and written informed consent was
obtained from each subject.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pepene CE, Ilie IR, Marian I and Duncea I:
Circulating osteoprotegerin and soluble receptor activator of
nuclear factor κB ligand in polycystic ovary syndrome:
Relationships to insulin resistance and endothelial dysfunction.
Eur J Endocrinol. 164:61–68. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Franik S, Eltrop SM, Kremer JA, Kiesel L
and Farquhar C: Aromatase inhibitors (letrozole) for subfertile
women with polycystic ovary syndrome. Cochrane Database Syst Rev.
5:CD0102872018.PubMed/NCBI
|
|
3
|
Broekmans FJ and Fauser BC: Diagnostic
criteria for polycystic ovarian syndrome. Endocrine. 30:3–11. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ding L, Gao F, Zhang M, Yan W, Tang R,
Zhang C and Chen ZJ: Higher PDCD4 expression is associated with
obesity, insulin resistance, lipid metabolism disorders, and
granulosa cell apoptosis in polycystic ovary syndrome. Fertil
Steril. 105:1330–1337.e3. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kosova G and Urbanek M: Genetics of the
polycystic ovary syndrome. Mol Cell Endocrinol. 373:29–38. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Urbanek M: The genetics of the polycystic
ovary syndrome. Nat Clin Pract Endocrinol Metab. 3:103–111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Shi
Y, Li Z, You L, Zhao J, Liu J, et al: Genome-wide association study
identifies susceptibility loci for polycystic ovary syndrome on
chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 43:55–59. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Diamanti-Kandarakis E and Piperi C:
Genetics of polycystic ovary syndrome: Searching for the way out of
the labyrinth. Hum Reprod Update. 11:631–643. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hossain MM, Cao M, Wang Q, Kim JY,
Schellander K, Tesfaye D and Tsang BK: Altered expression of miRNAs
in a dihydrotestosterone-induced rat PCOS model. J Ovarian Res.
6:362013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McCallie B, Schoolcraft WB and Katz-Jaffe
MG: Aberration of blastocyst microRNA expression is associated with
human infertility. Fertil Steril. 93:2374–2382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Zhao P, Qian D, Hu M, Zhang L, Shi
H and Wang B: MicroRNA-613 is downregulated in HCMV-positive
glioblastoma and inhibits tumour progression by targeting
arginase-2. Tumour Biol. 39:10104283177125122017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Q, Zhou L, Wang M, Wang N, Li C, Wang J
and Qi L: MicroRNA-613 impedes the proliferation and invasion of
glioma cells by targeting cyclin-dependent kinase 14. Biomed
Pharmacother. 98:636–642. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li B, Xie Z, Li Z, Chen S and Li B:
MicroRNA-613 targets FMNL2 and suppresses progression of colorectal
cancer. Am J Transl Res. 8:5475–5484. 2016.PubMed/NCBI
|
|
14
|
Kanafchian M, Esmaeilzadeh S, Mahjoub S,
Rahsepar M and Ghasemi M: Status of serum copper, magnesium, and
total antioxidant capacity in patients with polycystic ovary
syndrome. Biol Trace Elem Res. 193:111–117. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosenfield RL and Ehrmann DA: The
pathogenesis of polycystic ovary syndrome (PCOS): The hypothesis of
PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev.
37:467–520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang F, Ruan YC, Yang YJ, Wang K, Liang
SS, Han YB, Teng XM and Yang JZ: Follicular hyperandrogenism
downregulates aromatase in luteinized granulosa cells in polycystic
ovary syndrome women. Reproduction. 150:289–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belani M, Deo A, Shah P, Banker M, Singal
P and Gupta S: Differential insulin and steroidogenic signaling in
insulin resistant and non-insulin resistant human luteinized
granulosa cells-A study in PCOS patients. J Steroid Biochem Mol
Biol. 178:283–292. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lima PDA, Nivet AL, Wang Q, Chen YA,
Leader A, Cheung A, Tzeng CR and Tsang BK: Polycystic ovary
syndrome: Possible involvement of androgen-induced,
chemerin-mediated ovarian recruitment of monocytes/macrophages.
Biol Reprod. 99:838–852. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang C, Ma J, Wang W, Sun Y and Sun K:
Lysyl oxidase blockade ameliorates anovulation in polycystic ovary
syndrome. Hum Reprod. 33:2096–2106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sirotkin AV, Lauková M, Ovcharenko D,
Brenaut P and Mlyncek M: Identification of microRNAs controlling
human ovarian cell proliferation and apoptosis. J Cell Physiol.
223:49–56. 2010.PubMed/NCBI
|
|
22
|
Yao G, Yin M, Lian J, Tian H, Liu L, Li X
and Sun F: MicroRNA-224 is involved in transforming growth
factor-beta-mediated mouse granulosa cell proliferation and
granulosa cell function by targeting Smad4. Mol Endocrinol.
24:540–551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roth LW, McCallie B, Alvero R, Schoolcraft
WB, Minjarez D and Katz-Jaffe MG: Altered microRNA and gene
expression in the follicular fluid of women with polycystic ovary
syndrome. J Assist Reprod Genet. 31:355–362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murri M, Insenser M, Fernández-Durán E,
San-Millán JL and Escobar-Morreale HF: Effects of polycystic ovary
syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21,
miRNA-27b, miRNA-103, and miRNA-155 expression. J Clin Endocrinol
Metab. 98:E1835–E1844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ou Z, Wada T, Gramignoli R, Li S, Strom
SC, Huang M and Xie W: MicroRNA hsa-miR-613 targets the human LXRα
gene and mediates a feedback loop of LXRα autoregulation. Mol
Endocrinol. 25:584–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu Y, Tang L, Zhang Q, Zhang Z and Wei W:
MicroRNA-613 inhibits the progression of gastric cancer by
targeting CDK9. Artif Cells Nanomed Biotechnol. 46:980–984. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao R, Feng Q and Tan G: microRNA-613
exerts anti-angiogenic effect on nasopharyngeal carcinoma cells
through inactivating the AKT signaling pathway by down-regulating
FN1. Biosci Rep. 39:BSR20182196. 2019.doi: 10.1042/BSR20182196.
View Article : Google Scholar
|
|
28
|
Clemmons DR: Modifying IGF1 activity: An
approach to treat endocrine disorders, atherosclerosis and cancer.
Nat Rev Drug Discov. 6:821–833. 2007. View
Article : Google Scholar
|
|
29
|
Wang Q, Zhang F and Hong Y: Blocking of
autocrine IGF-1 reduces viability of human umbilical cord
mesenchymal stem cells via inhibition of the Akt/Gsk-3β signaling
pathway. Mol Med Rep. 17:4681–4687. 2018.PubMed/NCBI
|
|
30
|
Guarente L and Kenyon C: Genetic pathways
that regulate ageing in model organisms. Nature. 408:255–262. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Longo VD and Finch CE: Evolutionary
medicine: From dwarf model systems to healthy centenarians?
Science. 299:1342–1346. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tatar M, Bartke A and Antebi A: The
endocrine regulation of aging by insulin-like signals. Science.
299:1346–1351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Anisimov VN and Bartke A: The key role of
growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit
Rev Oncol Hematol. 87:201–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bartke A: Single-gene mutations and
healthy ageing in mammals. Philos Trans R Soc Lond B Biol Sci.
366:28–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi L, Liu S, Zhao W and Shi J: miR-483-5p
and miR-486-5p are down-regulated in cumulus cells of metaphase II
oocytes from women with polycystic ovary syndrome. Reprod Biomed
Online. 31:565–572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Homburg R, Pariente C, Lunenfeld B and
Jacobs HS: The role of insulin-like growth factor-1 (IGF-1) and IGF
binding protein-1 (IGFBP-1) in the pathogenesis of polycystic ovary
syndrome. Hum Reprod. 7:1379–1383. 1992. View Article : Google Scholar : PubMed/NCBI
|