Introduction
Pneumonia, one of the most common pediatric
respiratory diseases, is also the most prevalent cause of mortality
and morbidity for children <5 years old, especially newborns
(1–3). The World Health Organization reports
that pneumonia accounts for a third of newborn mortality worldwide
(4), and 1.1–1.4 million children
succumb to pneumonia worldwide every year (5). Among the types of pneumonia,
bronchopneumonia is the most common type in children and is a
leading cause of child mortality, resulting in 935,000 deaths in
children <5 years old in 2013 (6,7).
Despite the high mortality rate of bronchopneumonia, its underlying
molecular mechanisms remain to be elucidated.
In the last decade, the role of microRNAs
(miRNAs/miRs) in the development of pneumonia has been reported in
a number of studies (8–11). For instance, Gomez et al
(12) detected 1,100 miRNAs in an
S. pneumoniae pneumonia mouse model and identified that 31 miRNAs
were significantly increased and 67 miRNAs were decreased.
Additionally, miRNAs including miR-1247, miR-217 and miR-3941 have
been associated with pneumonia development (13–15). A
recent study reported that hsa-miR-409-3p is upregulated in whole
blood of adenovirus-infected children with pneumonia (16). However, to the best of our
knowledge, no study has reported the role and associated molecular
mechanisms of miR-409-3p in the development of
bronchopneumonia.
Suppressor of cytokine signaling (SOCS)3 is
considered as an anti-inflammation factor in a number of diseases,
including pneumonia (17). The
inhibition of SOCS3 may facilitate the M1 macrophage polarization
in childhood pneumonia (17).
Additionally, the inactivation of SOCS3 is considered to be
associated with the activation of NF-κB signaling, which is the key
factor of inflammatory signaling (18). In asthma, SOCS3 and NF-κB expression
is stimulated and associated with inflammation (19). Liu et al (20) identified that SOCS3 is a direct
target of miR-409-3p in astrocytes and in the pathogenesis of
experimental autoimmune encephalomyelitis mice. However, the
association between miR-409-3p and SOCS3 in bronchopneumonia is
unclear.
The present study aimed to investigate the role of
miR-409-3p in lipopolysaccharide (LPS)-induced BEAS-2B cells as an
in vitro model of bronchopneumonia, in order to improve the
understanding of the role of miR-409-3p in LPS-induced inflammation
and to provide novel research targets for bronchopneumonia
development.
Materials and methods
Cell culture and treatment
Immortalized human bronchial epithelial BEAS-2B
cells were obtained from the American Type Culture Collection. The
cells were cultured in Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc.) with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C and 5% CO2. BEAS-2B cells were
treated with 10 µM LPS (Sigma-Aldrich; Merck KGaA) for 6 h at the
same condition of 37°C and 5% CO2 to establish the in
vitro bronchopneumonia model (21). Untreated cells were used as
controls. For inhibition of JAK/STAT3 signaling, 10 µM Tofacitinib
(Sigma-Aldrich; Merck KGaA) was used to treat the BEAS-2B cells for
6 h at 37°C and 5% CO2 (22).
Transfection
For BEAS-2B cell transfection, the miR-409-3p
mimics, inhibitor and the corresponding negative controls (NCs), as
well as small interfering (si)RNAs against SOCS3 (si-SOCS3) and
si-NC, were synthesized by Shanghai GeneChem Co., Ltd.). Scrambled
sequences were used as NC. Cells were transfected with 50 nmol/l
miR-409-3p mimics, miR-409-3p inhibitor or si-SOCS3 at 37°C for 48
h using Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Transfection efficiency was determined after 48 h of transfection
by reverse transcription-quantitative (RT-q) PCR. The sequences
were as follows: miR-409-3p mimics: 5′-GAAUGUUGCUCGGUGAACCCCU-3′;
NC inhibitor: 5′-ACTACTGAGTGACAGTAGA-3′; miR-409-3p inhibitor:
5′-GAGCUACAGUGCUUCAUCUCA-3′; inhibitor NC:
5′-UUCUCCGAACGUGUCACGUTT-3′; si-SOCS3: sense,
5′-TTCTACATGGGGGGATAG-3′, antisense 5′-TGGTCCAGGAACTCCCGAAT-3′;
si-NC: sense 5′-UUCUCCGAACGUGUCACGUTT-3′, antisense
5′-CUUGAGGCUGUUGUCAUACTT-3′
Apoptosis
Briefly, BEAS-2B cells (4×105/well) were
harvested, trypsinized and seeded into 6-well plates. The cells
were stained by PI (20 µg/ml) for 20 min using an Annexin V-FITC
Apoptosis Detection kit (Sigma-Aldrich; Merck KGaA) according to
the manufacturer's instructions. Cell apoptosis was analyzed using
a FACSort flow cytometer (BD Biosciences) with Cell Quest software
5.1 (BD Biosciences). Early and late apoptotic cells were
considered for the apoptotic rate.
ELISA
Briefly, the cell suspension was centrifuged at
1,200 × g for 15 min at room temperature. The supernatants were
collected and the levels of TNF-α, IL-6 and IL-1β were evaluated by
ELISA using the following commercially available kits: Human TNF-α
ELISA kit (cat. no. ab181421; Abcam), human IL-6 ELISA kit (cat.
no. ab178013; Abcam) and Human IL-1β ELISA kit (cat. no. ab100562;
Abcam).
RT-qPCR
Total RNA was extracted from cells using
TRIzol® (Thermo Fisher Scientific, Inc.). The mirVana
miRNA isolation kit (Ambion; Thermo Fisher Scientific, Inc.) was
used for miRNA extraction according to the manufacturer's
instruction. RNA was converted to cDNA using the High Capacity cDNA
Reverse Transcription kit (Thermo Fisher Scientific, Inc.) for mRNA
and Taqman MicroRNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) for miRNA according to the
manufacturer's instructions. The PCR reactions were conducted in an
Applied Biosystems 7500 Real Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) using SYBR-Green PCR Master Mix
(Beijing Solarbio Science & Technology Co., Ltd.).
Thermocycling conditions were: Initial denaturation at 94°C for 30
sec, then 94°C for 5 sec, 60°C for 30 sec and 72°C for 30 sec (40
cycles). Primer sequences were as follows: miR-409 forward (F),
5′-GAATGTTGCTCGGTGA-3′ and reverse (R), 5′-GTGCAGGGTCCGAGGT-3′;
SOCS3 F, 5′-CCTGCGCCTCAAGACCTTC-3′ and R,
5′-GTCACTGCGCTCCAGTAGAA-3′; TNF-α F, 5′-ATGAGCACTGAAAGCATGATCCGG-3′
and R, 5′-GCAATGATCCCAAAGTAGACCTGCCC-3′; IL-6 F,
5′-ATGAACTCCTTCTCCACAAGCGC-3′ and R, 5′-GAAGAGCCCTCAGGCTGGACTG-3′;
IL-1β F, 5′-ATGGCAGAAGTACCTGAGCTCGC-3′ and R,
5′-ACACAAATTGCATGGTGAAGTCAGTT-3′; U6 F, 5′-CTCGCTTCGGCAGCACA-3′ and
R 5′-AACGCTTCACGAATTTGCGT-3′; GAPDH F, 5′-CCATGGAGAAGGCTGGGG-3′ and
R 5′-CAAAGTTGTCATGGATGACC-3′. U6 and GAPDH were used as internal
references for miRNAs and mRNAs, respectively. The relative
expression level was calculated using the 2−ΔΔCq method
(23). All experiments were
repeated in triplicate.
Dual-luciferase reporter assay
The binding region for SOCS3 3′-untranslated region
(UTR) and miR-409-3p was predicted using TargetScan 7.2 software
(http://www.targetscan.org). The
wild-type (WT) or mutant (MUT) 3′-UTR of SOCS3 was sub-cloned into
a pGL4.10 luciferase reporter vector (Promega Co.), followed by
co-transfection with either the vectors, miR-409-3p mimics,
inhibitors or the respective NCs using Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h of
transfection, luciferase assays were conducted using a Luciferase
Assay System (Promega Co.) and the relative luciferase activity was
calculated by normalization to Renilla luciferase
activity.
Western blotting
Briefly, proteins were extracted using RIPA buffer
(Vazyme Biotech Co., Ltd.) from BEAS-2B cells. The protein amount
was determined using a Bio-Rad protein assay reagent (Bio-Rad
Laboratories, Inc.). A total of 50 µg protein sample was subjected
to 10% SDS-PAGE. Samples were then transferred onto PVDF membranes.
After blocking with 5% non-fat milk for 1 h at room temperature,
membranes were incubated at 4°C overnight with the following
primary antibodies: Anti-SOCS3 (cat. no. ab16030; 1:1,000; Abcam),
anti-JAK1 (cat. no. ab133666; 1:1,000; Abcam) anti-phosphorylated
(p-)JAK1 (cat. no. ab138005; 1:1,000; Abcam), anti-STAT3 (cat. no.
ab119352; 1:5,000; Abcam), anti-p-STAT3 (cat. no. ab76315; 1:2,000;
Abcam), anti-cleaved caspase-3 (cat. no. ab2302; 1:500; Abcam),
anti-Bax (cat. no. ab32503; 1:1,000; Abcam), anti-Bcl-2 (cat. no.
ab32124; 1:1,000; Abcam) and anti-GAPDH (cat. no. ab8245; 1:500;
Abcam). The samples were then incubated with an HRP-conjugated goat
anti-rabbit IgG secondary antibody (cat. no. ab205718; 1:1,000;
Abcam) or an HRP-conjugated goat anti-mouse IgG H&L secondary
antibody (cat. no. ab205719; 1:1,000; Abcam) at 37°C for 45 min.
The blots were scanned and images were captured using the Super
Signal West Pico Chemiluminescent Substrate kit (Pierce; Thermo
Fisher Scientific, Inc.). Image-Pro Plus software 6.0 (Media
Cybernetics, Inc.) was used to calculate the relative protein
expression.
Statistical analysis
At least three independent experiments were
performed for all procedures. All statistical analyses were
performed using SPSS v22.0 (IBM Corp.). Comparisons among ≥3 groups
were performed using one-way ANOVA followed by Tukey's post-hoc
test. Comparison between two groups was made by t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
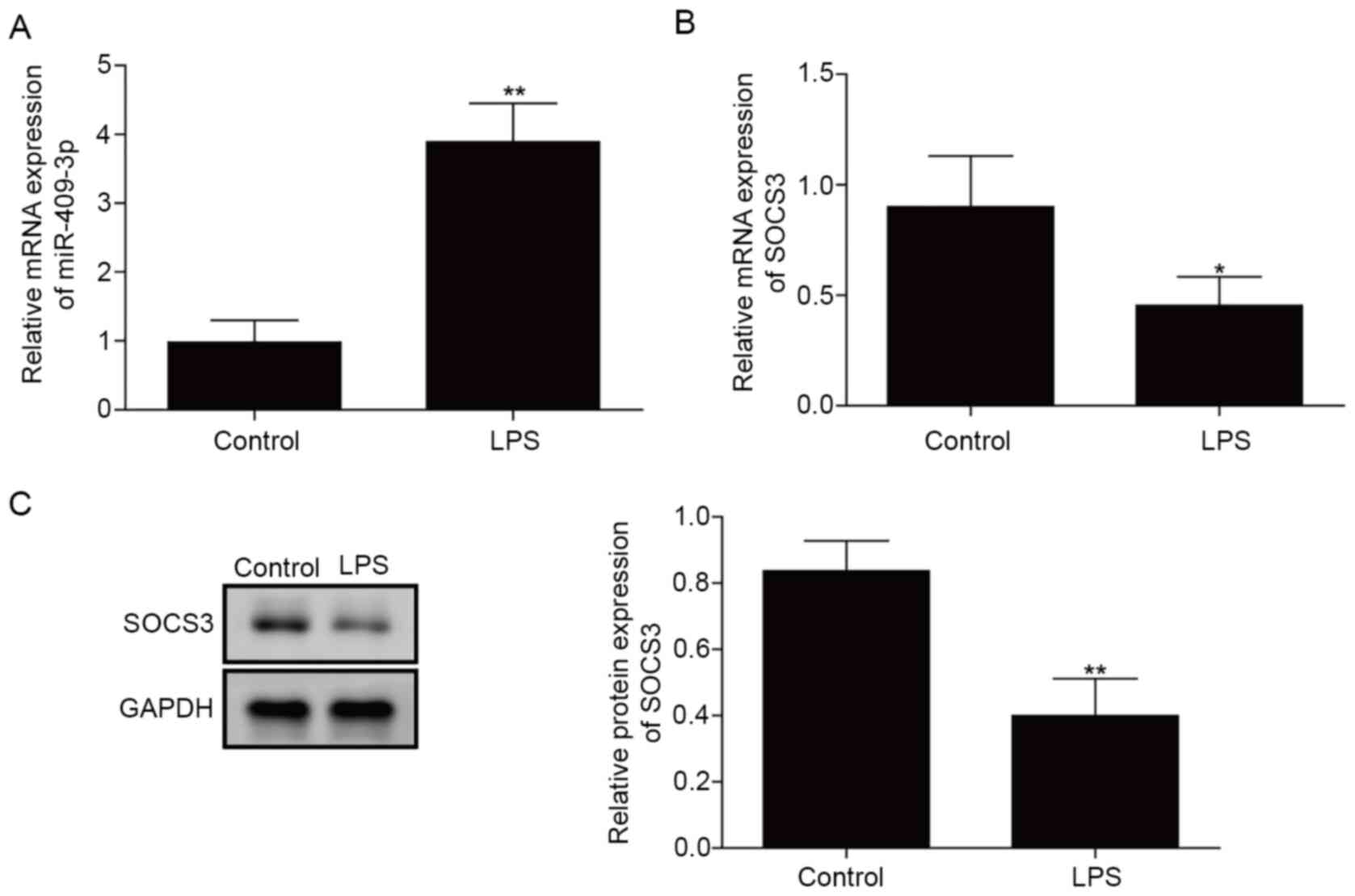
miR-409-3p expression is increased and
SOCS3 expression is downregulated in LPS-induced BEAS-2B cells
First, the expression levels of miR-409-3p and SOCS3
in the in vitro bronchopneumonia model were measured. As
demonstrated in Fig. 1A, miR-409-3p
expression was significantly upregulated in LPS-treated BEAS-2B
cells compared with in control cells (P<0.01). By contrast, mRNA
and protein levels of SOCS3 were significantly downregulated in
LPS-induced cells compared with in control cells (P<0.05;
Fig. 1B and C). These results
indicated that miR-409-3p and SOCS3 were abnormally expressed in
LPS-induced BEAS-2B cells.
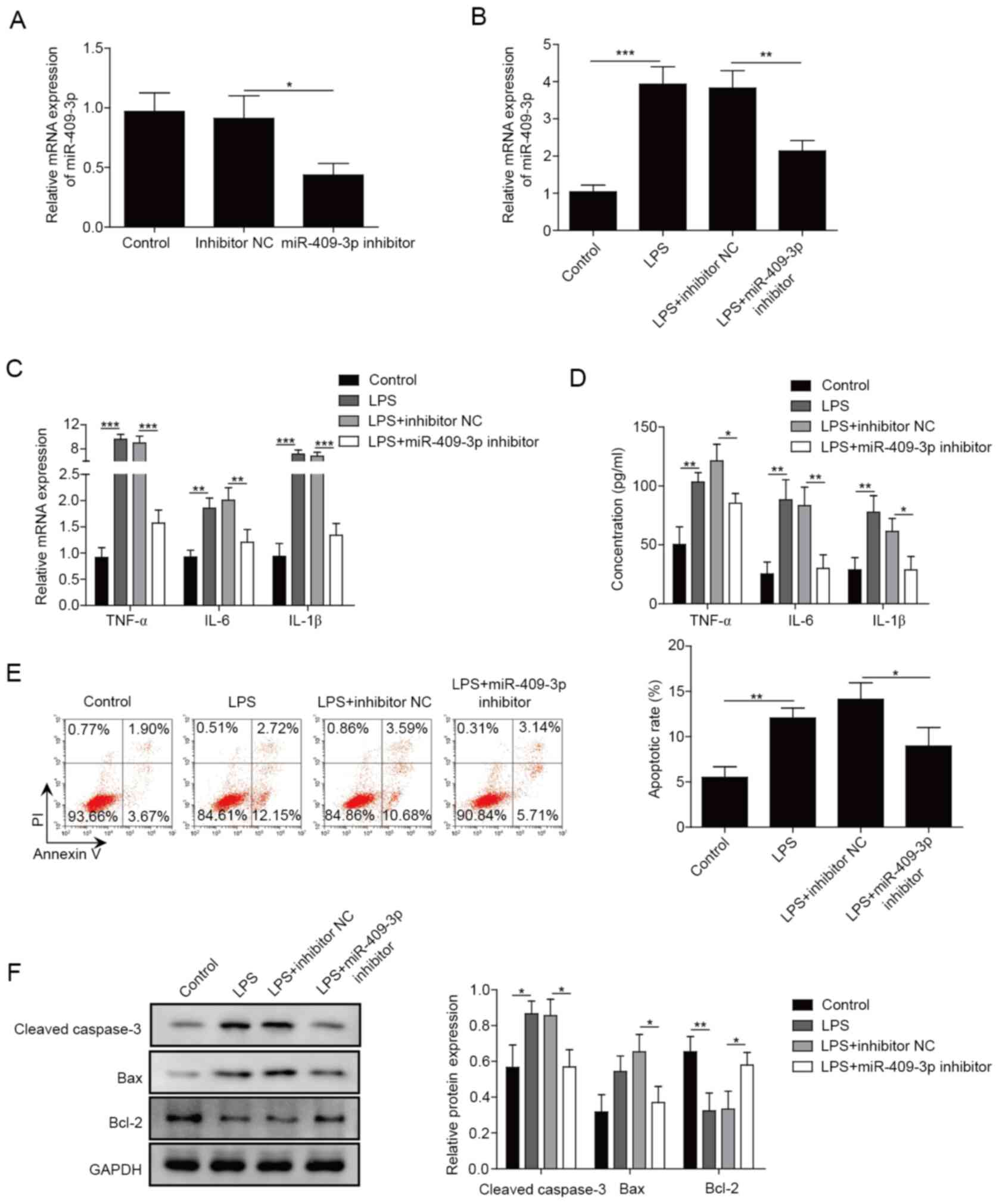
Inhibition of miR-409-3p suppresses
the LPS-induced inflammatory response in BEAS-2B cells
To further investigate the role of miR-409-3p in
LPS-induced inflammation, miR-409-3p inhibitor was used to
knockdown miR-409-3p expression, and the results demonstrated that
miR-409-3p expression was successfully suppressed in BEAS-2B cells
using the inhibitor (P<0.05; Fig.
2A). Additionally, the transfection of miR-409-3p inhibitor
resulted in inhibition of LPS-stimulated miR-409-3p expression in
BEAS-2B cells (P<0.01; Fig. 2B).
mRNA and protein expression levels of TNF-α, IL-6 and IL-1β were
significantly upregulated in LPS-induced BEAS-2B cells, and
inhibiting miR-409-3p significantly decreased these effects
(P<0.05; Fig. 2C and D).
Apoptosis analysis identified that LPS treatment significantly
enhanced the apoptosis rate; however, miR-409-3p inhibition was
able to rescue the LPS-induced apoptosis (P<0.05; Fig. 2E). Similar results were identified
for apoptosis-related proteins. The protein levels of cleaved
caspase-3 and Bax were markedly enhanced, while Bcl-2 expression
was significantly decreased in LPS-induced BEAS-2B cells, and these
effects were significantly reversed by inhibition of miR-409-3p
(P<0.05; Fig. 2F). The present
results suggested that knockdown of miR-409-3p inhibited
LPS-induced inflammation in BEAS-2B cells.
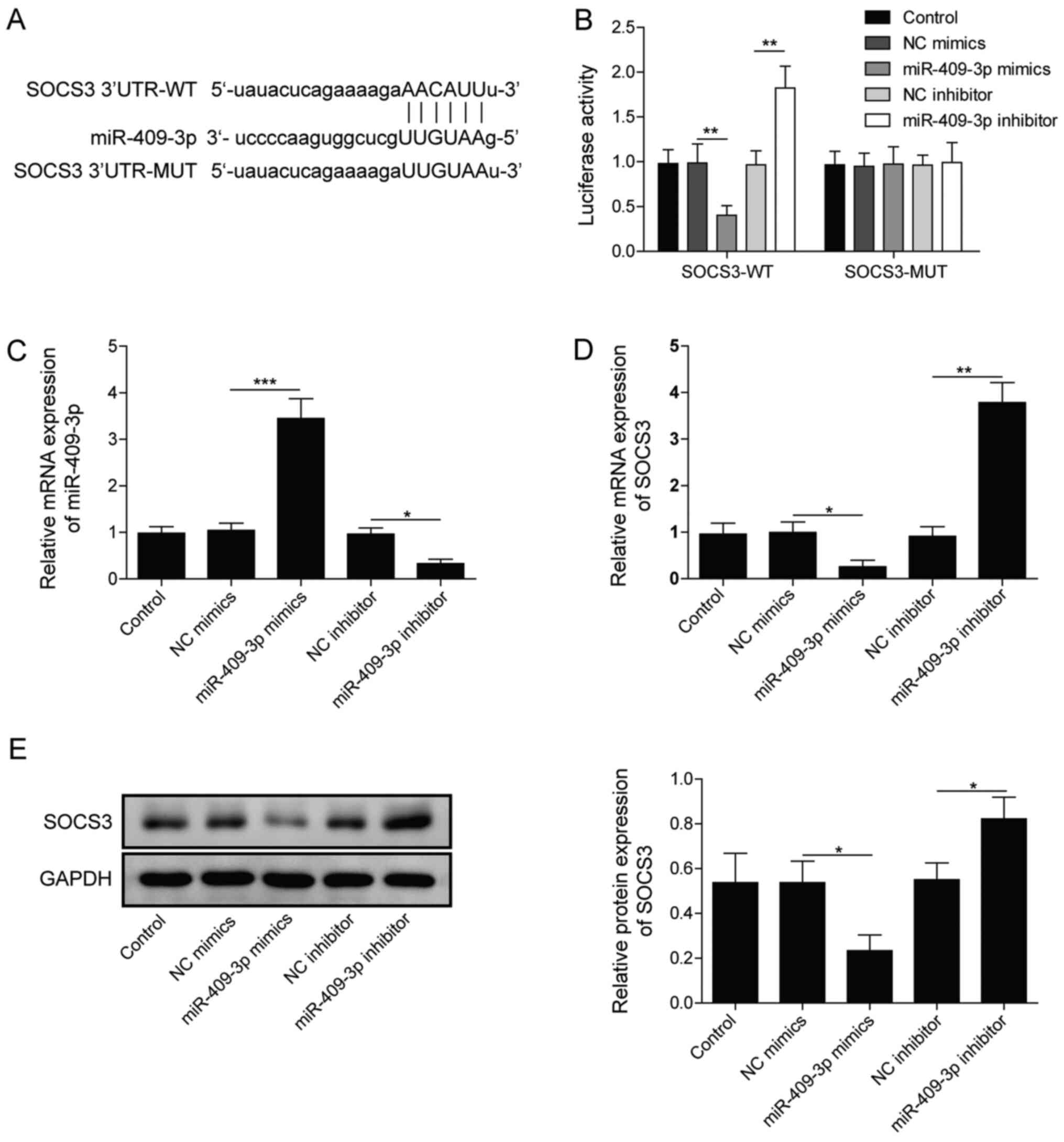
miR-409-3p directly targets and
negatively regulates SOCS3
The interaction between miR-409-3p and SOCS3 was
further explored. First, the binding between miR-409-3p and SOCS3
was predicted using bioinformatics (Fig. 3A). As demonstrated in Fig. 3B, miR-409-3p overexpression
significantly inhibited the relative luciferase activity, while
knockdown of miR-409-3p markedly enhanced the relative luciferase
activity in SOCS3-WT (P<0.01). However, no significant
difference was identified in SOCS3-MUT (Fig. 3B). The expression of miR-409-3p was
markedly downregulated by transfection of miR-409-3p inhibitor and
significantly upregulated following transfection with miR-409-3p
mimics (Fig. 3C). Meanwhile,
overexpressing miR-409-3p significantly inhibited mRNA and protein
levels of SOCS3 in BEAS-2B cells, while miR-409-3p inhibition led
to the opposite results (Fig. 3D and
E). The present results indicated that miR-409-3p directly
targeted SOCS3 and negatively regulated its expression.
miR-409-3p regulates LPS-induced
inflammation through SOCS3 in BEAS-2B cells
To further clarify the mechanisms for miR-409-3p in
LPS-induced BEAS-2B cells, cells were transfected with miR-409-3p
inhibitor, si-SOCS3 or miR-409-3p inhibitor and si-SOCS3. The
transfection efficiency of si-SOCS3 was confirmed by both RT-qPCR
and western blotting (Fig. 4A and
B). The results demonstrated that miR-409-3p expression was
significantly decreased by miR-409-3p inhibitor (P<0.05), but
was not affected by inhibition of SOCS3 using si-SOCS3 (Fig. 4C). Co-transfection of miR-409-3p
inhibitor and si-SOCS3 showed similar effects to mono-transfection
of miR-409-3p inhibitor. By contrast, inhibition of miR-409-3p
significantly enhanced SOCS3 expression (P<0.01; Fig. 4D). SOCS3 expression was
significantly decreased by transfection with si-SOCS3 and was then
reversed by miR-409-3p inhibitor (P<0.01; Fig. 4D). For TNF-α, IL-6 and IL-1β
expression, the LPS-induced upregulation of the inflammatory
factors was significantly decreased by suppression of miR-409-3p
compared with inhibitor NC group and was significantly increased by
si-SOCS3 compared with si-NC group. SOCS3 inhibition by
co-transfection of si-SOCS3 reversed the effects of miR-409-3p
inhibitor compared with cells only transfected with miR-409-3p
inhibitor (all P<0.05; Fig. 4E and
F). Inhibition of miR-409-3p suppressed the LPS-induced cell
apoptosis rate, which was increased by SOCS3-knockdown, and this
effect was reversed by inhibition of both miR-409-3p and SOCS3 in
LPS-induced BEAS-2B cells (P<0.05; Fig. 4G). Similarly, the LPS-induced
upregulation of cleaved caspase-3 and Bax was markedly decreased by
miR-409-3p inhibitor and the downregulation of Bcl-2 was notably
increased by miR-409-3p inhibitor, while si-SOCS3 transfection
resulted in the opposite effects (P<0.05; Fig. 4H). Co-transfection of si-SOCS3
markedly reversed the effects of transfection of miR-409-3p
inhibitor on apoptosis-related proteins. Additionally, protein
levels of p-JAK1 and p-STAT3 were significantly upregulated by LPS
treatment, which were then downregulated by miR-409-3p-knockdown
and significantly augmented by SOCS3-knockdown (P<0.05; Fig. 4I). Notably, the effects of
miR-409-3p inhibition on p-JAK1 and p-STAT3 levels were
significantly reversed by si-SOCS3 (P<0.05; Fig. 4I). The aforementioned results
suggested that miR-409-3p regulated LPS-induced inflammation by
regulating SOCS3 and that JAK1/STAT3 signaling may be involved.
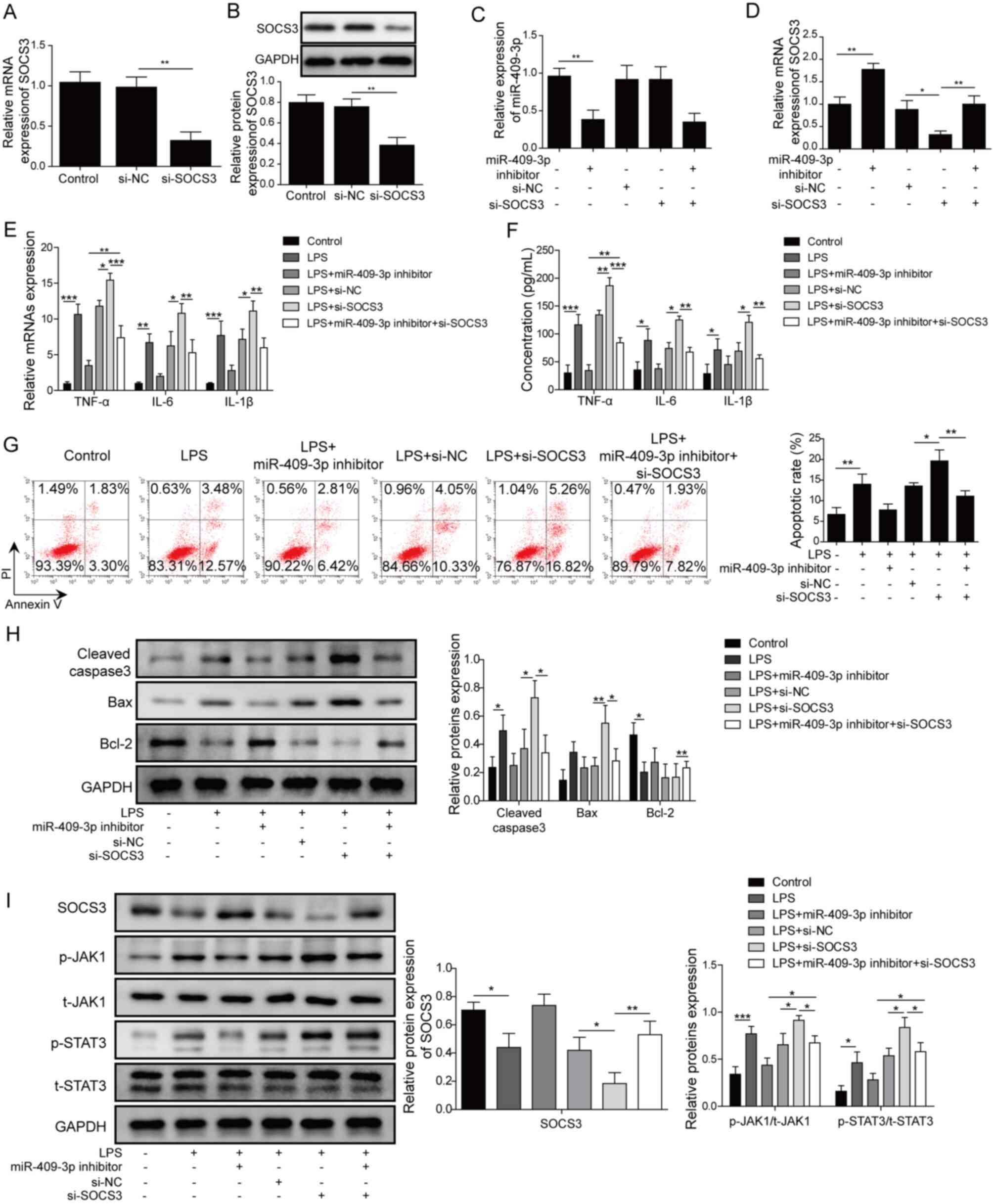 | Figure 4.miR-409-3p regulates LPS-induced
inflammation through SOCS3 in BEAS-2B cells. (A) mRNA and (B)
protein expression levels of SOCS3 in cells transfected with
si-SOCS3, si-NC, or control cells. (C) miR-409-3p and (D) SOCS3
expression in different groups of LPS-treated BEAS-2B cells was
determined by RT-qPCR. (E) mRNA expression levels of inflammatory
factors TNF-α, IL-6 and IL-1β were measured by RT-qPCR. (F) Protein
levels of TNF-α, IL-6 and IL-1β were determined using ELISA. (G)
Apoptosis was measured by flow cytometer analysis. (H)
Apoptosis-related proteins Bax, cleaved caspase-3 and Bcl-2 were
determined using western blotting. (I) Protein levels of SOCS3 and
JAK1/STAT3 signaling-related proteins in different groups of cells
were determined by western blotting. *P<0.05, **P<0.01,
***P<0.001. miR, microRNA; LPS, lipopolysaccharide; SOCS,
suppressor of cytokine signaling; RT-qPCR, reverse
transcription-quantitative PCR; p-, phosphorylated; t-, total; si,
small interfering; NC, negative control. |
miR-409-3p regulates inflammation
through SOCS3/JAK1/STAT3 in LPS-induced BEAS-2B cells
Finally, it was attempted to confirm the effect of
JAK1/STAT3 signaling on miR-409-3p/SOCS3 axis-regulated LPS-induced
inflammation. Inhibition of SOCS3 significantly increased p-JAK1
and p-STAT31 protein levels, which were then significantly
suppressed by treatment with Tofacitinib, a JAK/STAT3 inhibitor
(P<0.05; Fig. 5A). Protein and
mRNA levels of inflammatory factors TNF-α, IL-6 and IL-1β were
significantly enhanced by transfection with si-SOCS3; however,
treatment with Tofacitinib significantly decreased these effects
(P<0.01; Fig. 5B and C).
Additionally, the apoptosis rate was significantly increased by
si-SOCS3 in LPS-induced BEAS-2B cells (P<0.05), while
Tofacitinib decreased the apoptosis rates (P<0.01; Fig. 5D). The protein expression levels of
Bax and cleaved caspase-3 were significantly elevated by silencing
of SOCS3, while Bcl-2 expression was significantly decreased, and
these effects were reversed by Tofacitinib (P<0.01; Fig. 5E). These results indicated that
miR-409-3p regulated LPS-induced inflammation through
SOCS3/JAK1/STAT3 signaling in BEAS-2B cells.
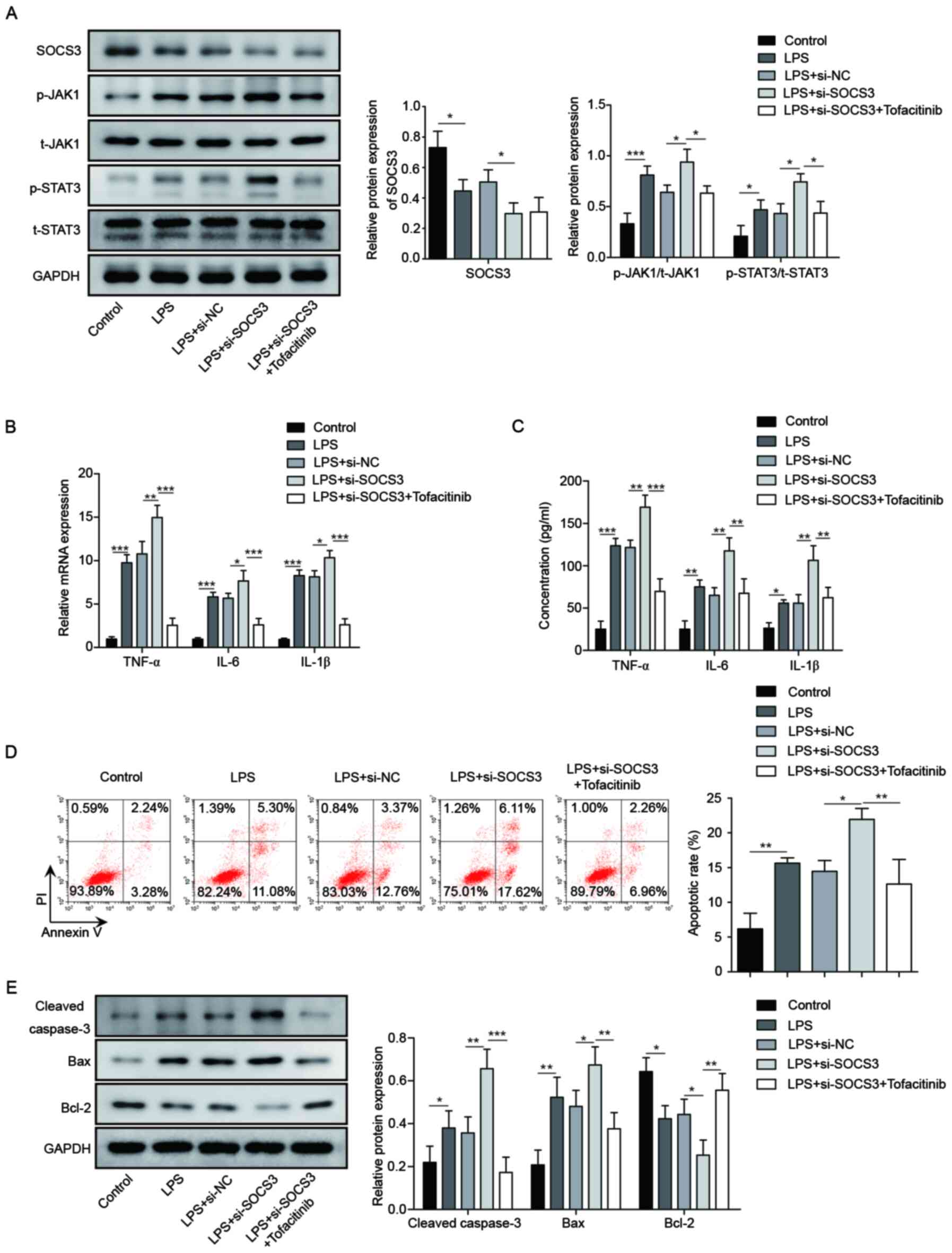 | Figure 5.miR-409-3p regulates inflammation
through SOCS3/JAK1/STAT3 in LPS-induced BEAS-2B cells. (A) Protein
levels of SOCS3 and JAK1/STAT3 signaling-related proteins in
different groups of LPS-treated BEAS-2B cells were determined by
western blotting. (B) mRNA expression levels of inflammatory
factors TNF-α, IL-6 and IL-1β were measured by RT-qPCR. (C) Protein
levels of TNF-α, IL-6 and IL-1β were determined using ELISA. (D)
Apoptosis was measured by flow cytometer analysis. (E)
Apoptosis-related proteins Bax, cleaved caspase-3 and Bcl-2 were
determined using western blotting. *P<0.05, **P<0.01,
***P<0.001. miR, microRNA; SOCS, suppressor of cytokine
signaling; LPS, lipopolysaccharide; p-, phosphorylated; t-, total;
si, small interfering; NC, negative control. |
Discussion
Despite previous studies on bronchopneumonia for
both diagnosis and treatment (6,7), the
molecular mechanisms for bronchopneumonia remain to be elucidated.
In recent years, the role of miRNAs in the development of
inflammatory responses, as wellas pneumonia, has been noted in
several studies (8–12). However, to the best of our
knowledge, no studies have reported the role of miR-409-3p in
bronchopneumonia. The present study identified that in LPS-induced
inflammation in BEAS-2B cells, miR-409-3p targeted and suppressed
SOCS3 expression and further influenced the inflammatory response
by regulating JAK1/STAT3 signaling.
It has been reported that miR-409-3p serves
important roles in a number of diseases, such as glioblastoma and
osteosarcoma (24,25). In addition, miR-409-3p in
inflammation-related diseases, as well as in pneumonia, has been
identified in several studies. For example, Dai et al
(26) noted that miR-409 expression
is upregulated in idiopathic thrombocytopenic purpura.
Additionally, miR-409 expression is upregulated in tissue
plasminogen activator-treated K562 cells and is considered as a
biomarker for megakaryocytopoiesis, which serves a crucial role in
inflammatory activation (27).
Similarly, the present study demonstrated that miR-409-3p
expression was elevated in LPS-induced BEAS-2B cells. Functionally,
it was observed that miR-409-3p inhibition improved LPS-induced
inflammation and stimulated apoptosis, suggesting that miR-409-3p
served anti-inflammatory functions in LPS-induced BEAS-2B
cells.
SOCS3 is considered an anti-inflammation factor. In
the present study, the data demonstrated that SOCS3 expression was
downregulated in LPS-induced BEAS-2B cells and inhibition of SOCS3
promoted LPS-induced inflammation. Other studies are consistent
with these findings. For instance, Kim et al (28) demonstrated that the activation of
SOCS3 leads to inactivation of the IL-6 signaling pathway. Dai
et al (29) demonstrated
that Kallikrein-binding protein suppressed LPS-induced inflammation
through upregulation of SOCS3. Mechanically, the present study
confirmed that SOCS3 was a direct target of miR-409-3p, which
exerted its function of anti-inflammation through inhibition of
SOCS3.
A number of studies have demonstrated that the
activation of JAK1/STAT3 signaling is a key process in inflammatory
response. Shien et al (30)
showed that the activation of the proinflammatory cytokine pathway
leads to activation of JAK1/STAT3. Aloin can inhibit LPS-induced
inflammation by suppressing JAK1/STAT1/3 activation (31). The association between SOCS3 and
JAK1/STAT3 signaling has also been reported. Andoh et al
(32) identified that
JAK1/STAT3/SOCS3 signaling is activated in inflammatory bowel
disease. Additionally, JAK/STAT/SOCS3 signaling is activated in
colon and rectal cancer (33).
Similarly, the present study verified that JAK1/STAT3 signaling was
activated in LPS-induced inflammation and that SOCS3 could regulate
the inflammatory response by regulating JAK1/STAT3 signaling in
LPS-induced BEAS-2B cells.
In conclusion, the present study used an in vitro
model to investigate the role of miR-409-3p in LPS-induced BEAS-2B
cells and revealed that inhibition of miR-409-3p improved
LPS-induced inflammation through SOCS3/JAK1/STAT3 signaling. The
present study may provide insight into the molecular mechanisms for
the role of miR-409-3p in LPS-induced inflammation, as well as in
the development of bronchopneumonia.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LH conceived the study and methodology, performed
bioinformatic analysis and wrote the original draft of the paper.
LT collected, analyzed and interpreted the data, supervised the
study, reviewed the manuscript, and was involved in drafting the
manuscript and revising it critically for important intellectual
content. Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LPS
|
lipopolysaccharide
|
|
miRNA/miR
|
microRNA
|
|
NC
|
negative controls
|
|
WT
|
wild-type
|
|
MUT
|
mutant
|
References
|
1
|
McAllister DA, Liu L, Shi T, Chu Y, Reed
C, Burrows J, Adeloye D, Rudan I, Black RE, Campbell H, et al:
Global, regional, and national estimates of pneumonia morbidity and
mortality in children younger than 5 years between 2000 and 2015: A
systematic analysis. Lancet Glob Health. 7:e47–e57. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu C, Xiang Q and Zhang H: Xianyu
decoction attenuates the inflammatory response of human lung
bronchial epithelial cell. Biomed Pharmacother. 102:1092–1098.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oumei H, Xuefeng W, Jianping L, Kunling S,
Rong M, Zhenze C, Li D, Huimin Y, Lining W, Zhaolan L, et al:
Etiology of community-acquired pneumonia in 1500 hospitalized
children. J Med Virol. 90:421–428. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garenne M, Ronsmans C and Campbell H: The
magnitude of mortality from acute respiratory infection in children
under 5 years in developing countries. World Health Stat Q.
45:180–191. 1992.PubMed/NCBI
|
|
5
|
Liu Z, Yu H and Guo Q: MicroRNA 20a
promotes inflammation via the nuclear factor κB signaling pathway
in pediatric pneumonia. Mol Med Rep. 17:612–617. 2018.PubMed/NCBI
|
|
6
|
Zec SL, Selmanovic K, Andrijic NL, Kadic
A, Zecevic L and Zunic L: Evaluation of drug treatment of
bronchopneumonia at the pediatric clinic in Sarajevo. Med Arh.
70:177–181. 2016. View Article : Google Scholar
|
|
7
|
Catry B, Govaere JLJ, Devriese L, Laevens
H, Haesebrouck F and Kruif AD: Bovine enzootic bronchopneumonia:
Prevalence of pathogens and its antimicrobial susceptibility.
Vlaams Diergeneeskd Tijdschr. 71:348–354. 2002.
|
|
8
|
Abd-El-Fattah AA, Sadik NAH, Shaker OG and
Aboulftouh ML: Differential microRNAs expression in serum of
patients with lung cancer, pulmonary tuberculosis, and pneumonia.
Cell Biochem Biophys. 67:875–884. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Foster PS, Plank M, Collison A, Tay HL,
Kaiko GE, Li J, Johnston SL, Hansbro PM, Kumar RK, Yang M, et al:
The emerging role of microRNAs in regulating immune and
inflammatory responses in the lung. Immunol Rev. 253:198–215. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Neudecker V, Yuan X, Bowser JL and
Eltzschig HK: MicroRNAs in mucosal inflammation. J Mol Med (Berl).
95 (Suppl 3):935–949. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang F, Zhang J, Yang D, Zhang Y, Huang
J, Yuan Y, Li X and Lu G: MicroRNA expression profile of whole
blood is altered in adenovirus-infected pneumonia children.
Mediators Inflamm. 2018:23206402018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gomez JC, Dang H, Kanke M, Hagan RS, Mock
JR, Kelada SNP, Sethupathy P and Doerschuk CM: Predicted effects of
observed changes in the mRNA and microRNA transcriptome of lung
neutrophils during S. pneumoniae pneumonia in mice. Sci Rep.
7:112582017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo J and Cheng Y: MicroRNA-1247 inhibits
lipopolysaccharides-induced acute pneumonia in A549 cells via
targeting CC chemokine ligand 16. Biomed Pharmacother. 104:60–68.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan J, Ye Z, Zhang N, Lou T and Cao Z:
MicroRNA 217 regulates interstitial pneumonia via IL 6. Biotechnol
Biotechnol Equip (7). 1–7. 2018.
|
|
15
|
Fei S, Cao L and Pan L: MicroRNA 3941
targets IGF2 to control LPS induced acute pneumonia in A549 cells.
Mol Med Rep. 17:4019–4026. 2018.PubMed/NCBI
|
|
16
|
Huang F, Zhang J, Yang D, Zhang Y, Huang
J, Yuan Y, Li X and Lu G: MicroRNA expression profile of whole
blood is altered in adenovirus infected pneumonia children.
Mediators Inflamm. 2018:23206402018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chi X, Ding B, Zhang L, Zhang J, Wang J
and Zhang W: lncRNA GAS5 promotes M1 macrophage polarization via
miR-455-5p/SOCS3 pathway in childhood pneumonia. J Cell Physiol.
234:13242–13251. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dhar K, Rakesh K, Pankajakshan D and
Agrawal DK: SOCS3 promotor hypermethylation and STAT3-NF-κB
interaction downregulate SOCS3 expression in human coronary artery
smooth muscle cells. Am J Physiol Heart Circ Physiol.
304:H776–H785. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mishra V, Baranwal V, Mishra RK, Sharma S,
Paul B and Pandey AC: Titanium dioxide nanoparticles augment
allergic airway inflammation and Socs3 expression via NF-κB pathway
in murine model of asthma. Biomaterials. 92:90–102. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X, Zhou F, Yang Y, Wang W, Niu L, Zuo
D, Li X, Hua H, Zhang B, Kou Y, et al: miR-409-3p and miR-1896
co-operatively participate in IL-17-induced inflammatory cytokine
production in astrocytes and pathogenesis of EAE mice via targeting
SOCS3/STAT3 signaling. Glia. 67:101–112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiuxia L and Jie M: Luteolin alleviates
LPS induced bronchopneumonia injury in vitro and in vivo by down
regulating microRNA 132 expression. Biomed Pharmacother.
106:1641–1649. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kjelgaardpetersen CF, Bayjensen AC,
Karsdal MA, Hägglund P and Thudium CS: Anti inflammatory inhibitors
targeting Jak and Ikk have an anabolic effect on type II collagen
turnover ex vivo. Annals of the Rheumatic Diseases. 75 (Suppl
2):185–186. 2016.
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khalil S, Fabbri E, Santangelo A, Bezzerri
V, Cantù C, Di Gennaro G, Finotti A, Ghimenton C, Eccher A,
Dechecchi M, et al: miRNA array screening reveals cooperative
MGMT-regulation between miR-181d-5p and miR-409-3p in glioblastoma.
Oncotarget. 7:28195–28206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Hou W, Jia J, Zhao Y and Zhao B:
miR-409-3p regulates cell proliferation and tumor growth by
targeting E74-like factor 2 in osteosarcoma. FEBS Open Bio.
7:348–357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai Y, Huang YS, Tang M, Lv TY, Hu CX, Tan
YH, Xu ZM and Yin YB: Microarray analysis of microRNA expression in
peripheral blood cells of systemic lupus erythematosus patients.
Lupus. 16:939–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Navarro F, Gutman D, Meire E, Cáceres M,
Rigoutsos I, Bentwich Z and Lieberman J: miR-34a contributes to
megakaryocytic differentiation of K562 cells independently of p53.
Blood. 114:2181–2192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim G, Ouzounova M, Quraishi AA, Davis A,
Tawakkol N, Clouthier SG, Malik F, Paulson AK, D'Angelo RC, Korkaya
S, et al: SOCS3-mediated regulation of inflammatory cytokines in
PTEN and p53 inactivated triple negative breast cancer model.
Oncogene. 34:671–680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dai Z, Lu L, Yang Z, Mao Y, Lu J, Li C, Qi
W, Chen Y, Yao Y, Li L, et al: Kallikrein-binding protein inhibits
LPS-induced TNF-α by upregulating SOCS3 expression. J Cell Biochem.
114:1020–1028. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shien K, Papadimitrakopoulou VA, Ruder D,
Behrens C, Shen L, Kalhor N, et al: JAK1/STAT3 activation through a
proinflammatory cytokine pathway leads to resistance to molecularly
targeted therapy in non small cell lung cancer. Mol Cancer Ther.
16:2234–2245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma Y, Tang T, Sheng L, Wang Z, Tao H,
Zhang Q, Zhang Y and Qi Z: Aloin suppresses lipopolysaccharide
induced inflammation by inhibiting JAK1?STAT1/3 activation and ROS
production in RAW264.7 cells. Int J Mol Med. 42:1925–1934.
2018.PubMed/NCBI
|
|
32
|
Andoh A, Shioya M, Nishida A, Bamba S,
Tsujikawa T, Kim-Mitsuyama S and Fujiyama Y: Expression of IL-24,
an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in
inflammatory bowel disease. J Immunol. 183:687–695. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Slattery ML, Lundgreen A, Kadlubar SA,
Bondurant KL and Wolff RK: JAK/STAT/SOCS-signaling pathway and
colon and rectal cancer. Mol Carcinog. 52:155–166. 2013. View Article : Google Scholar : PubMed/NCBI
|