Introduction
Autosomal dominant polycystic kidney disease
(ADPKD), one of the most common diseases of the kidney, has a high
incidence rate of 1/400 to 1/1,000 individuals (1), and is characterized by several
unregulated cysts of different sizes in the kidney, and often
progresses to kidney failure (2).
The disease is primarily caused by mutations in polycystin 1
transient receptor potential channel interacting (Pkd1; 85%)
and Pkd2 (15%), which encode the proteins polycystin-1 (PC1)
and polycystin-2 (PC2), respectively (3,4).
Previous studies have reported that mutant PC1 and PC2 decrease
intracellular Ca2+ levels and increase intracellular
cAMP levels (5–7). Increased intracellular cAMP stimulates
protein kinase A, which in turn promotes abnormal proliferation of
cyst epithelial cells and excessive secretion of cyst fluid
(6,8). There are multiple signaling pathways
associated with abnormal proliferation of cyst epithelial cells,
such as the Ras/MAPK pathway, mTOR pathway and Wnt pathway
(9–11). Signaling pathways associated with
excessive secretion of cyst fluid include those that increase the
activities of cystic fibrosis transmembrane conductance regulator
(CFTR), Na+-K+-ATPase and aquaporin 2
(12). Other signaling factors
include TGF-β/Smad2/3 (13) and
integrin-linked kinase (14), and
are associated with the pathological progression of fibrosis
throughout the course of ADPKD.
Except for lifelong hemodialysis or kidney
transplantation, treatments for ADPKD include decreasing
intracellular cAMP levels, prevention of abnormal cells
proliferation and inhibition of cyst fluid excessive secretion
(15). Somatostatin, and its
analogue octreotide, inhibit cyst development by decreasing
intracellular cAMP levels in the kidney and liver in patients with
ADPKD (16). Our previous studies
revealed that curcumin (Cur) (15)
and ginkgolide B (GB) (17) reduced
renal cyst cell proliferation by downregulating the activity of the
Ras/ERK1/2 signaling pathway. Small-molecule CFTR inhibitors
decrease cyst fluid secretion in PKD by inhibiting the function of
CFTR, which in turn stimulates chloride secretion, and thus cystic
fluid secretion (12). Other
treatments that decrease cyst growth in ADPKD include tolvaptan
(18,19), src inhibitors (20), Wnt inhibitors (21), mTOR inhibitors (22), pioglitazone (23,24),
triptolide (25), Ganoderma
triterpenes (26) and Quercetin
(27). Tolvaptan has been approved
for the treatment of ADPKD in Europe and other countries throughout
the world (28). However, the
majority of experimental treatments for ADPKD require further study
before they can be approved clinically.
Cur is a natural compound isolated from the
Traditional Chinese Medicine Curcuma longa L (29). Several studies have shown that Cur
exhibits notable anticancer, anti-inflammatory (30) and anti-oxidant effects, as well as
other beneficial properties (31,32).
Our previous study revealed that Cur inhibited cyst development
in vitro (15). GB is a
natural product isolated from the Chinese herbal medicine Ginkgo
biloba L (33), and has several
beneficial biological effects, including antiplatelet,
anti-inflammatory, antioxidant and neuroprotective activities
(34,35). The inhibitory effect of GB on cyst
formation was also reported in our previous study, both in in
vitro and in vivo models (17). Considering these inhibitory effects
of Cur and GB on cyst formation, it was hypothesized that Cur and
GB may exhibit a synergistic effect on the inhibition of
cystogenesis, and this synergistic effect may result from the
regulation of multiple signaling pathways. To assess this
hypothesis, in the present study, an in vitro Madin-Darby
canine kidney (MDCK) cyst model and an in vivo
kidney-specific Pkd1 knockout mouse model were used to
observe the effects of Cur combined with GB.
Materials and methods
Materials
Cur (Sigma-Aldrich; Merck KGaA; cat. no. C1386), GB
(Sigma-Aldrich; Merck KGaA; cat. no. G6910) and Forskolin (FSK;
Sigma-Aldrich; Merck KGaA; cat. no. F6886) were each dissolved in
100% DMSO to prepare a 100 mM stock solution and were stored
at-20°C.
Anti-phospho-(p-)EGFR (cat. no. sc-57542), anti-EGFR
(cat. no. sc-373746), anti-p-human epidermal growth factor receptor
2 (anti-p-Cerb-B2; cat. no. sc-81507), anti-Cerb-B2 (cat. no.
sc-33684), anti-H-Ras (cat. no. sc-35), anti-B-Raf (cat. no.
sc-5284), anti-Raf-1 (cat. no. sc-7267), anti-p-MEK-1/2 (cat. no.
sc-81503), anti-MEK-1/2 (cat. no. sc-81504), anti-p-ERK (cat. no.
sc-7383), anti-ERK-1/2 (cat. no. sc-514302), anti-p-JNK (cat. no.
sc-6254), anti-JNK (cat. no. sc-7345), anti-p-activator of S phase
kinase (ASK; cat. no. sc-166967), anti-ASK1 (cat. no. sc-5294),
anti-p-p38α (cat. no. sc-7973), anti-p38α/β (cat. no. sc-7972) and
anti-Actin (cat. no. sc-8432) were purchased from Santa Cruz
Biotechnology, Inc. Anti-p-PI3K (cat. no. 4228), anti-PI3K (cat.
no. 4292), anti-p-AKT (cat. no. 4060), anti-AKT (cat. no. 4691),
anti-p-mTOR (cat. no. 2971), anti-mTOR (cat. no. 2972),
anti-p-eukaryotic translation initiation factor 4E binding protein
1 (anti-p-4E-BP1; cat. no. 2855), anti-4E-BP1 (cat. no. 9452),
anti-p-p70S6 kinase (anti-p-p70S6k; cat. no. 9208), anti-p70S6k
(cat. no. 9202), anti-p-mitogen-activated protein kinase kinase
kinase 7 (anti-p-MAP3K7; cat. no. 9339), anti-MAP3K7 (cat. no.
4505), anti-p-mitogen-activated protein kinase kinase 3/6
(anti-p-MKK3/6; cat. no. 9231), anti-MKK3 (cat. no. 5674),
anti-p-MKK4 (cat. no. 9155) and anti-MKK4 (cat. no. 9152) were
purchased from Cell Signaling Technology, Inc. Horseradish
peroxidase (HRP) conjugated-goat anti-mouse IgG (H+L) (cat. no.
AP308P) and unconjugated goat anti-rabbit IgG (cat. no. AP132) were
purchased from Sigma-Aldrich (Merck KGaA).
MDCK cyst model
Type I MDCK cells (American Type Culture Collection;
cat. no. CCL-34) were cultured at 37°C in a humidified incubator
with 5% CO2 and 95% air in a 1:1 mixture of DMEM
(Mediatech, Inc.; cat. no. 07119003) and Ham's F-12 nutrient medium
(Mediatech, Inc.; cat. no. 30218008) supplemented with 10% FBS
(Hyclone; Cytiva), 100 U/ml penicillin and 100 µg/ml streptomycin.
MDCK cells (~400) were suspended in 0.4 ml ice-cold Minimum
Essential Medium (Mediatech, Inc.; cat. no. 12619015) containing
2.9 mg/ml collagen (PureCol; Inamed Biomaterials), 10 mM HEPES, 27
mM NaHCO3, 100 U/ml penicillin and 100 mg/ml
streptomycin (pH 7.4). The MDCK cell suspension was cultured in
24-well plates for 90 min, after which, the cells were treated at
37°C with the different compound combinations as follows: 10 µM
FSK; 0, 0.4, 2 or 10 µM Cur; and 0, 0.125, 0.5 or 2 µM GB for 4
days. Medium containing FSK and Cur or GB was changed every 12 h.
After cells were cultured for 4 days, MDCK cysts formed in the
continuous presence of 10 µM FSK at 37°C for 4 days.
To evaluate the inhibitory effect of Cur and GB on
cyst formation in the MDCK cyst model, different combinations of
Cur (0, 0.4, 2 or 10 µM) and GB (0, 0.125, 0.5 or 2 µM) were added
to the culture medium containing 10 µM FSK at 37°C from day 0 to
day 5. Medium containing FSK and the combination of Cur and GB was
changed every 12 h for 6 days. Cysts with a diameter >50 µm and
non-cyst cell colonies were counted under an Olympus confocal
microscopy (Olympus Corporation) on the 6th day (magnification,
×200). The MDCK cyst formation inhibitory rate was calculated as
follows: [(number of cysts in MDCK cyst model without
treatment-number of cysts in MDCK cyst model with treatment)/number
of cysts in MDCK cyst model without treatment] ×100%.
To determine inhibition of cyst growth mediated by
Cur and GB, different dose combinations of Cur (0, 0.4, 2 or 10 µM)
and GB (0, 0.125, 0.5 or 2 µM) were added to the culture medium
containing 10 µM FSK at 37°C from day 4 to day 9. Medium containing
FSK and the combination of Cur and GB was changed every 12 h for 6
days. Cysts were observed on days 4, 6, 8 and 10 using an in
situ tracking method (identified by markings on plates)
(17). To evaluate growth, cysts
with diameters >50 µm were measured on days 4, 6, 8 and 10 using
Image-Pro Plus version 6.0 (Media Cybernetics, Inc.). In total, ≥10
cysts/well and 3 wells/group were measured for each condition.
Pkd1flox/−; Ksp-Cre mouse
of ADPKD
Pkd1flox mice and kidney-specific
Cre (Ksp-Cre) transgenic mice were established as described
previously, and were housed at 22±3°C under 50±5% relative humidity
with a 12 h light/dark cycle with food and water available on
demand in the laboratory (17).
There was −15 Pa atmosphere pressure difference between inside and
outside laboratory. The animal use protocol has been reviewed and
approved by the Institutional Animal Care and Use Committee of
Shanxi Bethune Hospital. Pkd1Flox/−; Ksp-Cre mice
were used to evaluate the effects of Cur and GB on cyst inhibition.
The Pkd1Flox/−; Ksp-Cre homozygous mice generally
die within 15 days of birth (27).
Thus, treatments were performed only up until postnatal day 10.
Neonatal mice (postnatal day 0) were genotyped using genomic PCR. A
total of 96 homozygous mice (half male and half female; age, 1 day;
weight, 3.07±0.66 g) were randomly divided into three treatment
groups (Cur group, GB group, Cur and GB combination group) and PKD
mice group (2 µl × body weight DMSO + saline, 0.05 ml/injection). A
total of six heterozygous mice (half male and half female; age, 1
day; weight, 2.75±0.93 g) were used as the wild-type (WT) mice
group. Each group had six mice to ensure the reliability of
statistical analysis. The homozygous mice are characterized by
rapidly progressive disease, providing only a very small window for
treatment of the neonates (13). GB
(0, 20, 40 or 80 mg/kg) and Cur (0, 80, 160 and 320 mg/kg) were
injected subcutaneously in the back of neonatal mice once a day
from days 1–10 using a 1-ml insulin syringe. The mice were weighed
on day 11, and were anesthetized by intraperitoneal injection of
pentobarbital sodium (50 mg/kg). Then, mice were sacrificed by
cervical dislocation and decapitated. The kidney, liver and spleen
tissues were removed and weighed, and stored at 4°C in 10% formalin
solutions. The ratio of kidney weight to body weight was calculated
and analyzed. The kidney cysts were evaluated by the percentage of
cyst area to kidney area. The effects of Cur and GB on liver and
spleen were observed by measuring liver weight/body weight and
spleen weight/body weight. Blood samples (0.3 ml) were centrifuged
in heparinized Microtainer tubes at 3,000 × g for 10 min at 4°C.
Urea concentration was tested using urea assay kit (Bioassay
Systems). The blood urea nitrogen (BUN) (mg/dl) was calculated as
2.14 divided by urea (mg/dl).
Immunohistochemistry
Kidney tissue samples were fixed in 4% formaldehyde
at pH 7.4 and 4°C for 48 h, dehydrated, embedded in paraffin, and
stained with hematoxylin for 5 min and eosin for 3 min at room
temperature. To detect tissue localization and expression of EGFR
and Cerb-B2, tissues were sectioned at 5-µm in thickness and
stained as follows. The section was blocked by 1% hydrogen peroxide
in methanol for 10 min at 37°C. Antigens were retrieved by
microwaving in 10 mM citrate buffer (pH 6.0) at 95°C for 10 min.
Slides were blocked with 5% normal goat serum (Abcam; cat. no.
ab7481) at room temperature for 20 min. Then, the sections were
incubated in a humidified chamber at 4°C overnight with one of the
primary antibodies, including anti-EGFR (1:100) and anti-Cerb-B2
(1:100). The slides were washed three times with PBS, incubated at
37°C for 20 min with biotinylated second antibody (1:200), and
labeled for 20 min with HRP. Peroxidase activity was visualized
using a DAB concentrated kit (Bioswamp Life Science Lab; cat. no.
PAB180021). Samples were observed under an Olympus confocal
microscopy (Olympus Corporation) at ×400 magnification by two
experienced pathologists blinded to the conditions. Positive
staining was quantitatively analyzed using Image-Pro-Plus (version
6.0; Media Cybernetics, Inc.; http://www.mediacy.com/), and the average optical
density was the result of cumulative optical density divided by
total area. These results were statistically analyzed.
Western blotting
The kidney tissues were homogenized using a
homogenizer (FLUKO; FA25) at 8,000 × g for 2 min at 4°C, incubated
on ice for 20 min and lysed using an ultrasonic cell crusher
(Shanghai Hannuo Instrument Co., Ltd.; http://www.hanuo.com.cn/; HN-250M) for 3 min at 4°C in
a protein lysis buffer (10 mM triethanolamine at pH 7.6 and 250 mM
sucrose) and protease inhibitor cocktail (1 mM PMSF, 20 mM NaF and
1 mM Na3VO4). The kidney cell lysates were
centrifuged at 10,000 × g for 5 min at 4°C. The supernatant was
used immediately or stored at −80°C. Protein concentrations were
determined using a Bradford assay and 4 mg/ml BSA (Applygen
Technologies, Inc.; cat. no. P1512) was used as the protein
standard. Proteins (28 µg/lane) were loaded on a SDS-gel, resolved
using 10% SDS-PAGE and transferred to a PVDF membrane. Membranes
were blocked in 3% BSA/PBS at room temperature for 90 min. PVDF
membranes were subsequently incubated overnight at 4°C with
different primary antibodies at dilutions (anti-p-EGFR 1:1,000,
anti-EGFR 1:1,000, anti-p-Cerb-B2 1:1,000, anti-Cerb-B2 1:1,000,
anti-H-Ras 1:1,500, anti-B-Raf 1:1,000, anti-Raf-1 1:1,000,
anti-p-MEK-1/2 1:2,000, anti-MEK-1/2 1:2,000, anti-p-ERK 1:2,000,
anti-ERK-1/2 1:2,000, anti-p-JNK 1:1,500, anti-JNK 1:1,500,
anti-p-ASK 1:1,000, anti-ASK1 1:1,000, anti-p-p38α 1:1,500,
anti-p38α/β 1:1,500 and anti-actin 1:2,000, anti-p-PI3K 1:1,000,
anti-PI3K 1:1,000, anti-p-AKT 1:1,000, anti-AKT 1:1,000,
anti-p-mTOR 1:1,000, anti-mTOR 1:1,000, anti-p-4E-BP1 1:1,000,
anti-4E-BP1 1:1,500, anti-p-p70S6k 1:1,000, anti-p70S6k 1:1,000,
anti-p-MAP3K7 1:1,000, anti-MAP3K7 1:1,500, anti-p-MKK3/6 1:1,500,
anti-MKK3 1:1,500, anti-p-MKK4 1:2,000 and anti-MKK4 1:2,000) in
accordance with manufacturer's instructions (Santa Cruz
Biotechnology, Inc. or Cell Signaling Technology, Inc.). Then, PVDF
membranes were washed with TBS-Tween (0.1%) and incubated with
secondary antibodies at room temperature for 40 min. The specific
protein bands were visualized using an ECL chemiluminescence
detection kit. Intensity of the protein bands was measured by
densitometry and semi-quantified using Quantity One software
(version 4.6.2; Bio-Rad Laboratorires, Inc.; http://www.bio-rad.com/).
Statistical analysis
The experiment was repeated three times, and
experimental data were analyzed using SPSS version 16.0 (SPSS,
Inc.). Data are presented as the mean ± SEM. Comparison of
homogeneity of variance amongst multiple groups was performed using
a one-way ANOVA. Multiple comparisons were performed using a
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference. The inhibitory effect of Cur
combined with GB was evaluated using Jin's modified Bürgi's formula
(36). The formula was q=EAB/(EA +
EB-EA × EB), where EAB is the inhibitory effect of drug A combined
with drug B, and EA and EB are the effects of drug A and B,
respectively. A q-value between 0.85–1.15 indicates that the
effects of drug A and B are additive. A q-value >1.15 indicates
that the effects of drug A and B are synergistic, while a q-value
<0.85 indicates that the effects of drug A and B are
antagonistic (37).
Results
Cur combined with GB synergistically
inhibits cyst formation and growth in the MDCK cyst model
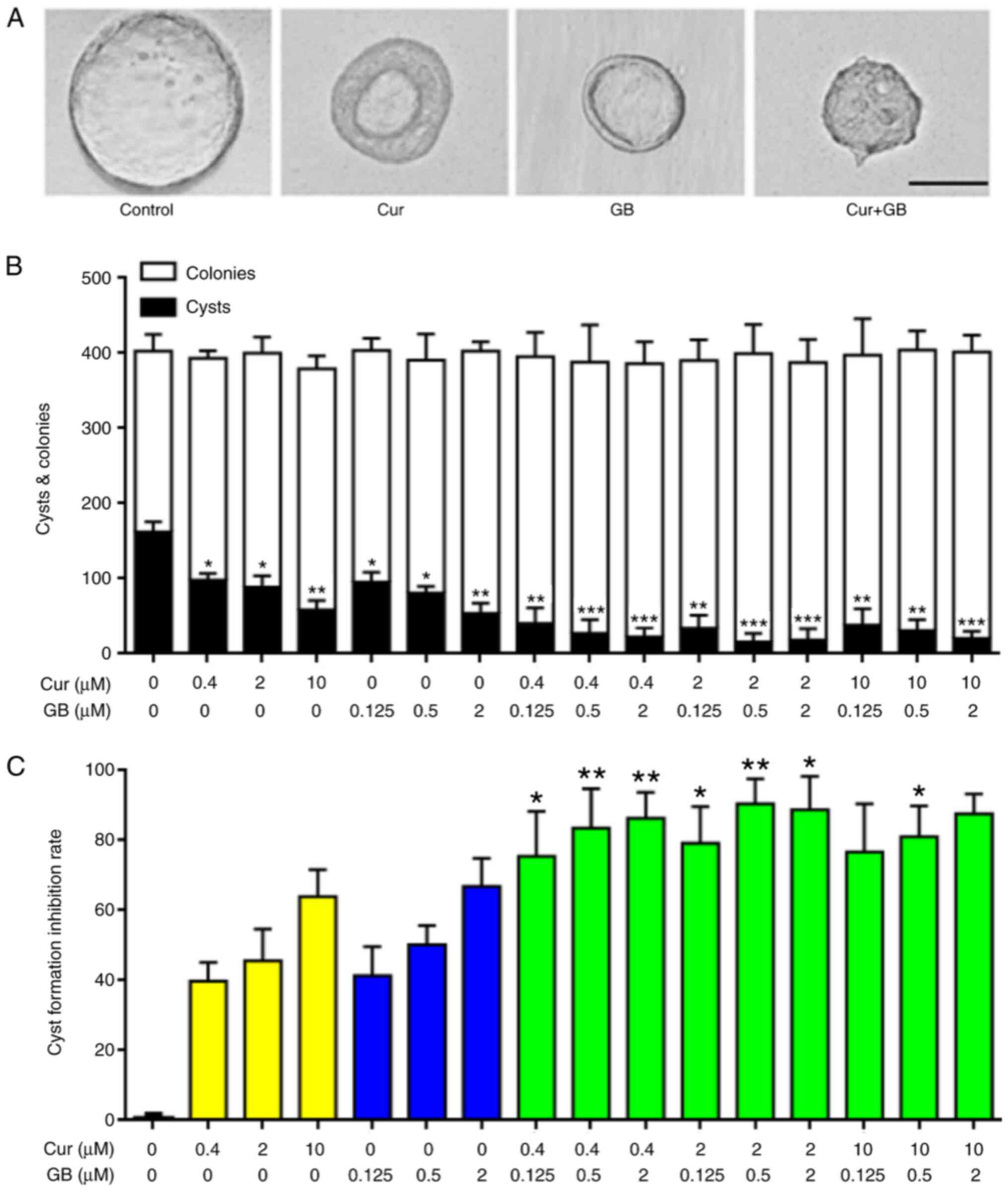
As presented in Fig. 1A
and B, the MDCK cells exposed to Cur primarily became small
thick-walled cysts; however, the MDCK cells exposed to GB became
small thin-walled cysts. These morphological differences suggested
that Cur and GB may mediate their effects via different mechanisms.
The MDCK cyst model was used to assess the inhibitory effect of Cur
combined with GB cyst formation in vitro. The combination of
Cur and GB significantly reduced the formation of MDCK cysts. The
maximum inhibitory rate of MDCK cysts treated with a combination of
2 µM Cur and 0.5 µM GB was as high as 90.46%, and the synergistic
effect (q>1.15) was significantly greater compared with either
Cur or GB alone (Fig. 1C;
P<0.01); the maximum MDCK cyst inhibitory rate of Cur and GB was
63.82 and 66.73%, respectively (Fig.
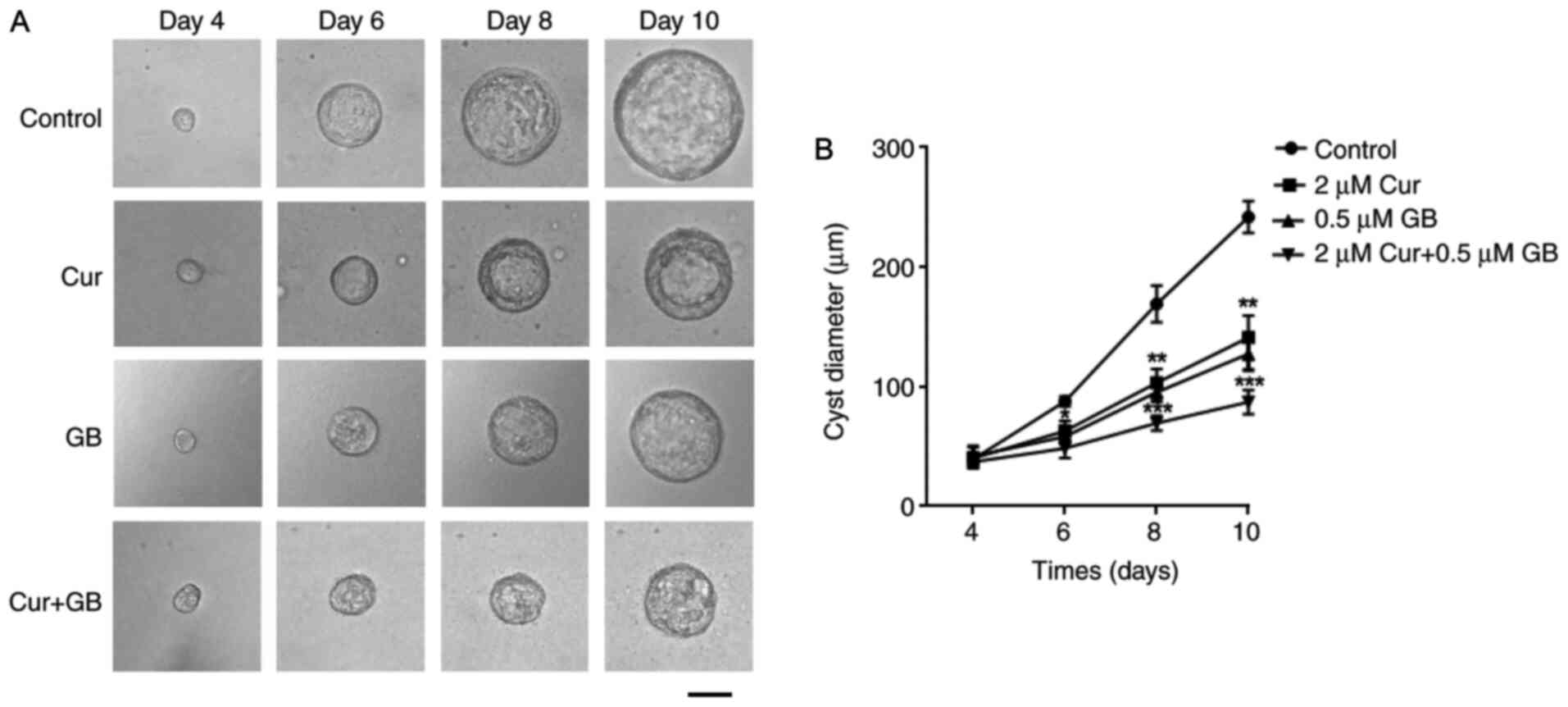
1C). The cysts formed on day 4 and were observed every 2 days
from days 4 to 10 (Fig. 2A; top
panel). The progressive growth of cysts was inhibited by treatment
with Cur and GB alone, as well as in combination (Fig. 2B).
Cur combined with GB synergistically
inhibits cyst enlargement in the PKD mouse model
The combination of Cur and GB significantly
decreased renal enlargement in mice (Fig. 3A). After 10 days of treatment, the
ratio of kidney weight to body weight of the PKD mice treated with
Cur or GB was significantly declined compared with the PKD mice
treated with DMSO + saline (Fig.
3B). The combination of Cur and GB was more effective compared
with Cur in decreasing the ratio of kidney weight to body weight of
the PKD mice. There was a downward tendency in the ratio of kidney
weight to body weight of the PKD mice treated with Cur combined
with GB, compared with GB. Hematoxylin and eosin-stained kidney
sections demonstrated that the percentage of cyst area to kidney
area was also decreased in PKD mice treated with Cur and/or GB
(Fig. 3A and C). The combination of
Cur and GB was more effective compared with Cur or GB alone in
decreasing the percentage of cyst area to kidney area of the PKD
mice. Although the kidney cyst expanded progressively, the BUN
levels suggested that renal function in PKD mice treated with Cur
and/or GB did not decrease as rapidly compared with that of the PKD
mice treated with DMSO + saline (Fig.
3E). There were no differences in the ratio of kidney weight to
body weight between the male and female mice in the same group
(Fig. 3D). Moreover, there was no
significant difference in spleen index or liver index between the
PKD mice, irrespective of treatments (Fig. 3F and G).
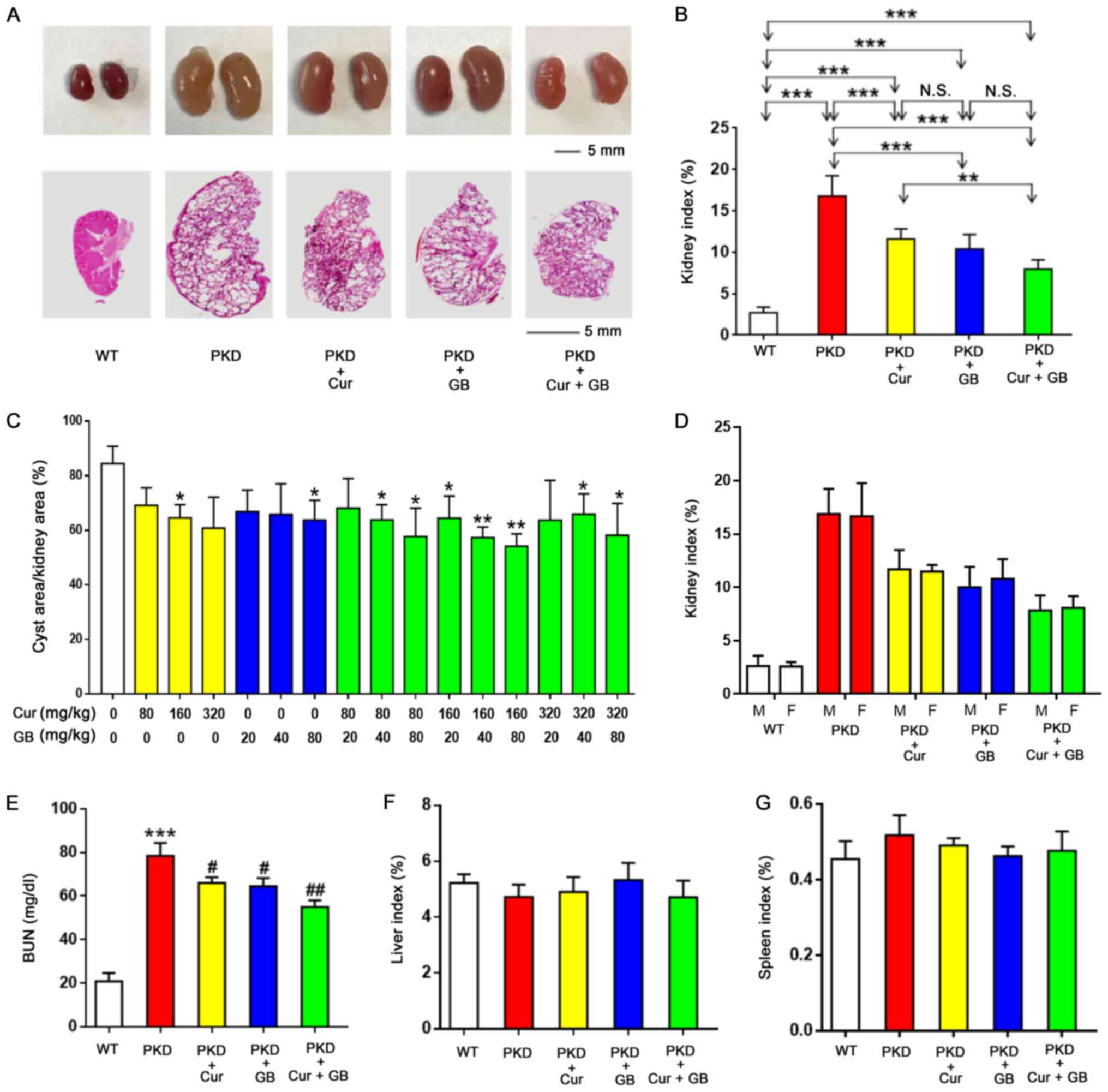 | Figure 3.Combination of Cur and GB
synergistically inhibits cyst enlargement in
Pkd1Flox/−; Ksp-Cre mice. (A) Representative
images (top panel) and hematoxylin and eosin staining images
(bottom panel) of the kidney (on postnatal day 11) of WT mice,
Pkd1Flox/−; Ksp-Cre mice treated with DMSO +
saline, 160 mg/kg Cur, 80 mg/kg GB and 160 mg/kg Cur combined with
80 mg/kg GB. (B) Kidney weight indexes of WT mice and
Pkd1Flox/−;Ksp-Cre mice treated with DMSO +
saline, 160 mg/kg Cur, 80 mg/kg GB and 160 mg/kg Cur combined with
80 mg/kg GB. Data are presented as the mean ± SEM, n=6. **P<0.01
and ***P<0.001. (C) Kidney cysts were evaluated as the
percentage of cyst area to kidney area in different combinations of
Cur and GB. Data are presented as the mean ± SEM, n=6. *P<0.05,
**P<0.01 vs. 0 mg/kg Cur + 0 mg/kg GB. (D) Kidney weight indexes
of male or female WT mice and Pkd1Flox/−;Ksp-Cre
mice treated with DMSO + saline, 160 mg/kg Cur, 80 mg/kg GB and 160
mg/kg Cur combined with 80 mg/kg GB. Data are presented as the mean
± SEM, n=6. (E) BUN levels in WT mice, Pkd1Flox/−;
Ksp-Cre mice treated with DMSO + saline, 160 mg/kg Cur, 80
mg/kg GB and 160 mg/kg Cur combined with 80 mg/kg GB. Data are
presented as the mean ± SEM, n=6. ***P<0.001 vs. WT mice;
#P<0.05 and ##P<0.01 vs. PKD mice. (F)
Liver weight indexes of PKD mice treated with or without 160 mg/kg
Cur and or 80 mg/kg GB. Data are presented as the mean ± SEM, n=6.
(G) Spleen weight indexes of PKD mice treated with or without 160
mg/kg Cur and or 80 mg/kg GB. Data are presented as the mean ± SEM,
n=6. WT, wild-type; N.S., no statistical significance; PKD,
polycystic kidney disease; Cur, curcumin; GB, ginkgolide B; M,
male; F, female; BUN, blood urea nitrogen. |
Cur and GB decrease the activity of
the EGFR/ERK1/2 signaling pathway
To further examine the mechanisms via which Cur and
GB inhibited cyst formation, the activity of the EGFR/ERK1/2
signaling pathway was assessed using western blotting and
immunohistochemistry on the kidneys obtained from the PKD mice
(Fig. 4A and B). The expression
levels of p-EGFR, p-Cerb-B2, H-Ras, B-Raf, p-MEK and p-ERK were
increased in the kidneys of the PKD mice. However, the expression
of Raf-1 was decreased in the kidneys of the PKD mice. The
inhibitory effect of Cur combined with GB on EGFR/ERK1/2 signaling
pathway was also examined (Fig.
4C). The expression levels of p-EGFR, p-Cerb-B2, H-Ras, B-Raf,
p-MEK and p-ERK were downregulated by Cur, GB and Cur combined with
GB, while the expression of Raf-1 was upregulated by GB and Cur
combined with GB (Fig. 4C). These
results suggested that Cur combined with GB inhibited the
development of renal cysts by regulating the EGFR/ERK1/2 signaling
pathway.
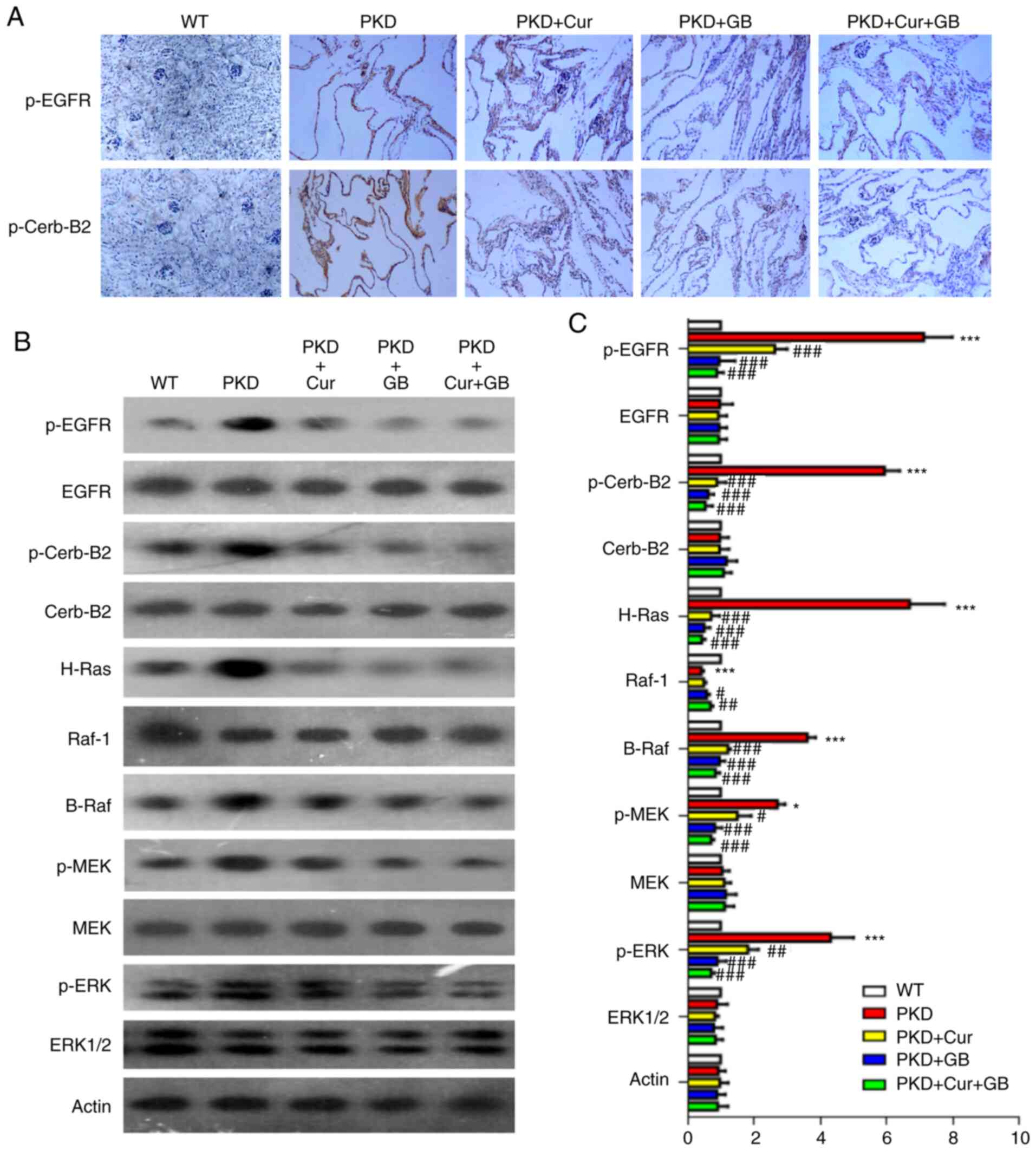 | Figure 4.Cur combined with GB regulates the
EGFR/ERK1/2 signaling pathway in Pkd1Flox/−;
Ksp-Cre mice. (A) Representative immunohistochemistry of p-EGFR
and p-Cerb-B2 in Pkd1Flox/−; Ksp-Cre mice treated
with 160 mg/kg Cur, 80 mg/kg GB and 160 mg/kg Cur combined with 80
mg/kg GB. Magnification, ×400. (B) Representative western blotting
of EGFR/ERK1/2 signaling proteins in Pkd1Flox/−;
Ksp-Cre mice treated with 160 mg/kg Cur, 80 mg/kg GB and 160
mg/kg Cur combined with 80 mg/kg GB. (C) Semi-quantitative analysis
of EGFR/ERK1/2 signaling protein expression levels in
Pkd1Flox/−; Ksp-Cre mice. Relative level refers
to the ratio of western blotting band density in different
treatment groups compared with that in WT group. Data are presented
as the mean ± SEM, n=6. *P<0.05 and ***P<0.001 vs. WT mice;
#P<0.05, ##P<0.01 and
###P<0.001 vs. PKD mice. WT, wild-type; PKD,
polycystic kidney disease; Cur, curcumin; GB, ginkgolide B; p-,
phosphorylated; Cerb-B2, human epidermal growth factor receptor
2. |
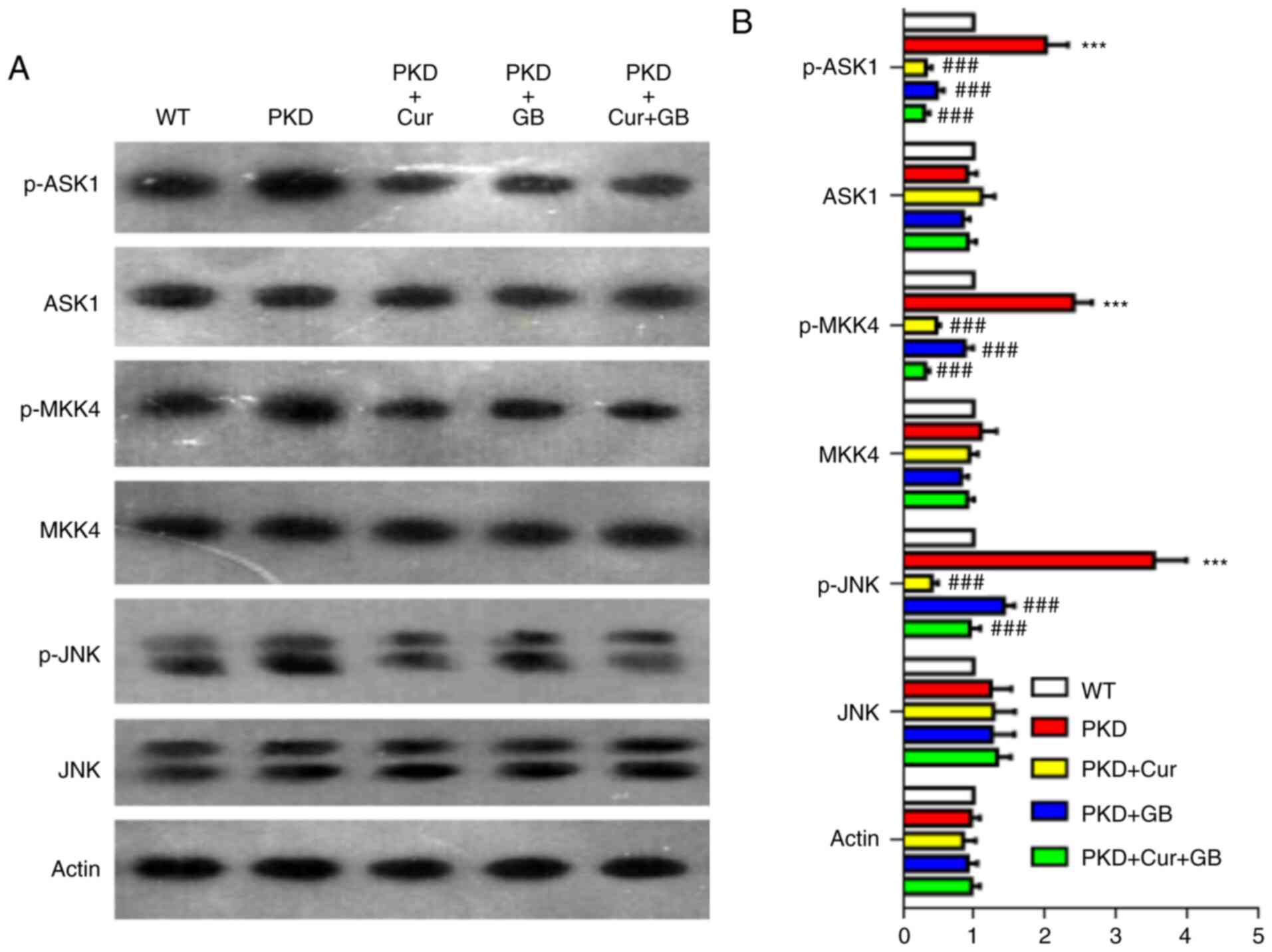
Cur and GB downregulate the ASK1/JNK
signaling pathway
To determine whether the JNK signaling pathway was
involved in cyst inhibition in vivo, the expression of the
ASK1/JNK signaling pathway was measured via western blotting in
kidneys of PKD mice treated with Cur, GB or Cur combined with GB
(Fig. 5A). The expression levels of
p-ASK1, p-MKK4 and p-JNK were increased in the kidneys of PKD mice,
and were downregulated by Cur, GB and Cur combined with GB
(Fig. 5B). Cur was more effective
compared with GB in inhibiting the JNK signaling pathway. These
results indicated that Cur combined with GB inhibited the
development of renal cysts by regulating the ASK1/JNK signaling
pathway.
GB downregulates the MAP3K7/p38
signaling pathway
The expression of the MAP3K7/p38 signaling pathway
was assessed via western blotting in kidneys of PKD mice treated
with Cur, GB or Cur combined with GB (Fig. 6A). The expression levels of
p-MAP3K7, p-MKK3 and p-p38 were increased in the kidneys of PKD
mice, and were significantly downregulated by GB treatment, as well
as by Cur combined with GB treatment. (Fig. 6B). There was no difference between
the mice treated with GB and Cur combined with GB. Cur failed to
inhibit the MAP3K7/p38 signaling pathway (Fig. 6B). There was also no difference
between the mice treated with and without Cur. These results
suggested that GB inhibited the development of renal cysts by
regulating the MAP3K7/p38 signaling pathway.
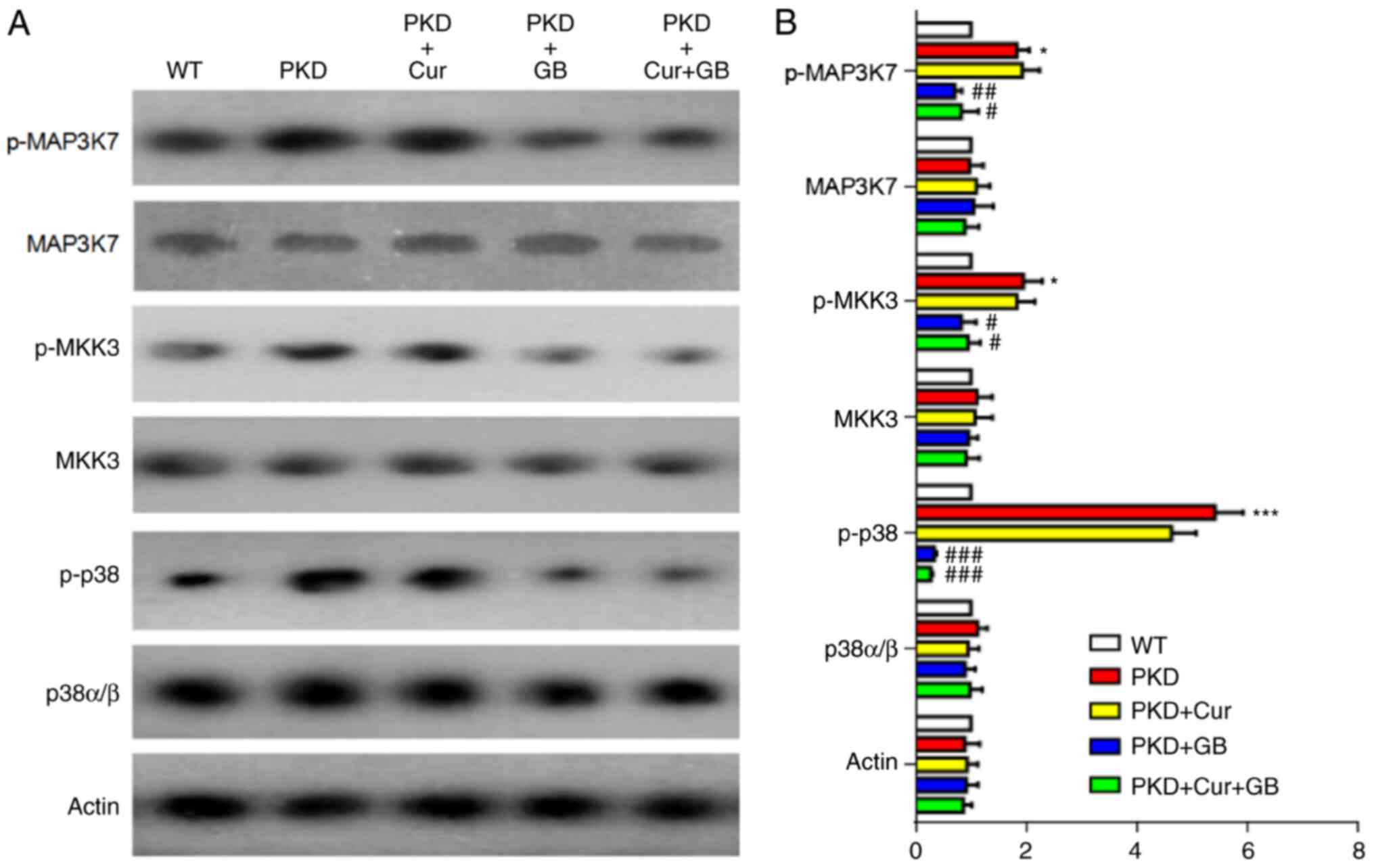 | Figure 6.Cur combined with GB regulates the
MAP3K7/p38 signaling pathway in Pkd1Flox/−;
Ksp-Cre mice. (A) Representative western blotting of MAP3K7/p38
signaling proteins in Pkd1Flox/−;Ksp-Cre mice
treated with 160 mg/kg Cur, 80 mg/kg GB and 160 mg/kg Cur combined
with 80 mg/kg GB. (B) Semi-quantitative analysis of MAP3K7/p38
signaling protein expression levels in Pkd1Flox/−;
Ksp-Cre mice. Relative level refers to the ratio of western
blotting band density in different treatment groups compared with
that in WT group. Data are presented as the mean ± SEM, n=6.
*P<0.05 and ***P<0.001 vs. WT mice; #P<0.05,
##P<0.001 and ###P<0.001 vs. PKD mice.
WT, wild-type; PKD, polycystic kidney disease; Cur, curcumin; GB,
ginkgolide B; p-, phosphorylated; MAP3K7, mitogen-activated protein
kinase kinase kinase 7; MKK, mitogen-activated protein kinase
kinase. |
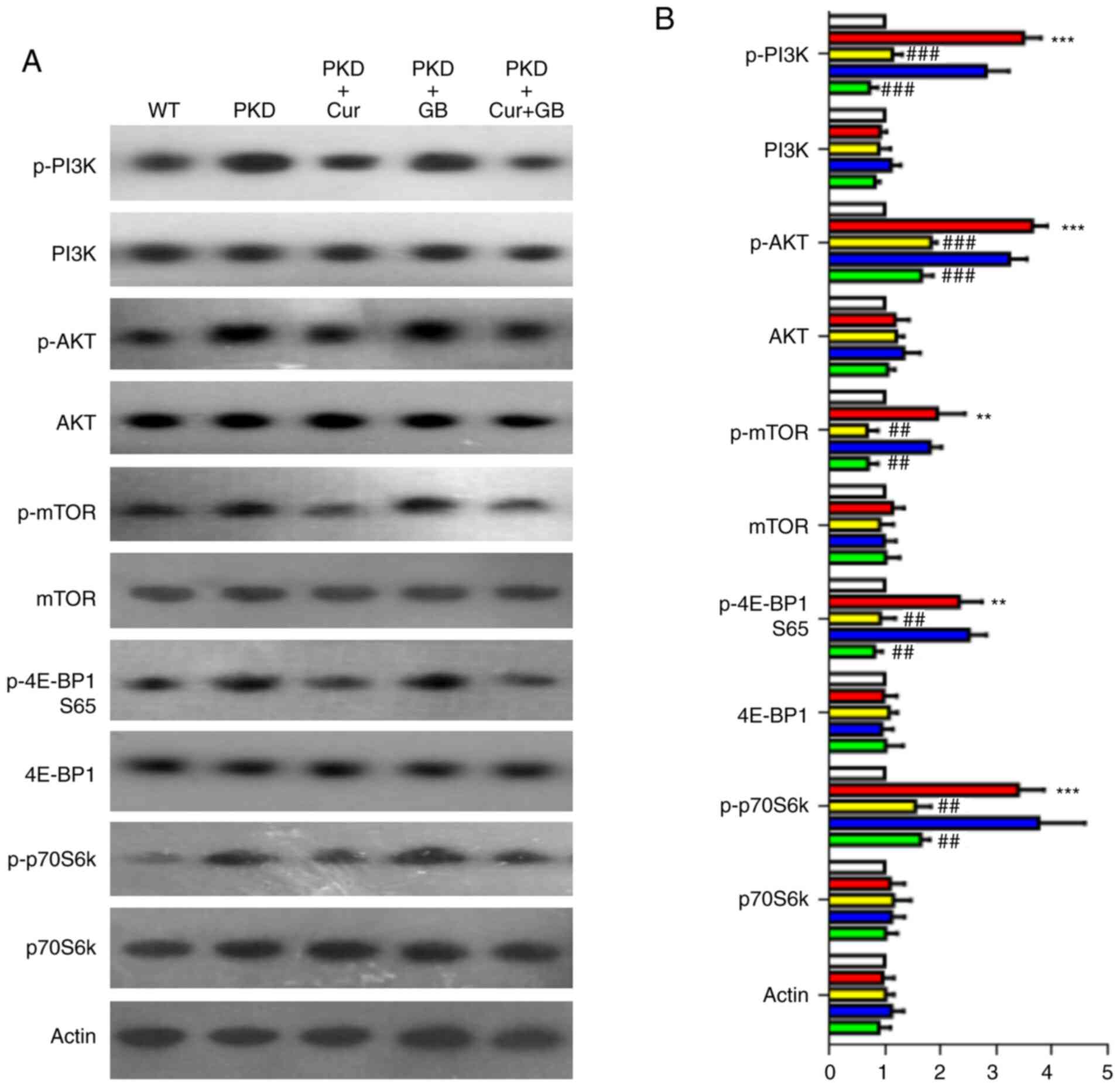
Cur downregulates the PI3K/mTOR
signaling pathway
The PI3K/mTOR pathway has been reported to be
associated with abnormal proliferation of cyst epithelial cells
(38). In the present study, the
expression levels of p-PI3K, p-AKT, p-mTOR, p-4E-BP1 (S65) and
p-p70S6k were assessed using western blotting on the kidney samples
of PKD mice treated with Cur, GB or Cur combined with GB (Fig. 7A). The expression levels of p-PI3K,
p-AKT, p-mTOR, p-4E-BP1 (S65) and p-p70S6k were increased in the
kidneys of the PKD mice. However, the expression levels of p-PI3K,
p-AKT, p-mTOR, p-4E-BP1 (S65) and p-p70S6k were significantly
decreased by Cur. GB failed to downregulate these signaling factors
(Fig. 7B). There was no synergistic
effect when GB and Cur were used in combination, suggesting that
Cur affected the expression levels of p-PI3K, p-AKT, p-mTOR,
p-4E-BP1 (S65) and p-p70S6k alone. These results indicated that Cur
inhibited the development of renal cysts by downregulating the
PI3K/mTOR signaling pathway.
Discussion
Curcuma longa is an essential ingredient in
numerous Southeast Asian countries, where it is frequently used in
curries (39). Cur is a type of
rhizome extracted from Curcuma longa (40). The estimated consumption of
Curcuma longa in South Asia is 1–2 g/day or higher (41), which corresponds to 31.49–62.98 mg
Cur. However, the amount of Cur in the daily diet in South Korea is
only 2.7–14.8 mg (42). Thus, the
Cur consumption in some countries may not be sufficient to achieve
the biological effects of Cur. Cur has several beneficial
pharmacological activities (43),
including anti-oxidant, anti-fibrotic and anti-angiogenic
properties. Our previous study reported that Cur inhibited cyst
development in an MDCK cyst model and embryonic kidney cyst model
by downregulating the activity of the ERK signaling pathway
(15).
Ginkgo is one of the oldest plants on earth,
and it is colloquially referred to as a living fossil plant
(44). GB is a type of terpene
lactone compound extracted from GB (45). GB has several potential beneficial
properties, including antiplatelet, anti-inflammatory and
neuroprotective effects (46). Our
previous study revealed GB also inhibited cyst growth in
vivo and in vitro (17).
The mechanism via which GB inhibits cyst formation is associated
with the ERK signaling pathway (17).
To improve cyst inhibition, the combined effect of
Cur and GB was assessed in the present study using a MDCK cyst
model and Pkd1Flox/−; Ksp-Cre mice. The combined
effect of Cur and GB on cyst inhibition was greater compared with
that of either compound alone, both in vitro and in
vivo. The MDCK cells exposed to Cur primarily became small
thick-walled cysts, whereas the MDCK cells treated with GB
primarily became small thin-walled cysts. Therefore, it was
hypothesized that Cur combined with GB may be more effective
compared with Cur or GB alone in inhibiting cyst formation. MDCK
cells treated with Cur combined with GB often formed cell colonies,
and the inhibition of MDCK cyst formation rate of Cur combined with
GB was 90.46%. The combination of Cur and GB significantly
decreased renal enlargement, but had no effect on the weight of the
liver and spleen in the Pkd1Flox/−; Ksp-Cre mice.
The maximum dose of Cur in the treatment of PKD mice in the present
study was 320 mg/kg, which is relatively large. The United States
Food and Drug Administration has approved Cur as ‘Generally
Recognized As Safe’ (47). One
study (41) revealed that rats
treated with 3,500 mg/kg Cur per day for 90 days did not show any
adverse effect. The human dose equivalent is 26.02 mg/kg, which is
determined by dividing the mouse dose (320 mg/kg) by the conversion
factor for the species (that of mice is 12.3) according to previous
study (48). Though the doses used
in vivo cannot be reached in humans via the daily diet, 500
mg doses of Cur twice daily have been used to treat rheumatoid
arthritis with fewer adverse effects in clinical trial (49).
It was hypothesized that Cur and GB may regulate
their effects on cyst formation by blocking multiple signaling
pathways. The EGFR family includes EGFR, Cerb-B2, human epidermal
growth factor receptor 3 (HER3) and HER4 (50). EGFR also serves a key role in the
development of PKD (51). Some
studies (52–54) revealed that the overexpression of
EGFR promoted the progression of PKD by increasing the
proliferation of cyst epithelial cells. The inhibitors of EGFR and
its downstream signaling pathway can block the excessive
proliferation of cyst epithelial cells, including EGFR inhibitor
(55), Raf inhibitor (56), MEK inhibitor (57), ERK inhibitor (57) and mTOR inhibitor (58). The present study found that the
EGFR/ERK1/2 signaling pathway was activated in the kidneys of PKD
mouse. Moreover, the current study examined the effect of Cur, GB
and Cur combined with GB on the EGFR/ERK1/2 pathway by detecting
the expression and/or phosphorylation levels of signaling proteins,
including EGFR, Ras, Raf, MEK and ERK. The results demonstrated
that the expression of B-Raf was increased, while the levels of
Raf-1 were decreased in Pkd1Flox/−; Ksp-Cre mice.
Thus, B-Raf and Raf-1 may be the turn-on and turn-off switches of
EGFR/ERK1/2 pathway. Both Cur and GB downregulated the expression
levels of EGFR and the downstream B-Raf/ERK1/2 signaling pathway,
while GB upregulated the expression of Raf-1.
The MAPK signaling pathways include not only ERK1/2,
but also JNK and p38-MAPK (59).
The JNK signaling pathway regulates cell proliferation and
apoptosis (60). It has been
reported that Pkd1 regulates the apoptosis of renal
epithelial cells via JNK activation (61). In Pkd1Flox/−;
Ksp-Cre mice, renal epithelial cells continuously proliferated
due to loss of apoptosis regulated by Pkd1, and this
proliferation induces ‘compensatory’ apoptosis mediated by JNK in
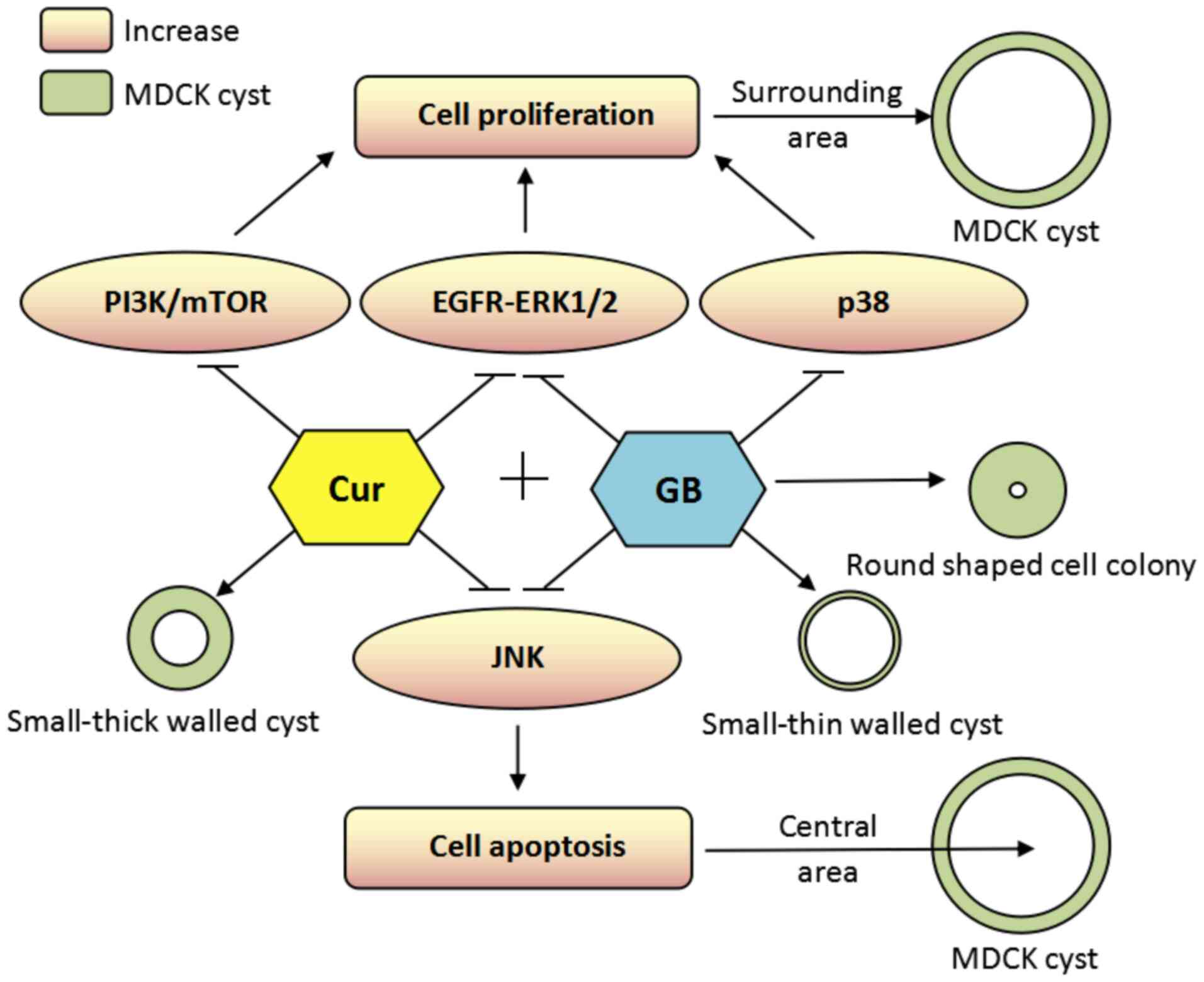
an attempt to re-establish normal renal structure (61). It was proposed that a cyst structure
may be formed as a result of central area cell apoptosis mediated
by JNK and surrounding area cell proliferation mediated by ERK, p38
and other signaling factors (Fig.
8). Cur and GB inhibit the overactive JNK cascade in
Pkd1Flox/−; Ksp-Cre mice. Cur is more effective
compared with GB in inhibiting the JNK signaling pathway, which
leads to thick-walled cysts after treatment with Cur and
thin-walled cysts after GB treatment. It has been reported that the
activation of p38 MAPK is associated with cyst cell proliferation
(62). In the present study, the
expression of p-p38 was increased in the renal tissue of
Pkd1Flox/−; Ksp-Cre mice. Therefore, GB may
inhibit cyst cell proliferation by downregulating the p38 MAPK
signaling pathway, including p-MAP3K7, p-MKK3 and p-p38 expression
levels in the kidneys of Pkd1Flox/−; Ksp-Cre
mice. Moreover, Cur failed to inhibit the MAP3K7/p38 signaling
pathway.
mTOR serves an important role in the regulation of
cyst cell proliferation in ADPKD (58). The present study also evaluated the
effects of Cur and GB on the mTOR pathway. Unexpectedly, Cur
inhibited the PI3K/mTOR pathway, including p-PI3K, p-AKT, p-mTOR,
p-4E-BP1 (S65) and p-p70S6k, in the kidneys of
Pkd1Flox/−;Ksp-Cre mice. However, GB was not able
to block the PI3K/mTOR pathway. The cyst surrounding cell
proliferation may be regulated by EGFR/ERK1/2, p38 and mTOR
(6). Cur reduced the cyst diameter
by blocking EGFR/ERK1/2 and PI3K/mTOR signaling pathways. Moreover,
GB decreased cyst diameter by downregulating EGFR/ERK1/2 and p38
signaling pathways.
However, there were several limitations in this
study. First, it was not clear which signaling pathway had the
largest effect on cyst formation, and the specific interactions
between the identified signaling pathways were not studied.
Considering that there are numerous types of combinations of Cur
and GB, it is difficult to use specific inhibitors to prove their
inhibition is indeed as a result of EGFR/ERK1/2, JNK and PI3K/mTOR
signaling pathways. Our previous studies (15,17)
and other studies (63,64) have confirmed that the mechanism of
Cur or GB is associated with ERK1/2 and mTOR signaling pathways.
Accordingly, the mechanism of Cur and GB is credible, but at
present, it is complex to identify the most important relative
signaling pathway. Thus, future studies will optimize the
combination of Cur and GB, and use specific inhibitors to clarify
the key mechanism of inhibition of cystogenesis. Secondly, average
daily consumption of Cur and GB in humans may be insufficient to
exert notable biological effects (42,65).
Considering the oral bioavailability of Cur in mice is very low,
the present study administered Cur subcutaneously. Cur has no
obvious adverse effect on human health (31), while GB only affects early-stage
embryonic development in mice (66). Therefore, a Cur and GB combination
may have risks of various interactions and side-effects, which
should be further researched. Although clinical trials have
demonstrated that low or similar doses of Cur or GB can be used to
treat certain kidney diseases (67), there is no direct evidence showing
that GB and Cur administration reached the kidney in the present
study. GB is mainly excreted in urine (68), but Cur is metabolized in the liver
and excreted in feces (69). Thus,
the pharmacokinetics of Cur and GB will further studied. Finally,
the present study did not assess any specific markers of renal
fibrosis and cyst derivation. The rapid development of ADPKD is
associated with progressive fibrosis (14), and epithelial changes lead to
fibrosis in ADPKD (70). ERK and
JNK/p38 MAPK, PI3K/Akt pathways are activated in fibrosis (70). In the present study, it was not
possible to confirm fibrosis development and determine which cysts
were derived from which tubules, as more advanced technologies and
methods are required to examine these factors, in which specialist
staining of fibrosis and various tubules is performed to track
these over longer periods of time.
In conclusion, the present study demonstrated that
Cur combined with GB inhibited cystogenesis more effectively
compared with either treatment alone, both in vitro and
in vivo. The molecular mechanism via which Cur and GB
reduced cyst formation was found to be mediated by regulation of
different signaling pathways, including EGFR/ERK1/2, JNK, p38 and
mTOR. The novel combination of Cur and GB may serve as a more
effective treatment for ADPKD.
Acknowledgements
The authors gratefully acknowledge the numerous
contributions made by Professor Yanmei Du, Dr Dan Bao, Mrs Xiuping
Xu, Mr Ligang Yang, Mrs Hui Yu, Mr Yang Liu (Ping An Healthcare and
Technology Company Limited) and Dr Xiaojun Ren (Shanxi Bethune
Hospital).
Funding
The present study was supported by the National
Natural Science Foundation of China grant (grant no. 81302828), the
Shanxi Province Science Foundation for Youths (grant no.
2013021037-3), the Taiyuan Science and Technology Fund Foundation
grant (grant no. 12016911) and the Natural Science Foundation of
Shanxi Province Health and Family Planning Commission (grant no.
2017093).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, JG and XY designed the study, performed the MDCK
cyst model and the animal experiment, and drafted the manuscript.
TL helped perform the MDCK cyst model and animal experiment. BY and
AA participated in the design and coordination of the study. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The animal use protocol has been reviewed and
approved by the Institutional Animal Care and Use Committee of
Shanxi Bethune Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Corradi V, Gastaldon F, Caprara C,
Giuliani A, Martino F, Ferrari F and Ronco C: Predictors of rapid
disease progression in autosomal dominant polycystic kidney
disease. Minerva Med. 108:43–56. 2017.PubMed/NCBI
|
|
2
|
Chebib FT, Sussman CR, Wang X, Harris PC
and Torres VE: Vasopressin and disruption of calcium signalling in
polycystic kidney disease. Nat Rev Nephrol. 11:451–464. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harris PC and Torres VE: Genetic
mechanisms and signaling pathways in autosomal dominant polycystic
kidney disease. J Clin Invest. 124:2315–2324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Su Q, Hu F, Ge X, Lei J, Yu S, Wang T,
Zhou Q, Mei C and Shi Y: Structure of the human PKD1/PKD2 complex.
Science. 361:eaat98192018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Douguet D, Patel A and Honoré E: Structure
and function of polycystins: Insights into polycystic kidney
disease. Nat Rev Nephrol. 15:412–422. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malekshahabi T, Khoshdel Rad N, Serra AL
and Moghadasali R: Autosomal dominant polycystic kidney disease:
Disrupted pathways and potential therapeutic interventions. J Cell
Physiol. 234:12451–12470. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gattone VH, Chen NX, Sinders RM, Seifert
MF, Duan D, Martin D, Henley C and Moe SM: Calcimimetic inhibits
late-stage cyst growth in ADPKD. J Am Soc Nephrol. 20:1527–1532.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Torres VE: Cyclic AMP, at the hub of the
cystic cycle. Kidney Int. 66:1283–1285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calvet JP: Strategies to inhibit cyst
formation in ADPKD. Clin J Am Soc Nephrol. 3:1205–1211. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shibazaki S, Yu Z, Nishio S, Tian X,
Thomson RB, Mitobe M, Louvi A, Velazquez H, Ishibe S, Cantley LG,
et al: Cyst formation and activation of the extracellular regulated
kinase pathway after kidney specific inactivation of Pkd1. Hum Mol
Genet. 17:1505–1516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pandey P, Brors B, Srivastava PK, Bott A,
Boehn SNE, Groene HJ and Gretz N: Microarray-based approach
identifies microRNAs and their target functional patterns in
polycystic kidney disease. BMC Genomics. 9:6242008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang B, Sonawane ND, Zhao D, Somlo S and
Verkman AS: Small-molecule CFTR inhibitors slow cyst growth in
polycystic kidney disease. J Am Soc Nephrol. 19:1300–1310. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He J, Zhou H, Meng J, Zhang S, Wang S,
Shao G, Jin W, Geng X, Zhu S and Yang B: Cardamonin retards
progression of autosomal dominant polycystic kidney disease via
inhibiting renal cyst growth and interstitial fibrosis. Pharmacol
Res. 155:1047512020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raman A, Reif GA, Dai Y, Khanna A, Li X,
Astleford L, Parnell SC, Calvet JP and Wallace DP: Integrin-linked
kinase signaling promotes cyst growth and fibrosis in polycystic
kidney disease. J Am Soc Nephrol. 28:2708–2719. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao J, Zhou H, Lei T, Zhou L, Li W, Li X
and Yang B: Curcumin inhibits renal cyst formation and enlargement
in vitro by regulating intracellular signaling pathways. Eur J
Pharmacol. 654:92–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higashihara E, Nutahara K, Okegawa T,
Tanbo M, Mori H, Miyazaki I, Nitatori T and Kobayashi K: Safety
study of somatostatin analogue octreotide for autosomal dominant
polycystic kidney disease in Japan. Clin Exp Nephrol. 19:746–752.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou H, Gao J, Zhou L, Li X, Li W, Li X,
Xia Y and Yang B: Ginkgolide B inhibits renal cyst development in
in vitro and in vivo cyst models. Am J Physiol Renal Physiol.
302:F1234–F1242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Torres VE, Chapman AB, Devuyst O,
Gansevoort RT, Perrone RD, Koch G, Ouyang J, McQuade RD, Blais JD,
Czerwiec FS, et al: Tolvaptan in later-stage autosomal dominant
polycystic kidney disease. N Engl J Med. 377:1930–1942. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reif GA, Yamaguchi T, Nivens E, Fujiki H,
Pinto CS and Wallace DP: Tolvaptan inhibits ERK-dependent cell
proliferation, Cl-secretion, and in vitro cyst growth of human
ADPKD cells stimulated by vasopressin. Am J Physiol Renal Physiol.
301:F1005–F1013. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tesar V, Ciechanowski K, Pei Y, Barash I,
Shannon M, Li R, Williams JH, Levisetti M, Arkin S and Serra A:
Bosutinib versus placebo for autosomal dominant polycystic kidney
disease. J Am Soc Nephrol. 28:3404–3413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li A, Xu Y, Fan S, Meng J, Shen X, Xiao Q,
Li Y, Zhang L, Zhang X, Wu G, et al: Canonical Wnt inhibitors
ameliorate cystogenesis in a mouse ortholog of human ADPKD. JCI
Insight. 3:e958742018. View Article : Google Scholar
|
|
22
|
Cabrera-López C, Bullich G, Martí T,
Català V, Ballarín J, Bissler JJ, Harris PC, Ars E and Torra R:
Insight into response to mTOR inhibition when PKD1 and TSC2 are
mutated. BMC Med Genet. 16:392015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muto S, Aiba A, Saito Y, Nakao K, Nakamura
K, Tomita K, Kitamura T, Kurabayashi M, Nagai R, Higashihara E, et
al: Pioglitazone improves the phenotype and molecular defects of a
targeted Pkd1 mutant. Hum Mol Genet. 11:1731–1742. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Raphael KL, Strait KA, Stricklett PK,
Baird BC, Piontek K, Germino GG and Kohan DE: Effect of
pioglitazone on survival and renal function in a mouse model of
polycystic kidney disease. Am J Nephrol. 30:468–473. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leuenroth SJ, Bencivenga N, Chahboune H,
Hyder F and Crews CM: Triptolide reduces cyst formation in a
neonatal to adult transition Pkd1 model of ADPKD. Nephrol Dial
Transplant. 25:2187–2194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su L, Liu L, Jia Y, Lei L, Liu J, Zhu S,
Zhou H, Chen R, Lu HAJ and Yang B: Ganoderma triterpenes retard
renal cyst development by downregulating Ras/MAPK signaling and
promoting cell differentiation. Kidney Int. 92:1404–1418. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu Y, Teng T, Wang H, Guo H, Du L, Yang
B, Yin X and Sun Y: Quercetin inhibits renal cyst growth in vitro
and via parenteral injection in a polycystic kidney disease mouse
model. Food Funct. 9:389–396. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chebib FT, Perrone RD, Chapman AB, Dahl
NK, Harris PC, Mrug M, Mustafa RA, Rastogi A, Watnick T, Yu ASL and
Torres VE: A practical guide for treatment of rapidly progressive
ADPKD with tolvaptan. J Am Soc Nephrol. 29:2458–2470. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan FS, Sun JL, Xie WH, Shen L and Ji HF:
Neuroprotective effects and mechanisms of curcumin-cu(II) and
-zn(II) complexes systems and their pharmacological implications.
Nutrients. 10:282017. View Article : Google Scholar
|
|
30
|
Zhao NJ, Liao MJ, Wu JJ and Chu KX:
Curcumin suppresses Notch1 signaling: Improvements in fatty liver
and insulin resistance in rats. Mol Med Rep. 17:819–826.
2018.PubMed/NCBI
|
|
31
|
Bandgar BP, Hote BS, Jalde SS and Gacche
RN: Synthesis and biological evaluation of novel curcumin analogues
as anti-inflammatory, anti-cancer and anti-oxidant agents. Med Chem
Res. 21:3006–3014. 2012. View Article : Google Scholar
|
|
32
|
Adahoun MA, AlAkhras MH, Jaafar MS and
Bououdina M: Enhanced anti-cancer and antimicrobial activities of
curcumin nanoparticles. Artif Cells Nanomed Biotechnol. 45:98–107.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu W, Cao L, Zhao Y, Xiao W and Xiao B:
Comparing the role of Ginkgolide B and Ginkgolide K on cultured
astrocytes exposed to oxygen?glucose deprivation. Mol Med Rep.
18:4417–4427. 2018.PubMed/NCBI
|
|
34
|
Li R, Chen B, Wu W, Bao L, Li J and Qi R:
Ginkgolide B suppresses intercellular adhesion molecule-1
expression via blocking nuclear factor-kappaB activation in human
vascular endothelial cells stimulated by oxidized low-density
lipoprotein. J Pharmacol Sci. 110:362–369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shu ZM, Shu XD, Li HQ, Sun Y, Shan H, Sun
XY, Du RH, Lu M, Xiao M, Ding JH and Hu G: Ginkgolide B protects
against ischemic stroke via modulating microglia polarization in
mice. CNS Neurosci Ther. 22:729–739. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou YY, Wang HY, Tang ZG and Ma DL: Two
new formulae for evaluating the effectiveness of drug combinations
and the revision of Bürgi's and Jin's modified Bürgi's formulae.
Zhongguo Yao Li Xue Bao. 5:217–221. 1984.(In Chinese). PubMed/NCBI
|
|
37
|
Pan YY, Xu SP and Wei W: Effect of
combined nimesulide and adriamycin on proliferation and apoptosis
in hepatocellular carcinoma cell line HepG_2. Chin Pharmacol Bull.
22:884–887. 2006.
|
|
38
|
Liu Y, Pejchinovski M, Wang X, Fu X,
Castelletti D, Watnick TJ, Arcaro A, Siwy J, Mullen W, Mischak H
and Serra AL: Dual mTOR/PI3K inhibition limits PI3K-dependent
pathways activated upon mTOR inhibition in autosomal dominant
polycystic kidney disease. Sci Rep. 8:55842018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ng TP, Chiam P, Lee T, Chua H, Lim L and
Kua EH: Curry consumption and cognitive function in the elderly. Am
J Epidemiol. 164:898–906. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Van Nong H, Hung LX, Thang PN, Chinh VD,
Vu LV, Dung PT, Van Trung T and Nga PT: Fabrication and vibration
characterization of curcumin extracted from turmeric (Curcuma
longa) rhizomes of the northern Vietnam. Springerplus.
5:11472016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Basnet P and Skalko-Basnet N: Curcumin: An
anti-inflammatory molecule from a curry spice on the path to cancer
treatment. Molecules. 6:4567–4598. 2011. View Article : Google Scholar
|
|
42
|
Youngjoo K: Estimation of curcumin intake
in Korea based on the Korea national health and nutrition
examination survey (2008–2012). Nutr Res Pract. 8:589–594. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maheshwari RK, Singh AK, Gaddipati J and
Srimal RC: Multiple biological activities of curcumin: A short
review. Life Sci. 78:2081–2087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guan R, Zhao Y, Zhang H, Fan G, Liu X,
Zhou W, Shi C, Wang J, Liu W, Liang X, et al: Draft genome of the
living fossil Ginkgo biloba. Gigascience. 5:492016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang S, Ouyang B, Aa J, Geng J, Fei F,
Wang P, Wang J, Peng Y, Geng T, Li Y, et al: Pharmacokinetics and
tissue distribution of ginkgolide A, ginkgolide B, and ginkgolide K
after intravenous infusion of ginkgo diterpene lactones in a rat
model. J Pharm Biomed Anal. 126:109–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zheng PD, Mungur R, Zhou HJ, Hassan M,
Jiang SN and Zheng JS: Ginkgolide B promotes the proliferation and
differentiation of neural stem cells following cerebral
ischemia/reperfusion injury, both in vivo and in vitro. Neural
Regen Res. 13:1204–1211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Agrawal S: Curcumin and its protective and
therapeutic uses. Natl J Physiol Pharm Pharmacol. 6:1–8. 2016.
View Article : Google Scholar
|
|
48
|
Anroopb N and Shery J: A simple practice
guide for dose conversion between animals and human. J Basic Clin
Pharm. 7:27–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chandran B and Goel A: A randomized, pilot
study to assess the efficacy and safety of curcumin in patients
with active rheumatoid arthritis. Phytother Res. 26:1719–1725.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lyu H, Han A, Polsdofer E, Liu S and Liu
B: Understanding the biology of HER3 receptor as a therapeutic
target in human cancer. Acta Pharm Sin B. 8:503–510. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zeng F and Harris RC: The ErbB receptors
and their ligands in PKD, an overview. Curr Signal Trans Ther.
5:170–180. 2010. View Article : Google Scholar
|
|
52
|
Zheleznova NN, Wilson PD and Staruschenko
A: Epidermal growth factor-mediated proliferation and sodium
transport in normal and PKD epithelial cells. Biochim Biophys Acta.
1812:1301–1313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wilson SJ, Amsler K, Hyink DP, Li X, Lu W,
Zhou J, Burrow CR and Wilson PD: Inhibition of HER-2(neu/ErbB2)
restores normal function and structure to polycystic kidney disease
(PKD) epithelia. Biochim Biophys Acta. 1762:647–655. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Streets AJ, Magayr TA, Huang L, Vergoz L,
Rossetti S, Simms RJ, Harris PC, Peters DJM and Ong ACM: Parallel
microarray profiling identifies ErbB4 as a determinant of cyst
growth in ADPKD and a prognostic biomarker for disease progression.
Am J Physiol Renal Physiol. 312:F577–F588. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Torres VE, Sweeney WE, Wang X, Qian Q,
Harris PC, Frost P and Avner ED: EGF receptor tyrosine kinase
inhibition attenuates the development of PKD in Han:SPRD rats.
Kidney Int. 64:1573–1579. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yamaguchi T, Reif GA, Calvet JP and
Wallace DP: Sorafenib inhibits cAMP-dependent ERK activation, cell
proliferation, and in vitro cyst growth of human ADPKD cyst
epithelial cells. Am J Physiol Renal Physiol. 299:F944–F951. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Spirli C, Okolicsanyi S, Fiorotto R,
Fabris L, Cadamuro M, Lecchi S, Tian X, Somlo S and Strazzabosco M:
ERK1/2-dependent vascular endothelial growth factor signaling
sustains cyst growth in polycystin-2 defective mice.
Gastroenterology. 138:360–371. e367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
De Stephanis L, Bonon A, Varani K, Lanza
G, Gafà R, Pinton P, Pema M, Somlo S, Boletta A and Aguiari G:
Double inhibition of cAMP and mTOR signalling may potentiate the
reduction of cell growth in ADPKD cells. Clin Exp Nephrol.
21:203–211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Uzgare AR, Kaplan PJ and Greenberg NM:
Differential expression and/or activation of P38MAPK, erk1/2, and
jnk during the initiation and progression of prostate cancer.
Prostate. 55:128–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hui L, Bakiri L, Mairhorfer A, Schweifer
N, Haslinger C, Kenner L, Komnenovic V, Scheuch H, Beug H and
Wagner EF: p38alpha suppresses normal and cancer cell proliferation
by antagonizing the JNK-c-Jun pathway. Nat Genet. 39:741–749. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nishio S, Hatano M, Nagata M, Horie S,
Koike T, Tokuhisa T and Mochizuki T: Pkd1 regulates immortalized
proliferation of renal tubular epithelial cells through p53
induction and JNK activation. J Clin Invest. 115:910–918. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu Y, Dai B, Xu C, Fu L, Hua Z and Mei C:
Rosiglitazone inhibits transforming growth factor-β1 mediated
fibrogenesis in ADPKD cyst-lining epithelial cells. PLoS One.
6:e289152011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Leonhard WN, Der Wal AV, Novalic Z, Kunnen
SJ, Gansevoort RT, Breuning MH, Heer ED and Peters DJ: Curcumin
inhibits cystogenesis by simultaneous interference of multiple
signaling pathways: In vivo evidence from a Pkd1-deletion model. Am
J Physiol Renal Physiol. 300:F1193–F1202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Aguiari G, Catizone L and Senno LD:
Multidrug therapy for polycystic kidney disease: A review and
perspective. Am J Nephrol. 37:175–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lieberman HR, Kellogg MD, Fulgoni VL III
and Agarwal S: Moderate doses of commercial preparations of Ginkgo
biloba do not alter markers of liver function but moderate alcohol
intake does: A new approach to identify and quantify biomarkers of
‘adverse effects’ of dietary supplements. Regul Toxicol Pharmacol.
84:45–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shiao N and Chan W: Injury effects of
ginkgolide B on maturation of mouse oocytes, fertilization, and
fetal development in vitro and in vivo. Toxicol Lett. 188:63–69.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hu Y, Mou L, Yang F, Tu H and Lin W:
Curcumin attenuates cyclosporine Ainduced renal fibrosis by
inhibiting hypermethylation of the klotho promoter. Mol Med Rep.
14:3229–3236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen W, Liang Y, Xie L, Lu T, Liu X and
Wang G: Pharmacokintics of the ginkgo bfollowing intravenous
administration of ginkgo B emulsion in rats. Biol Pharm Bull.
30:1–5. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fadus MC, Lau C, Bikhchandani J and Lynch
HT: Curcumin: An age-old anti-inflammatory and anti-neoplastic
agent. J Tradit Complement Med. 7:339–346. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Norman JT: Fibrosis and progression of
autosomal dominant polycystic kidney disease (ADPKD). Biochim
Biophys Acta. 1812:1327–1336. 2011. View Article : Google Scholar : PubMed/NCBI
|