Introduction
Hepatic fibrosis is a pathological change caused by
various endogenous and exogenous damaging factors, such as
inflammation, bacteria, viral infections, alcohol toxicity, drug
toxicity and genetic factors, resulting in chronic liver damage
(1). Hepatic fibrosis manifests in
the abnormal proliferation of connective tissue in the liver,
excessive deposition of extracellular matrix (ECM) and the
activation of hepatic stellate cells (HSCs), which is an inevitable
stage for numerous chronic liver diseases, including viral
hepatitis, alcoholic fatty liver and cholestatic liver disease
progressing to cirrhosis (2). The
activation of HSCs serves a crucial role in the progression of
hepatic fibrosis (3). Under normal
physiological conditions, HSCs remain stationary, with low
proliferative activity and a reduced ability to synthesize
collagen; however, HSCs can be activated and converted into
myofibroblasts under the stimuli of damaging factors (4). Myofibroblasts can secrete
fibroblast-promoting proteins, such as TGF-β, connective tissue
growth factor and tissue inhibitors of metalloproteinases (TIMP),
resulting in the generation of ECM, such as collagen, fibronectin
and laminin, thus serving a key role in the occurrence of hepatic
fibrosis (5). In China, ~300
million individuals suffer from viral hepatitis (primarily caused
by hepatitis B virus), non-alcoholic fatty liver disease and
alcoholic hepatitis (6). Delayed
treatment of hepatic fibrosis can lead to the progression of
various liver diseases to cirrhosis, thus increasing the risk of
concurrent acute/chronic liver failure and HCC (7). Early treatment with antifibrosis drugs
can effectively reverse the process and has become a research
hotspot in hepatic diseases (8).
In recent years, a large number of studies have
demonstrated that long non-coding (lnc)RNAs are involved in the
occurrence and development of hepatic fibrosis by regulating
fibrosis-related cell signaling pathways and activating HSCs
(9–11). lncRNAs are a type of RNA that are
>200 nucleotides in length and lack protein coding abilities
(10). At first, lncRNAs were
considered to be the ‘noise’ of genome transcription with no
biological function. However, increasing research indicated that
lncRNAs are involved in numerous physiological and pathological
processes, including cell differentiation, development,
tumorigenesis, migration and organ and tissue fibrosis (12,13).
lncRNA AK021443 is a newly identified lncRNA, and the expression
levels were found to be increased in tissues of patients with HCC
(14), whereas AK021443 knockdown
inhibits HCC cell proliferation, invasion and migration (15). However, whether AK021443
participates in the regulation of hepatic fibrosis is not
completely understood.
lncRNA research has primarily focused on tumors;
however, increasing research has been conducted to investigate the
role of lncRNAs in fibrotic diseases (16,17). A
recent study suggested that lncRNAs are specifically expressed in a
variety of fibrotic tissues and serve an important regulatory role
in the occurrence and development of fibrosis (9). Therefore, identifying the regulatory
mechanism underlying lncRNAs in fibrotic diseases and their
potential value in the prevention and treatment of fibrosis is of
significance for the clinical treatment of fibrotic diseases.
The aim of the present study was to investigate the
effects of AK021443 overexpression on HSC proliferation and
activation in the LX-2 cell line.
Materials and methods
Cell culture
The human LX-2 HSC cell line (American Type Culture
Collection) was cultured in RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Wisent, Inc.) and 1%
streptomycin/penicillin antibiotics at 37°C with 5% CO2.
The medium was replaced every other day. At 80–90% confluence,
cells were passaged and cells in the logarithmic growth phase were
used for subsequent experiments.
Plasmid construction and
transfection
Recombinant full-length human AK021443 cDNA that was
cloned into the pcDNA3.1 vector (pcDNA-AK021443) was designed and
synthesized by Shanghai GenePharma Co., Ltd. The pcDNA3.1 empty
vector was used as a negative control (Shanghai GenePharma Co.,
Ltd.). Cells at the density of 2×106/ml were transfected
with 2 µg/ml plasmids using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. At 72 h post-transfection, cells were
used for subsequent experiments.
Cell proliferation assay
To determine cell proliferation, cells were cultured
in 96-well plates and transfected with indicated vectors. At 0, 24,
48 and 72 h post-transfection, cell proliferation was assessed
using the Cell Counting Kit-8 (CCK-8) assay (Beyotime Institute of
Biotechnology) according to the manufacturer's instructions.
Briefly, 10 µl CCK-8 working solution was added to each well and
incubated for 2 h at 37°C. Absorbance was measured at a wavelength
of 450 nm using a microplate reader.
Cell cycle analysis
Flow cytometry was performed to analyze the effect
of AK021443 on the cell cycle. Briefly, LX-2 cells were fixed with
70% ethanol overnight at 4°C and incubated with 0.5 mg/ml RNaseA
(Thermo Fisher Scientific, Inc.) at 37°C for 30 min. Following
incubation with 25 µg/ml propidium iodide (Thermo Fisher
Scientific, Inc.) on ice for 1 h in the dark, the cell cycle
distribution was analyzed using a FACSCalibur flow cytometer (BD
Biosciences). Data were analyzed using a flow cytometry software
(iSort Automated Cell Sorter A.0; Thermo Fisher Scientific,
Inc).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from LX-2 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA (1 µg) was reverse transcribed into cDNA (37°C for
15 min and 85°C for 5 sec) using the PrimeScript RT reagent kit
with gDNA Eraser (Takara Biotechnology Co., Ltd.). Subsequently,
qPCR was performed using the TB Green Fast qPCR mix (Takara
Biotechnology Co., Ltd.). The following primers were used for qPCR:
AK021443 forward, 5′-CTTGAACCCAGAAGACAGG-3′ and reverse,
5′-ATGGAACATTAGAGGTAGCAC-3′; and β-actin forward,
5′-ATCGTGCGTGACATTAAGGAGAAG-3′ and reverse,
5′-AGGAAGGAAGGCTGGAAGAGTG-3′. The following thermocycling
conditions were used for qPCR: Initial denaturation at 95°C for 2
min; followed by 40 cycles at 95°C for 20 sec, 58°C for 20 sec and
72°C for 20 sec. mRNA expression levels were quantified using the
2−ΔΔCq method (18) and
normalized to the internal reference gene β-actin.
Western blotting
Total protein was extracted from LX-2 cells using
RIPA buffer (Beyotime Institute of Biotechnology) and quantified
using the bicinchoninic acid protein assay kit (Thermo Fisher
Scientific, Inc.). Equal amounts of protein (50 µg) were separated
via 8–12% SDS-PAGE and transferred onto PVDF membranes (Bio-Rad
Laboratories, Inc.). The membranes were blocked with TBS-0.05%
Tween-20 containing 5% skimmed milk at room temperature for 2 h.
Subsequently, the membranes were incubated overnight at 4°C with
primary antibodies targeted against: cyclin D1 (cat. no. ab16663;
1:200; Abcam), cyclin-dependent kinase 2 (CDK2; cat. no. ab32147;
1:1,000; Abcam), p21 (cat. no. ab109520; 1:5,000; Abcam),
E-cadherin (cat. no. ab40772; 1:10,000; Abcam), N-cadherin (cat.
no. ab76011; 1:10,000; Abcam), vimentin (cat. no. ab92547; 1:5,000;
Abcam), snail (cat. no. ab216347; 1:1,000; Abcam), TIMP1 (cat. no.
ab211926; 1:1,000; Abcam), collagen1 (cat. no. ab34710; 1:10,000;
Abcam), endothelin 1 (ET-1; cat. no. ab113697; 1:250; Abcam),
matrix metallopeptidase (MMP)2 (cat. no. ab92536; 1:5,000; Abcam),
MMP9 (cat. no. ab38898; 1:1,000; Abcam) and GAPDH (cat. no.
sc-32233; 1:10,000; Santa Cruz Biotechnology, Inc.). Following
primary incubation, the membranes were incubated with a goat
anti-rabbit IgG horseradish peroxidase-conjugated secondary
antibody (cat. no. ab205718; 1:10,000; Abcam) at room temperature
for 2 h. Protein bands were visualized using an enhanced
chemiluminescence kit (Cytiva) and ImageJ software (v1.4; National
Institutes of Health) was utilized to analyze the intensity of each
protein band. GAPDH was used as the loading control.
Immunofluorescence (IF)
LX-2 cells cultured on slides were fixed with 4%
paraformaldehyde for 20 min at room temperature (RT), blocked with
5% bovine serum albumin (Beyotime Institute of Biotechnology) at RT
for 30 min and incubated with an anti-collagen1 primary antibody
(cat. no. ab34710; 1:200; Abcam) overnight at 4°C. After washing
with PBS, cells were incubated with a FITC-conjugated goat
anti-rabbit secondary antibody (cat. no. ab6717; 1:5,000; Abcam) in
the dark at 37°C for 1.5 h. After washing with PBS, the slides were
incubated with DAPI (RT, 5 min) for nuclear staining. Stained
slides were observed using a confocal fluorescent microscope
(magnification, ×400).
Detection of inflammatory factors and
oxidative stress level
The levels of the proinflammatory cytokines, TGF-β
(cat. no. ab100647), interleukin (IL)-1β (cat. no. ab100562),
platelet derived growth factor (PDGF; cat. no. ab184860) and
epidermal growth factor (EGF; cat. no. ab217772) were detected
using ELISA kits from Abcam according to the manufacturer's
protocol. Briefly, standard samples and cell supernatants were
added into wells with corresponding antibody coating and incubated
for 2 h at room temperature. Subsequently, conjugate was added to
the wells and incubated for 2 h at room temperature, followed by 50
µl stop solution to terminate the reaction. Absorbance was measured
at a wavelength of 450 nm using a SpectraMax 340 microplate reader
(Molecular Devices LLC). A cellular reactive oxygen species (ROS)
assay kit (cat. no. ab186027; Abcam) was used to determine ROS
levels. Briefly, transfected cells were plated
(4×104/100 µl per well) in a 96-well plate overnight.
Subsequently, cells were stained with ROS red stock solution for 30
min at room temperature. Absorbance was measured using a microplate
reader and ROS levels were determined by the fluorescence increase
at Ex/Em=520/605 nm (19).
Statistical analysis
All experiments were repeated at least three times
and statistical analyses were performed using GraphPrism software
(version 5.0; GraphPad Prism, Inc.). Data are presented as the mean
± SD. Comparisons among groups were analyzed using one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
AK021443 overexpression promotes LX-2
proliferation
To investigate the potential role of lncRNA AK021443
in hepatic fibrosis in vitro, AK021443 was overexpressed in
the LX-2 HSC cell line to observe alterations to cellular molecular
processes associated with hepatic fibrosis. The results verified
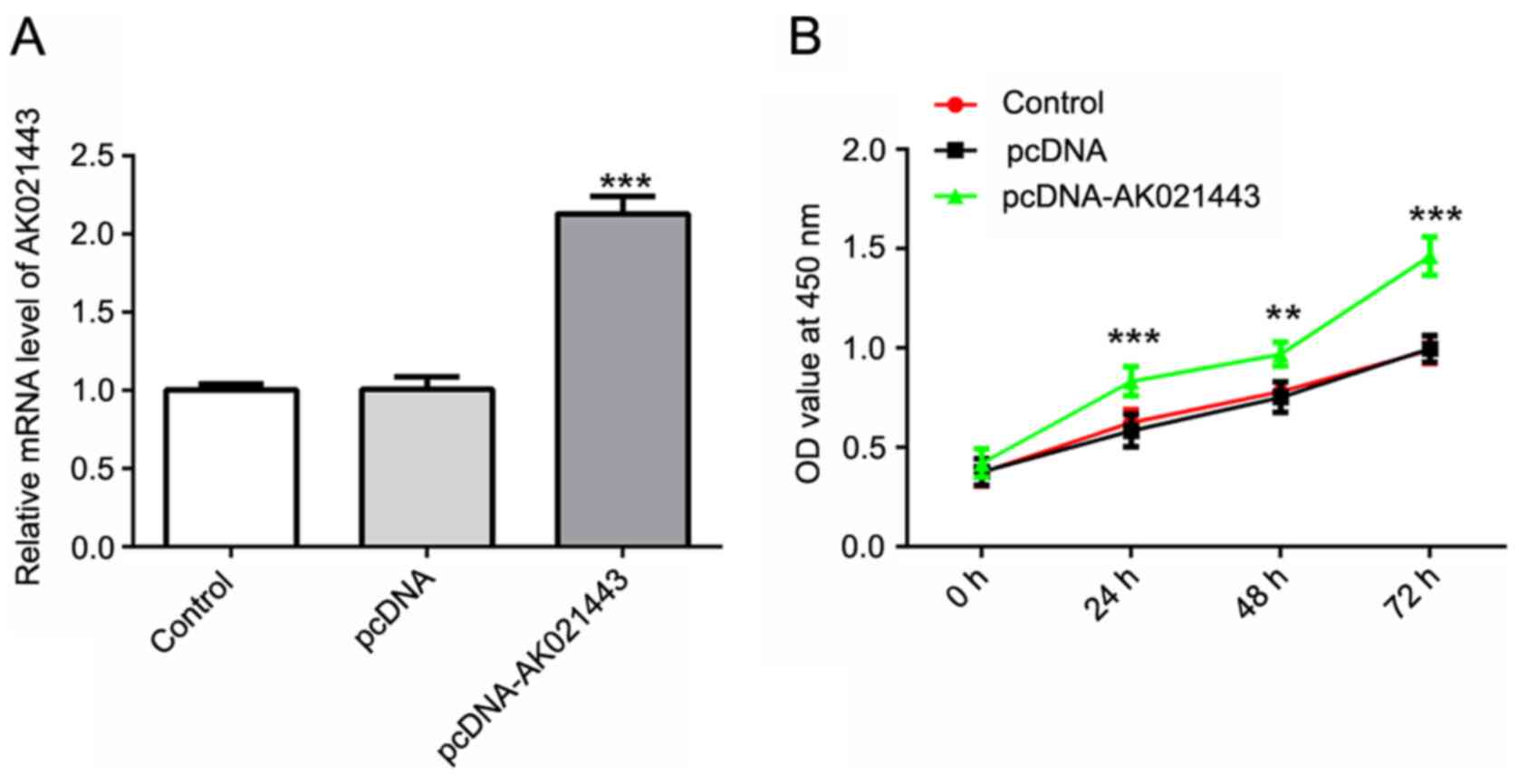
the successful overexpression of AK021443 in cells transfected with
pcDNA-AK021443 compared with cells transfected with pcDNA (Fig. 1A).
Cell proliferation was assessed using a CCK-8 assay,
flow cytometry and western blotting. At 0, 24, 48 and 72 h
post-transfection, cell viability was assessed by performing a
CCK-8 assay. The results suggested that AK021443 overexpression
significantly increased cell proliferation from 24 h in a
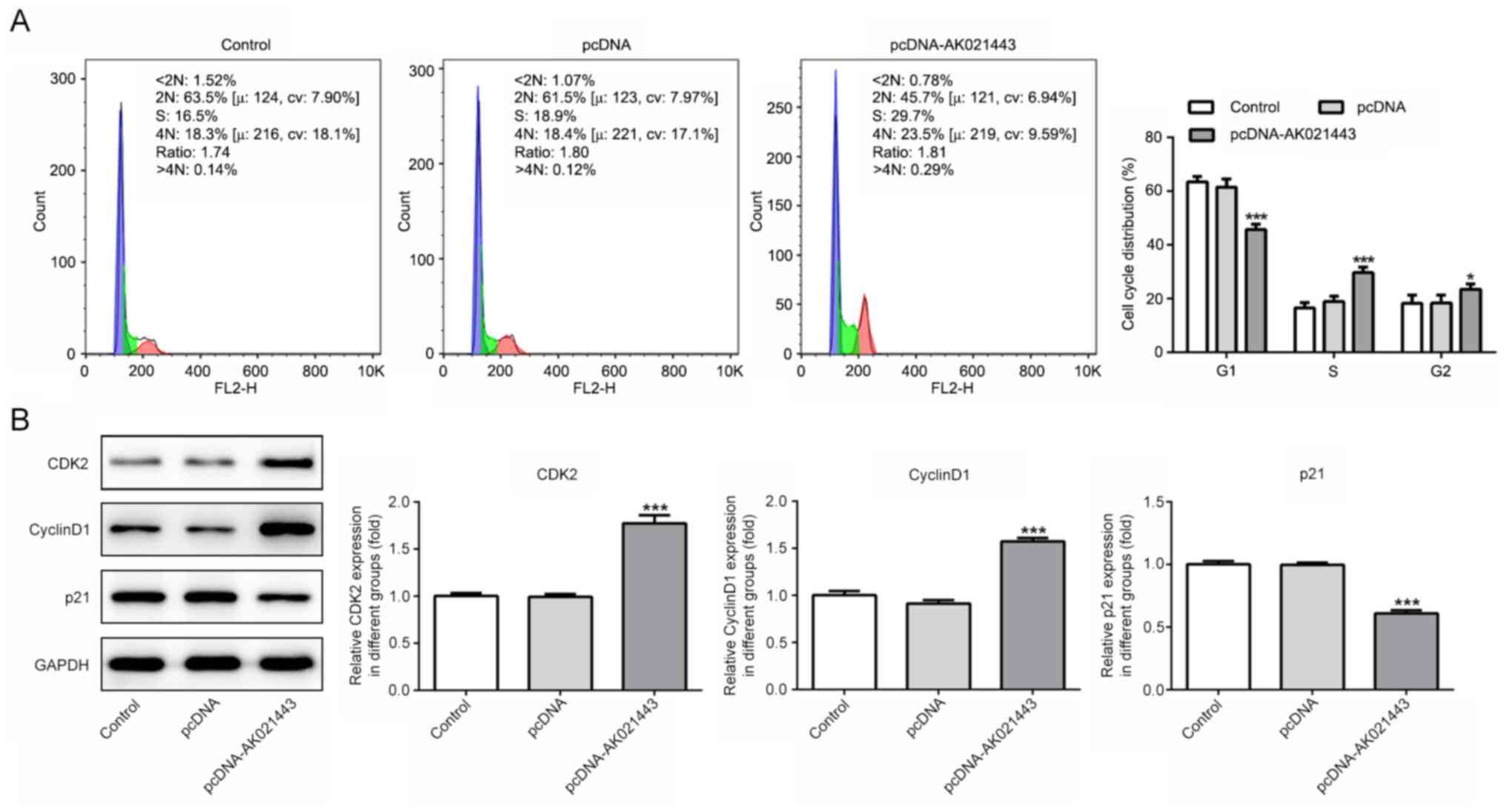
time-dependent manner compared with the pcDNA group (Fig. 1B). Flow cytometry was conducted to
detect the cell cycle distribution, and the results indicated that
pcDNA-AK021443 significantly decreased the proportion of
G1 cells, but significantly increased the proportion of
S and G2 phase cells compared with the pcDNA group
(Fig. 2A). Moreover, the protein
expression levels of cyclin-dependent kinase 2 (CDK2) and cyclin D1
were significantly increased, whereas p21 expression levels were
significantly decreased by pcDNA-AK021443 compared with pcDNA
(Fig. 2B). Therefore, the results
indicated that AK021443 could increase LX-2 cell proliferation.
AK021443 overexpression triggers the
activation and conversion of LX-2 cells into myofibroblasts
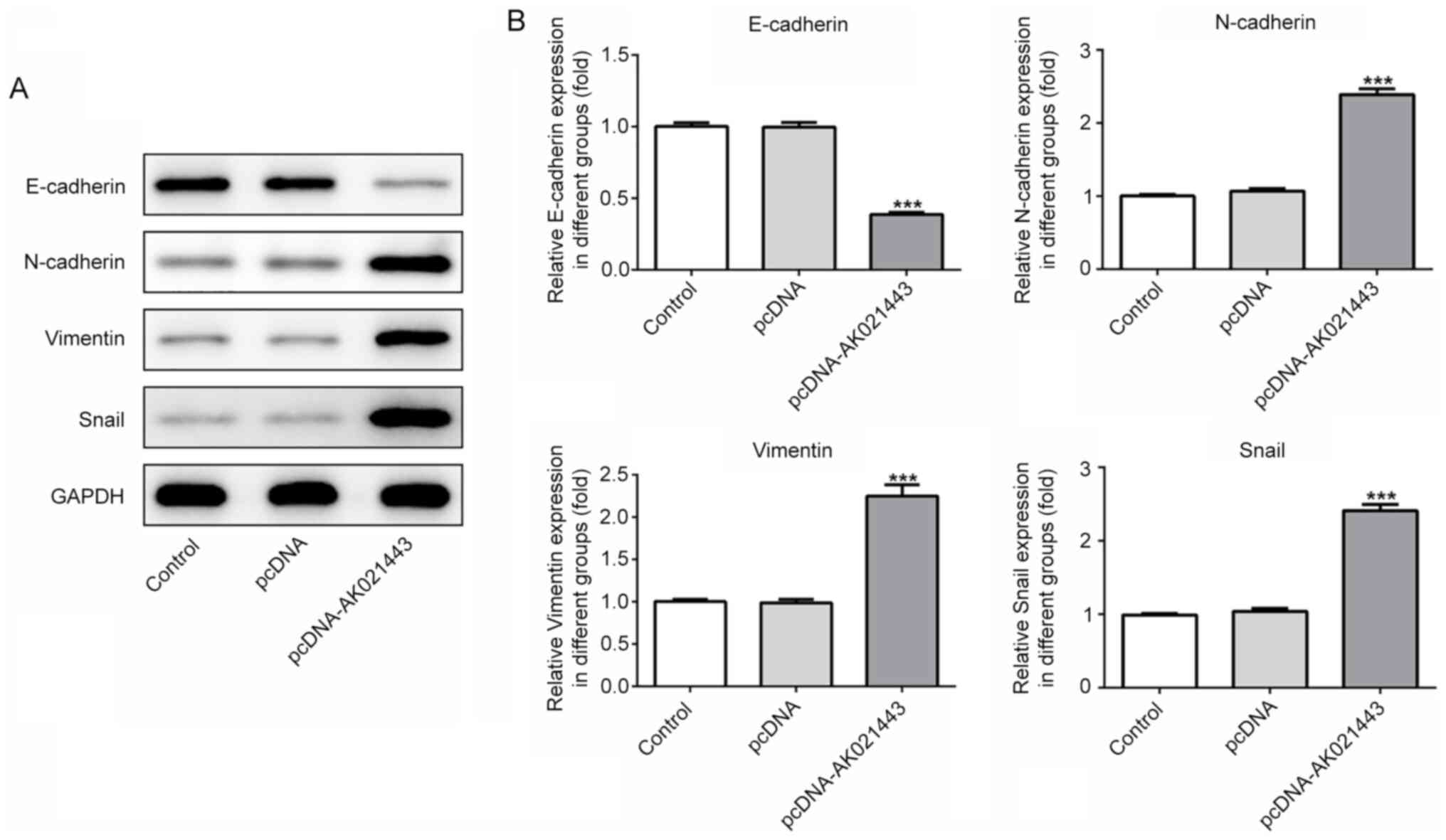
Subsequently, to investigate whether AK021443 could
induce the activation of LX-2 cells and their conversion into
myofibroblasts, the expression levels of proteins associated with
epithelial-mesenchymal transition (EMT) and ECM were detected. The
protein expression level of E-cadherin was significantly decreased,
whereas N-cadherin, vimentin and snail protein expression levels
were significantly increased in AK021443-overexpression LX-2 cells
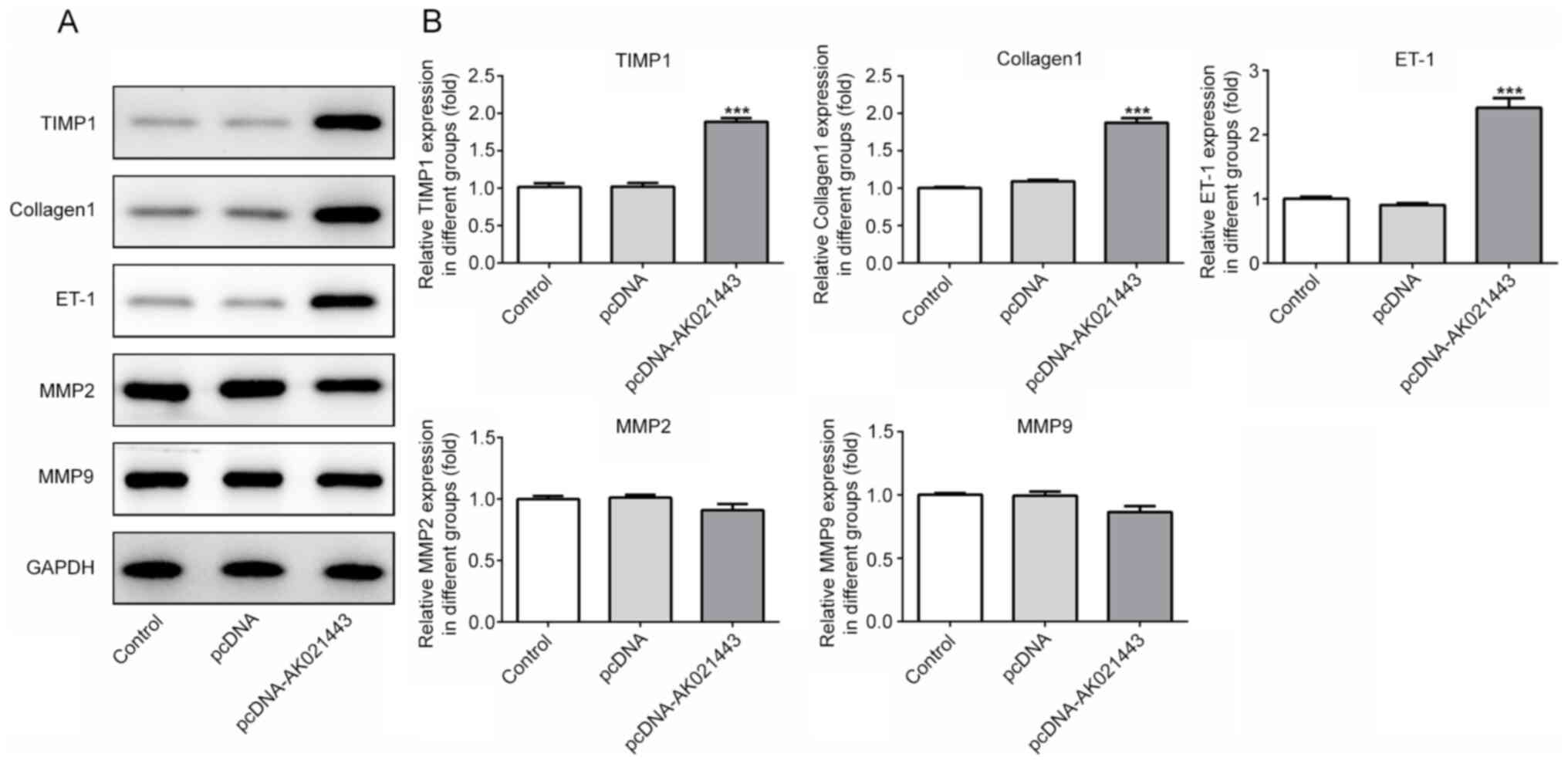
compared with pcDNA-transfected cells (Fig. 3). AK021443 overexpression also
significantly increased the protein expression levels of TIMP1,
collagen1 and ET-1, but slightly reduced the expression levels of
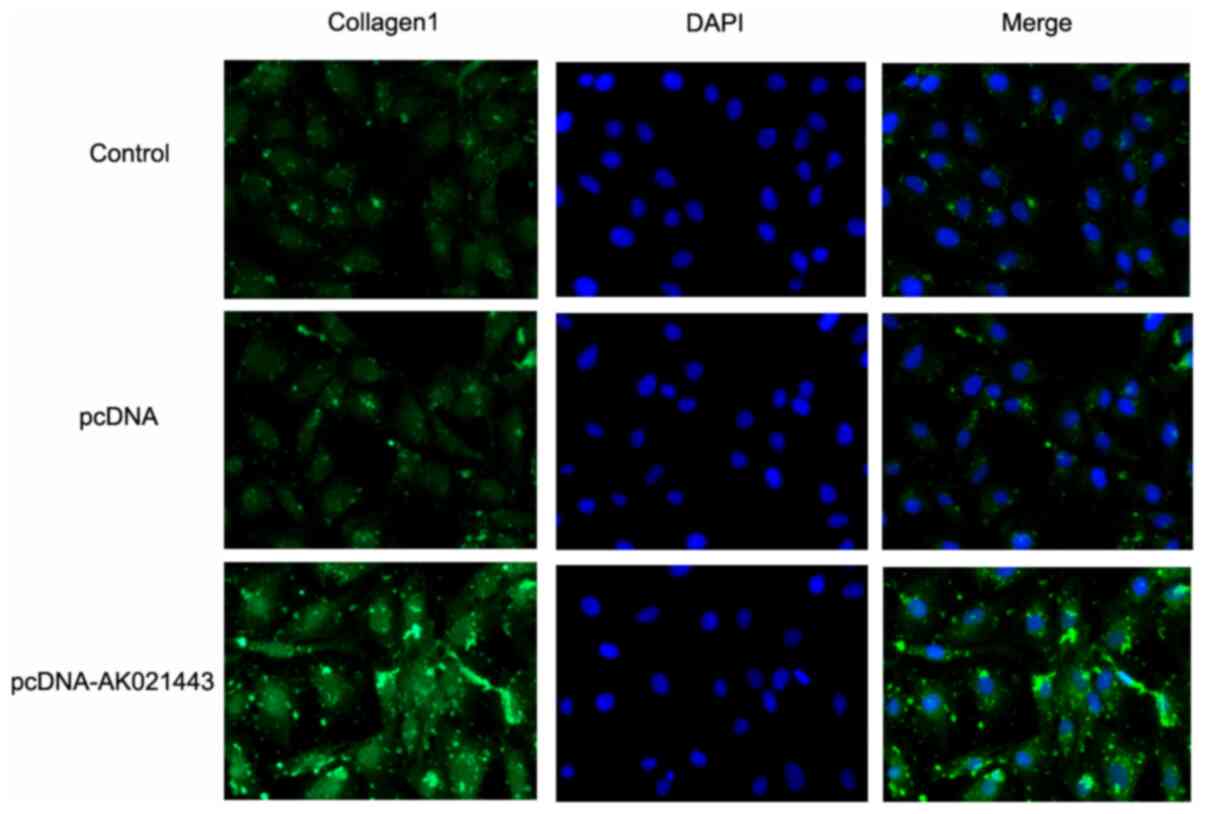
MMP2 and MMP9 compared with the pcDNA group (Fig. 4). In addition, IF staining was
performed to assess collagen1 expression in LX-2 cells transfected
with pcDNA-AK021443. The results suggested that pcDNA-AK021443
increased the levels of collagen1 compared with pcDNA, which was
consistent with the western blotting results (Fig. 5). The results demonstrated that
AK021443 may function to trigger EMT and ECM deposition in
HSCs.
AK021443 overexpression enhances the
generation of the inflammatory cytokines in LX-2 cells
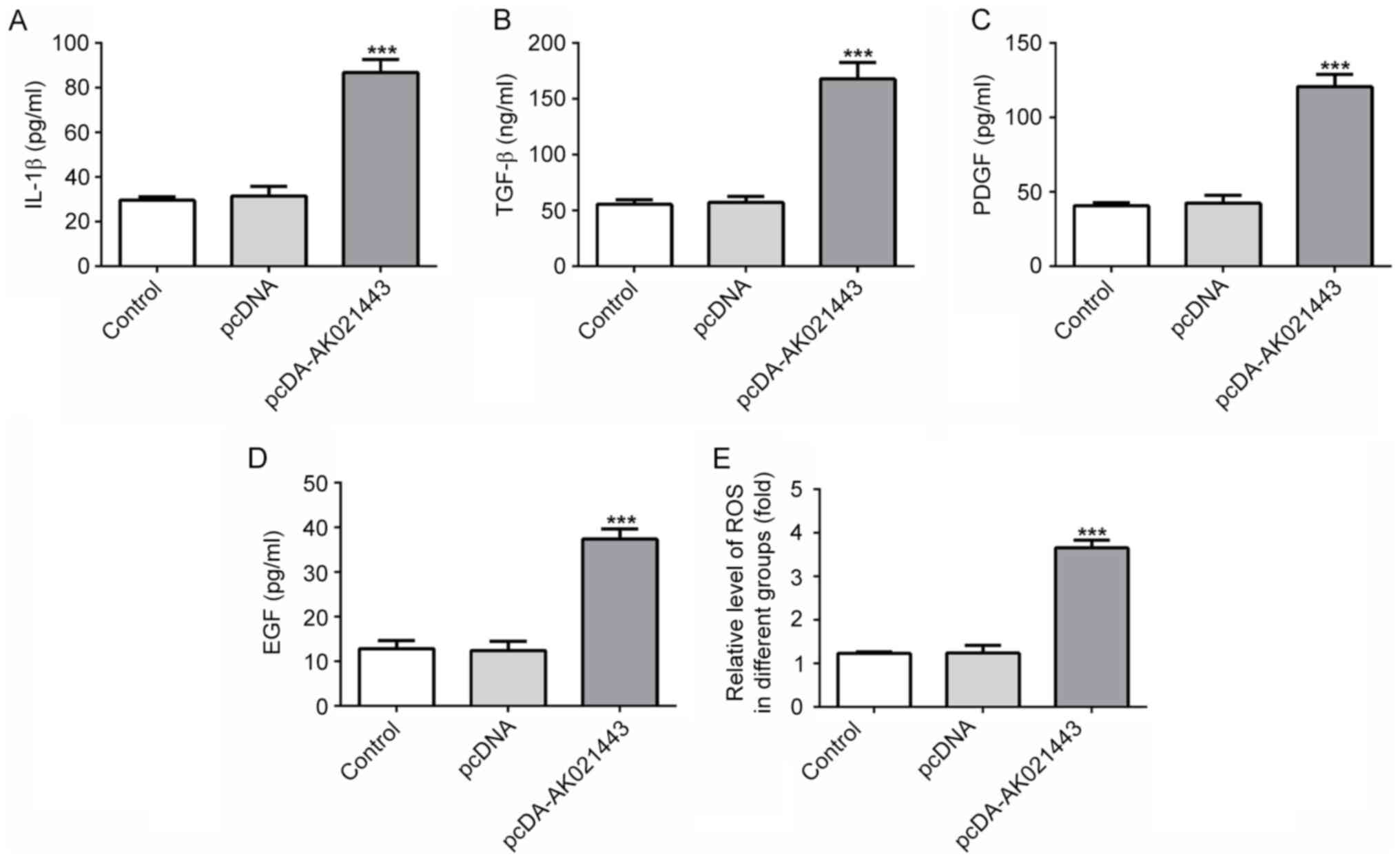
Alterations to the inflammatory response in LX-2
cells were investigated. The results indicated that the generation
of IL-1β, TGF-β, PDGF, EGF and ROS was increased by AK021443
overexpression compared with the pcDNA group (Fig. 6). The results suggested that
AK021443 might display a stimulatory effect on the release of
inflammatory cytokines in LX-2 cells.
Discussion
To the best of our knowledge, the present study
investigated the association between lncRNA AK021443 and hepatic
fibrosis for the first time. The results suggested that
AK021443-overexpression LX-2 cells displayed enhanced
proliferation, EMT, ECM deposition and inflammatory responses
compared with pcDNA-transfected cells, demonstrating the potential
role of AK021443 in promoting hepatic fibrosis development.
Extensive studies have revealed that lncRNAs serve a
vital role in hepatic fibrosis. For example, lncRNA metastasis
associated lung adenocarcinoma transcript 1 promoted HSC activation
by blocking the silent information regulator 1, which mediated the
inhibition of the TGF-β signaling pathway in the progression of
hepatic fibrosis (20,21). In addition, the expression of lncRNA
activated by TGF-β was increased in the liver tissues and plasma of
patients with liver fibrosis, and competed with TGF-β receptor II
and Smad2 to bind to microRNA-425-5p to promote collagen formation
and HSC activation, thereby affecting the occurrence of hepatic
fibrosis (22). Considering the
wide regulatory effects of lncRNAs in hepatic fibrosis and the
promoting effect of lncRNA AK021443 in HCC, the present study
hypothesized that AK021443 may also serve a potential role in
hepatic fibrosis.
Hepatic fibrosis is a chronic pathological process,
and the activation of HSCs, the resident perisinusoidal cell type,
is typically considered to be a pivotal step in hepatic fibrosis
(23). HSCs activated by
inflammation-related chemokines were identified as proliferative
cells that could transform into fibroblasts (24). Fibroblasts excessively secrete and
express collagen, resulting in excessive ECM deposition, thereby
contributing to fibrosis (25).
Therefore, suppressing HSC activation may serve as a potential
therapeutic target for hepatic fibrosis (26). The aim of the present study was to
investigate the effects of AK021443 overexpression on HSC
activation in the LX-2 cell line.
Firstly, the results indicated that AK021443
overexpression significantly increased cell proliferation, the
proportion of S phase cells and the protein expression levels of
CDK2 and cyclin D1, but decreased p21 expression levels compared
with the pcDNA group. The cyclin/CDK/CDK inhibitor (CDKI) network
serves an important role in the regulation of the G1/S
phase during the cell cycle, among which, the cyclin/CDK complex
can facilitate the initiation of the cell cycle and promote cell
proliferation. By contrast, CDKI p21 can bind to the cyclin/CDK
complex, including cyclin D/CDK4/6 and cyclin E/CDK2, thereby
arresting the cell cycle in the G1 phase, leading to the
inhibition of DNA replication and cell proliferation (27). Therefore, alterations to cell
proliferation and cell cycle distribution observed in the present
study suggested that AK021443 promoted HSC proliferation.
Subsequently, based on the aforementioned results,
which suggested that AK021443 enhanced HSC proliferation, whether
AK021443 could induce the transformation of HSC into fibroblasts
was investigated. AK021443 overexpression decreased E-cadherin
expression and increased the expression of N-cadherin, vimentin and
snail compared with the pcDNA group. These proteins are associated
with EMT, whereby epithelial cells lose cell adhesion-associated
junction proteins, such as E-cadherin and occludin, and gain
mesenchymal markers, such as N-cadherin, vimentin and snail, to
promote EMT (28). EMT refers to
the complex process of epithelial cell transformation into
mesenchymal cells. During the process, the polarity of epithelial
cells disappears, and invasive and migratory abilities increase,
accompanied by the downregulation of epithelial markers and the
upregulation of mesenchymal markers (29). On the other hand, the expression
levels of TIMP1, collagen1 and ET-1 were significantly increased,
whereas MMP2 and MMP9 expression levels were reduced by AK021443
overexpression compared with the pcDNA group. TIMP, collagen and
ET-1 are markers of ECM, the deposition of which serves a
profibrotic role in the development of fibrosis (25). MMP2 and MMP9, which can be inhibited
by TIMPs, hydrolyze collagen (30).
In summary, the results indicated that AK021443 might function as a
promoter of HSC transformation into fibroblasts.
Moreover, the results indicated that
AK021443-overexpression cells displayed significantly increased
release of IL-1β, TGF-β, PDGF, EGF and ROS compared with the pcDNA
group. These cytokines participate in the inflammatory response and
fibrillogenesis initiated by endogenous and exogenous damage
factors during fibrosis, and the release of these cytokines can
aggravate inflammation, further promoting fibrosis (7). Moreover, TGF-β is a potent stimulator
of the synthesis of ECM proteins in fibrogenic cells and can
stimulate other signaling pathways to regulate fibroblast function
(31). The excessive generation of
ROS can result in oxidative stress damage, which serves an
important role in the development of liver fibrosis (32). In liver fibrosis, HSC activation is
induced by the increased generation of oxidative free radicals
(33). Therefore, the results
indicated that AK021443 induced HSC cell activation and their
transformation into myofibroblasts.
In summary, the results of the present study
provided a comprehensive understanding of the role of AK021443 in
hepatic fibrosis, indicating that AK021443 promoted HSC cell
activation, proliferation and transformation into myofibroblast by
regulating EMT processes and ECM deposition. The present study
provided novel insights into the role of AK021443 in the occurrence
and development of hepatic fibrosis in vitro. Collectively,
the results indicated that AK021443 upregulation may serve as a
promising marker for hepatic fibrosis and AK021443 inhibition may
serve as a potential therapeutic approach for hepatic fibrosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY and ZM contributed to the conception and design
of the study, and acquired and analyzed the data. YY drafted the
manuscript and revised it critically for important intellectual
content. Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou WC, Zhang QB and Qiao L: Pathogenesis
of liver cirrhosis. World J Gastroenterol. 20:7312–7324. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang CY, Yuan WG, He P, Lei JH and Wang
CX: Liver fibrosis and hepatic stellate cells: Etiology,
pathological hallmarks and therapeutic targets. World J
Gastroenterol. 22:10512–10522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Higashi T, Friedman SL and Hoshida Y:
Hepatic stellate cells as key target in liver fibrosis. Adv Drug
Deliv Rev. 121:27–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tacke F and Weiskirchen R: Update on
hepatic stellate cells: Pathogenic role in liver fibrosis and novel
isolation techniques. Expert Rev Gastroenterol Hepatol. 6:67–80.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang FS, Fan JG, Zhang Z, Gao B and Wang
HY: The global burden of liver disease: The major impact of China.
Hepatology. 60:2099–2108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Manka P, Zeller A and Syn WK: Fibrosis in
chronic liver disease: An update on diagnostic and treatment
modalities. Drugs. 79:903–927. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sui M, Jiang X, Chen J, Yang H and Zhu Y:
Magnesium isoglycyrrhizinate ameliorates liver fibrosis and hepatic
stellate cell activation by regulating ferroptosis signaling
pathway. Biomed Pharmacother. 106:125–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng H, Wan LY, Liang JJ, Zhang YQ, Ai WB
and Wu JF: The roles of lncRNA in hepatic fibrosis. Cell Biosci.
8:632018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong Z, Li S, Wang X, Si L, Ma R, Bao L
and Bo A: lncRNA GAS5 restrains CCl4-induced hepatic
fibrosis by targeting miR-23a through the PTEN/PI3K/Akt signaling
pathway. Am J Physiol Gastrointest Liver Physiol. 316:G539–G550.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen X, Guo H, Xu J and Wang J: Inhibition
of lncRNA HULC improves hepatic fibrosis and hepatocyte apoptosis
by inhibiting the MAPK signaling pathway in rats with nonalcoholic
fatty liver disease. J Cell Physiol. 234:18169–18179. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu J, Bai J, Zhang X, Lv Y, Gong Y, Liu L,
Zhao H, Yu F, Ping Y, Zhang G, et al: A comprehensive overview of
lncRNA annotation resources. Brief Bioinform. 18:236–249.
2017.PubMed/NCBI
|
|
13
|
Yu F, Geng W, Dong P, Huang Z and Zheng J:
LncRNA-MEG3 inhibits activation of hepatic stellate cells through
SMO protein and miR-212. Cell Death Dis. 9:10142018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li YC, Wang D and Zhu GY: Increased
expression of long noncoding RNA AK021443 predicts worse clinical
outcome in hepatocellular carcinoma. Eur Rev Med Pharmacol Sci.
22:4855–4860. 2018.PubMed/NCBI
|
|
15
|
Yang J, Li J, Liu B, Zhang R, Gu F, Zhao J
and Cheng S: Long noncoding RNA AK021443 promotes cell
proliferation and migration by regulating epithelial-mesenchymal
transition in hepatocellular carcinoma cells. DNA Cell Biol.
37:481–490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumar MM and Goyal R: LncRNA as a
therapeutic target for angiogenesis. Curr Top Med Chem.
17:1750–1757. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Z, Jiang S, Shang J, Jiang Y, Dai Y,
Xu B, Yu Y, Liang Z and Yang Y: LncRNA: Shedding light on
mechanisms and opportunities in fibrosis and aging. Ageing Res Rev.
52:17–31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang H, Shen H, Li J and Guo LW:
SIGMAR1/Sigma-1 receptor ablation impairs autophagosome clearance.
Autophagy. 15:1539–1557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun L, Fan Z, Chen J, Tian W, Li M, Xu H,
Wu X, Shao J, Bian Y, Fang M and Xu Y: Transcriptional repression
of SIRT1 by protein inhibitor of activated STAT 4 (PIAS4) in
hepatic stellate cells contributes to liver fibrosis. Sci Rep.
6:284322016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee FT, Mountain AJ, Kelly MP, Hall C,
Rigopoulos A, Johns TG, Smyth FE, Brechbiel MW, Nice EC, Burgess AW
and Scott AM: Enhanced efficacy of radioimmunotherapy with
90Y-CHX-A′-DTPA-hu3S193 by inhibition of epidermal growth factor
receptor (EGFR) signaling with EGFR tyrosine kinase inhibitor
AG1478. Clin Cancer Res. 11:7080s–7086s. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu N, Niu X, Wang Y, Du H, Wang B, Du J,
Li Y, Wang R, Zhang Y, Zhao S, et al: Role of LncRNA-activated by
transforming growth factor beta in the progression of hepatitis C
virus-related liver fibrosis. Discov Med. 22:29–42. 2016.PubMed/NCBI
|
|
23
|
Trautwein C, Friedman SL, Schuppan D and
Pinzani M: Hepatic fibrosis: Concept to treatment. J Hepatol. 62 (1
Suppl):S15–S24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duval F, Moreno-Cuevas JE, Gonzalez-Garza
MT, Rodriguez-Montalvo C and Cruz-Vega DE: Protective mechanisms of
medicinal plants targeting hepatic stellate cell activation and
extracellular matrix deposition in liver fibrosis. Chin Med.
9:272014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baglieri J, Brenner DA and Kisseleva T:
The role of fibrosis and liver-associated fibroblasts in the
pathogenesis of hepatocellular carcinoma. Int J Mol Sci.
20:17232019. View Article : Google Scholar
|
|
26
|
Liu WH, Song FQ, Ren LN, Guo WQ, Wang T,
Feng YX, Tang LJ and Li K: The multiple functional roles of
mesenchymal stem cells in participating in treating liver diseases.
J Cell Mol Med. 19:511–520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Georgakilas AG, Martin OA and Bonner WM:
p21: A two-faced genome guardian. Trends Mol Med. 23:310–319. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang CH, Zhu XD, Ma DN, Sun HC, Gao DM,
Zhang N, Qin CD, Zhang YY, Ye BG, Cai H, et al: Flot2 promotes
tumor growth and metastasis through modulating cell cycle and
inducing epithelial-mesenchymal transition of hepatocellular
carcinoma. Am J Cancer Res. 7:1068–1083. 2017.PubMed/NCBI
|
|
29
|
Yamada S, Fuchs BC, Fujii T, Shimoyama Y,
Sugimoto H, Nomoto S, Takeda S, Tanabe KK, Kodera Y and Nakao A:
Epithelial-to-mesenchymal transition predicts prognosis of
pancreatic cancer. Surgery. 154:946–954. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wynn TA: Common and unique mechanisms
regulate fibrosis in various fibroproliferative diseases. J Clin
Invest. 117:524–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rockey DC, Bell PD and Hill JA: Fibrosis-a
common pathway to organ injury and failure. N Engl J Med.
373:962015.PubMed/NCBI
|
|
32
|
Birben E, Sahiner UM, Sackesen C, Erzurum
S and Kalayci O: Oxidative stress and antioxidant defense. World
Allergy Organ J. 5:9–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qiu YN, Wang GH, Zhou F, Hao JJ, Tian L,
Guan LF, Geng XK, Ding YC, Wu HW and Zhang KZ: PM2.5 induces liver
fibrosis via triggering ROS-mediated mitophagy. Ecotoxicol Environ
Saf. 167:178–187. 2019. View Article : Google Scholar : PubMed/NCBI
|