Introduction
As the most frequently diagnosed malignancy in men
≥65 years old, prostate cancer (PCa) is characterized by high
morbidity and mortality rates worldwide (1,2). The
current morbidity of PCa is as high as 31.1 per 100,000 in the
population, accounting for ~15% of all cancer cases worldwide
(3). Several factors have been
reported to be involved in the development of PCa, including family
history, genetic inheritance and age (4). Radiotherapy, alone or combined with
androgen deprivation therapy, represents a standard treatment
regimen for patients with PCa (5).
However, despite the advancements which have been achieved in local
tumor control, a considerable percentage of patients with PCa still
undergo tumor recurrence (6);
thereafter, these patients quickly progress to an incurable stage
of PCa, with the overall survival merely lasting longer than 3–4
months (7).
MicroRNAs (miRNAs/miRs) are a subtype of non-coding
RNA of 22 nucleotides in length (8), which have been demonstrated to be
crucial post-transcriptional regulators (9). miRNAs have been identified to serve
various roles during cancer progression; for example, miRNAs that
promote tumorigenesis are termed ‘OncomiRs’, while miRNAs that
suppress tumorigenesis are referred to as tumor suppressors
(10,11). Numerous physiological functions,
including stemness maintenance, cell apoptosis, proliferation,
migration and invasion, are regulated by miRNAs (12). In particular, miR-583 has been
discovered to have a role in several types of human disease; for
instance, miR-583 was demonstrated to be important for Zheng
differentiation in chronic hepatitis B (13); downregulated expression levels of
miR-583 were discovered in the sera of patients with congestive
heart failure (14); and miR-583
was reported to negatively regulate the differentiation of natural
killer cells through silencing IL2 receptor γ (15). In addition, miR-583 expression
levels were found to be upregulated in a good recovery group
compared with a poor recovery group following human stroke
(16). miR-583 was also identified
to be involved in circular (circ)RNA_104515 and circ_100291/miRNA
interactions in hepatocellular carcinoma (17). Furthermore, in 2017, a meta-analysis
indicated that miR-583 expression levels were downregulated in
recurrent PCa samples compared with non-recurrent cases (18). However, the function of miR-583 in
PCa has not been reported, which will be explored in the present
study. It was concluded that miR-583 may inhibit the proliferation
and invasion of PCa cells by targeting JAK1.
Materials and methods
Patient samples
Tumor tissues and matched adjacent normal tissues
were obtained from 21 patients with PCa (aged between 57 and 75
years old) who had been treated at The First Affiliated Hospital of
Kunming Medical University (Kunming, China) between January 2015
and June 2017. Participants who had undergone surgical procedures
for tissue extraction were included in the present study. None of
the participants had received radiotherapy or chemotherapy prior to
surgery. Written informed consent was provided by all patients. The
use of tissues from patients with PCa for scientific research was
approved by the ethics committee of The First Affiliated Hospital
of Kunming Medical University.
Cell culture
A human normal prostate epithelial cell line
(RWPE-1) and 4 human PCa cell lines (LNCaP, C4-2, DU145 and PC3)
were purchased from the American Type Culture Collection. All cell
lines were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), and maintained at 37°C with 5%
CO2.
Cell transfection
The miR-583 mimic (50 nM,
5′-CAUUACCCUGGAAGGAGAAAC-3′) and miR-negative control (NC, 50 nM,
5′-UCGCUUGGUGCAGGUCGGG-3′) mimic were purchased from Guangzhou
RiboBio Co., Ltd. pcDNA3 NC (empty vector, 2 µg) and pcDNA3-JAK1 (2
µg) overexpression vectors were purchased from Addgene, Inc. DU145
and PC3 cell lines (3×105 cells/well) were transiently
transfected with each transfectant using Lipofectamine®
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. After incubation at 37°C
for 48 h, cells were harvested for subsequent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from PCa cell lines and
tissues using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
concentration of extracted RNA in each sample was assessed using a
NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc.).
Total RNA was reverse transcribed into cDNA using a PrimeScript™ RT
Master mix (Takara Biotechnology Co., Ltd.) at 37°C for 15 min and
85°C for 5 sec. qPCR was subsequently performed using a SYBR Green
qPCR Master mix (Takara Biotechnology Co., Ltd.) on a CFX96 Touch
Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.) with
the following thermocycling conditions: Denaturation at 94°C for 2
min, followed by 35 cycles of 94°C for 5 sec and 60°C for 30 sec.
The expression levels were quantified using the 2−ΔΔCq
method (19). GAPDH and U6 were
used as the reference genes for JAK1 and miR-583, respectively. The
primers used in the present study were as follows: JAK1 forward,
5′-GTCTTAGACCCCAGCCACAG-3′ and reverse, 5′-CCCCTTCCACAAACTCTTCC-3′;
GAPDH forward, 5′-ATGACCCCTTCATTGACCTCA-3′ and reverse,
5′-GAGATGATGACCCTTTTGGCT-3′; U6 forward,
5′-TGCGGGTGCTCGCTTCGCAGC-3′ and reverse, 5′-CCAGTGCAGGGTCCGAGGT-3′;
and miR-583 forward, 5′-CATTACCCTGGAAGGAGAAAC-3′ and reverse,
5′-GTTTCTCCTTCCAGGGTAATG-3′.
Western blotting
Total protein was extracted from PCa cell lines
using RIPA lysis buffer [Roche Diagnostics (Shanghai) Co., Ltd.].
Protein concentration in each sample was determined using a BCA
protein assay kit (Beyotime Institute of Biotechnology) and protein
(20 µg per lane) was separated via SDS-PAGE on 10% gel. The
separated proteins were subsequently transferred onto a PVDF
membrane and blocked using 5% non-fat milk at room temperature for
1 h. The membranes were then incubated with the following primary
antibodies at 4°C for 12 h: Anti-JAK1 (cat. no. 3344; 1:1,000),
anti-STAT3 (cat. no. 12640; 1:1,000), anti-phosphorylated (p)-STAT3
(cat. no. 9145; 1:1,000) and anti-GAPDH (cat. no. 2118; 1:1,000)
(all Cell Signaling Technology, Inc.). Following the primary
antibody incubation and washing with TBS with 0.1% Tween-20 three
times for 5 min each, the membranes were incubated with a
HRP-conjugated anti-rabbit IgG secondary antibody (cat. no. 7074;
1:1,000; Cell Signaling Technology, Inc.) at room temperature for 2
h. GAPDH was used as the internal reference protein. The
immunoreactive bands were visualized using ECL reagents (Beyotime
Institute of Biotechnology). The densitometric analysis was
performed using ImageJ software (version 1.46; National Institutes
of Health).
MTT assay
DU145 and PC3 cells (3×103 cells/well)
were seeded into 96-well plates and incubated at 37°C for 24, 48 or
72 h. Subsequently, 20 µl MTT solution (5 mg/ml) was added/well
prior to incubation at 37°C for 4 h. Following the incubation, the
culture medium and MTT solution were replaced with 150 µl DMSO
(Sigma-Aldrich; Merck KGaA) to dissolve the purple formazan.
Finally, the absorbance at 570 nm was measured using an ELISA
microplate reader (Thermo Fisher Scientific, Inc.).
Transwell assay
For the invasion assay, DU145 and PC3 cells
(1×104 cells/well) were plated in serum-free RPMI-1640
medium into the upper chambers of Transwell plates (Corning, Inc.)
precoated with Matrigel for 4 h at 37°C (Corning, Inc.). The lower
chamber was filled with 500 µl RPMI-1640 medium supplemented with
20% FBS. Following incubation at 37°C for 24 h, the invasive DU145
and PC3 cells were fixed with 100% methanol for 15 min at room
temperature and stained with 0.1% crystal violet for 20 min at room
temperature. Stained cells were visualized using a light microscope
(magnification, ×100).
Dual luciferase reporter assay
The binding sites between JAK1 and miR-583 were
predicted using the bioinformatics analysis tool, TargetScan 7.1
(http://www.targetscan.org/vert_71/).
The wild-type (WT) and mutant (MUT) type of the JAK1
3′-untranslated region (UTR), which was generated using the
QuickChange Site-Directed Mutagenesis kit (Agilent Technologies,
Inc.) were cloned into the pGL3 vector (Promega Corporation). The
WT (0.4 mg) and MUT (0.4 mg) vectors were co-transfected with the
miR-583 mimic (20 nM) or miR-NC mimic (20 nM) into DU145 and PC3
cells (1×104 cells/well) using Lipofectamine 2000.
Following incubation at 37°C for 48 h, the relative luciferase
activity in each group was measured using a Dual Luciferase
Reporter assay system (Promega Corporation). Firefly luciferase
activity was normalized to Renilla (Promega Corporation)
luciferase activity.
Statistical analysis
Statistical analysis was performed using GraphPad
6.0 software (GraphPad Software, Inc.). All experiments were
performed in triplicate and data are presented as the mean ± SD. An
unpaired Student's t-test was used for the comparisons between two
groups in the PCa cell lines, whereas a paired Student's t-test was
applied for the comparisons between the PCa tissues and the
adjacent normal tissues. Statistical differences between ≥3 groups
were determined using a one-way ANOVA followed by a
Student-Newman-Keuls test or Tukey's post hoc test for multiple
comparisons. Pearson's correlation analysis was performed to
determine the correlation between miR-583 and JAK1 mRNA expression
levels in PCa tissues. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-583 expression levels are
downregulated, while JAK1 expression levels are upregulated, in PCa
cell lines
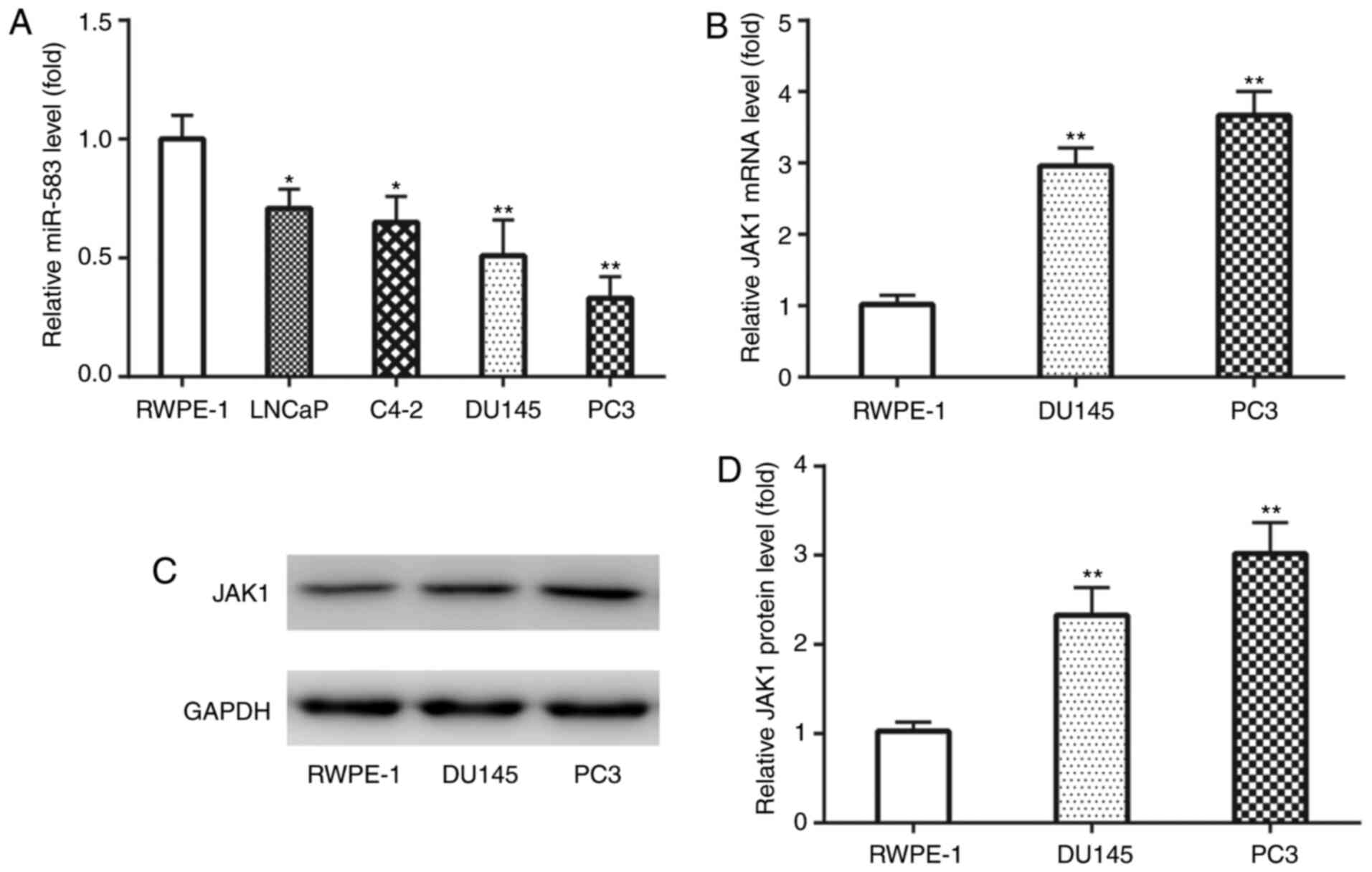
The expression levels of miR-583 and JAK1 were first
analyzed in PCa cell lines. RT-qPCR analysis revealed that the
expression levels of miR-583 were significantly downregulated in
LNCaP, C4-2, DU145 and PC3 cell lines compared with the RWPE-1 cell
line (Fig. 1A). Since DU145 and PC3
cell lines exhibited markedly lower miR-583 expression levels
compared with LNCaP and C4-2 cells, DU145 and PC3 cell lines were
used for subsequent experiments.
Conversely, RT-qPCR analysis demonstrated that JAK1
mRNA expression levels were significantly upregulated in DU145 and
PC3 cell lines compared with the RWPE-1 cell line (Fig. 1B). Similarly, western blotting also
revealed that JAK1 protein expression levels were also
significantly upregulated in DU145 and PC3 cell lines compared with
the RWPE-1 cell line (Fig. 1C and
D). These results indicated the potential underlying
involvement of miR-583 and JAK1 in the development of PCa.
miR-583 inhibits the proliferation and
invasion of PCa cell lines, which is partially abolished by
JAK1
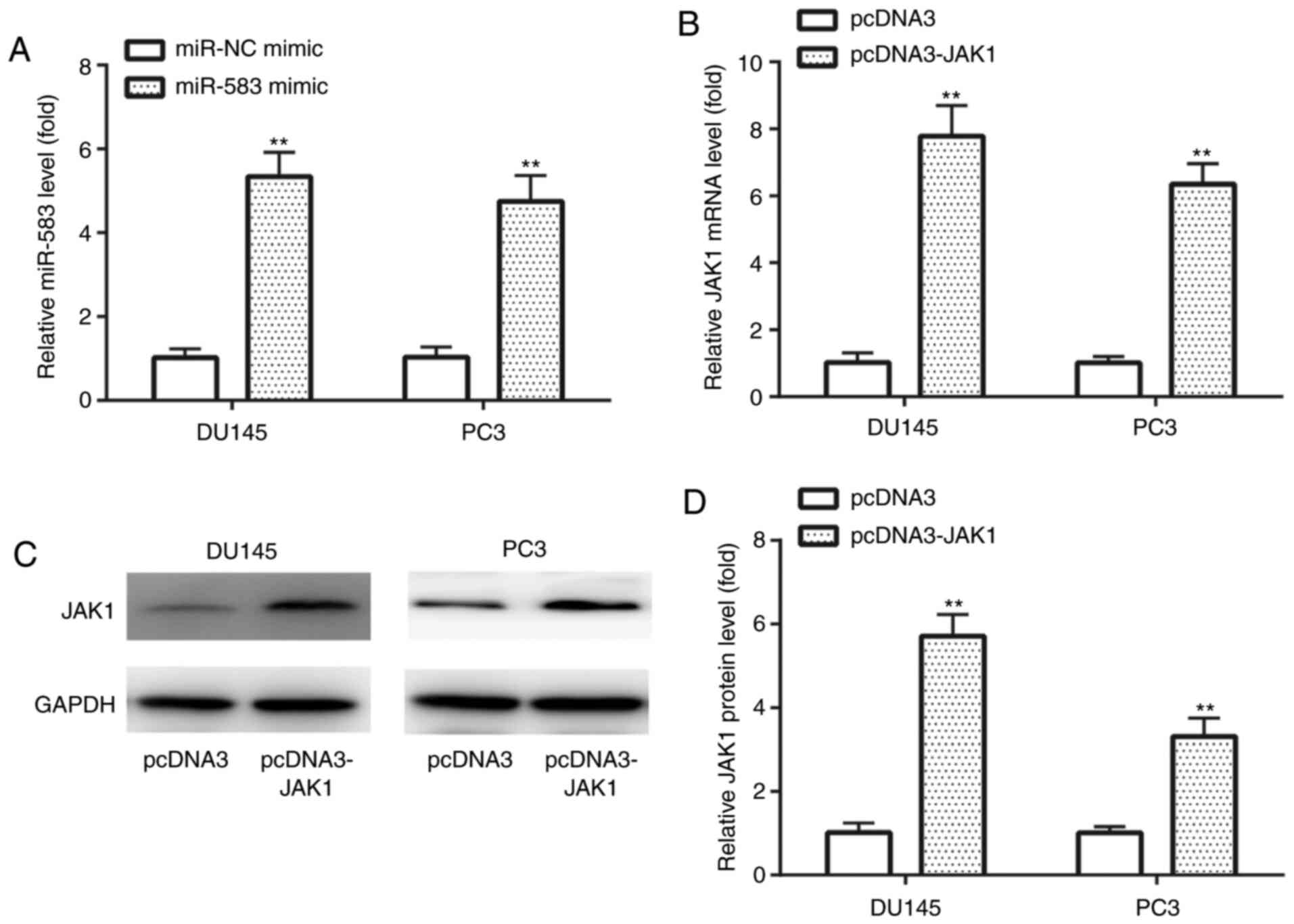
In DU145 and PC3 cell lines, RT-qPCR analysis
discovered that miR-583 expression levels were significantly
upregulated in the miR-583 mimic group compared with the miR-NC
mimic group (Fig. 2A). In addition,
RT-qPCR was also used to demonstrate that JAK1 mRNA expression
levels were significantly upregulated in the pcDNA3-JAK1 group
compared with the pcDNA3 group (Fig.
2B). Similarly, western blotting revealed that JAK1 protein
expression levels were significantly upregulated in the pcDNA3-JAK1
group compared with the pcDNA3 group in both cell lines (Fig. 2C and D). These findings indicated
the successful transfection of miR-583 mimic and pcDNA-JAK1 into
DU145 and PC3 cell lines.
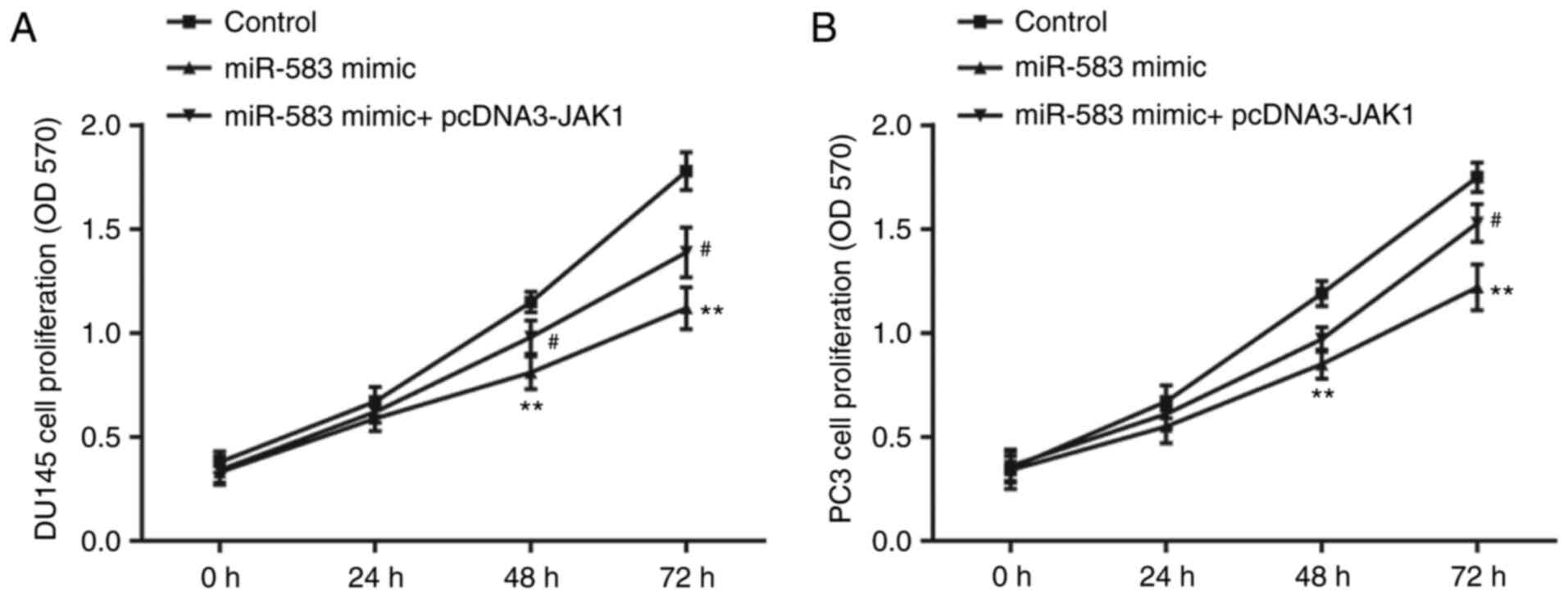
In the DU145 cell line, the results of the MTT assay
demonstrated that the proliferation was significantly decreased by
the miR-583 mimic compared with the control (miR-NC mimic +
pcDNA3), which was then subsequently partially reversed by the
co-transfection with pcDNA3-JAK1 (Fig.
3A). The results of the MTT assay in the PC3 cell line showed a
similar trend in the proliferative ability of each group (Fig. 3B).
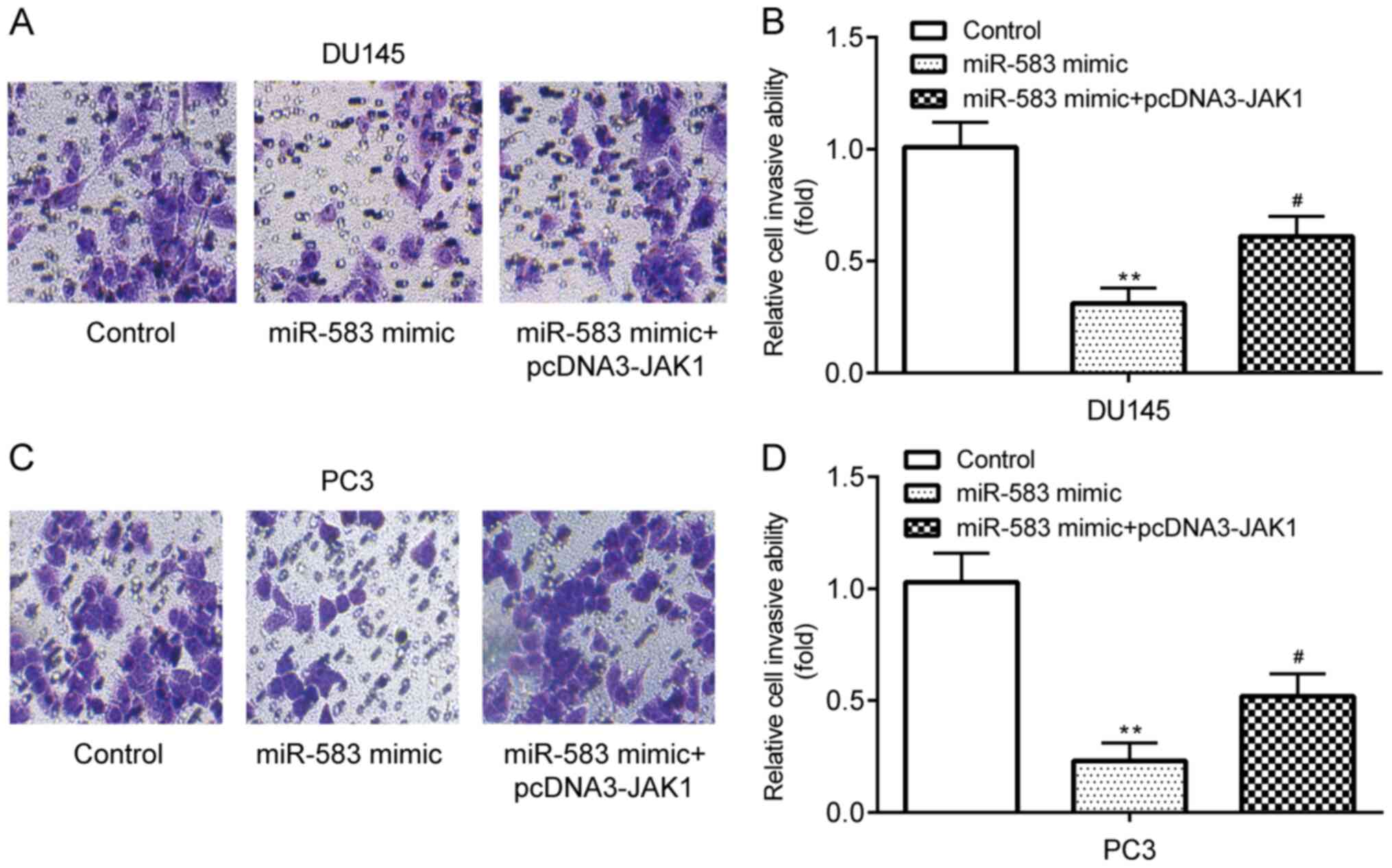
Transwell assays demonstrated that DU145 cell
invasion was significantly inhibited following the transfection
with the miR-583 mimic compared with the control group (miR-NC
mimic + pcDNA3), which was subsequently partially reversed by the
co-transfection with pcDNA3-JAK1 (Fig.
4A and B). The PC3 cell invasive ability exhibited similar
trends to the DU145 cell line following each transfection (Fig. 4C and D). These findings indicated
the potential involvement of miR-583 and JAK1 in the proliferation
and invasion of PCa; however, the responsible molecules and the
association between miR-583 and JAK1 remains undetermined.
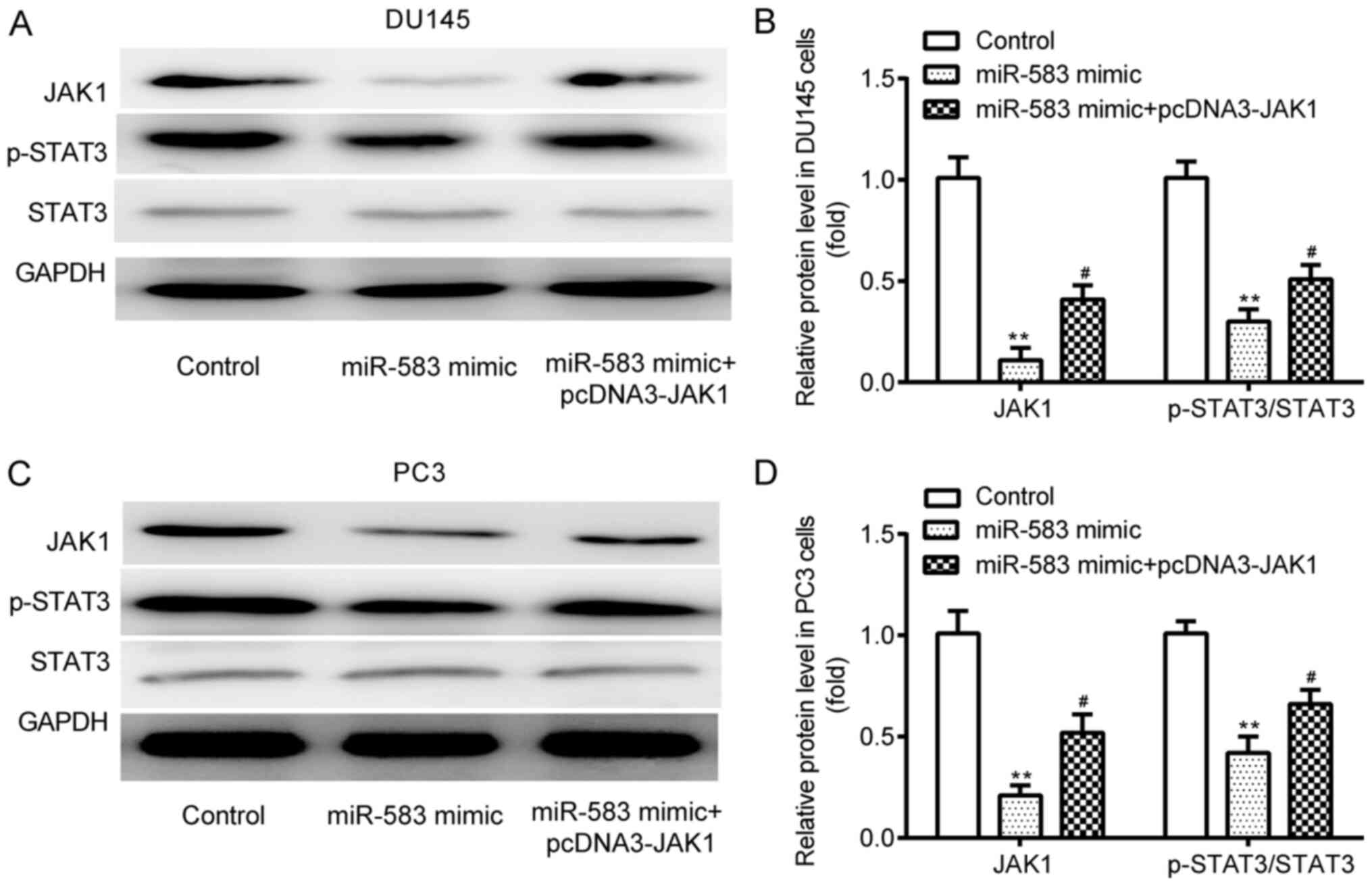
miR-583 downregulates JAK1 and p-STAT3
expression levels, which is partially reversed by JAK1
overexpression
In the DU145 cell line, western blotting revealed
that JAK1 and p-STAT3 expression levels were significantly
downregulated by the miR-583 mimic compared with the control
(miR-NC mimic + pcDNA3); however, these expression levels were
subsequently significantly upregulated by the co-transfection with
pcDNA3-JAK1 (Fig. 5A and B). No
significant differences were observed in the expression levels of
STAT3 between the 3 groups. Similar trends were obtained in the
expression levels of JAK1, p-STAT3 and STAT3 in the PC3 cell line
between the three groups (Fig. 5C and
D).
JAK1 is a target of miR-583
The complementary binding sequences between position
186–193 of the JAK1 3′-UTR and miR-583 are presented in Fig. 6A. In DU145 (Fig. 6B) and PC3 (Fig. 6C) cells, the binding between
position 186–193 of the WT JAK1 3′-UTR and miR-583 was confirmed
using dual luciferase reporter assays, exhibiting that the relative
luciferase activity in the WT JAK1 vectors co-transfected with
miR-583 mimic was significantly lower than that of miR-NC mimic,
whereas no significant difference was observed between the MUT JAK1
vectors co-transfected with miR-583 mimic and miR-NC mimic. The
complementary binding sequences between position 1,301–1,307 of the
WT JAK1 3′-UTR and miR-583 are shown in Fig. 6D. In DU145 (Fig. 6E) and PC3 (Fig. 6F) cells, the binding between
position 1,301–1,307 of the WT JAK1 3′-UTR and miR-583 was
confirmed using dual luciferase reporter assays, exerting that the
relative luciferase activity in the WT JAK1 vectors co-transfected
with miR-583 mimic was decreased compared with miR-NC mimic,
whereas no significant difference was observed between the MUT JAK1
vectors co-transfected with miR-583 mimic and miR-NC mimic.
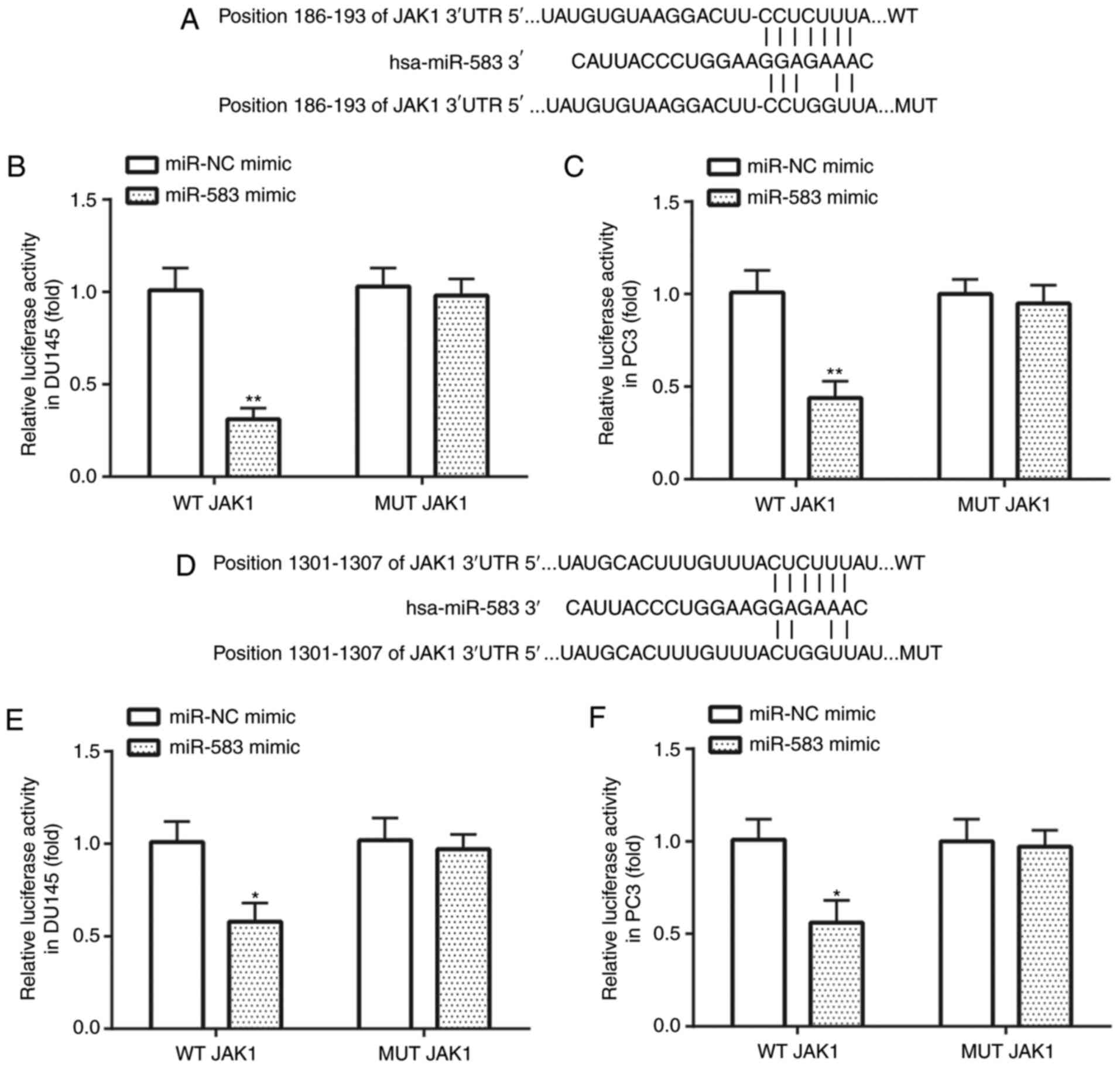 | Figure 6.JAK1 is targeted by miR-583 in
prostate cancer cell lines. (A) The binding between position
186–193 of the WT JAK1 3′-UTR and miR-583. In (B) DU145 and (C) PC3
cell lines, the transfection with the miR-583 mimic repressed the
relative luciferase activity in cells transfected with WT, but not
MUT JAK1. (D) The binding between position 1,301–1,307 of the WT
JAK1 3′-UTR and miR-583. In (E) DU145 and (F) PC3 cells, the
transfection with the miR-583 mimic repressed the relative
luciferase activity in cells transfected with WT, but not MUT JAK1.
*P<0.05, **P<0.01 vs. miR-NC mimic + WT JAK1. miR, microRNA;
NC, negative control; UTR, untranslated region; WT, wild-type; MUT,
mutant. |
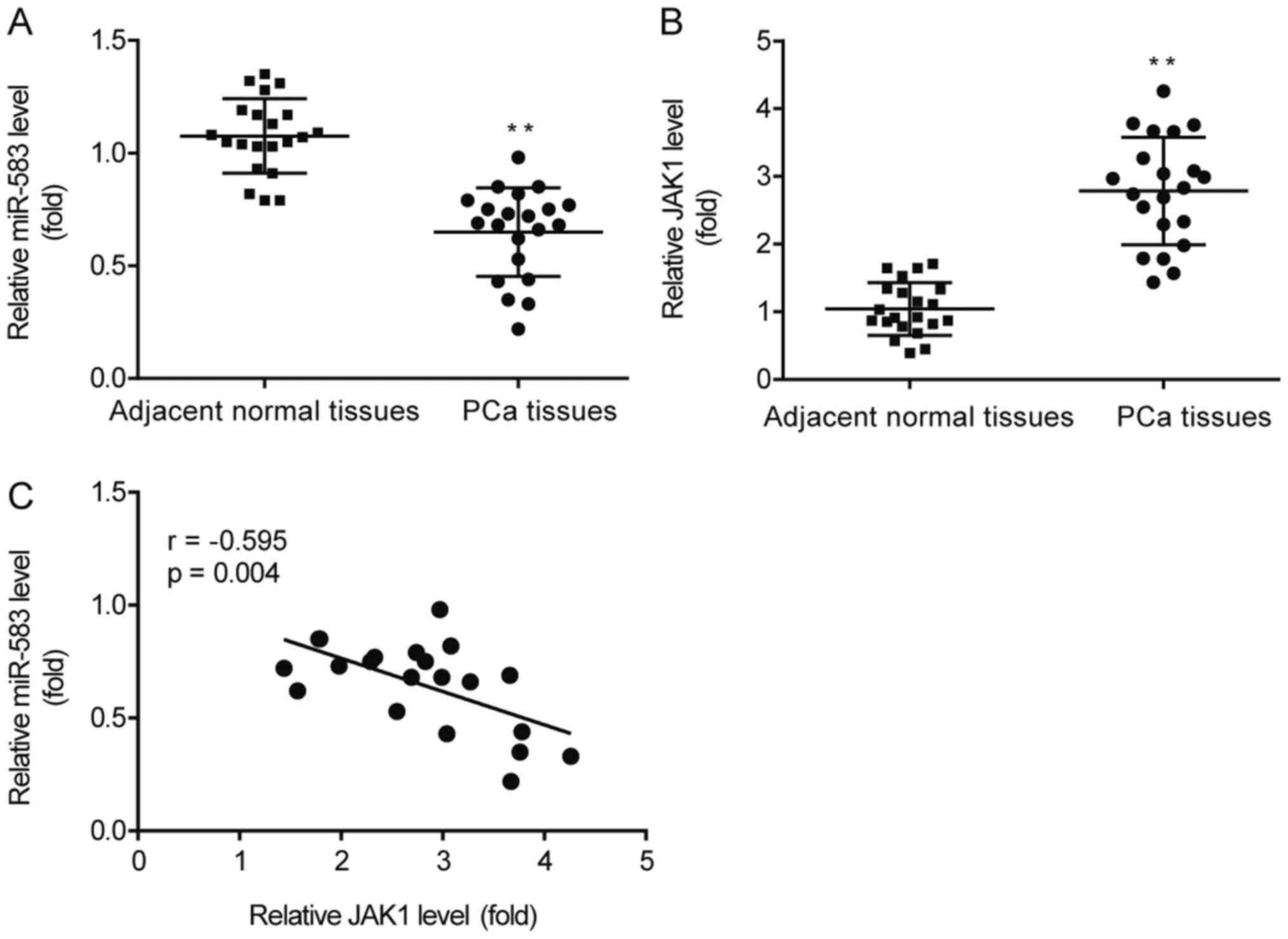
miR-583 expression levels are
negatively correlated with JAK1 expression levels in PCa
tissues
RT-qPCR analysis revealed that miR-583 expression
levels were significantly downregulated (Fig. 7A), while JAK1 expression levels were
significantly upregulated (Fig.
7B), in PCa tissues compared with the adjacent normal tissues.
In addition, in the PCa tissues, a negative correlation was
identified between miR-583 and JAK1 expression levels (r=−0.595;
P=0.004), with a 95% confidential interval of −0.817 to −0.220
(Fig. 7C).
Discussion
PCa, which is characterized with high morbidity and
mortality rates, is the most common type of malignancy to be
diagnosed in men (2). Despite the
advancements in medical technology, the treatment options available
for PCa remain unsatisfactory and tumor recurrence is common in
patients with PCa (6), therefore,
the overall survival rate is ~3–4 months (7). Therefore, it is of great significance
to investigate novel therapeutic targets for PCa.
To date, numerous non-coding RNAs have been
demonstrated to have a crucial role in various types of cancer,
including PCa (20). For example,
miR-215-5p repressed the metastasis of PCa through targeting
phosphoglycerate kinase 1 (21);
miR-1272 reduced the migration and invasion of PCa by targeting
huntingtin interaction protein 1 (22); and the inhibition of miR-4286
inhibited the proliferation and promoted the apoptosis of PCa by
targeting spalt like transcription factor 1 (23). As for the role of miR-583 in PCa, to
the best of our knowledge, only one meta-analysis report exists
reporting its downregulation in recurrent PCa samples compared with
non-recurrent cases (18). The
findings of the present study revealed that miR-583 expression
levels were downregulated in PCa tissues and cell lines, further
indicating its potential tumor suppressive role in the development
of PCa. However, to the best of our knowledge, until now, the
function of miR-583 and the mRNA targets for miR-583 in PCa have
not been reported.
Metastasis is the primary cause for PCa-related
death (24) and miRNAs have been
demonstrated to regulate multiple steps of the metastatic process
in PCa by targeting their specific mRNAs (25). Consequently, the roles of oncogenic
mRNAs, which have also been associated with the metastasis of PCa,
should be further investigated. For instance, miR-448 was
discovered to suppress metastasis in pancreatic ductal
adenocarcinoma by targeting the JAK1/STAT3 signaling pathway
(26); miR-214 inhibited
proliferation and invasion in lung cancer by targeting JAK1
(27); and miR-769-5p reduced the
migration and invasion of oral squamous cell carcinoma by targeting
the JAK1/STAT3 signaling pathway (28). Moreover, the knockdown of JAK1
suppressed the IL-6-induced induction of PCa metastasis (29). However, to the best of our
knowledge, no previous studies have determined whether miR-583
functions through regulating JAK1 in PCa. Consistent with the
aforementioned reports concerning the potential oncogenetic role of
JAK1 during the progression of pancreatic ductal adenocarcinoma,
lung cancer, oral squamous cell carcinoma, as well as PCa, the
results of the current study also revealed that JAK1 expression
levels were upregulated in PCa tissue and cells, which were found
to be repressed and targeted by miR-583.
It is commonly known that the JAK/STAT3 signaling
pathway plays crucial roles in the progression of PCa. miR-17 has
been demonstrated to inhibit PCa cell proliferation and induce PCa
cell apoptosis by inactivating the JAK/STAT3 signaling pathway
(30). Furthermore. capsazepine has
been reported to inhibit tumor growth and cell survival of PCa by
inactivation of JAK/STAT3 signaling (31), and miR-124 can reduce the invasion
and proliferation of PCa cells by inhibiting the activation of
JAK/STAT3 signaling pathway (32).
However, to the best of our knowledge, the relationship between
miR-583 and JAK1 in PCa metastases remained unknown until the
present study.
The findings of the present study indicated that
miR-583 mimic may inhibit PCa proliferation and invasion by
targeting JAK1 and inactivating the p-STAT3 signaling pathway, thus
providing a potential novel therapeutic target for patients with
PCa.
Acknowledgements
Not applicable.
Funding
The present study is funded by Zhejiang Natural
Science Foundation (grant no. LY19H050006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL and JC performed the experiments and analyzed the
data. JC conceived and designed the study, and supervised and wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The use of tissues from patients with PCa for
scientific research was approved by the ethics committee of The
First Affiliated Hospital of Kunming Medical University. Written
informed consent was provided by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Perkins C, Balma D, Garcia R; Members of
the Consensus Group, ; Susan G: Komen for the cure: Why current
breast pathology practices must be evaluated. A Susan G. Komen for
the Cure white paper: June 2006. Breast J. 13:443–447. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hass GP, Delongchamps N, Brawley OW, Wang
CY and de la Roza G: The worldwide epidemiology of prostate cancer:
Perspectives from autopsy studies. Can J Urol. 15:3866–3871.
2008.PubMed/NCBI
|
|
4
|
Cancer Genome Atlas Research Network, .
The molecular taxonomy of primary prostate cancer. Cell.
163:1011–1025. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. Part 1:
Screening, diagnosis, and local treatment with curative
intent-update 2013. Eur Urol. 65:124–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tetreault-Laflamme A and Crook J: Options
for salvage of radiation failures for prostate cancer. Semin Radiat
Oncol. 27:67–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sternberg CN: Novel hormonal therapy for
castration-resistant prostate cancer. Ann Oncol. 23 (Suppl
10):x259–x263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pasquinelli AE: MicroRNAs and their
targets: Recognition, regulation and an emerging reciprocal
relationship. Nat Rev Genet. 13:271–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki H, Maruyama R, Yamamoto E and Kai
M: Epigenetic alteration and microRNA dysregulation in cancer.
Front Genet. 4:2582013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramalho-Carvalho J, Fromm B, Henrique R
and Jerónimo C: Deciphering the function of non-coding RNAs in
prostate cancer. Cancer Metastasis Rev. 35:235–262. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stefani G and Slack FJ: A ‘pivotal’ new
rule for microRNA-mRNA interactions. Nat Struct Mol Biol.
19:265–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Guan Y, Lu YY, Hu YY, Huang S and
Su SB: Circulating miR-583 and miR-663 refer to ZHENG
differentiation in chronic hepatitis B. Evid Based Complement
Alternat Med. 2013:7513412013.PubMed/NCBI
|
|
14
|
Cakmak HA, Coskunpinar E, Ikitimur B,
Barman HA, Karadag B, Tiryakioglu NO, Kahraman K and Vural VA: The
prognostic value of circulating microRNAs in heart failure:
Preliminary results from a genome-wide expression study. J
Cardiovasc Med (Hagerstown). 16:431–437. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yun S, Lee SU, Kim JM, Lee HJ, Song HY,
Kim YK, Jung H, Park YJ, Yoon SR, Oh SR, et al: Integrated
mRNA-microRNA profiling of human NK cell differentiation identifies
MiR-583 as a negative regulator of IL2Rγ expression. PLoS One.
9:e1089132014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edwardson MA, Zhong X, Fiandaca MS,
Federoff HJ, Cheema AK and Dromerick AW: Plasma microRNA markers of
upper limb recovery following human stroke. Sci Rep. 8:125582018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiong DD, Dang YW, Lin P, Wen DY, He RQ,
Luo DZ, Feng ZB and Chen G: A circRNA-miRNA-mRNA network
identification for exploring underlying pathogenesis and therapy
strategy of hepatocellular carcinoma. J Transl Med. 16:2202018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pashaei E, Pashaei E, Ahmady M, Ozen M and
Aydin N: Meta-analysis of miRNA expression profiles for prostate
cancer recurrence following radical prostatectomy. PLoS One.
12:e01795432017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beermann J, Piccoli MT, Viereck J and Thum
T: Non-coding RNAs in development and disease: Background,
mechanisms, and therapeutic approaches. Physiol Rev. 96:1297–1325.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen JY, Xu LF, Hu HL, Wen YQ, Chen D and
Liu WH: MiRNA-215-5p alleviates the metastasis of prostate cancer
by targeting PGK1. Eur Rev Med Pharmacol Sci. 24:639–646.
2020.PubMed/NCBI
|
|
22
|
Rotundo F, Cominetti D, EI Bezawy R,
Percio S, Doldi V, Tortoreto M, Zuco V, Valdagni R, Zaffaroni N and
Gandellini P: miR-1272 exerts tumor-suppressive functions in
prostate cancer via HIP1 suppression. Cells. 9:4352020. View Article : Google Scholar
|
|
23
|
Li Z, Zhao S, Wang H, Zhang B and Zhang P:
miR-4286 promotes prostate cancer progression via targeting the
expression of SALL1. J Gene Med. e31272019.(Epub ahead of print).
PubMed/NCBI
|
|
24
|
Sartor O and de Bono JS: Metastatic
prostate cancer. N Engl J Med. 378:645–657. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bhagirath D, Yang TL, Dahiya R and Saini
S: MicroRNAs as regulators of prostate cancer metastasis. Adv Exp
Med Biol. 1095:83–100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu DL, Zhang T, Wu K, Li Y, Wang J, Chen
J, Li XQ, Peng XG, Wang JN and Tan LG: MicroRNA-448 suppresses
metastasis of pancreatic ductal adenocarcinoma through targeting
JAK1/STAT3 pathway. Oncol Rep. 38:1075–1082. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen X, Du J, Jiang R and Li L:
MicroRNA-214 inhibits the proliferation and invasion of lung
carcinoma cells by targeting JAK1. Am J Transl Res. 10:1164–1171.
2018.PubMed/NCBI
|
|
28
|
Zhou Y, Xu XM and Feng Y: MiR-769-5p
inhibits cancer progression in oral squamous cell carcinoma by
directly targeting JAK1/STAT3 pathway. Neoplasma. 67:528–536. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu L, Talati P, Vogiatzi P, Romero-Weaver
AL, Abdulghani J, Liao Z, Leiby B, Hoang DT, Mirtti T, Alanen K, et
al: Pharmacologic suppression of JAK1/2 by JAK1/2 inhibitor AZD1480
potently inhibits IL-6-induced experimental prostate cancer
metastases formation. Mol Cancer Ther. 13:1246–1258. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dai H, Wang C, Yu Z, He D, Yu K, Liu Y and
Wang S: MiR-17 regulates prostate cancer cell proliferation and
apoptosis through inhibiting JAK-STAT3 signaling pathway. Cancer
Biother Radiopharm. 33:103–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JH, Kim C, Baek SH, Ko JH, Lee SG,
Yang WM, Um JY, Sethi G and Ahn KS: Capsazepine inhibits JAK/STAT3
signaling, tumor growth, and cell survival in prostate cancer.
Oncotarget. 8:17700–17711. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu Z, Huang W, Chen B, Bai PD, Wang XG and
Xing JC: Up-regulation of miR-124 inhibits invasion and
proliferation of prostate cancer cells through mediating JAK-STAT3
signaling pathway. Eur Rev Med Pharmacol Sci. 21:2338–2345.
2017.PubMed/NCBI
|