Introduction
Inflammation is induced in response to various
stimuli such as palmitate and lipopolysaccharides (LPS). Palmitate,
a C16 saturated fatty acid, plays an important role in low dose
inflammation in obesity and many other metabolic diseases (1). LPS, an important component of the
outer membrane of gram-negative bacteria, is the key immune
activator against bacterial infection. LPS can bind to TLR4
(toll-like receptor 4) and activate the MAP kinase and NF-κB
pathway, which can subsequently increase the expression of various
pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6 and
interferon-β. Even though palmitate is not a TLR4 agonist (2), it can activate TLR4, MAP kinase, and
the NF-κB pathway (3). Furthermore,
palmitate treatment not only increases the generation of reactive
oxygen species (ROS) in the mitochondria, but also increases
calcium release from the endoplasmic reticulum (ER) leading to
mitochondrial dysfunction and cell death (1). LPS also has similar effects that
induce ROS generation, mitochondrial dysfunction and cell death
(4).
The inflammasome could be triggered by many signals
such as potassium efflux, ROS generation in mitochondria, etc.
(5). The NOD-like receptor pyrin
domain containing 3 (NLRP3), the apoptosis-associated speck-like
protein containing a caspase-recruitment domain (ASC), and the
pro-caspase-1 are assembled, leading to auto-activation of
caspase-1 upon its cleavage which subsequently cleaves its
substrates, IL-1β and IL-18 (5).
IL-1β, a pro-inflammatory cytokine, plays an important role in
inflammatory responses while IL-18 activates inflammatory responses
as well as protects against invading various microorganisms
(6). The inflammasome complex
formation is induced in response to various stimuli such as LPS
(7), palmitate (8), and anthrax toxin (9); it is also formed in case of many
inflammatory disease such as alcoholic hepatitis (10), liver injury (11), lung injury (12), and several cancers (13). Thus, formation of the inflammasome
complex is a novel process involved in regulating many inflammatory
responses.
Gryllus bimaculatus (GB) is an edible insect
that can be used as an alternative protein source. Previously, GB
extract has been shown to inhibit inflammation in several disease
models such as that of chronic arthritis (14) and alcohol-induced steatohepatitis
(15). Furthermore, GB has
exhibited glucose lowering effect in streptozotocin-induced
diabetic mice (16). Therefore, GB
could be used as a functional food source for patients with chronic
inflammation or metabolic diseases.
In this study, we evaluated the effects of GB
extract on LPS or palmitate-induced production of pro-inflammatory
cytokines, inflammasome formation, ER stress, ROS generation, and
cell death.
Materials and methods
Materials
The following materials were purchased as indicated:
i) LPS, palmitate, SB203580, SP600125, PD98059,
2′,7′-dichlorofluorescin diacetate (DCF-DA) and anti-α-tubulin
antibody (T9026) from Sigma-Aldrich; Merck KGaA; ii) anti-p65
(8242), anti-phospho-IκB (2859), anti-Lamin B2 (13823),
anti-phosphor-p38 (4511), anti-p38 (8690), anti-phospho-JNK (9255),
anti-JNK (9252), anti-phospho-ERK (4370), anti-ERK (4695),
anti-phospho-protein kinase RNA-like ER kinase (PERK; 3179),
anti-PERK (3192), anti-phospho-eukaryotic initiation factor 2α
(eif2α; 3597), anti-eif2α (5324), anti-caspase-3 (9664),
anti-cleaved-PARP1 (5625) and NLRP3 (15101) antibodies from Cell
Signalling Technology, Inc.; iii) anti-caspase-1 (sc-56036) and
anti-ACS (sc-22514-R) antibodies from Santa Cruz Biotechnology,
Inc.; iv) IL-1β antibody (NB600-633) from Novus Biologicals; v)
pyrrolidinedithiocarbamate ammonium (PDTC) from Biovision; vi)
Alexa-Fluor 488 antibody (A-11008) from Thermo Fisher Scientific,
Inc.; vii) 4,6-Diamidino-2-phenylindole dihydrochloride (DAPI) from
Merck (D9542); and viii) anti-mouse-HRP (horseradish peroxidase,
115-036-003) and anti-rabbit-HRP (111-035-003) antibodies from
Jackson Laboratory.
Gryllus bimaculatus extract
GB extract was generated as per the previous study
(15). Briefly, it was dried,
ground, extracted overnight using 70% ethanol, and evaporated.
Prepared samples were dissolved in PBS and frozen at −20°C until
further use.
Fatty acid analysis
GB were snap frozen at −40°C by lyophilization for
three days (Freezone 4.5; Labconco) and ground into fine powder.
Heptane (1 ml), 2 ml methylation mixture
(MeOH/benzene/dimethoxypropane/sulphuric acid in the ratio of
39:20:5:2, v/v), and 0.2 mg of pentadecanoic acid (internal
standard) were added to the GB samples and shaken gently at 80°C
for 2 h (BioFree Co.). Then, the samples were cooled down to room
temperature and the supernatant containing fatty acid methyl esters
were centrifuged for 1 min. The fatty acid composition in the
supernatant was determined using the Agilent 7890A (Agilent
Technologies, Inc.) and DB-23 60 mm, 0.25 mm, 0.25 µm (Agilent
Technologies, Inc.). The injection volume was 1 µl with 1:50 split
mode. The flow rates were as follows: hydrogen flame gas, 35
ml/min; helium carrier gas, 35 ml/min, and mixed gas, 350 ml/min.
The corresponding column oven temperatures were as follows: 50°C, 1
min, 25°C/min to 200°C, 3°C/min to 230°C, and 18 min as previously
described (17).
In vitro LPS and palmitate
treatment
RAW264.7 cells were grown in Dulbecco's modified
Eagles medium (HyClone) supplemented with 10% fetal bovine serum
and 1% penicillin/streptomycin (HyClone). LPS (50 ng/ml) or 250 µM
palmitate were used to induce inflammation as previously described
(7,18,19)
and 50 µg/ml LPS or 2 mM palmitate were used to induce cell death
(20, 21).
Enzyme-linked immunosorbent assay
(ELISA)
At 30 h, after pre-treatment of RAW264.7 cells with
100–200 µg/ml GB extract and another 18 h co-incubation with 50
ng/ml LPS or 250 µM palmitate, TNF-α, IL-1β and IL-6 levels in the
culture medium were measured using ELISA kits (TNF-α, IL-1β and
IL-6 Mouse ELISA kits; Komabiotech) as per manufacturer's
instructions.
Western blotting
RAW264.7 cells were lysed using RIPA buffer (50 mM
Tris–Cl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium
deoxycholate, and 0.1% SDS) containing protease and phosphatase
inhibitors (Sigma-Aldrich; Merck KGaA). The lysates were then
incubated on ice for 30 min and centrifuged (10,000 × g, 10 min,
4°C); protein levels in the supernatant were measured using the
Protein Assay Dye Reagent (Bio-Rad Laboratories). Using SDS-PAGE,
50 µg proteins were separated on 8–15% SDS polyacrylamide gels and
transferred to nitrocellulose membranes (Bio-Rad Laboratories).
Membranes were blocked using 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) in TBST (TBS with 0.1% Tween-20) for 1
h and incubated with primary antibodies (1:1,000 dilution)
overnight at 4°C. Secondary antibodies were incubated for 1 h at
room temperature. Protein bands were detected using the EzWestLumi
Plus Reagents (ATTO Corporation) on the Chemidoc MP imaging system
(Bio-Rad Laboratories).
Separation of nuclear and cytoplasmic
fractions
The nuclear and cytoplasmic fractions were separated
as previously described (22).
Briefly, RAW264.7 cells were lysed using a fractionation buffer (50
mM HEPES, pH 7.4, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM
dithiothreitol, and 0.1% NP-40) containing protease and phosphatase
inhibitors (Sigma-Aldrich; Merck KGaA). After 30 min incubation on
ice, the lysates were centrifuged (4,000 × g, 5 min, 4°C), and the
pellet was further sonicated in lysis buffer (20 mM HEPES, pH 7.4,
150 mM NaCl, 12.5 mM glycerophosphate, 1.5 mM MgCl2, 2
mM EGTA, 10 mM NaF, 2 mM dithiothreitol, 1 mM
Na3VO4, 0.5%, and Triton X-100) containing
protease and phosphatase inhibitors to obtain the nuclear fraction.
The supernatant was centrifuged (12,500 × g, 5 min, 4°C) to obtain
the cytoplasmic fraction.
Immunohistochemistry
RAW264.7 cells were co-treated with GB extract (100
or 200 µg/ml) and palmitate (250 µM) or LPS (50 ng/ml) for 18 h,
after which the cells were fixed with cold methanol for 10 min.
Fixed cells were blocked with horse serum for 1 h and then
incubated with p65 antibody (1:200) overnight at 4°C. After
washing, Alexa-Fluor 488 secondary antibody (1:500) was incubated
with the cells at room temperature in the dark for 1 h. DAPI was
used to label nuclei for 10 min, and cells were mounted with vector
shield mount media solution (Vector Laboratories). The p65
fluorescence and DAPI signals were viewed using a confocal
microscope (LSM 710; Carl Zeiss).
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was performed as previously described
(23). Briefly, total mRNA was
extracted from the RAW264.7 cells using the RNAiso Plus reagent
(Takara) and cDNA was synthesized using the PrimeScript™ RT Reagent
kit with gDNA Eraser (Takara). qPCR was performed using the
SYBR® Premix Ex Taq™ II, ROX Plus (Takara) in a Bio-Rad
CFX96 System (Bio-Rad). Relative gene expression was calculated
using the 2−ΔΔCt method (24). Primer sequences are listed in
Table I.
 | Table I.Primers used for quantitative
PCR. |
Table I.
Primers used for quantitative
PCR.
| Gene | Primer
sequences | (Ref.) |
|---|
| TNF-α | F:
5′-CTGTAGCCCACGTCGTAGC-3′ | (22) |
| (mouse) | R:
5′-TTGAGATCCATGCCGTTG-3′ |
|
| IL-1β | F:
5′-TGTAATGAAAGACGGCACACC-3′ | (22) |
| (mouse) | R:
5′-TCTTCTTTGGGTATTGCTTGG-3′ |
|
| IL-6 | F:
5′-TCCAGTTGCCTTCTTGGGAC-3′ | (22) |
| (mouse) | R:
5′-GTACTCCAGAAGACCAGAGG-3′ |
|
| GAPDH | F:
5′-CGACTTCAACAGCAACTCCCACTCTTCC-3′ | (22) |
| (mouse) | R:
5′-TGGGTGGTCCAGGGTTTCTTACTCCTT-3′ |
|
MTT assay
Cell death was evaluated using the MTT
(3-(4,5,-dimethylthiazol-2,5-diphenyl tetrazolium bromide) assay
(25). RAW264.7 cells were seeded
onto 96-well plates at a density of 5×104 cells/ well.
After treatment with either chemicals (SB203580, SP600125, PDTC) or
GB extract, the cells were treated with MTT solution (0.5 mg/ml
final concentration) and further incubated for 4 h. The assay was
terminated by adding 50 µl dimethyl sulfoxide (DMSO) to dissolve
the purple formazan crystals. Solubilized formazan was quantified
spectrophotometrically at 540 nm.
Detection of ROS
RAW264.7 cells were pre-treated with GB extract
followed by either LPS or palmitate treatment. To quantify ROS,
DCF-DA was added at a final concentration of 10 µM and incubated
for 30 min in the dark. The fluorescent intensity of DCF was
measured at 485 nm (excitation) and 535 nm (emission) using a
confocal microscope (LSM 710).
Statistical analysis
All the experiments were repeated independently in
triplicates and the data was expressed as mean ± standard error of
the mean (SEM). Statistical significance was calculated using
one-way ANOVA followed by Tukey's post-hoc test (GraphPad Prism
6.0; GraphPad Software, Inc.). P<0.05 was considered to indicate
a statistically significant difference.
Results
Fatty acids composition of Gryllus
bimaculatus extract
First, we examined the fatty acid composition of the
GB extract. It was found to contain two major saturated fatty
acids, palmitic acid (52.2 mg/g) and stearic acid (15.2 mg/g) and
two main unsaturated fatty acids, oleic acid (60.5 mg/g) and
linoleic acid (60.4 mg/g) as listed in Table II.
 | Table II.Fatty acid composition of the
Gryllus bimaculatus extract. |
Table II.
Fatty acid composition of the
Gryllus bimaculatus extract.
| Fatty acid | Concentration
(mg/g) |
|---|
| Caproic acid
(hexanoic acid) |
0.376 |
| Lauric acid
(dodecanoic acid) |
0.195 |
| Myristic acid
(tetradecanoic acid) |
1.660 |
| Palmitic acid
(hexadecanoic acid) | 52.162 |
| Palmitoleic
acid |
3.876 |
| Margaric acid
(heptadecanoic acid) |
0.708 |
| Stearic acid
(octadecanoic acid) | 15.159 |
| Oleic acid | 60.570 |
| Linoleic acid | 60.411 |
| Alpha-linolenic
acid (ALA) |
1.170 |
| Arachidic acid
(eicosanoic acid) |
1.608 |
Gryllus bimaculatus extract has an
inhibitory effect on LPS or palmitate-induced production of
inflammatory cytokines
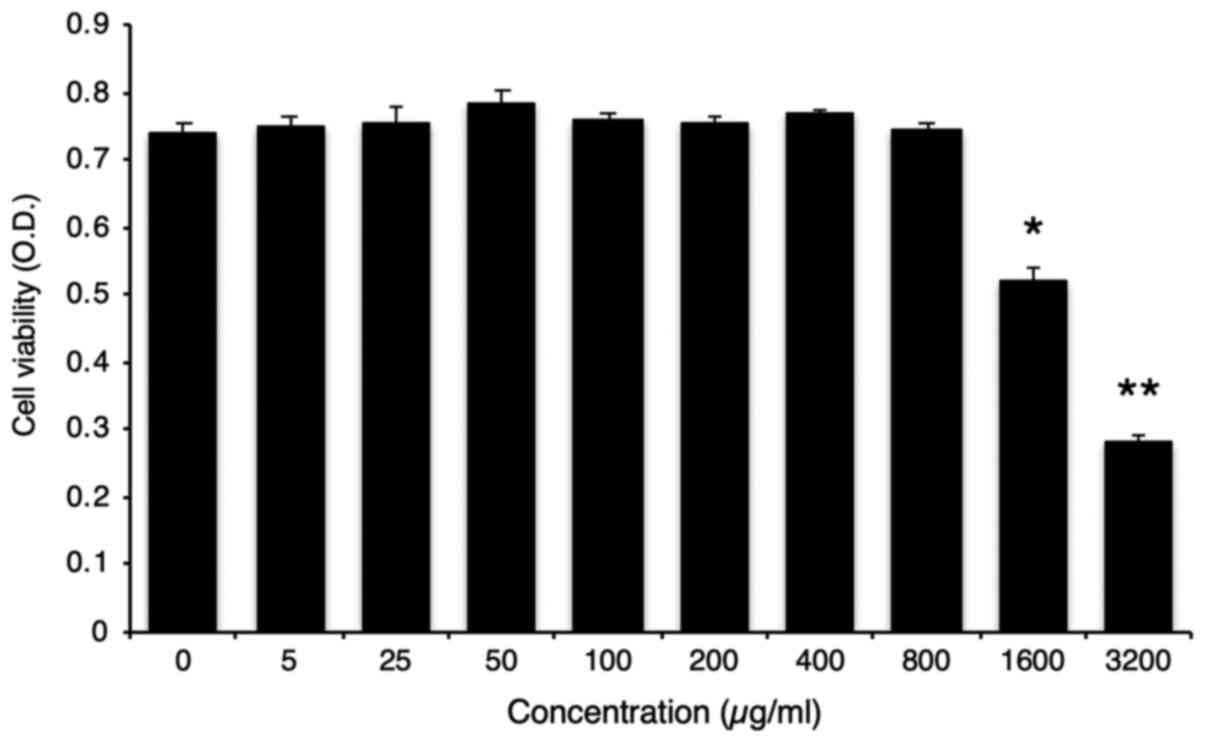
We examined the cell viability in vitro using
various concentrations of the GB extract; less than 800 µg/ml of
the GB extract did not affect the viability of RAW264.7 cells
(Fig. 1). Previous studies showed
that 40–100 µg/ml of the GB extract could reduce nitrite, TNF-α,
and IL-6 in the LPS-treated kupffer cells (15). Therefore, we used 100 and 200 µg/ml
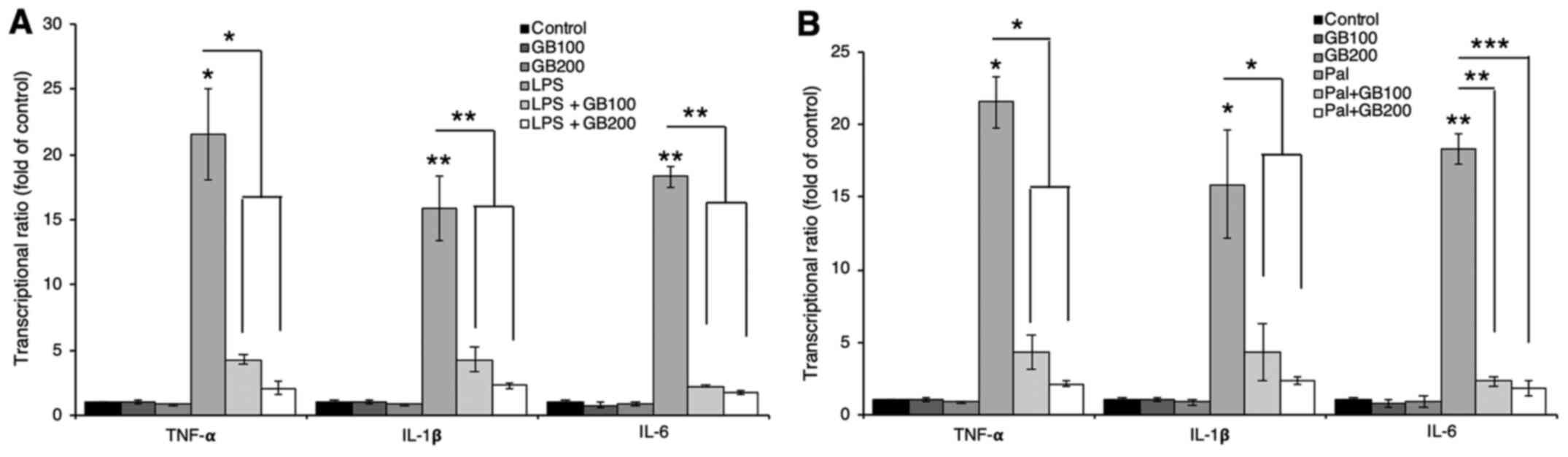
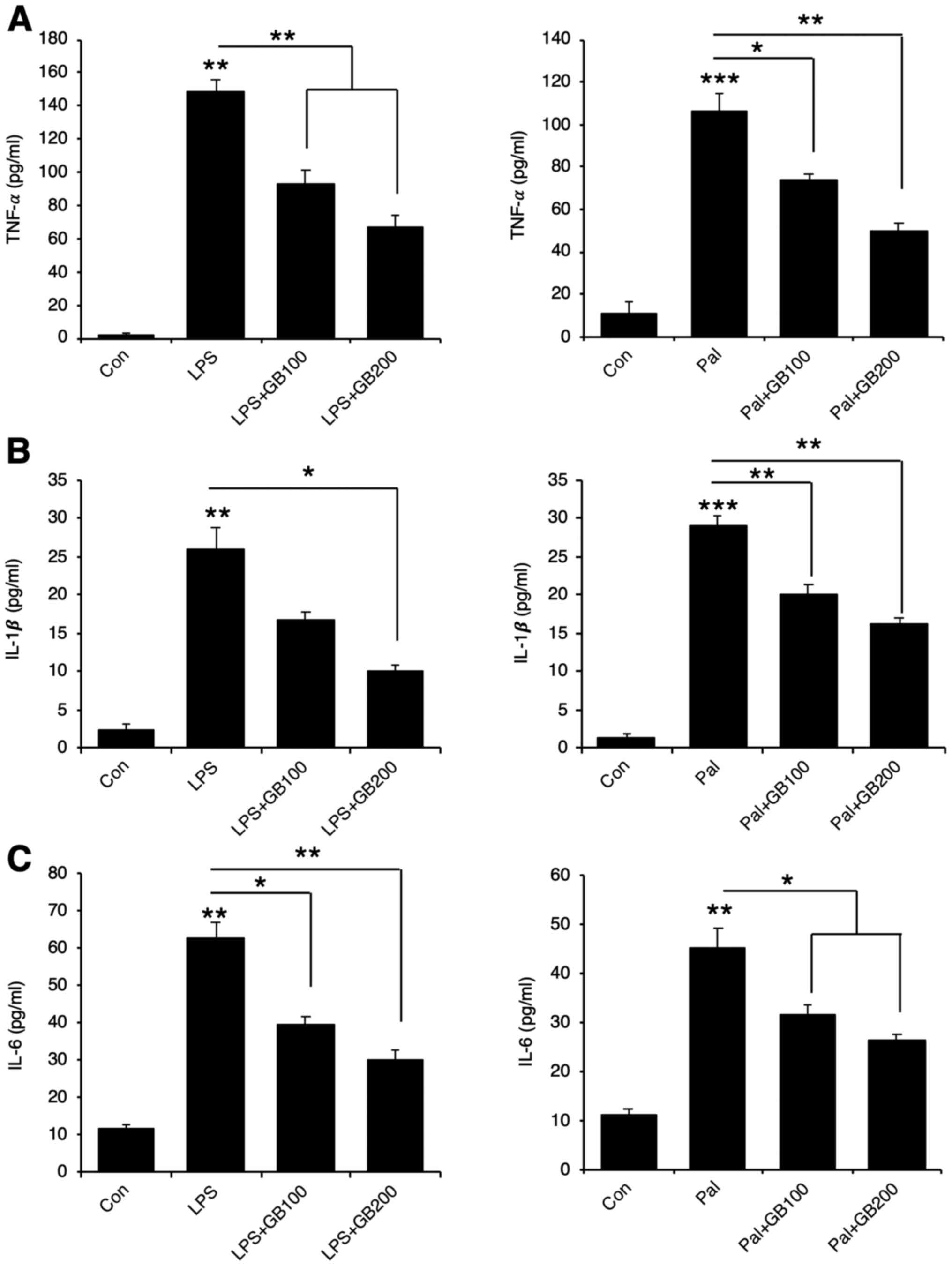
of GB in this study. To examine if GB extract has any
anti-inflammatory effect upon LPS and palmitate treatment, the
cells were pre-treated with GB extract and then with either LPS or
palmitate. LPS or palmitate treatment induced pro-inflammatory
cytokine production including TNF-α, IL-1β, and IL-6 while GB
extract pre-treatment inhibited the pro-inflammatory cytokine mRNA
levels (Fig. 2A and B) and their
secretion (Fig. 3A-C).
Gryllus bimaculatus extract has an
inhibitory effect on LPS or palmitate-induced activation of NF-κB
and MAP kinase
Previous studies showed that MAP kinases and NF-κB
signalling pathways play an important role in the production of LPS
or palmitate-induced inflammatory cytokines (18,22,26).
Therefore, both these pathways, NF-κB signalling (p65 translocation
into nucleus and phosphorylation of IκB) and MAP kinases (p38, JNK,
ERK) were examined. LPS or palmitate treatment elevated p65
translocation, IκB phosphorylation (Fig. 4A and B) as well as phosphorylation
of MAP kinases (p38, JNK, ERK) (Fig.
4C); though they were reduced upon pre-treatment with GB
extract (Fig. 4A-C). To further
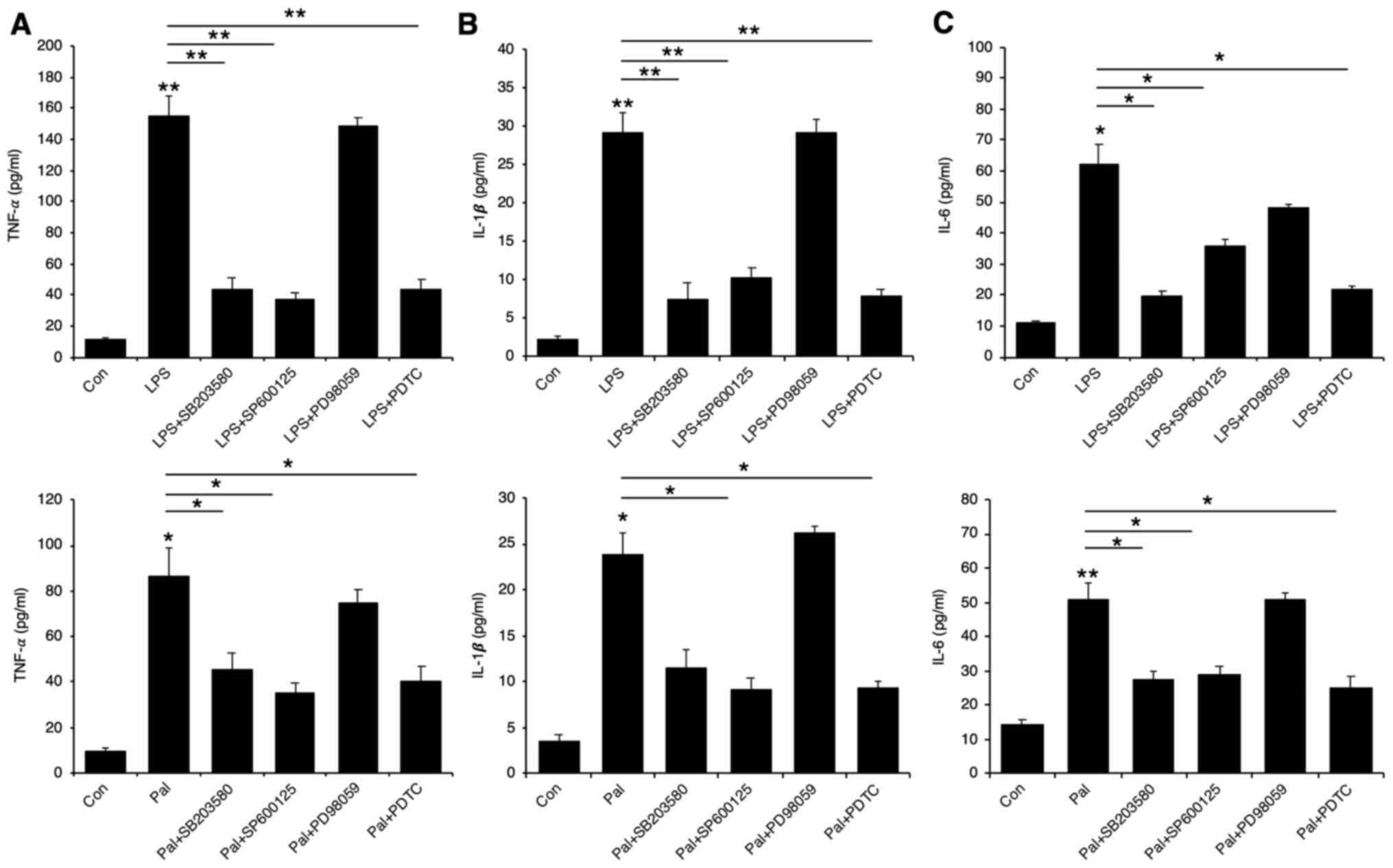
examine if MAP kinase and NF-κB activation involve any
pro-inflammatory cytokine production, we pre-treated the cells with
either p38 inhibitor (SB203580), JNK inhibitor (SP600125), ERK
inhibitor (PD98059), or NF-κB inhibitor (PDTC). A reversal in the
LPS and palmitate induced elevation of pro-inflammatory cytokine
production was observed with respect to p38, JNK, and NF-κB
inhibitors, but not ERK inhibitor (Fig.
5A-C).
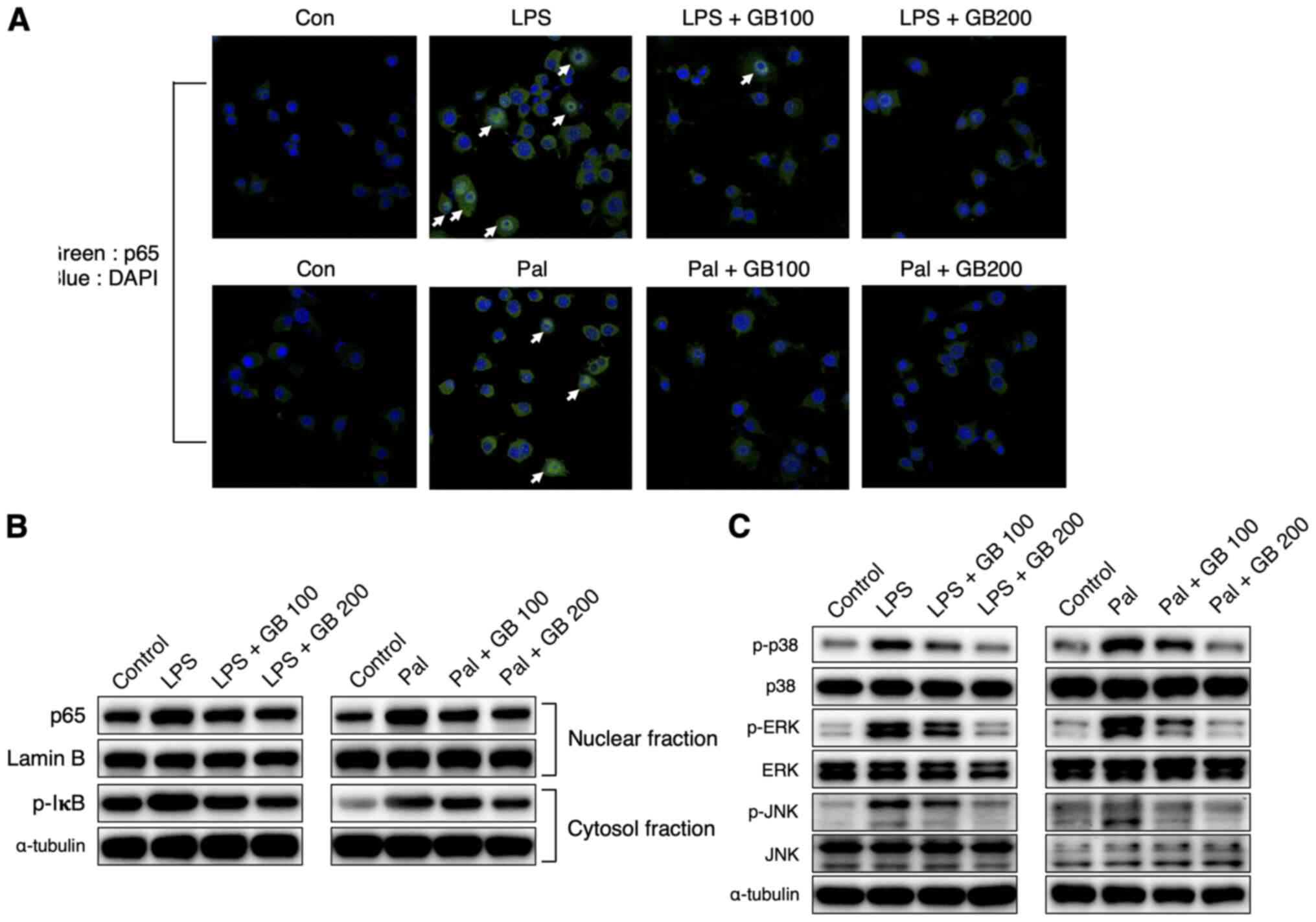 | Figure 4.GB extract decreases NF-κB
signalling, as well as MAP kinase phosphorylation. (A) After
co-treatment with LPS (50 ng/ml), Pal (250 µM) and GB extract (100
and 200 µg/ml), translocation of p65 was determined using a p65
antibody and an Alexa-Fluor 488-conjugated anti-rabbit antibody
(magnification, ×400). Nuclei were counterstained with DAPI. p65
translocation into the nucleus is marked with arrows.
Representative western blots were performed for (B) p65 in the
nuclear fraction and IκB phosphorylation in the cytosol fraction,
and (C) MAP kinase (p38, JNK and ERK) phosphorylation. All the
experiments were performed in triplicates. GB, Gryllus
bimaculatus; LPS, lipopolysaccharide; Pal, palmitate; Con,
control; p-, phosphorylated. |
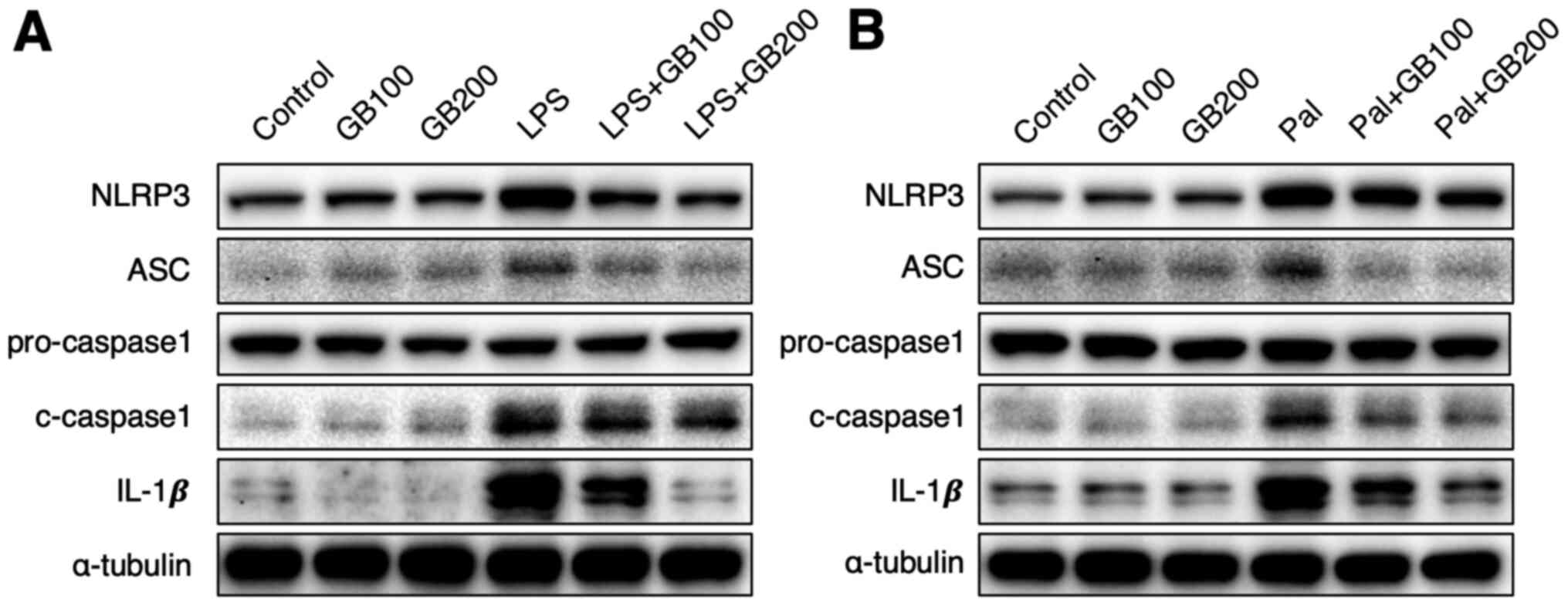
Gryllus bimaculatus extract has an
inhibitory effect on LPS or palmitate-induced NLRP3 inflammasome
activation
Previous studies showed that, both palmitate and LPS
induce NLRP3 inflammasome activation (7,27), we
therefore examined the inflammasome components in this study-
NLRP3, ASC, pro-caspase-1, cleaved-caspase1 (c-caspase-1), and
IL-1β. LPS or palmitate treatment increased the expression of all
the components while GB extract reduced them, suggesting that the
GB extract can reduce inflammasome formation (Fig 6A and B). Interestingly, GB extract
did not affect expression of pro-caspase-1.
Gryllus bimaculatus extract has an
inhibitory effect on LPS or palmitate-induced ROS generation and
endoplasmic reticulum (ER) stress
Since both LPS and palmitate are known to increase
ROS generation (1,4,28), we
examined if GB extract affects ROS generation upon LPS or palmitate
treatment. As expected, both LPS and palmitate increased ROS
generation, while GB reduced it (Fig.
7A). Furthermore, GB also reduced the LPS or palmitate-induced
ER stress markers- PERK and eif2α phosphorylation (Fig. 7B and C).
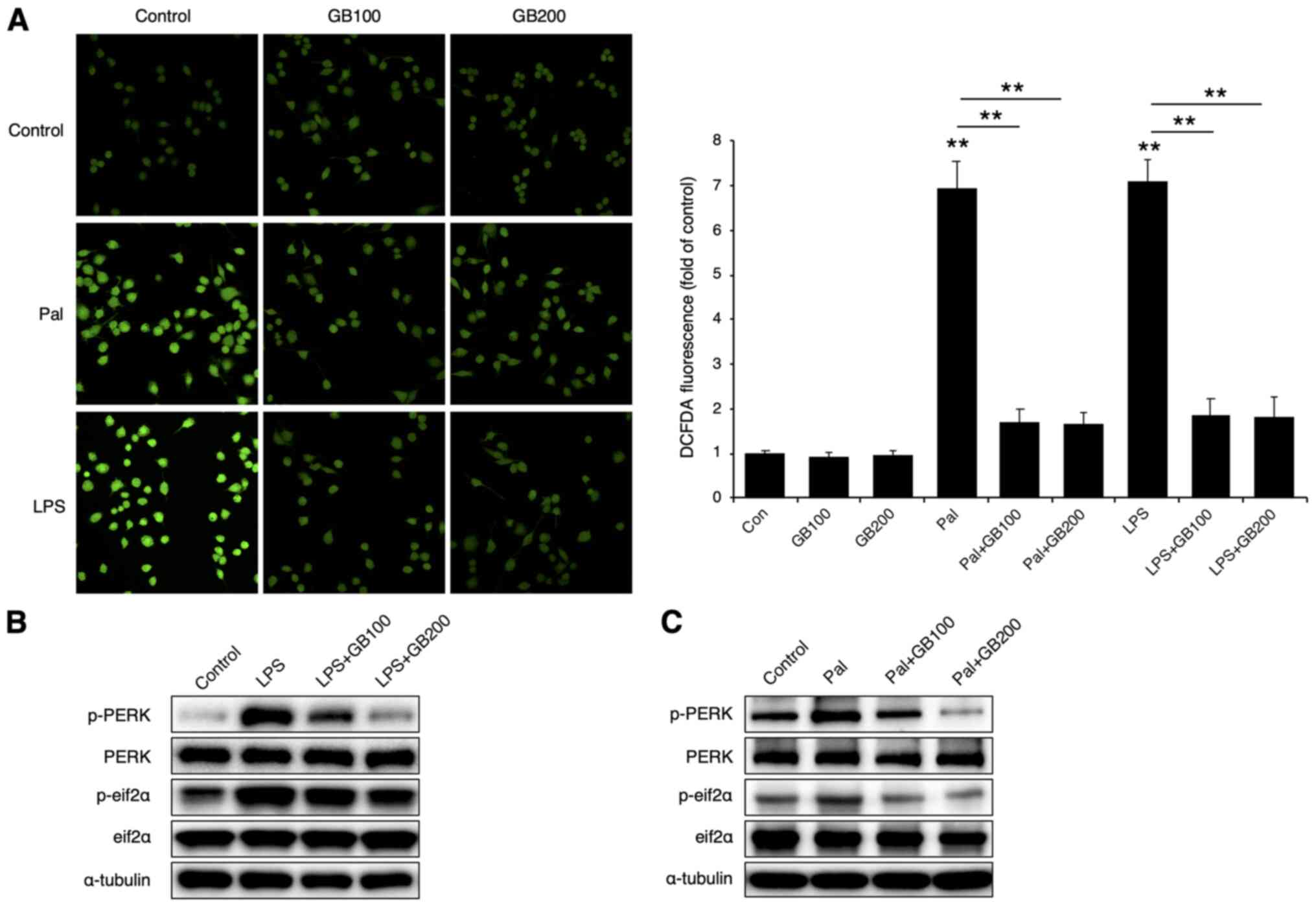 | Figure 7.GB extract decreases LPS- or
Pal-induced generation of ROS and endoplasmic reticulum stress. (A)
After co-treatment with LPS (50 ng/ml), Pal (250 µM) and GB extract
(100 and 200 µg/ml), ROS generation was evaluated (left panel;
magnification, ×400) and quantified (right panel). Representative
western blots of the indicated proteins in (B) LPS- (50 ng/ml) or
(C) Pal-treated (250 µM) RAW264.7 cells co-incubated with the GB
extract (100 and 200 µg/ml). The values are expressed as the mean ±
SEM (n=3). **P<0.01. GB, Gryllus bimaculatus; LPS,
lipopolysaccharide; Pal, palmitate; Con, control; ROS, reactive
oxygen species; p-, phosphorylated; DCFDA, 2′,7′-dichlorofluorescin
diacetate; PERK, protein kinase RNA-like ER kinase; eif2α,
eukaryotic initiation factor 2α. |
Gryllus bimaculatus extract has an
inhibitory effect on LPS or palmitate-induced cell death
Finally, we examined the role of GB extract in cell
death upon exposure to high doses of LPS or palmitate. LPS or
palmitate treatment induced cell death with increased cleavage of
caspase-3 (c-caspase-3) and PARP1 (c-PARP1). Co-treatment with GB
extract reduced cell death and also decreased cleavage of caspase-3
and PARP1 (Fig. 8A). Since GB
extract reduced phosphorylation of MAP kinases (p38, JNK) and NF-κB
pathway, we also treated the cells with these inhibitors and
examined cell death. These inhibitors were observed to reduce cell
death as well as caspase-3 and PARP1 cleavage (Fig. 8B). Therefore, GB appears to have a
protective role in LPS or palmitate-induced cell death.
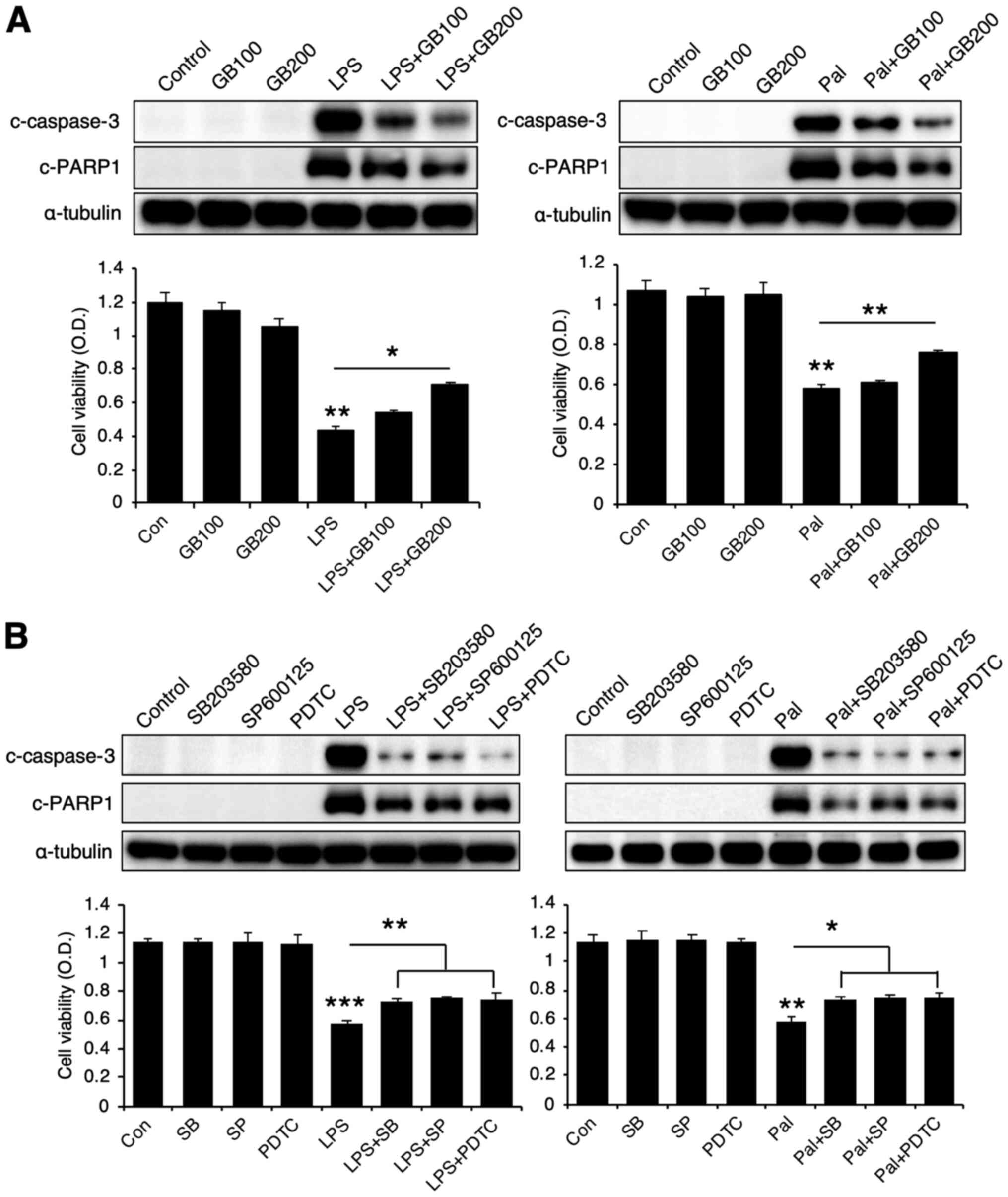 | Figure 8.GB extract protects against LPS- or
Pal-induced cytotoxicity. (A) Representative western blots of
c-caspase-3 and c-PARP1, and MTT cell viability in LPS- (50 µg/ml)
or Pal-treated (2 mM) RAW264.7 cells co-incubated with GB extract
(100 and 200 µg/ml). (B) Representative western blots of
c-caspase-3 and c-PARP1, and MTT cell viability in LPS- (5 µg/ml)
or Pal-treated (2 mM) RAW264.7 cells co-incubated with either 10 µM
SB203580 (p38 inhibitor), 10 µM SP600125 (JNK inhibitor) or 10 µM
PDTC (NF-κB inhibitor). The values are expressed as the mean ± SEM
(n=3). *P<0.05; **P<0.01; ***P<0.001. GB, Gryllus
bimaculatus; LPS, lipopolysaccharide; Pal, palmitate; Con,
control; c-, cleaved; PDTC, pyrrolidinedithiocarbamate ammonium;
OD, optical density. |
Discussion
Inflammation plays an important role in many
diseases such as arthritis, steatohepatitis and cancers;
macrophages have a central role in inflammation. In the present
study, we examined the effect of GB extract on LPS or
palmitate-induced production of pro-inflammatory cytokines and
formation of the inflammasome complex in RAW264.7 cells. GB extract
exhibited a protective role in production of pro-inflammatory
cytokines through inhibition of MAP kinase and NF-κB signalling
pathway. IκB is phosphorylated by activating IκB kinase in response
to an inflammatory stimulus followed by translocation of an
activated p65 from the cytosol to the nucleus (29); activated p65 in the nucleus in turn
increases the transcription of pro-inflammatory cytokines. MAP
kinases (p38, JNK, ERK) play diverse roles in many cellular
processes ranging from proliferation to differentiation and cell
death. Among the three MAP kinases, only p38 and JNK reduced the
production of all the pro-inflammatory cytokines, suggesting that
ERK pathway may be playing a minor role in the process.
Furthermore, GB also had an inhibitory role in the formation of
inflammasome complex. Inflammasome is equipped by the formation of
NLRP3, ASC and pro-caspase-1 complex (6). GB extract reduced the expression of
NLRP3, ASC, c-caspase-1 and IL-1β upon LPS or palmitate treatment.
NLRP3 expression is low but can be increased by TLR activation in a
NF-κB dependent manner (6). Since
GB extract has inhibitory effects on NF-κB pathway, it can also
reduce inflammasome formation.
ROS generation upon palmitate treatment is due to
the partial inhibition of mitochondrial complexes I and III
(1). LPS also inhibited
mitochondrial complex I (30) and
increased ROS generation (4). GB
extract reduced both LPS-induced and palmitate-induced ROS
generation. Oleic acid has been shown to downregulate
palmitate-induced ROS generation and cell death, in a CD36
dependent manner (31); therefore,
oleic acid in GB extract might improve palmitate-induced
mitochondrial complex I inhibition and reduce ROS generation.
Furthermore, oleic acid and linoleic acid also have protective
effects against palmitate-induced ER stress (32–34).
These unsaturated fatty acids are known to inhibit inflammation via
several mechanisms. For example, linoleic acid and oleic acid
prevent inflammation by activating PPAR-γ (35,36)
and inhibiting NF-κB signalling (37,38).
Therefore, unsaturated fatty acids in the GB extract might prevent
LPS or palmitate-induced NF-κB activation, ER stress, and
subsequent production of pro-inflammatory cytokines and
inflammasome complex formation.
Some edible insects have also been known to inhibit
inflammation when taken as food. For example, Tenebrio
molitor and Allomyrina dichotoma reduce not only the
high fat diet-induced steatohepatitis, but also hepatic
inflammation (39). GB also has
protective effects against alcohol-induced liver damage (15) and chronic arthritis (14). Tenebrio molitor has
antioxidant and anti-inflammatory effects, and its composition is
well studied; it contains various essential amino acids,
unsaturated fatty acids, tocopherol and squalene (40) which may be contributing to these
beneficial effects. Tenebrio molitor, Gryllodes sigillatus
and Schistocerca gregaria have anti-inflammatory and
antioxidant effects (41).
Similarly, GB also has high amounts of unsaturated fatty acid,
which might affect the anti-inflammatory effect, reduce ROS
generation and decrease LPS or palmitate-induced cell death.
In conclusion, GB may potentially be one of the
beneficial foods for controlling macrophage inflammatory processes,
ROS generation, ER stress and cell death; it may also prevent
diseases related to oxidative stress and inflammation. Thus, it can
be used as a functional food in conditions of oxidative stress and
inflammation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education (grant no.
NRF-2019R1I1A1A01041076).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WJP and JSH contributed to the conception and design
of the study. WJP and JSH performed the experiments and confirmed
the authenticity of the raw data. WJP contributed to the
acquisition of data and wrote the manuscript. WJP and JSH reviewed
and edited the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ASC
|
apoptosis-associated speck-like
protein containing a caspase-recruitment domain
|
|
ER
|
endoplasmic reticulum
|
|
GB
|
Gryllus bimaculatus
|
|
LPS
|
lipopolysaccharide
|
|
NLRP3
|
NOD-like receptor family pyrin domain
containing 3
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Korbecki J and Bajdak-Rusinek K: The
effect of palmitic acid on inflammatory response in macrophages: An
overview of molecular mechanisms. Inflamm Res. 68:915–932. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lancaster GI, Langley KG, Berglund NA,
Kammoun HL, Reibe S, Estevez E, Weir J, Mellett NA, Pernes G,
Conway JR, et al: Evidence that TLR4 is not a receptor for
saturated fatty acids but mediates lipid-induced inflammation by
reprogramming macrophage metabolism. Cell Metab. 27:1096–1110.e5.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen C, Ma W, Ding L, Li S, Dou X and Song
Z: The TLR4-IRE1α pathway activation contributes to
palmitate-elicited lipotoxicity in hepatocytes. J Cell Mol Med.
22:3572–3581. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu W, Zhang X, Wu H, Zhou Q, Wang Z, Liu
R, Liu J, Wang X and Hai C: HO-1 is essential for
tetrahydroxystilbene glucoside mediated mitochondrial biogenesis
and anti-inflammation process in LPS-treated RAW264.7 macrophages.
Oxid Med Cell Longev. 2017:18185752017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rathinam VA, Vanaja SK and Fitzgerald KA:
Regulation of inflammasome signaling. Nat Immunol. 13:333–342.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Zoete MR, Palm NW, Zhu S and Flavell
RA: Inflammasomes. Cold Spring Harb Perspect Biol. 6:a0162872014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baek HS, Min HJ, Hong VS, Kwon TK, Park
JW, Lee J and Kim S: Anti-inflammatory effects of the novel PIM
kinase inhibitor KMU-470 in RAW 264.7 cells through the
TLR4-NF-κB-NLRP3 pathway. Int J Mol Sci. 21:51382020. View Article : Google Scholar
|
|
8
|
Zhou H, Feng L, Xu F, Sun Y, Ma Y, Zhang
X, Liu H, Xu G, Wu X, Shen Y, et al: Berberine inhibits
palmitate-induced NLRP3 inflammasome activation by triggering
autophagy in macrophages: A new mechanism linking berberine to
insulin resistance improvement. Biomed Pharmacother. 89:864–874.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wickliffe KE, Leppla SH and Moayeri M:
Anthrax lethal toxin-induced inflammasome formation and caspase-1
activation are late events dependent on ion fluxes and the
proteasome. Cell Microbiol. 10:332–343. 2008.PubMed/NCBI
|
|
10
|
Peng Y, French BA, Tillman B, Morgan TR
and French SW: The inflammasome in alcoholic hepatitis: Its
relationship with Mallory-Denk body formation. Exp Mol Pathol.
97:305–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woolbright BL and Jaeschke H: Role of the
inflammasome in acetaminophen-induced liver injury and acute liver
failure. J Hepatol. 66:836–848. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Xu C, Chen X, Shi Q, Su W and
Zhao H: SOCS-1 Suppresses inflammation through inhibition of NALP3
inflammasome formation in smoke inhalation-induced acute lung
injury. Inflammation. 41:1557–1567. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moossavi M, Parsamanesh N, Bahrami A,
Atkin SL and Sahebkar A: Role of the NLRP3 inflammasome in cancer.
Mol Cancer. 17:1582018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahn MY, Han JW, Hwang JS, Yun EY and Lee
BM: Anti-inflammatory effect of glycosaminoglycan derived from
Gryllus bimaculatus (a type of cricket, insect) on
adjuvant-treated chronic arthritis rat model. J Toxicol Environ
Health A. 77:1332–1345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hwang BB, Chang MH, Lee JH, Heo W, Kim JK,
Pan JH, Kim YJ and Kim JH: The edible insect Gryllus
bimaculatus protects against gut-derived inflammatory responses
and liver damage in mice after acute alcohol exposure. Nutrients.
11:8572019. View Article : Google Scholar
|
|
16
|
Park SA, Lee GH, Lee HY, Hoang TH and Chae
HJ: Glucose-lowering effect of Gryllus bimaculatus powder on
streptozotocin-induced diabetes through the AKT/mTOR pathway. Food
Sci Nutr. 8:402–409. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garcés R and Mancha M: One-step lipid
extraction and fatty acid methyl esters preparation from fresh
plant tissues. Anal Biochem. 211:139–143. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ajuwon KM and Spurlock ME: Palmitate
activates the NF-kappaB transcription factor and induces IL-6 and
TNFalpha expression in 3T3-L1 adipocytes. J Nutr. 135:1841–1846.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoon J, Um HN, Jang J, Bae YA, Park WJ,
Kim HJ, Yoon MS, Chung IY and Jung Y: Eosinophil activation by
Toll-like receptor 4 ligands regulates macrophage polarization.
Front Cell Dev Biol. 7:3292019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wehinger S, Ortiz R, Díaz MI, Aguirre A,
Valenzuela M, Llanos P, Mc Master C, Leyton L and Quest AF:
Phosphorylation of caveolin-1 on tyrosine-14 induced by ROS
enhances palmitate-induced death of beta-pancreatic cells. Biochim
Biophys Acta. 1852:693–708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vogel SN, Marshall ST and Rosenstreich DL:
Analysis of the effects of lipopolysaccharide on macrophages:
Differential phagocytic responses of C3H/HeN and C3H/HeJ
macrophages in vitro. Infect Immun. 25:328–336. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim MH, Ahn HK, Lee EJ, Kim SJ, Kim YR,
Park JW and Park WJ: Hepatic inflammatory cytokine production can
be regulated by modulating sphingomyelinase and ceramide synthase
6. Int J Mol Med. 39:453–462. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oh AR, Sohn S, Lee J, Park JM, Nam KT,
Hahm KB, Kim YB, Lee HJ and Cha JY: ChREBP deficiency leads to
diarrhea-predominant irritable bowel syndrome. Metabolism.
85:286–297. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morgan DM: Tetrazolium (MTT) assay for
cellular viability and activity. Methods Mol Biol. 79:179–183.
1998.PubMed/NCBI
|
|
26
|
Tang S, Shen XY, Huang HQ, Xu SW, Yu Y,
Zhou CH, Chen SR, Le K, Wang YH and Liu PQ: Cryptotanshinone
suppressed inflammatory cytokines secretion in RAW264.7 macrophages
through inhibition of the NF-κB and MAPK signaling pathways.
Inflammation. 34:111–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Chen Y, Li X and Zhang Y, Gulbins
E and Zhang Y: Enhancement of endothelial permeability by free
fatty acid through lysosomal cathepsin B-mediated Nlrp3
inflammasome activation. Oncotarget. 7:73229–73241. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang HL, Lin SW, Lee CC, Lin KY, Liao CH,
Yang TY, Wang HM, Huang HC, Wu CR and Hseu YC: Induction of
Nrf2-mediated genes by Antrodia salmonea inhibits ROS
generation and inflammatory effects in
lipopolysaccharide-stimulated RAW264.7 macrophages. Food Funct.
6:230–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu X, Gao H, Hou Y, Yu J, Sun W, Wang Y
and Chen X, Feng Y, Xu QM and Chen X: Dihydronortanshinone, a
natural product, alleviates LPS-induced inflammatory response
through NF-κB, mitochondrial ROS, and MAPK pathways. Toxicol Appl
Pharmacol. 355:1–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duarte S, Arango D, Parihar A, Hamel P,
Yasmeen R and Doseff AI: Apigenin protects endothelial cells from
lipopolysaccharide (LPS)-induced inflammation by decreasing
caspase-3 activation and modulating mitochondrial function. Int J
Mol Sci. 14:17664–17679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim DH, Cho YM, Lee KH, Jeong SW and Kwon
OJ: Oleate protects macrophages from palmitate-induced apoptosis
through the downregulation of CD36 expression. Biochem Biophys Res
Commun. 488:477–482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu X, Zeng X, Chen X, Luo R, Li L, Wang
C, Liu J, Cheng J, Lu Y and Chen Y: Oleic acid protects
insulin-secreting INS-1E cells against palmitic acid-induced
lipotoxicity along with an amelioration of ER stress. Endocrine.
64:512–524. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Dong L, Yang X, Shi H and Zhang
L: α-Linolenic acid prevents endoplasmic reticulum stress-mediated
apoptosis of stearic acid lipotoxicity on primary rat hepatocytes.
Lipids Health Dis. 10:812011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Katsoulieris E, Mabley JG, Samai M, Green
IC and Chatterjee PK: alpha-Linolenic acid protects renal cells
against palmitic acid lipotoxicity via inhibition of endoplasmic
reticulum stress. Eur J Pharmacol. 623:107–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu Y, Correll PH and Vanden Heuvel JP:
Conjugated linoleic acid decreases production of pro-inflammatory
products in macrophages: Evidence for a PPAR gamma-dependent
mechanism. Biochim Biophys Acta. 1581:89–99. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Medeiros-de-Moraes IM,
Gonçalves-de-Albuquerque CF, Kurz AR, Oliveira FM, de Abreu VH,
Torres RC, Carvalho VF, Estato V, Bozza PT, Sperandio M, et al:
Omega-9 Oleic acid, the main compound of olive oil, mitigates
inflammation during experimental sepsis. Oxid Med Cell Longev.
2018:60534922018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng WL, Lii CK, Chen HW, Lin TH and Liu
KL: Contribution of conjugated linoleic acid to the suppression of
inflammatory responses through the regulation of the NF-kappaB
pathway. J Agric Food Chem. 52:71–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Harvey KA, Walker CL, Xu Z, Whitley P,
Pavlina TM, Hise M, Zaloga GP and Siddiqui RA: Oleic acid inhibits
stearic acid-induced inhibition of cell growth and pro-inflammatory
responses in human aortic endothelial cells. J Lipid Res.
51:3470–3480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee JY, Im AR, Shim KS, Ji KY, Kim KM, Kim
YH and Chae S: Beneficial effects of insect extracts on
nonalcoholic fatty liver disease. J Med Food. 23:760–771. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Son YJ, Choi SY, Hwang IK, Nho CW and Kim
SH: Could defatted Mealworm (Tenebrio molitor) and Mealworm
Oil Be Used as Food Ingredients? Foods. 9:402020. View Article : Google Scholar
|
|
41
|
Zielińska E, Baraniak B and Karaś M:
Antioxidant and Anti-inflammatory activities of hydrolysates and
peptide fractions obtained by enzymatic hydrolysis of selected
heat-treated edible insects. Nutrients. 9:9702017. View Article : Google Scholar
|