Introduction
Gouty arthritis (GA) is a non-specific inflammatory
reaction resulting from urate crystal deposition in joints and
surrounding soft tissues (1). It is
a common metabolic disease that is directly related to
hyperuricemia (2). Gout is common
in North America and Western Europe, with prevalence of 1–4%
(3). Acute GA is paroxysmal in
nature and often presents as redness, swelling, heat and pain in
single joints, limiting mobility; in addition, prominent
inflammatory and immune responses are activated. The pain is
usually excruciating (it may feel like being cut with a knife or
being bitten), requiring medications for relief. The first-line
drugs for GA include nonsteroidal anti-inflammatory drugs,
colchicine and glucocorticoids (4).
Although these Western medicines display good efficacy for treating
single episodes of acute GA, they are not so beneficial to the
overall prognosis of patients who experience multiple episodes of
acute GA owing to the adverse effects on gastrointestinal, hepatic
and renal systems after repeated use (5). Therefore, a more rational and
effective treatment approach for GA is needed in a clinical
setting.
To improve clinical treatment, a combination of
Western and Chinese medicines may be useful. The dialectical
classification of GA in Chinese medicine is ‘damp-heat
obstruction’, and the treatment is focused on eliminating the heat
and dampness as well as improving circulation and detoxification
(6). Representative treatments for
acute GA include simiaosan as the main component combined with
other herbal medicines (6). Our
group has previously evaluated the clinical characteristics of 104
patients with acute GA and found that the time required for pain
relief was significantly shorter for patients treated with
simiaosan + Celebrex (5.76±1.43 days) compared with patients
treated with Celebrex alone (8.01±1.57 days; P<0.05). Decrease
in various parameters, such as the serum uric acid level,
erythrocyte sedimentation rate and C-reactive protein, was greater
in the simiaosan + Celebrex group compared with the control group
(P<0.05) (7).
Acute GA is an acute inflammatory process induced by
monosodium urate (MSU) crystals and the monocyte/macrophage system
serves a critical role in GA onset, progression and remission
(8). MSU crystals interact with
resident monocytes/macrophages, which release inflammatory factors,
leading to neutrophil chemotaxis and cascading reactions. In
particular, IL-1β is a key factor in MSU crystal-induced
inflammation (9). Previous studies
have revealed that nucleotide-binding oligomerization domain-like
receptor 3 (NALP3) promotes IL-1β production and serves an
essential role in the onset of gout (10,11).
TGF-β1 is considered an important anti-inflammatory factor
implicated in the spontaneous remission of gout (12). An animal model of acute GA was
established to investigate the therapeutic efficacy of simiaosan
(7). Using this model, the effects
of simiaosan on the NALP3/IL-1β pathway were evaluated. The results
served as evidence supporting the use of Chinese medicine for the
treatment of acute GA (7).
Materials and methods
Experimental animals
A total of 25 specific-pathogen-free (SPF)-grade
male Sprague-Dawley (SD) rats (weight, 200–250 g; age, 8 weeks)
were purchased from Changzhou Cavens Lab Animal Co., Ltd., China
[license number: SCXK (Xiang) 2016-0002]. Rats (Jiangsu Sinorda
Biomedicine Co., Ltd.) were maintained at 20–26°C with 40–70%
humidity, 12-h light/dark cycles, and free access to food and
water. All methods were approved by the Ethics Committee of Tongde
Hospital of Zhejiang Province.
Main reagents and instruments
Sodium urate (cat. no. S16N8I46625; Shanghai Yuanye
Bio-Technology Co., Ltd.); Rat IL-1β ELISA kit (cat. no. m1003057;
Shanghai Enzyme-linked Biotechnology Co., Ltd.); Rat TGF-β1 ELISA
kit (cat. no. m1002856; Shanghai Enzyme-linked Biotechnology Co.,
Ltd.); TRIzol® Reagent (Thermo Fisher Scientific, Inc.);
Ultrapure RNA Extraction kit (cat. no. CW0581M; CWBIO); HiFiScript
cDNA First-Strand Synthesis kit (cat. no. CW2569M; CWBIO);
UltraSYBR mixture (cat. no. CW0957M; CWBIO); PVDF membrane (cat.
no. IPVH00010; EMD Millipore); mouse monoclonal Anti-GAPDH (cat.
no. TA-08; OriGene Technologies, Inc.; 1:2,000); rabbit polyclonal
anti-NALP3 (cat. no. bs-6655R; BIOSS; 1:300); Hypersensitive
luminescent solution (cat. no. 340776: Thermo Fisher Scientific,
Inc.); microplate reader (RT-6100; Rayto Life and Analytical
Sciences Co., Ltd.); PCR instrument (TCT8-II; Shanghai Tocan
Biotechnology Co., Ltd.); ultrasensitive chemiluminescence imaging
system (Chemi Doc XRS+; Bio-Rad Laboratories, Inc.); fluorescence
PCR instrument (CFX Connect real-time; Bio-Rad Laboratories, Inc.);
vertical gel electrophoresis instrument (DYY-6C; Beijing Liuyi
Instrument Factory); and light microscope (CX41; Olympus
Corporation).
Simiaosan powder consisted of the following
components: The dried bark of Phellodendron chinense
Schneid. (class Dicotyledonae, family Rutaceae), inner bark layer
of Berberis, which is processed into fried
Atractylodes (class Dicotyledon, order Chrysanthemum, family
Compositae), Semen Coicis, the seed of Coix (order Poales,
family Poaceae) and Achyranthes, a perennial herb (order
Caryophyllales, family Amaranthaceae). The herbal medicine
simiaosan was prepared by combining 9 g of Phellodendron
bark, 12 g of fried Atractylodes, 30 g of Semen Coicis and
15 g of two-toothed Achyranthes root in a rice cooker. After
cooking in boiling water for 30 min at 9 o'clock in the morning,
the liquid was retained. Subsequently, the medicine was cooked
again in boiling water for 30 min in the afternoon and the liquid
was again retained. The liquids from two preparations were mixed
and concentrated to 0.55 g/ml.
Construction of acute GA models
SD rats were weighed and 330 mg/kg 10% chloral
hydrate was administered via intraperitoneal injection. None of the
SD rats exhibited decreased appetite, mental weakness, or clinical
manifestations of peritonitis, including abdominal wall tension,
palpation avoidance, or resistance. There were no abnormal
mortalities during the trial. The joint circumference of the right
hind ankle was measured while bent at a 90°angle in all rats at the
same location by wrapping a thread around the joint and was
recorded as the circumference before modeling (0 h). SD rats were
fixed in a supine position and the joint of the right hind ankle
was disinfected with iodophor. A 6-mm injection needle was inserted
into the dorsum of the joint at a 45°angle into the inner surface
of the tibial tendon until a space was identified. Subsequently,
100 µl of 3.0% sodium urate solution was injected (a preparation of
3% sodium urate: 0.06 g of sodium urate was weighed and dissolved
in 2 ml of physiological saline to form a 3% sodium urate
suspension, which was mixed by agitation). The injection continued
until swelling of the contralateral joint capsule was observed and
the acute GA rat model was constructed.
Construction of the NALP3
overexpression vector
The cDNA fragment of NALP3 was PCR amplified
using restriction enzyme site (ClaI/ClaI) (Bsu15I
(ClaI); cat. no. ER0141; Thermo Fisher Scientific, Inc.)
NALP3 was cloned into the PDS_166 pAd-CMV-GFPa1-IRES vector
(cat. no. D6950-01; Omega Bio-Tek, Inc.). DH5α cells (50 ml;
2×109 cells/ml; cat. no. 9057; Takara Bio, Inc.) were
transfected with 1 ng plasmids encoding the NALP3s for 16 h
(media-Luria-Bertani; cat. no. HB0128; Qingdao Hope Bio-Technology
Co., Ltd.). The plasmid NALP3-PDS_166 pAd-CMV-GFPa1-IRES was
separated by 1% agarose gel electrophoresis. Following enzyme
digestion, expansion was continued to ensure a high purity and
in vitro cell transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), following the manufacturer's instructions. The
NALP3 adenovirus expression solution was obtained and processed
using an adenovirus packaging system.
Experimental groups
The 25 SPF-grade SD rats were randomly divided into
five groups: i) Normal control; ii) model + saline (saline was
administered intragastrically at a concentration of 10.8 ml/kg for
two consecutive weeks); iii) model + simiaosan (simiaosan at a
concentration of 0.55 g/ml was administered intragastrically at a
dose of 5.94 g/kg, a concentration of 10.8 ml/kg, for two
consecutive weeks); iv) model + NALP3-overexpressing adenovirus +
simiaosan (the joint cavity was injected with 10 µl of
NALP3-overexpressing adenovirus and simiaosan at a concentration of
0.55 g/ml was administered intragastrically at a dose of 5.94 g/kg,
a concentration of 10.8 ml/kg, for two consecutive weeks); and v)
model + empty vector adenovirus + simiaosan (the joint cavity was
injected with 10 µl of empty vector adenovirus and simiaosan at a
concentration of 0.55 g/ml was administered intragastrically at a
dose of 5.94 g/kg, a concentration of 10.8 ml/kg, for two
consecutive weeks).
Quantitative fluorescence (QF)
PCR
The joint fluid was extracted from the rats in the
control, NALP3-overexpressing adenovirus and empty vector
adenovirus groups. The cells were isolated and RNA was extracted
with Ultrapure RNA Extraction kit (cat. no. CW0581M; Beijing ComWin
Biotech Co., Ltd.) from the joint fluid in each group. Following
RNA extraction, 0.2 ml of chloroform was added and the mixture was
centrifuged at 4°C and 13,400 × g for 15 min. The colorless aqueous
phase was transferred to the same volume of 70% pre-cooled ethanol
and mixed. The solution was added to the adsorption column of the
collection tube for centrifugation and drying. The adsorption
column was added to a new 1.5-ml pre-cooled RNase-free centrifuge
tube. RNase-free water (35 µl) was added to fully dissolve the RNA.
The RNA was obtained after two rounds of centrifugation at 13,400 ×
g at 4°C for 1 min and was stored at −80°C. The extracted RNA was
used to synthesize cDNA using a reverse transcription kit,
following the manufacturer's protocol. The cDNA was used as a
template for real-time PCR. GAPDH was used as an internal
control to calculate the relative expression levels of NALP3
in each group. Primer information is presented in Table I.
 | Table I.Primer information. |
Table I.
Primer information.
| Primer name | Primer sequence
(5′-3′) | Primer length
(bp) | Amplicon length
(bp) | Annealing temperature
(°C) |
|---|
| NALP3 F |
AGCCTCAGGGCACCAAA | 17 | 443 | 57.8 |
| NALP3 R |
GGGATGAAGCACATAGTAAACAG | 23 |
|
|
| GAPDH F |
GCAAGTTCAACGGCACAG | 18 | 141 | 58.6 |
| GAPDH R |
CGCCAGTAGACTCCACGAC | 19 |
|
|
The QF-PCR mixture was comprised of 9.5 µl of
RNase-free dH20, 1 µl of cDNA/DNA, 1 µl of upstream
primer, 1 µl of downstream primer and 12.5 µl of 2X GoldStar Taq
MasterMix (cat. no. CW0957M; Beijing ComWin Biotech Co.).
The QF-PCR protocol was as follows: 40 cycles of
predenaturation at 95°C for 3 min, denaturation at 95°C for 10 sec,
annealing at 53°C for 30 sec and extension at 72°C for 30 sec,
followed by a final extension at 72°C for 10 min. Bio-Rad CFX
Manager (version 3.0; Bio-Rad Laboratories, Inc.) was used to
analyze the data.
Histopathological analysis
Synovial tissue samples obtained from the joint
cavity were rinsed under running water for 2 h and then dehydrated
using a graded ethanol series at 70, 80 and 90%. A mixture with
equal volumes of pure alcohol and xylene was added to the tissue
samples and the samples were incubated for 15 min, followed by the
addition of xylene I for 15 min and xylene II for 15 min at 20–26°C
(until the tissues were translucent). A mixture comprising equal
volumes of xylene and paraffin wax was then added for 15 min.
Paraffin I and paraffin II were added and the samples were
incubated for 50–60 min to allow wax infiltration at 20–26°C. The
tissues were then paraffin-embedded and sectioned into 4-µm thick
sections. The paraffin sections were heated and then dewaxed and
hydrated with distilled water. When the sections were hydrated,
they were placed in an 0.5% aqueous solution of hematoxylin and
stained for 3 min. Hydrochloric acid in ethanol was added for 15
sec for differentiation, followed by quick rinsing with water,
addition of bluing solution for 15 sec, rinsing with water, 0.5%
eosin staining for 3 min at 20–26°C, rinsing with water,
dehydration, clearing, and sealing with neutral resin.
Subsequently, synovial membranes were observed in three randomly
selected regions using a light microscope (magnification,
×200).
Determination of serum IL-1 and TGF-1
levels by ELISA
The IL-1 and TGF-1 contents in the serum of rats in
each group were detected using an ELISA kit. Optical density (OD)
at 450 nm was measured using a microplate reader. The concentration
of the standard substance and OD values were used to generate a
standard curve and to obtain a linear regression equation. The OD
values for each sample were then used to calculate the contents of
IL-1 and TGF-1 (pg/ml).
Determination of NALP3 level in joint
fluid by western blotting
Ankle fluid from the hind foot and lower leg of rats
in each group was collected. Following lysis centrifugation, the
supernatant was absorbed and the protein was extracted by RIPA
lysis buffer (cat. no. C1053; Applygen Technologies, Inc.). A BCA
kit was used to determine the protein concentration. Following
total protein (4 µg per lane) denaturation and electrophoresis (12%
SDS-PAGE), samples were transferred onto a PVDF membrane, which was
blocked with 5% fat-free milk at 4°C overnight, and then incubated
with a rabbit polyclonal anti-NALP3 primary antibody (cat. no.
bs-6655R; BIOSS; 1:300) at 4°C overnight. Subsequently, the
membranes were incubated with a HRP-conjugated Affinipure goat
anti-rabbit IgG (H+L) secondary antibody (cat. no. ZB-2301; OriGene
Technologies, Inc.; 1:2,000) at 20–26°C for 2 h, followed by
exposure and development (hypersensitive luminescent solution; cat.
no. RJ239676; Thermo Fisher Scientific, Inc.). Quantity One
software (version 4.5; Bio-Rad Laboratories, Inc.) was used to
analyze the gray values for each antibody strip, draw standard
curves and calculate the protein concentrations of the samples in
µg/µl.
Animal care
Animal care and all experimental procedures were
performed in accordance with the Guide for the Care and Use of
Laboratory Animals published by the US National Institutes of
Health (publication no. 85-23, revised 1996). The handling of rats
and all experimental procedures were approved by the Institutional
Animal Care and Use Committee of Tongde Hospital of Zhejiang
Province. The following humanitarian thresholds were used: i)
Weight loss (rats losing 15–20% of their body weight rapidly); ii)
loss of appetite (rats that did not eat for 24–36 h or ate only a
small amount of food for 3 days); and iii) body organ infection
(abnormal physical indicators and blood tests indicated the failure
of drug treatment and the development of systemic disease). At the
end of the experiment, after collecting the body fluids and tissues
samples, all rats were euthanized by complete anesthesia with
inhalation of 3% halothane followed by cervical dislocation.
Mortality was confirmed by checking whether the heart of the
animals had stopped completely and the pupils were dilated.
Statistical analysis
All data were analyzed using SPSS 19.0 (IBM Corp.).
One-way ANOVA followed by Bonferroni's post hoc test was used to
determine significant differences among rat groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Pathological validation of model
establishment
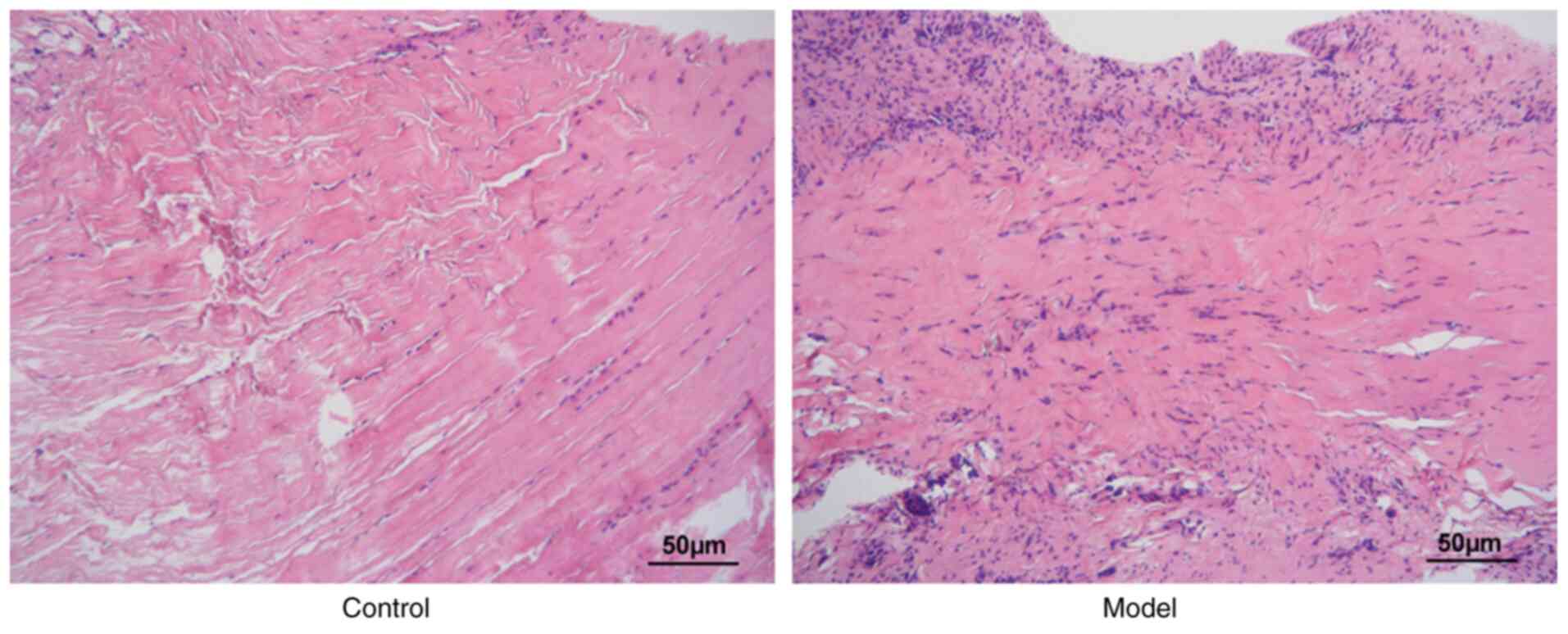
As revealed in Fig.
1, the synovial tissues in the model group exhibited more
extensive inflammatory cell infiltration compared with the normal
control group, indicating successful establishment of the
experimental model.
Construction of the NALP3
overexpression vector and validation of the transfection
efficiency
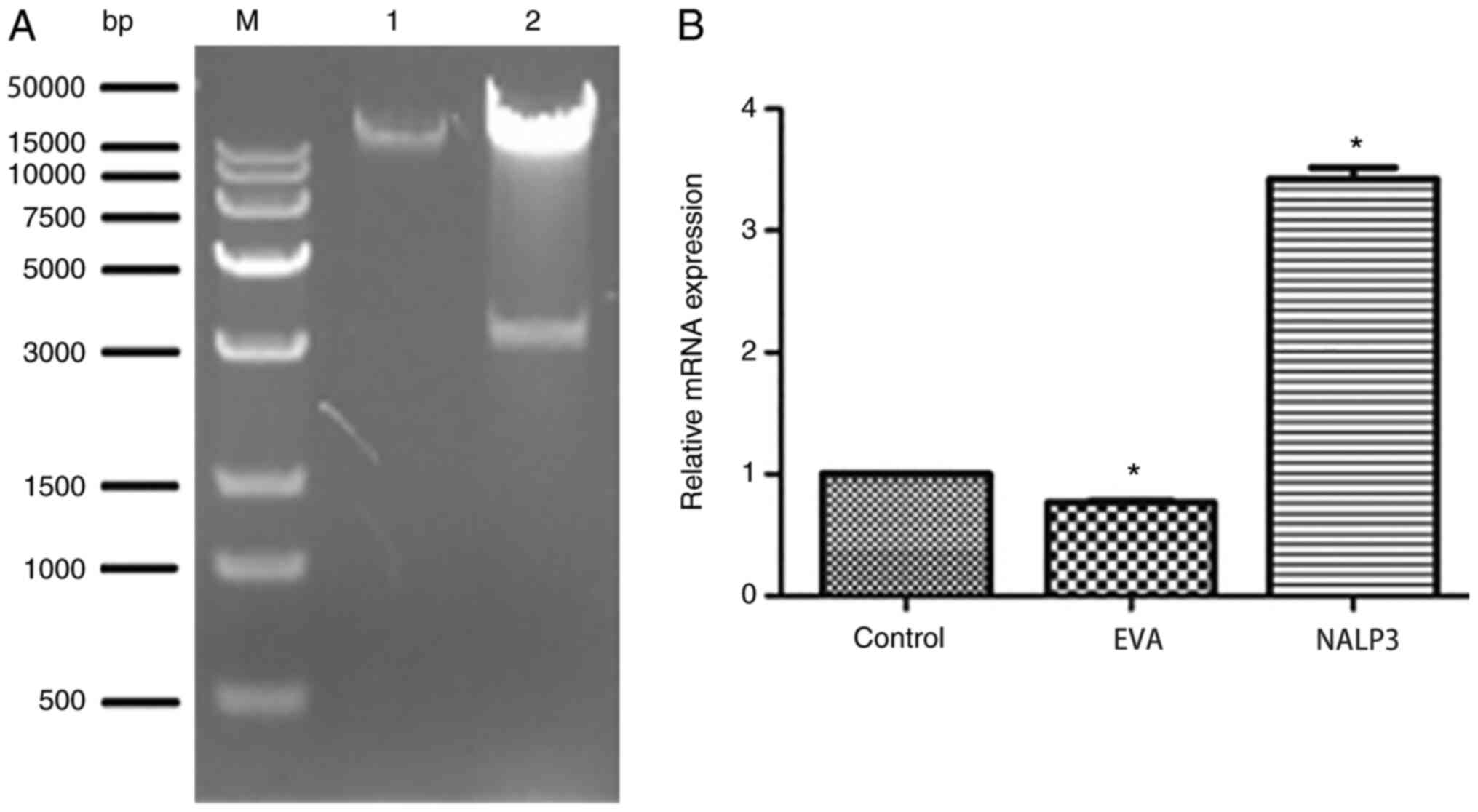
The expected band sizes for the plasmid
NALP3-PDS_166 pAd-CMV-GFPa1-IRES cleaved by ClaI were 35,184
and 3,120 bp. As revealed in Fig.
2A, the electrophoresis results were consistent with the
anticipated values, demonstrating that the correct plasmid was
obtained. As revealed in Fig. 2B,
the expression of NALP3 in the NALP3-overexpression group was
significantly higher compared with the empty vector adenovirus
group (P<0.05).
Hematoxylin and eosin (H&E)
staining
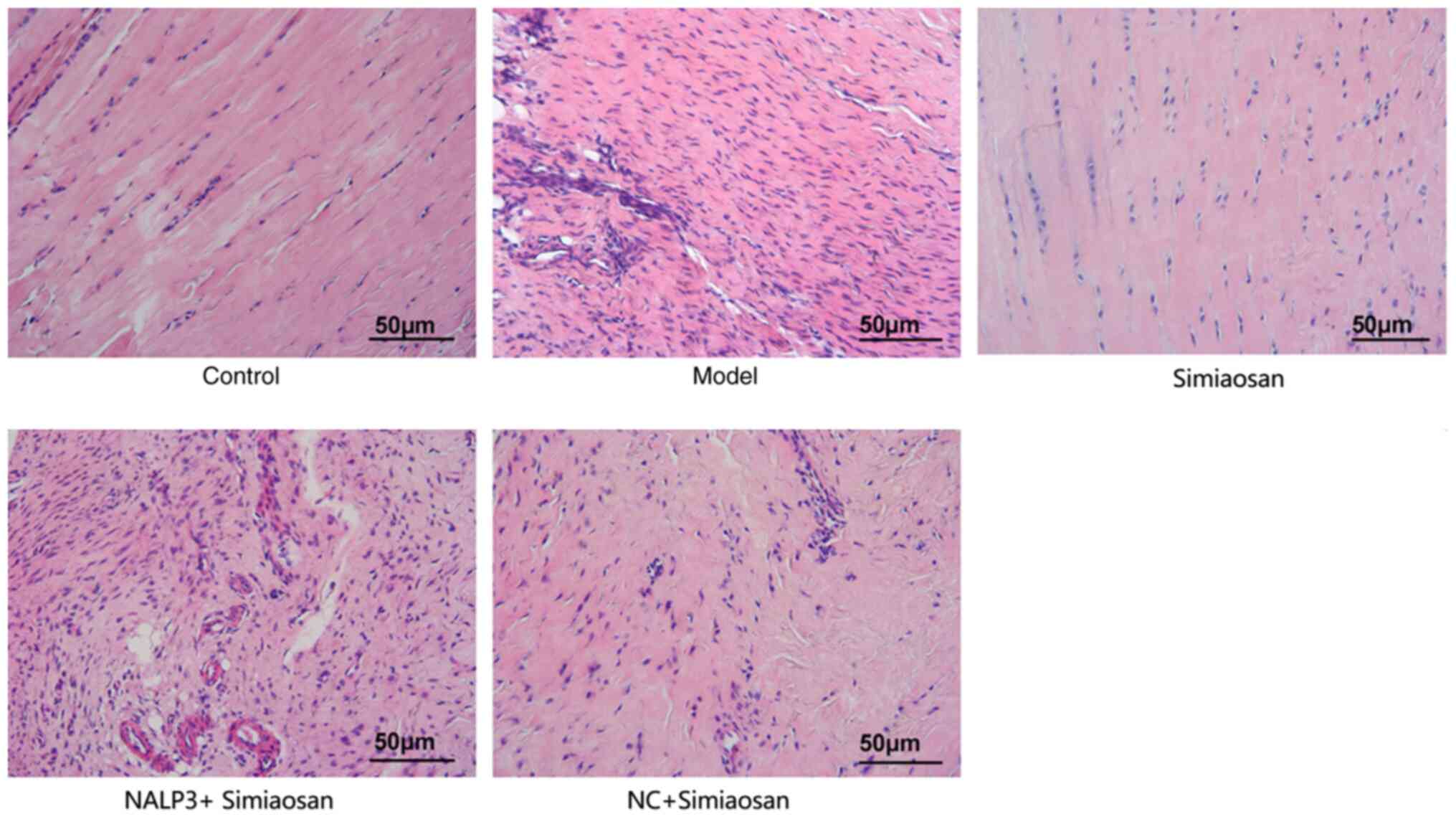
As revealed in Fig.
3, the synovial tissues in the model group exhibited extensive
inflammatory cell infiltration. The synovial tissues in the model +
simiaosan group were neatly arranged without discernable
infiltration of inflammatory cells, unlike those in the model
group. The model + NALP3-overexpressing adenovirus + simiaosan
group exhibited a significantly higher inflammatory cell count and
the model + empty vector adenovirus + simiaosan group exhibited a
lower extent of inflammatory cell infiltration compared with the
model group. These findings indicated that simiaosan may reduce
inflammatory cell infiltration, whereas NALP3 aggravates the
inflammatory response in tissues.
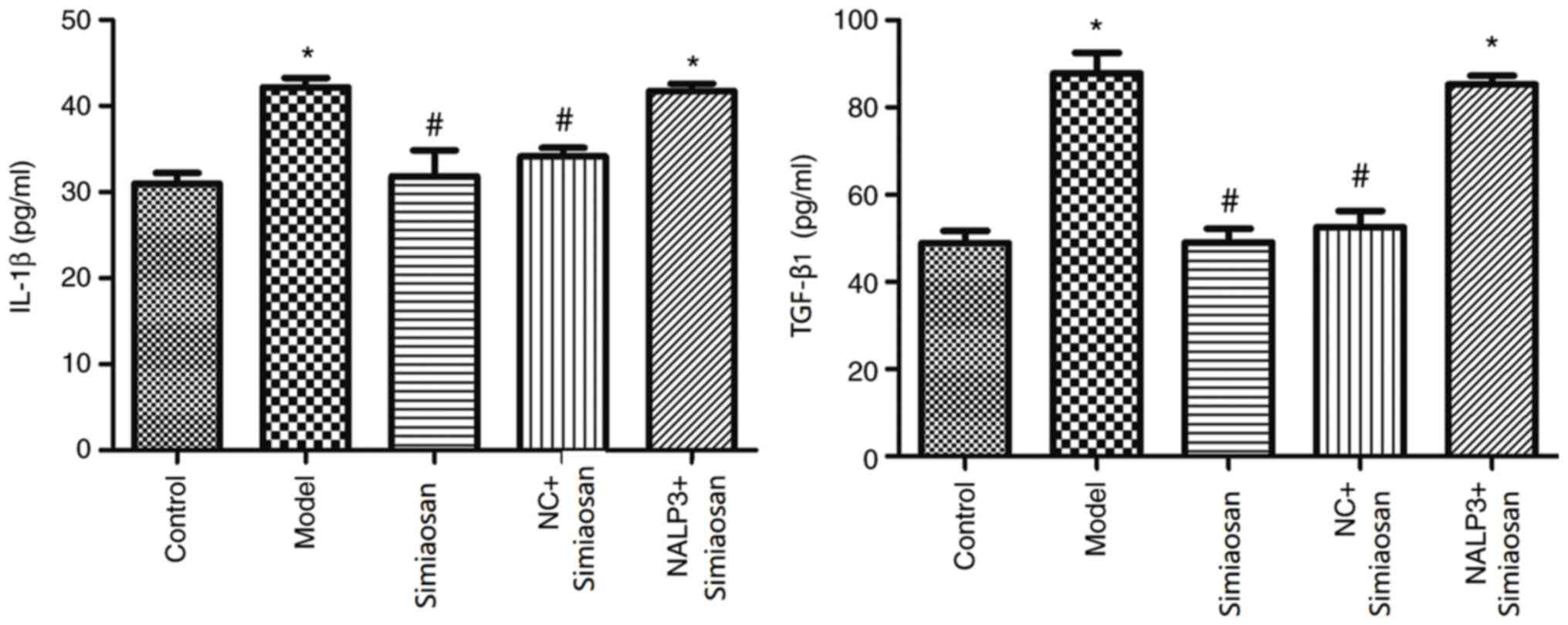
Expression of IL-1β and TGF-β1
As revealed in Fig.
4, compared with the levels in the normal control group, the
expression levels of IL-1β and TGF-β1 were significantly higher in
the model and the model + NALP3-overexpressing adenovirus +
simiaosan group (P<0.05). Compared with the levels in the model
group, the expression levels of IL-1β and TGF-β1 were significantly
lower in the model + simiaosan and the model + empty vector
adenovirus + simiaosan groups (P<0.05).
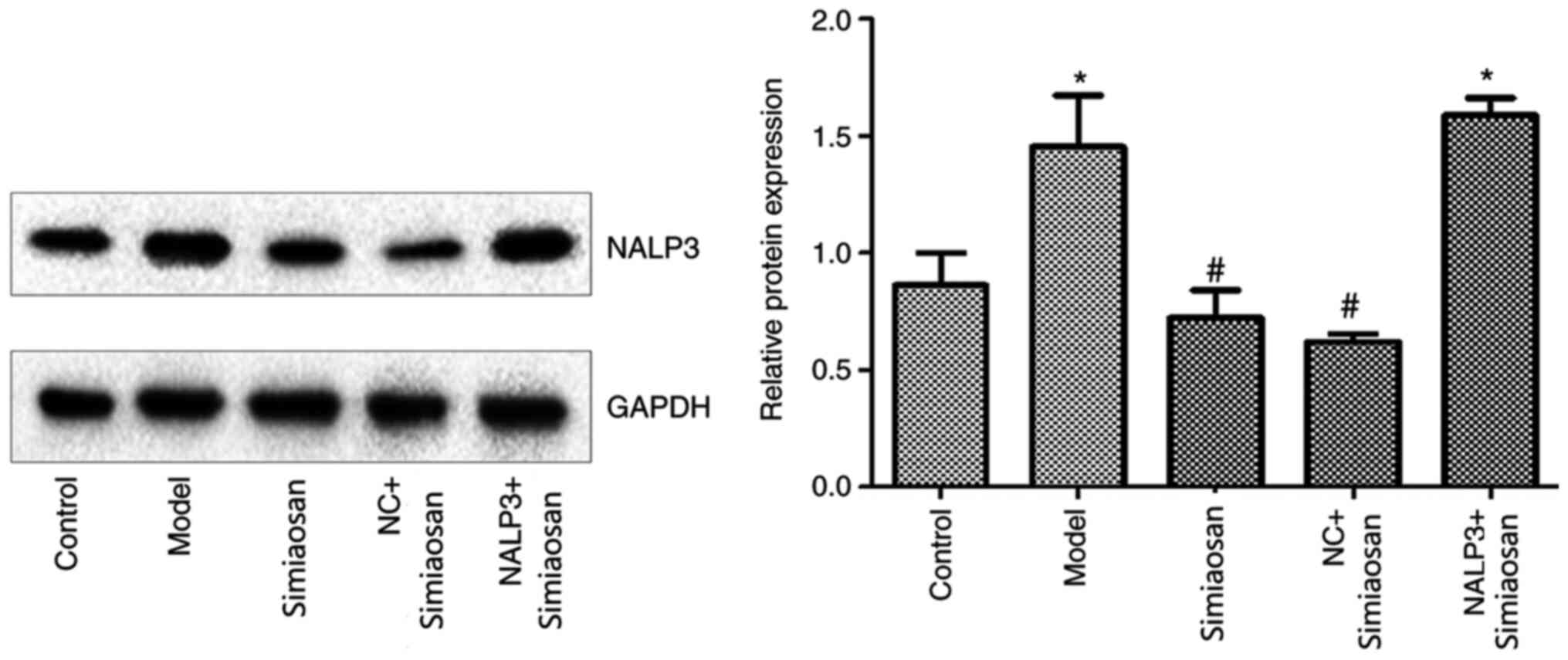
Expression of NALP3
As revealed in Fig.
5, the expression of NALP3 was significantly higher in the
model and the model + NALP3-overexpressing adenovirus + simiaosan
groups compared with the model control group (P<0.05). The
expression of NALP3 was significantly lower in the model +
simiaosan and the model + empty vector adenovirus + simiaosan
groups compared with the model group (P<0.05).
Discussion
GA, one of the most common forms of autoimmune
arthritis, is characterized biochemically by hyperuricemia, leading
to the repeated deposition of MSU in joint cavities and synovial
tissue (1). The development of GA
can be roughly divided into two phases. The first phase involves
the processing and maturation of IL-1β, in which mononuclear
phagocytes phagocytose and internalize MSU, leading to the
activation of NALP3 complex and the synthesis, processing and
release of IL-1β. The processing and maturation of IL-1β are
primarily related to the activity of the NALP3 complex. The second
phase comprises IL-1β-induced recruitment. In this phase, a series
of chemical factors and inflammatory mediators are generated via
the activation of ILReceptor/myeloid differentiation primary
response 88 on the surface of non-bone marrow-derived cells
(13,14). This induces neutrophil chemotaxis
toward the joint cavity, leading to an acute inflammatory process
characterized by neutrophil infiltration, that is, the onset of a
GA episode (15).
IL-1β is an inflammatory factor synthesized by
monocytes or macrophages. The precursor form is implicated in
certain chronic inflammatory diseases, such as rheumatoid arthritis
(16). However, studies have found
that compared to other inflammatory factor inhibitors (such as
TNF-α inhibitors), IL-1 inhibitors are less effective in improving
the clinical symptoms of rheumatoid arthritis, indicating that
IL-1β does not serve a key role in the pathogenesis of rheumatoid
arthritis (17,18). However, a previous study revealed
that IL-1β may serve a critical role in the pathogenesis of GA
(19). Liu (20) reported that simiaosan can
effectively relieve the symptoms of GA.
In the present study, an acute GA model was
established using sodium urate. The synovial tissue of the model
group exhibited extensive inflammatory cell infiltration,
indicating successful establishment of the experimental model.
Similarly, extensive inflammatory cell infiltration was observed in
the synovial tissue of the model group and treatment with simiaosan
improved the condition of synovial tissues, whereas NALP3
aggravated the inflammatory response. Furthermore, NALP3 expression
was significantly reduced in the simiaosan group. These results
demonstrated that the beneficial effects of simiaosan on GA are
mediated by NALP3 inhibition.
A recent study on the treatment of gout arthritis by
inhibiting the activation of NALP3 complex also confirmed the
important role of NALP3 complex in the inflammatory process of gout
(21). In the present study,
simiaosan downregulated the expression of IL-1β and TGF-β1, while
the overexpression of NALP3 led to increased levels of IL-1β and
TGF-β1. These findings indicated that simiaosan can alleviate the
symptoms of GA to a certain extent by downregulating the
NALP3/IL-1β pathway. In summary, simiaosan can alleviate the
symptoms of GA and may exert its effect by regulating the
NALP3/IL-1β signaling pathway.
Acknowledgements
Not applicable.
Funding
This study was supported by the Zhejiang TCM Science
and Technology Project (grant no. 2017ZA023).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and GH participated in performing the
experiments, collecting data and drafted the manuscript. HD
performed the statistical analysis and participated in the design
of the present study. YL participated in the design of the present
study and helped to draft the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was carried out in accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal
experiments were performed in accordance with protocols approved by
the Institutional Animal Care and Use Committee of Tongde Hospital
of Zhejiang Province (approval no. DW2016035).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tausche AK and Aringer M: Gouty arthritis.
Z Rheumatol. 75:885–898. 2016.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cleophas MC, Crisan TO and Joosten LA:
Factors modulating the inflammatory response in acute gouty
arthritis. Curr Opin Rheumatol. 29:163–170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuo CF, Grainge MJ, Zhang W and Doherty M:
Global epidemiology of gout: Prevalence, incidence and risk
factors. Nat Rev Rheumatol. 11:649–662. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilson L and Saseen JJ: Gouty arthritis: A
review of acute management and prevention. Pharmacotherapy.
36:906–922. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ge N, Peng XJ and Zheng YX: Drugs and
evaluation for the treatment of acute phase gout. Chinese General
Practice. 6:477–478. 2005.
|
|
6
|
Wang YJ: Guiding Journal of Traditional
Chinese Medicine and Pharmacy. 3:89–90. 2015.
|
|
7
|
Zhang XZ, Hu G, Huang MC and Ying L:
Clinical observation on 104 cases of acute gouty arthritis treated
by Simiaosan. Zhejiang J Trad Chin Med. 53:31–32. 2018.
|
|
8
|
Jovanovic DV, Boumsell L, Bensussan A,
Chevalier X, Mancini A and Di Battista JA: CD101 expression and
function in normal and rheumatoid arthritis-affected human T cells
and monocytes/macrophages. J Rheumatol. 38:419–428. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitroulis I, Kambas K and Ritis K:
Neutrophils, IL-1β, and gout: Is there a link? Semin Immunopathol.
35:501–512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martinon F, Pétrilli V, Mayor A, Tardivel
A and Tschopp J: Gout-associated uric acid crystals activate the
NALP3 inflammasome. Nature. 440:237–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang G, Lee HE, Moon SJ, Ko KM, Koh JH,
Seok JK, Min JK, Heo TH, Kang HC, Cho YY, et al: Direct binding to
NLRP3 pyrin domain as a novel strategy to prevent NLRP3-driven
inflammation and gouty arthritis. Arthritis Rheumatol.
72:1192–1202. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steiger S and Harper JL: Neutrophil
cannibalism triggers transforming growth factor β1 production and
self regulation of neutrophil inflammatory function in monosodium
urate monohydrate crystal-induced inflammation in mice. Arthritis
Rheum. 65:815–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martin WJ, Walton M and Harper J: Resident
macrophages initiating and driving inflammation in a monosodium
urate monohydrate crystal-induced murine peritoneal model of acute
gout. Arthritis Rheum. 60:281–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CJ, Shi Y, Hearn A, Fitzgerald K,
Golenbock D, Reed G, Akira S and Rock KL: MyD88-dependent IL-1
receptor signaling is essential for gouty inflammation stimulated
by monosodium urate crystals. J Clin Invest. 116:2262–2271. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang QB, Qing YF, Yin CC, Zhou L, Liu XS,
Mi QS and Zhou JG: Mice with miR-146a deficiency develop severe
gouty arthritis via dysregulation of TRAF 6, IRAK 1 and NALP3
inflammasome. Arthritis Res Ther. 20:452018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lopez-Castejon G and Brough D:
Understanding the mechanism of IL-1β secretion. Cytokine Growth
Factor Rev. 22:189–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takakubo Y, Barreto G, Konttinen YT, Oki H
and Takagi M: Role of innate immune sensors, TLRs, and NALP3 in
rheumatoid arthritis and osteoarthritis. J Long Term Eff Med
Implants. 24:243–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dinarello CA and van der Meer JW: Treating
inflammation by blocking interleukin-1 in humans. Semin Immunol.
25:469–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sil P, Wicklum H, Surell C and Rada B:
Macrophage-derived IL-1β enhances monosodium urate
crystal-triggered NET formation. Inflamm Res. 66:227–237. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu MY: Clinical study of Jiawei Si Miao
SAN in the treatment of hyperuricemia and acute gouty arthritis.
Liaoning J Trad Chin Med (Chinese Science and Technology Journal
Database). 38:675–677. 2011.
|
|
21
|
Deng W, Yang Z, Yue H, Ou Y, Hu W and Sun
P: Disulfiram suppresses NLRP3 inflammasome activation to treat
peritoneal and gouty inflammation. Free Radic Biol Med. 152:8–17.
2020. View Article : Google Scholar : PubMed/NCBI
|