Introduction
Gastric cancer is one of the commonest types of
cancers worldwide (1), with
widespread geographic variation in its prevalence in East Asia,
South and Central America and Eastern Europe (2). Gastric cancer has a poor prognosis
because of the likelihood of recurrence following curative surgery;
recurrence occurs in ~30–50% of patients despite the use of
different surgical strategies, including D2 lymphadenectomy and D2
gastrectomy (3,4). Peritoneal recurrence occurs in ~10–45%
of patients following curative surgery (3,5).
Multiple strategies have been developed for peritoneal recurrence,
including adjuvant chemotherapy (4,6),
neoadjuvant chemotherapy (7) and
adjuvant chemoradiation (8);
however, none of them successfully lower the rate of distant
metastasis.
Hyperthermic intraperitoneal chemotherapy (HIPEC) is
an adjunctive strategy to surgery. It is performed by employing a
heated solution containing cytotoxic drugs and intended to destroy
tumor cells that remain following tumor removal or existing tumor
cells in the peritoneum (which can later lead to recurrence)
(9). Hyperthermia is critical for
HIPEC, in which the malignant tumor is exposed to temperatures
≥40–43°C, which induce apoptosis of tumor cells mainly by
disrupting cytoskeleton components, changing membrane permeability
and inhibiting tumor cell growth (10). However, the exact mechanism of the
positive therapeutic effects of hyperthermia remains to be
elucidated.
It has been known for over three decades that cancer
cells present markedly more sensitivity to mild hyperthermia than
normal or nontumor cells (11,12).
Several mechanisms have been proposed for how hyperthermia kills
tumor cells, including disruption of plasma membrane protein and
cytoskeletal distribution, induction of reactive oxygen species,
disturbance of mitochondrial membrane potential, promotion of cell
apoptosis and arrest of cell cycle phases (13,14).
All these hyperthermic effects may be due to a change in global
gene expression in tumor cells. Studies have evaluated heat
shock-induced changes in global gene expression in tumor cells and
identify several genes involved in the regulation of apoptosis, the
cell cycle and cell structure/maintenance (15,16),
including kinesin family member 11 (KIF11), cyclin-dependent kinase
6 (CDK6), PAGE family member 2 (PAGE2), NIMA-related kinase 2
(NEK2) and karyopherin subunit α 4 (KPNA4). These findings have
raised further questions regarding the roles of these genes in
gastric cancer cells and in hyperthermia-induced cell damage.
The cell cycle kinase CDK6 has been recognized not
only as a typical cyclin-dependent kinase but also as a
transcriptional regulator of a number of genes and its effects may
be dependent on or independent of its kinase activity (15,16).
CDK6 tightly regulates the G1 to S cell cycle transition
and negatively regulates cell differentiation (17). In gastric cancer cells, CDK6 has
been reported to interact with EGFR and promote malignant
behaviors, including cell proliferation, migration and invasion,
while increasing chemoresistance (18). As CDK6 is also upregulated by
hyperthermia treatment, it was hypothesized that CDK6 may be
induced by hyperthermia and regulate tumor progression.
The purpose of the present study was to investigate
whether effects on CDK6 induced by hyperthermia are involved in
regulating malignant behaviors and hyperthermia-induced cell
injury. By identifying the potential roles of CDK6 in
hyperthermia-induced cell injury, the results suggested CDK6 as a
promising therapeutic target for enhancing sensitivity to
hyperthermia in gastric cancer.
Materials and methods
Cell culture
The Human gastric cancer cell lines SH-10-TC, HGC-27
and epithelial cell line GES-1 were bought from the American Type
Culture Collection and frozen in liquid nitrogen (~-196°C) in the
Department of Gastrointestinal Surgery, the First Affiliated
Hospital of Zhengzhou University (Zhengzhou, China). They were
cultured in DMEM (Thermo Fisher Scientific, Inc.) supplemented with
10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin (Thermo
Fisher Scientific, Inc.). Cells were maintained at 37°C in 5%
CO2 and passaged every 3–4 days.
Gene expression profiling interactive
analysis (GEPIA)
The relative expression levels in gastric cancer
samples of KIF11, CDK6, stromal antigen 2 (STAG2), NEK2 and KPNA4
compared with adjacent non-tumor samples were analyzed in GEPIA
(gepia.cancer-pku.cn; release date, 2017). The mRNA levels of these
genes in 407 gastric cancer samples and 211 adjacent non-tumor
samples were obtained from The Cancer Genome Atlas (TCGA) database.
Statistical analyses were performed using an unpaired Student's
t-test for two independent groups and the cut-off was
P<0.01.
Western blot analysis
Rabbit monoclonal anti-human KIF11 antibody (cat.
no. ab254298), rabbit anti-human CDK6 antibody (cat. no. ab124821),
goat polyclonal anti-human STAG2 antibody (cat. no. ab4463), rabbit
anti-human NEK2 antibody (cat. no. ab227958), goat anti-human KPNA4
antibody (cat. no. ab6039), rabbit anti-pan-AKT antibody (cat. no.
ab8805), rabbit anti-AKT (Ser473) antibody (cat. no. ab81283),
rabbit anti-AKT (Thr308) antibody (cat. no. ab38449) and mouse
monoclonal anti-β-actin antibody (cat. no. ab8226) were bought from
Abcam, diluted at 1:1,000 and used following the manufacturer's
instructions.
Cells were lyzed using a SoniConvert®
sonicator (DocSense) to obtain cell lysate following the
manufacturer's instruction. Total protein was quantitatively
measured using a BCA kit (Sigma-Aldrich; Merck KGaA). Total protein
(30 µg) was fractionated using 6–12% gradient Tris-glycine gels and
transferred onto PVDF membranes. The PVDF membranes were blocked in
5% milk/Tris-buffered saline (TBS) buffer at room temperature for
30 min followed by an incubation with primary antibodies at 4°C
overnight. After washing with PBS-T (containing 0.1% Tween-20)
three times, PVDF membranes were incubated with HRP-conjugated
secondary antibodies for another 1 h. The following secondary
antibodies were used (all 1:5,000; Abcam): Goat anti-mouse IgG
(HRP) secondary antibody (cat. no. ab97040); goat anti-rat IgG
(HRP) secondary antibody (cat. no. ab7097); and donkey anti-goat
IgG (HRP) secondary antibody (cat. no. ab7125). Blots were
developed using the Pierce ECL Western Blotting Substrate (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Protein expression was semi-quantified via
densitometry using ImageJ software (version-2.0; National
Institutes of Health) with β-actin as the loading control.
Short interfering (si)RNA
transfection
Knockdown of mRNA level of KIF11, CDK6, PAG2, NEK2
and KPNA4 were achieved by transient transfection of cells with
(si)RNA duplexes. siRNA duplexes of KIF11 (cat. no. 4390844), CDK6
(cat. no. 4390824), PAG2 (cat. no. 289806), NEK2 (cat. no. 143612)
and KPNA4 (cat. no. 143404) were purchased from Thermo Fisher
Scientific, Inc. The commercial specific siRNAs were provided by
Thermo Fisher Scientific, Inc.; negative control (siNC) sense
5′-GUUCAAUAUUAUCAAGCGGUU-3′ and antisense
5′-CCGCUUGAUAAUAUUGAACUU-3′ was employed as negative control
without specific target. siRNA transfection was performed by
following manufacturer's protocol. Briefly, 5×105 cells
were grown in 2 ml of serum-free medium. A siRNA/transfection
reagent complex was formed at room temperature by combining siRNA
oligomer (50 nM) with 5 µl (2 µg/ml) Lipofectamine® 2000
transfection reagent (Thermo Fisher Scientific, Inc.) in 0.5 ml
OptiMEM medium (Thermo Fisher Scientific, Inc.) and this was
applied to the cells for 48 h until they were harvested. Cells
transfected with NC siRNA were considered as control cells.
Successful knockdown of target gene was confirmed by performing
quantitative PCR (qPCR).
Apoptosis assay
The apoptosis of cells was analyzed performing
Annexin V-FITC/propidium iodide (PI)-double staining followed by
fluorescence-activated cell sorting analysis using Annexin V
Apoptosis Detection kit FITC (cat. no. 88-8005-72; eBioscience;
Thermo Fisher Scientific, Inc.). Briefly, 5×104 cells
were grown in a 6-well plate. Following 37°C or 42°C pre-incubation
for 2 h and another 24-h incubation under 37°C, cells were
trypsinized, washed with PBS, resuspended in 200 µl PBS with 10 µl
RNAase (10 mg/ml) and incubated at 37°C for 30 min. At the end of
incubation, 50 µl Annexin V-FITC/PI labeling solution (BD
Biosciences) was added and cells were analyzed for apoptosis using
a flow cytometer (BD FACS Canto II; BD Biosciences). The results
were analyzed using FlowJo software (version 9.7.4; Tree Star,
Inc.). Apoptotic cells were defined as Annexin
V−FITC+ cells.
Cell cycle analysis
To analyze cell cycle phases by quantification of
DNA content, cells harvested by trypsin were washed with ice-cold
PBS for three times to remove the medium. The cells were fixed with
ice-cold 70% ethanol overnight at 4°C. Fixed cells were pelleted
under 1,000 × g centrifugation and the supernatant was removed
followed by three washes with ice-cold PBS. Cells were incubated
with final concentration of 100 µg/ml RNase A and 40 µg/ml PI
(Beyotime Institute of Biotechnology) for 15 min in dark. The cells
were analyzed by a 3 laser Navios flow cytometer (Beckman Coulter,
Inc.) and the results were analyzed using ModFit analysis software
program (version 4.0; Verity Software House, Inc.).
Reverse transcription-qPCR
(RT-qPCR)
To isolate total RNA from cells, TRIzol®
(Thermo Fisher Scientific, Inc.) was used according to the
manufacturer's instructions. Subsequently, total RNA (1 µg) was
reverse transcribed into cDNA using a High-Capacity cDNA Reverse
Transcription kit (cat. no. 4368814, Thermo Fisher Scientific,
Inc.) according to the manufacturer's instruction. qPCR was
performed using PowerUp SYBR Green Master Mix (cat. no. A25918;
Thermo Fisher Scientific, Inc.) and an ABI 7500 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and the following
thermocycling conditions: 95°C for 10 min, 40 cycles of 95°C for 30
sec, 55°C for 30 sec and 72°C for 1 min. The following primers were
used for qPCR: CDK6 forward, 5′-GCTGACCAGCAGTACGAATG-3′ and
reverse, 5′-GCACACATCAAACAACCTGACC-3′; AKT forward,
5′-AGCGACGTGGCTATTGTGAAG-3′ and reverse,
5′-GCCATCATTCTTGAGGAGGAAGT-3′; β-actin forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′. mRNA expression levels were quantified
using the 2−ΔΔCq method (19) and normalized to the internal
reference gene β-actin.
High-content screening (HCS)-based
functional assay
The numbers of cells of HGC-27 following CDK6
knockdown or inhibition were observed for 4 days using an
ImageXpress®Micro XLS High-Content Screening (HCS)
system (Molecular Devices, LLC), according to the manufacturer's
protocols. Briefly, at 24 h post-transfection, cells were plated in
96-well plates and imaged every 24 h. Then the images were analyzed
using MetaXpress software (version 3.1; Molecular Devices,
LLC).
Migration and invasion
Transwell permeable plates with 8 µm pores (Corning
Life Sciences) was used for analyzing cell migration and invasion.
For measuring migration ability, cells (5×103) were
suspended and plated into upper chamber (8 µm pore size; Corning
Life Sciences). For measuring invasion ability, cells
(5×103) suspended in serum-free DMEM were plated into
the upper chamber (8 µm pore size; Corning Life Sciences), which
was precoated with Matrigel at room temperature for 4 h
(Sigma-Aldrich; Merck KGaA). The low chamber contained 500 µl of
serum-free DMEM medium supplemented with 1% FBS. To the upper
chamber, 1×104 cells were added suspended in serum-free
medium. After 24 h the cells in the chamber were fixed with 4%
paraformaldehyde at room temperature for 10 min and stained with
0.25% crystal violet (Sigma-Aldrich; Merck KGaA) at room
temperature for 30 min. Migratory and invading cells were
visualized using an X71 (U-RFL-T) fluorescence microscope (Olympus
Corporation; magnification, ×40).
Statistical analysis
All experiments in the present study were repeat
three times independently. An unpaired Student's t-test or one-way
ANOVA followed by Tukey's post hoc test was used to compare two or
multiple groups, respectively. All data in this study was presented
as the mean ± standard deviation. P<0.05 was considered to
indicate a statistically significant difference.
Results
Association between potential
hyperthermic-responding proteins and gastric cancer
As tumor cells demonstrate significantly more
sensitivity to hyperthermia (11,12),
the present study aimed to investigate the potential association
between hyperthermic responding proteins and gastric cancer.
According to a previous study (20), mRNA levels of KIF11, CDK6, STAG2,
NEK2 and KPNA4 are stimulated by hyperthermic incubation in cancer
cells. Thus, the present study investigated their expression levels
in gastric cancer samples by employing GEPIA to analyze RNA
sequencing data in TCGA. In GEPIA, the expression levels of these
five genes was analyzed in cancer samples (n=407) compared with
adjacent non-tumor samples (n=211). In general, the five genes
presented higher expression levels in cancer samples compared with
adjacent non-tumor samples (Fig.
1A). To confirm whether the high expression of these genes
existed in gastric cancer cell lines, gastric cancer cell lines
SH-10-TC, HGC-27 were used. Compared with human gastric epithelial
cells GES-1, protein levels of KIF11, CDK6, STAG2, NEK2 and KPNA4
were significantly higher in gastric cancer cell lines (Fig. 1B and C). These data indicated that
KIF11, CDK6, STAG2, NEK2 and KPNA4 were potentially involved in
gastric cancer progression and involved in regulating hyperthermic
response.
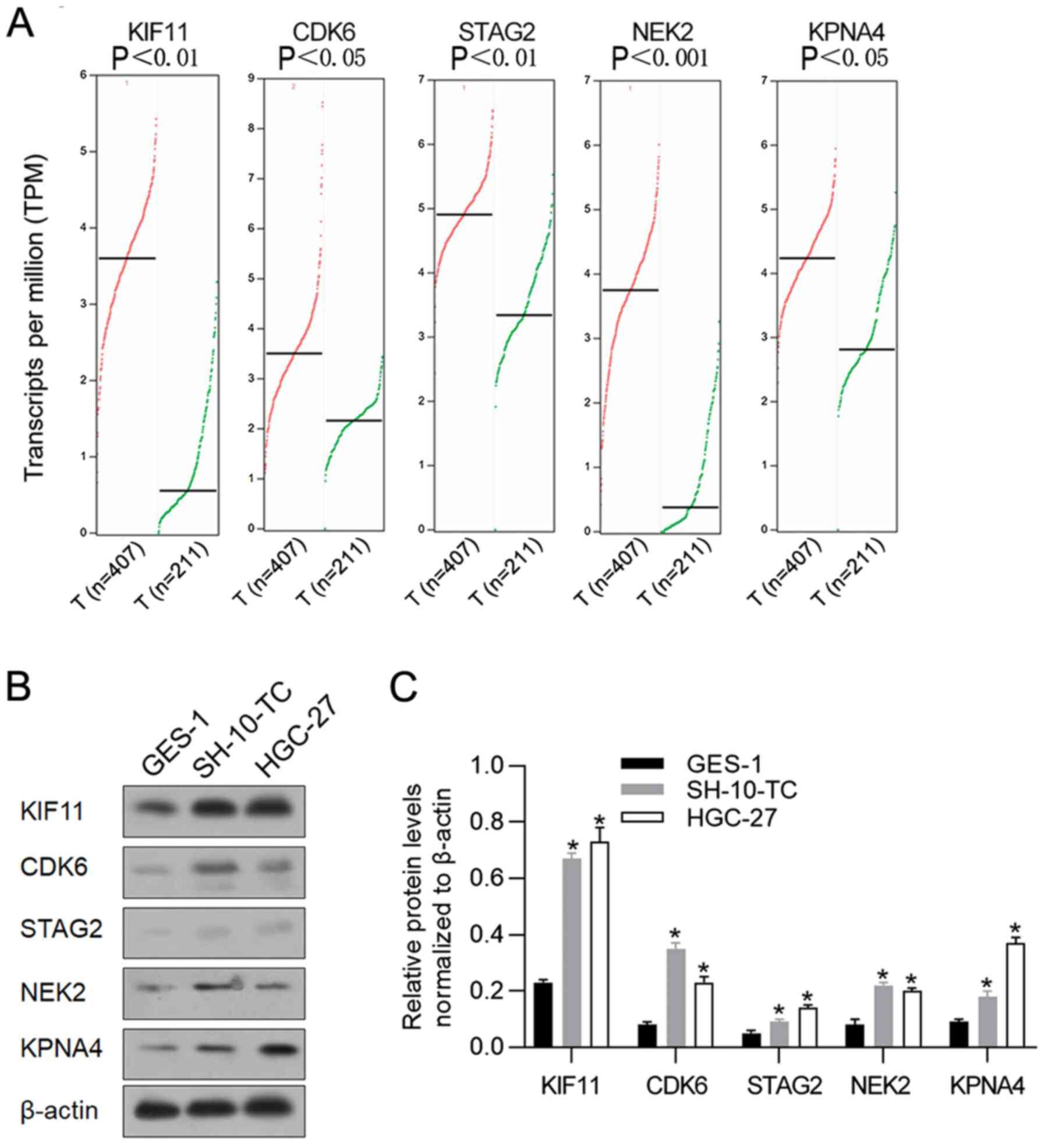 | Figure 1.Expression levels of KIF11, CDK6,
STAG2, NEK2 and KPNA4 in gastric cancer samples and cell lines. (A)
the GEPIA database revealed that KIF11, CDK6, STAG2, NEK2 and KPNA4
expression levels were significantly upregulated in gastric cancer
tissues (n=407) compared with adjacent tissues (n=211). (B) Western
blot analysis was performed to detect relative protein levels of
KIF11, CDK6, STAG2, NEK2 and KPNA4 in gastric cancer cell lines
SH-10-TC and HGC-27, compared with gastric epithelial cell line
GES-1. (C) Relative protein levels of KIF11, CDK6, STAG2, NEK2 and
KPNA4 in gastric cancer cell lines SH-10-TC and HGC-27 were
normalized to β-actin. *P<0.05, vs. GES-1 group. KIF11, kinesin
family member 11; CDK6, cyclin-dependent kinase 6; STAG2, stromal
antigen 2; NEK2, NIMA-related kinase 2; KPNA4, karyopherin subunit
α 4; GEPIA Gene Expression Profiling Interactive Analysis. |
CDK6 may protect gastric cancer cells
from hyperthermic-induced apoptotic cell death
To further investigate the role of hyperthermic
responding protein in hyperthermia treatment, instead of
overexpression due to high endogenous level, KIF11, CDK6, STAG2,
NEK2 and KPNA4 were knocked down by transfecting specific siRNAs
(Fig. 2A). Cells were pretreated at
37°C or 42°C for 2 h and incubated under 37°C for another 24 h,
followed by apoptotic analysis using Annexin V-FITC/PI double
staining. As shown in Fig. 2B and
C, 2-h pretreatment of hyperthermia induced cell apoptosis and
knockdown of both KIF11 and CDK6 significantly increased apoptotic
induction of hyperthermia. Knockdown of the other three proteins,
STAG2, NEK2 and KPNA4, failed to notably affect apoptosis induced
by hyperthermic pretreatment, indicating that KIF11 and CDK6 may be
critical for regulating hyperthermic-induced cell death. Markedly,
knockdown of KIF11 promoted apoptotic cell death under 37°C,
indicating that its effect on apoptosis may not be related to
hyperthermia treatment and thus the present study focused on
CDK6.
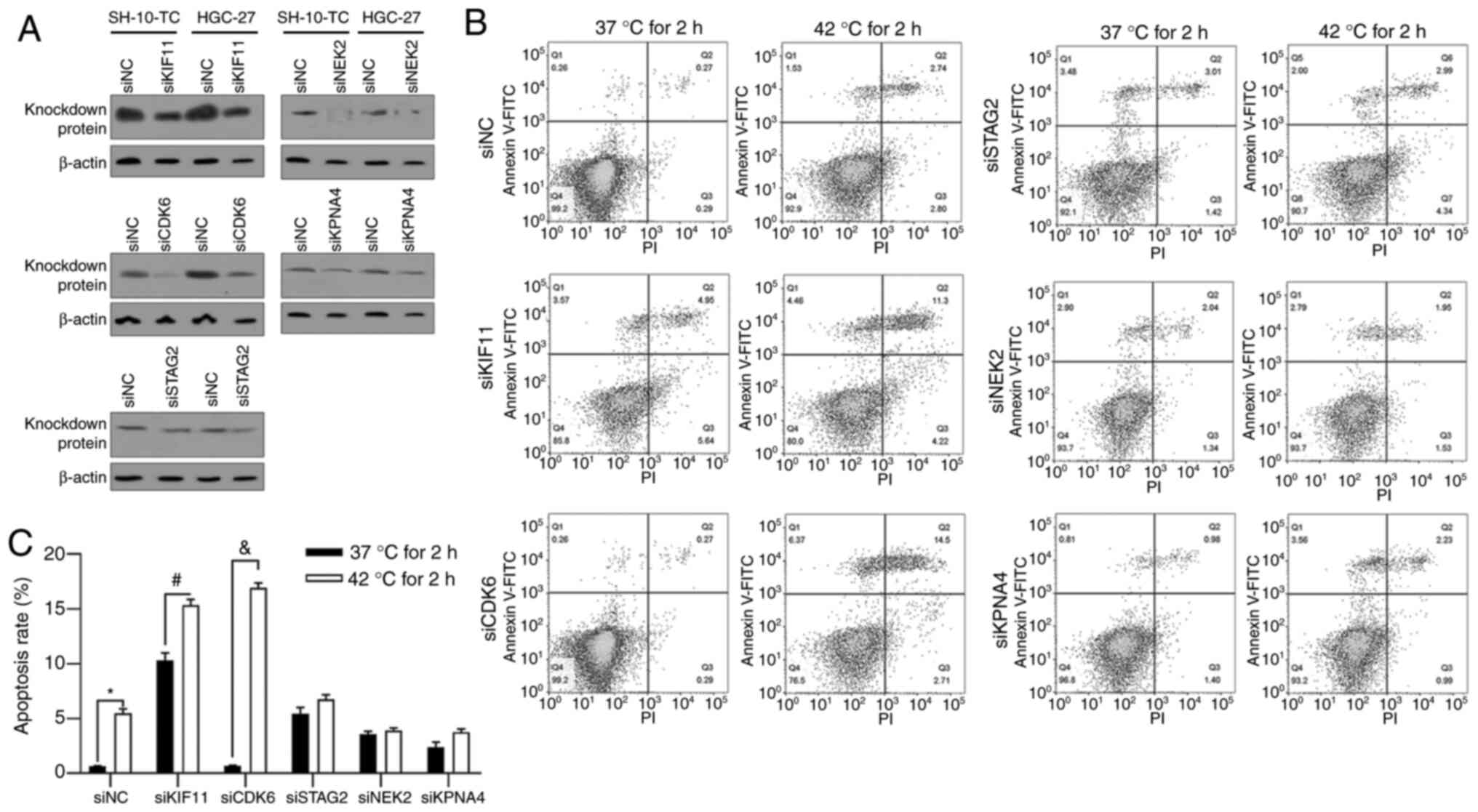 | Figure 2.Regulation of KIF11, CDK6, STAG2, NEK2
and KPNA4 on hyperthermic-induced apoptosis in HGC-27 cells. (A) At
48 h following siRNA transfection, protein levels of KIF11, CDK6,
STAG2, NEK2 and KPNA4 were analyzed by western blotting to confirm
the knockdown efficacy. (B and C) Following 2 h pre-incubation at
37°C or 42°C, cell apoptosis was analyzed by performing Annexin
V-FITC/PI double staining followed by follow cytometric analysis.
*P<0.05 vs. siNC/37°C 2 h-pre-incubated group;
#P<0.05 vs. siKIF11/37°C 2 h-pre-incubated group;
&P<0.05 vs. siCDK6/37°C 2 h-pre-incubated group.
KIF11, kinesin family member 11; CDK6, cyclin-dependent kinase 6;
STAG2, stromal antigen 2; NEK2, NIMA-related kinase 2; KPNA4,
karyopherin subunit α 4; PI, propidium iodide; si, short
interfering; NC, negative control. |
CDK6 is induced by hyperthermia
treatment and exerts a pro-survival effect with hyperthermia
To examine the expression level of CDK6 in HCG-27
cells under hyperthermia, RT-qPCR was performed to detect CDK6 mRNA
after 0, 6 and 12 h and western blot analysis was performed to
detect CKD6 protein after 0, 12 and 24 h. As shown in Fig. 3A and B, hyperthermia significantly
increased CDK6 transcriptionally at 6 and 12 h time points
following 2-h hyperthermia treatment and an increasing trend in
protein level was also observed after 12 and 24 h. Palbociclib, a
CDK6 inhibitor against its phosphorylating activity, was employed
to access the effect of CDK6 on the regulation of cell behavior
under hyperthermia. As shown in Fig.
3C, CDK6 knockdown and inhibition increased apoptotic cell
death under hyperthermia, demonstrating that CDK6 protects cells
from hyperthermic-induced apoptosis, at least partly, depending on
its phosphorylation activity. To investigate its critical effect on
cell cycle processes, cell cycle distribution was analyzed
following CDK6 knockdown or inhibition. CDK6 knockdown and
inhibition both blocked cell cycle at G1/G0
(Fig. 3D).
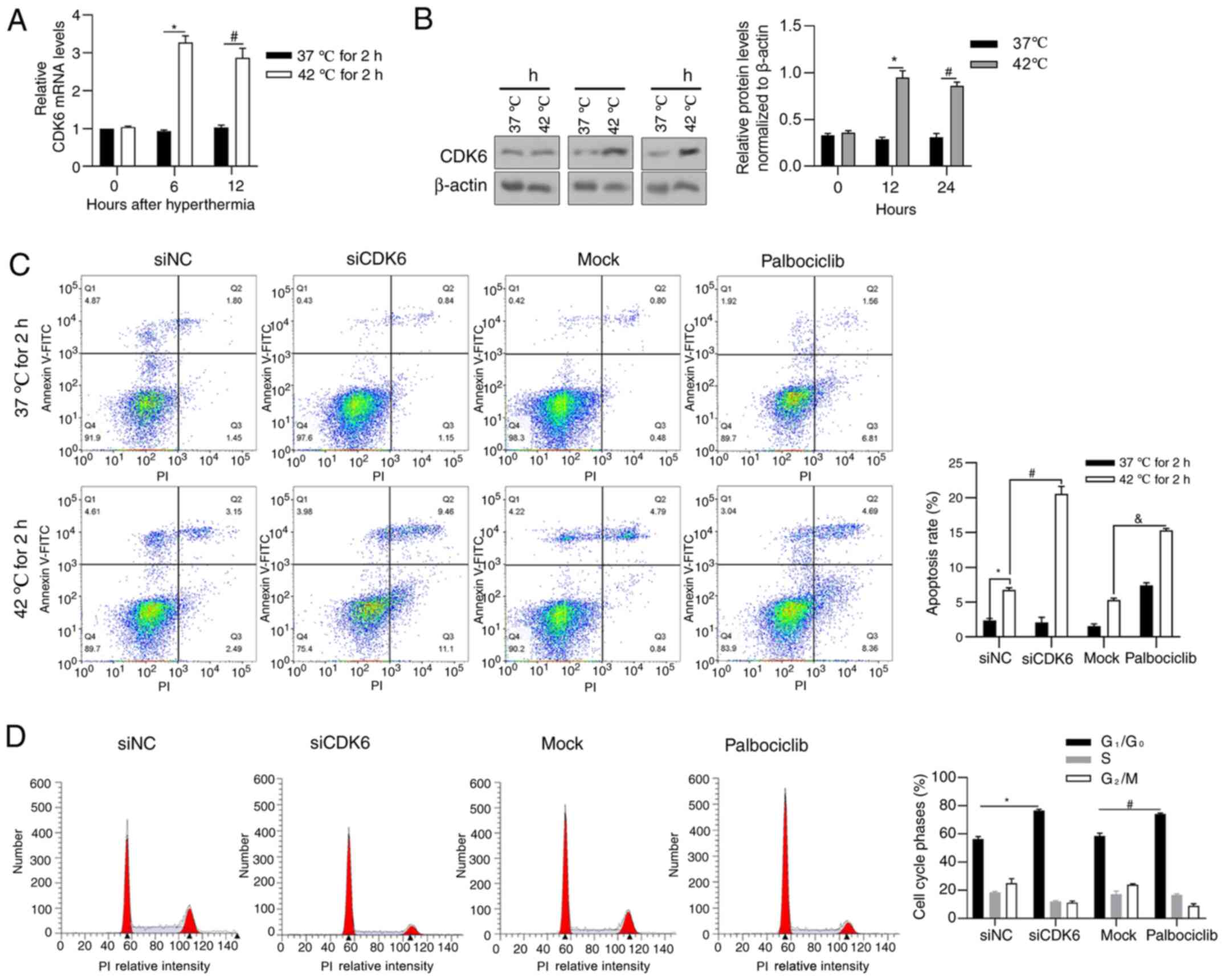 | Figure 3.CDK6 is transcriptionally induced by
hyperthermia and exerts pro-survival effect on HGC-27 cells. (A)
Reverse transcription-quantitative PCR was performed to detect CDK6
mRNA levels following 0, 6, 12 h of hyperthermia treatment.
*P<0.05 vs. 37°C for 2/6 h extra incubation;
#P<0.05, vs. 37°C for 2/12 h extra incubation. (B)
Western blotting was performed to detect CDK6 protein following 0,
12 and 24 h of hyperthermia treatment. *P<0.05 vs. 37°C for 12 h
group#P<0.05, vs. 37°C for 24 h group. (C) Following
CDK6 knockdown or inhibition by adding Palbociclib, the apoptosis
rate was measured by performing Annexin-FICT/PI double staining.
*P<0.05 vs. siNC/37°C for 2 h group; #P<0.05, vs.
siNC/42°C for 2 h group; &P<0.05 vs. mock/42°C
for 2 h group. (D) The effect of CDK6 knockdown or inhibition on
cell cycle distribution without hyperthermia treatment was measured
by performing PI staining followed by flow cytometry analysis.
*P<0.05 vs. siNC group; #P<0.05 vs. mock group.
CDK6, cyclin-dependent kinase 6; PI, propidium iodide; si, short
interfering; NC, negative control. |
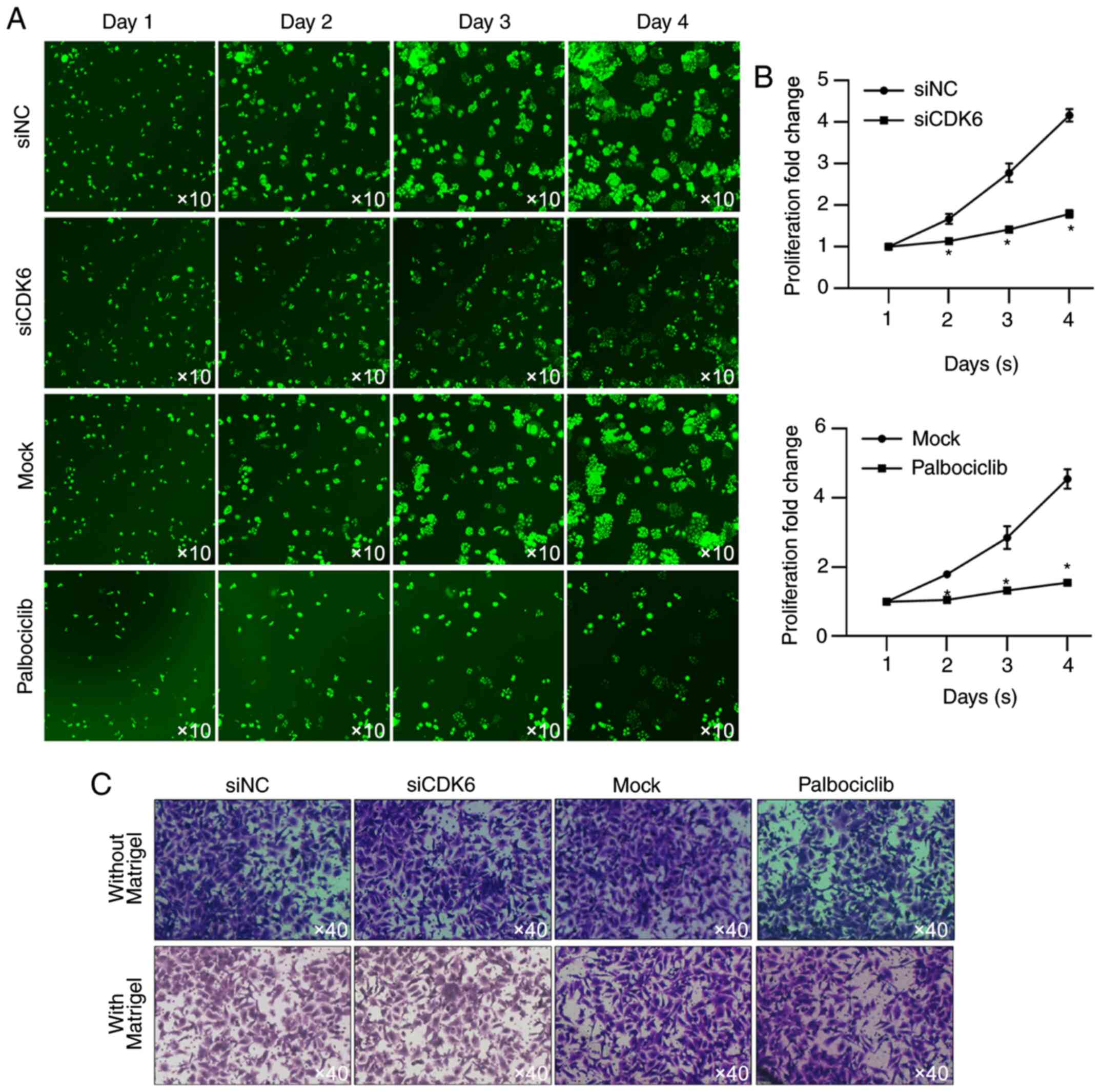
To further examine the effect of CDK6 on
proliferation, HSC-based cell viability assay was performed. As
shown in Fig. 4A and B, the
presence of CDK6 and activity of CDK6 are critical for maintaining
proliferation in HGC-27 cells. Whether CDK6 affected other
malignant behaviors, including migration and invasion was also
assessed. When incubated in serum-free medium, CDK6 failed to
affect cell proliferation (Fig.
4C), indicating that CDK6 mainly regulated HGC-27 via its
regulatory role in proliferation.
Hyperthermia suppresses expression and
phosphorylation of protein kinase B (AKT) and can increase CDK6
transcriptionally
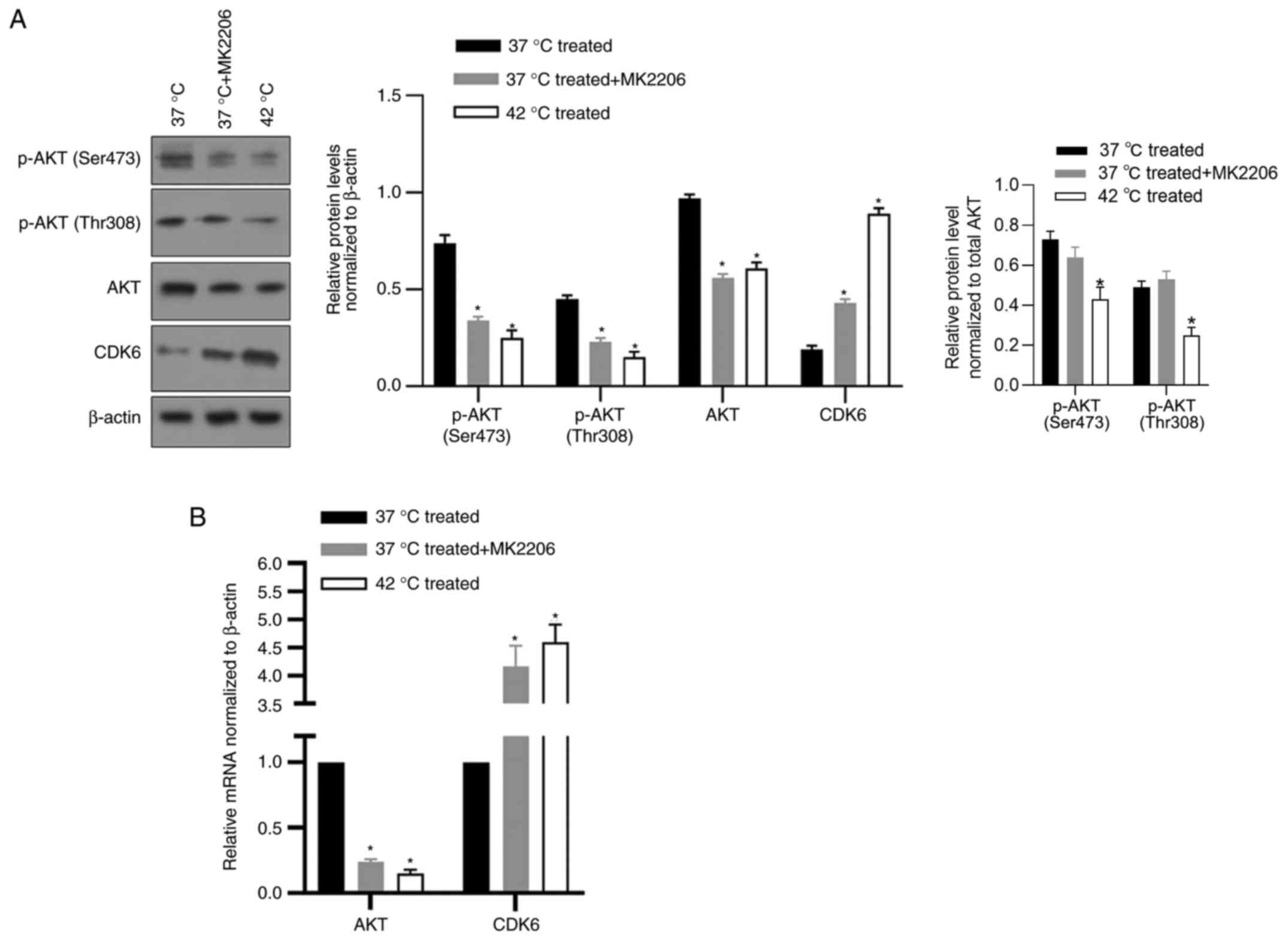
To investigate how CDK6 was regulated by
hyperthermia in cancer cells, the potential role of
serine/threonine kinase AKT, which is reported to transcriptionally
inhibit CDK6 in breast cancer cells (21) and be inhibited by hyperthermia
(22) was examined. An AKT
inhibitor, MK2206, which inhibits phosphorylation of AKT at Ser473
and Thr308, was used to assess the regulation of AKT on CDK6. As
shown in Fig. 5A, 42°C treatment
and addition of MK2206 significantly decreased AKT protein level.
AKT phosphorylation at Ser473 and Thr308 increased CDK6 protein
level (Fig. 5A), which indicated
that AKT activity inhibited by hyperthermia may be a critical
regulator of CDK6. To assess whether AKT transcriptionally
regulated CDK6, RT-qPCR was performed following hyperthermia
treatment or the addition of MK2206 and it was found that the
expression level of AKT was negatively associated with CDK6,
confirming its transcriptional regulation on CDK6 (Fig. 5B).
Discussion
The present study identified that CDK6 was highly
expressed in gastric cancer cells compared with gastric epithelial
cells and is further overexpressed in response to hyperthermia.
Notably, CDK6 was upregulated in gastric cancer patient tissues and
in gastric cancer cell lines. When CDK6 expression was
downregulated, increased cell apoptosis induced by hyperthermia
treatment and an inhibitory effect on cell proliferation via
blockade of the cell cycle at the G1 phase was observed,
but there were no effects on migration or invasion. It was further
observed that hyperthermia treatment inhibited AKT expression and
phosphorylation, which may be critical for the upregulation of
CDK6.
In global gene expression affected by heat
shock-induced change, KIF11, CDK6, PAGE2, NEK2 and KPNA4 are
reported to be markedly changed in the regulation of apoptosis, the
cell cycle and cell structure and/or maintenance (15,16).
Their relative expression, as measured from TCGA database, showed
no association between clinicopathological features, due to the
lack of online data. In further investigation, it is hoped to
perform these analyses.
The cell cycle kinase CDK6 has been recognized as
not only functioning as a typical cyclin-dependent kinase but also
acting as a transcriptional regulator with distinct properties from
its close homologue CDK4 (23,24).
As a transcriptional regulator, CDK6 controls the transcription of
numerous genes in both a kinase activity-dependent and -independent
manner (25) and regulates
malignant behaviors in several types of cancers (21,26,27).
Scheicher et al (28)
reported that in leukemic stem cells, CDK6 transcriptionally
regulates Egr1, a critical factor for hematopoietic stem cell and
leukemic stem cell activation and thus results in leukemia
tumorigenesis. It has also been reported that CDK6 physically
interacts with forkhead box O3 (FOXO3) and thus stabilizes it to
cause resistance of epithelial ovarian cancer cells to
platinum-induced cell death (27).
CDK6 is also found to exert critical regulatory roles in
tumorigenesis by acting as a kinase and antagonizing the
p53-induced antitumor response (26). Taken together, these findings show
that CDK6 is intricately involved in tumor regulation, indicating
its potential role in regulating hyperthermia-induced cell
damage.
In addition to determining the effect of CDK6 on
malignant behaviors, the effect of CDK6 on hyperthermia-induced
cell apoptosis was investigated. The presence of CDK6 inhibited
hyperthermia-induced cell apoptosis and hyperthermia treatment
significantly upregulated CDK6 protein levels. These findings
indicated that upregulation of CDK6 in gastric cancer tissues
compared with adjacent tissues could predict poor prognosis due to
potential insensitivity to hyperthermia and chemotherapy,
supporting the idea that CDK6 could represent a viable therapeutic
target.
In breast cancer cells, the CDK6 promoter is
transcriptionally regulated by the FOXO3/bromodomain-containing
protein 4 complex, which is phosphorylated and activated by AKT
(21). The addition of the AKT
inhibitor MK2206 transcriptionally stimulated CDK6 expression. In
the present study, the addition of MK2206 and siRNA-mediated AKT
knockdown in HGC-27 cells significantly increased CDK6 mRNA and
protein levels, which is consistent with previous findings
(21). Narikawa et al
(22) reported that hyperthermia
treatment suppresses AKT signaling by decreasing its protein level
and phosphorylation. The hypothesis of the present study was that
hyperthermia may upregulate CDK6 by causing AKT suppression. HGC27
is an unique gastric cancer cell line derived from lymph node
metastasis, therefore the results should be validated by further
investigation in other cell lines.
In conclusion, the associations between the
expression of several genes and sensitivity to hyperthermia in
gastric cancer cells has been detailed. The analyses of the present
study revealed that CDK6 had a role in promoting cell proliferation
and in preventing hyperthermia-induced cell apoptosis in gastric
cancer cells. It is hypothesized that CDK6 was upregulated in
gastric cancer cells and promoted both malignancy and resistance to
hyperthermia, which are known to promote the development and
progression of cancer as well as failure of therapy. The present
findings support the hypothesis that CDK6 is a potential
therapeutic target in the treatment of gastric cancer.
Acknowledgements
The authors would like to thanks for Mr. Tao Hong
(Sichuan University) for language editing and the suggestions for
statistical analysis.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
GL, HZ and QD designed the experiments. GL, HL, TL,
YL and HY performed the experiments. GL and QD were responsible for
data collection and performed statistical analysis. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thomassen I, van Gestel YR, van Ramshorst
B, Luyer MD, Bosscha K, Nienhuijs SW, Lemmens VE and de Hingh IH:
Peritoneal carcinomatosis of gastric origin: A population-based
study on incidence, survival and risk factors. Int J Cancer.
134:622–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Angelica M, Gonen M, Brennan MF,
Turnbull AD, Bains M and Karpeh MS: Patterns of initial recurrence
in completely resected gastric adenocarcinoma. Ann Surg.
240:808–816. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bang YJ, Kim YW, Yang HK, Chung HC, Park
YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, et al: Adjuvant
capecitabine and oxaliplatin for gastric cancer after D2
gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled
trial. Lancet. 379:315–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smalley SR, Benedetti JK, Haller DG,
Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson
JA, Jessup JM, et al: Updated analysis of SWOG-directed intergroup
study 0116: A phase III trial of adjuvant radiochemotherapy versus
observation after curative gastric cancer resection. J Clin Oncol.
30:2327–2333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yurttas C, Hoffmann G, Tolios A, Haen SP,
Schwab M, Königsrainer I, Königsrainer A, Beckert S and Löffler MW:
Systematic review of variations in hyperthermic intraperitoneal
chemotherapy (HIPEC) for peritoneal metastasis from colorectal
cancer. J Clin Med. 7:5672018. View Article : Google Scholar
|
|
10
|
Tao Y, Guo Y, Liu W, Zhang J, Li X, Shen
L, Ru Y, Xue Y, Zheng J, Liu X, et al: AKT inhibitor suppresses
hyperthermia-induced Ndrg2 phosphorylation in gastric cancer cells.
Braz J Med Biol Res. 46:394–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Habash RW, Bansal R, Krewski D and Alhafid
HT: Thermal therapy, part 2: Hyperthermia techniques. Crit Rev
Biomed Eng. 34:491–542. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Habash RW, Bansal R, Krewski D and Alhafid
HT: Thermal therapy, part 1: An introduction to thermal therapy.
Crit Rev Biomed Eng. 34:459–489. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roti RJ: Cellular responses to
hyperthermia (40–46 degrees C): Cell killing and molecular events.
Int J Hyperthermia. 24:3–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koutcher JA, Barnett D, Kornblith AB,
Cowburn D, Brady TJ and Gerweck LE: Relationship of changes in pH
and energy status to hypoxic cell fraction and hyperthermia
sensitivity. Int J Radiat Oncol Biol Phys. 18:1429–1435. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tabuchi Y, Wada S, Furusawa Y, Ohtsuka K
and Kondo T: Gene networks related to the cell death elicited by
hyperthermia in human oral squamous cell carcinoma HSC-3 cells. Int
J Mol Med. 29:380–386. 2012.PubMed/NCBI
|
|
16
|
Tabuchi Y, Takasaki I, Wada S, Zhao QL,
Hori T, Nomura T, Ohtsuka K and Kondo T: Genes and genetic networks
responsive to mild hyperthermia in human lymphoma U937 cells. Int J
Hyperthermia. 24:613–622. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malumbres M and Barbacid M: Mammalian
cyclin-dependent kinases. Trends Biochem Sci. 30:630–641. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia Y, Zhao LM, Bai HY, Zhang C, Dai SL,
Lv HL and Shan BE: The tumor-suppressive function of miR-1296-5p by
targeting EGFR and CDK6 in gastric cancer. Biosci Rep.
39:BSR201815562019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amaya C, Kurisetty V, Stiles J, Nyakeriga
AM, Arumugam A, Lakshmanaswamy R, Botez CE, Mitchell DC and Bryan
BA: A genomics approach to identify susceptibilities of breast
cancer cells to ‘fever-range’ hyperthermia. BMC Cancer. 14:812014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Duan Z, Guo W, Zeng L, Wu Y, Chen
Y, Tai F, Wang Y, Lin Y, Zhang Q, et al: Targeting the
BRD4/FOXO3a/CDK6 axis sensitizes AKT inhibition in luminal breast
cancer. Nat Commun. 9:52002018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Narikawa M, Umemura M, Tanaka R, Fujita T,
Yokoyama U, Ishigami T, Kimura K, Tamura K and Ishikawa Y: Acute
hyperthermia inhibits TGF-β1-induced cardiac fibroblast activation
via suppression of Akt signaling. Sci Rep. 8:62772018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kollmann K, Heller G, Schneckenleithner C,
Warsch W, Scheicher R, Ott RG, Schäfer M, Fajmann S, Schlederer M,
Schiefer AI, et al: A kinase-independent function of CDK6 links the
cell cycle to tumor angiogenesis. Cancer Cell. 24:167–181. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hydbring P, Malumbres M and Sicinski P:
Non-canonical functions of cell cycle cyclins and cyclin-dependent
kinases. Nat Rev Mol Cell Biol. 17:280–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tigan AS, Bellutti F, Kollmann K, Tebb G
and Sexl V: CDK6-a review of the past and a glimpse into the
future: From cell-cycle control to transcriptional regulation.
Oncogene. 35:3083–3091. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bellutti F, Tigan AS, Nebenfuehr S,
Dolezal M, Zojer M, Grausenburger R, Hartenberger S, Kollmann S,
Doma E, Prchal-Murphy M, et al: CDK6 antagonizes p53-induced
responses during tumorigenesis. Cancer Discov. 8:884–897. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dall'Acqua A, Sonego M, Pellizzari I,
Pellarin I, Canzonieri V, D'Andrea S, Benevol S, Sorio R, Giorda G,
Califano D, et al: CDK6 protects epithelial ovarian cancer from
platinum-induced death via FOXO3 regulation. EMBO Mol Med.
9:1415–1433. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scheicher R, Hoelbl-Kovacic A, Bellutti F,
Tigan AS, Prchal-Murphy M, Heller G, Schneckenleithner C,
Salazar-Roa M, Zöchbauer-Müller S, Zuber J, et al: CDK6 as a key
regulator of hematopoietic and leukemic stem cell activation.
Blood. 125:90–101. 2015. View Article : Google Scholar : PubMed/NCBI
|