Introduction
The components of ginger root (Zingiber
officinale Roscoe, Zingiberaceae) are widely used for various
medicinal purposes all over the world (1). One of the well-known effects of ginger
is relief of gastrointestinal symptoms, including hyperemesis
gravidarum and nausea (2). Several
bioactive compounds - that is, 6-, 8- and 10-gingerols, and
6-shogaol - have been identified in ginger (3,4). These
bioactive compounds function as antagonists of cholinergic and
serotonergic receptors (5) and, in
turn, might induce prevention of hyperemesis gravidarum and nausea
(2). Meanwhile, one of the
generally acknowledged beneficial effects of ginger consumption is
an induction of a ‘warm sensation.’ Recently, we demonstrated that
ginger powder might affect human metabolism in vivo
(6). Interestingly, 6-, 8- and
10-gingerols, and 6-shogaol act as regulators of transient receptor
potential (TRP) cationic channels, including TRP cation channel
subfamily V member 1 (TRPV1), TRP canonical 5 (TRPC5), and TRP
ankyrin 1 (TRPA1) (7–9).
TRP cationic channels are nonselective channels and
are activated by chemicals and temperature (heat) (10,11).
In particular, TRPV1 functions as a sensor for heat >42°C and a
capsaicin receptor causing a burning sensation under stimulation of
capsaicin that is the ‘hot’ ingredient in chili peppers. Recently,
we showed that moderate heat (39.5°C) or capsaicin activates
protein kinases, upregulates the expression of heat shock proteins
(HSPs), and induces morphological changes in mouse fibroblast cells
(12–15). If the components of ginger affect
cells in a similar manner as heat or capsaicin by activating TRPV1,
it is postulated that these components can regulate protein
kinases, HSP expression, and cell morphology. However, the effects
of such components on cells have not been fully elucidated.
In this study, to determine whether ginger powder
extracts (GPE) modify cell functions, we conducted various in
vitro experiments in NIH3T3 mouse fibroblast cells. We
investigated the effects of GPE on cellular responses; for
instance, activation of Akt-mammalian target of rapamycin (mTOR)
signaling and mitogen-activated protein kinases (MAPKs), cell
morphology and migration, levels of HSPs, and heat tolerance - in
mouse fibroblast cells.
Materials and methods
Chemicals
Dried ginger powder was provided by Sunsho
Pharmaceutical Co., Ltd. Dulbecco's modified Eagle's medium (DMEM)
was obtained from Wako Pure Chemical Industries, Ltd., whereas
fetal bovine serum (FBS) was obtained from Invitrogen; Thermo
Fisher Scientific, Inc.. Anti-phospho-mTOR (Ser2448) rabbit
antibody (#2971), anti-mTOR rabbit antibody (#2983),
anti-phospho-Akt (Ser473) rabbit antibody (#9271), anti-Akt rabbit
antibody (#9272), anti-phospho-specific p38 mitogen-activated
protein kinase (p38 MAPK) (Thr180/Tyr182) rabbit antibody (#9211),
anti-p38 MAPK rabbit antibody (#9212), anti-phospho-specific
extracellular signal-regulated kinase (ERK1/2) (Thr202/Tyr204)
(20G11) rabbit antibody (#4376), anti-ERK1/2 rabbit antibody
(#9102), anti-heat shock factor 1 (HSF1) rabbit antibody (#4356),
anti-HSP90 (E289) rabbit antibody (#4875), anti-HSP70 rabbit
antibody (#4872), anti-HSP40 rabbit antibody (#4868),
anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) rabbit
antibody (#2118), and horseradish peroxidase (HRP)-conjugated
anti-rabbit IgG (#7074) were purchased from Cell Signaling
Technology, Inc.. Meanwhile, EzWestBlue was purchased from ATTO
Corp..
Preparation and characterization of
GPE
GPE was extracted from dried ginger powder (Sunsho
Pharmaceutical Co., Ltd. Shizuoka, Japan) with 95% ethanol and dry
down with N2 gas. Then, residues were dissolved in
dimethyl sulfoxide (DMSO). The active components of GPE used in
this study were characterized using high-performance liquid
chromatography (HPLC). The active components of GPE were measured
as described by Yu et al (4)
and Tao et al (3) with a
slight modification. Briefly, HPLC was combined with electrospray
ionization/tandem mass spectrometry (LC-ESI-MS/MS) in a TSQ Quantum
mass spectrometer (Thermo Fisher Scientific, Inc.). HPLC was
conducted in a Luna 3u C18 (2) 100
Å LC column (100×2.0 mm; Phenomenex) at 30°C. Samples were eluted
with a mobile phase composed of acetonitrile-methanol (4:1,v/v) and
water-acetic acid (100:0.1, v/v) in a 20:80 ratio for 5 min, then
ramped up to a 100:0 ratio after 10 min, and held for 5 min at a
flow rate of 0.2 ml/min. MS/MS analyses were conducted in positive
ion mode, and 6-, 8-, 10- and 12-gingerols and 6-, 8- and
10-shogaols were detected and quantified with selected reaction
monitoring. Peaks were selected, and their areas were calculated
using Xcalibur 2.1 software (TThermo Fisher Scientific, Inc.). The
main active components in GPE are summarized in Table I.
 | Table I.Main bioactive components in ginger
powder extracts (100 µg) used in the present study. |
Table I.
Main bioactive components in ginger
powder extracts (100 µg) used in the present study.
| Bioactive
components | Weight, µg |
|---|
| 6-Gingerol | 23.68 |
| 8-Gingerol | 3.80 |
| 10-Gingerol | 5.22 |
| 12-Gingerol | 0.15 |
| 6-Shogaol | 51.38 |
| 8-Shogaol | 7.18 |
| 10-Shogaol | 4.58 |
For treatment of cells, GPE stock solutions
dissolved in DMSO at the concentrations of 0.001, 0.01, 0.1, and
1.0 mg/ml were prepared and stored at −20°C until use. The
respective GPE stock solutions were diluted 1:1,000 (v/v) in the
cell culture medium. Resultantly, the final concentration of GPE in
culture medium used in each experiment was 0.001, 0.01, 0.1, and
1.0 mg/ml, respectively.
Cell culture
NIH3T3 mouse fibroblast cells were provided by Dr
Nobuhiko Komine (Kanazawa University). The cells were maintained in
DMEM containing 10% FBS at 37°C in a 5% CO2
incubator.
Western blotting
Western blotting was performed as described
previously (16). Briefly, proteins
were extracted from cells, and protein concentrations were
determined using Pierce BCA protein assay kit (Thermo Fisher
Scientific, Inc.) according to the manufactures protocol. Equal
amounts of protein (30 µg) were separated from each sample using
10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE). The resolved proteins were transferred onto
polyvinylidene fluoride (PVDF) membranes, which were incubated with
primary antibodies (1:1,000), followed by incubation with
HRP-linked secondary antibodies (1:2,000).
Actin filament staining
To evaluate the actin cytoskeletons, cells were
fixed in 3.7% (v/v) formaldehyde in Dulbecco's phosphate-buffered
saline (PBS) and processed as described previously (17). F-actin was visualized with
tetramethylrhodamine (TRITC)-labeled phalloidin under an inverted
EVOS fluorescence microscope (Life Technologies Japan).
Cell viability assay
Cell viability was analyzed using the Cell Counting
Kit-8 (Wako Pure Chemical Industries, Ltd.) as described previously
(16). NIH3T3 cells were seeded in
96-well plates at a density of 1×103 cells/well. After
24 h of incubation, the cells were treated with 0.001–1.0 µg/ml of
GPE for 2 days. Next, the cells were incubated with 10 µl CCK-8 for
3 h at 37°C. The absorbance of the colored formazan product
produced by mitochondrial dehydrogenases in metabolically active
cells was recorded at 450 nm. Cell viability was expressed as a
ratio of the absorbance obtained in treated wells relative to that
in untreated (control: 0.1% DMSO) wells.
Wound healing assay (cell
migration)
A wound healing assay was performed to evaluate the
migration ability of the cells. Cells were passaged into 35-mm
dishes. When the cells reached to the 90% confluence, an injury
line of 2 mm width was drawn using a pipet tip. The dishes were
rinsed with PBS and incubated with DMEM. The images were obtained
after 16 h of incubation and the wound closure was measured.
Severe heat shock treatment
The cells were exposed to 45°C temperature for 30
min after a 2-day treatment with or without GPE at 37°C in 5%
CO2. One day after the heat shock treatment, cell
viability was analyzed using the Cell Counting Kit-8 as described
above.
Statistical analysis
Data are presented as the mean ± standard error of
the mean (SEM) from at least three independent experiments.
Statistical analysis was performed using a Student's unpaired
t-test or a Kruskal-Wallis nonparametric analysis of variance
(ANOVA) with Dunn's post hoc test, and results were considered
statistically significant when P<0.05 or P<0.01.
Results
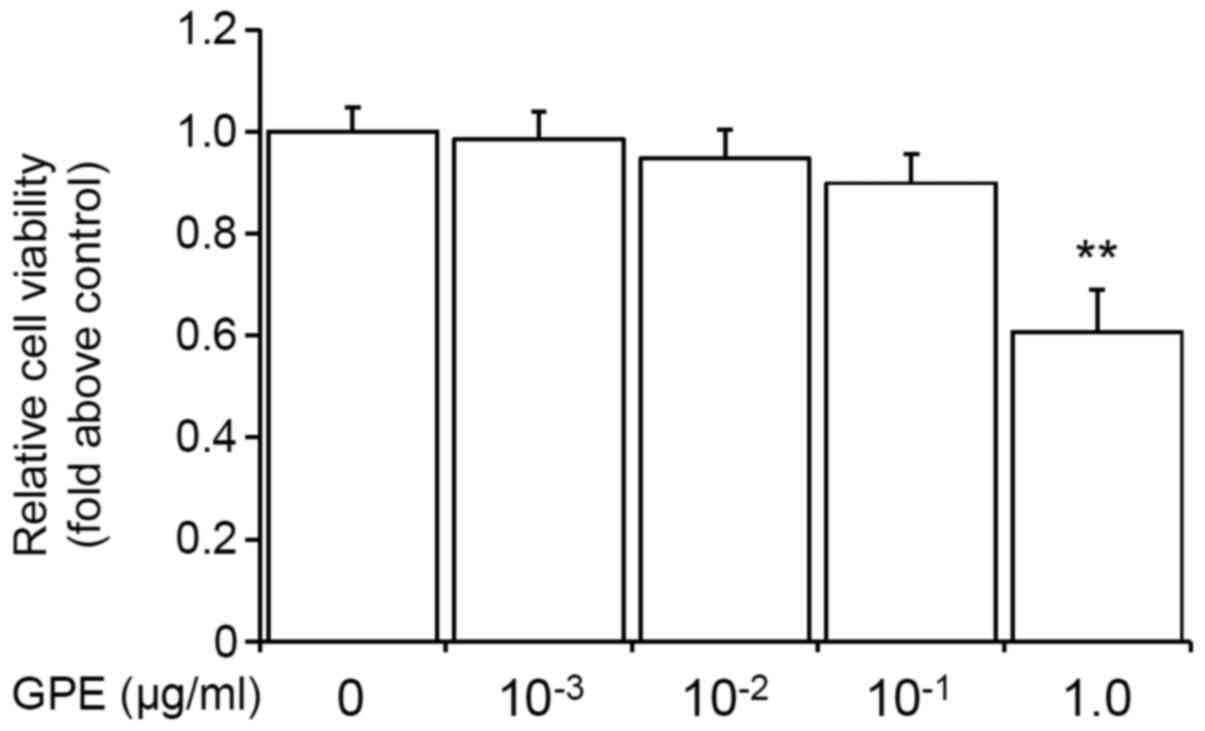
Low doses of GPE do not affect cell
viability in mouse fibroblast cells
Several previous reports have shown that the
ingredients of GPE, gingerol and shogaol have toxic effects on the
cells (18–20). However, in this study, we found that
low doses of GPE (0.001–0.1 µg/ml) had a minimal effect on cell
viability. As shown in Fig. 1, the
low doses (0.001–0.1 µg/ml) of GPE did not significantly decrease
the cell viability. Therefore, we used 0.001–0.1 µg/ml doses of GPE
for the subsequent experiments.
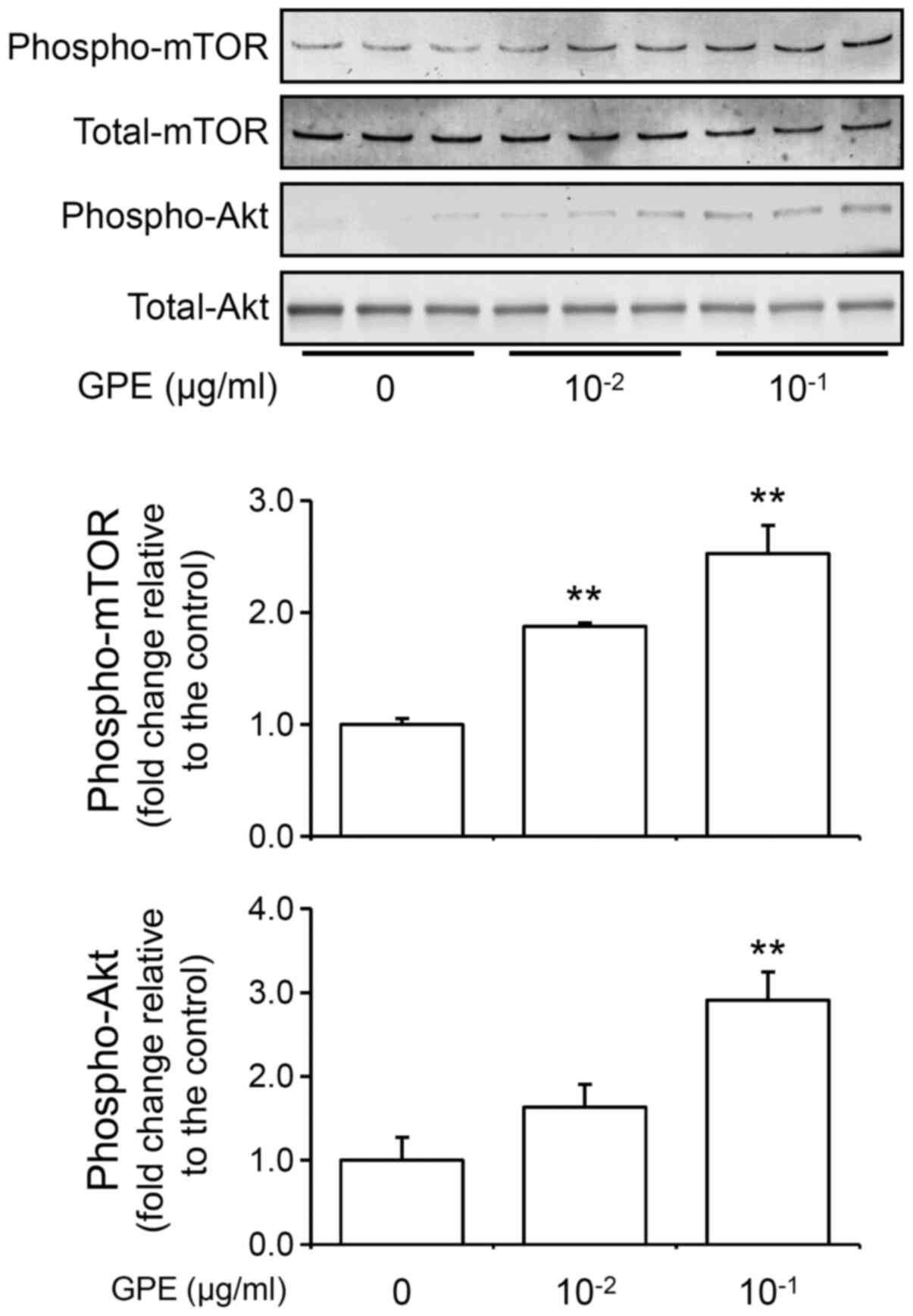
GPE activate Akt/mTOR signaling
To determine whether GPE activates Akt/mTOR
signaling pathway, we detected phosphorylated Akt (phospho-Akt) and
phosphorylated mTOR (phospho-mTOR) by western blot. Western blot
analysis revealed that the GPE increased phospho-Akt and
phospho-mTOR levels in NIH3T3 cells (Fig. 2). These results indicate that the
GPE activates Akt/mTOR signaling pathway. These result indicate
that the activation of Akt-mTOR signaling and increase in
intracellular phosphatidylinositol 3-phosphate (PtdIns3P or PI3P).
PI3P stimulates Rho family small G proteins (Rho, Rac, and Cdc42)
as well as Akt (17,21), and, in turn, regulates cell
morphology and cell migration.
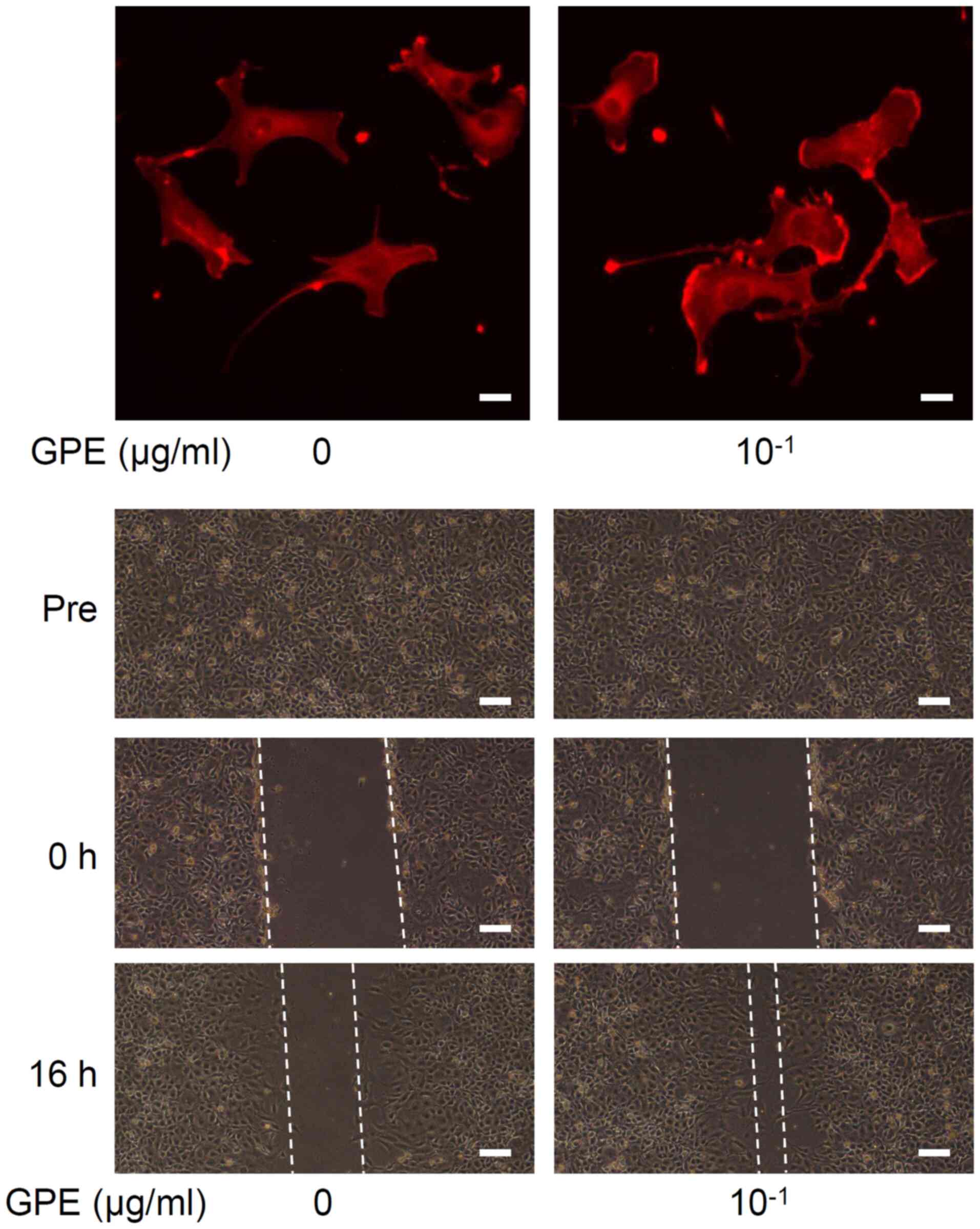
GPE regulate cell morphology and
stimulate cell migration
Microscopic examination indicated that GPE changed
cell morphology and promoted cell migration (Fig. 3). We observed that lamellipodia
formation occurred at the cell edges (Fig. 3, upper), which is known to
facilitate cell migration (22). In
fact, a 16-h treatment with GPE narrowed the wound area in
vitro (Fig. 3, lower),
indicating acceleration of cell migration.
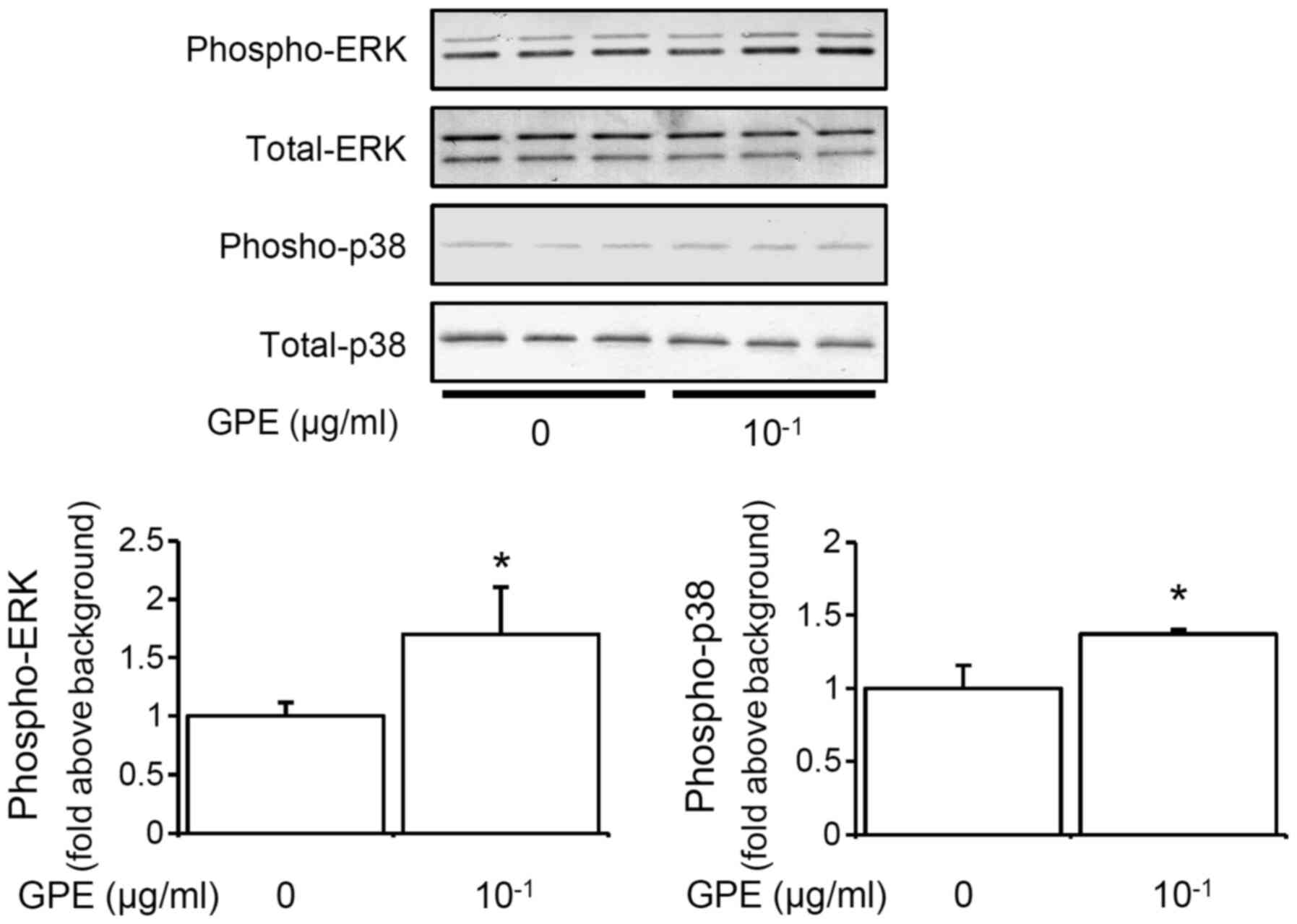
GPE activate ERK and p38 MAPK in mouse
fibroblast cells
MAPKs, including ERK and p38 MAPK, play crucial
roles in the transduction from extracellular stimuli to
intracellular signaling (15,22).
Next, we tested the effects of GPE on ERK and p38 MAPK in the
cells. A 10-min treatment with GPE increased the phosphorylation of
ERK and p38 MAPK in a dose-dependent manner (Fig. 4), indicating that the GPE activates
ERK and p38 MAPK.
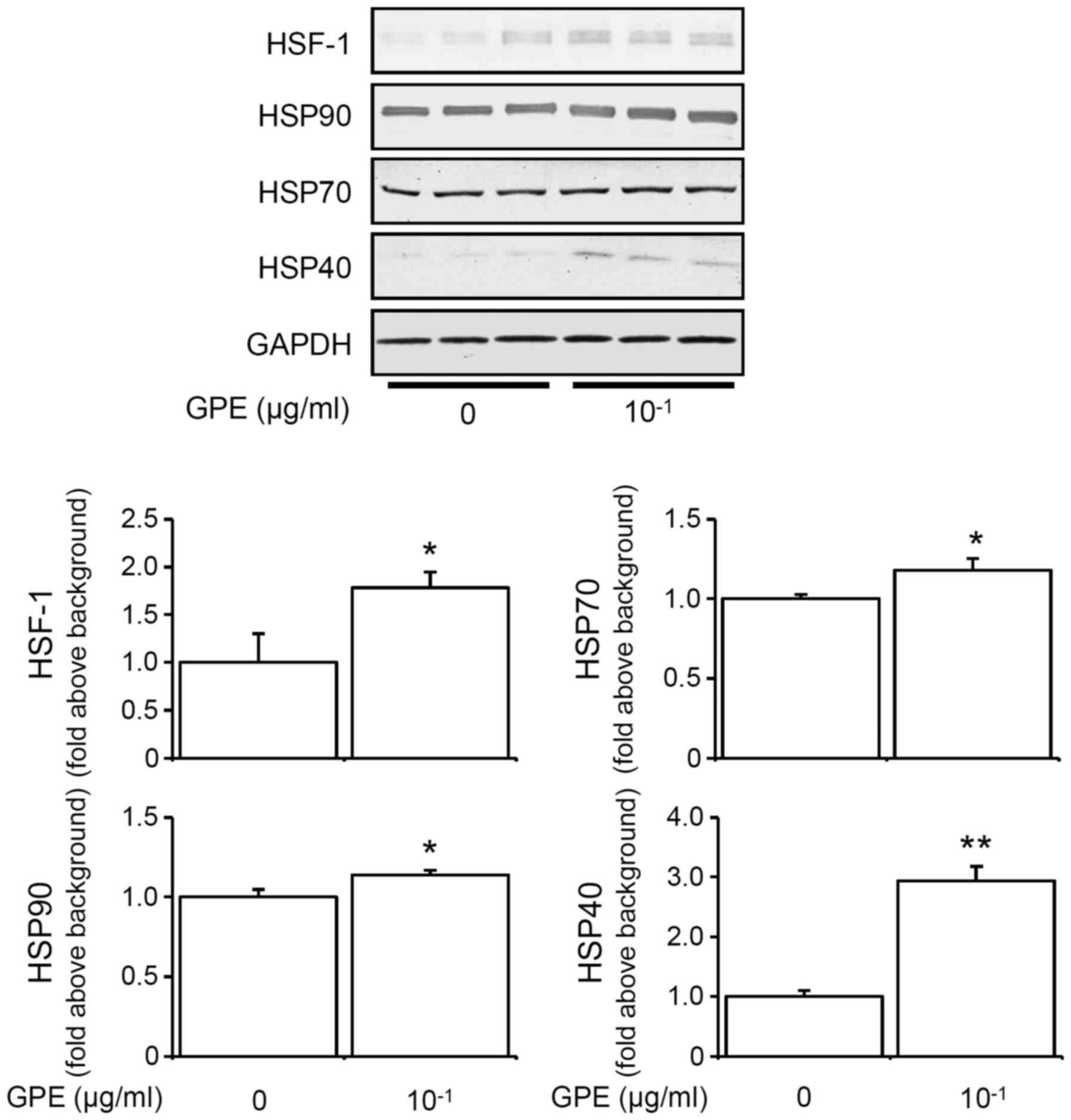
GPE induces HSF1, HSP90, HSP70 and
HSP40 expression
Continuous exposure to heat induces the upregulation
of HSPs in vitro (23,24).
Previously, we had also shown that continuous 2-day exposure to
moderate heat increased HSP70 and HSP90 expressions (12). We therefore speculated whether GPE
upregulates HSPs expressions in mouse fibroblast cells.
Interestingly, the expression of HSF1, HSP90, HSP70, and HSP40 was
increased after a 2-day continuous treatment with GPE in NIH3T3
cells (Fig. 5). These results
indicated that ginger might induce upregulation of HSPs similar to
the effect of heat exposure.
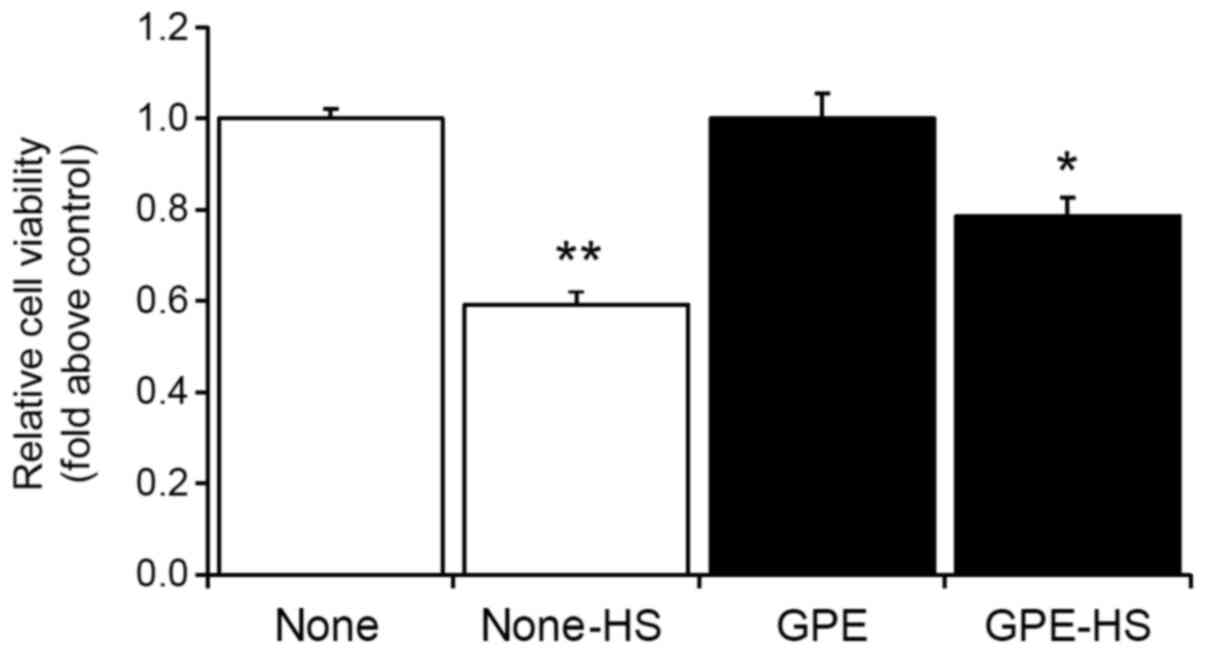
GPE attenuates severe heat
shock-induced cell death
HSPs play an important role in protecting cells from
environmental stressors such as heat shock (14,15,23,24).
Thus, we examined whether a continuous 2-day treatment with GPE can
improve heat tolerance by measuring cell viability after severe
heat shock in mouse fibroblast cells. The cells were incubated at
45°C for 30 min (severe heat shock) after an initial 2-day
treatment with GPE at 37°C. Cell viability was determined after 1
day of severe heat shock.
Results showed that although severe heat shock
decreased cell viability, GPE treatment was able to attenuate heat
shock-induced cell death in mouse fibroblast cells (Fig. 6). This result suggested that GPE may
facilitate heat tolerance in vitro.
Discussion
Several bioactive compounds-6-, 8-, 10-, and
12-gingerols and 6-, 8- and 10-shogaols-were identified in GPE
(Table I). However, the effects of
these bioactive compounds on cells have not been fully elucidated
in vitro. The results of our investigation provide evidence
that GPE activated phosphoinositide 3-kinase (PI3K)-Akt-mTOR and
MAPKs, facilitated cell migration and expression levels of HSPs,
and attenuated heat shock-induced cell death in mouse fibroblast
cells.
Treatment with GPE (~0.1 µg/ml) did not affect cell
viability, indicating that GPE (~0.1 µg/ml) is nontoxic to mouse
fibroblast cells, in contrast to several reports showing that the
ingredients of ginger extracts, such as gingerol and shogaol, have
cytotoxic activity (18–20). The discrepancy in the findings
regarding the cytotoxic activity of ginger might be attributable to
the difference in the doses of GPE used. In our study, the dose
used was ~0.1 µg/ml, whereas Akimoto et al (18) used ~25 µg/ml. Interestingly, the
total levels of free 6-, 8- and 10-gingerols and 6-shogaol in serum
60 min after the oral intake of GPE in humans were <0.1 µg/ml
(25). Therefore, the physiological
response to lower doses of ginger constituents-rather than higher
doses-is likely to be of greater importance. The doses of GPE
(0.01–0.1 µg/ml) used in our study were appropriate for elucidating
the effect of ginger on cells.
Actin assembly and cell migration are regulated by
the activity of a number of signaling molecules (15,22).
Lamellipodia are formed by actin assembly at the edge of a cell in
the direction of migration. PI3K activation is known to
phosphorylate Akt (Akt activation) and induce lamellipodia
formation (15,22). After Akt activation by PI3K, Akt
phosphorylates mTOR (15,22,26).
Here, we have shown that GPE phosphorylate Akt and mTOR in mouse
fibroblast cells, indicating that they activate the PI3K-Akt-mTOR
pathway. Moreover, GPE facilitate lamellipodia formation occurring
at cell edges and narrow wound areas, indicating acceleration of
cell migration. These results suggested that ginger might play a
valuable role in wound healing, erosion, or ulcer. In fact, a
recent study showed that the Japanese herbal medicine
Hangeshashinto, which contains ginger, enhances oral keratinocyte
migration to facilitate healing of oral ulcerative mucositis
(6). The components contained in
ginger may regulate actin assembly and cell migration via the
Akt-mTOR pathway. However, there are many other signal cascades
that control cytoskeletal polymerization and cell migration other
than Akt-mTOR pathway, e.g., Rho-A/Rho-kinase signaling pathway
(27,28). The impact of GPE on actin assembly-
and cell migration-related signal cascades other than Akt-mTOR
pathway should be investigated in the future.
In general, the activation of Akt pathway is related
not only to migration but also to proliferation of various types of
cells, such as mononuclear macrophages and epithelial cells
(29,30). Also, we have previously shown that
direct exposure to mild heat increases neural stem/progenitor cells
(NSC/NPCs) proliferation concomitant with the upregulation of Akt
phosphorylation (31). Since GPE
may affect various types of cell proliferation via activation of
Akt pathway, the effects of GPE on cell proliferation e.g.,
mononuclear macrophages, epithelial cells and NSC/NPCs, should be
investigated as a continuation of this study.
GPE activated ERK and p38 MAPK. ERK signaling is a
crucial regulator of growth, differentiation, and migration
(32), whereas p38 MAPK is
generally known as the principal stress-activated protein kinase,
and the p38 MAPK pathway regulates HSP transcription (33) through the activation of HSF-1
(34). In this study, GPE increased
the levels of HSF-1, and HSPs (HSP90, HSP70, and HSP40),
concomitant with activation of p38 MAPK.
HSPs play an essential chaperoning role that helps
cells maintain cellular protein homeostasis and prevent apoptosis
under diverse forms of stress (24,35–37).
Previously, the upregulation of HSP70 and HSP90 has been shown to
cause the development of heat tolerance in mouse fibroblast cells
(12,14,15).
In this study, GPE attenuated heat shock-induced cell death. These
data indicate that ginger may facilitate heat tolerance similar to
the effect of heat exposure.
However, our data did not show which receptor
mediated ginger-induced cell functions. The bioactive compounds in
ginger have been reported to function as antagonists of cholinergic
and serotonergic receptors (5) or
activators of TRPV1, TRPVC5, and TRPA1 (7–9).
Interestingly, TRPV1 and TRPA1 act as thermo-sensors. In this
study, we showed that GPE upregulate HSP levels in a similar manner
to heat in mouse fibroblast cells. These lines of evidence indicate
that ginger might moderate cell functions through TRPs, including
TRPV1 and TRPA1 (Fig. 7).
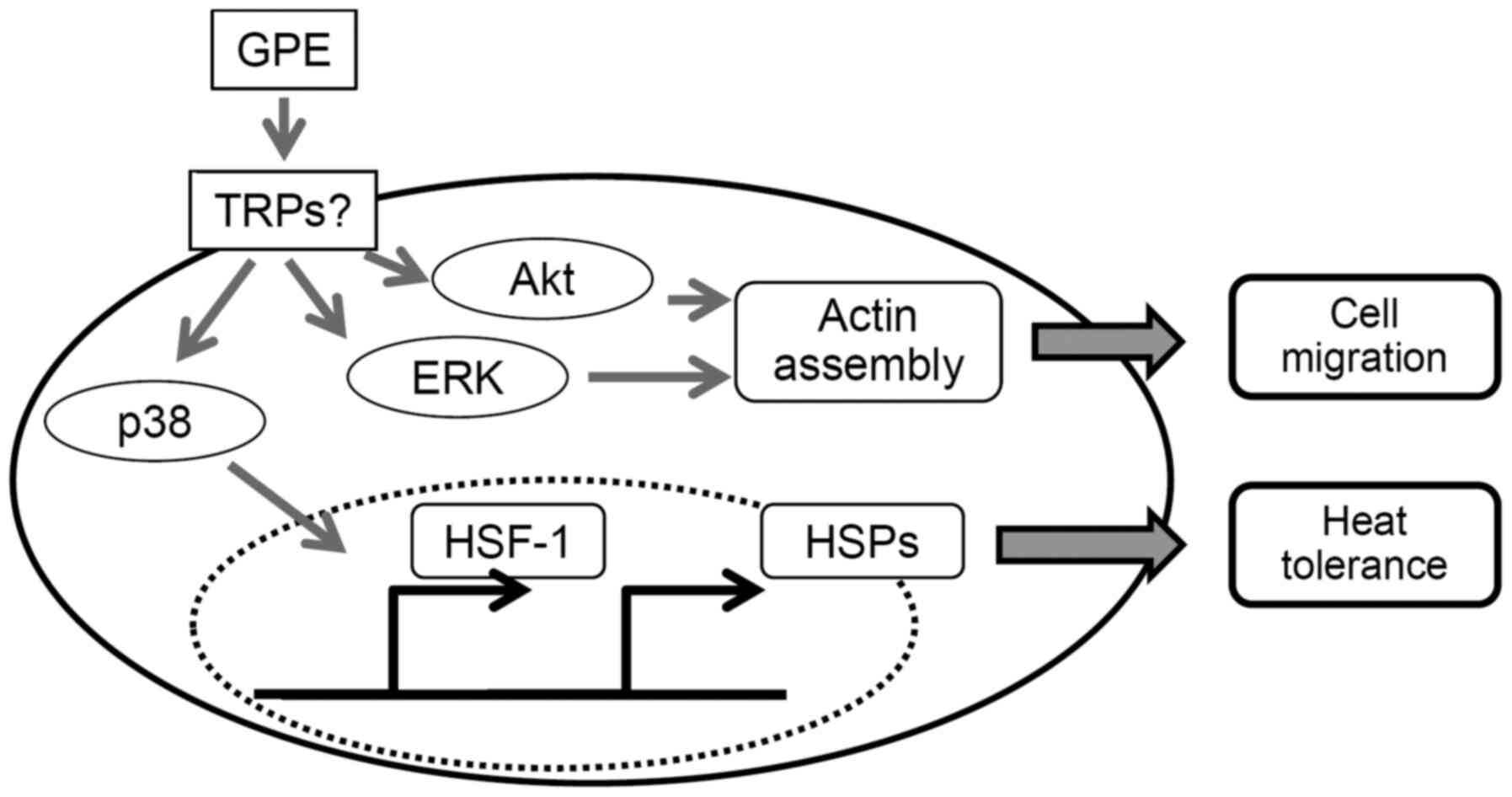 | Figure 7.Schematic diagram showing how GPE
facilitate cell migration and heat tolerance in mouse fibroblast
cells. GPE activate protein kinases, including Akt, ERK and p38
MAPK. Akt and ERK accelerate cell migration though actin assembly.
p38 MAPK increases the expression levels of HSPs through HSF-1
activation and, in turn, facilitates heat tolerance. GPE, ginger
powder extracts; TRPs, transient receptor potential cationic
channels; ERK, extracellular signal-regulated kinase; MAPK,
mitogen-activated protein kinase; HSF-1, heat shock factor-1; HSP,
heat shock protein. |
In this study, we showed that GPE accelerate cell
migration and prevent heat shock-induced cell death in
vitro. These results suggest that ginger might play a valuable
role in wound and ulcer healing, preventing erosion, as well as
resisting heat shock.
Acknowledgements
Not applicable.
Funding
This study was supported by Grants-in-Aid for
Science and Culture (grant nos. 25282021, 26650173, 15KT0003,
16K13013 and 17H01963) from the Ministry of Education, Culture,
Sports, Science, and Technology of Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NS designed the study and prepared the manuscript.
NS, MK, KM and ES conducted the experiments. MM, TW and HN analyzed
the data. NS, MK and KM assessed the authenticity of the data. NS
obtained funding. AY and OS contributed to interpretation of data
and supervised the study. NS, MK, KM, AY and OS revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ali BH, Blunden G, Tanira MO and Nemmar A:
Some phytochemical, pharmacological and toxicological properties of
ginger (Zingiber officinale Roscoe): A review of recent
research. Food Chem Toxicol. 46:409–420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McParlin C, O'Donnell A, Robson SC, Beyer
F, Moloney E, Bryant A, Bradley J, Muirhead CR, Nelson-Piercy C,
Newbury-Birch D, et al: Treatments for hyperemesis gravidarum and
nausea and vomiting in pregnancy: A systematic review. JAMA.
316:1392–1401. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tao Y, Li W, Liang W and Van Breemen RB:
Identification and quantification of gingerols and related
compounds in ginger dietary supplements using high-performance
liquid chromatography-tandem mass spectrometry. J Agric Food Chem.
57:10014–10021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu Y, Zick S, Li X, Zou P, Wright B and
Sun D: Examination of the pharmacokinetics of active ingredients of
ginger in humans. AAPS J. 13:417–426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pertz HH, Lehmann J, Roth-Ehrang R and Elz
S: Effects of ginger constituents on the gastrointestinal tract:
Role of cholinergic M3 and serotonergic 5-HT3 and 5-HT4 receptors.
Planta Med. 77:973–978. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyano K, Eto M, Hitomi S, Matsumoto T,
Hasegawa S, Hirano A, Nagabuchi K, Asai N, Uzu M, Nonaka M, et al:
The Japanese herbal medicine Hangeshashinto enhances oral
keratinocyte migration to facilitate healing of
chemotherapy-induced oral ulcerative mucositis. Sci Rep.
10:6252020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dedov VN, Tran VH, Duke CC, Connor M,
Christie MJ, Mandadi S and Roufogalis BD: Gingerols: A novel class
of vanilloid receptor (VR1) agonists. Br J Pharmacol. 137:793–798.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim YS, Hong CS, Lee SW, Nam JH and Kim
BJ: Effects of ginger and its pungent constituents on transient
receptor potential channels. Int J Mol Med. 38:1905–1914. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin Y, Dong Y, Vu S, Yang F, Yarov-Yarovoy
V, Tian Y and Zheng J: Structural mechanisms underlying activation
of TRPV1 channels by pungent compounds in gingers. Br J Pharmacol.
176:3364–3377. 2019.PubMed/NCBI
|
|
10
|
Bandell M, Macpherson LJ and Patapoutian
A: From chills to chilis: Mechanisms for thermosensation and
chemesthesis via thermoTRPs. Curr Opin Neurobiol. 17:490–497. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Venkatachalam K and Montell C: TRP
channels. Annu Rev Biochem. 76:387–417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sugimoto N, Katakura M, Matsuzaki K,
Nakamura H, Yachie A and Shido O: Capsaicin partially mimics heat
in mouse fibroblast cells in vitro. Naunyn Schmiedebergs Arch
Pharmacol. 390:281–289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sugimoto N, Matsuzaki K, Katakura M,
Nakamura H, Ueda Y, Yachie A and Shido O: Heat attenuates
sensitivity of mammalian cells to capsaicin. J Biochem Mol Toxicol.
33:e222882019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sugimoto N, Shido O, Matsuzaki K, Katakura
M, Hitomi Y, Tanaka M, Sawaki T, Fujita Y, Kawanami T, Masaki Y, et
al: Long-term heat exposure prevents hypoxia-induced apoptosis in
mouse fibroblast cells. Cell Biochem Biophys. 70:301–307. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugimoto N, Shido O, Matsuzaki K,
Ohno-Shosaku T, Hitomi Y, Tanaka M, Sawaki T, Fujita Y, Kawanami T,
Masaki Y, et al: Cellular heat acclimation regulates cell growth,
cell morphology, mitogen-activated protein kinase activation, and
expression of aquaporins in mouse fibroblast cells. Cell Physiol
Biochem. 30:450–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leu H, Sugimoto N, Shimizu M, Toma T, Wada
T, Ohta K and Yachie A: Tumor necrosis factor-α modifies the
effects of Shiga toxin on glial cells. Int Immunopharmacol.
38:139–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugimoto N, Takuwa N, Yoshioka K and
Takuwa Y: Rho-dependent, Rho kinase-independent inhibitory
regulation of Rac and cell migration by LPA1 receptor in
Gi-inactivated CHO cells. Exp Cell Res. 312:1899–1908. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akimoto M, Iizuka M, Kanematsu R, Yoshida
M and Takenaga K: Anticancer effect of ginger extract against
pancreatic cancer cells mainly through reactive oxygen
species-mediated autotic cell death. PLoS One. 10:e01266052015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kotowski U, Kadletz L, Schneider S, Foki
E, Schmid R, Seemann R, Thurnher D and Heiduschka G: 6-shogaol
induces apoptosis and enhances radiosensitivity in head and neck
squamous cell carcinoma cell lines. Phytother Res. 32:340–347.
2018. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao C, Oh JH, Oh IG, Park CH and Chung JH:
[6]-Shogaol inhibits melanogenesis in B16 mouse melanoma cells
through activation of the ERK pathway. Acta Pharmacol Sin.
34:289–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ueda Y, Ii T, Aono Y, Sugimoto N, Shinji
S, Yoshida H and Sato M: Membrane dynamics induced by a
phosphatidylinositol 3,4,5-trisphosphate optogenetic tool. Anal
Sci. 35:57–63. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Le Clainche C and Carlier MF: Regulation
of actin assembly associated with protrusion and adhesion in cell
migration. Physiol Rev. 88:489–513. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matozaki M, Saito Y, Yasutake R, Munira S,
Kaibori Y, Yukawa A, Tada M and Nakayama Y: Involvement of Stat3
phosphorylation in mild heat shock-induced thermotolerance. Exp
Cell Res. 377:67–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morotomi T, Kitamura C, Okinaga T,
Nishihara T, Sakagami R and Anan H: Continuous fever-range heat
stress induces thermotolerance in odontoblast-lineage cells. Arch
Oral Biol. 59:741–748. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyamoto M, Matsuzaki K, Katakura M, Hara
T, Tanabe Y and Shido O: Oral intake of encapsulated dried ginger
root powder hardly affects human thermoregulatory function, but
appears to facilitate fat utilization. Int J Biometeorol.
59:1461–1474. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sugimoto N, Katakura M, Matsuzaki K,
Sumiyoshi E, Yachie A and Shido O: Chronic administration of
theobromine inhibits mTOR signal in rats. Basic Clin Pharmacol
Toxicol. 124:575–581. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qi Y, Liang X, Dai F, Guan H, Sun J and
Yao W: RhoA/ROCK pathway activation is regulated by AT1 receptor
and participates in smooth muscle migration and dedifferentiation
via promoting actin cytoskeleton polymerization. Int J Mol Sci.
21:53982020. View Article : Google Scholar
|
|
28
|
Katoh M and Katoh M: Molecular genetics
and targeted therapy of WNT-related human diseases (Review). Int J
Mol Med. 40:587–606. 2017.PubMed/NCBI
|
|
29
|
Manning BD and Toker A: AKT/PKB signaling:
Navigating the network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi X, Wang J, Lei Y, Cong C, Tan D and
Zhou X: Research progress on the PI3K/AKT signaling pathway in
gynecological cancer (Review). Mol Med Rep. 19:4529–4535.
2019.PubMed/NCBI
|
|
31
|
Hossain ME, Matsuzaki K, Katakura M,
Sugimoto N, Mamun AA, Islam R, Hashimoto M and Shido O: Direct
exposure to mild heat promotes proliferation and neuronal
differentiation of neural stem/progenitor cells in vitro. PLoS One.
12:e01903562017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gong X, Luo T, Deng P, Liu Z, Xiu J, Shi H
and Jiang Y: Stress-induced interaction between p38 MAPK and HSP70.
Biochem Biophys Res Commun. 425:357–362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Westerheide SD, Raynes R, Powell C, Xue B
and Uversky VN: HSF transcription factor family, heat shock
response, and protein intrinsic disorder. Curr Protein Pept Sci.
13:86–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sugimoto N, Matsuzaki K, Ishibashi H,
Tanaka M, Sawaki T, Fujita Y, Kawanami T, Masaki Y, Okazaki T,
Sekine J, et al: Upregulation of aquaporin expression in the
salivary glands of heat-acclimated rats. Sci Rep. 3:17632013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Creagh EM, Sheehan D and Cotter TG: Heat
shock proteins-modulators of apoptosis in tumour cells. Leukemia.
4:1161–1173. 2000. View Article : Google Scholar
|
|
37
|
Horowitz M and Assadi H: Heat
acclimation-mediated cross-tolerance in cardioprotection: Do HSP70
and HIF-1alpha play a role? Ann N Y Acad Sci. 1188:199–206. 2010.
View Article : Google Scholar : PubMed/NCBI
|