Introduction
Glioma (GM) is the commonest type of primary brain
tumor (1). Based on the
histopathologic classification formulated by the World Health
Organization, glioma can be graded into four phases (I–IV)
(2). The overall survival rate of
GM patients is only ~14 months (3).
Although novel therapies against GM have been applied, the
situation is still grim for patients (4). At present, the pathogenesis of GM
remains elusive.
Circular RNAs (circRNAs) are characterized by a
closed-loop structure with limited protein coding capacity
(5). The majority of these
covalently closed loop circRNAs are produced with exons via the
head-to-tail junction (6).
Previously, they were believed to be functionless owing to errors
in splicing (7). With the
development of high-throughput RNA sequencing, they were identified
as important factors in numerous biological processes (8). They are relatively stable and
resistant to ribonuclease R digestion, making them better
biomarkers for diagnosis and treatment than linear RNAs (9). Accumulating evidence suggests that
circRNAs can be regulators in the development and progression of
cancers (10,11). Nevertheless, the effects and
molecular mechanisms of circRNAs in GM are not fully understood and
thus need to be extensively and systemically investigated.
Previously, Wang et al (12) performed circRNA sequencing and
identified several dysregulated circRNAs in GM tissues relative to
their normal counterparts. The present study aimed to identify the
potential therapeutic target for GM from the view of circRNAs.
Materials and methods
Clinical specimens
A total of 74 pairs of GM/matched noncancerous
specimens (Table SI) were
harvested from patients at the Second Affiliated Hospital of
Qiqihar Medical University, Heilongjiang, China, between January
2013 and January 2015. A validation cohort consisting of 74 GM
patients (age range: 23–71 years old; 49 male, 25 female) was
selected according to the following inclusion criteria: i) Patients
were diagnosed with GM by preoperative imaging examination
(Fig. 1A) and postoperative
pathology; ii) patients who underwent radical resection with a
clear surgical margin; iii) patients with available follow-up
information; iv) patients with a survival time of more than 1
month; v) none of the patients received anticancer treatment before
the surgery; and vi) patients who had no history of other
malignancies. Exclusion criteria were patients with serious
diseases or severe chronic diseases, such as cardiovascular and
cerebrovascular diseases. The research was authorized by the Ethics
Committee of our hospital (approval number: KY2020-012) and written
informed consent was acquired from each patient.
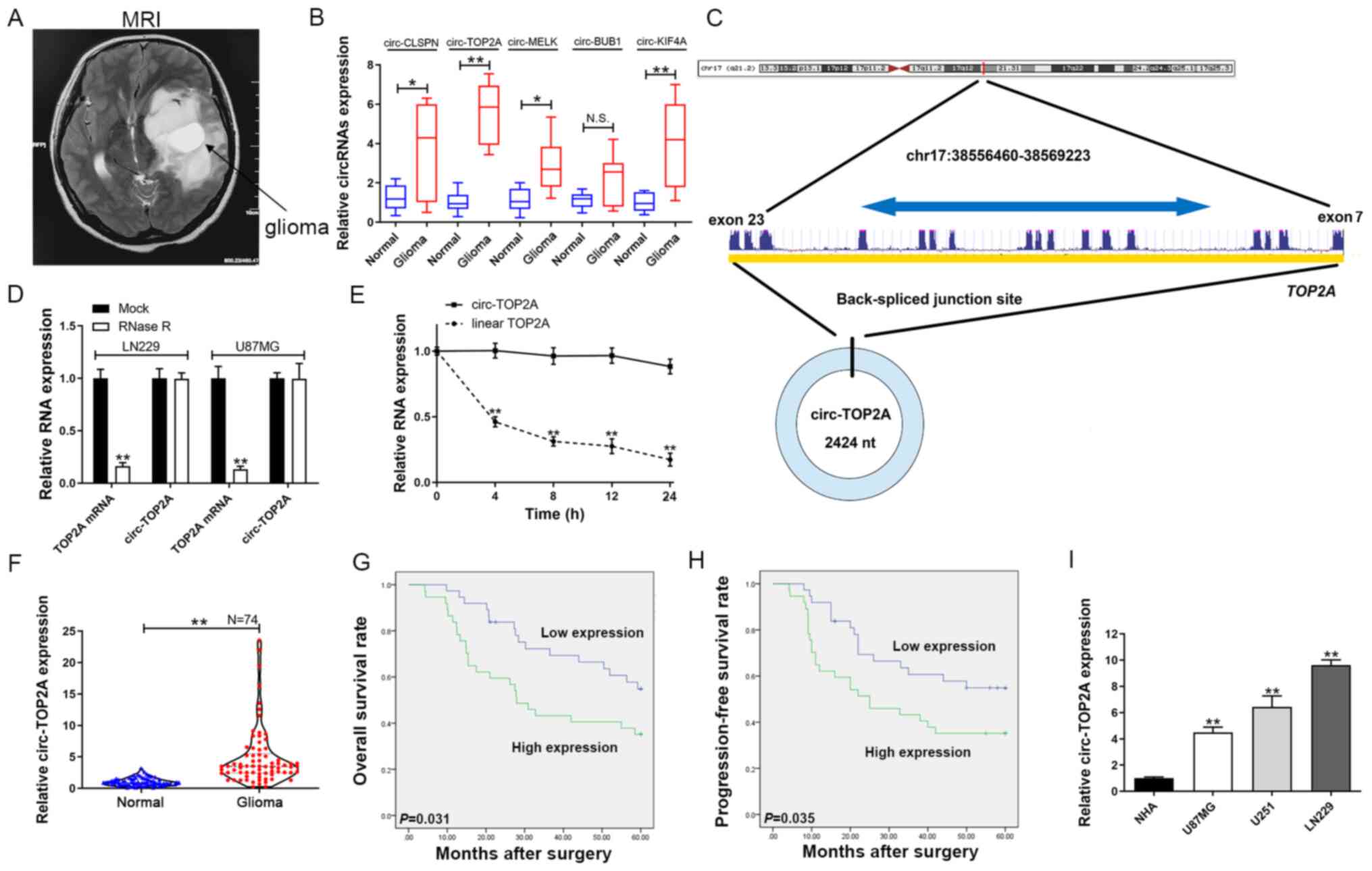 | Figure 1.Circ-TOP2A expression in GM tissues
and cells and its clinical importance. (A) Representative image of
GM by magnetic resonance imaging. (B) Circ-CLSPN, circ-TOP2A,
circ-MELK, circ-BUB1 and circ-KIF4A expression was evaluated by
RT-qPCR in GM tissues/adjacent normal tissues. (C) Schematic
representation of circ-TOP2A formation. (D) Circ-TOP2A was
resistant to RNase R digestion in GM cells. (E) Relative circ-TOP2A
and linear TOP2A mRNA expression at different time points. (F)
Circ-TOP2A expression in 74 pairs of GM tissues/adjacent normal
tissues by RT-qPCR. (G) Kaplan-Meier analysis with log-rank test
for overall survival in GM patients according to circ-TOP2A
expression. (H) Kaplan-Meier analysis with log-rank test for
progression-free survival in GM patients according to circ-TOP2A
expression. (I) Relative expression of circ-TOP2A in GM and normal
cells by RT-qPCR. D, E and I: The data are shown as the mean ±
standard deviation (n=3). *P<0.05, **P<0.01. Circ, circRNA;
GM, glioma; MRI, magnetic resonance imaging; RT-qPCR, reverse
transcription-quantitative PCR; NHA, normal human astrocytes. |
Cell lines and transfection
LN229, U251 and U87MG (cat. no. TCHu138,
glioblastoma of unknown origin) were provided by the Chinese
Academy of Sciences and normal human astrocytes (NHA; cat. no.
CC-2565) were acquired from Lonza Group Ltd. All the cells were
cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) with
10% fetal bovine serum (FBS, Invitrogen; Thermo Fisher Scientific,
Inc.) in a cell incubator (37°C, 5% CO2). The sequences
of circ-TOP2A and SUSD2 were synthesized and cloned into the pcDNA
3.1 circRNA mini vector and pcDNA 3.1 vector, respectively. Short
interfering (si)RNAs specific against circ-TOP2A and SUSD2, miR-346
mimics and inhibitors were purchased from GenePharma (Shanghai,
China). The plasmids and small RNAs were transfected using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Serum-free medium (125 µl) was used to dilute 5 µl of
Lipofectamine® 3000 in a 1.5 ml EP tube. Meanwhile, 5 µl
of siRNA (20 µM) or 2.5 µg of plasmid vector with 5 µl of P3000™
reagent was diluted in 125 µl serum-free medium. After five min of
incubation at room temperature, the reagents in the two tubes were
combined. After 15–20 min, the mixtures were added into a 2.5-cm
dish filled with serum-free medium. Following 8 h of incubation,
the medium was replaced with medium containing 10% FBS.
Transfection was confirmed by reverse transcription-quantitative
(RT-q)PCR at 48 h after transfection. The targeted sequences of the
siRNAs specifically targeting circ-TOP2A were: si-circ-TOP2A-1,
5′-ATGCAACTCTATGACATGGAT-3′ and si-circ-TOP2A-2,
5′-CATGCAACTCTATGACATGGA-3′.
RT-qPCR
RT-qPCR was performed as previously described
(13). Briefly, total RNA was
extracted with the TRIzol® (Thermo Fisher Scientific,
Inc.) method according to routine RNA extraction procedures in the
laboratory according to the manufacturer's protocol. The isolated
RNA was reverse transcribed into cDNA (Roche Diagnostics) in
accordance with the manufacturer's instructions. Then, RT-qPCR
assay was conducted on a 7500 fast Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using SYBR Green Master
(Roche Diagnostics) according to the manufacturer's instructions.
The reaction volume was 50 µl. The thermocycling conditions were as
follows: 90°C for 5 min, 90°C for 15 sec and 60°C for 30 sec for 40
cycles. U6 and GAPDH were used as internal controls. PCR primers
were as follows: circ-TOP2A, forward, 5′-GGCAGAGAGAGTTGGACTACAC-3′
and reverse, 5′-CTTCTCCATTGAAGGGCTTGAG-3′; U6, forward,
5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse,
5′-GGAACGCTTCACGAATTTG-3′ and GAPDH, forward
5′-GGGAGCCAAAAGGGTCAT-3′ and reverse, 5′-GAGTCCTTCCACGATACCAA-3′.
Each reaction was performed in triplicate and results were
calculated using the 2−ΔΔCq method (14).
Actinomycin D treatment
A total of 2.5×105 cells were inoculated
in 6-well plates and cultured for 48 h at 37°C. Then, the
transcriptional inhibitor actinomycin D (EMD Millipore) was added
to the culture medium at 2 mg/ml for 0, 4, 8, 12 and 24 h at 37°C.
Then, total RNA was extracted, and RT-qPCR was used to detect the
expression levels of circ-TOP2Aand TOP2A mRNA, as
aforementioned.
Western blotting
Proteins were isolated using RIPA buffer (Beijing
Solarbio Science & Technology Co., Ltd.). The protein
concentration was detected using a BCA detection kit (Beijing
Solarbio Science & Technology Co., Ltd.). Then, 30 µg samples
were separated by 10% SDS-PAGE and transferred onto PVDF membranes.
After immersion in 5% skimmed milk at 22–25°C for 2 h, the membrane
was incubated with anti-SUSD2 (1:2,000; cat. no. ab182147) and
anti-GAPDH (1:10,000; cat. no. ab181602; both Abcam) primary
antibodies overnight at 4°C. After incubation with the
HRP-conjugated secondary antibody (1:5,000; cat. no. ZB-2306;
OriGene Technologies, Inc.) for 2 h at room temperature, the
membrane was visualized using an ECL kit (Beyotime Institute of
Biotechnology) using a full-automatic chemiluminescence imaging
analysis system (Tanon Science and Technology Co., Ltd.).
Densitometry was analyzed using ImageJ software (National
Institutes of Health).
Subcellular fractionation test
A PARIS kit (Thermo Fisher Scientific, Inc.) was
used to separate RNAs in the cytoplasmic and nuclear fractions.
RT-qPCR was performed as aforementioned, with U6 and GAPDH as the
nuclear and cytoplasmic controls, respectively.
RNA immunoprecipitation (RIP)
RIP was conducted using the Magna RIP RNA-Binding
Protein immunoprecipitation kit (EMD Millipore) in accordance with
the manufacturer's protocols. After transfection for 48 h, GM cells
were lysed with RIP lysis buffer. Afterward, cell lysates were
incubated with magnetic beads conjugated with anti-Ago2 (1:50; cat.
no. ab186733; Abcam), or anti-IgG at 4°C for 8 h. The beads were
then washed and incubated with Proteinase K to remove the proteins.
After purification, the enrichment of circ-TOP2A was tested using
RT-qPCR.
RNA pulldown assay
The biotin-labeled circ-TOP2A probe targeting the
junction sequence of circ-TOP2A and oligonucleotide probes (500
pmol) were designed and synthesized in vitro by Wuhan
GeneCreate Biological Engineering Co., Ltd. and used for incubation
with cell lysates at 4°C overnight. Then, the complex was incubated
with streptavidin-conjugated magnetic beads (Invitrogen; Thermo
Fisher Scientific, Inc.) at 22–25°C for 2 h. After purification and
RNA extraction (TRIzol method) (15), the enrichment of circ-TOP2A and miRs
was measured using RT-qPCR.
Dual-luciferase reporter assay
The circular RNA interactome database (v2020-01-30;
circinteractome.nia.nih.gov) was used
for predicting the miRs potentially interacting with circ-TOP2A
(16). The binding relationship of
the 3′-untranslated region (UTR) of SUSD2 and miR-346 was predicted
by starBase v2.0 (17). The region
sequence of circ-TOP2A or SUSD2 3′-UTR that contained the binding
site (wt) as well as the mutated region sequence (mut) were
amplified and cloned into the pmirGLO luciferase vector (Promega
Corporation). 293T cells were co-transfected with miR-346 mimics
and plasmids containing the 3′-UTRs of wild-type or mutant
sequences of the miR binding site in SUSD2 or wild-type or mutant
sequences of circ-TOP2A using Lipofectamine 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's instructions. After transfection for 36 h, the
relative luciferase signals of the cells were measured using a
Dual-Luciferase Reporter Assay System (Promega Corporation). The
specific target activity was expressed as the relative activity
ratio of firefly luciferase to Renilla luciferase. The
experiment was repeated three times independently.
Cell Counting Kit-8 (CCK-8)
Cell viability was assessed using the CCK-8 assay
(Tiangen Biotech Co., Ltd.) according to the manufacturer's
instructions. In brief, the cells were collected and the
concentration was adjusted to 2×103 cells/well before
they were maintained in a cell incubator for 0, 24, 48, 72 and 96
h. The cells were then seeded in 96-well plates at 1,500 cells per
well. After the indicated specific treatments, 10 µl of CCK-8 was
supplied to the wells and maintained at 37°C for 2 h. Then, the
absorbance of each well at 450 nm was tested using a microplate
reader (Thermo Fisher Scientific, Inc.).
Colony forming test
LN229 and U87MG cells were plated into 6-well plates
(1,000 cells/well) with medium containing 10% FBS and incubated at
37°C. After ~10 days, the colonies were fixed with 4%
paraformaldehyde for 20 min at room temperature and stained with
crystal violet solution (Sigma-Aldrich; Merck KGaA) for another 20
min at room temperature. Finally, images were captured of the
colonies and they were counted manually.
Apoptosis detection
Cell apoptosis assays were performed by flow
cytometry (Applied Biosystems; Thermo Fisher Scientific, Inc.).
First, GM cells were seeded in 6-well plates before collection by
centrifuging at 1,000 × g for 3 min at room temperature. The cells
were washed twice with precooled PBS and the concentration was
adjusted to 5×105−5×106 cells with 400 µl of
staining buffer (10 mM Hepes/NaOH, pH 7.4; 140 mM NaCl and 2.5 mM
CaCl2) containing 5 µl of Annexin V labeled with
fluorescein isothiocyanate and 5 µl of PI (Annexin V-FITC apoptosis
kit; BD Biosciences). Following a 20-minute incubation at 22–25°C
in the dark, the treated cells were subjected to apoptosis assays
by flow cytometry (FACScan; BD Biosciences). FlowJo v10 software
(Tree Star, Inc.) was used for apoptosis analysis. The percentage
of early + late apoptotic cells was calculated as the apoptotic
rate.
Transwell experiments
A Transwell plate containing an 8-µm pore size
filter (BD Biosciences) was used to detect the invasion and
migration of cells. The cells were suspended in RPMI-1640 with 0.1%
FBS and supplied to the top compartment at a density of 10,000
cells per well. For the cell invasion assay, Matrigel was
pre-cooled at 4°C overnight and coated on the upper side of the
membrane. Then, the chambers were placed in a 24-well plate and 600
µl of medium containing 10% FBS was added to each well. After
incubation in a cell incubator at 37°C for 24 h, the cells on the
upper side of the membrane were removed with cotton swabs and the
migrated/invaded cells in the lower membrane of the chambers were
fixed by paraformaldehyde at room temperature for 20 min and then
stained with crystal violet for 20 min at room temperature. Images
were captured from five randomly selected fields of view
(magnification, ×200) under a light microscope.
Data analysis
All results are presented as the mean ± standard
deviation and were analyzed with GraphPad Prism 8.0 (GraphPad
Software, Inc.) and SPSS 22.0 (IBM Corp.). Unpaired Student's t
test was used to compare the significance of differences between
two groups. For comparisons between cancerous and adjacent normal
tissues, paired t test was used. One-way ANOVA with post hoc
Tukey's test was used to compare the significance of differences
among three or more groups. Kaplan-Meier analysis with log-rank
test was applied to measure the overall survival rate (from surgery
to death) and progression-free survival (from surgery to tumor
recurrence/metastasis) of GM patients. The Cancer Genome Atlas
(TCGA) and Pearson's correlation analysis was used to assess the
correlation between SUSD2 and miR-346 expression levels, as well as
patient survival data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Circ-TOP2A is overexpressed in GM and
is associated with poor prognosis
A total of five circRNAs, circ-CLSPN, circ-TOP2A,
circ-MELK, circ-BUB1 and circ-KIF4A, were selected from the
sequencing data (12). Total RNA
was isolated from 10 pairs of GM and normal tissues to detect the
expression of circRNAs. As shown in Fig. 1B, circ-TOP2A was the most
upregulated circRNA expressed in GM tissues compared with its the
normal counterparts. Therefore, it was chosen for further study.
Circ-TOP2A (circ_0043548) was mapped to chr17:38556460-38569223. It
was spliced from exons 7–23 of TOP2A. The spliced variant of
circ-TOP2A was 2424 nucleotides long (Fig. 1C). It was determined that circ-TOP2A
was more stable than TOP2A mRNA (Fig.
1D). In addition, total RNA was extracted to detect the
expression of circ-TOP2A and linear TOP2A mRNA following treatment
with actinomycin D at different time points. Linear TOP2A showed a
shorter half-life compared with circ-TOP2A (Fig. 1E). RT-qPCR demonstrated that
circ-TOP2A was markedly elevated in GM specimens compared with
noncancerous samples (Fig. 1F). To
explore the prognostic value of circ-TOP2A, patients were divided
into high- and low-circ-TOP2A groups according to the median
expression level of circ-TOP2A in cancer tissues. As illustrated in
Fig. 1G and H, Kaplan-Meier curves
revealed that overall survival rate (P=3.1×10−2) and
progression-free survival (P=3.5×10−2) were lower in the
high-circ-TOP2A group compared with the low-circ-TOP2A group.
Furthermore, the expression of circ-TOP2A was found to be
overexpressed in GM cell lines compared with NHA cells (Fig. 1I).
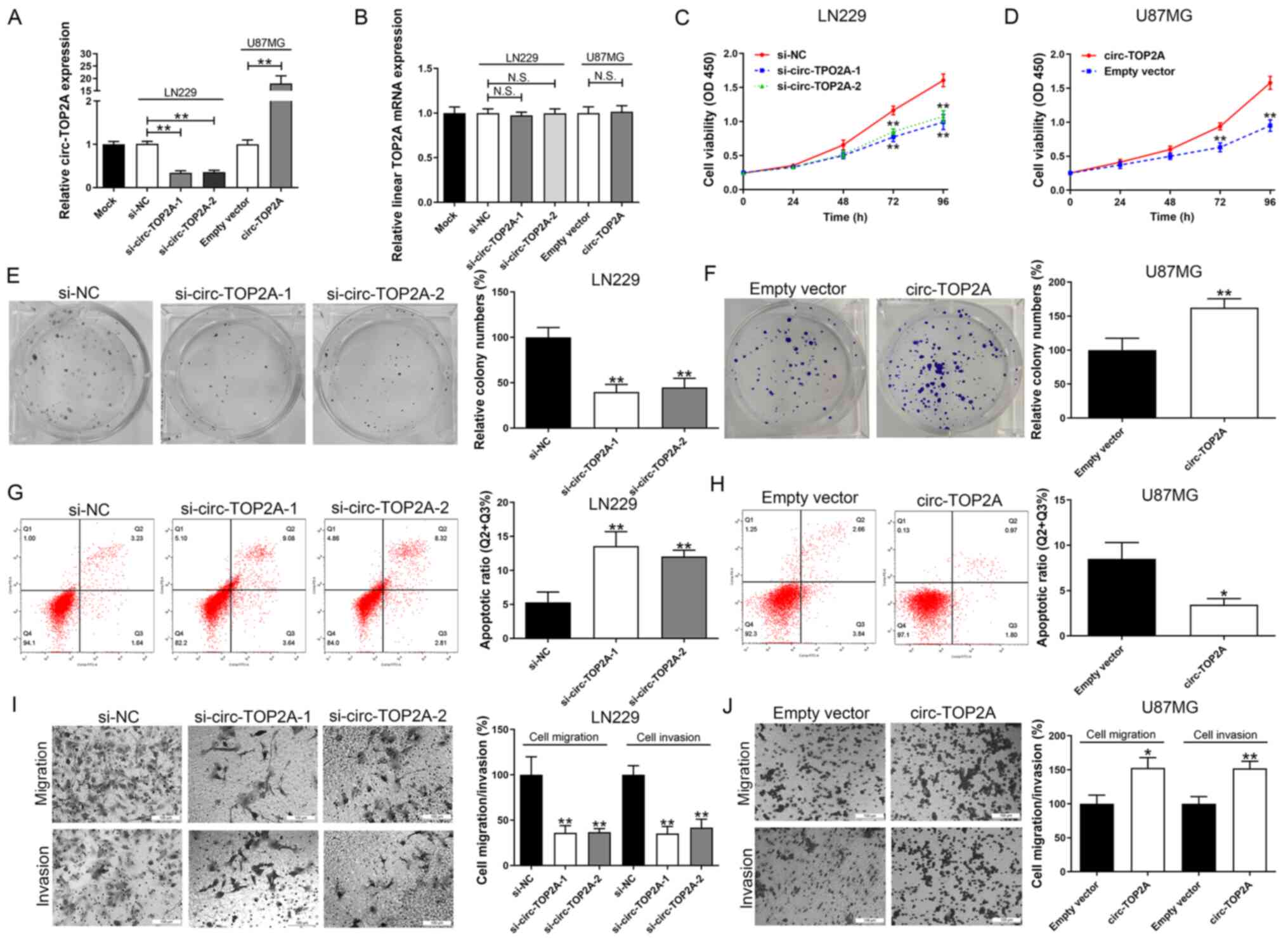
Circ-TOP2A promotes GM cell
proliferation and aggressiveness
The cellular function of circ-TOP2A by
down-/up-regulation of circ-TOP2A was then examined in GM cell
lines. LN229 cells were used for the knockdown study due to their
high expression of circ-TOP2A. The silencing efficiencies of
si-circ-TOP2A-1 and si-circ-TOP2A-2 were favorable (Fig. 2A). Circ-TOP2A expression was
ectopically expressed in U87MG cells due to its lowest level of
circ-TOP2A among the enrolled GM cell lines (Fig. 2A). As presented in Fig. 2B, transfection with circ-TOP2A
siRNAs or vector could not down-/up-regulate TOP2A mRNA expression
levels. Functionally, silencing of circ-TOP2A significantly impeded
cell viability and clone-forming ability in LN229 cells (Fig. 2C and E). Overexpression of
circ-TOP2A caused the opposite effect in U87MG cells (Fig. 2D and F). Additionally, down and
upregulation of circ-TOP2A respectively triggered or inhibited cell
apoptosis in GM cells (Fig. 2G and
H). In addition, circ-TOP2A downregulation suppressed cell
migration and invasion in LN229 cells (Fig. 2I). In contrast, overexpression of
circ-TOP2A exerted the opposite effect in U87MG cells (Fig. 2J).
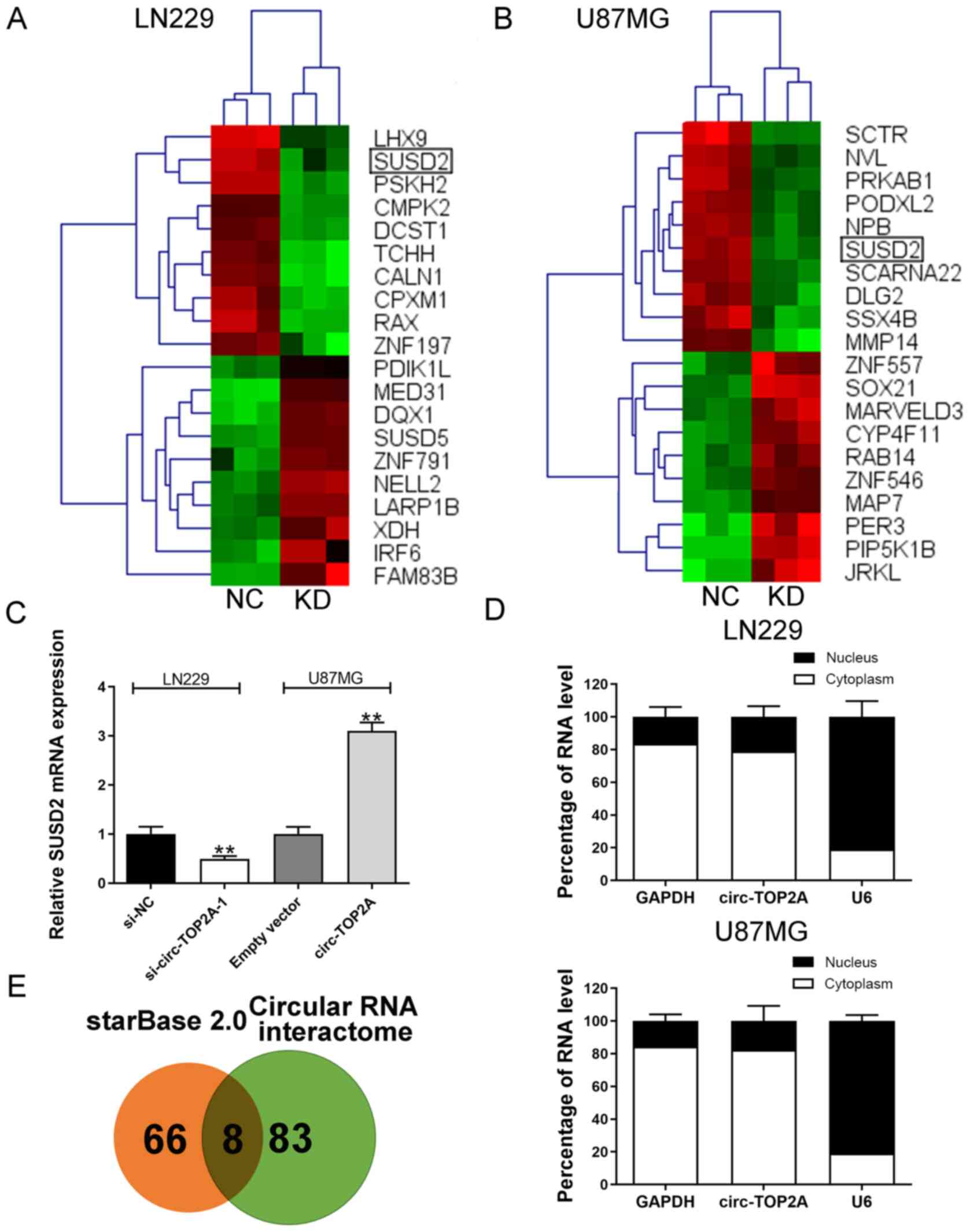
Circ-TOP2A elevates SUSD2 expression
by sponging miR-346 in GM cells
RNA-Seq was conducted and generated a heatmap for
the 10 most differentially expressed mRNAs in circ-TOP2A-knockdown
and control cells. SUSD2 was a common gene target of circ-TOP2A in
LN229 and U87MG cells (Fig. 3A and
B). Additionally, RT-qPCR validated that circ-TOP2A could
positively regulate SUSD2 expression levels (Fig. 3C). Furthermore, a subcellular
distribution assay illustrated that circ-TOP2A was primarily
localized in the cytoplasm of GM cells (Fig. 3D). The starBase v2.0 database
predicted 72 miRs that potentially bind with SUSD2 3′-UTR. The
circular RNA interactome database predicted 89 miRs that may be
sponged by circ-TOP2A. miR-217, miR-383-5p, miR-23c, miR-346,
miR-494-3p, miR-23b-3p, miR-873-5p and miR-1286 were identified as
common miRs in the two databases (Fig.
3E). As shown in Fig. 4A,
circ-TOP2A was markedly enriched in the anti-ago2
immunoprecipitated pool compared with the anti-IgG pool. In
addition, knockdown of circ-TOP2A partly weakened this binding
ability. Circ-TOP2A was then pulled down using a specific probe in
LN229 and U87MG cells. Ectopic expression of circ-TOP2A could
enhance this efficiency (Fig. 4B).
RNA pulldown assays indicated that only miR-346 could interact with
circ-TOP2A in LN229 cells (Fig.
4C). Coefficient correlation analysis uncovered a negative
association between SUSD2 mRNA and miR-346 expression
(P=8.0×10−3). The data also showed that the expression
of SUSD2 mRNA was positively linked to circ-TOP2A expression
(P=3.0×10−3; Fig. 4D and
E). Similarly, TCGA data showed a negative correlation between
SUSD2 mRNA and miR-346 in GM tissue samples
(P=7.19×10−11; Fig. 4F).
Kaplan-Meier curves demonstrated that the patients with high
expression of SUSD2 had a worse overall survival rate
(P=6.6×10−6; Fig. 4F).
Pearson's correlation analysis indicated a negative association
between SUSD2 and miR-346 expression levels (Fig. 4G). Conversely, miR-346 expression in
GM tissues correlated with favorable prognosis analyzed by the TCGA
dataset (P=1.1×10−2). As expected, miR-346
mimics/inhibitor could significantly up/downregulated miR-346
expression (Fig. 4I). It was
further confirmed that SUSD2 vector/si-SUSD2 could effectively
contribute to SUSD2 increase/decrease (Fig. 4J). In addition, SUSD2 mRNA
expression was negatively regulated by miR-346 (Fig. 4J). Furthermore, miR-346 expression
in GM cell lines was generally decreased, which is conversely
associated with circ-TOP2A (Fig.
1I) and SUSD2 expression levels (Fig. 4K and L). To verify the target
binding between circ-TOP2A and miR-346, a dual-luciferase reporter
gene test was performed in constructed wild-type and mutant
circ-TOP2A (Fig. 4M). As shown in
Fig. 4N, miR-346 markedly decreased
the luciferase intensity in wild-type circ-TOP2A but had no effect
on binding motif mutations. In addition, the binding ability
between the 3′-UTR of SUSD2 and miR-346 was further verified by a
dual-luciferase reporter gene test (Fig. 4M and N).
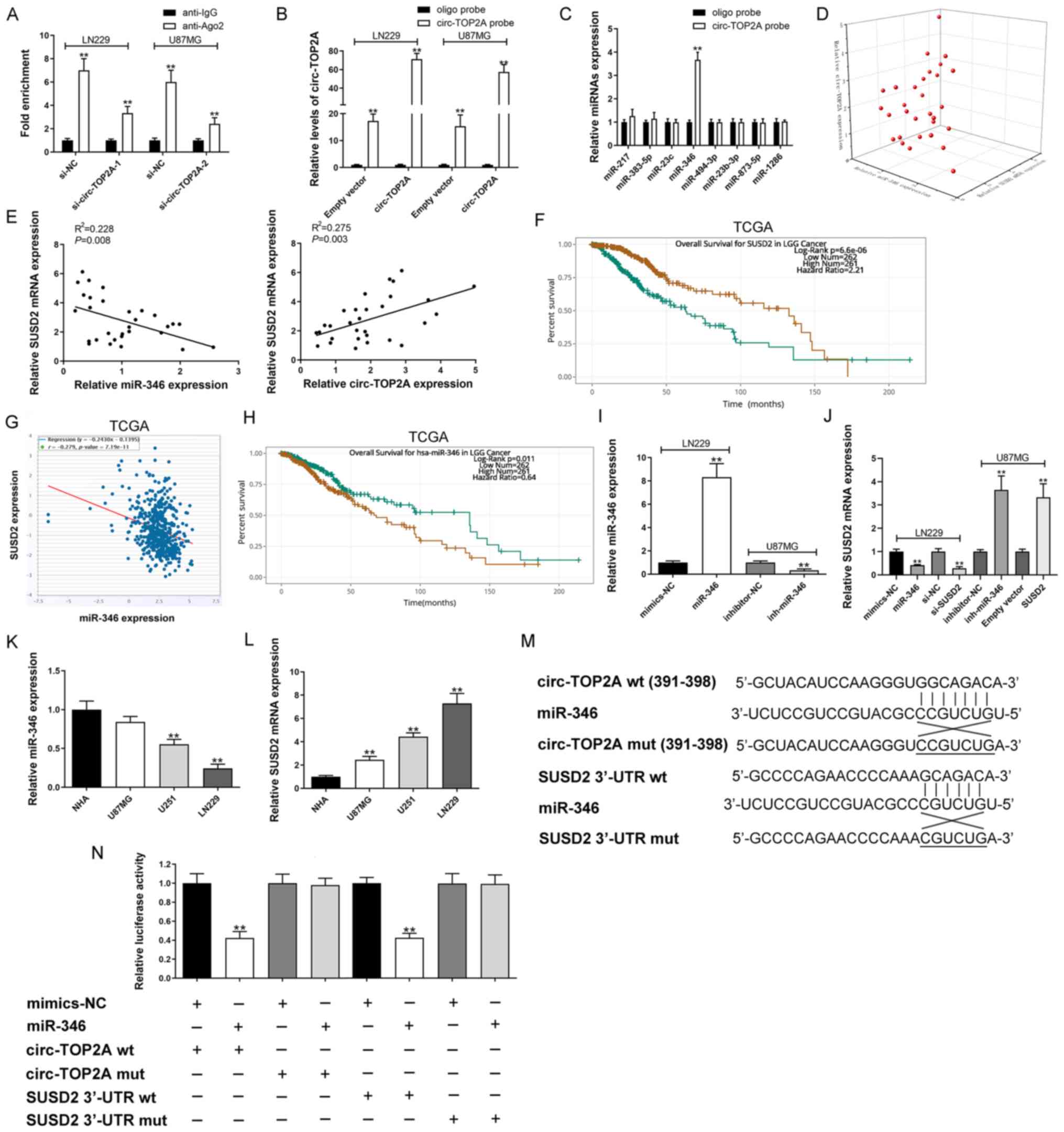 | Figure 4.Circ-TOP2A sponges miR-346 to
upregulate SUSD2 expression in GM. (A) Ago2-RNA immunoprecipitation
assay for circ-TOP2A levels in LN229 and U87MG cells following
transfection. (B) Lysates prepared from LN229 and U87MG cells
following transfection were subjected to RNA pull-down assay. (C)
RT-qPCR for miR-217, miR-383-5p, miR-23c, miR-346, miR-494-3p,
miR-23b-3p, miR-873-5p and miR-1286 expression in LN229 cell
lysates. (D and E) Correlation among circ-TOP2A, miR-346 and SUSD2
mRNA in GM samples. (F) Kaplan-Meier analysis of overall survival
in GM patients according to SUSD2 expression by TCGA data. (G)
Correlation analysis of SUSD2 and miR-346 expression in GM/normal
tissues by TCGA data. (H) Kaplan-Meier analysis of overall survival
in GM patients according to miR-346 expression by TCGA data. (I)
miR-346 expression was detected by RT-qPCR after
down-/up-regulating miR-346 in LN229 and U87MG cells. (J) SUSD2
mRNA expression was detected by RT-qPCR following transfection in
LN229 and U87MG cells. (K) Relative expression of miR-346 in GM and
normal cells by RT-qPCR. (L) Relative expression of SUSD2 mRNA in
GM and normal cells by RT-qPCR. (M) Schematic illustration of
circ-TOP2A-wt/mut and SUSD2 3′-UTR-wt/mut luciferase reporter
vectors. (N) The binding ability between circ-TOP2A/SUSD2 3′-UTR
and miR-346 was detected by dual-luciferase reporter assay in 293T
cells. A-C, I-L and N: The data are shown as the mean ± standard
deviation (n=3). **P<0.01. Circ, circRNA; RT-qPCR, reverse
transcription-quantitative PCR; miR, microRNA; GM, glioma; wt,
wild-type; mut, mutant; TCGA, The Cancer Genome Atlas; UTR,
untranslated region; NC, negative control. |
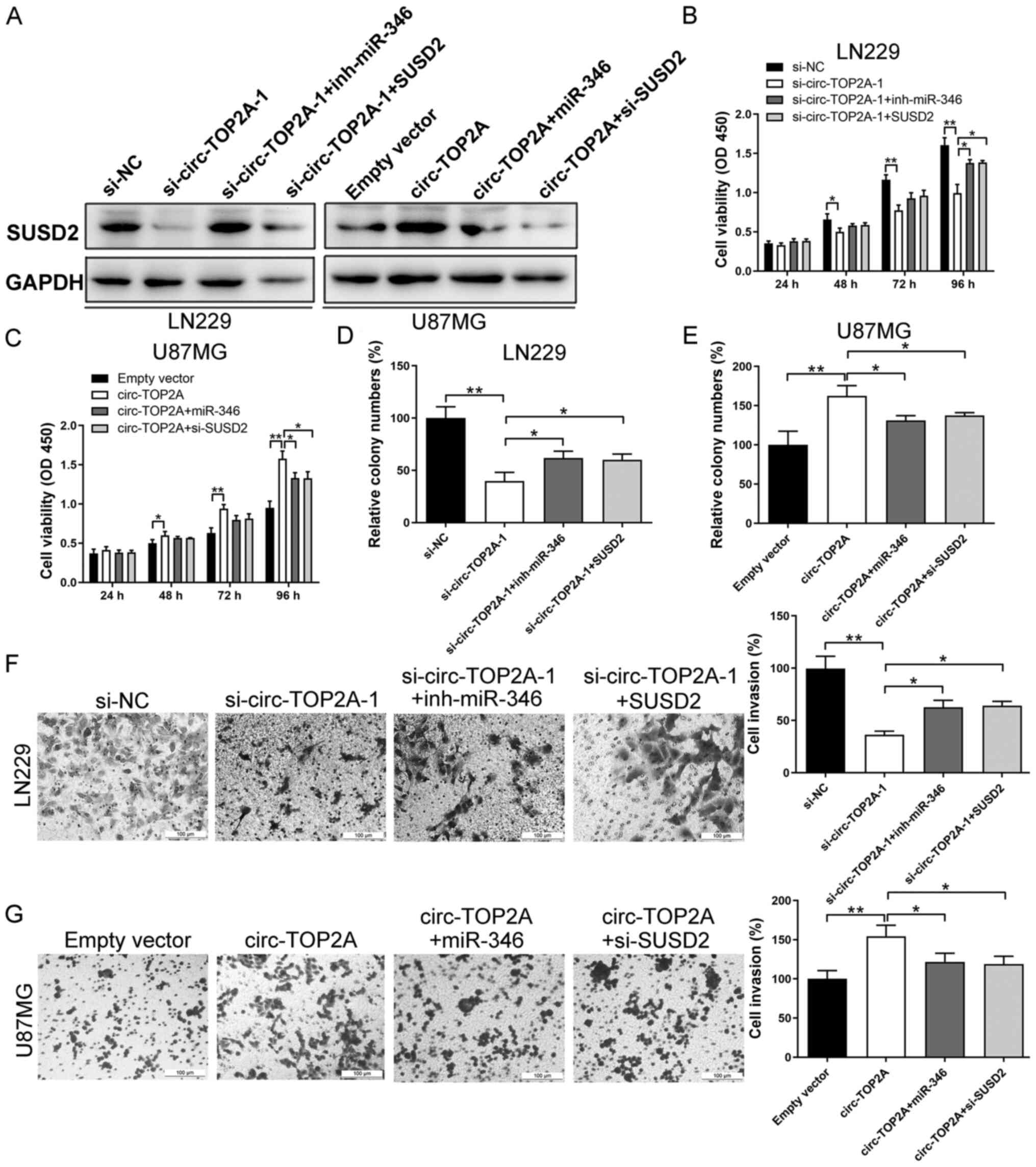
Circ-TOP2A/miR-346/SUSD2 signaling is
critical for GM cell function
Rescue experiments were performed to reveal the
mechanisms of circ-TOP2A in GM. As shown in Fig. 5A, the protein level of SUSD2 was
decreased in the si-circ-TOP2A-1 group and changes in SUSD2
expression were reversed by co-transfection with miR-346 inhibitor
or SUSD2 vector. In addition, SUSD2 expression was elevated
following transfection with the circ-TOP2A-overexpressing vector in
U87MG cells. After co-transfection with miR-346 mimics or si-SUSD2,
the protein expression of SUSD2 was partly inhibited (Fig. 5A). Additionally, relative expression
of circ-TOP2A was decreased following transfection with
si-circ-TOP2A-1. Si-circ-TOP2A-1 co-transfection with miR-346
inhibitor or SUSD2 vector did not alter circ-TOP2A expression in
LN229 cells. Similarly, circ-TOP2A vector led to increased
expression of circ-TOP2A. Further co-transfection with miR-346
mimics or si-SUSD2 had no effect on circ-TOP2A expression in U87MG
cells (Fig. S1A). miR-346
expression was unchanged after knockdown or overexpression of
circ-TOP2A, which implied that circ-TOP2A sponges miR-346 to
inhibit its functions rather than expression levels.
Co-transfection with miR-346 inhibitor and miR-346 mimics markedly
decreased and increased miR-346 expression, respectively (Fig. S1B).
CCK-8, colony formation and Transwell assays
indicated that the inhibitory effects of si-circ-TOP2A on cell
proliferation and invasion were attenuated by co-transfection with
miR-346 inhibitor or SUSD2 vector (Fig.
5B, D and F). Overexpression of the circ-TOP2A vector led to
increased cell viability, clone-forming ability and invasive
potential in U87MG cells. These malignant behaviors were partially
rescued after co-transfection with miR-346 mimics or si-SUSD2
(Fig. 5C, E and G). Taken together,
these findings revealed that the circ-TOP2A mediated miR-346/SUSD2
axis triggered GM carcinogenesis.
Discussion
Tumorigenesis and metastasis are complicated
processes involving the activation of oncogenes and the
inactivation of tumor suppressor genes. CircRNAs are reported to
regulate gene expression at epigenetic, transcriptional and
posttranscriptional levels, mediating the initiation and metastasis
of tumors (10,18,19).
Circ-TOP2A was screened as an upregulated circRNA in GM tissue
samples compared with noncancerous tissues (12). The current study further identified
that the expression of circ-TOP2A was upregulated in GM tissues and
cell lines (LN229, U251 and U87MG). The results were obtained based
on 74 GM tissue samples. It was identified that high expression of
circ-TOP2A in GM specimens correlated with adverse prognosis and
high progression-free survival. However, due to the limited number
of patients recruited, the independent prognostic role of
circ-TOP2A was not explored in this study.
Functional experiments indicated the oncogenic role
of circ-TOP2A in mediating GM cell growth, migration and
invasiveness. RNA-Seq of circ-TOP2A-depleted LN229 and U87MG cells
revealed SUSD2 as a target of circ-TOP2A. In addition to
interacting with proteins, circRNAs can regulate target gene
expression indirectly via competitive binding with miRs (20). For instance, circ-CSPP1 acts as a
competing endogenous RNA (ceRNA) to contribute to colorectal
carcinoma cell epithelial-mesenchymal transition and liver
metastasis by upregulating collagen, type I, α 1 (21). The localization of circRNAs suggests
how they exert their functions. Circ-TOP2A was dominantly
distributed in the cytoplasm, which suggested that its mechanism
functions at the post-transcriptional level. miR-346 was further
demonstrated as a novel target of circ-TOP2A. miR-346 is reported
to target and inhibit the expression of a number of classical
oncogenes and then suppress the progression of a number of cancers
(22,23). Previously, miR-346 was reported as a
tumor suppressor in GM by directly targeting NFIB (24). The present study validated the tumor
suppressive function of miR-346 in GM and found that a miR-346
inhibitor could reverse the anticancer effects of circ-TOP2A
knockdown. These results demonstrated that circ-TOP2A facilitates
GM growth and aggressiveness, at least in part via repression of
the function of miR-346. SUSD2, a type I transmembrane protein that
localizes to the cell surface, has been reported to be upregulated
in a number of tumors and to promote cancer progression and
metastasis (25,26). However, some studies have indicated
that upregulated SUSD2 impedes cell progression in several
malignancies, suggesting a possible tumor suppressive role of SUSD2
(27,28). These previous studies indicate that
SUSD2 might have a complex function in different malignancies.
Consistent with the studies on breast cancer and ovarian cancer
(25,26), SUSD2 was obviously increased in GM
tissues and cells compared with matched control groups. Notably,
the present study also demonstrated that overexpression of SUSD2
acted as a tumorigenic function in circ-TOP2A-downregulated GM
cells, suggesting the cancerogenic function of SUSD2 in GM.
Collectively, circ-TOP2A promoted GM proliferation and
aggressiveness through the miR-346/SUSD2 signaling pathway.
In conclusion, the results of the present study
suggested that circ-TOP2A was increased, circular and stable in GM
cells. Notably, upregulation of circ-TOP2A exerted its functions by
facilitating cell growth, migration and invasion, while inhibiting
apoptosis in GM cells by targeting miR-346/SUSD2 signaling.
Therefore, circ-TOP2A may be a potential prognostic
biomarker/therapeutic target for patients with GM.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CZ, XZ and GL analyzed and interpreted patient data
regarding the hematological disease and transplant. JS, XL and LL
performed the in vitro assay. JS wrote the manuscript. JS
and XL confirmed the authenticity of raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The research was authorized by the Ethics Committee
of The Second Affiliated Hospital of Qiqihar Medical University
(approval number: KY2020-012) and written informed consent was
acquired from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Farina P, Lombardi G, Bergo E, Roma A and
Zagonel V: Treatment of malignant gliomas in elderly patients: A
concise overview of the literature. Biomed Res Int.
2014:7342812014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wen PY and Huse JT: 2016 World Health
Organization classification of central nervous system tumors.
Continuum (Minneap Minn). 23:1531–1547. 2017.PubMed/NCBI
|
|
3
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gusyatiner O and Hegi ME: Glioma
epigenetics: From subclassification to novel treatment options.
Semin Cancer Biol. 51:50–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salzman J: Circular RNA expression: Its
potential regulation and function. Trends Genet. 32:309–316. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eger N, Schoppe L, Schuster S, Laufs U and
Boeckel JN: Circular RNA Splicing. Adv Exp Med Biol. 1087:41–52.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanger HL, Klotz G, Riesner D, Gross HJ
and Kleinschmidt AK: Viroids are single-stranded covalently closed
circular RNA molecules existing as highly base-paired rod-like
structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han B, Chao J and Yao H: Circular RNA and
its mechanisms in disease: From the bench to the clinic. Pharmacol
Ther. 187:31–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Belousova EA, Filipenko ML and Kushlinskii
NE: Circular RNA: New regulatory molecules. Bull Exp Biol Med.
164:803–815. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen B and Huang S: Circular RNA: An
emerging non-coding RNA as a regulator and biomarker in cancer.
Cancer Lett. 418:41–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu Y, Yao Y, Zhong X, Leng K, Qin W, Qu L,
Cui Y and Jiang X: Downregulated circular RNA hsa_circ_0001649
regulates proliferation, migration and invasion in
cholangiocarcinoma cells. Biochem Biophys Res Commun. 496:455–461.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang R, Zhang S, Chen X, Li N, Li J, Jia
R, Pan Y and Liang H: EIF4A3-induced circular RNA MMP9 (circMMP9)
acts as a sponge of miR-124 and promotes glioblastoma multiforme
cell tumorigenesis. Mol Cancer. 17:1662018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu H, Wang Y, Zhang T, Zhang C, Liu Y, Li
G, Zhou D and Lu S: Association of LncRNA-GACAT3 with MRI features
of breast cancer and its molecular mechanism. J BUON. 24:2377–2384.
2019.PubMed/NCBI
|
|
14
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mannhalter C, Koizar D and Mitterbauer G:
Evaluation of RNA isolation methods and reference genes for RT-PCR
analyses of rare target RNA. Clin Chem Lab Med. 38:171–177. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dudekula DB, Panda AC, Grammatikakis I, De
S, Abdelmohsen K and Gorospe M: CircInteractome: A web tool for
exploring circular RNAs and their interacting proteins and
microRNAs. RNA Biol. 13:34–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Yao Y, Leng K, Ji D, Qu L, Liu Y and
Cui Y: Increased expression of circular RNA circ_0005230 indicates
dismal prognosis in breast cancer and regulates cell proliferation
and invasion via miR-618/CBX8 signal pathway. Cell Physiol Biochem.
51:1710–1722. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu Y, Yao Y, Gao P and Cui Y: Upregulated
circular RNA circ_0030235 predicts unfavorable prognosis in
pancreatic ductal adenocarcinoma and facilitates cell progression
by sponging miR-1253 and miR-1294. Biochem Biophys Res Commun.
509:138–142. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Q, Shi L, Shi K, Yuan B, Cao G, Kong
C, Fu J, Man Z, Li X, Zhang X, et al: CircCSPP1 functions as a
ceRNA to promote colorectal carcinoma cell EMT and liver metastasis
by upregulating COL1A1. Front Oncol. 10:8502020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bai N, Peng E, Qiu X, Lyu N, Zhang Z, Tao
Y, Li X and Wang Z: circFBLIM1 act as a ceRNA to promote
hepatocellular cancer progression by sponging miR-346. J Exp Clin
Cancer Res. 37:1722018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu W, Qian J, Ma L, Ma P, Yang F and Shu
Y: MiR-346 suppresses cell proliferation through SMYD3 dependent
approach in hepatocellular carcinoma. Oncotarget. 8:65218–65229.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Xu J, Zhang J, Zhang J, Zhang J and
Lu X: MicroRNA-346 inhibits the growth of glioma by directly
targeting NFIB. Cancer Cell Int. 19:2942019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hultgren EM, Patrick ME, Evans RL, Stoos
CT and Egland KA: SUSD2 promotes tumor-associated macrophage
recruitment by increasing levels of MCP-1 in breast cancer. PLoS
One. 12:e01770892017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu Y, Miao C, Jin C, Qiu C, Li Y, Sun X,
Gao M, Lu N and Kong B: SUSD2 promotes cancer metastasis and
confers cisplatin resistance in high grade serous ovarian cancer.
Exp Cell Res. 363:160–170. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng Y, Wang X, Wang P, Li T, Hu F, Liu
Q, Yang F, Wang J, Xu T and Han W: SUSD2 is frequently
downregulated and functions as a tumor suppressor in RCC and lung
cancer. Tumour Biol. 37:9919–9930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sheets JN, Patrick ME and Egland KA: SUSD2
expression correlates with decreased metastasis and increased
survival in a high-grade serous ovarian cancer xenograft murine
model. Oncotarget. 11:2290–2301. 2020. View Article : Google Scholar : PubMed/NCBI
|