Introduction
Vascular dementia (VD) is one of the leading causes
of dementia following Alzheimer's disease (AD), which accounted for
15% of all dementia cases following AD in 2015 worldwide (1). At present, there is a shortage of
accepted treatments and diagnostic approaches for the management of
VD (2). VD is characterized by
neurocognition dysfunction and behavioral alterations indicative of
cognitive impairment (3). Basic and
clinical studies have demonstrated that progressive neuronal loss
is closely related to dementia (4).
Atherosclerosis, infarction and arteriosclerosis are markers of
neurodegeneration in VD (5).
Although different drugs, including donepezil and galantamine, are
effective in curing AD, the effects of the drugs significantly vary
among individuals when used as VD therapies (6). The impact of molecular mechanisms on
brain studies has been increasingly investigated, and several
researchers developing novel therapeutic strategies for dementia
have focused on microRNA (miRNA/miR)-based mechanisms aiming to
identify further targets for the diagnosis of VD (7–9).
miRNAs are single-stranded RNAs, ~22 nucleotides in
length, that can mediate mRNA expression (10). miRNAs are associated with the
regulation of type 2 diabetes mellitus, ischemic stroke, AD and VD
(8). For instance, downregulation
of angiogenic miR-126 leads to cognitive impairment in VD model
mice (11). miR-150 has been
reported to serve a role in inflammation and different neurological
syndromes in association with the potential downstream targets
(12). Moreover, miR-150 is one of
the miRNAs correlated with mice displaying cognitive deficits and
advanced neuropathology (13),
indicating a possible correlation with neuronal dysfunction. In
addition, long non-coding RNA myocardial infarction associated
transcript knockdown is responsible for progressive neuronal loss
and neurodegeneration, as well as behavioral deficits in AD, which
is associated with miR-150 overexpression (14). However, how miR-150 functions in VD,
as well as its role in mediating neuronal apoptosis in vitro
and cognitive function in vivo are not completely
understood. Homeobox (HOX)A1, a member of the HOX family, is
involved in the development of vertebrates, as well as in multiple
diseases, such as atherosclerosis (15). The targeting relationship between
miR-339-5p and HOXA1 has been observed in ischemic brain damage
(16). However, to the best of our
knowledge, an association between miR-150 and HOXA1 has not been
previously reported. Furthermore, the role of HOXA1 in VD is not
completely understood. Therefore, the present study hypothesized
that miR-150 may serve as a regulator of VD by interacting with
HOXA1. To investigate the hypothesis, miR-150 knockdown was
established using miR-150 antagomiR, with an attempt to utilize
miRNAs as biomarkers and develop a novel miRNA-based therapy for
patients with VD.
Materials and methods
Ethics statement
The present study was approved by the Laboratory
Animal Care and Use Committee of Heilongjiang Provincial Hospital
(approval no. KY-2018-197) and performed according to the
Guidelines for the Care and Use of Laboratory Animals published by
the National Institutes of Health (17).
Establishment of VD model rats
A total of 48 male Sprague-Dawley rats (weight,
180–220 g; age, 6–7 weeks) were provided by the Laboratory Animal
Center of Heilongjiang Provincial Hospital. Rats were housed at
25±1°C with 50–70% humidity, 12-h light/dark cycles, and free
access to food and water. They were acclimated to the housing
conditions for 7 days before modeling.
To establish VD model rats, rats were subjected to
global cerebral ischemia via permanent occlusion of the bilateral
common carotid arteries (2-VO) from day 7 to day 35 as previously
described (18,19). Rats were anesthetized with an
intraperitoneal injection (0.15 ml/100 g) of 100 mg/kg ketamine and
10 mg/kg xylazine. Subsequently, rats were placed on a stereotactic
device. The bilateral common carotid arteries were separated and
permanently ligated with small diameter nylon sutures. Rats in the
control group underwent the same procedure, but the common carotid
arteries were not ligated. The body temperature of the rats was
monitored during the whole process and maintained at 37°C. After
surgery, rats were returned to the cages and fed normally.
To determine the effects of miR-150 on cognitive
function and hippocampal neurons in VD model rats, rats were
subjected to intracerebroventricular (ICV) injections of miR-150
antagomiR (5′-CCCCUCUGGUCAACCAGUCACA-3′) or antagomiR negative
control (NC, 5′-TTCTCCGAACGTGTCACGT-3′) from day 36 to day 38. Both
sequences were from Guangzhou RiboBio Co., Ltd. Rats were divided
into the following groups (n=12 per group): i) Control; ii) VD
model; iii) antagomiR NC (treated with 2-VO and antagomiR NC); and
iv) miR-150 antagomiR (treated with 2-VO and miR-150 antagomiR).
Rats received one ICV injection a day for 3 consecutive days. Rats
in the miR-150 antagomiR group received an injection of 200 pmol
miR-150 antagomiR dissolved in 5 µl sterile double distilled water
(ddH2O). Rats in the antaogmiR NC group received an
injection of 200 pmol antagomiR NC dissolved in 5 µl
ddH2O. ICV injections were administered through both
sides of the lateral ventricle. Based on the rat brain atlas, two
small holes were drilled into both sides of the skull using
surgical drills. Stereotaxic coordinates for the ICV injection were
as follows: Anterior, −0.8 mm; lateral, 1.5 mm; and depth, −4.5
mm.
Morris water maze (MWM) test
A water maze with a black circular pool (diameter, 2
m) was filled with opaque water by adding black edible pigments and
maintained at 25±1°C. A diving escape platform (diameter, 20 cm;
depth below water, 2.0 cm) was located in the center of the first
quadrant. Before training, a pupillary light reflex test was
performed in all rats. Rats with impaired pupillary light reflex
were excluded from the experiment to prevent visual factors
affecting the test. Behavioral training consisting of three trials
per day for 5 days was conducted. For each behavioral training
trial, rats were placed in the water facing the lateral wall and
each rat was allowed to find the platform within 120 sec. If the
platform was not found within 120 sec, they were guided to the
platform and allowed to rest for at least 20 sec (the escape
latency was set to 120 sec in this situation). The DigBehav-Morris
water maze video analysis system (Mobile Benchmark Software
Technology Co., Ltd.) was used to monitor escape latencies. At the
end of the experiment, rats were euthanized with an intraperitoneal
injection of 150 mg/kg sodium pentobarbital. Following euthanasia,
animal death was confirmed by observing the lack of heartbeat,
respiratory arrest, pupil dilation, and lack of nerve reflex.
Reverse transcription-quantitative PCR
(RT-qPCR)
Target gene expression levels were measured via
RT-qPCR. Total RNA was extracted from rat hippocampus tissues and
cells using the PureLink RNA mini kit (Thermo Fisher Scientific,
Inc.). Total RNA was reverse transcribed into cDNA using
TransScript® Reverse Transcriptase (cat. no. T101-02;
Beijing Transgen Biotech Co., Ltd.), according to the
manufacturer's protocol. Subsequently, qPCR was performed using
Power SYBR™ Green PCR Master Mix (cat. no. 4367659; Thermo Fisher
Scientific, Inc.) on a LightCycler 96 [Roche Diagnostics (Shanghai)
Co., Ltd.]. The reaction system (20 µl) consisted of 10 µl SYBR
premix Extaq, 0.6 µl forward and reverse primers, 1 µl cDNA
template (with 10X-dilution) and 7.8 µl deionized water. The
conditions for RT-qPCR were as follows: Pre-denaturation at 95°C
for 10 min, 40 cycles of denaturation at 95°C for 5 sec, annealing
at 60°C for 30 sec, and extension at 95°C for 10 sec. The sequences
of the primers used for qPCR are presented in Table I. GAPDH and U6 were used as internal
controls, the 2−ΔΔCq method was used to calculate the
expression of miRNA and mRNA (20).
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5′→3′) |
|---|
| miR-150 | F:
CTCCCAACCCTTGTACCA |
|
| R:
GAACATGTCTGCGTATCTC |
| HOXA1 | F:
AGCCACCAAGAAGCCTGTCGTT |
|
| R:
TTGACCCACGTAGCCGTACTCT |
| U6 | F:
CTCGCTTCGGCAGCACAT |
|
| R:
TTTGCGTGTCATCCTTGCG |
| GAPDH | F:
CATCACTGCCACCCAGAAGACTG |
|
| R:
ATGCCAGTGAGCTTCCCGTTCAG |
| Apaf1 | F:
CACGAGTTCGTGGCATATAGGC |
|
| R:
GGAAATGGCTGTCGTCCAAGGA |
| p53 | F:
CCTCAGCATCTTATCCGAGTGG |
|
| R:
TGGATGGTGGTACAGTCAGAGC |
| Cyto C | F:
GAGGCAAGCATAAGACTGGACC |
|
| R:
ACTCCATCAGGGTATCCTCTCC |
Triphenyltetrazolium chloride (TTC)
staining
Briefly, brain tissues were rapidly excised from
rats and frozen at −20°C for 5 min. Subsequently, tissues were cut
into 5–6 uniform coronal sections (2 mm), which were water-bathed
in 2% TTC (Sigma-Aldrich; Merck KGaA) at 37°C in the dark for 30
min. Following fixation with 4% phosphate-buffered formalin at room
temperature for 30 min, sections were observed under light
microscope (CX33; Olympus Corporation) and photographed directly
with a camera. The infarct area was analyzed using ImageJ software
(V1.46, National Institutes of Health).
Immunohistochemistry (IHC)
The extracted rat brain tissues were fixed with 1%
paraformaldehyde at room temperature for 30 min, hydrated with 50,
70, 80, 95 and 100% ethanol for 30 min each, followed by blocking
in xylene containing 50% ethanol for 2 h, paraffin-embedding, and
sectioning (5 µm). Paraffin-embedded sections were dewaxed twice
with xylene for 10 min each and rehydrated with gradient ethanol
(95, 70 and 50%). After washing under running water for 10 sec, the
sections were treated with 0.03% hydrogen peroxide-methanol
solution for 10 min and then heated in a microwave oven for 10 min
after adding 0.01 M citrate buffer (pH 6.0). Subsequently, tissue
sections were sealed with 10% bovine serum albumin at room
temperate for 30 min and incubated with anti-c-fos (cat. no.
ab222669; 1:2,000; Abcam) and anti-HOXA1 (cat. no. ab230513;
1:5,000; Abcam) primary antibodies overnight at 4°C. Following
primary incubation, tissue sections were incubated with the
secondary goat anti-rabbit IgG (cat. no. ab6721; 1:1,000; Abcam) or
goat anti-rat IgG (cat. no. ab6734; 1:1,000; Abcam) for 30 min at
room temperature. Following thorough washing with PBS, chromogen
detection was performed using diaminobenzidine (Beijing Solarbio
Science & Technology Co., Ltd.). All sections were scanned
using a Panoramic MIDI high-resolution pathology slice scanner
(3DHistech, Ltd.) and analyzed using ImageJ with FIJI installed
(National Institutes of Health). To calculate the positive cell
rate, the area of density (AOD) was calculated according to the
following formula: AOD=integrated optical density/total area.
Nissl staining
Hippocampal neurons were observed by performing
Nissl staining according to a previously described protocol
(21). Sections were fixed with 1%
paraformaldehyde at room temperature for 30 min, rehydrated in
distilled water and stained with 0.5% crystal violet solution for
10 min. Brain sections were imaged using a DM5000B bright-field
microscope (magnification, ×400; Leica Microsystems GmbH). Normal
neurons with visible nuclei in the CA1 subdomain of the hippocampal
region were circled, counted within an unbiased counting field
(200×130 µm) in each group and expressed as the number of
cells/mm2 in the CA1 region of the hippocampus. The
number of neurons was assessed in five brain sections from each rat
at different depths on the left and right sides.
Hematoxylin and eosin (H&E)
staining
Brain tissues were fixed with 1% paraformaldehyde at
room temperature for 30 min, embedded in paraffin, cut into 5-µm
thick sections and heated at 60°C for 25 min. After rehydration
using graded alcohols (95, 70 and 50%), tissue sections were rinsed
in distilled water, stained using in 0.5% hematoxylin solution for
15 min at room temperature, then washed, and stained with eosin
solution in 95% ethanol for 3–5 min at room temperature.
Pathological alterations to neurons in the CA1 region of the
hippocampus were observed using an optical microscope (Olympus
Corporation). Scores of post 2VO surgery-related injuries were
observed under a light microscope (CX33, Olympus Corporation) at
×200 magnification in five brain regions per rat were evaluated. A
damage score based on the system proposed by Vannucci et al
(22) was used to assess the tissue
sections: 0, no damage; 1, focal loss of a single neuron; 2,
multiple damage in the hippocampus; 3, multiple or large foci of
neuronal loss with edema and reactive glial hyperplasia, but
without cystic infarction; and 4, severe cystic and non-cystic
infarction, edema, myelin pallor and reactive glial
hyperplasia.
TUNEL staining
The apoptotic index of rat hippocampal tissues was
evaluated using the TUNEL apoptosis detection kit (Roche
Diagnostics) according to the manufacturer's protocol. Briefly,
after being fixed with 1% paraformaldehyde at room temperature for
30 min, paraffin-embedded sections were dewaxed and rehydrated in a
gradient ethanol (95, 70 and 50%). Subsequently, tissue sections
were detached with trypsin at room temperature for 40 min. Tissue
sections were then incubated with TUNEL reaction buffer at 37°C for
1 h in humidity (45–65%). The sections were immersed in 0.5%
hematoxylin solution for 15 min at room temperature for the nuclear
staining. Following washing with PBS, TUNEL-positive cells and
normal cells in each group were observed under five fields of view
using a light microscope (magnification ×200; CX33; Olympus
Corporation).
Quantification of lactic dehydrogenase
(LDH) content and Caspase-3 activity
The level of LDH was determined using a cytotoxic
LDH assay kit (Dojindo Molecular Technologies, Inc.) according to
the manufacturer's protocol. Briefly, the tissues were ground on
ice with TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) at a volume ratio of 1:10, followed by centrifugation at
161,2.8 × g to extract the supernatant. The brain tissue lysate was
plated into 96-well plates at 10 µl per well, and 100 µl working
solution was added to each well. Following incubation for 30 min in
the dark at room temperature, 50 µl termination solution was added.
The level of LDH was assessed by measuring the absorbance at a
wavelength of 490 nm using a microplate reader. LDH activity unit
(mU/ml) was calculated according to the following formula: [optical
density (OD) value sample-OD value blank
control]/(OD value standard tube-OD value
standard blank control tube) × standard
concentration.
The brain tissue lysate was prepared as described
above. Caspase-3 activity in rat brain tissue lysate was assessed
using the Caspase-Glo®3 assay (cat. no. G8981; Promega
Corporation), a luminescence-based test system that measures
caspase-3 activity, according to the manufacturer's protocol. The
luminescence signal was quantified using a microplate fluorometer
at 405 nm.
Cell culture
PC12 cells (Procell Life Science & Technology
Co., Ltd.) were cultured in RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin solution (Thermo
Fisher Scientific, Inc.) at 37°C with 5% CO2. Cell
culture medium was renewed every 3 days.
PC12 cells were seeded (5×106 cells/well)
in 6-well plates with complete medium. At 80% confluence, cells
were transfected with miR-150 mimic, mimic negative control (NC),
miR-150 inhibitor, inhibitor NC, miR-150 mimic + empty vector,
miR-150 mimic + HOXA1, miR-150 inhibitor + small interfering RNA
(si) Scramble (Scr), miR-150 inhibitor + si-HOXA1-#1 or miR-150
inhibitor + si-HOXA1-#2 (the final concentration was 50 ng/µl). The
following sequences were used: miR-150 mimic,
5′-CCCCUCUGGUCAACCAGUCACA-3′; miR-150 inhibitor,
5′-AGAGGGTTGGGAACATGGTCAC-3′; NC for both miR-150 mimic and
inhibitor, 5′-TCTCCCAACCCTTGTCCAGTG-3′; si-HOXA1-#1,
CCGGCCCTCGGACCATAGGATTACACTCGAGTGTAATCCTATGGTCCGAGGGTTTTTG;
si-HOXA1-#2,
CCGGCCGCTGTTTACTCTGGAAATCCTCGAGGATTTCCAGAGTAAACAGCGGTTTTTG; si-Scr,
GGCCGGGAGCCTAGGTATCCTAATGGTACGAGGCTAGCTGGGATC; and HOXA1,
5′-TGGCTTTTGAAGGGAGTTCTGATTTTTCTTCTCCGGCCCCA-3′. For transfection,
miR-mimics, miR-inhibitors, siRNAs and vectors were centrifuged at
447.2 × g for 3 min at 4°C, and then dissolved in 125 µl diethyl
pyrocarbonate water. Subsequently, 4 µl miR-mimic, miR-inhibitor,
siRNA or vector solution was mixed with 7.5 µl
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), incubated for 5 min at room temperature, allowed
to stand for 20 min at room temperature, and then added to each
well of the 6-well plate. After 48 h of transfection, the medium
was changed to medium supplemented with 10% FBS and antibiotics,
followed by another incubation for 48 h. At 70% confluence, cells
were passaged and cultured. Stable cell lines were screened using
10 µg/ml puromycin or 250 µg/ml G418. Following a 2-week
maintenance (5 µg/ml puromycin or 200 µg/ml G418), miR-150 or HOXA1
expression levels were detected via RT-qPCR and western blotting,
respectively.
PI/Hoechst 33342 staining
PI/Hoechst 33342 staining was performed according to
the manufacturer's protocol. PC12 cells were seeded at
5×103 cells/well and incubated with both 10 µg/ml
Hoechst 33342 (cat. no. C1017; Beyotime Institute of Biotechnology)
and 10 µg/ml PI (cat. no. ST512; Beyotime Institute of
Biotechnology) at 37°C for 15 min, respectively. Stained cells were
observed in five randomly selected fields of view using a
fluorescence microscope to calculate the number of apoptotic cells
using ImageJ software (V1.46; National Institutes of Health).
Flow cytometry
Cell apoptosis was detected via flow cytometry using
the Annexin V/PI kit (BD Biosciences). Briefly, PC12 cells were
seeded at 5×103 cells/well and resuspended in 1X binding
buffer. Subsequently, PC12 cells were stained with Annexin V and PI
at 37°C for 30 min in a dark room. FACS analysis was performed
using a FACSCalibur flow cytometer (BD Biosciences) using FlowJo
V10.6.2 (BD Biosciences). Early apoptotic cells are in the lower
right quadrant, while late apoptotic cells in the upper right
quadrant.
Western blotting
Total protein was isolated from brain tissues and
PC12 cells using RIPA assay lysis buffer (cat. no. P0013C; Beyotime
Institute of Biotechnology) on ice. Total protein was quantified
using the BCA method. Proteins (20 µg) were separated via 10%
SDS-PAGE and transferred onto PVDF membranes (EMD Millipore).
Following blocking with 5% skimmed milk for 2 h at 37°C, the
membranes were incubated overnight at 4°C with primary antibodies
targeted against: HOXA1 (cat. no. ab230513; 1:5,000; Abcam),
apoptotic peptidase activating factor 1 (Apaf1; cat. no. 8969;
1:2,000; Cell Signaling Technology, Inc.), p53 (cat. no. 2524;
1:5,000; Cell Signaling Technology, Inc.), Cytochrome C (Cyto C;
cat. no. 11940; 1:2,000; Cell Signaling Technology, Inc.) and GAPDH
(cat. no. ab8245; 1:5,000; Abcam). Subsequently, the membranes were
incubated with HRP-conjugated secondary antibody against IgG (cat.
no. ab6721; 1:10,000; Abcam) for 2 h at 37°C. Following washing
with TBS with 0.1% Tween-20, protein bands were visualized using an
enhanced chemiluminescence detection system (Thermo Fisher
Scientific, Inc.). Semi-quantification was conducted using Gel-Pro
Analyzer version 4.0 (Media Cybernetics, Inc.).
Dual-luciferase reporter gene
assay
PicTar (https://pictar.mdc-berlin.de/) was used to verify
whether HOXA1 was the direct target gene of miR-150.
Dual-luciferase reporter assays were performed to verify the
interaction between miR-150 and the 3′-untranslated region (UTR) of
HOXA1. The wild-type (WT) HOXA1 sequence from HOXA1 mRNA (including
the predicted binding sites of miR-150) was amplified and inserted
into the pLUC dual-luciferase vector (Promega Corporation) to
construct the pmirGLO-HOXA1-WT reporter vector. The presumed
miR-150 binding site in HOXA1 3′-UTR was mutated using GeneArt TM
site-directed mutagenesis PLUS system (cat. no. A14604; Thermo
Fisher Scientific, Inc.). The mutant type (MUT) HOXA1 3′-UTR was
inserted into the pLUC vector to develop the pLUC-HOXA1-MUT
reporter vector. 293T cells (American Type Culture Collection) were
seeded into a 96-well plate at 5×103 cells/well and
co-transfected with 50 ng pmirGLO-HOXA1-WT or pLUC-HOXA1-MUT and 50
pmol miR-150 mimic (5′-CCCCUCUGGUCAACCAGUCACA-3′) or NC mimic
(5′-TTCTCCGAACGTGTCACGT-3′, both from Guangzhou RiboBio Co., Ltd.).
Transfection was performed at 37°C for 24 h using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). At 48 h post-transfection, luciferase activities
were measured using the Dual-Luciferase Reporter Assay System
(Promega Corporation) and the firefly luciferase activity value was
compared against the Renilla luciferase activity value.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 22.0; IBM Corp.). Data are presented as the mean
± SD. To assess the normality of the data, the Kolmogorov-Smirnov
test was used. Comparisons between two groups were analyzed using
an unpaired Student's t-test. Comparisons among multiple groups
were analyzed using one-way ANOVA or two-way mixed ANOVA followed
by Tukey's post hoc test. All experiments were repeated three
times. P<0.05 was considered to indicate a statistically
significant difference.
Results
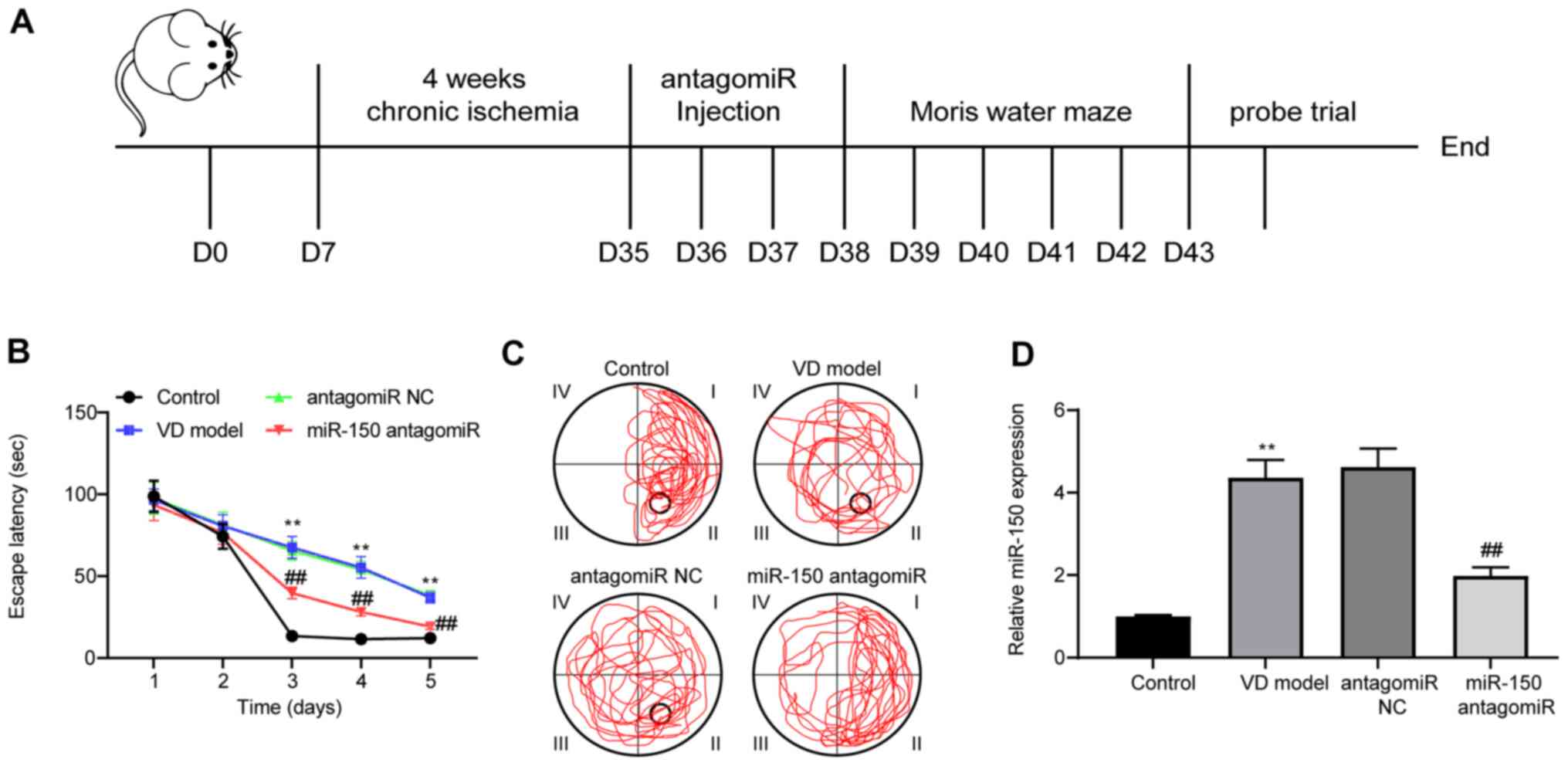
miR-150 knockdown strengthens the
learning and memorial abilities of VD model rats
VD model rats were established by performing 2-VO
surgery. After 35 days, rats were injected with miR-150 antagomiR
or antagomiR NC. To verify whether miR-150 altered the spatial
learning and memory abilities of VD model rats, the MWM test was
performed at 3 days post-ICV injection (Fig. 1A). During MWM training, the escape
latency of rats was notably reduced in a time-dependent manner,
indicating that the platform could be found with training (Fig. 1B). Compared with the control group,
the escape latency of the rats in the VD model group was
significantly increased. By contrast, the escape latency of the
rats in the miR-150 antagomiR group was significantly shorter
compared with the antagomiR NC model group.
On the 5th day of the MWM test, the number of VD
model rats crossing the platform was notably decreased compared
with the control group; however, rats treated with miR-150
antagomiR displayed the opposite results (Fig. 1C). miR-150 expression levels in rat
brain tissues were measured (Fig.
1D). Compared with the control group, miR-150 expression levels
were significantly increased in the brain tissues of VD model rats.
miR-150 expression was significantly downregulated by miR-150
antagomiR treatment compared with the antagomiR NC group. As
indicated by the MWM test, spatial learning abilities were impaired
when rats were exposed to 4-week chronic ischemia. Moreover,
miR-150 knockdown alleviated cognitive dysfunction, and enhanced
learning and memory abilities in VD model rats.
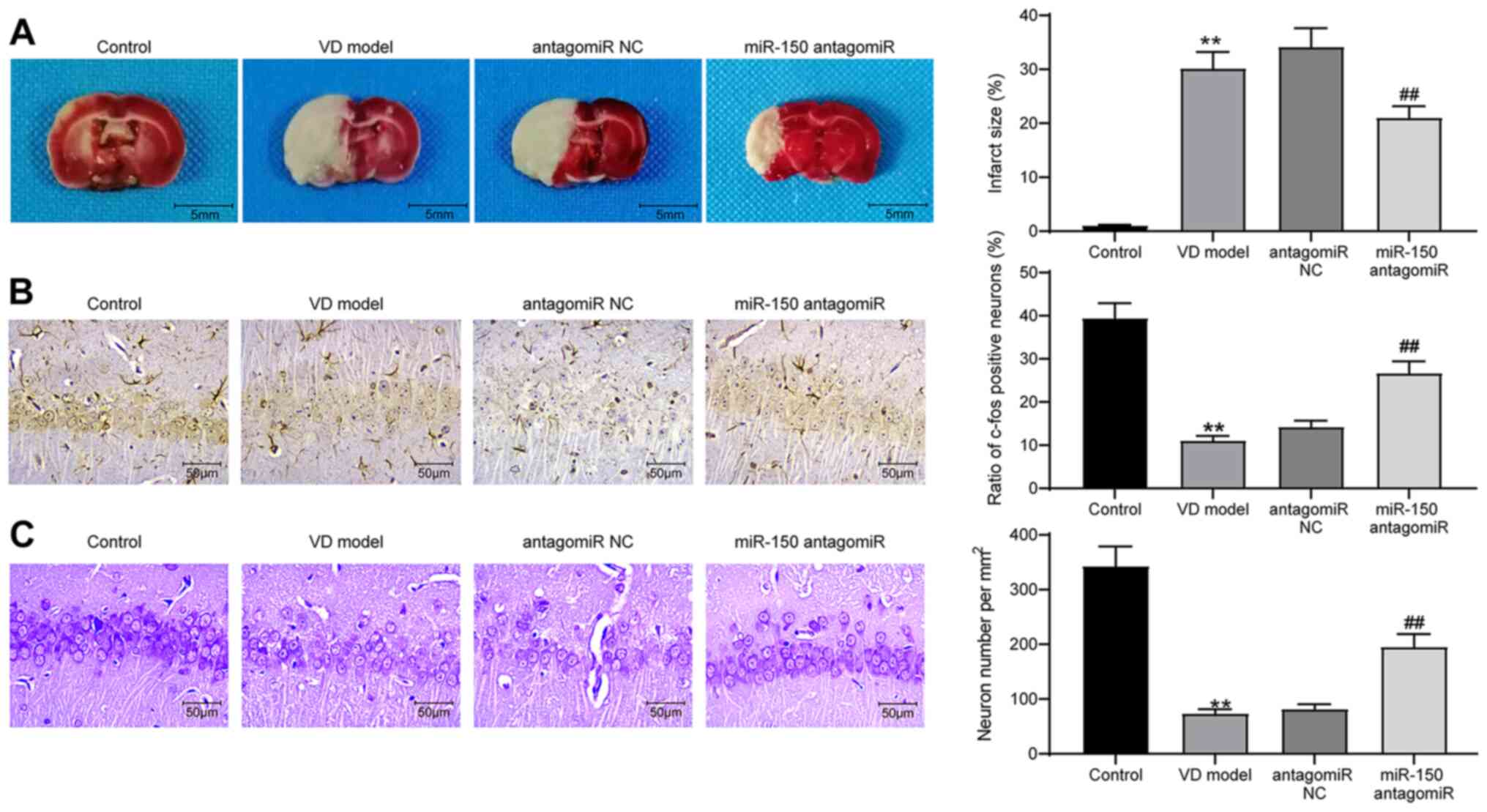
miR-150 knockdown improves hippocampal
neuron activity
Subsequently, brain tissues were extracted from rats
for histological staining and detection of protein expression. The
infarct area in brain tissues was measured by performing TTC
staining. The infarct area was significantly increased in the brain
tissues of VD model rats compared with control rats, whereas
miR-150 knockdown in brain tissues significantly reduced 2-VO
operation-induced cerebral infarction compared with the antagomiR
NC group (Fig. 2A). Moreover, IHC
was conducted to detect the expression of c-fos protein in
hippocampal tissues of the CA1 region in brain tissues (Fig. 2B). The IHC results demonstrated that
c-fos protein expression was significantly reduced in the
hippocampal tissues of VD model rats compared with control rats;
however, miR-150 knockdown significantly reversed VD-induced
downregulation of c-fos protein expression compared with the
antagomiR NC group. Subsequently, Nissl staining was performed to
assess the number of Nissl bodies in rat hippocampal tissues to
measure hippocampal neuron activity. Compared with the control
group, the VD model group displayed significantly suppressed rat
hippocampal neuron activity, which was restored by miR-150
antagomiR treatment (Fig. 2C).
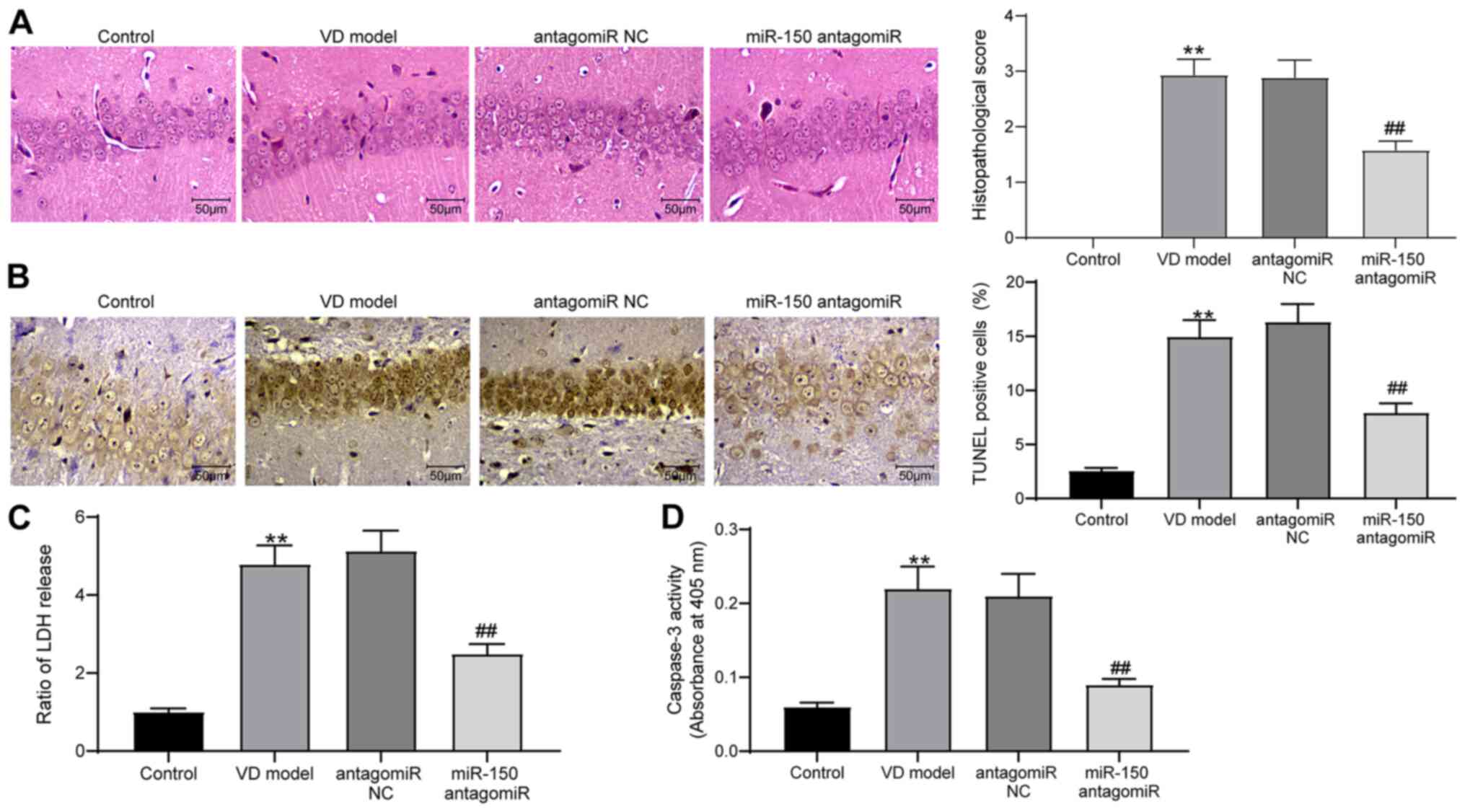
miR-150 knockdown suppresses
hippocampal neuron apoptosis in VD model rats
H&E staining was performed to detect the
pathological structure of rat brain tissues (Fig. 3A). Compared with the control group,
pathological injury in the brain tissues of VD model rats was
significantly increased, which was accompanied by blurred cell
boundaries. miR-150 knockdown significantly relieved the level of
pathological injury in VD model rats compared with the antagomiR NC
group. The TUNEL staining results indicated that miR-150 antagomiR
significantly inhibited 2-VO surgery-induced cell apoptosis in
hippocampal tissues in the CA1 region compared with the antagomiR
NC group (Fig. 3B). Additionally,
the content of LDH and activity of Caspase-3 in rat brain tissues
were assessed using LDH and Caspase-3 kits, respectively (Fig. 3C and D). The content of LDH and
activity of Caspase-3 in VD model rat brain tissues were
significantly increased compared with the control group. miR-150
knockdown significantly inhibited VD-induced alterations to LDH
content and Caspase-3 activity compared with the antagomiR NC
group.
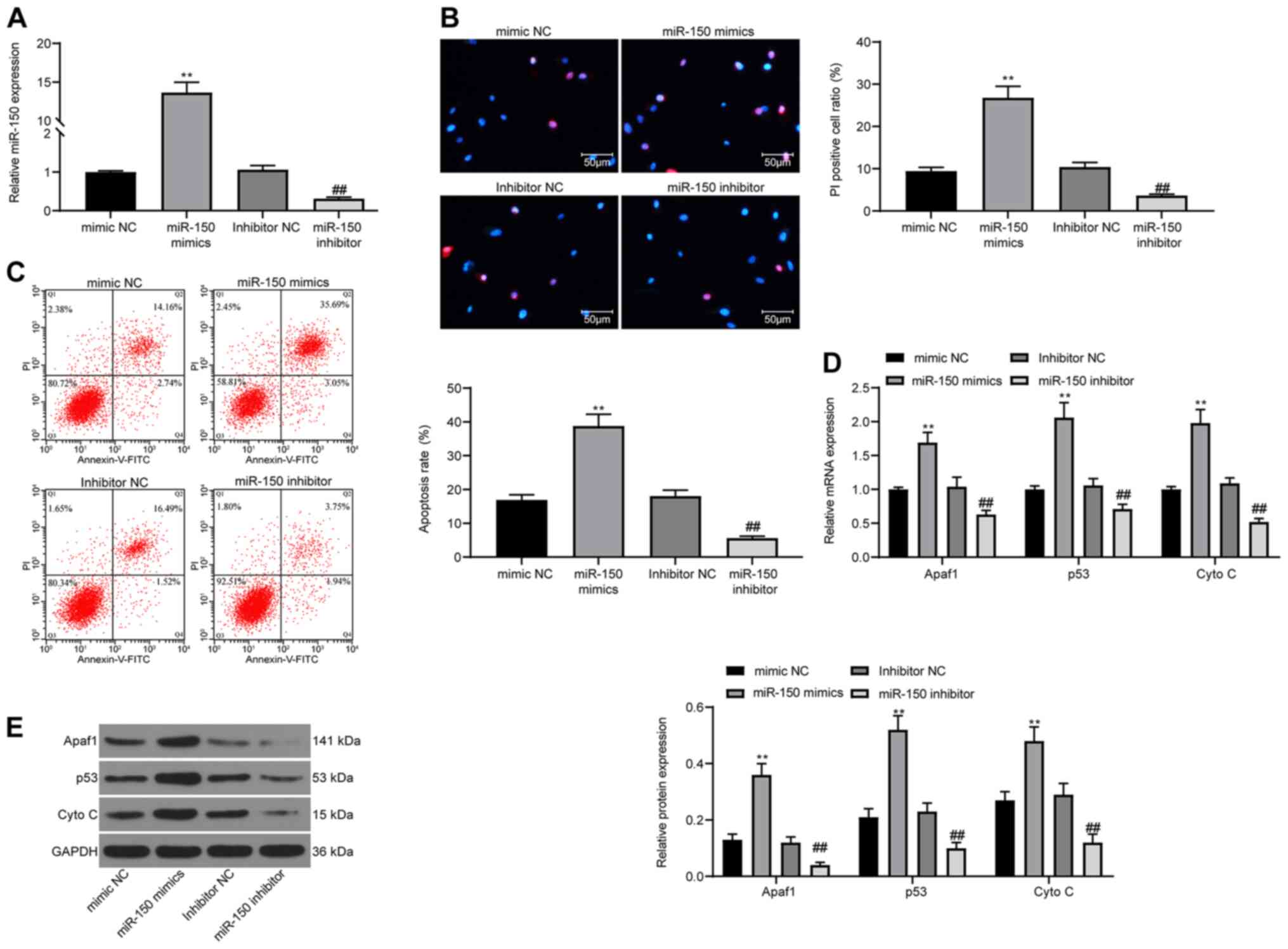
miR-150 overexpression promotes PC12
cell apoptosis
To validate the role of miR-150 in neurons in
vitro, PC12 cells were used in the present study. miR-150
expression was overexpressed or knocked down in PC12 cells.
Transfection efficiencies were assessed via RT-qPCR (Fig. 4A). Subsequently, cell apoptosis was
detected via Hoechst 33342/PI staining. The results demonstrated
that miR-150 overexpression significantly promoted cell apoptosis
compared with the mimic NC group, whereas miR-150 knockdown
significantly reduced cell apoptosis compared with the inhibitor NC
group (Fig. 4B). The detection of
cell apoptosis via flow cytometry displayed the same experimental
results (Fig. 4C). Furthermore,
RT-qPCR and western blotting were performed to detect the
expression levels of apoptosis-related proteins Apaf1, p53 and Cyto
C (Fig. 4D and E). miR-150
overexpression significantly increased the expression levels of
apoptosis-related proteins compared with the mimic NC group,
whereas miR-150 knockdown displayed the opposite effects on protein
expression compared with the inhibitor NC group.
miR-150 negatively modulates HOXA1
expression via direct binding
To explore the downstream molecular mechanism
underlying miR-150, PicTar was used to predict the possible targets
of miR-150. PicTar identified a potential binding relationship
between miR-150 and HOXA1 (Fig.
5A). To evaluate the direct effect of miR-150 on HOXA1
expression, a dual-luciferase reporter gene pLUC-HOXA1 with an
miR-150 target site was constructed (Fig. 5B). miR-150 overexpression
significantly decreased the luciferase activity of HOXA1-WT in 293T
cells compared with the mimic control group (Fig. 5C). Therefore, western blotting and
IHC were performed to detect HOXA1 expression in the hippocampus of
the CA1 region in rat brain tissues. HOXA1 expression in VD model
rats was significantly reduced compared with control rats, and
VD-induced downregulation of HOXA1 expression was significantly
reversed by miR-150 antagomiR treatment compared with the antagomiR
NC group (Fig. 5D and E). HOXA1
expression in PC12 cells was further analyzed via RT-qPCR and
western blotting. miR-150 overexpression significantly decreased
HOXA1 expression compared with the mimic NC group, whereas miR-150
knockdown significantly increased HOXA1 expression compared with
the inhibitor NC group (Fig. 5F and
G).
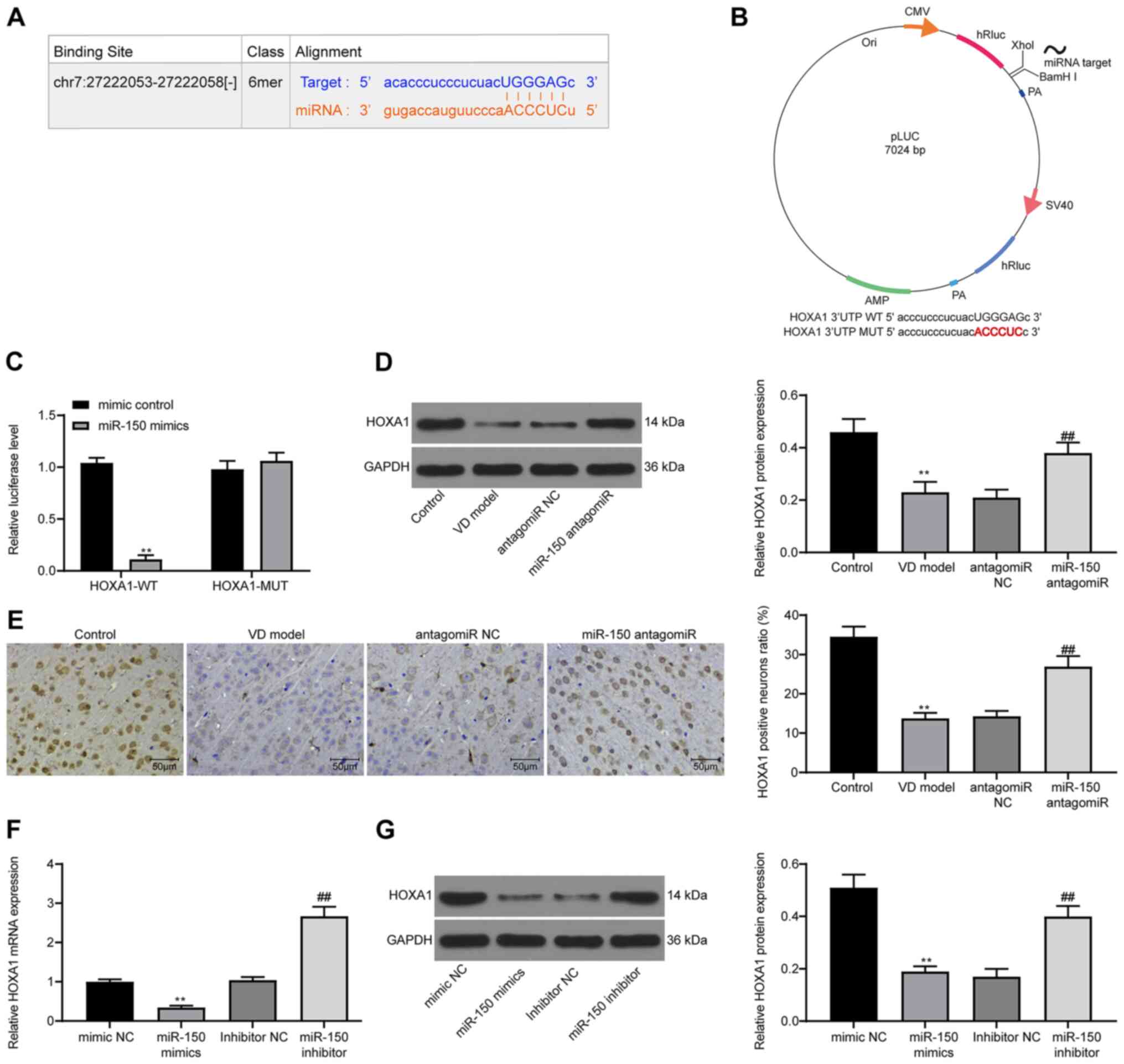 | Figure 5.miR-150 negatively regulates HOXA1
expression. (A) PicTar predicted the binding site between HOXA1
3′UTR and miR-150. (B) Establishment of pLUC-HOXA1-MUT/WT reporter
vectors containing miR-150 target sites. (C) Dual-luciferase
reporter gene assays were determined to assess the interaction
between miR-150 and HOXA1. (D) HOXA1 protein expression levels in
rat brain tissues were determined via western blotting. (E) HOXA1
positive cells in rat brain tissues were observed via
immunohistochemistry. HOXA1 (F) mRNA and (G) protein expression
levels in PC12 cells were determined via reverse
transcription-quantitative PCR and western blotting, respectively.
Experiments were repeated three times. Data are presented as the
mean ± standard deviation. An unpaired Student's t-test was used to
analyze the data presented (C). One-way ANOVA followed by Tukey's
post hoc test was used to analyze the data presented (D-G).
**P<0.01 vs. mimic control, control or mimic NC;
##P<0.01 vs. antagomiR NC or inhibitor NC. miR,
microRNA; HOXA1, homeobox A1; UTR, untranslated region; MUT,
mutant; WT, wild-type; NC, negative control; CMV, Cytomegalovirus;
VD, vascular dementia. |
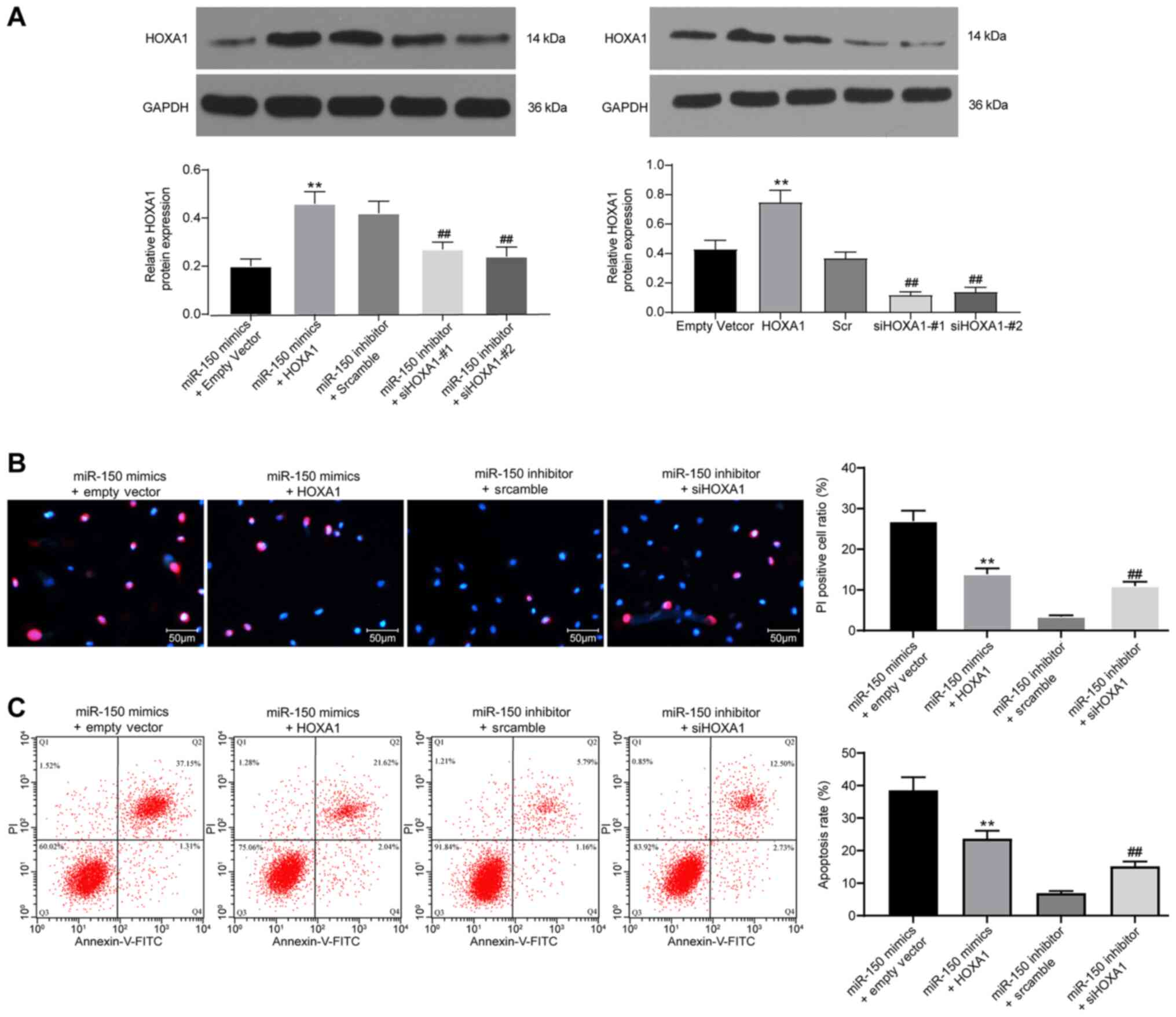
HOXA1 overexpression suppresses
miR-150-induced PC12 cell apoptosis
Based on the aforementioned results, HOXA1 was
overexpressed in miR-150-overexpression PC12 cells, but knocked
down in miR-150-knockdown PC12 cells. Western blotting was
performed to assess the transfection efficiency of HOXA1
overexpression and knockdown (Fig.
6A). HOXA1 overexpression significantly increased HOXA1
expression levels compared with cells transfected with empty
vector. HOXA1 expression in cells transfected with siHOXA1-#1 or
siHOXA1-#2 was significantly lower compared with cells transfected
with Scr. The aforementioned results demonstrated successful
transfection of the HOXA1 overexpression vector, siHOXA1-#1 and
si-HOXA1#2. The Hoechst 33342/PI double staining and flow cytometry
results demonstrated that compared with the empty vector group,
HOXA1 overexpression significantly inhibited cell apoptosis in
miR-150-overexpression PC12 cells (Fig.
6B and C). By contrast, compared with the Scr group, HOXA1
knockdown significantly promoted miR-150-knockdown PC12 cell
apoptosis.
Discussion
The present study investigated whether miR-150
knockdown displayed an inhibitory effect on VD via targeting HOXA1
in vivo and in vitro. VD model rats were established
using 2-VO methods. PC12 cells are also often used as a cell model
for neuronal studies (23), thus
PC12 cells were used in the present study. The results suggested
that chronic ischemia-induced VD model rats displayed learning
disorders after 4 weeks, which implied that the VD rat model was
successfully established.
Initially, the effects of miR-150 on cognitive
function and hippocampal neurons in VD model rats were explored. VD
model rats were subjected to ICV injections of miR-150 antagomiR,
followed by the detection of cognitive functions by performing MWM
tests. miR-150 antagomiR significantly shortened the escape latency
of VD model rats compared with the antagomiR NC group. A previous
study reported that escape latency is usually prolonged following
VD modeling (24). Therefore,
miR-150 knockdown should improve cognitive function in VD model
rats. Furthermore, compared with antagomiR NC, miR-150 antagomiR
elevated c-fos protein expression levels and increased the number
of Nissl bodies in the brain tissue of VD model rats. C-fos is
aberrantly expressed during neuronal activity processes and an
infarction is considered as a biomarker for VD (25). Furthermore, ischemic model rats
display a reduced density of Nissl bodies in neurons (26). Collectively, the results of the
present study demonstrated that compared with the antagomiR NC
group, miR-150 knockdown enhanced the activity of hippocampal
neurons. Furthermore, compared with the antagomiR NC group, miR-150
knockdown significantly reduced fibrosis levels, LDH contents and
Caspase-3 activities in the brain tissues of VD model rats. It has
been reported that cerebrovascular levels vary with altered
expression of fibrotic degrees (27). LDH levels are an indicator of
oxidative stress, and a reduction in LDH levels is associated with
alleviation of VD (28).
Improvement of VD is also linked to reduced oxidative stress and
apoptosis in the hippocampus of VD model rats, as well as decreased
levels of Caspase-3 (29). Overall,
the results of the present study suggested that miR-150 knockdown
displayed therapeutic effects on VD model rats.
Subsequently, how miR-150 affected neurons in
vitro was investigated. miR-150 antagomiR significantly
downregulated Apaf1, p53 and Cyto C expression levels compared with
antagomiR NC. p53 and Apaf1 are proapoptotic genes (30). Moreover, apoptosis can be further
enhanced when p53 proteins are combined with Cyto C (31). The aforementioned results supported
the hypothesis that miR-150 knockdown suppresses cell apoptosis.
Since ligustrazine is considered as a neuroprotective agent in the
management of VD disease by suppressing PC12 cell apoptosis
(32), it was also hypothesized
that miR-150 knockdown might protect against VD by inhibiting PC12
cell apoptosis. A previous study demonstrated that miR-150
knockdown functions in the survival of cerebral cortical neurons
(33). In the present study,
compared with the empty vector group, HOXA1 overexpression
significantly inhibited miR-150 mimic-induced PC12 cell apoptosis,
which suggested that miR-150 might mediate the biological function
of PC12 cells via HOXA1.
In the present study, further mechanistic
investigations indicated that miR-150 targeted HOXA1. Gavalas et
al (34) reported that HOXA1 or
HOXB1 null mutants cause developmental disorders in the hindbrain.
Subsequently, in 2007, Paraguison et al (35) demonstrated that polyhistidine
variants of HOXA1 lead to decreased PBX homeobox 1 activity and
inhibition of neuronal differentiation. In 2008, Martinez-Ceballos
and Gudas (36) highlighted the
necessity of HOXA1 in retinoic acid-induced embryonic stem cell
differentiation to neurons. The aforementioned results indicated
that HOXA1 is closely related to neuronal activity and development.
A previous study reported that loss of HOXA1 could lead to a
deficit in the neural crest (37).
Of note, HOXA1 is regulated by different miRNAs in various
diseases. For instance, miR-99a-5p regulates atherosclerotic
progression via downregulation of HOXA1 mRNA and protein expression
levels (15). Additionally,
miR-10a-5p overexpression can suppress the HOXA1 expression in
osteoarthritis (38). However,
studies on the interaction between miR-150 and HOXA1 are
limited.
The present study investigated the role of miR-150
in VD. Specifically, compared with the antagomiR NC group, miR-150
knockdown remarkably alleviated cognitive deficits and suppressed
neuron apoptosis in the brain tissues of VD model rats. The results
of the present study indicated that miR-150 knockdown might serve
as a potential novel treatment strategy in VD-induced cognitive
impairment. To the best of our knowledge, the present study
identified an association between miR-150 and HOXA1 for the first
time, and explored the effects of the miR-150/HOXA1 axis in VD
in vitro and in vivo. Moreover, miR-150 might mediate
other genes or signaling pathways in VD; therefore, the mechanisms
underlying miRNA-modulated molecules in VD require further
investigation. However, a limitation of this study includes the use
of PC12 cells, which is a noradrenergic cell line derived from a
rat pheochromocytoma, as the control. A more appropriate control
cell line in future studies may further verify the authenticity of
these results.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Scientific
Research Project of Heilongjiang Health Committee (grant nos.
2017-500 and 2017-496).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW and XX contributed to designing the study and
preparing the manuscript. HZ analyzed the data and edited the
manuscript. XZ acquired and analyzed the data, and edited the
manuscript. ZG conceptualized the study and reviewed the
manuscript. CW, XX and HZ confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Laboratory
Animal Care and Use Committee of Heilongjiang Provincial Hospital
(approval no. KY-2018-197) and performed according to the
Guidelines for the Care and Use of Laboratory Animals published by
the National Institutes of Health (17).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
O'Brien JT and Thomas A: Vascular
dementia. Lancet. 386:1698–1706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jellinger KA and Attems J: Is there pure
vascular dementia in old age? J Neurol Sci. 299:150–154. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalaria RN: The pathology and
pathophysiology of vascular dementia. Neuropharmacology.
134:226–239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han F: Cerebral microvascular dysfunction
and neurodegeneration in dementia. Stroke Vasc Neurol. 4:105–107.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wolters FJ and Ikram MA: Epidemiology of
vascular dementia. Arterioscler Thromb Vasc Biol. 39:1542–1549.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farooq MU, Min J, Goshgarian C and
Gorelick PB: Pharmacotherapy for vascular cognitive impairment. CNS
Drugs. 31:759–776. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vijayan M, Kumar S, Bhatti JS and Reddy
PH: Molecular links and biomarkers of stroke, vascular dementia,
and Alzheimer's disease. Prog Mol Biol Transl Sci. 146:95–126.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vijayan M and Reddy PH: Non-Coding RNAs
based molecular links in type 2 diabetes, ischemic stroke, and
vascular dementia. J Alzheimers Dis. 75:353–383. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan M and Bi X: Therapeutic and
diagnostic potential of microRNAs in vascular cognitive impairment.
J Mol Neurosci. 70:1619–1628. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toyama K, Spin JM, Deng AC, Huang TT, Wei
K, Wagenhäuser MU, Yoshino T, Nguyen H, Mulorz J, Kundu S, et al:
MicroRNA-mediated therapy modulating blood-brain barrier disruption
improves vascular cognitive impairment. Arterioscler Thromb Vasc
Biol. 38:1392–1406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu P, Venkat P, Chopp M, Zacharek A, Shen
Y, Ning R, Liang L, Li W, Zhang L, Landschoot-Ward J, et al: Role
of microRNA-126 in vascular cognitive impairment in mice. J Cereb
Blood Flow Metab. 39:2497–2511. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cohen A, Zinger A, Tiberti N, Grau GER and
Combes V: Differential plasma microvesicle and brain profiles of
microRNA in experimental cerebral malaria. Malar J. 17:1922018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryan B, Williams JM and Curtis MA: Plasma
MicroRNAs are altered early and consistently in a mouse model of
tauopathy. Neuroscience. 411:164–176. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang Q, Shan K, Qun-Wang X, Zhou RM, Yang
H, Liu C, Li YJ, Yao J, Li XM, Shen Y, et al: Long non-coding
RNA-MIAT promotes neurovascular remodeling in the eye and brain.
Oncotarget. 7:49688–49698. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han Z, Guan Y, Liu B, Lin Y, Yan Y, Wang
H, Wang H and Jing B: MicroRNA-99a-5p alleviates atherosclerosis
via regulating Homeobox A1. Life Sci. 232:1166642019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao J, He L and Yin L: lncRNA NEAT1 binds
to MiR-339-5p to increase HOXA1 and alleviate ischemic brain damage
in neonatal mice. Mol Ther Nucleic Acids. 20:117–127. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guide for the Care and Use of
Laboratory Animals. National Academies Press; Washington, DC:
1996
|
|
18
|
Wang XR, Shi GX, Yang JW, Yan CQ, Lin LT,
Du SQ, Zhu W, He T, Zeng XH, Xu Q and Liu CZ: Acupuncture
ameliorates cognitive impairment and hippocampus neuronal loss in
experimental vascular dementia through Nrf2-mediated antioxidant
response. Free Radic Biol Med. 89:1077–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiao LY, Wang XR, Yang JW, Ye Y, Zhu W,
Cao Y, Ma SM and Liu CZ: Acupuncture prevents the impairment of
hippocampal LTP through β1-AR in vascular dementia rats. Mol
Neurobiol. 55:7677–7690. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Cui SS, Wallace AE, Hannesson DK,
Schmued LC, Saucier DM, Honer WG and Corcoran ME: Relations between
brain pathology and temporal lobe epilepsy. J Neurosci.
22:6052–6061. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vannucci RC, Towfighi J and Vannucci SJ:
Secondary energy failure after cerebral hypoxia-ischemia in the
immature rat. J Cereb Blood Flow Metab. 24:1090–1097. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu C, Guo Y, Zhao F, Qin H, Lu H, Fang L,
Wang J and Min W: Potential mechanisms mediating the protective
effects of a peptide from walnut (Juglans mandshurica Maxim.)
against hydrogen peroxide induced neurotoxicity in PC12 cells. Food
Funct. 10:3491–3501. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao Y, Shen H, Li R, Zhou X, Xiao H and
Yan J: A novel octapeptide derived from G protein-coupled receptor
124 improves cognitive function via pro-angiogenesis in a rat model
of chronic cerebral hypoperfusion-induced vascular dementia. Drug
Des Devel Ther. 13:3669–3682. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Summers PM, Hartmann DA, Hui ES, Nie X,
Deardorff RL, McKinnon ET, Helpern JA, Jensen JH and Shih AY:
Functional deficits induced by cortical microinfarcts. J Cereb
Blood Flow Metab. 37:3599–3614. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shang Y, Cheng J, Qi J and Miao H:
Scutellaria flavonoid reduced memory dysfunction and neuronal
injury caused by permanent global ischemia in rats. Pharmacol
Biochem Behav. 82:67–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tong XK and Hamel E: Simvastatin restored
vascular reactivity, endothelial function and reduced string vessel
pathology in a mouse model of cerebrovascular disease. J Cereb
Blood Flow Metab. 35:512–520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Lu F, Li W, Qin L, Yao Y, Ge X, Yu
Q, Liang X, Zhao D, Li X and Zhang J: Edaravone injection reverses
learning and memory deficits in a rat model of vascular dementia.
Acta Biochim Biophys Sin (Shanghai). 49:83–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun M, Shen X and Ma Y: Rehmannioside A
attenuates cognitive deficits in rats with vascular dementia (VD)
through suppressing oxidative stress, inflammation and apoptosis.
Biomed Pharmacother. 120:1094922019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao F, Li H, Cao F, Chen X, Liang Y and
Qiu L: Short-term developmental toxicity and potential mechanisms
of the herbicide metamifop to zebrafish (Danio rerio) embryos.
Chemosphere. 236:1245902019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen X, Zhu Q, Xu X, Shen S, Zhang Y and
Mo R: Sequentially site-specific delivery of apoptotic protein and
tumor-suppressor gene for combination cancer therapy. Small.
15:e19029982019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao T, Fu Y, Sun H and Liu X:
Ligustrazine suppresses neuron apoptosis via the Bax/Bcl-2 and
caspase-3 pathway in PC12 cells and in rats with vascular dementia.
IUBMB Life. 70:60–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lv H, Li J and Che YQ: MicroRNA-150
contributes to ischemic stroke through its effect on cerebral
cortical neuron survival and function by inhibiting ERK1/2 axis via
Mal. J Cell Physiol. 234:1477–1490. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gavalas A, Studer M, Lumsden A, Rijli FM,
Krumlauf R and Chambon P: Hoxa1 and Hoxb1 synergize in patterning
the hindbrain, cranial nerves and second pharyngeal arch.
Development. 125:1123–1136. 1998.PubMed/NCBI
|
|
35
|
Paraguison RC, Higaki K, Yamamoto K,
Matsumoto H, Sasaki T, Kato N and Nanba E: Enhanced autophagic cell
death in expanded polyhistidine variants of HOXA1 reduces
PBX1-coupled transcriptional activity and inhibits neuronal
differentiation. J Neurosci Res. 85:479–487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martinez-Ceballos E and Gudas LJ: Hoxa1 is
required for the retinoic acid-induced differentiation of embryonic
stem cells into neurons. J Neurosci Res. 86:2809–2819. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gouti M, Briscoe J and Gavalas A: Anterior
Hox genes interact with components of the neural crest
specification network to induce neural crest fates. Stem Cells.
29:858–870. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma Y, Wu Y, Chen J, Huang K, Ji B, Chen Z,
Wang Q, Ma J, Shen S and Zhang J: miR-10a-5p promotes chondrocyte
apoptosis in osteoarthritis by targeting HOXA1. Mol Ther Nucleic
Acids. 14:398–409. 2019. View Article : Google Scholar : PubMed/NCBI
|