Introduction
Colorectal cancer (CRC) is one of the most common
malignant tumors, leading to ~700,000 cancer-related mortalities
each year worldwide (1). In China,
the International Agency for Research on Cancer reported that CRC
accounts for 6.3% of all malignancy-related mortalities in 2012
(2). Despite advances in
therapeutic methods, almost half of patients with CRC still
experience tumor metastasis or recurrence after treatment, which
causes a poor overall survival (OS) (3). Therefore, elucidation of the potential
mechanisms of CRC proliferation and metastasis is important for
improving the treatment and prognosis of patients with CRC.
MicroRNAs (miRNAs/miRs) are a class of small
non-coding RNAs (18–25 nucleotides in length) that act as
post-transcriptional regulators of gene expression by direct
binding to the 3′ untranslated regions (3′UTRs) of target mRNAs;
this binding induces mRNA degradation and/or translational
repression (4). miRNAs participate
in a number of biological processes, such as cell cycle
progression, proliferation, invasion, migration and cell metabolism
(5–8). The regulatory role of miR-592 is
context-dependent. For instance, miR-592 functions as a tumor
suppressor in breast cancer (9),
non-small cell lung cancer (10)
and glioma (11), but as an
oncogene in gastric cancer (12).
Moreover, contrary results have been observed in CRC. Fu et
al (13) reported an oncogenic
role of miR-592 in CRC by targeting forkhead box O3A (FoxO3A).
However, Liu et al (14)
revealed that miR-592 can inhibit the proliferation of CRC cells by
suppressing cyclin D3 expression.
Therefore, the present study aimed to further verify
miR-592 expression and investigate its regulatory mechanism in
CRC.
Materials and methods
Clinical samples
A total of 35 paired CRC tissues and adjacent normal
tissues (ANTs) were collected from patients who underwent surgical
resection at The Affiliated Huai'an No. 1 People's Hospital of
Nanjing Medical University (Huai'an, China) between January 2017
and June 2019. The healthy tissue was >2 cm away from the CRC
tissue. All samples were confirmed by three pathologists
independently and stored at −80°C until use. The inclusion criteria
for the patients to be enrolled in the study were as follows: i)
Patients with CRC whose histologic slides were identified by two
independent pathologists; and ii) patients with CRC who had not
been treated before surgery, such as chemoradiotherapy. Otherwise,
patients were excluded. The age of patients with CRC ranged between
43 and 76 years old. Written informed consent was obtained from
each participant, and the study was approved by the Ethics
Committees of The Affiliated Huai'an No. 1 People's Hospital of
Nanjing Medical University.
Cell culture and transfection
The normal colonic epithelial cell (FHC) and CRC
cell lines (HCT8, HT29, HCT116, SW480 and SW620) were obtained from
The Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences. Cells were cultured in DMEM that was supplemented with
10% FBS (both from Invitrogen; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin and 100 µg/ml streptomycin in a humidified
atmosphere containing 5% CO2 at 37°C. All cells had been
authenticated using short tandem repeat profiling. As conducted in
previous studies (15,16), the present study performed
subsequent experiments using two cancer cells (HT29 and SW480).
The miR-592 inhibitor and negative control (NC) were
designed by Shanghai GenePharma Co., Ltd., and their corresponding
core sequences were as follows: miR-592 inhibitor,
5′-ACATCATCGCATATTGACACAA-3′ and NC, 5′-TTCTCCGAACGTGTCACGTTTC-3′.
The small interfering (si)RNA of secreted protein acidic and rich
in cysteine (SPARC; siSPARC) was generated from Guangzhou Ribobio
Co., Ltd., and the corresponding sequences were
5′-AACAAGACCUUCGACUCUUCC-3′ (siSPARC) and
5′-GCUCACAGCUCAAUCCUAAUC-3′ (siNC). Both miR-NC and siNC were
non-targeting. The transfection was conducted at a concentration of
100 nM using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C for 6 h according to the
manufacturer's protocol. After transfection for 48 h, subsequent
experimentations were conducted.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was isolated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instruction. RT-qPCR of miR-592 was performed
using a Hairpin-it™ miRNA Normalization RT-PCR Quantitation kit
(Shanghai GenePharma Co., Ltd.), and U6 snRNA was regarded as the
internal control. The following thermocycling conditions of miRNA
RT-qPCR were as follows: Initial denaturation at 95°C for 3 min, 40
cycles of denaturation at 95°C for 15 sec, annealing and elongation
at 62°C for 34 sec. The RT-qPCR primer sequences were as follows:
miR-592 forward, 5′-ACGTTGTGTCAATATGCGATGA-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′; and U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′. cDNA was synthesized using the
PrimeScript RT-PCR kit (Takara Biotechnology Co., Ltd.), and
RT-qPCR for SPARC transcript was performed in 20 µl reactions using
the TB Green® Fast qPCR mix kit (Takara Biotechnology
Co., Ltd.). GAPDH was used as the internal control. The following
thermocycling conditions of mRNA RT-qPCR were as follows: Initial
denaturation at 95°C for 5 min, 40 cycles of denaturation at 95°C
for 10 sec, annealing at 60°C for 30 sec, and elongation at 72°C
for 30 sec. The primers used were as follows: SPARC forward,
5′-GTGCAGAGGAAACCGAAGAG-3′ and reverse, 5′-AGTGGCAGGAAGAGTCGAAG-3′;
and GAPDH forward, 5′-GAGTCAACGGATTTGGTCGT-3′ and reverse,
5′-TTGATTTTGGAGGGATCTCG-3′. Relative miR-592 expression was
measured using the 2−ΔΔCq method (17).
Immunoblotting analysis
CRC cells were washed and lysed in RIPA lysis buffer
(Nanjing KeyGen Biotech Co., Ltd.). Subsequently, the protein was
determined using a BCA protein content detection kit (Nanjing
KeyGen Biotech Co., Ltd.) and boiled for 5 min. Denatured protein
(10 µg/lane) was separated via SDS-PAGE on 10% gel, and then
transferred to PVDF membranes. The membranes were blocked with TBS
with Tween-20 (0.1%) containing 5% skimmed milk at room temperature
for 1 h and subsequently incubated with primary antibodies (rabbit
polyclonal SPARC antibody, 1:1,000, cat. no. 15274-1-AP,
ProteinTech Group, Inc.; and rabbit polyclonal GAPDH antibody,
1:5,000, cat. no. 10494-1-AP, ProteinTech Group, Inc.) overnight at
4°C. The membranes were then incubated with anti-IgG secondary
antibodies conjugated to horseradish peroxidase at room temperature
for 1 h [HRP-conjugated Goat anti-rabbit IgG (H+L); 1:5,000; cat.
no. SA00001-2; ProteinTech Group, Inc.]. Bands were visualized
using the ECL reagent (Nanjing KeyGen Biotech Co., Ltd.).
Plasmid construction and luciferase
reporter assay
Both wild-type (Wt) and mutant (Mut) SPARC 3′UTRs
were amplified via PCR and cloned into the pMIR-Report plasmid
(Ambion; Thermo Fisher Scientific, Inc.). Wt 3′UTRs were achieved
from gDNA and Mut 3′UTRs were chemically synthesized (Shanghai
GeneChem Co., Ltd.). Taq Plus DNA polymerase (Vazyme Biotech Co.,
Ltd.) were used in PCR. The primers used were as follows: Forward,
5′-GAAACTGCCTTCCTGGGTGA-3′ and reverse,
5′-GGAACCATACACTCCCTGTGT-3′. The thermocycling conditions were as
follows: Initial denaturation at 98°C for 5 min, 40 cycles of
denaturation at 98°C for 10 sec, annealing at 60°C for 30 sec, and
elongation at 72°C for 1 min. For the luciferase assay, 293T cells
(The Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences) were cultured in 24-well plates and co-transfected with
plasmids along with miR-592 inhibitor (100 nM) or NC (100 nM) at
37°C for 48 h. Transfection was conducted using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Cells were collected after 48 h, and the
luciferase reporter activity was analyzed using a Dual luciferase
Reporter assay system (Promega Corporation) in which Renilla
luciferase was used for normalization.
Cell Counting Kit (CCK)-8 assay
The proliferative ability of cells was measured
using the CCK-8 assay (Nanjing KeyGen Biotech Co., Ltd.), according
to the manufacturer's instructions. Briefly, HT29 and SW480 cells
were plated into a 96-well plate at a density of 1.0×103
cells/well and incubated at 37°C for 24, 48 and 72 h. Then, the
absorbance was measured at 450 nm on a microplate reader (Infinite
M200 PRO; Tecan Group, Ltd.).
A 5-Ethynyl-2′-deoxyuridine (EdU) incorporation
assay. The EdU incorporation assay was conducted using an EdU DNA
Cell Proliferation kit (Guangzhou RiboBio Co., Ltd.). After
incubation with EdU (1X) 37°C for 2 h, HT29 and SW480 cells were
fixed with paraformaldehyde (4%) at room temperature for 30 min
followed by staining with Apollo Dye Solution at room temperature
for 30 min. Then, the cells were mounted with Hoechst (1X) at room
temperature for 30 min. Finally, cells were imaged and counted
using an Olympus FSX100 fluorescence microscope (Olympus
Corporation) at ×200 magnification.
Migration assay
HT29 and SW480 cells were seeded into 6-well plates
in DMEM without FBS and grown until 90% confluency for 24 h. Then,
the cells were scratched using 200-µl pipette tips and washed twice
with PBS. Next, cells were cultured in an incubator containing 5%
CO2 at 37°C. Images were captured after 0 and 24 h under
an Olympus FSX100 light microscope (Olympus Corporation) at ×200
magnification. Images were subsequently analyzed by ImageJ software
(v1.53; National Institutes of Health).
Transwell invasion assay
The top chambers of Transwell chambers (8-µm pore
size; BD Biosciences) were coated with Matrigel mix (BD
Biosciences) at 37°C for 2 h and 1.5×105 cells were
seeded on the upper chamber containing 100 µl DMEM without FBS.
Then, 500 µl DMEM with 10% FBS was added to the bottom chamber.
After being cultured at 37°C for 24 h, cells that passed through
the Matrigel were fixed with 100% methanol at room temperature for
20 min and stained with crystal violet (0.1%) at room temperature
for 30 min. Finally, cells were counted under an Olympus FSX100
light microscope (Olympus Corporation) at ×200 magnification.
Immunohistochemistry analysis
The tissue samples were fixed with 4%
paraformaldehyde at room temperature for 24 h and embedded in
paraffin. Then, slices (5 µm thickness) were washed twice with
phosphate buffered saline at room temperature for 5 min each time
and incubated with 10% goat serum (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 30 min. Immunohistochemistry analysis
was performed on paraffin-embedded sections using a primary
antibody against SPARC (1:1,000; cat. no. HPA003020; Sigma-Aldrich;
Merck KGaA) overnight at 4°C. After being washed three times,
sections were incubated with a horseradish peroxidase-conjugated
IgG (1:1,000; cat. no. ab7090; Abcam) at room temperature for 1 h.
In total, three high power fields (magnification, ×400) were
randomly selected from CRC tissues using an Olympus FSX100 light
microscope (Olympus Corporation). For histological scoring, the
degree of positivity was initially classified according to scoring
both the proportion of positively stained tumor cells and the
staining intensities. Scores representing the proportion of
positively stained tumor cells were graded as follows: i) 0, ≤10%;
ii) 1, 11–25%; iii) 2, 26–50%; iv) 3, 51–75%; and v) 4, >75%.
The intensity of staining was determined as follows: i) 0, no
staining; ii) 1, weak staining (light yellow); iii) 2, moderate
staining (yellow brown); and iv) 3, strong staining (brown). The
reactivity degree was assessed by ≥2 pathologists
independently.
Immunofluorescence analysis
For the immunofluorescence assay, cells were fixed
in 4% paraformaldehyde at room temperature for 20 min,
permeabilized using 0.5% Triton X-100 at room temperature for 20
min, blocked with 5% goat serum at room temperature for 2 h, and
incubated with a primary antibody against SPARC (1:1,000; cat. no.
HPA003020; Sigma-Aldrich; Merck KGaA) overnight at 4°C. After being
washed three times, cells were incubated with a secondary antibody
(1:500; cat. no. ab150081; Abcam) at room temperature for 1 h.
Coverslips were counterstained with DAPI at room temperature for 5
min and imaged with a confocal laser scanning microscope (Olympus
FV1000; Olympus Corporation) at ×400 magnification.
Bioinformatics analysis
The differentially expressed miRNAs and mRNAs in CRC
deposited in the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) were analyzed
using the online tool GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/). Three GEO
datasets for miRNA expression in CRC (GSE128446, GSE126093 and
GSE125961) (18) were found using
the key words ‘microRNA’ and ‘colorectal cancer’. The putative
targets of miR-592 were identified using the TargetScan (v7.2)
database (http://www.targetscan.org/vert_72/) and the miRDB
database (http://mirdb.org). Gene Ontology (GO)
(19) and Kyoto Encyclopedia of
Genes and Genomes (KEGG) (18)
analyses were conducted on the Database for Annotation,
Visualization and Integrated Discovery website (https://david.ncifcrf.gov). The protein-protein
interaction (PPI) network was constructed using Search Tool for the
Retrieval of Interacting Genes/Proteins (https://string-db.org) and Cytoscape (v3.8.2;
http://cytoscape.org). Analysis of functional
modules and hub genes were explored using the MCODE and Cytohubba
tools in Cytoscape. The expression and location of SPARC protein in
CRC cells (RH-30) revealed by immunohistochemistry and
immunofluorescence were further examined using The Human Protein
Atlas (https://www.proteinatlas.org)
database (20).
Statistical analysis
Data were obtained from three independent
experiments and are presented as the mean ± SD. Statistical
analysis of the difference between groups was performed using SPSS
22.0 (IBM, Corp.) or GraphPad Prism 8.0 (GraphPad Software, Inc.)
using the Student's paired or unpaired t-test or one-way ANOVA,
followed by Tukey's post hoc test. The association between miR-592
expression and the clinicopathological characteristics of patients
with CRC was assessed using the χ2 test. The correlation
between miR-592 expression and SPARC expression was assessed via
Pearson correlation coefficients. The Kaplan-Meier method with the
log-rank test was used to calculate the OS rate. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-592 expression is significantly
elevated in CRC and is associated with the prognosis of
patients
The GEO database was first used to investigate the
expression level of miR-592 in CRC. In total, three GEO datasets
for miRNA expression in CRC (GSE128446, GSE126093, GSE125961)
(18) were found using the key
words ‘microRNA’ and ‘colorectal cancer’. The characteristics of
these GEO datasets are listed in Table
I. As presented in Fig. 1A-C,
miR-592 expression was significantly upregulated in CRC tissues
compared with that in healthy tissues. Moreover, RT-qPCR analysis
demonstrated that miR-592 expression was significantly elevated in
CRC tissues and cell lines compared with normal tissues and FHC
cells, respectively (Fig. 1D and
E). The expression of miR-592 in lymphatic metastatic nodules
was higher compared with that in primary tumor sites (Fig. 1D).
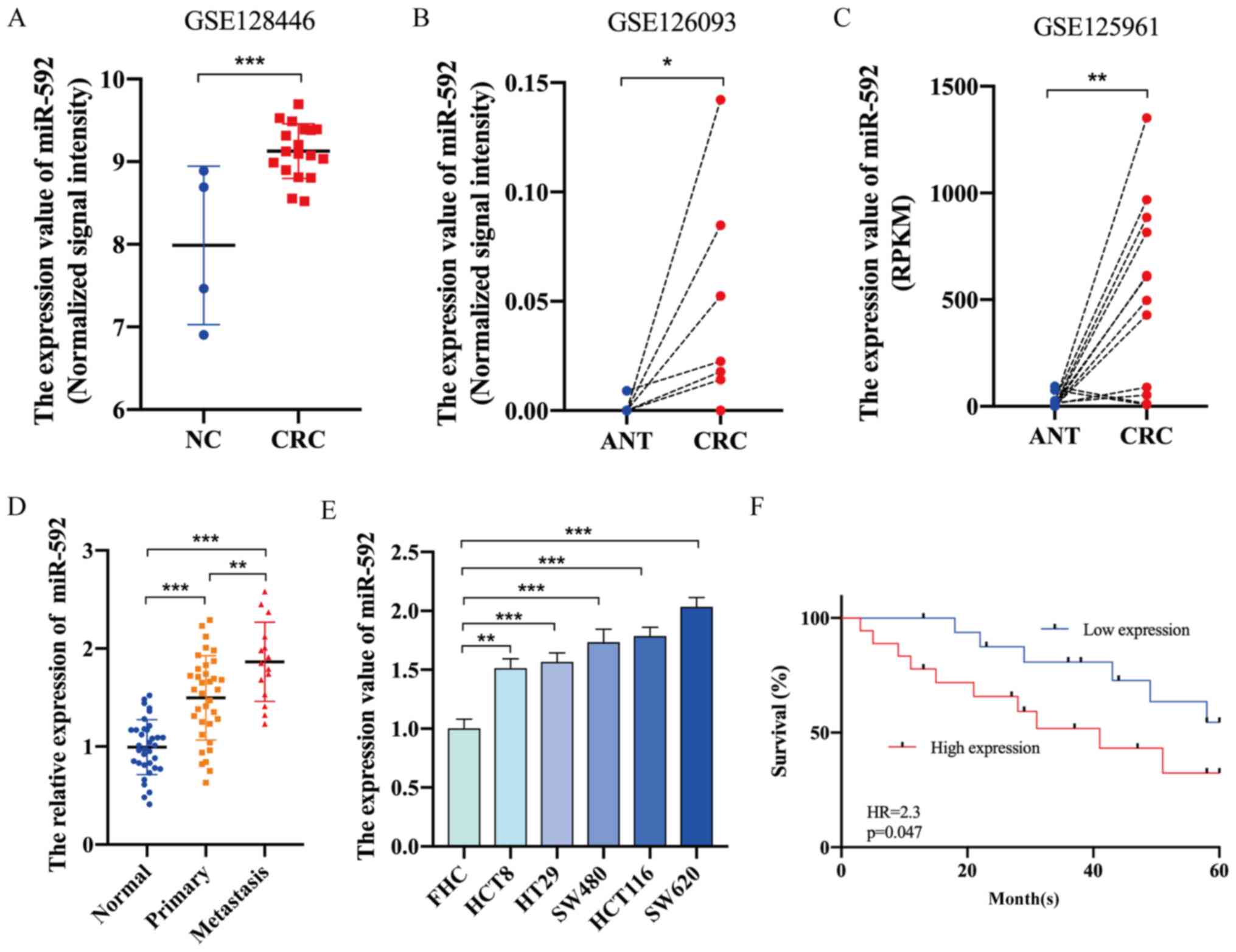 | Figure 1.miR-592 expression is significantly
upregulated in CRC and is associated with the prognosis of
patients. Expression level of miR-592 in CRC tissues and normal
tissues based on analysis of (A) GSE128446, (B) GSE126093 and (C)
GSE125961. (D) miR-592 expression in clinical normal tissues,
primary CRC tissues and metastatic nodules. (E) Expression of
miR-592 in CRC cell lines and FHC cells. (F) Kaplan-Meier curve
analysis of patients with CRC. *P<0.05, **P<0.01,
***P<0.001. NC, negative control; CRC, colorectal cancer; ANT,
adjacent normal tissues; miR, microRNA; HR, hazard ratio; RPKM,
Reads Per Kilobase per Million. |
 | Table I.Detailed characteristics of Gene
Expression Omnibus datasets enrolled in this study. |
Table I.
Detailed characteristics of Gene
Expression Omnibus datasets enrolled in this study.
| Characteristics
Organism | GSE128446 Homo
sapiens | GSE126093 Homo
sapiens | GSE125961 Homo
sapiens | GSE126092 Homo
sapiens |
|---|
| Experiment type | Non-coding RNA
profiling by array | Non-coding RNA
profiling by array | Non-coding RNA
profiling by high throughput sequencing | Expression profiling
by array |
| Platform | GPL14767 | GPL18058 | GPL16791 | GPL121047 |
| Sample size | Four normal tissues
and 18 CRC tissues | 10 paired ANTs and
CRC tissues | 10 paired ANTs and
CRC tissues | 10 paired ANTs and
CRC tissues |
| BioProject | PRJNA527778 | PRJNA521013 | PRJNA518096 | PRJNA521015 |
To assess the association between miR-592 expression
and the clinicopathologic characteristics of patients with CRC,
patients were categorized into the miR-592 high group (n=18) and
low group (n=17) according to the median value of miR-592
expression. As presented in Table
II, miR-592 expression was significantly associated with TNM
stage (P=0.018) and lymphatic metastasis (P=0.041). Furthermore,
Kaplan-Meier survival analysis indicated that patients with CRC
with higher miR-592 expression may have shorter OS times (hazard
ratio =2.3; P=0.047) (Fig. 1F).
 | Table II.Associations between the
clinicopathological characteristics of patients with colorectal
cancer and miR-592 expression levels. |
Table II.
Associations between the
clinicopathological characteristics of patients with colorectal
cancer and miR-592 expression levels.
|
|
| miR-592
expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Number | High | Low | P-value |
|---|
| Sex |
|
Male | 24 | 13 | 11 |
|
|
Female | 11 | 5 | 6 | 0.730 |
| Age, year |
|
>60 | 23 | 12 | 11 |
|
|
≤60 | 12 | 6 | 6 | 0.900 |
| TNM stage |
| I +
II | 17 | 5 | 12 |
|
| III +
IV | 18 | 13 | 5 | 0.018 |
| Histology
grade |
|
Well | 12 | 5 | 7 |
|
|
Moderate + poor | 23 | 13 | 10 | 0.320 |
| Lymphatic
metastasis |
| No | 20 | 8 | 12 |
|
|
Yes | 15 | 10 | 5 | 0.041 |
| Tumor size, cm |
|
≤4.5 | 19 | 7 | 12 |
|
|
>4.5 | 16 | 11 | 5 | 0.092 |
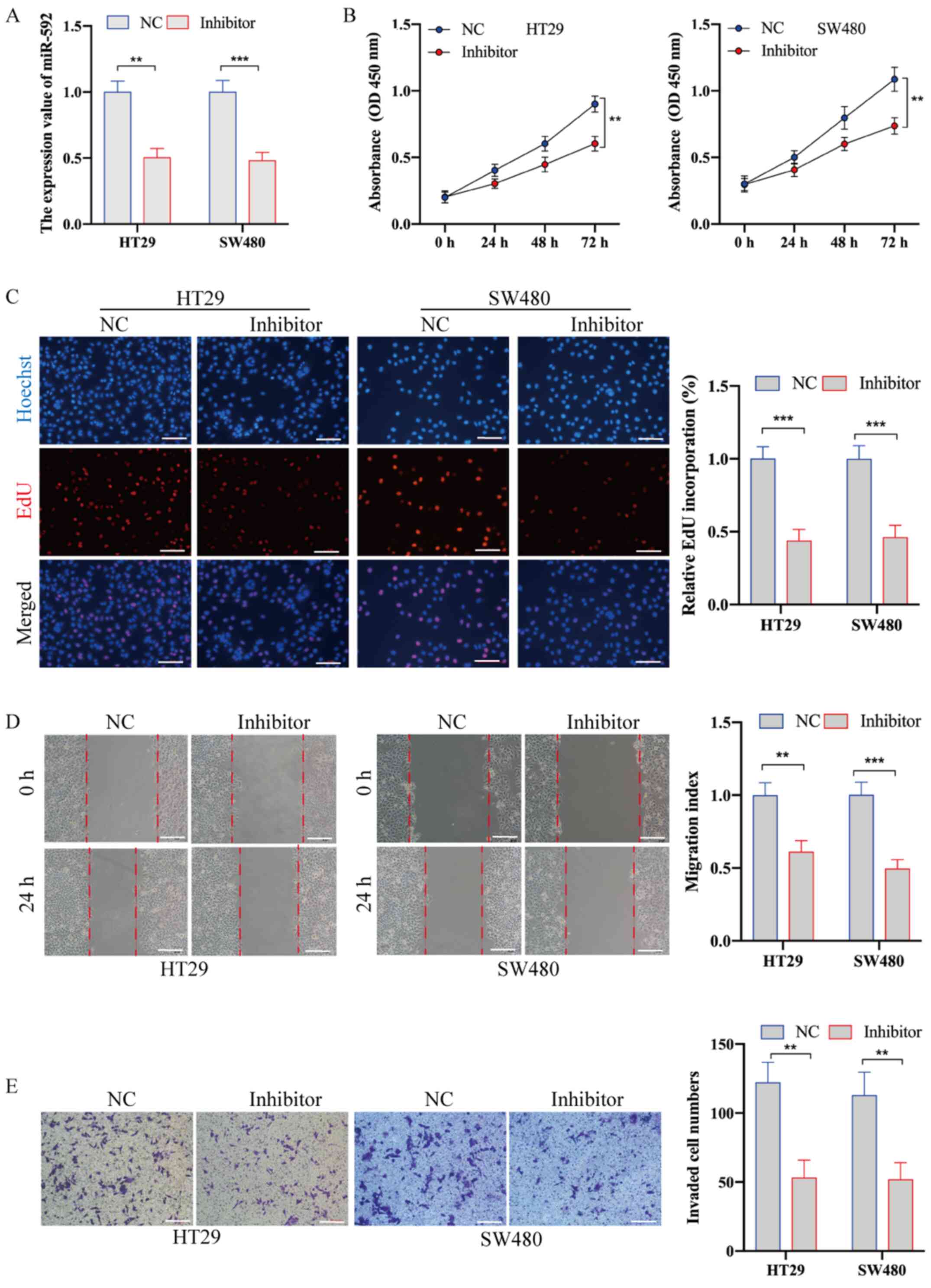
miR-592 knockdown suppresses the
proliferation, migration and metastasis of CRC cells
To examine the biological role of miR-490-3p in CRC,
HT29 and SW480 cells were transfected with miR-592 inhibitor and
NC. RT-qPCR analysis was used to confirm the transfection
efficiency (Fig. 2A). The CCK-8 and
EdU incorporation assays demonstrated that miR-592 knockdown
significantly inhibited the proliferative ability of CRC cells
(Fig. 2B and C). In addition, the
wound healing assay identified that, compared with NC transfection,
miR-592 inhibitor transfection significantly decreased cell
migration (Fig. 2D). The Transwell
invasion assay also demonstrated that the invasive ability of CRC
cells transfected with inhibitor was significantly decreased
compared with that of cells transfected with NC (Fig. 2E).
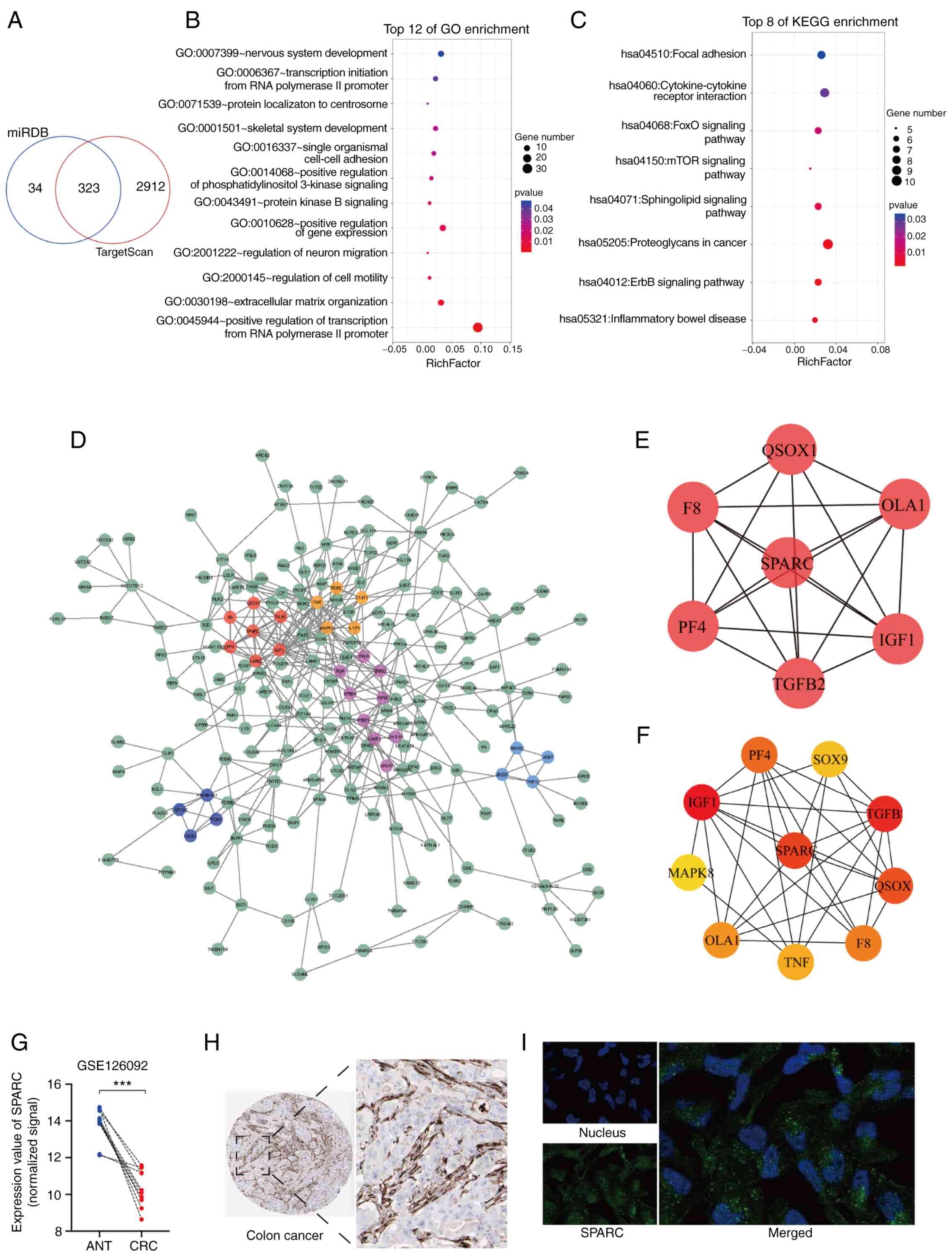
Analysis of putative targets of
miR-592
To identify the targets of miR-592, the current
study first overlapped predicted results searched in the TargetScan
and miRDB algorithms, and 323 putative targets were found (Fig. 3A). GO analysis of these targets
indicated that miR-592 participated in various biological
processes, such as ‘positive regulation of transcription’,
‘extracellular matrix organization’ and ‘regulation of cell
motility’ (Fig. 3B). KEGG analysis
identified that the targets of miR-592 were significantly
associated with several signaling pathways, including the ‘ErbB
signaling pathway’, ‘mTOR signaling pathway’ and ‘FoxO signaling
pathway’ (Fig. 3C). In addition, a
PPI network of miR-592 targets was constructed, among which five
functional modules were discovered using the MCODE tool (Fig. 3D). Cytohubba analysis revealed seven
hub genes among the PPI network (Fig.
3E).
It has been reported that one miRNA may regulate the
expression of multiple genes and vice versa (21). Considering the upregulation of
miR-592 in CRC, it was suggested that these targets with
downregulated expression in CRC may be regulated by miR-592. It was
found that SPARC, one of the hub genes, was significantly
downregulated in CRC tissues compared with that in ANTs based on
GSE126092 analysis (Fig. 3G).
However, the expression of the other six hub genes was either
upregulated or undifferentiated in CRC tissues compared with ANTs
(data not shown). Additionally, SPARC belonged to a functional
module in the PPI network (Fig.
3F). Immunohistochemistry analysis demonstrated that SPARC was
barely expressed in CRC cells but was highly enriched in the tumor
microenvironment (Fig. 3H). In
addition, immunofluorescence analysis identified that SPARC was
mainly located in the vesicles of RH-30 tumor cells (Fig. 3I).
SPARC is a direct target of miR-592 in
CRC
To identify SPARC as the direct target of miR-592 in
CRC, its expression was firstly evaluated. RT-qPCR analysis
demonstrated that SPARC expression was significantly downregulated
in CRC tissues and cell lines compared with ANTs and FHC cells,
respectively (Fig. 4A and B).
Furthermore, a weak negative correlation was identified between
miR-592 expression and SPARC expression in CRC tissues (Fig. 4C). When the miR-592 inhibitor was
transfected into HT29 and SW480 cells, the expression of SPARC at
both the transcriptional and protein levels was significantly
upregulated in CRC cells (Fig. 4D and
E). Moreover, the direct regulatory role of miR-592 on SPARC
expression was assessed using a dual-luciferase assay, in which
Renilla luciferase was used for normalization. Interestingly, the
SPARC 3′UTR sequence had two binding sites of miR-592 with a high
conserved score. Therefore, several luciferase reporter plasmids
were constructed with Wt and Mut1-3 SPARC 3′UTRs (Fig. 4F). As presented in Fig. 4G, after co-transfecting with miR-592
inhibitor, the luciferase activity was significantly elevated in
luciferase reporter groups with Wt and Mut1-2 SPARC 3′UTRs, but not
in the luciferase reporter group with Mut3 SPARC 3′UTRs, which
suggested that miR-592 can directly bind to two sites in SPARC
3′UTRs.
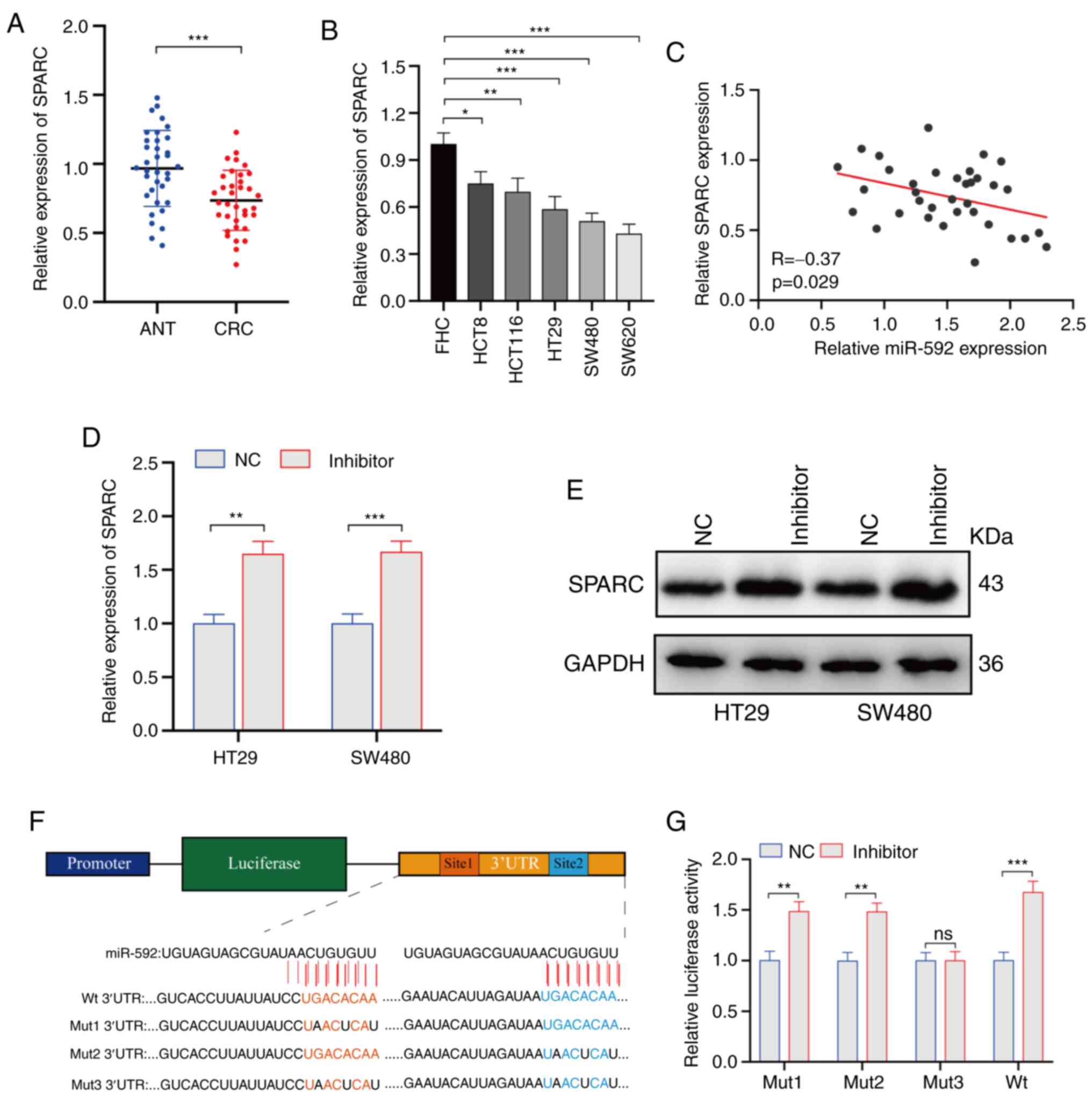 | Figure 4.Identifying SPARC as a direct target
of miR-592 in CRC. (A) Expression of miR-592 in clinical CRC
tissues and ANTs. (B) Expression of miR-592 in CRC cell lines and
FHC cells. (C) Correlation between miR-592 expression and SPARC
expression in CRC tissues. (D) Transfection effect of the miR-592
inhibitor on SPARC expression in CRC cells, (E) as determined via
western blotting. (F) Construction of luciferase reporter plasmids
with Wt or Mut binding sites. (G) Luciferase activity of luciferase
reporter plasmids co-transfected with miR-592 inhibitor or NC.
*P<0.05, **P<0.01, ***P<0.001. NC, negative control; CRC,
colorectal cancer; Wt, wild-type; Mut, mutant; UTR, untranslated
region; ANT, adjacent normal tissues; miR, microRNA; SPARC,
secreted protein acidic and rich in cysteine; ns, not
significant. |
Silencing SPARC significantly reverses
the effect of miR-592 knockdown on CRC cells
To investigate the regulatory role of SPARC in CRC,
SPARC expression was inhibited by transfecting cells with siSPARC.
Western blotting results demonstrated that siSPARC effectively
suppressed SPARC expression (Fig.
5A). The cellular phenotypes indicated that SPARC knockdown
significantly promoted the proliferation, migration and invasion of
CRC cells (Fig. 5B-E).
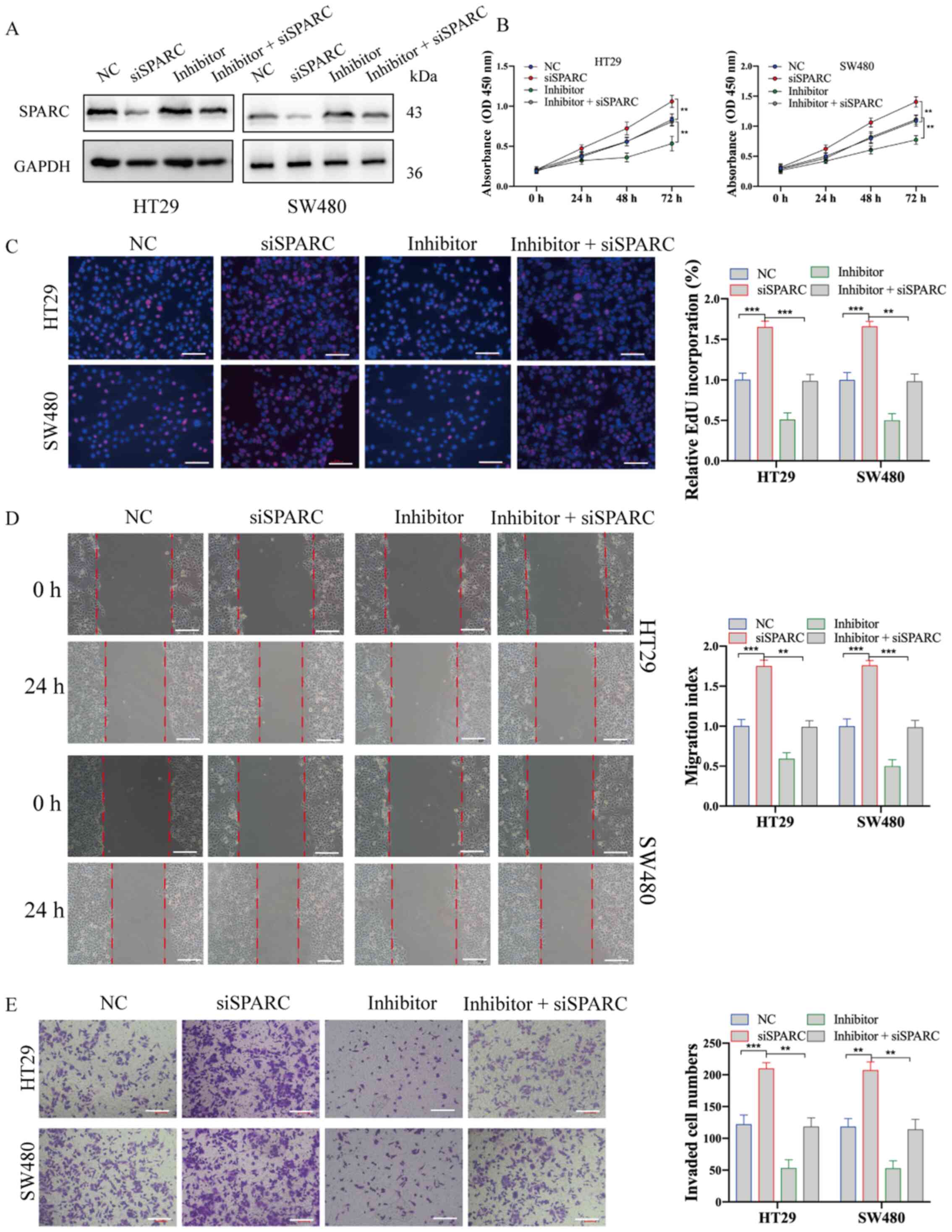 | Figure 5.Knockdown of SPARC significantly
reverses the effect of miR-592 knockdown on CRC cells. (A)
Transfection effect of siSPARC and miR-592 inhibitor on SPARC
expression in CRC cells. (B) Cell Counting Kit-8 and (C)
incorporation assays demonstrated that siSPARC significantly
promoted CRC cell proliferation, but co-transfecting with the
miR-592 inhibitor reversed such effects. (D) Wound healing assay
results indicated that siSPARC significantly promoted CRC cell
migration, but co-transfecting with miR-592 inhibitor reversed
these effects. (E) Transwell invasion assay results identified that
siSPARC significantly promoted CRC cell invasion, but
co-transfecting with miR-592 inhibitor reversed such effects. Scale
bar, 100 µm. **P<0.01, ***P<0.001. NC, negative control; CRC,
colorectal cancer; si, small interfering RNA; miR, microRNA; OD,
optical density; SPARC, secreted protein acidic and rich in
cysteine; EdU, 5-Ethynyl-2′-deoxyuridine |
Subsequently, CRC cells were co-transfected with
siSPARC and miR-592 inhibitor to investigate whether the oncogenic
role of miR-592 was directly mediated by SPARC inhibition (Fig. 5A). The CCK-8 and EdU incorporation
assays suggested that SPARC knockdown significantly reversed the
effect of miR-592 knockdown on cell proliferation (Fig. 5B and C). Consistently, the migration
and Transwell invasion assays identified that both the migratory
and invasive abilities, which were suppressed by miR-592 knockdown,
were restored after SPARC knockdown in HT29 and SW480 cells
(Fig. 5D and E).
Discussion
Dysregulated miR-592 has been reported to serve a
vital role in the malignant progression of multiple cancer types,
such as hepatocellular carcinoma and prostate cancer (22–24).
Liu et al (25) revealed
that miR-592 expression was upregulated in clinical CRC tissues.
Consistently, the present study demonstrated that miR-592
expression was significantly upregulated in CRC tissues and cell
lines compared with ANTs and FHC cells, respectively. Furthermore,
patients with CRC with higher miR-592 expression had a poorer
prognosis compared with those with low expression. Fu et al
(13) reported that miR-592 can
promote the proliferation and metastasis of CRC cells, in part, by
targeting FoxO3A. In the present study, SPARC was identified as
another target of miR-592 in CRC. Moreover, it was found that
miR-592 acted as an oncogene by directly suppressing SPARC
expression in CRC. Liu et al (25) also discovered that serum miR-592
expression was elevated in patients with CRC compared with healthy
individuals and was significantly decreased after radical surgery.
Therefore, it was hypothesized that CRC cells may secrete
intracellular miR-592 into a circulating system, which offers a
novel potential diagnostic biomarker of CRC. However, a contrary
report regarding the low expression of miR-592 in CRC was
previously published (14), and it
was suggested that this phenomenon may be attributed to the
heterogeneity of CRC tissues and the small sample size used in this
previous study.
The promoter of SPARC has been reported to be
epigenetically hypermethylated, which results in decreased SPARC
expression in CRC (26). The
present study demonstrated that SPARC expression was also
controlled by miRNA at the post-transcription level. Furthermore,
the immunohistochemistry analysis identified that SPARC was highly
enriched in the tumor microenvironment. In line with the current
findings, SPARC was previously found to be upregulated in the tumor
stroma, and fibroblast-derived SPARC can promote the invasiveness
of CRC cells (27). Interestingly,
the immunofluorescence analysis indicated that SPARC was mainly
located in the vesicles of RH-30 tumor cells. Emerging evidence has
revealed that cancer cells can release cellular RNAs into
peripheral blood through vesicles, such as exosomes (28,29).
Therefore, it was hypothesized that SPARC may also be located in
the vesicles of CRC cells and that the downregulation of SPARC in
CRC cells may also be attributed to their enrichment in secretory
vehicles.
There are several limitations in the present study.
First, the number of clinical samples was relatively small. Second,
the current study did not further investigate the expression or
regulatory role of miR-592 and SPARC in the tumor microenvironment.
Third, the association between SPARC and secretory vehicles in CRC
will be examined in future studies.
In conclusion, the present findings demonstrated
that miR-592 expression was significantly upregulated in CRC, and
that miR-592 overexpression contributed to the promotion of cell
proliferation, migration and metastasis by directly suppressing
SPARC expression, which provides a novel potential therapeutic
target for patients with CRC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The GEO datasets analyzed during the current study are
available in the GEO repository, (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE128446;
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE126093;
http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE125961).
Authors' contributions
CG and ZP designed the present study and drafted the
initial manuscript. RX and WS acquired the clinical samples and
performed RT-qPCR. Both RX and ZP performed the statistical
analysis. CG and WS assessed the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research and
Ethical Committee at The Affiliated Huai'an No. 1 People's Hospital
of Nanjing Medical University, and written informed consent was
provided by all patients before the start of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brody H: Colorectal cancer. Nature.
521:S12015. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mai D, Ding P, Tan L, Zhang J, Pan Z, Bai
R, Li C, Li M, Zhou Y, Tan W, et al: PIWI-interacting RNA-54265 is
oncogenic and a potential therapeutic target in colorectal
adenocarcinoma. Theranostics. 8:5213–5230. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dalmay T: Mechanism of miRNA-mediated
repression of mRNA translation. Essays Biochem. 54:29–38. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao H, Zheng C, Wang Y, Hou K, Yang X,
Cheng Y, Che X, Xie S, Wang S, Zhang T, et al: miR-1323 promotes
cell migration in lung adenocarcinoma by targeting Cbl-b and is an
early prognostic biomarker. Front Oncol. 10:1812020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Molist C, Navarro N, Giralt I, Zarzosa P,
Gallo-Oller G, Pons G, Magdaleno A, Moreno L, Guillén G, Hladun R,
et al: miRNA-7 and miRNA-324-5p regulate alpha9-Integrin expression
and exert anti-oncogenic effects in rhabdomyosarcoma. Cancer Lett.
477:49–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gharib E, Nasri Nasrabadi P and Reza Zali
M: miR-497-5p mediates starvation-induced death in colon cancer
cells by targeting acyl-CoA synthetase-5 and modulation of lipid
metabolism. J Cell Physiol. 235:5570–5589. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu X, He B, Xu T, Pan Y, Hu X, Chen X and
Wang S: MiR-490-3p functions as a tumor suppressor by inhibiting
oncogene VDAC1 expression in colorectal cancer. J Cancer.
9:1218–1230. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hou W, Zhang H, Bai X, Liu X, Yu Y, Song L
and Du Y: Suppressive role of miR-592 in breast cancer by
repressing TGF-β2. Oncol Rep. 38:3447–3454. 2017.PubMed/NCBI
|
|
10
|
Li Z, Li B, Niu L and Ge L: miR-592
functions as a tumor suppressor in human non-small cell lung cancer
by targeting SOX9. Oncol Rep. 37:297–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao S, Chen J, Wang Y, Zhong Y, Dai Q,
Wang Q and Tu J: MiR-592 suppresses the development of glioma by
regulating Rho-associated protein kinase. Neuroreport.
29:1391–1399. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He Y, Ge Y, Jiang M, Zhou J, Luo D, Fan H,
Shi L, Lin L and Yang L: MiR-592 promotes gastric cancer
proliferation, migration, and invasion through the PI3K/AKT and
MAPK/ERK signaling pathways by targeting Spry2. Cell Physiol
Biochem. 47:1465–1481. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu Q, Du Y, Yang C, Zhang D, Zhang N, Liu
X, Cho WC and Yang Y: An oncogenic role of miR-592 in tumorigenesis
of human colorectal cancer by targeting Forkhead Box O3A (FoxO3A).
Expert Opin Ther Targets. 20:771–782. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Z, Wu R, Li G, Sun P, Xu Q and Liu Z:
MiR-592 inhibited cell proliferation of human colorectal cancer
cells by suppressing of CCND3 expression. Int J Clin Exp Med.
8:3490–3497. 2015.PubMed/NCBI
|
|
15
|
Cheng L, Xing Z, Zhang P and Xu W: Long
non-coding RNA LINC00662 promotes proliferation and migration of
breast cancer cells via regulating the miR-497-5p/EglN2 axis. Acta
Biochim Pol. 67:229–237. 2020.PubMed/NCBI
|
|
16
|
Chen Y, Wu N, Liu L, Dong H and Liu X:
microRNA-128-3p overexpression inhibits breast cancer stem cell
characteristics through suppression of Wnt signalling pathway by
down-regulating NEK2. J Cell Mol Med. 24:7353–7369. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Z, Ren R, Wan D, Wang Y, Xue X, Jiang
M, Shen J, Han Y, Liu F, Shi J, et al: Hsa_circ_101555 functions as
a competing endogenous RNA of miR-597-5p to promote colorectal
cancer progression. Oncogene. 38:6017–6034. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Colwill K and Gräslund S; Renewable
Protein Binder Working Group, : A roadmap to generate renewable
protein binders to the human proteome. Nat Methods. 8:551–558.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Slattery ML, Herrick JS, Mullany LE,
Samowitz WS, Sevens JR, Sakoda L and Wolff RK: The co-regulatory
networks of tumor suppressor genes, oncogenes, and miRNAs in
colorectal cancer. Genes Chromosomes Cancer. 56:769–787. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang W, Zhang H, Tang M, Liu L, Zhou Z,
Zhang S and Wang L: MicroRNA-592 targets IGF-1R to suppress
cellular proliferation, migration and invasion in hepatocellular
carcinoma. Oncol Lett. 13:3522–3528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia YY, Zhao JY, Li BL, Gao K, Song Y, Liu
MY, Yang XJ, Xue Y, Wen AD and Shi L: miR-592/WSB1/HIF-1α axis
inhibits glycolytic metabolism to decrease hepatocellular carcinoma
growth. Oncotarget. 7:35257–35269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lv Z, Rao P and Li W: MiR-592 represses
FOXO3 expression and promotes the proliferation of prostate cancer
cells. Int J Clin Exp Med. 8:15246–15253. 2015.PubMed/NCBI
|
|
25
|
Liu M, Zhi Q, Wang W, Zhang Q, Fang T and
Ma Q: Up-regulation of miR-592 correlates with tumor progression
and poor prognosis in patients with colorectal cancer. Biomed
Pharmacother. 69:214–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheetham S, Tang MJ, Mesak F, Kennecke H,
Owen D and Tai IT: SPARC promoter hypermethylation in colorectal
cancers can be reversed by 5-Aza-2′deoxycytidine to increase SPARC
expression and improve therapy response. Br J Cancer. 98:1810–1819.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Drev D, Harpain F, Beer A, Stift A, Gruber
ES, Klimpfinger M, Thalhammer S, Reti A, Kenner L, Bergmann M, et
al: Impact of fibroblast-derived SPARC on invasiveness of
colorectal cancer cells. Cancers (Basel). 11:112019. View Article : Google Scholar
|
|
28
|
Deng Z, Wu J, Xu S, Chen F, Zhang Z, Jin A
and Wang J: Exosomes-microRNAs interacted with gastric cancer and
its microenvironment: A mini literature review. Biomarkers Med.
14:141–150. 2020. View Article : Google Scholar
|
|
29
|
Kim SM, Yang Y, Oh SJ, Hong Y, Seo M and
Jang M: Cancer-derived exosomes as a delivery platform of
CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J
Control Release. 266:8–16. 2017. View Article : Google Scholar : PubMed/NCBI
|