Introduction
Medicinal plants and their derivatives have emerged
as functional food materials globally because they contain diverse
bioactive phytochemicals with potential immunomodulatory effects on
the human body. Therefore, the demand for functional foods and
beverages has rapidly increased (1,2).
Active ingredients in functional foods include polyunsaturated
fatty acids, peptides, flavonoids, and phenolic compounds, which
help prevent obesity, diabetes, and cancer (3,4).
Cirsium setidens (Dunn) Nakai, a wild
perennial herb popularly known as gondre, is mainly found in the
Kangwon province of South Korea (5). Its extract is commonly used as a food
product in Korea, China, Japan, Canada, USA, and Australia
(6). All parts of Cirsium
spp., including roots, are enriched in phytochemicals, such as
flavonoids, phenolics, sterols, and alkaloids, and utilized in the
treatment of liver and kidney inflammation and bleeding, as well as
other hepatic disorders (7–9). In addition, the aerial parts of C.
chanroenicum are used to treat fever and to improve
detoxification and blood circulation, in Chinese medicine (10). Leaves and stems are rich sources of
calcium, proteins, and vitamins. In the case of gondre,
pectolinarin, pectolinarigenin, apigenin, and luteolin are the
essential phytochemicals that can regulate various physiological
activities (11). Pectolinarin has
anti-tumor, anti-cancer, anti-oxidant, anti-inflammatory, and
hepatoprotective properties, whereas pectolinarigenin has
anti-oxidant and anti-inflammatory effects (12–14).
Stem cells are considered a promising source for
regenerative medicine and tissue engineering, because of their
multi-lineage differentiation potential and self-renewal properties
(15,16). For instance, human mesenchymal stem
cells (hMSCs) are used in cell-based therapies to treat various
diseases. Nevertheless, microenvironment conditions, such as
oxidative stress, low oxygen level, inflammation, and limited
nutrient supply, restrict clinical trials of stem cells (17).
Human dental mesenchymal stem cells can be obtained
from different dental tissues, such as the periodontal ligament,
dental pulp, periapical follicle, and apical papilla. These cells
have the potential for multi-lineage differentiation, and can be
exploited to regenerate a desired cell type for tissue engineering
applications (18,19).
This study aimed to investigate the differentiation
potential of human periodontal ligament stem cells (hPDLSCs) in the
presence of gondre powder. Thus, a methanol extraction was
performed to determine the major bioactive components of the gondre
powder, and the extracted material was analyzed with nuclear
magnetic resonance (NMR) and matrix-assisted laser
deposition/ionization (time-of-flight) MALDI-TOF mass spectrometry.
The results indicated that pectolinarin is the chief component of
the gondre extract. Enhanced mineralization occurred in hPDLSCs in
the presence of gondre powder via the activation of
osteogenesis-related genes and proteins. Therefore, gondre can be
used as a functional food material for improving metabolism and
cellular homeostasis.
Materials and methods
Extraction and characterization of the
methanol extract of C. setidens
The gondre powder was obtained from the Department
of Food Science and Biotechnology, Kangwon National University,
Republic of Korea. The extraction of bioactive phytochemicals was
performed as described by Jeong et al (11). Briefly, methanol was added to
sufficient quantities of dried gondre powder at room temperature,
with continuous mechanical stirring for 4 h. After that, the
mixture was filtered to remove undesired particles, and repeated
extraction was performed three times. The obtained solution was
centrifuged (4,000 × g/10°C) for 30 min, and the supernatant was
separated for further analysis. The chemical structures of the
phytochemicals present in the methanol extract were elucidated with
1H-NMR (JNM-ECZ400S/L1) in DMSO-d6 solvent,
and MALDI-TOF mass spectrometry (Bruker Autoflex speed
TOF/TOF).
Culture of hPDLSCs
Human third molars were collected from three young
males (18–22 years). This protocol was approved by the
Institutional Review Board of the Dental Hospital, Seoul National
University (Seoul, Republic of Korea; IRB Number 05004), and
written consent was obtained from each patient. The primary cell
culture was established according to the procedure described by Jin
and Choung (20). In brief, hPDLSCs
were gradually separated from the extracted third molar and treated
with a solution of 3 mg/ml collagenase type 1 (Worthington Biochem)
and 4 mg/ml dispase (Boehringer-Mannheim) at 37°C for 1 h. The cell
suspension was obtained by passing the solution through a 40-µm
strainer (Falcon-BD Labware). The derived cells were cultured in
alpha-modified Eagle's medium (α-MEM, Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), 100 µmol/l ascorbic acid
2-phosphate (Sigma-Aldrich; Merck KGaA), 2 mM glutamine, 100 U/ml
penicillin, and 100 µg/ml streptomycin (Biofluids), and incubated
at 37°C in 5% CO2. The medium was changed after 24 h,
followed by changes every 3–4 days. Only primary cells at passages
2 or 3 were used for the proliferation and differentiation
studies.
Flow cytometry analysis
The expression of mesenchymal stem cell-associated
surface markers at passage 3 was analyzed with flow cytometry, to
characterize the immunophenotype. Approximately 1×106
cells were fixed with 3.7% paraformaldehyde (Sigma-Aldrich; Merck
KGaA) for 10 min and then re-suspended in phosphate-buffered saline
(PBS) solution (Welegene) containing 1% bovine serum albumin (BSA)
(ICN Biomedicals), for 30 min to block the nonspecific
antibody-binding sites. Next, the cells were incubated with
specific antibodies against CD34, CD13, CD90, and CD146 at 4°C for
1 h, followed by incubation with fluorescent secondary antibodies
at room temperature for 1 h. All antibodies were purchased from BD
Biosciences. The percentages of CD13, CD90, and CD146-positive, as
well as CD34-negative cells, were measured with a
fluorescence-activated cell sorting (FACS) caliber flow cytometer
(Becton Dickinson Immunocytometry Systems). The data were analyzed
using the Cell Quest Pro software (BD Biosciences).
Cytotoxicity and migration assay
Cell proliferation and cytotoxicity were measured
using the colorimetric
3-(4,5-dimethylthazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay kit (Promega Corp.). Briefly, the cells were seeded in
96-well plates (1×104 cells per well), cultured for the
desired periods, and then treated with various concentrations of
gondre powder (0.01, 0.05, 0.1, 0.2, and 0.25%) for the required
time periods. These concentrations have proliferative effects, as
reported earlier (21). Medium
without gondre was considered a negative control. The dye solution
(15 µl) was added at the end of the treatment, followed by further
incubation in 5% CO2 at 37°C for 4 h. The solubilized
formazan product was obtained by adding 100 µl of a
solubilization/stop solution in the media. The formed products were
quantitated using an ELISA plate reader at 595 nm (with readings at
655 nm as reference). All experiments were performed in triplicate,
and values are expressed as mean ± standard deviation (SD).
We performed migration assays to assess the defect
healing potential of hPDLSCs in the presence of gondre. To this
end, cells were seeded in 6-well plates and allowed to grow to
90–100% confluence. Next, the cell monolayer was wounded with a
plastic tip (1 mm), and washed with PBS (twice) to remove cell
debris. Scratched cells were incubated either with or without
gondre. Cell migration towards the wounded area was monitored after
time intervals of 0, 12, 24 and 48 h, using a light microscope
(Olympus U-SPT; Olympus Corporation). Cell migration was quantified
by measuring the distance traveled by migrating cells from the
wound edge (starting point at t=0) to the furthest migration
point.
Multi-lineage differentiation of
hPDLSCs
The cells were cultured in osteogenic, chondrogenic,
adipogenic, and neurogenic differentiation media for 21 days
(Gibco; Thermo Fisher Scientific, Inc.) to examine the osteogenic,
chondrogenic, adipogenic, and neurogenic differentiation potential
of hPDLSCs, respectively, when stimulated with an appropriate
supplement. After 21 days of treatment, cells were stained with 2%
alizarin red S stain (ARS) at pH 4.2, 1% alcian blue, 0.3% oil red
O dye (all three from Sigma-Aldrich; Merck KGaA), and Nissl stain.
Stained cells were visualized under an inverted light microscope to
detect the calcified matrix, proteoglycans, fat vacuoles, and Nissl
bodies, which are indicators of osteogenic, chondrogenic,
adipogenic, and neurogenic differentiation, respectively.
In vitro differentiation and
mineralization
Cells (4×104) were cultured in a 24-well
plate with α-MEM containing 10% FBS until they reached 50% to 60%
confluency. The cells were fixed with 10% formalin solution (Duksan
Chemical Co., Gyeonggi-do), followed by an incubation with 0.1%
Triton X-100 for 5 min. Then, the incubated cells were stained with
a leukocyte ALP kit (Sigma-Aldrich; Merck KGaA) according to the
manufacturer's protocols. Mineralized nodules were detected by
staining with 2% ARS at pH 4.2 on treatment day 14. For
mineralization, cells were cultured in an osteogenic
differentiation medium with 50 µg/ml ascorbic acid, 10 mM
β-glycerophosphate, and 100 nM dexamethasone (Sigma-Aldrich; Merck
KGaA) for 14 days.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
We utilized RT-qPCR to evaluate the expression of
osteogenesis-related genes in hPDLSCs (22,23).
Briefly, cells (1×106) were cultured in a 60-mm culture
dish for 2 weeks under differentiation induction conditions, and
RNA was isolated from the treated cells using an RNeasy Mini kit
(Qiagen) according to the manufacturer's instructions. Next, cDNA
was synthesized from 2 µg of total RNA using reverse transcriptase
(Superscript II Preamplification System; Invitrogen; Thermo Fisher
Scientific, Inc.). SYBR-Green PCR Master Mix (ABI Prism 7500;
Applied Biosystems; Thermo Fisher Scientific, Inc.) was used for
RT-qPCR. The experiment conditions were as follows: 40 cycles of
denaturation for 15 sec at 95°C and 1 min of amplification at 60°C.
All reactions were run in triplicate, and normalized to the
housekeeping gene hypoxanthine-guanine phosphoribosyl transferase
(HPRT). The cycle threshold values were calculated and compared to
assess gene expression levels in control and gondre-treated groups.
The relative mRNA expression levels in hPDLSCs and their
gondre-treated counterparts were plotted in a histogram. We
evaluated the expression levels of collagen 1 (Col1), runt-related
transcription factor 2 (Runx2), bone sialoprotein (BSP), and
alkaline phosphatase (ALP) and HPRT. The specific primer sets used
for this analysis are listed in Table
I.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | GenBank no. | Sequences |
|---|
| Col1 | NM007742 | F:
5′-GCTCCTCTTAGGGGCCACT-3′ |
|
|
| R:
5′-CCACGTCTCACCATTGGGG-3′ |
| Runx2 | NM_001146038 | F:
5′-CGCACGACAACCGCACCAT-3′ |
|
|
| R:
5′-CAGCACGGAGCACAGGAAGTT-3′ |
| BSP | L09555 | F:
5′-AACTTTTATGTCCCCCGTTGA-3′ |
|
|
| R:
5′-TGGACTGGAAACCGTTTCAGA-3′ |
| ALP | NM007431 | F:
5′-CCAACTCTTTTGTGCCAGAGA-3′ |
|
|
| R:
5′-GGCTACATTGGTGTTGAGCTTTT-3′ |
| HPRT | NM_000194 | F:
5′-GGCTATAAGTTCTTTGCTGACCTG-3′ |
|
|
| R:
5′-CCACAGGGACTAGAACACCTGCTA-3′ |
Western blot analysis
Gondre-induced protein expression levels were
determined with western blotting. For this, cells were lysed with
RIPA buffer containing 1 mM phenylmethylsulfonyl fluoride,
centrifuged, and collected in clean Eppendorf tubes. Protein
concentration was analyzed using the BSA protein assay kit (Bio-Rad
Laboratories, Inc.). An equal volume of protein (25 µg) was
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to a polyvinylidene difluoride
membrane (GE Healthcare). Primary antibodies against Runx2 and
osterix (OSX) were purchased from Abcam. The blots were developed
using horseradish peroxidase-conjugated secondary antibodies (Cell
Signaling Technology, Inc.), and visualized using a
gel-documentation imaging system (Chemi-Doc XRS+Imaging System;
Bio-Rad Laboratories, Inc.). Blots were quantified using the ImageJ
software (ImageJ v1.8, NIH), and normalized to α-tubulin.
Statistical analysis
Statistical analysis was performed with one-way
ANOVA using the Origin Pro 9.0 software. All experiments were
performed in triplicate (n=3), and the results are expressed as
mean OD ± SD. To compare significant differences between control
and experimental groups, Tukey's post hoc analysis was performed.
P<0.05 was considered to indicate a statistically significant
difference.
Results and Discussion
Characterization of the extract
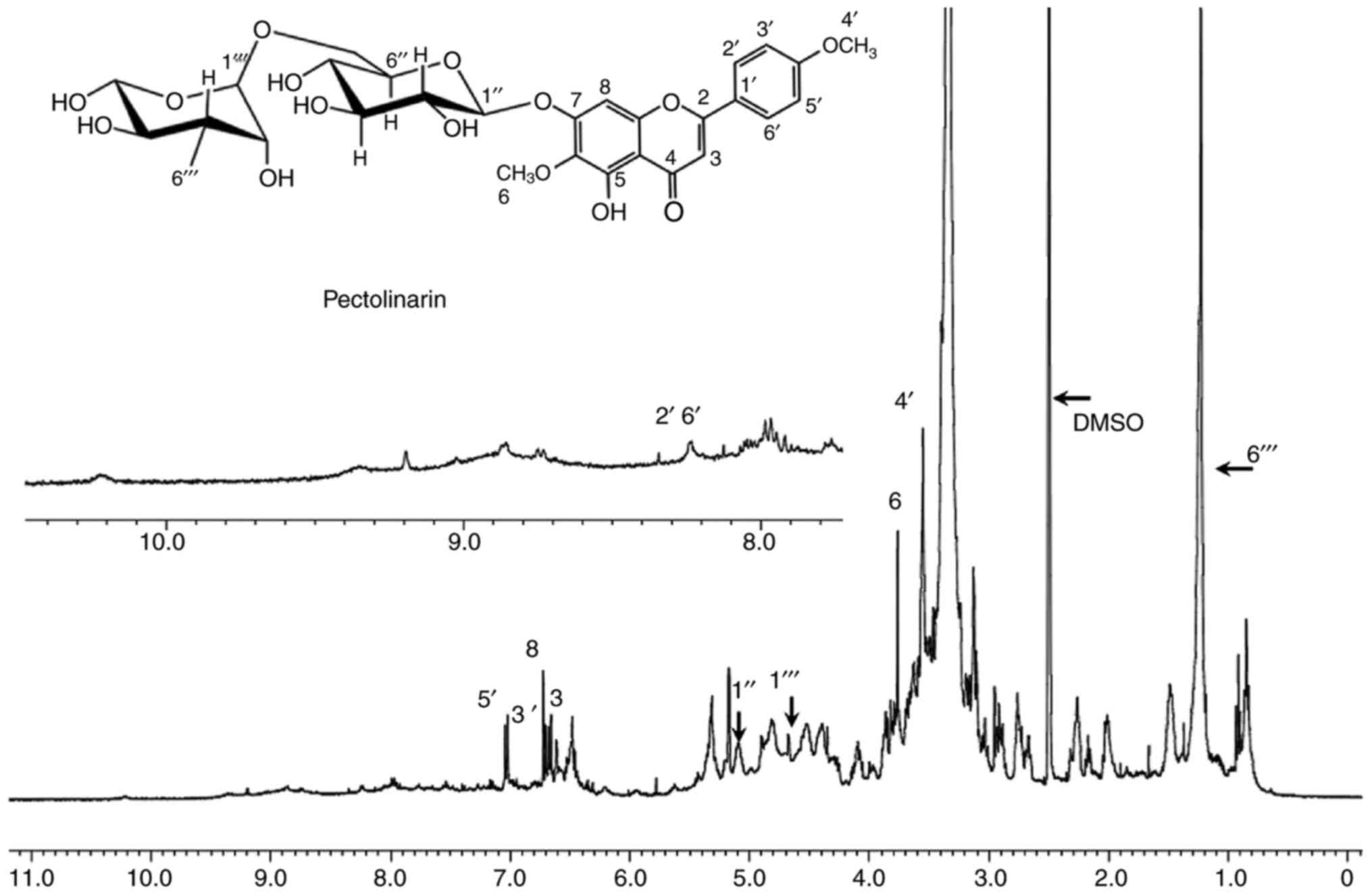
The proton spectrum is a powerful analytical tool
used to identify the different kinds of protons present in a
structure. This information provides substantial support for
explaining the chemical composition of a certain material. The
1H-NMR spectrum of the methanol-extracted sample is
shown in Fig. 1. The NMR spectrum
resembled the previously reported pattern of pectolinarin, which
suggests that the extracted material primarily consisted of
pectolinarin (10,12). The proton NMR spectrum exhibits
several peaks in the chemical shift (δ) region of 0.83–2.2 ppm,
which correspond to the methyl and methylene hydrogen moieties
(24). Chemical shifts of other
protons due to different chemical environments are indicated in
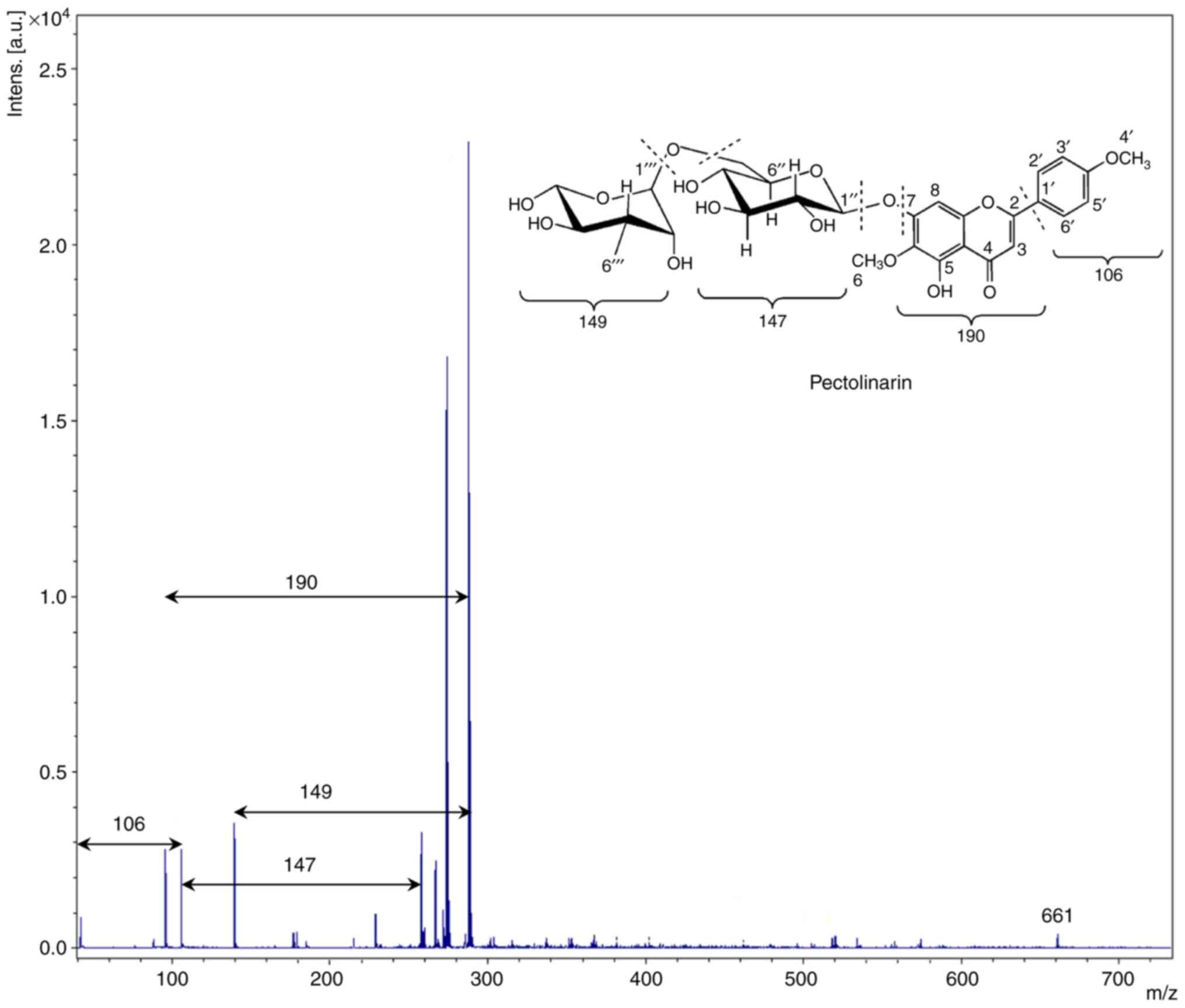
detail in the proton spectrum. The MALDI-TOF mass spectrum of the
methanol-extracted sample with a 2,5-Dihydroxybenzoic acid
(2,5-DHB)-assisted matrix is shown in Fig. 2. It is well established that the
2,5-DHB matrix enhances the intensity of the signals in the
MALDI-TOF spectrometry (25).
Interestingly, the gap between the two fragmented peaks is not
constant. This is attributed to the presence of different chemical
moieties in the extracted sample, which are associated with various
linkages. The gap between the two peaks was ~147 and 149 Da,
suggesting the presence of glucose and rhamnose units in the
structure, respectively. It can also be inferred that it was linked
with a glycosidic linkage, which was cleaved during the
measurement. The appearance of a signal at ~288 shows the presence
of a flavone structure in the methanol extract, which is linked to
the glucose unit. It is not always necessary to consider the
highest m/z peak ratio as the molecular ion species
[M+H]+ in the positive mode, and [M-H]− in a
negative way, mainly because of the formation of molecular
complexes ([2M+H]+ or [2M-H]−) or adducts
with solvents or acid molecules (26). Based on the data obtained by
1H-NMR and MALDI-TOF, the methanol extract of gondre
predominantly consisted of the pectolinarin chemical moiety.
Characterization of the hPDLSCs
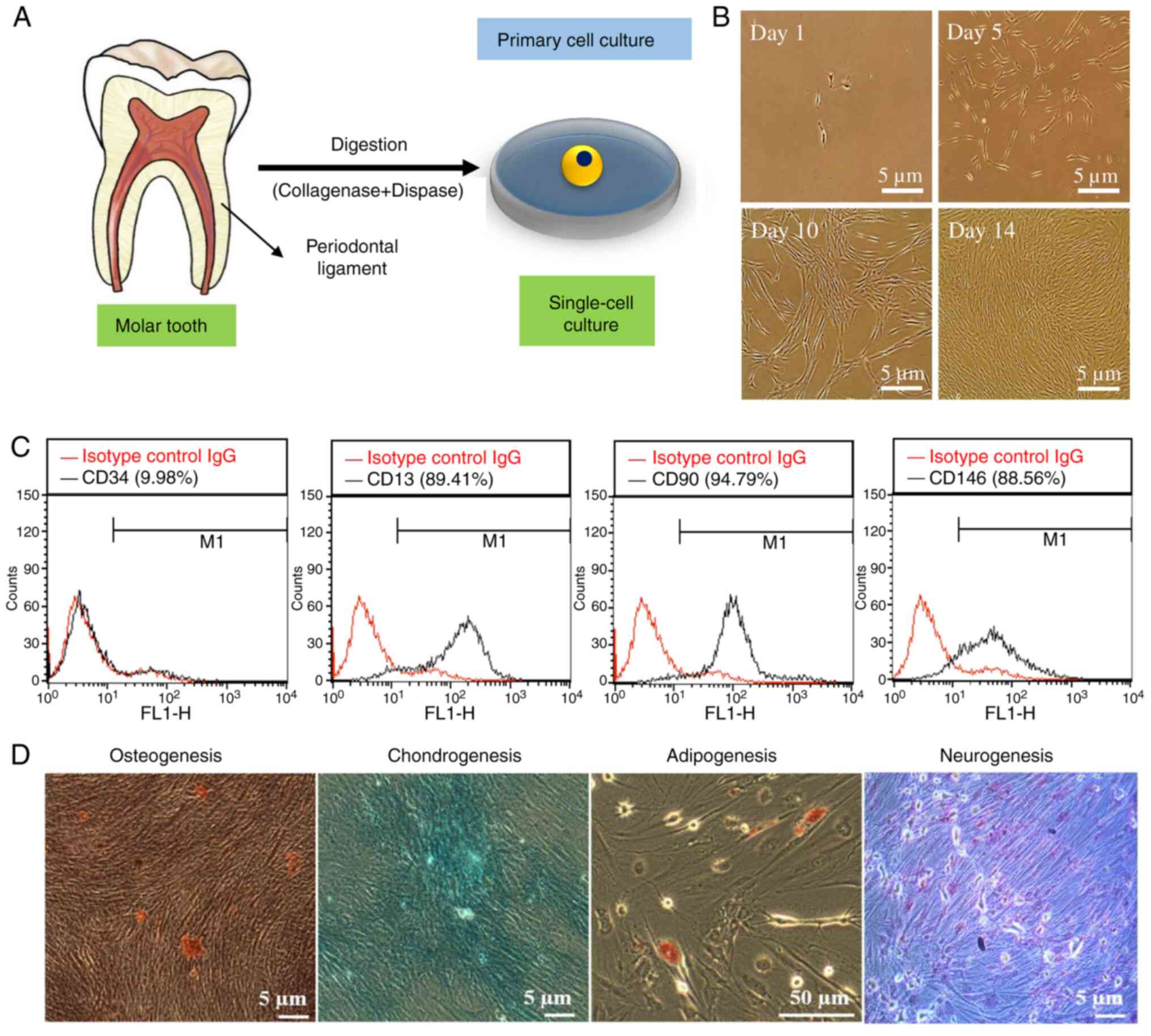
A schematic illustration of the presence of
periodontal ligament cells in the human molar tooth and of their
isolation for primary cell culture is presented in Fig. 3A. Cultured cell morphology after
different time intervals is shown in Fig. 3B. Stem cells are characterized by
their pluripotentiality and their expression of different surface
markers, and their fate is profoundly affected by growth factors,
cytokines, and the surrounding microenvironments (27,28).
These factors can promote self-renewal and dedifferentiation of
stem cells. This ability is known as the stemness potential
(29). As shown in Fig. 3C, we evaluated the stemness
potential of hPDLSCs through FACS analysis. Not only can hPDLSCs be
differentiated into cementoblasts, osteoblasts, adipocytes, and
chondrocytes (30), but they also
exhibit superior bone cell formation properties, compared with
human dental pulp stem cells and human periapical follicular stem
cells (31). FACS results showed
enhanced expression (~90%) of stem cell-related surface markers,
such as CD13, CD90, and CD146, indicating the stemness potential of
cultured hPDLSCs. The low expression (~10%) of CD34 surface markers
in hPDLSCs supports their stemness potential as well. Fig. 3D shows the multi-lineage
differentiation potential of hPDLSCs after 21 days of culture in
diverse induction media, which was determined by monitoring the
expression of specific markers. The presence of mineralized matrix,
proteoglycan, fat vacuoles, and Nissl bodies, as detected by
staining with ARS, alcian blue, oil red O dye, and Nissl stain,
indicates the osteogenic, chondrogenic, adipogenic, and neurogenic
potential of hPDLSCs, respectively (10).
Cell viability and migration
Cytotoxicity is one of the important criteria that
determine whether a substance can be used in the biomedical field,
since materials should be nontoxic and biodegradable (32). The cytotoxic effects of gondre
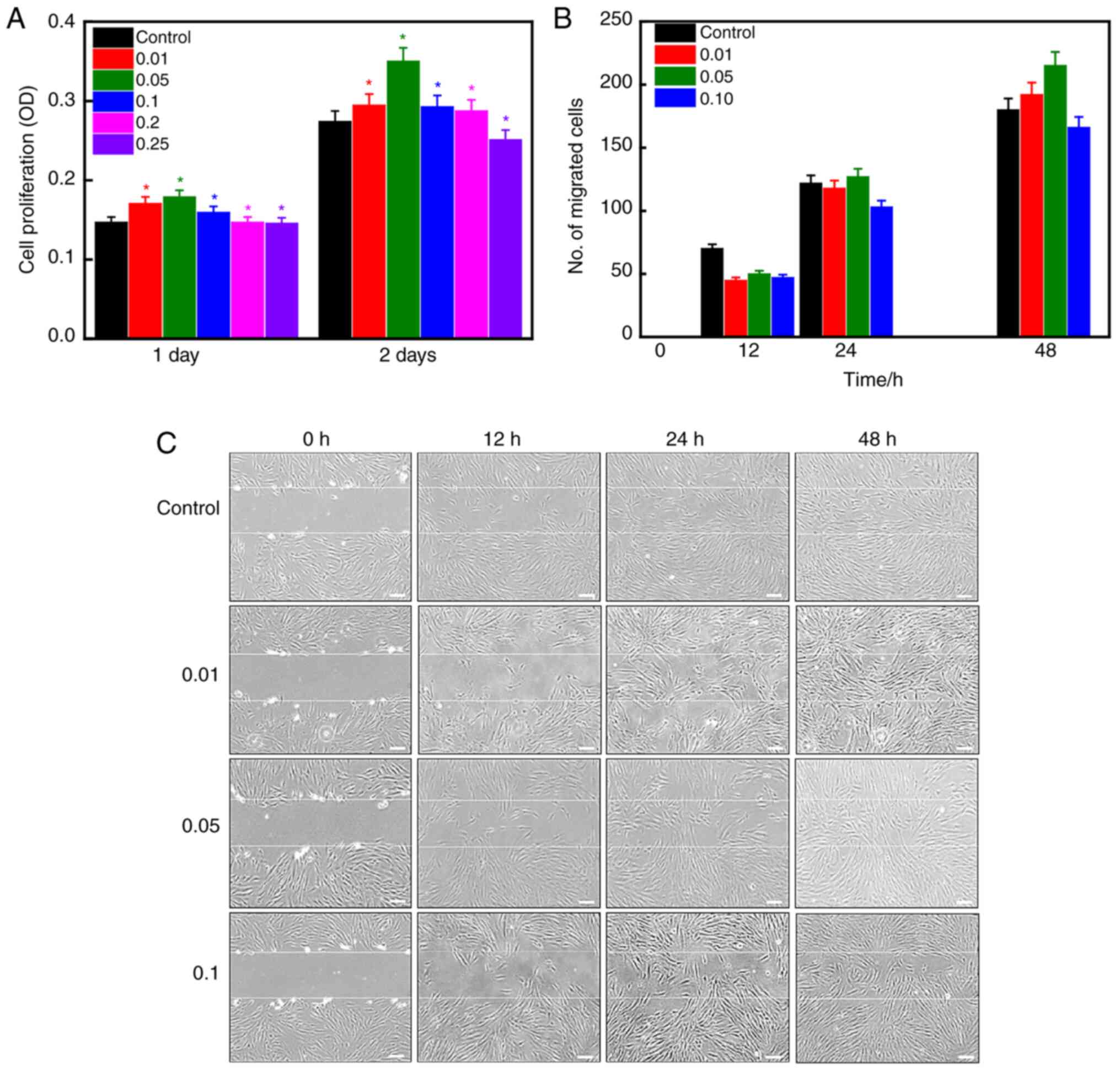
powder on hPDLSCs were evaluated using the MTT assay, as presented
in Fig. 4A. Cells cultured in
medium without gondre were used as control. An increase in cell
viability in gondre-treated hPDLSCs, compared with control ones,
was evidenced after 24 h of incubation, suggesting that there is
biocompatibility. Furthermore, a significant improvement in cell
viability was observed after 48 h of incubation, indicating their
improved biocompatibility. This shows that gondre has no adverse
effects on hPDLSCs. Among 0.01, 0.05, 0.1, 0.2, and 0.25% gondre
concentrations, 0.05% elicited the highest cell viability,
demonstrating that it is a suitable concentration for improved
biocompatibility. Lee et al (15) also reported increased viability of
hMSCs in the presence of gondre, after inducing oxidative stress
with H2O2 for 8 h. The authors noted that
cell viability was significantly enhanced in the presence of C.
setidens, compared to untreated conditions, which evidenced the
protective effects of C. setidens against
H2O2-induced oxidative stress (15).
Migration is essential for living cells, as it
contributes to healthy development, and immune responses, as well
as to disease processes, such as cancer metastasis and inflammation
(33). Stem cell migration occurs
not only during embryonic development but also in adult tissues to
maintain homeostasis and repair damage. The migration ability of
stem cells has tremendous therapeutic significance in the field of
regenerative tissue applications (34). Fig. 4B
and C shows the migration potential of hPDLSCs in the presence
of different concentrations of gondre at indicated time intervals.
Although cell migration towards the wounded area was initially slow
in the presence of gondre compared with control conditions, after
24 h, this tendency increased, and a higher accumulation of cells
was observed in gondre-treated conditions, compared to the control.
Moreover, a 0.05% concentration of gondre exhibited a high number
of cells migrating towards the wound area. Cell viability and
migration tendency results indicated that the 0.05% gondre
concentration was the optimal concentration to improve cellular
activity.
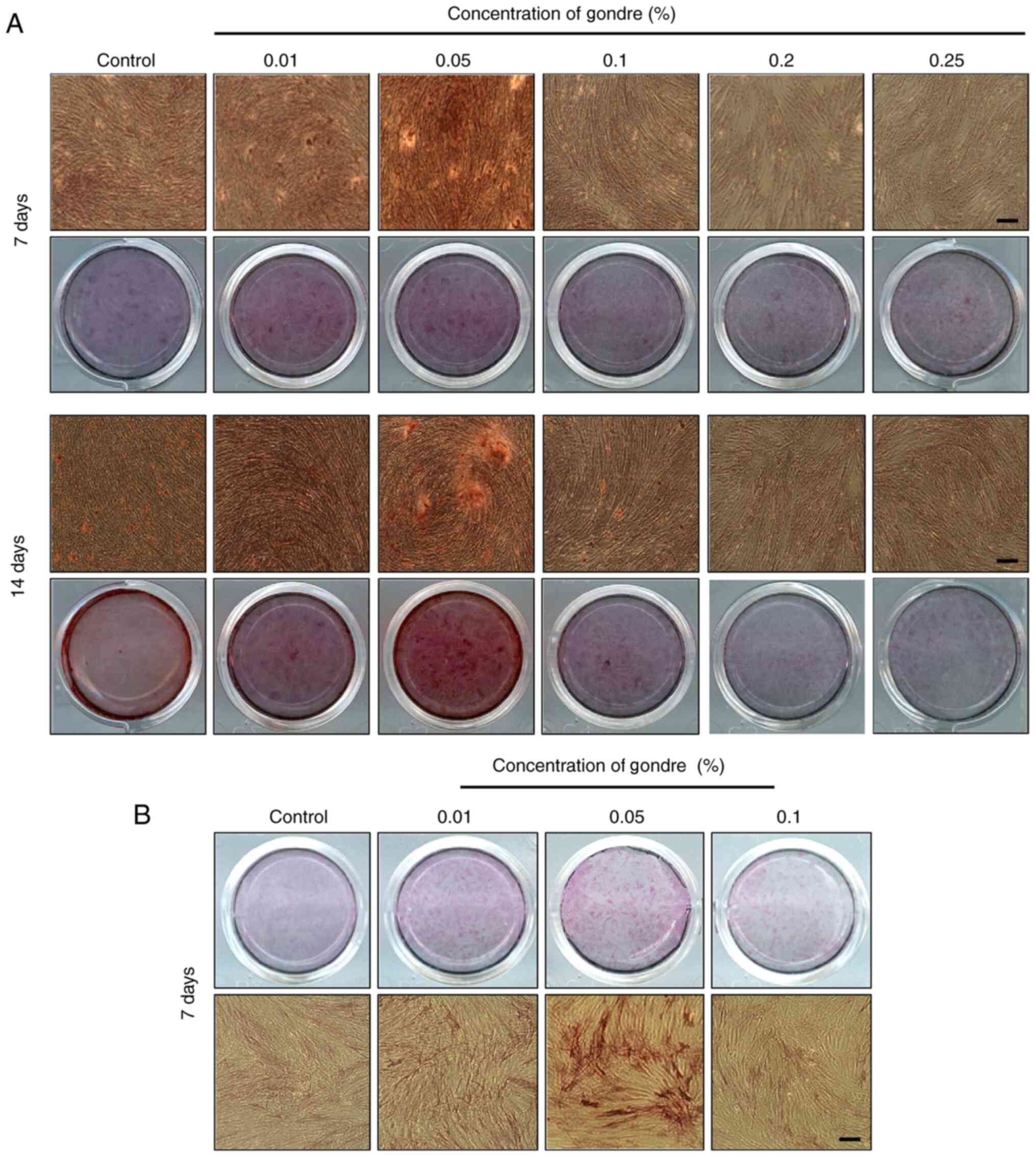
Mineralization and ALP activity
Stem cells are the most prominent cells in tissue
engineering applications, owing to their differentiation potential.
Even though they can be differentiated into osteoblasts,
chondrocytes, adipocytes, and other cells (35,36),
their differentiation ability is profoundly affected by local
biological conditions (37).
Gondre-induced mineralization of hPDLSCs was examined through the
ARS staining process after 7 and 14 days of incubation, and the
results are shown in Fig. 5A. A
more intense color was observed in gondre-treated cells compared
with those in control conditions, which demonstrated better
mineralization potential. This parameter is extensively affected by
the biomaterial's concentration in the differentiation medium
(38). Among experimental
conditions (0, 0.01, 0.05, 0.1, 0.2, and 0.25%), a gondre
concentration of 0.05% showed greater potential for mineralization
after 7 days of treatment. A similar trend was also observed after
14 days of treatment, indicating that 0.05% is the optimal
concentration for mineralization.
Different dosages of gondre may have different
effects on stem cells. For instance, it has been reported that
lower concentrations of this traditional herb enhance the metabolic
efficacy and alkaline phosphatase activity, whereas high
concentrations remarkably decrease cell density (3). Fig. 5B
shows the ALP activity of hPDLSCs in the presence of gondre after
14 days of treatment. ALP activity was higher in the gondre-treated
groups than in the control group, and this difference was
significant in the 0.05% gondre-treated cells. Since ALP is an
important gene marker that suggests the presence of preosteoblasts
and osteoblasts for bone mineral production at the time of
differentiation (23,39), more intensely stained cells in the
presence of gondre confirm a higher osteogenic potential of
gondre-treated cells compared to control ones. It is well known
that osteoclast resorption and osteoblast formation are two
important processes that influence overall bone metabolic
activity.
Osteogenic gene and protein
expression
Bone formation is a complex biological process that
involves several osteogenesis-associated genes (22). The expression of
osteogenesis-related genes (Col1, Runx2, BSP, and ALP) in the
presence of gondre after 7 and 14 days of treatment is shown in
Fig. 6A and B. Untreated cells (no
gondre) were considered as controls. Col1, the most abundant
protein found in the bone matrix, is vital during the proliferation
of osteoblast cells (40). The
expression of the Col1 biomarker from hPDLSCs in the presence of
gondre evidences bone cell formation. Moreover, its expression was
high in gondre-treated cells, compared to control ones, after 14
days of treatment, suggesting their greater osteogenic potential.
Runx2 is another osteogenic marker that is expressed during
osteogenesis. Since it is an early transcription marker,
osteogenesis cannot occur without it. The results showed that its
expression was higher in the gondre-treated cells than in the
control ones. Moreover, Runx2 expression was high after 14 days of
treatment with 0.05% gondre. The expression and activity of Runx2
are severely affected by other transcription factors and
protein-protein or protein-DNA interactions. However,
overexpression of the Runx2 factor facilitates bone resorption
(35). BSP comes from a ‘small
integrin-binding ligand N-linked glycoprotein’ (SIBLING) family,
which occurs in bone and dentin. SIBLING plays a significant role
in bone development, healing, remodeling, and mineralization
(41). Higher expression levels of
BSP in the presence of gondre, compared to those in control
conditions, indicated a higher osteogenic efficiency.
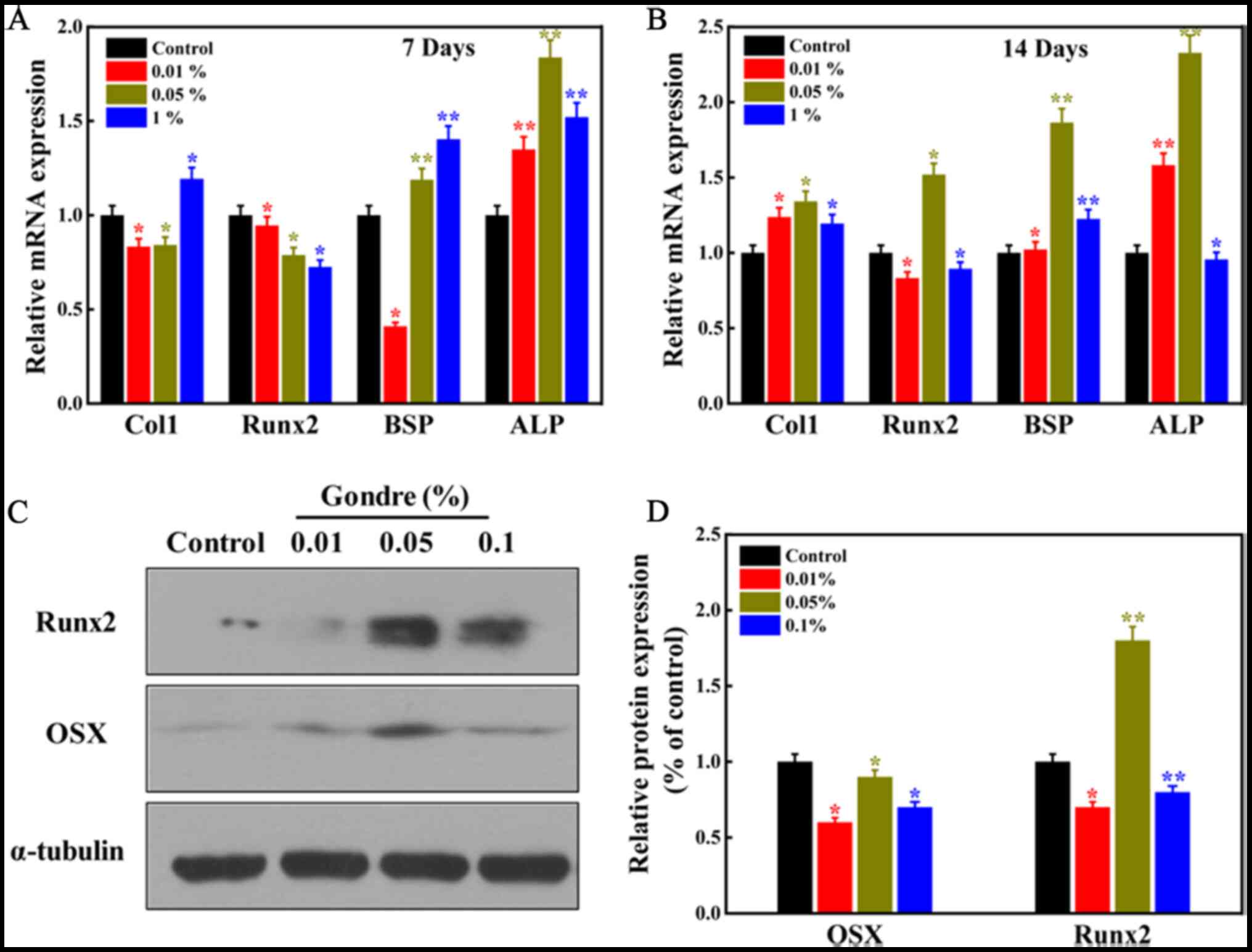 | Figure 6.Evaluation of the expression of
osteogenesis-specific gene markers and proteins in the presence of
gondre powder. (A and B) The relative expression level of mRNA in
the presence of different concentrations of gondre after 7, and
after 14 days of treatment, respectively. Data were normalized to
the housekeeping gene HPRT. (C) Western blot analysis of Runx2 and
OSX following gondre (0, 0.01, 0.05, and 0.1%) treatment. (D)
Western blotting was quantified using the ImageJ software, and data
were normalized to α-tubulin. The data are mean ± SD of triplicate
experiments (n=3). *P<0.05, **P<0.01 vs. Control. Col1,
collagen 1; Runx2, runt-related transcription factor 2; BSP, bone
sialoprotein 2; ALP, alkaline phosphatase; OSX, osterix. |
Osteogenesis is widely affected by the nature,
concentration, and size of the biomaterials present in the
osteogenic media (42). Bi et
al (43) tested the osteogenic
potential of surface-functionalized gold nanoparticles (Au NPs)
with different surface charges in the presence of hMSCs. They
observed that Au NPs with hydroxyl groups exhibited higher
osteogenic properties than others due to the presence of a hydroxyl
moiety, which facilitates bone formation and growth, thus inducing
osteogenesis. The hydroxyl groups also promote ECM growth, which
contributes to osteogenesis (43).
A similar result was also reported by Chen et al (44), with green tea catechins. In the
present work, the abundant hydroxyl groups in pectolinarin
facilitate apatite deposition in the matrix, as observed in the ALP
activity and mineralization test, which causes greater
osteogenesis. Cellular activity was higher at the 0.05%
concentration compared to the others; hence, more apatite
deposition occurred at this concentration, leading to superior
osteogenesis.
Understanding the interaction mechanisms between
hPDLSCs and gondre at the cellular and molecular level will help
utilize the herb-derived material for osteogenic differentiation of
cells in tissue engineering applications. Thus, the material
characteristics and mechano-transduction pathways provide the
primary molecular mechanics that affect cellular activities
(45). It has been reported that
nanomaterial-incorporated platforms display improved cellular
activities, owing to their nano-topographical surface geometry,
which promote cell proliferation and differentiation (46). Therefore, the abundance of hydroxyl
groups in gondre promotes the improved osteogenic differentiation
of hPDLSCs via their surface chemistry. As presented in
Fig. 6C and D, changes in protein
expression of hPDLSCs in the presence of gondre were evaluated with
western blotting. Results showed that protein expression of Runx2
was higher in gondre-treated cells than in control ones, further
confirming their superior osteogenic potential. In particular,
0.05% gondre-treated cells presented the highest levels compared to
the other groups. Runx2, also known as Cbfal or AML3 transcription
factor, is an essential transcription factor that regulates the
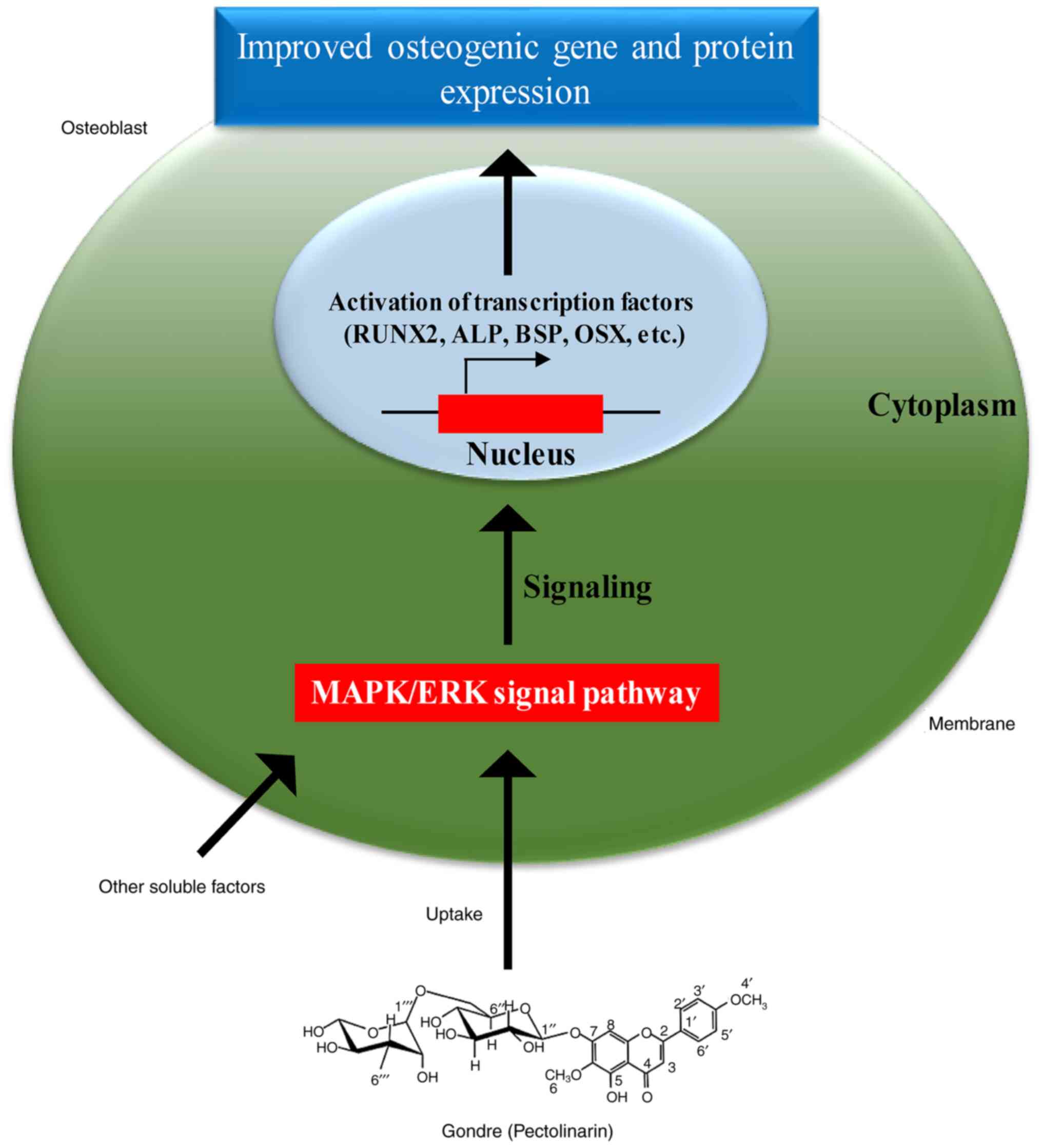
osteogenic differentiation of stem cells (47). Based on the improved osteogenic
activity and protein expression in the presence of gondre, a
hypothetical pathway is presented in Fig. 7. It has been reported that the
mitogen-activated protein kinase (MAPK)/extracellular regulated
protein kinase (ERK) signaling pathway may play a crucial role in
osteogenic differentiation and bone cell development by
accelerating Runx2 phosphorylation and transcriptional activity
(47). Previously, it was reported
that gondre extract had a positive role in
H2O2-induced oxidative damage protection in
hMSCs by signaling through MAPK/ERK pathway (15). Therefore, we assumed that the
MAPK/ERK signaling pathway facilitates superior osteogenic
differentiation in the presence of gondre, by activating Runx2
phosphorylation and transcriptional activity. The lack of proper
molecular mechanism to support these findings will be the future
direction of this work. In this study, a concentration of 0.05%
gondre is optimal and can be explored as an osteogenic substrate
for tissue engineering applications, especially in bone
tissues.
In this study, we extracted the phytochemicals
present in gondre, using methanol, and characterized them via
MALDI-TOF mass spectrometry and 1H-NMR spectroscopy. It
was observed that the methanol extract of gondre primarily
consisted of pectolinarin. hPDLSCs were used to evaluate gondre
cytotoxicity. The stemness potential of the hPDLSCs was evaluated
using the FACS technique. Interestingly, the isolated hPDLSCs
maintained their stemness potential. Notably, higher cell viability
was observed in the presence of gondre, compared to the control,
and viability was extensively affected by gondre content in the
media. Among these concentrations (0.01, 0.05, 0.1, 0.2, and
0.25%), 0.05% exhibited greater cell viability than the other
concentrations. The cell migration study also supports that 0.05%
is an ideal concentration for improved cellular activity. A more
intense mineralized nodule formation occurred in the presence of
0.05% gondre concentration, and the control showed greater
osteogenic potential. In addition, a significant enhancement in
osteogenesis-related gene expression was observed in the presence
of 0.05% concentration of gondre, compared to the other
concentrations, after 7 and 14 days of treatment, suggesting its
potential use in tissue engineering applications. Based on these
findings, we concluded that gondre was effective for enhanced
osteogenesis, and can be useful as a natural, edible, and
osteogenic agent.
Acknowledgements
The authors acknowledge the Central Instrumentation
Laboratory, Kangwon National University, Chuncheon for providing
the proton NMR and mass spectroscopy facility.
Funding
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant no.
2018R1A6A1A03025582) and the National Research Foundation of Korea
(grant no. NRF-2019R1D1A3A03103828). The study was also supported
by the Innovative Cultured Meat Technology Development Alchemist
Project (grant no. 20012439) funded by the Ministry of Trade,
Industry, and Energy (MoTIE, Korea).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SDD and DKP conceptualized the study, designed the
methodology and validated the study. SDD, BJ, SIC and OHL performed
formal analysis. SDD wrote the original draft. DKP reviewed and
edited the manuscript. KTL conceptualized the work, acquired
funding and gave the final approval of the paper. SDD and KTL
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the Dental Hospital, Seoul National University
(Seoul, Republic of Korea; IRB Number 05004), and written consent
was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ozen AE, Pons A and Tur JA: Worldwide
consumption of functional foods: A systematic review. Nutr Rev.
70:472–481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biella CA, Salvador MJ, Dias DA,
Dias-Baruffi M and Pereira-Crott LS: Evaluation of immunomodulatory
and anti-inflammatory effects and phytochemical screening of
Alternanthera tenella Colla (Amaranthaceae) aqueous
extracts. Mem Inst Oswaldo Cruz. 103:569–577. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cho BY, Park MR, Lee JH, Ra MJ, Han KC,
Kang IJ and Lee OH: Standardized Cirsium setidens Nakai
ethanolic extract suppresses adipogenesis and regulates lipid
metabolisms in 3T3-L1 adipocytes and C57BL/6J mice fed high-fat
diets. J Med Food. 20:763–776. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Daliri EBM, Choi SI, Cho BY, Jo HY, Kim
SH, Chelliah R, Rubab M, Kim JH, Oh HT, Lee OH, et al: Biological
activities of a garlic-Cirsium setidens Nakai blend
fermented with Leuconostoc mesenteroides. Food Sci Nutr.
7:2024–2032. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee WB, Kwon HC, Cho OR, Lee KC, Choi SU,
Baek NI and Lee KR: Phytochemical constituents of Cirsium
setidens Nakai and their cytotoxicity against human cancer cell
lines. Arch Pharm Res. 25:628–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung HA, Kim YS and Choi JS: Quantitative
HPLC analysis of two key flavonoids and inhibitory activities
against aldose reductase from different parts of the Korean
thistle, Cirsium maackii. Food Chem Toxicol. 47:2790–2797.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee HB, Kwak JH, Zee OP and Yoo SJ:
Flavonoids from Cirsium rhinoceros. Arch Pharm Res.
17:273–277. 1994. View Article : Google Scholar
|
|
8
|
Yim SH, Kim HJ and Lee IS: A polyacetylene
and flavonoids from Cirsium rhinoceros. Arch Pharm Res.
26:128–131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JC, Hur JM, Park JG, Kim SC, Park JR,
Choi SH and Choi JW: Effects of methanol extract of Cirsium
japonicum var. ussuriense and its principle,
hispidulin-7-O-neohesperidoside on hepatic alcohol-metabolizing
enzymes and lipid peroxidation in ethanol-treated rats. Phytother
Res. 18:19–24. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lim H, Son KH, Chang HW, Bae K, Kang SS
and Kim HP: Anti-inflammatory activity of pectolinarigenin and
pectolinarin isolated from Cirsium chanroenicum. Biol Pharm
Bull. 31:2063–2067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeong HC, Shim YS, Rhee YK, Choi SY, Hong
HD, Chung J, Han MJ and Cho CW: Quantification of marker compounds
in Cirsium setidens Nakai by HPLC-DAD. Food Sci Biotechnol.
22:1481–1486. 2013. View Article : Google Scholar
|
|
12
|
Yoo YM, Nam JH, Kim MY, Choi J and Park
HJ: Pectolinarin and pectolinarigenin of Cirsium setidens
prevent the hepatic injury in rats caused by D-galactosamine via an
antioxidant mechanism. Biol Pharm Bull. 31:760–764. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martínez-Vázquez M, Ramírez Apan TO,
Lastra AL and Bye R: A comparative study of the analgesic and
anti-inflammatory activities of pectolinarin isolated from
Cirsium subcoriaceum and linarin isolated from Buddleia
cordata. Planta Med. 64:134–137. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu S, Luo X, Li D, Zhang J, Qiu D, Liu W,
She L and Yang Z: Tumor inhibition and improved immunity in mice
treated with flavone from Cirsium japonicum DC. Int
Immunopharmacol. 6:1387–1393. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee JH, Jung HK, Han YS, Yoon YM, Yun CW,
Sun HY, Cho HW and Lee SH: Antioxidant effects of Cirsium
setidens extract on oxidative stress in human mesenchymal stem
cells. Mol Med Rep. 14:3777–3784. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: Μesenchymal stem cells: Τheir
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amiri F, Jahanian-Najafabadi A and
Roudkenar MH: In vitro augmentation of mesenchymal stem cells
viability in stressful microenvironments: In vitro augmentation of
mesenchymal stem cells viability. Cell Stress Chaperones.
20:237–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jo YY, Lee HJ, Kook SY, Choung HW, Park
JY, Chung JH, Choung YH, Kim ES, Yang HC and Choung PH: Isolation
and characterization of postnatal stem cells from human dental
tissues. Tissue Eng. 13:767–773. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin B and Choung PH: Recombinant human
plasminogen activator inhibitor-1 accelerates odontoblastic
differentiation of human stem cells from apical papilla. Tissue Eng
Part A. 22:721–732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huifang G, Jiang Y and Wang MH:
Synergistic anti-diabetic effect of Cirsium setidens
combined with other plants in vitro and in vivo. Korean J Plant
Resour. 28:752–758. 2015. View Article : Google Scholar
|
|
22
|
Kim HB, Jin B, Patel DK, Kim JW, Kim J,
Seonwoo H and Lim KT: Enhanced osteogenesis of human mesenchymal
stem cells in presence of single-walled carbon nanotubes. IEEE
Trans Nanobioscience. 18:463–468. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dutta SD, Patel DK, Seo YR, Park CW, Lee
SH, Kim JW, Kim J, Seonwoo H and Lim KT: In vitro biocompatibility
of electrospun poly (ε-caprolactone)/cellulose
nanocrystals-nanofibers for tissue engineering. J Nanomater.
2019:20615452019. View Article : Google Scholar
|
|
24
|
Florencio-Silva R, Sasso GRdS, Sasso-Cerri
E, Simões MJ and Cerri PS: Biology of bone tissue: Structure,
function, and factors that influence bone cells. BioMed Res Int.
2015:4217462015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seo YR, Patel DK, Shin WC, Sim WS, Lee OH
and Lim KT: Structural elucidation and immune-enhancing effects of
novel polysaccharide from Grifola frondosa. BioMed Res Int.
2019:75686092019. View Article : Google Scholar
|
|
26
|
Cuyckens F and Claeys M: Mass spectrometry
in the structural analysis of flavonoids. J Mass Spectrom. 39:1–15.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ouspenskaia T, Matos I, Mertz AF, Fiore VF
and Fuchs E: WNT-SHH antagonism specifies and expands stem cells
prior to niche formation. Cell. 164:156–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo G, von Meyenn F, Santos F, Chen Y,
Reik W, Bertone P, Smith A and Nichols J: Naive pluripotent stem
cells derived directly from isolated cells of the human inner cell
mass. Stem Cell Reports. 6:437–446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aponte PM and Caicedo A: Stemness in
cancer: Stem cells, cancer stem cells, and their microenvironment.
Stem Cells Int. 2017:56194722017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gay IC, Chen S and MacDougall M: Isolation
and characterization of multipotent human periodontal ligament stem
cells. Orthod Craniofac Res. 10:149–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park JY, Jeon SH and Choung PH: Efficacy
of periodontal stem cell transplantation in the treatment of
advanced periodontitis. Cell Transplant. 20:271–285. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
O'brien FJ: Biomaterials & scaffolds
for tissue engineering. Mater Today. 14:88–95. 2011. View Article : Google Scholar
|
|
33
|
Justus CR, Leffler N, Ruiz-Echevarria M
and Yang LV: In vitro cell migration and invasion assays. J Vis
Exp. 88:e510462014.
|
|
34
|
de Lucas B, Pérez LM and Gálvez BG:
Importance and regulation of adult stem cell migration. J Cell Mol
Med. 22:746–754. 2018.PubMed/NCBI
|
|
35
|
Phinney DG: Functional heterogeneity of
mesenchymal stem cells: Implications for cell therapy. J Cell
Biochem. 113:2806–2812. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bianco P, Cao X, Frenette PS, Mao JJ,
Robey PG, Simmons PJ and Wang CY: The meaning, the sense and the
significance: Translating the science of mesenchymal stem cells
into medicine. Nat Med. 19:35–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giuliani N, Lisignoli G, Magnani M, Racano
C, Bolzoni M, Palma BD, Spolzino A, Manferdini C, Abati C, Toscani
D, et al: New insights into osteogenic and chondrogenic
differentiation of human bone marrow mesenchymal stem cells and
their potential clinical applications for bone regeneration in
pediatric orthopaedics. Stem Cells Int. 2013:3125012013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Simões LR, Maciel GM, Brandão GC, S Filho
JD, Oliveira AB and Castilho RO: Chemical constituents of
Distictella elongata (Vahl) Urb. (Bignoniaceae). An Acad
Bras Cienc. 85:873–879. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dutta SD, Hexiu J, Patel DK, Ganguly K and
Lim KT: 3D-printed bioactive and biodegradable hydrogel scaffolds
of alginate/gelatin/cellulose nanocrystals for tissue engineering.
Int J Biol Macromol. 167:644–658. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Niu C, Yuan K, Ma R, Gao L, Jiang W, Hu X,
Lin W, Zhang X and Huang Z: Gold nanoparticles promote osteogenic
differentiation of human periodontal ligament stem cells via the
p38 MAPK signaling pathway. Mol Med Rep. 16:4879–4886. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bouet G, Bouleftour W, Juignet L,
Linossier MT, Thomas M, Vanden-Bossche A, Aubin JE, Vico L, Marchat
D and Malaval L: The impairment of osteogenesis in bone
sialoprotein (BSP) knockout calvaria cell cultures is cell density
dependent. PLoS One. 10:e01174022015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li JJ, Kawazoe N and Chen G: Gold
nanoparticles with different charge and moiety induce differential
cell response on mesenchymal stem cell osteogenesis. Biomaterials.
54:226–236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bi X, You Z, Gao J, Fan X and Wang Y: A
functional polyester carrying free hydroxyl groups promotes the
mineralization of osteoblast and human mesenchymal stem cell
extracellular matrix. Acta Biomater. 10:2814–2823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen CH, Ho ML, Chang JK, Hung SH and Wang
GJ: Green tea catechin enhances osteogenesis in a bone marrow
mesenchymal stem cell line. Osteoporos Int. 16:2039–2045. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao C, Tan A, Pastorin G and Ho HK:
Nanomaterial scaffolds for stem cell proliferation and
differentiation in tissue engineering. Biotechnol Adv. 31:654–668.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang G, Zheng L, Zhao H, Miao J, Sun C,
Ren N, Wang J, Liu H and Tao X: In vitro assessment of the
differentiation potential of bone marrow-derived mesenchymal stem
cells on genipin-chitosan conjugation scaffold with surface
hydroxyapatite nanostructure for bone tissue engineering. Tissue
Eng Part A. 17:1341–1349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Deng ZL, Sharff KA, Tang N, Song WX, Luo
J, Luo X, Chen J, Bennett E, Reid R, Manning D, et al: Regulation
of osteogenic differentiation during skeletal development. Front
Biosci. 13:2001–2021. 2008. View
Article : Google Scholar : PubMed/NCBI
|