Introduction
Alzheimer's disease (AD) is the most common form of
dementia present in the aging population worldwide and the number
of patients with AD is predicted to rise (1). According to the World Alzheimer Report
2018 (2), of the 50 million
individuals in the world with dementia, two thirds were diagnosed
with AD. While there have been numerous attempts to develop an
effective drug for treating AD, only two types of drugs are
currently available. One such type is cholinesterase inhibitors,
such as donepezil, which function to prevent acetylcholinesterase
from breaking down acetylcholine (3); therefore, signals are able to be
transmitted between nerve cells. The other type of drug is an
N-methyl-D-aspartic acid receptor antagonist (memantine), which
acts to relieve the damage to nerve cells caused by excessive
amounts of released glutamate (4).
However, the currently available drugs are only able to provide
symptomatic relief (5). Due to the
high socioeconomic costs associated with patients with AD worldwide
(6,7), the development of effective therapies
to treat AD is of great significance.
Although the fundamental mechanisms underlying AD
remain unknown, two classic pathological proteins, β-amyloid (Aβ)
and tau, are reported to be involved (8). Aβ accumulates and forms amyloid
plaques, which causes damage to the synapses (9). In addition to Aβ, hyperphosphorylated
tau forms neurofibrillary tangles (NFT) inside neurons, and these
tau aggregations block the neuronal transport system (10). The ubiquitin (Ub)-proteasome system
(UPS) is a sophisticated mechanism for intracellular protein
degradation and turnover (11). Ub
is a polypeptide that can label substrate proteins via the covalent
attachment of multiple Ub molecules, such as Ub-activating enzyme
E1 (UbE1) and Ub-conjugating enzyme E2 (UbE2). In addition, the 26S
proteasome serves a major role in the degradation of Ub-conjugated
proteins (12). Dysregulation of
the UPS is of particular interest in the pathogenesis of AD, and
emerging evidence has suggested that aberrant UPS activity may
contribute to the disorder of Aβ and tau degradation (13,14).
Yuan-zhi-san (YZS) is an herbal drug in Traditional
Chinese Medicine that has been clinically applied to treat
dementia. Previous studies have revealed that YZS and its
components are effective and safe in relieving some symptoms and
improving cognitive impairment in patients with AD (15–17).
Our previous study reported that YZS could improve learning and
memory abilities in a D-galactose-induced aging mouse model, which
was achieved, in part, by the attenuation of oxidative stress
(18). A similar result was also
reported by Jin et al (19).
Moreover, our previous study using another scopolamine-induced
mouse model of dysmnesia further validated the memory-improving
ability of YZS (20). Qiang et
al (21) observed that YZS
relieved AD by regulating acetylcholine receptor activity and by
binding to Aβ. Furthermore, nearly 180 active ingredients of YZS
demonstrating multi-component and multi-target characteristics have
been screened, and a specific database of the active ingredients
has been established (21).
However, whether YZS has an impact on UPS-mediated tau degradation
remains to be elucidated.
The present study aimed to investigate the effects
of YZS on learning and memory abilities, as well as AD pathology,
in an Aβ-induced AD rat model. Moreover, it was determined whether
YZS affected the phosphorylation of tau protein, and whether its
potential therapeutic effects were achieved by modulating the
UPS.
Materials and methods
Animals
Sprague-Dawley rats (n=40; equal number of males and
females; weight, 200±20 g; age, 7 weeks) were obtained from Chengdu
Dashuo Experimental Animal Co., Ltd. (animal license no.
SYXK-2017-179). Animals were housed under controlled conditions of
22±2°C and 50±10% humidity, with a 12-h light/dark cycle and ad
libitum access to standard rodent chow and water. The present
study was approved by the Medical Ethics Committee of Chengdu
University of Traditional Chinese Medicine (Chengdu, China;
approval no. 2017-02).
Drugs
Aβ1–40 was purchased from Sigma-Aldrich;
Merck KGaA (cat. no. A1075); donepezil was obtained from Shanxi
Aark Pha. Ltd. (drug approval. no. H20030583; http://www.800pharm.com/shop/groupId_101169.html).
YZS was composed of 12 g Radix polygala (Yuan Zhi),
12 g Rhizomacoptidis (Huang Lian), 15 g Sclerotium poriaecocos (Fu
Ling), 9 g Radix ginseng (Ren Shen) and 18 g Acori Tatarinowii
Rhizoma (Shi Chang Pu), and was prepared into a standardized
granule formula by Sichuan Neo-Green Pharmaceutical Technology
Development Co., Ltd.
AD animal model establishment and
treatment
Sprague-Dawley rats were randomly divided into four
groups: i) Sham group (n=10; treated with vehicle); ii) AD model
group (n=10; treated with Aβ1–40 and vehicle); iii) AD
model + donepezil group (n=10; treated with Aβ1–40 and
donepezil); and iv) AD model + YZS group (n=10; treated with
Aβ1–40 and YZS). The Aβ-induced AD rat model was
established as described previously (22–24).
Following anesthesia with intraperitoneal injection of 0.45%
pentobarbital sodium (40 mg/kg), rats were secured on a stereotaxic
device. Subsequently, referring to the Paxinos and Watson rat brain
atlas (25), the sham-operated rats
received a saline injection into the bilateral hippocampus CA1,
whereas the other AD rats received 5 µg Aβ1–40 solution.
The solution was infused at a speed of 0.5 µl/min, and the needle
remained in place for 5 min. A total of 200,000 U/ml penicillin-G
was administered intramuscularly to rats once daily for 7 days
after surgery. Following the procedure, 6 g/kg YZS, 0.45 mg/kg
donepezil or vehicle (equivalent dose of saline) were
intragastrically administered to the rats once daily for 8
consecutive weeks.
Morris water maze test
Rats were subjected to the Morris water test for the
spatial navigation task (escape latency) and a probe trial in the
last 5 days of the drug administration period. The training period
was performed for the first 3 days, and the orientation navigation
experiment was conducted on day 4. A video tracking system was used
to monitor the whole procedure, which was assessed by two
independent investigators in a blinded manner. A hidden platform
was placed 2-cm beneath the surface of the water in one pool
quadrant. When the rats had successfully located the hidden
platform, they were left on the platform for 10 sec. Rats were
manually guided to the platform and remained there for 10 sec if
they failed to find the platform within 90 sec, wherein the escape
latency was recorded as 90 sec. A probe test was performed on day 5
to evaluate memory consolidation.
Hematoxylin & eosin (H&E)
staining
Following anesthetization with 0.45% pentobarbital
sodium, rats were sacrificed via transcardial perfusion with 0.9%
saline (pH 7.4). Hippocampi were harvested, fixed in 4%
paraformaldehyde solution for >24 h at 4°C, dehydrated in graded
alcohol series (30, 50, 70, 95 and 100%) and embedded in paraffin.
Samples were cut into 5-µm sections and then stained with H&E
(hematoxylin at room temperature for 5 min; eosin at room
temperature for 1 min) to examine the pathological alterations.
Images were captured under a light microscope (IX71; Olympus
Corporation) at a magnification of ×400.
Golgi-Cox staining
After transcardial perfusion with 0.9% saline,
hippocampi were collected and immersed in a vial containing
Golgi-Cox solution (prepared solution from FD Rapid GolgiStain™
kit; cat. no. PK401; FD Neurotechnologies, Inc.). The vial was kept
in the dark and the Golgi-Cox solution was replaced every alternate
day for 2 weeks. Subsequently, the samples were transferred to a
vial containing 30% sucrose solution and stored at 4°C in the dark
for 6 days. The samples were cut into 100-µm sections and then
rinsed with distilled water. Subsequently, the sections were
treated with 22.5% ammonium hydroxide solution for 30 min and then
with 5% thiosulphate solution for 30 min at room temperature. After
rinsing with distilled water for 2 min, routine glass slides
containing the sections were processed. Images were captured under
a light microscope (Eclipse E100; Nikon Corporation) at
magnification of ×600. The density of the dendritic spines was
measured using Image-pro plus 6.0 (Media Cybernetics, Inc.).
Transmission electron microscopy
A small portion of the hippocampus was fixed in 2.5%
glutaraldehyde in cacodylate buffer (pH 7.4) at 4°C for 4 h and
were post-fixed in 2% osmium tetroxide at 4°C for 1 h in the same
buffer. Subsequently, the tissues were routinely dehydrated in
acetone, infiltrated and embedded in epoxy resin-filled capsules.
Finally, 70-nm ultrathin sections were prepared with an LKB
ultramicrotome and counterstained with 2% aqueous uranyl acetate
for 10 min and 0.8% lead citrate for 2 min at room temperature. The
ultrastructure of nerve cells was observed and imaged under a
transmission electron microscope (TEM; magnification, ×2,500 and
×6,000; H-7650; Hitachi Ltd.).
Immunohistochemistry
Brain tissues were fixed in 4% paraformaldehyde at
4°C for 24 h, dehydrated and embedded in paraffin.
Paraffin-embedded specimens were sliced into 3-µm sections, dewaxed
and rehydrated. After routine peroxidase blocking for 10 min and
blocking with 5% BSA (cat. no. G5001; Wuhan Servicebio Technology
Co., Ltd.) for 30 min at room temperature, the sections were
incubated with antibodies against Tau5 (1:100; cat. no. MAB361; EMD
Millipore), phosphorylated (p)-Tau (Ser199; 1:100; cat. no.
44-734G; Invitrogen; Thermo Fisher Scientific, Inc.), p-Tau
(Thr231; 1:100; cat. no. 11110; Signalway Antibody LLC) and p-Tau
(Ser396; 1:100; cat. no. 11102; Signalway Antibody LLC) overnight
at 4°C. The sections were then exposed to horseradish peroxidase
(HRP)/Fab polymer-conjugated secondary antibodies (solution from
the kit; cat. no. PV-6000-D; OriGene Technologies, Inc.) at room
temperature for 30 min, followed by staining with
3,3′-diaminobenzidine solution at room temperature for 5 min and
counterstaining with hematoxylin at room temperature for 20 sec. To
evaluate the protein expression levels, three visual fields were
randomly selected from each slice under a light microscope
(magnification, ×400). Images were acquired and analyzed using
Image Pro Plus 6.0 software (Media Cybernetics, Inc.).
Quantification of expression levels was determined using mean
integrated optical density (IOD).
Western blotting
Hippocampal sections were homogenized in pre-cooled
RIPA buffer (cat. no. G2002; Wuhan Servicebio Technology Co., Ltd.)
containing a protease inhibitor cocktail, and homogenates were then
incubated on ice for 10 min and centrifuged at 16,128 × g at 4°C
for 15 min. The protein concentration was determined using a
commercial BCA kit (cat. no. P0010S; Beyotime Institute of
Biotechnology). Proteins (30 µg) were separated by SDS-PAGE on
8–10% gels and transferred onto PVDF membranes. The membranes were
blocked with 5% skimmed milk in Tris-buffered saline containing
0.05% Tween-20 (TBST) at room temperature for 1 h and incubated at
4°C overnight with primary antibodies against UbE1a/b (1:1,000;
cat. no. 4891; Cell Signaling Technology, Inc.), UbE2a (1:500; cat.
no. 11080-1-AP; ProteinTech Group, Inc.), carboxyl terminus of
Hsc70-interacting protein (CHIP; 1:1,000; cat. no. 2080; Cell
Signaling Technology, Inc.), Ub C-236 terminal hydrolase L1
(UCH-L1; 1:1,000; cat. no. 14730-1-AP; ProteinTech Group, Inc.),
26S proteasome (1:500; cat. no. sc-65755; Santa Cruz Biotechnology,
Inc.) and β-actin (1:4,000; cat. no. 200068-8F10; Chengdu Zen
BioScience Co., Ltd.). After washing three times with TBST, the
membranes were incubated at room temperature for 1 h with
HRP-conjugated secondary antibodies (1:3,000; cat. nos. GB23301 and
GB23303; Wuhan Servicebio Technology Co., Ltd.). An enhanced
chemiluminescence kit (cat. no. NEL103E001EA; PerkinElmer, Inc.)
was used to detect the bands using a Molecular Imager PharosFX Plus
system (Bio-Rad Laboratories, Inc.), and the bands were analyzed
and semi-quantified using Quantity One software system (ver. no.
4.6.9; Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analyses were performed using SPSS 17.0
software (SPSS, Inc.). Differences between groups were evaluated
using one-way ANOVA followed by Tukey's or Dunnett's T3 post hoc
tests. Data are presented as the mean ± SD from three independent
experiments. P<0.05 was considered to indicate a statistically
significant difference.
Results
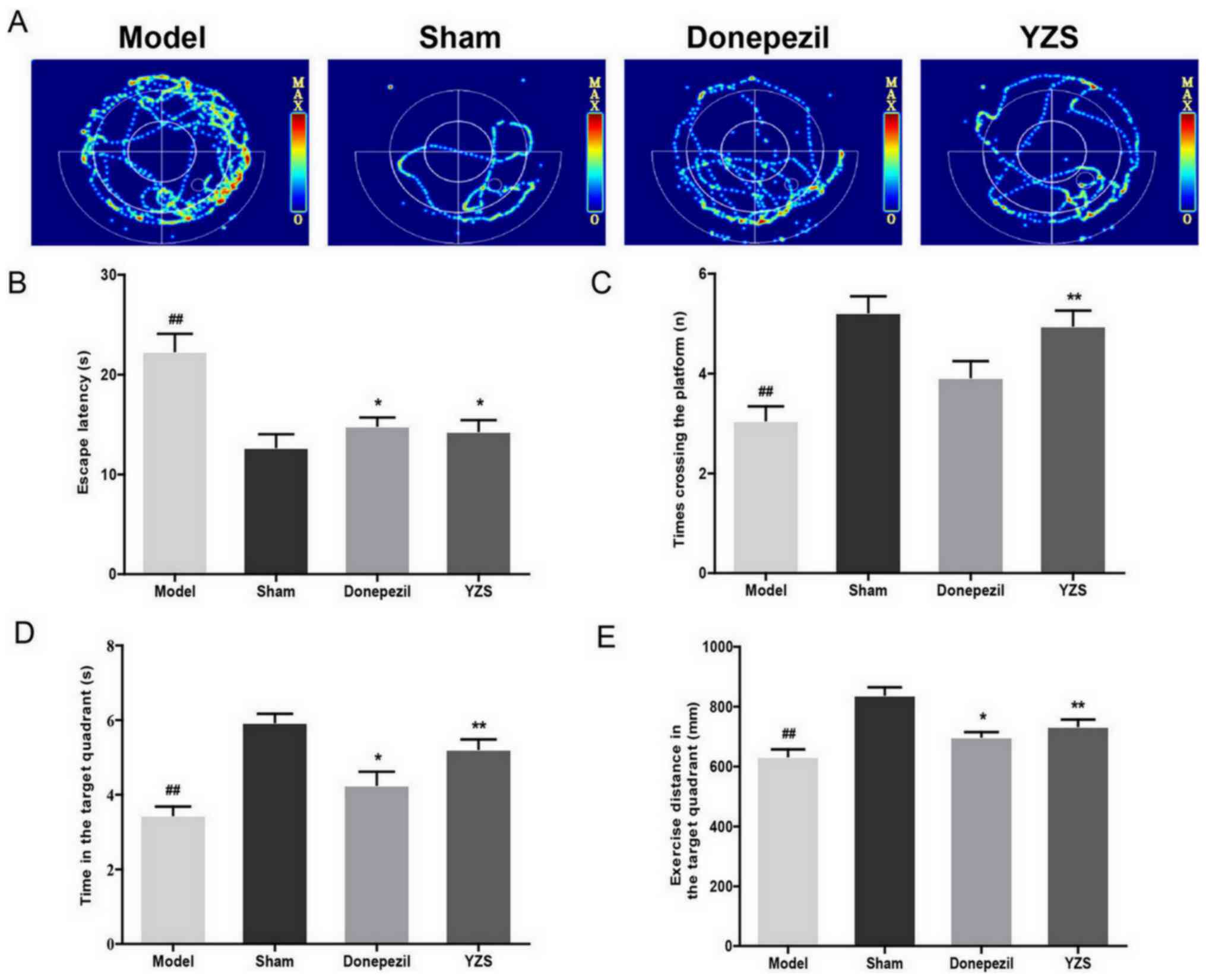
YZS rescues the memory deficit of AD
rats
The memory and learning performances of YZS-treated
AD rats were examined using a Morris water maze test. As expected,
the mean escape latency of all rats declined during the training
period. On day 4, AD rats demonstrated a significantly longer
escape latency in the orientation navigation experiment compared
with that of sham-operated rats (P<0.01). It was demonstrated
that, similar to the effects of donepezil administration, YZS
administration shortened the escape latency (P<0.05; Fig. 1B). In the probe test, YZS-treated AD
rats had greater crossing times (P<0.01; Fig. 1C), increased time in the target
quadrant (P<0.01; Fig. 1D) and
lengthier swimming routes (P<0.01; Fig. 1E) compared with those of AD rats.
These results indicated the rescuing effect of YZS on the memory
and learning abilities of AD rats.
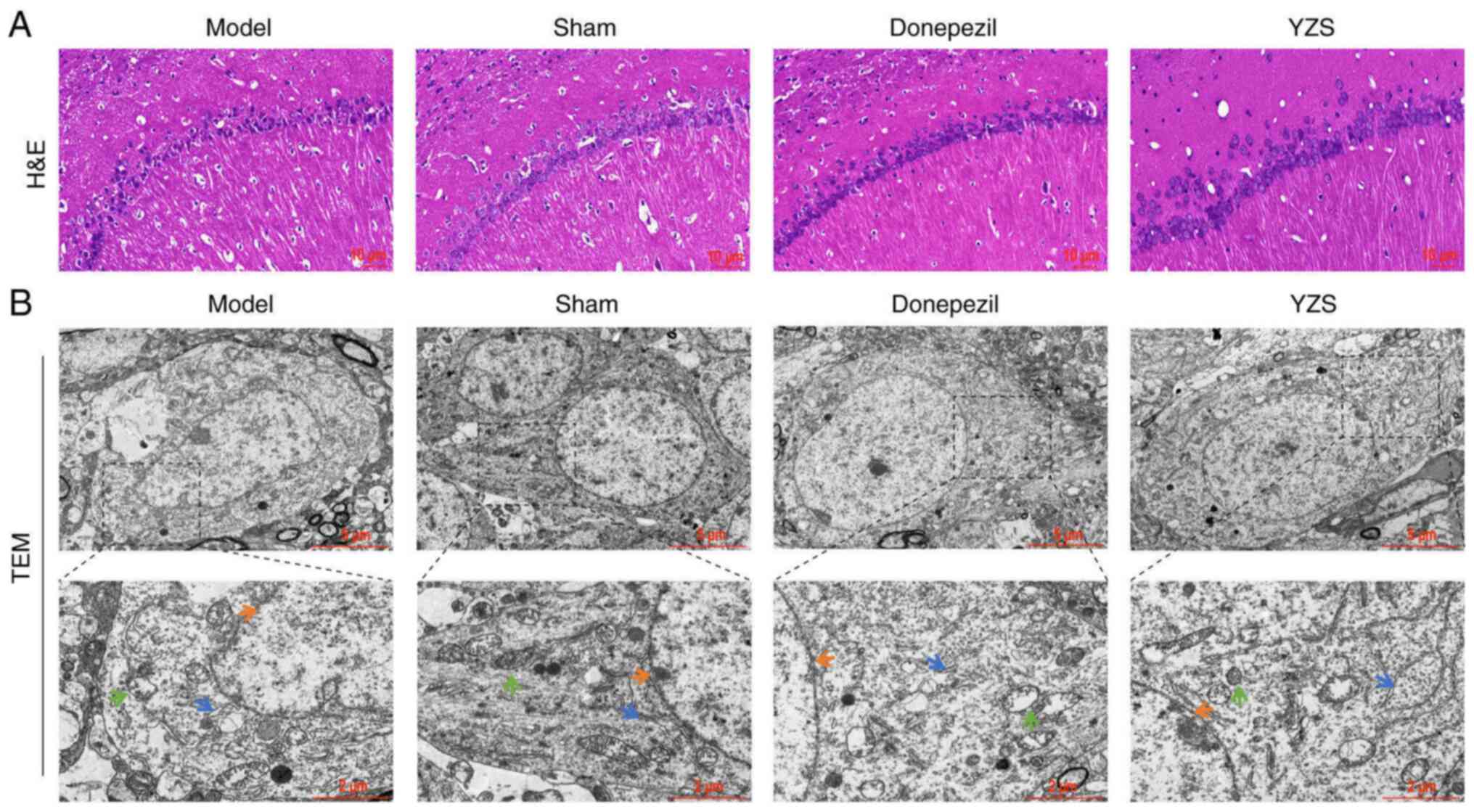
YZS ameliorates AD histopathology and
ultrastructural abnormalities
H&E staining indicated that the number of
neurons in the CA1 region was markedly lower in AD rats compared
with in the sham-operated rats (Fig.
2A). Both donepezil- and YZS-treated AD rats had notably
increased numbers of neurons. These results suggested that YZS
could protect against neuronal loss in AD rats.
The ultrastructural examination of nerve cells in
the hippocampus using a TEM demonstrated a visible difference
between AD and sham-operated rats. In nerve cells from AD rats, the
nuclei were swollen and shaped like grapes, along with chromatin
condensation and nuclear membrane invagination. The presence of
swollen mitochondria with broken cristae and an expanded
endoplasmic reticulum was also observed. However, both donepezil
and YZS intervention appeared to reverse the aberrant morphological
ultrastructure in terms of nuclei, mitochondria and endoplasmic
reticulum (Fig. 2B). Taken
together, these findings indicated that YZS could effectively
restore the number of neurons and reduce ultrastructural
abnormalities in the brain of AD rats.
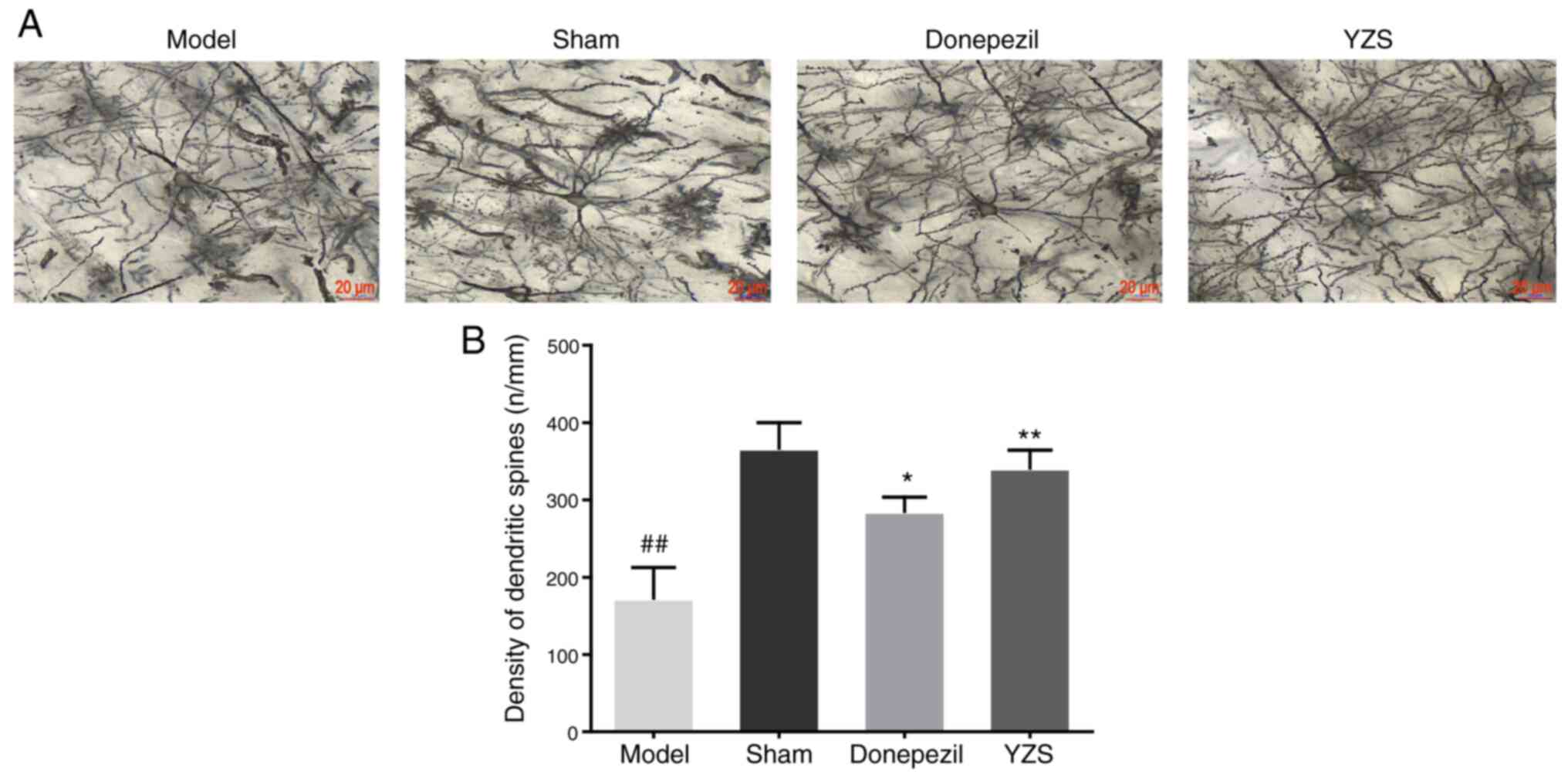
YZS enhances the density of dendritic
spines in AD rats
To further examine the neuroprotective effect of YZS
against Aβ-induced AD, the density of dendritic spines was measured
using Golgi-Cox staining (Fig. 3A).
AD rats had a significantly lower density of dendritic spines
compared with in sham-operated rats (P<0.01; Fig. 3B). Moreover, YZS treatment could
protect against the loss of dendritic spines in the brain of AD
rats, which was reflected by an increased density of dendritic
spines in AD rats treated with YZS or donepezil (P<0.01;
Fig. 3B). Notably, the protective
capability of YZS on dendritic spines appeared stronger compared
with that of donepezil.
YZS inhibits the hyperphosphorylation
of tau protein in AD rats
Tau is a microtubule-associated protein that, in a
hyperphosphorylated state, is considered to self-assemble into a
paired helical filament, and ultimately contribute to the NFT
pathology of AD (10). The results
of the present study detected no significant differences in the
expression of total tau5 protein among all rats (P>0.05;
Fig. 4A and B). Conversely, YZS
administration significantly inhibited the hyperphosphorylation of
tau protein at Ser199 and Thr231 sites (P<0.01; Fig. 4A, C and E). Although p-Tau Ser396
was identified to be hyperphosphorylated in AD rats, the
hyperphosphorylation level of p-Tau Ser396 was barely reduced by
YZS treatment (P>0.05 Fig. 4A and
D). These results indicated that YZS could suppress
hyperphosphorylation of tau protein (Ser199 and Thr231), thereby
decreasing its self-assembly into paired helical filaments and
reducing the NFT pathology in AD rats.
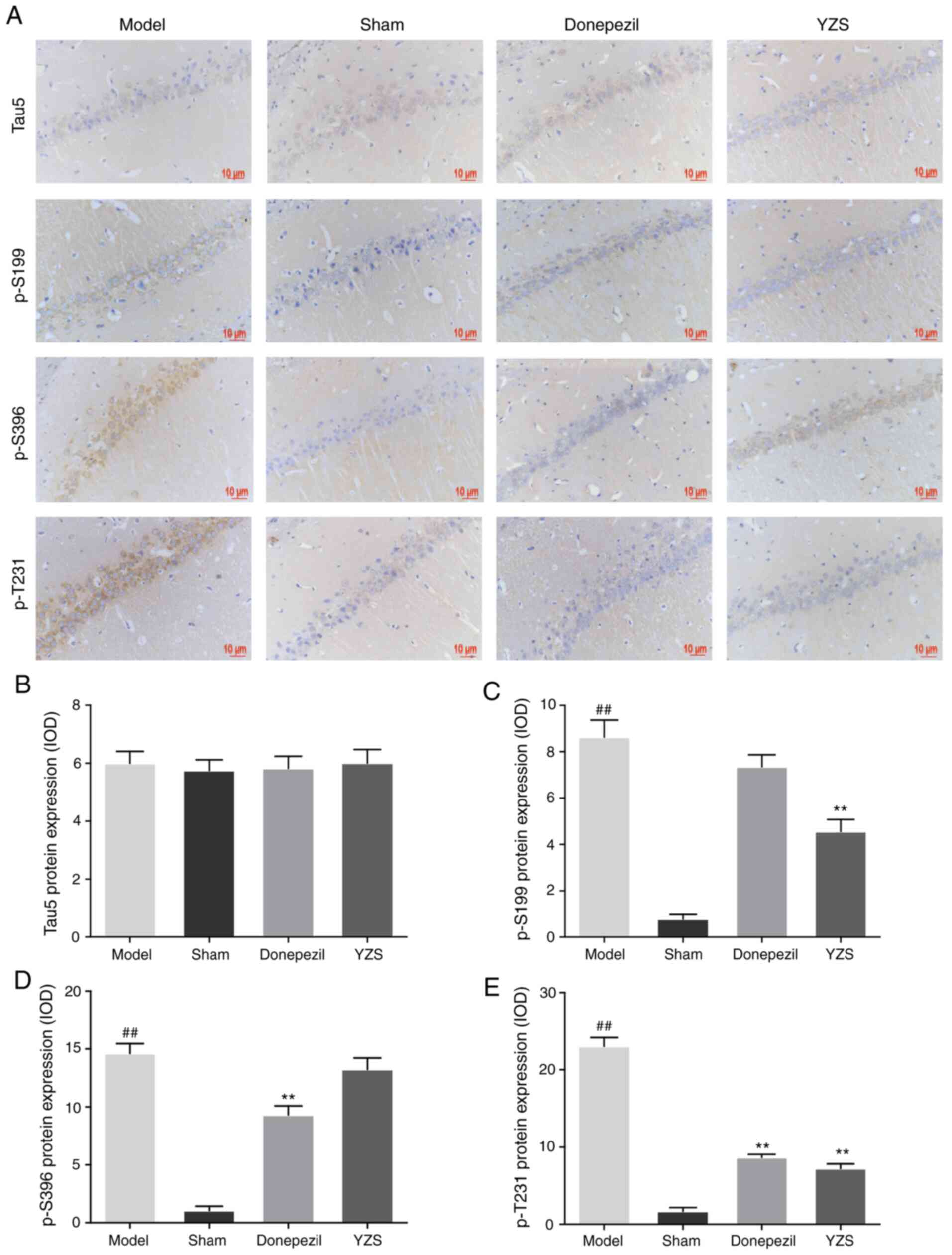 | Figure 4.Effects of YZS on the expression
levels of Tau5, p-S199, p-S396 and p-T231 in Alzheimer's disease
model rats. (A) Representative immunohistochemistry images
demonstrating the expression levels of Tau5, p-S199, p-S396 and
p-T231 in the CA1 region from each group (magnification, ×400).
Analysis of (B) Tau5, (C) p-S199, (D) p-S396 and (E) p-T231 protein
expression levels in each group. Data are presented as the mean ±
SD (n=10). ##P<0.01 vs. Sham group; *P<0.05,
**P<0.01 vs. Model group. p-S199, phosphorylated-Tau (Ser199);
p-S396, phosphorylated-Tau (Ser396); p-T231, phosphorylated-Tau
(Thr231); YZS, Yuan-zhi-san. |
YZS modulates the expression levels of
UPS-related proteins in AD rats
The UPS is essential for degrading misfolded and
damaged intracellular proteins, including tau degradation, and it
has been reported that an impaired UPS is involved in AD
pathogenesis (26). Thus, it was
investigated whether YZS could restore functionality of the UPS by
impacting several vital enzymes involved in the complex enzymatic
cascade of UPS, including UbE1a/b, UbE2a, CHIP, UCH-L1 and 26S
proteasome. Western blot analysis identified that the protein
expression levels of UbE1a/b, UbE2a, CHIP, UCH-L1 and 26S
proteasome were all significantly downregulated in the AD model
rats (P<0.01 or P<0.05; Fig.
5A-E), which suggested an impaired enzymatic cascade of the
UPS. When compared with the AD model rats, these enzymes were
upregulated in the brains of AD rats co-treated with YZS or
donepezil (P<0.01; Fig. 5A-E).
These findings indicated that YZS could, at least in part,
effectively upregulate the protein expression levels of UbE1a/b,
UbE2a, CHIP, UCH-L1 and 26S proteasome, which may facilitate
restoration of the UPS to remove tau accumulation.
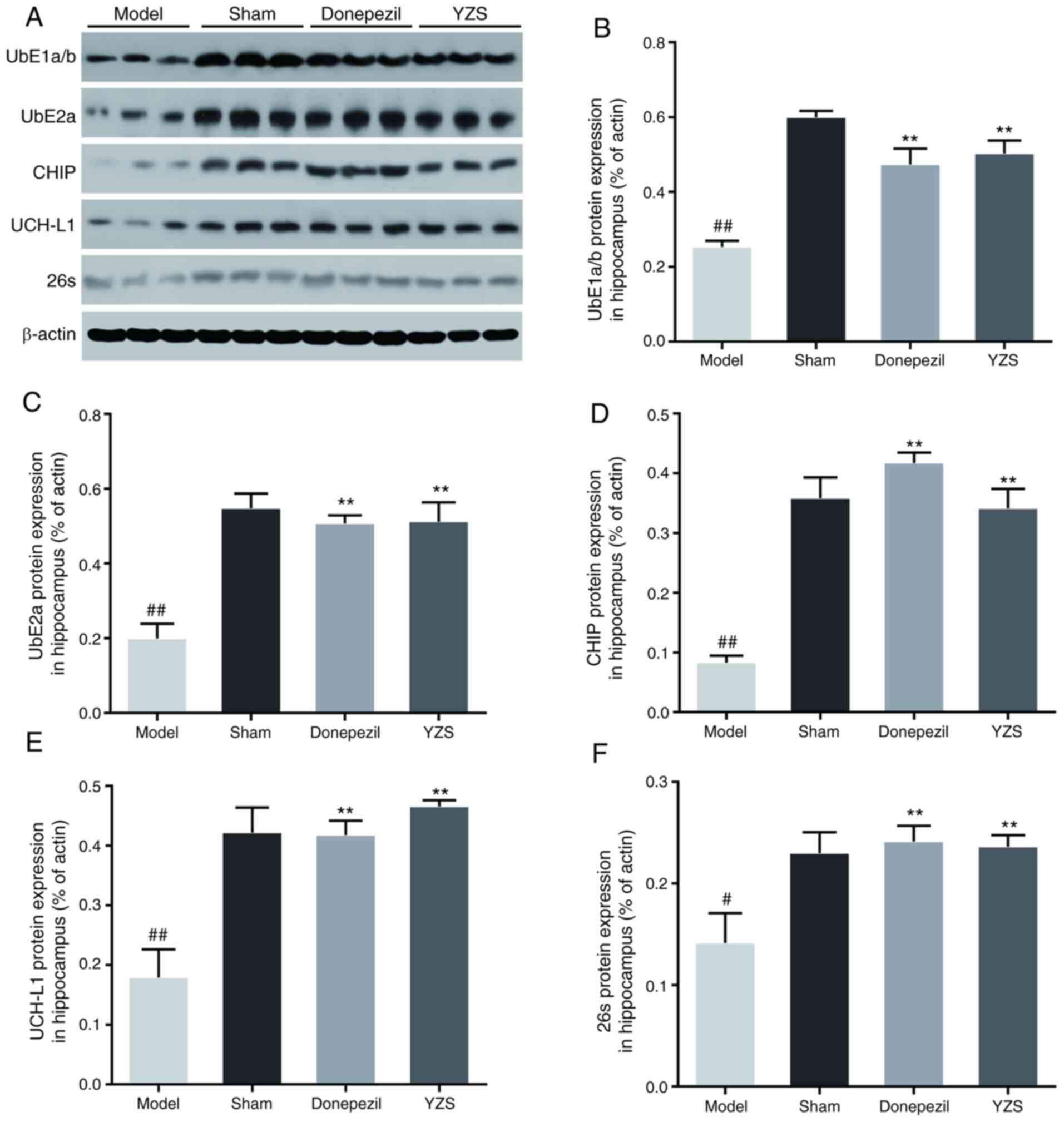 | Figure 5.Effects of YZS on the protein
expression levels of UbE1a/b, UbE2a, CHIP, UCH-L1 and 26S
proteasome in Alzheimer's disease model rats. (A) Representative
western blotting images of UbE1a/b, UbE2a, CHIP, UCH-L1 and 26S
proteasome in each group. Semi-quantification of (B) UbE1a/b, (C)
UbE2a, (D) CHIP, (E) UCH-L1 and (F) 26S proteasome expression
levels in each group. Data are presented as the mean ± SD (rats
n=3). #P<0.05, ##P<0.01 vs. Sham group;
**P<0.01 vs. Model group. UbE1a/b, ubiquitin-activating enzyme
E1a/b; UbE2a, ubiquitin-conjugating enzyme E2a; CHIP, carboxyl
terminus of Hsc70-interacting protein; UCH-L1, ubiquitin C-236
terminal hydrolase L1; YZS, Yuan-zhi-san. |
Discussion
AD is mainly characterized by a progressive loss of
memory and the development of cognitive deficits, leading to
profound dementia. It has been suggested that the two pathological
hallmarks of AD include extracellular Aβ plaques and intracellular
tau tangles (8). Due to the huge
socioeconomic burden caused by AD globally (6,7),
discovering potential effective therapies to treat AD is of great
importance. Several studies have revealed the neuroprotective
effects of YZS, with its multi-component and multi-target
characteristics (18–21).
To verify the present hypothesis that YZS may
enhance learning and memory abilities partly by restoring
UPS-mediated tau degradation, an Aβ-induced AD rat model treated
with YZS was established. The present results demonstrated that
treatment with YZS, similar to donepezil, improved learning and
memory, and restored the number of neurons and reversed
ultrastructural abnormalities. Furthermore, YZS could protect
against the loss of dendritic spines in the brains of AD rats.
These findings suggested that YZS may serve as an effective herbal
formula that brings relief to AD rats.
In the past two decades, a positive association has
been identified between pathological tau aggregation and the
progression of AD (27).
Ultrastructurally, when tau, a microtubule-associated protein, is
in an abnormally hyperphosphorylated state it is consequently
incapable of binding to microtubules and thus self-assembles into
paired helical filaments. Studies have reported that paired helical
filaments serve as the major fibrous component of NFTs found in
cell bodies and apical dendrites (10). Abundant NFTs distribute in the nerve
cells that undergo degeneration and their degree of abundance has
been shown to be closely associated with the severity of AD
(28). In the current study,
hyperphosphorylated tau protein at Ser199, Ser396 and Thr231 sites
was observed in AD model rats. Tau hyperphosphorylation at Ser199
and Thr231 was significantly decreased following YZS treatment,
which may contribute to the remission of AD pathology. However, it
was revealed that YZS treatment exhibited little effect on tau
hyperphosphorylation at Ser396; a possible reason for this is that
p-Tau Ser396 may not be a potential target of YZS.
The UPS is essential for degrading misfolded and
damaged intracellular proteins, including tau degradation.
Therefore, the present study investigated whether YZS was capable
of regulating the expression of UPS-related molecules, which are
critical for functionality of the UPS and may contribute to tau
degradation. Ub-activating enzymes (UbE1) form a thioester bond
with Ub via an ATP-dependent mechanism. Subsequently, Ub is shifted
to Ub-conjugating enzymes (UbE2), which function as scaffold
proteins that favor the interaction between Ub ligase (E3) and the
target substrate protein (11),
allowing the ligase to transfer the Ub from E2 to the substrate
protein (11). Polyubiquitinated
proteins are subsequently recognized and degraded by the 26S
proteasome into small peptides (12). It has been reported that the 26S
proteasome can degrade phosphorylated and non-phosphorylated tau
proteins (29). In the UPS, CHIP,
an E3 ligase, facilitates UPS-mediated tau degradation, and the
activity of CHIP has been reported to be impaired in the human AD
brain (30,31), suggesting that CHIP inhibition may
lead to tau accumulation and exacerbation of AD pathology.
Moreover, UCH-L1 is a protease belonging to the deubiquitinating
enzyme family, which removes Ub from Ub chains or the substrate
(32). It has been established that
inhibition of UCH-L1 activity may decrease the microtubule-binding
ability and increase the phosphorylation of tau protein (33). The present study demonstrated that
the expression levels of UbE1a/b, UbE2a, CHIP, UCH-L1 and 26S
proteasome were significantly decreased in the brains of AD rats,
which indicated an impaired enzymatic cascade of the UPS. Moreover,
co-administration with YZS partly increased the expression levels
of these enzymes, suggesting that YZS may be beneficial in
restoring functionality of the UPS, so as to regain the ability to
degrade ubiquitinated and hyperphosphorylated tau protein. Future
studies should focus on in vitro experiments to further
understand the molecular mechanisms underlying the regulatory
effect of YZS on the UPS.
In conclusion, the present study demonstrated that
YZS may improve the learning and memory abilities, and reduce the
severity of AD pathology in an Aβ-induced AD rat model.
Furthermore, it was identified that YZS could suppress the
hyperphosphorylation of tau protein, which may be partially
associated with its beneficial role in restoring functionality of
the UPS.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81704024), the Sichuan
Science and Technology Program (grant no. 2021YJ0435), the
‘Xing-lin Scholars’ Project of Chengdu University of TCM (grant
nos. QNXZ2019014 and QNXZ2019017) and the ‘Hundred Talents Program’
of the Hospital of Chengdu University of Traditional Chinese
Medicine (grant nos. 20-Y01 and 20-Q05).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL, JHZ and YZ designed the study. JG and PJX
carried out the experiments. JRY and YG interpreted the results of
the experiments. YWH and DYG organized the database and conducted
the statistical analysis. BL and PJX prepared the manuscript. YZ
and JHZ confirmed the authenticity of all the raw data. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Chengdu University of Traditional Chinese Medicine
(approval no. 2017-02).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reitz C, Brayne C and Mayeux R:
Epidemiology of Alzheimer disease. Nat Rev Neurol. 7:137–152. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang HN, Wang MR, Chen XL, Xu YJ, Li J,
Wang HL and Du J: Research on the content construction of dementia
management in community based on Delphi method. Chin Gen Pract.
23:2072–2079. 2020.
|
|
3
|
Haake A, Nguyen K, Friedman L,
Chakkamparambil B and Grossberg GT: An update on the utility and
safety of cholinesterase inhibitors for the treatment of
Alzheimer's disease. Expert Opin Drug Saf Feb. 19:147–157. 2020.
View Article : Google Scholar
|
|
4
|
Titova NV: Memantine: From the original
brand to generics. Zh Nevrol Psikhiatr Im S S Korsakova.
117:136–143. 2017.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patterson C: World Alzheimer report
2018-The state of the art of dementia research: New frontiers.
Alzheimer's Disease International (ADI). 1–48. 2018.
|
|
6
|
El-Hayek YH, Wiley RE, Khoury CP, Daya RP,
Ballard C, Evans AR, Karran M, Molinuevo JL, Norton M and Atri A:
Tip of the Iceberg: Assessing the global socioeconomic costs of
Alzheimer's disease and related dementias and strategic
implications for stakeholders. J Alzheimers Dis. 70:323–341. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jia J, Wei C, Chen S, Li F, Tang Y, Qin W,
Zhao L, Jin H, Xu H, Wang F, et al: The cost of Alzheimer's disease
in China and re-estimation of costs worldwide. Alzheimers Dement.
14:483–491. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roberson ED and Mucke L: 100 years and
counting: Prospects for defeating Alzheimer's disease. Science.
314:781–784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H and Zheng Y: β amyloid hypothesis
in Alzheimer's disease: Pathogenesis, prevention, and management.
Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 41:702–708. 2019.(In Chinese).
PubMed/NCBI
|
|
10
|
Goedert M: Tau protein and the
neurofibrillary pathology of Alzheimer's disease. Trends Neurosci.
16:460–465. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tramutola A, Di Domenico F, Barone E,
Perluigi M and Butterfield DA: It is all about (U)biquitin: Role of
altered ubiquitin-proteasome system and UCHL1 in Alzheimer disease.
Oxid Med Cell Longev. 2016:27560682016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Al Mamun AA, Uddin MS, Kabir MT, Khanum S,
Sarwar MS, Mathew B, Rauf A, Ahmed M and Ashraf GM: Exploring the
promise of targeting ubiquitin-proteasome system to combat
Alzheimer's disease. Neurotox Res. 38:8–17. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee MJ, Lee JH and Rubinsztein DC: Tau
degradation: The ubiquitin-proteasome system versus the
autophagy-lysosome system. Prog Neurobiol. 105:49–59. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sulistio YA and Heese K: The
ubiquitin-proteasome system and molecular chaperone deregulation in
Alzheimer's disease. Mol Neurobiol. 53:905–931. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu G, He WB, Zhao ZQ, Chu SF and Chen NH:
Screening of core herbal combinations in anti-Alzheimer
prescriptions by using Traditional Chinese Medicine Inheritance
System. Chin J Exp Traditional Med Formulae. 22:223–228. 2016.
|
|
16
|
Zheng MY: Treatment of Alzheimer's
dementia in Taiyin people with shiqiupu Yuanzhi powder. J Med Pharm
Chin Minorities. 16:16–17. 2010.
|
|
17
|
Sun ZL, Wang MJ, Wang LJ, Wang F and Li B:
Theoretical discussion on treatment of senile dementia by
supplementing Qi, inducing resuscitation and clearing away heart
fire. J Basic Chin Med. 23:1374–1375+1401. 2017.
|
|
18
|
Guo J, Li B, Wang ZC, Wu YX, Yang Q and
Chen X: Effects of yuanzhi powder on learning and memory ability
and oxidative stress level of D-galactose induced aging mice. Chin
Arch Traditional Chin Med. 37:2144–2147+2314. 2019.
|
|
19
|
Jin YS, Li ML, Jin X, Zhao M, Lu HJ and Xu
QS: Efects of Shichangpuyuanzhisan on learning and memory abilities
in mice model with Alzeimer's disease. J Med Sci Yanbian Uni.
37:108–111. 2014.
|
|
20
|
Li B, Sun ZL, Chen GR, Zheng YQ, He XJ, Li
GM, Yang R, Zou SZ and Chen LW: Effects of Yuanzhi san on ethology
and cerebral acetylcholinesterase activity of memory disorder mouse
model induced by scopolamine. J Guangzhou Uni Traditional Chin Med.
34:733–736. 2017.
|
|
21
|
Qiang WJ, Chen Y, He FY, Xiao MF, Cai WY,
Dai YF, Yang Q, Li YJ, Weng XG, Li Q, et al: Molecular biological
mechanisms of Yuan Zhi powder in the treatment of Alzheimer's
disease: An analysis based on network pharmacology. Digital Chin
Med. 1:90–101. 2018. View Article : Google Scholar
|
|
22
|
Lin J, Gao S, Wang T, Shen Y, Yang W, Li Y
and Hu H: Ginsenoside Rb1 improves learning and memory ability
through its anti-inflammatory effect in Aβ1–40 induced
Alzheimer's disease of rats. Am J Transl Res. 11:2955–2968.
2019.PubMed/NCBI
|
|
23
|
Zhao J, Lu S, Yu H, Duan S and Zhao J:
Baicalin and ginsenoside Rb1 promote the proliferation and
differentiation of neural stem cells in Alzheimer's disease model
rats. Brain Res. 1678:187–194. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu HY, Cui ZH, Li HQ, Wang YR, Chen X, Li
JH, Xv DM and Zheng GQ: Fumanjian, a classic Chinese herbal
formula, can ameliorate the impairment of spatial learning and
memory through apoptotic signaling pathway in the hippocampus of
rats with Aβ 1–40-induced Alzheimer's disease. Evid Based
Complement Alternat Med. 2014:9429172014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paxinos G and Watson C: The rat brain in
stereotaxic coordinates. 6th edition. Amsterdam Boston Academic
Press/Elsevier; 2007
|
|
26
|
Gentier RJ and van Leeuwen FW: Misframed
ubiquitin and impaired protein quality control: An early event in
Alzheimer's disease. Front Mol Neurosci. 8:472015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao Y and Tan L, Yu JT and Tan L: Tau in
Alzheimer's disease: Mechanisms and therapeutic strategies. Curr
Alzheimer Res. 15:283–300. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lacosta AM, Insua D, Badi H, Pesini P and
Sarasa M: Neurofibrillary tangles of Aβx-40 in Alzheimer's disease
brains. J Alzheimers Dis. 58:661–667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang JY, Liu SJ, Li HL and Wang JZ:
Microtubule-associated protein tau is a substrate of
ATP/Mg(2+)-dependent proteasome protease system. J Neural Transm
(Vienna). 112:547–555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Keck S, Nitsch R, Grune T and Ullrich O:
Proteasome inhibition by paired helical filament-tau in brains of
patients with Alzheimer's disease. J Neurochem. 85:115–122. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Keller JN, Hanni KB and Markesbery WR:
Impaired proteasome function in Alzheimer's disease. J Neurochem.
75:436–439. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nijman SM, Luna-Vargas MP, Velds A,
Brummelkamp TR, Dirac AM, Sixma TK and Bernards R: A genomic and
functional inventory of deubiquitinating enzymes. Cell.
123:773–786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie M, Han Y, Yu QT, Wang X, Wang SH and
Liao XM: UCH-L1 inhibition decreases the microtubule-binding
function of tau protein. J Alzheimers Dis. 49:353–363. 2016.
View Article : Google Scholar : PubMed/NCBI
|