Introduction
MicroRNAs (miRNAs) are small non-coding RNAs which
regulate gene expression at the post-transcriptional level in
eukaryotes (1). miR-21 levels are
comparatively high in mammalian cells and upregulated in
association with human colon cancer and chronic lymphocytic
leukemia cells (2,3). This seems to be an extraordinary
property of miR-21 since in a profiling of 540 clinical samples of
cancer patients it was the only constantly upregulated miRNA
(4). The sum of miR-21
characteristics may lead to its utilization as a diagnostic and
prognostic biomarker for diverse types of cancer and as a potential
therapeutic target (5). In result
most studies on miR-21 focused on its association with cancer and
clinical application. However, miR-21 expression also controls
osteoblast-mediated bone formation and osteoclast-related bone
remodeling. For instance, miR-21 can promote and reduce osteogenic
differentiation in MC3T3-E1 cells (6) and human adipose mesenchymal cells
(7), respectively. Alternatively,
miR-21 was highly upregulated during osteoclast differentiation
(8) and regulates RANKL-induced
osteoclastogenesis (9). In
addition, miR-21 was found at increased levels in sera and bone
tissue of osteoporotic patients, and high levels of miR-21 support
fracture healing in preclinical models (10). Mouse models have provided further
insights into the biological function of miR-21 in vivo.
Knockout mice of miR-21 showed delayed early healing
of alveolar socket following tooth extraction (11), impaired bone regeneration of
maxillary bone defects (12), and
enhanced inflammatory osteolysis upon ligature-induced
periodontitis (13). Orthodontic
tooth movement is impaired in miR-21 knockout mice (14–16),
likely because miR-21 deficiency inhibits osteoclast function
(17). During tooth development,
particularly during amelogenesis, miR-21-5p turned out to be
differentially expressed (18).
However, the consequences of miR-21 knockout on the tooth phenotype
and the corresponding alveolar bone have not been investigated so
far.
There is a large variation of the shape in molar
crowns making the metameric evaluation difficult. Consequently
geometric morphometrics (GMM), a multivariate statistical technique
to provide a comprehensive description of morphology aspects based
on landmarks (19) was introduced
to the field (20,21). GMM was originally used in
anthropology (22,23) but also applied in clinical research
on orthodontic tooth movement (24). GMM is now also increasingly
implemented in mouse genetics to define a phenotype. For example,
the craniofacial shape of Twist1+/− mice and wild-type
controls was analyzed by GMM. Twist1+/− mice showed a
consistent pattern of craniofacial dysmorphology affecting all
major regions of the skull (25).
GMM also revealed that Panx3 knockout mice have shorter diaphyseal
shafts compared with wild-type littermates, and relatively larger
areas of muscle attachment sites (24), overall supporting the use of GMM to
identify anatomical changes of bones.
GMM has been used to investigate the tooth phenotype
in mouse models. For example, phylogeny and adaptation affect the
shape of molars of insular mice (26). Mouse dietary groups can be
distinguished with the use of a GMM based on first upper molars
(27). Likewise, the impact of p63
on tooth and jaw development was reported based on GMM (28). GMM was further applied to determine
the impact of BMP7 on the shape of molars in mutant mice (29). Considering the involvement of miR-21
in tooth development and the alveolar bone to follow the anatomy of
the teeth, we hypothesized that miR-21 affects the anatomy of the
tooth and consequently also the dimensions of the alveolar
bone.
Materials and methods
Animals and microcomputed
tomography
miR-21 knockout mice were backcrossed six times into
the C57BL/6J background and maintained by breeding heterozygous
animals. Mice were fed standard chow and kept under controlled
lighting conditions (12 h light, 12 h dark) at the Division of
Biomedical Research at the Medical University of Vienna. Animal
experiments were approved by the local animal welfare committee and
the Austrian Federal Ministry for Science (GZ
BMWFW-66.009/0080-WF/V/3b/2017). We had skulls of 5 female and 3
male littermates, each miR-21 knockout (KO) and corresponding
wild-type (WT) controls. Mice were sacrificed at an age between 39
and 66 weeks with carbon dioxide (at a displacement of volume at
10–30% / per min ensuring a standardized increase and a homogeneous
distribution of the gas inside the modified cage in October 2015).
After cessation of respiratory and cardiac movements (observation
after ≥10 min, at room air) decapitation was performed. The heads
were fixed in 4% formaldehyde for 48 h and transferred into 70%
ethanol. µCT with an isometric voxel size of 17.2 µm was carried
out using a µCT50 device (Scanco Medical AG). The scanning of the
skulls was done at 70 kVp/100 µAs with an integration time of 500
msec.
Data acquisition
Bone and tooth surfaces of these skulls were
generated by using the half maximum height value (HMHV) as
threshold in each individual. The gray values of the tissue of
interest and the adjacent material are measured and the value half
way between the highest and lowest value is used as threshold for
the surface reconstruction. The HMHV give the value most close to
reality and the best standardization for possible differences in
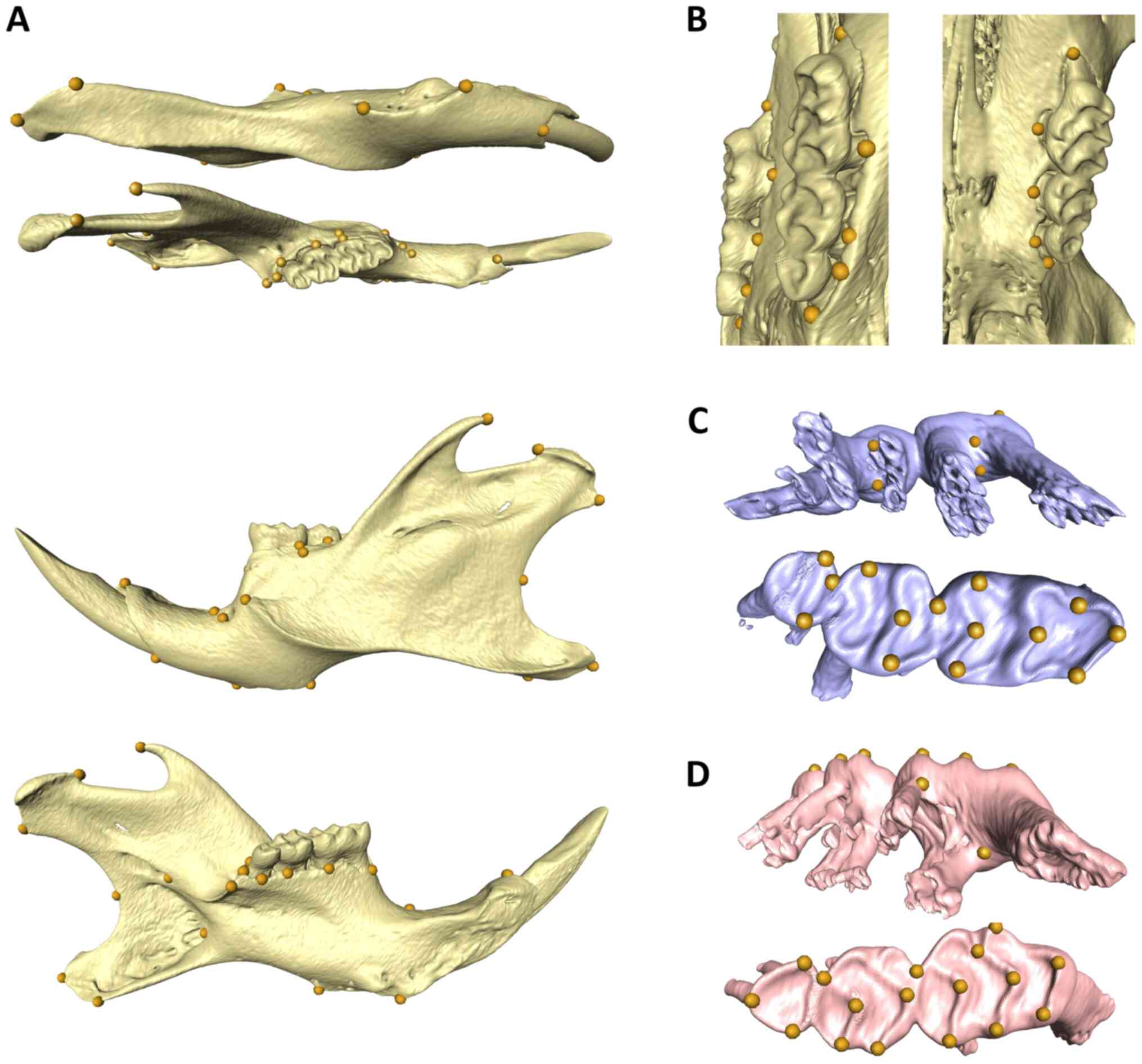
density. We established six different landmark sets for the
different regions, i) upper teeth, ii) lower teeth, iii) upper
alveolar bone, iv) lower alveolar bone, v) the mandible as a whole
and vi) landmarks for jaw lengths. A total of 36 and 38 landmarks
were placed according to the anatomical structure on the occlusal
surface and root furcation of mandibular and maxillary molars.
There are also 8 landmarks on each upper and lower alveolar bone
per side, 17 landmarks on each side of the mandible (Fig. 1). Surface reconstruction and
landmark coordinate retrieval were performed using Amira (Version
6.1; Visage Imaging Inc). For the dental jaw distance, distance
between the temporomandibular joint and the cusps of the first
lower and upper molar were calculated. For the skeletal distance,
distance between the temporomandibular joint and the lower alveolar
inner point of the lower incisor and the prosthion were calculated
for the skeletal distance.
Statistical analysis
We transferred the coordinates of the landmarks into
a text file in morphologika format for analysis in the EVAN Toolbox
1.71 (www.evan-society.org). A generalized
Procrustes analysis was performed to superimpose the landmark
configurations, quantify centroid size, and calculate the
Procrustes distances and Procrustes shape coordinates. Separate
principal component analysis (PCA) with and without the natural log
of centroid sizes provided a representation of morphometric
variation across groups. We used the decision procedure of
Bookstein, when 2Nlog(a/g) exceeds ‘2’ the
principal component is considered for interpretation below ‘2’ the
principle component is considered to only express noise (30,31).
Asymmetry which is described by Procrustes distance between any
form and its reflected relabeling was used as an additional
quantification of perturbed development via the formulas for total,
directional, and fluctuating asymmetry (32). Normality tests and according to the
outcome unpaired t-tests or Mann-Whitney tests were performed using
GraphPad Prism version 8.0.0 for Windows, GraphPad Software,
www.graphpad.com.
Results
Generalized Procrustes analysis of
molars and the dimension of the alveolar bone reveals reduced sizes
in miR-21 knockout mice
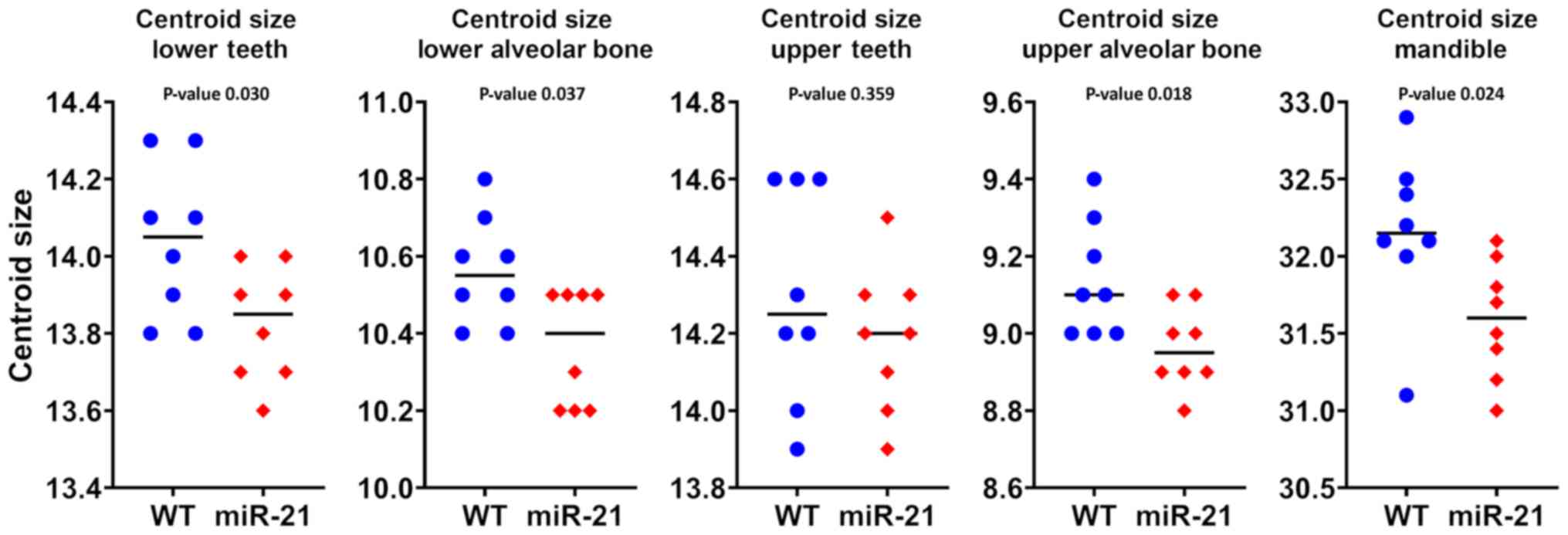
To compare the size of molars a generalized
Procrustes analysis was performed. Based on the 36 and 38 landmarks
placed on mandibular and maxillary molars, respectively, the molars
in the mandible (P=0.02) but not on the maxilla (P=0.36) were
significantly smaller in the miR-21 knockout mice compared to the
wild-type controls. In line with these observations, the dimension
of the alveolar bone of the mandible (P=0.03) and of the maxilla
(P=0.02) was smaller in the miR-21 knockout mice when compared to
the wild-type littermates. In addition, the dimension of the
mandible was reduced as a consequence of the lack of miR-21
(P=0.02). Fig. 2 shows the
statistical values of centroid size. Taken together, the absence of
miR-21 causes smaller molars and the respective alveolar bone in
the mandible and in the maxilla.
Principal component analysis to assess
changes in form and shape
We performed the PCA with (form space) and without
(shape space) the natural log of centroid sizes, which integrates
size and shape information to find a difference in the variance
between miR-21 knockout mice and corresponding wild-type controls.
The two groups were similar in form and shape at all anatomical
sites investigated (lower molars, lower alveolar bone, upper
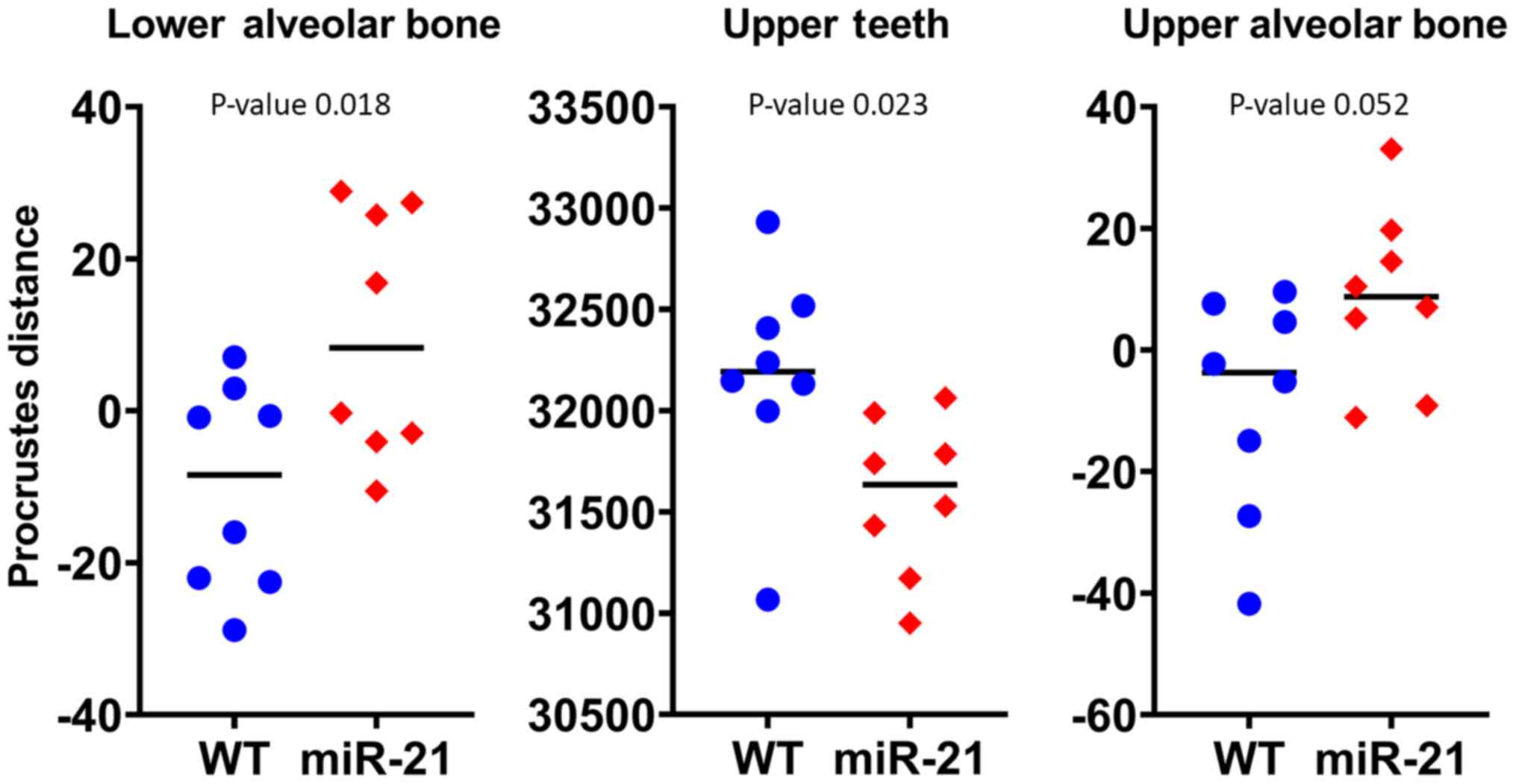
molars, upper alveolar bone, and mandible). In Table I, the variance and noise criterion
of principal component 1 and 2 are shown. The Procrustes distance
values on the PC1 for the lower alveolar bone are significantly
different with size (P=0.01) (Fig.
3).
 | Table I.Variance in form and shape and noise
criterion. |
Table I.
Variance in form and shape and noise
criterion.
| A, PC1 |
|---|
|
|---|
| Site | Variance in form
(%) | 2Nln (a/g) | Variance in shape
(%) | 2Nln (a/g) |
|---|
| Lower molars | 34.3 | 1.8 | 25.9 | 1.3 |
| Upper molars | 42.8 | 7.0a | 19.2 | 0.2 |
| Lower alveo-lar
bone | 35.8 | 3.5a | 23.1 | 0.7 |
| Upper alveo-lar
bone | 33.0 | 2.3a | 21.5 | 0.5 |
| Mandible | 24.8 | 0.7 | 20.1 | 0.2 |
|
| B, PC2 |
|
| Site | Variance in form
(%) | 2Nln
(a/g) | Variance in
shape (%) | 2Nln
(a/g) |
|
| Lower molars | 17.3 | 1.3 | 14.4 | 0.2 |
| Upper molars | 10.8 | 0.1 | 15.0 | 0.2 |
| Lower alveo-lar
bone | 13.8 | 0.3 | 15.4 | 0.1 |
| Upper alveo-lar
bone | 15.3 | 0.5 | 15.1 | 0.3 |
| Mandible | 16.4 | 0.3 | 16.0 | 0.2 |
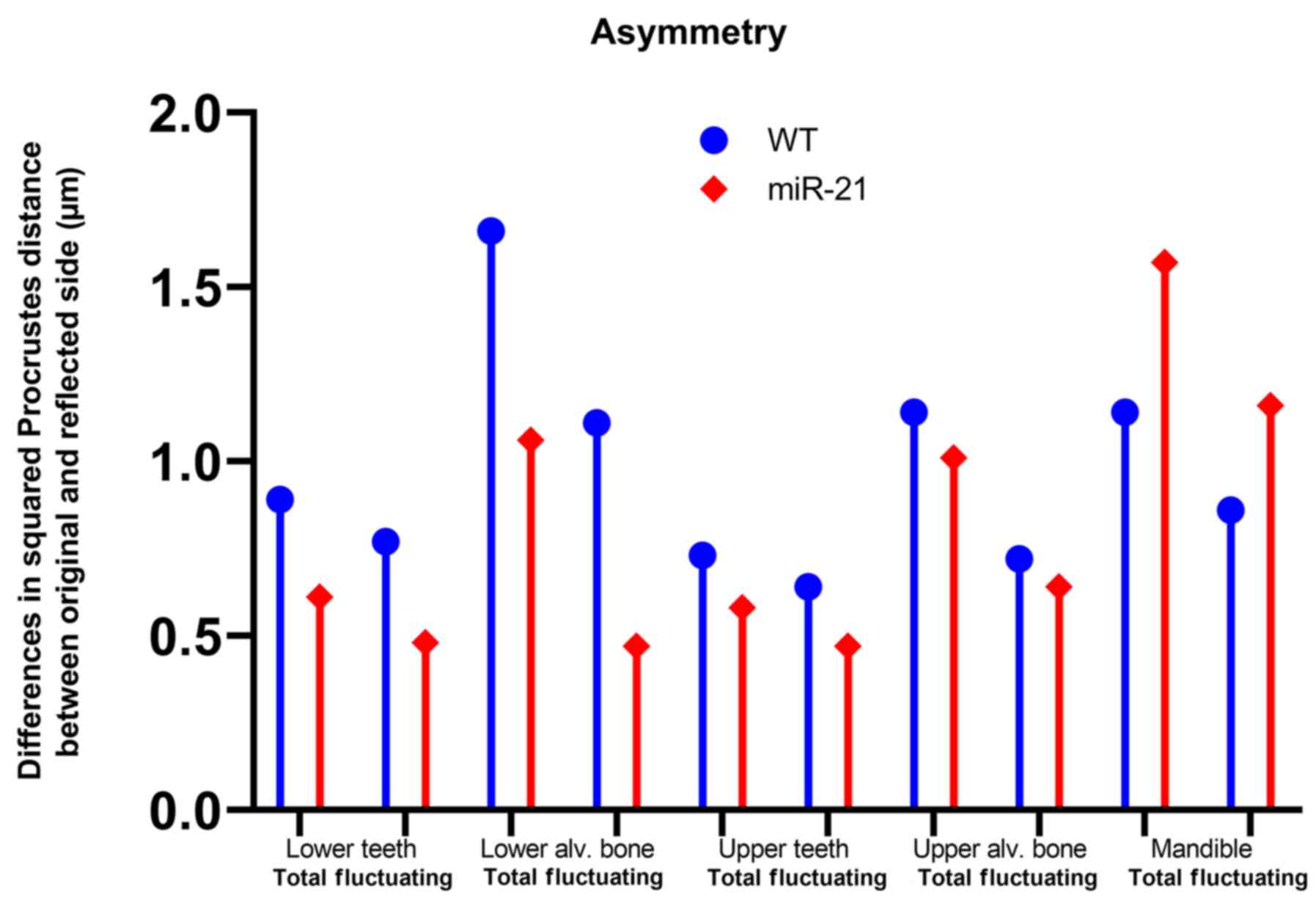
Asymmetry analysis as a parameter for
developmental stress and instability
The values presented are by their nature of
calculation (32) group values,
since directional and fluctuating asymmetry depend on the squared
Procrustes distance between the original mean and the reflected
mean of the landmarks. For that reason, significance values are not
provided and the values should be interpreted as means. miR-21
knockout reduced the fluctuating asymmetry of the molars in the
mandible and the maxilla by 38 and 27%, respectively. Fluctuating
asymmetry of the respective alveolar bone was also reduced in
miR-21 knockout mice by 58 and 12% (Fig. 4)
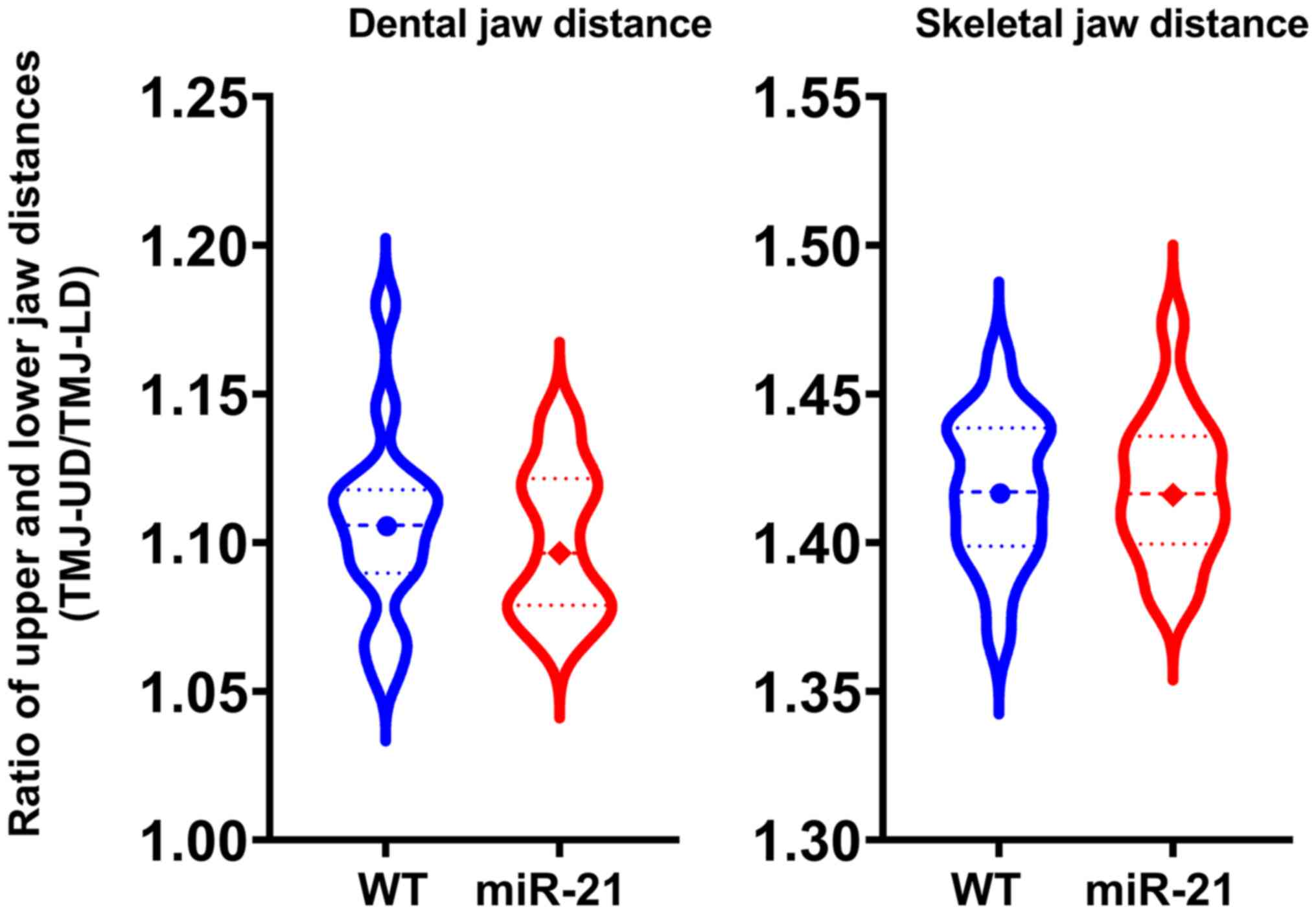
Dental and skeletal jaw length as a
parameter for genetic diseases
Jaw lengths of miR-21 knockout mice showed no
differences in either dental or skeletal length when compared to
the wild-type group (Fig. 5;
P=0.72, P=0.95).
Discussion
Considering that microRNAs are involved in tooth
development (33) and the notion
that miR-21 is differentially expressed during this process,
particularly during amelogenesis, it is reasonable to suggest that
miR-21 knockout mice generate a tooth phenotype. However, miR-21
knockout mice show a normal skeletal phenotype suggesting that, if
at all, only marginal changes in tooth morphology can be expected.
Bearing in mind that metameric evaluation of teeth is problematic,
it requires GMM to reveal if miR-21 plays indeed a role during
tooth development. According to this landmark-based statistical
method, we demonstrate that the molars and the alveolar bone in the
mandible and in the maxilla are smaller in the miR-21 knockout mice
when compared to the wild-type controls. No changes in shape were
noticed. miR-21 knockout also reduced the fluctuating asymmetry of
the molars in both, the mandible and the maxilla suggesting a
moderate effect on tooth development. Dental and skeletal jaw
length can be influenced by genetic diseases (34), however, dental and skeletal jaw
length showed no difference between the groups. Taken together, our
data suggest that miR-21 affects, even though at a moderate level,
molar development and the dimensions of the corresponding alveolar
bone in mice.
Our findings basically support the ground tenor that
microRNAs regulate tooth morphogenesis by fine-tuning the signaling
network (33). Our research is
among the pioneer study using GMM to identify the impact of miRNAs
on tooth and alveolar bone morphology. The present study extends
the use of GMM in mouse dental research such as phylogeny and shape
adaptation of molars of insular mice and to distinguish dietary
groups based on first upper molars. We applied GMM not only on
molars but also on the respective alveolar bone in mouse models.
This research is consistent with previous work with GMM to detect
size shape and fluctuating asymmetry of the mandible and teeth in
mouse models (35,36). Fluctuating asymmetry is considered
to be the product of developmental stress and instability, caused
by both genetic and environmental stressors (37). A gene defect which results in the
absence of a protein may cause developmental stress or uncanalized
development leading to the inability of an organism to compensate
(canalize) this stress which is often associated with excess
morphological variance or higher asymmetry (37,38).
miR-21 knockout mice present here with less asymmetry which is the
opposite. One reason to explain this could be that miR21 does not
code for a protein but as a micro RNA is a regulator by itself. The
interaction with other regulating mechanism could result in an
imbalance of tissue apposition and therefore higher asymmetry in
the wild-type. A second reason could be the smaller size of the
more symmetrical tissue. At the level of hard tissue, failure to
produce perfect symmetry is manifested by different apposition
rates, different tooth eruption and suture fusion times in the
developing organism. With less tissue mass, there seems to be a
lower probability of failure in the symmetric apposition of tissue.
The opposite was found in Sost KO mice where more bone mass lead to
higher asymmetry (34).
A study limitation is that the presented work
remains descriptive; thus, we have no explanation about the
underlying molecular and cellular mechanisms that cause the smaller
size of teeth in miR-21 knockout mice. However, new hypotheses
originate from our cross search of genes regulated by miR-21 by
miRWalk2.0 and genes associated with GO terms by amigo.geneontology.org (39) for ‘tooth’: Four target genes were
identified, namely peroxisome proliferator-activated receptor alpha
(PPARA), endoribonuclease dicer (DICER1), activating transcription
factor 2 (ATF2), and osteoprotegerin (TNFRSF11B, OPG).
Interestingly, and similar to the dental phenotype of miR-21
knockout mice, a slight decrease in the size of the molars was
observed in PPARA knockout mice. Thus, miR-21 might exert its
function during tooth development by modulation of PPARA
translational activity. DICER1 is involved in the biogenesis of
most small RNAs, including miR-21 and plays a central role in tooth
development. ATF2 activation occurs in the late secretion phase of
ameloblasts apical to the transition zone of rat incisors OPG
production by the dental follicle likely affects the alveolar bone
resorption needed for tooth eruption. The present observations are
a primer for a more detailed analysis on the expression changes of
the putative target genes in miR-21 mice, with a particular focus
on the cell involved in tooth formation (40).
Matrix metalloproteinases (MMPs) might offer another
link between miR-21 and tooth anatomy. Mice deficient for MMP14,
the membrane-type 1 metalloproteinase (MT1-MMP), have impaired
tooth eruption and root elongation (41), and the expression of MT1-MMP was
enhanced by miR-21 mimics in mesenchymal cells (42). Also, MMP20 and kallikrein-related
peptidase 4 (KLK4) are required to harden enamel (43). There are potential miRNA-binding
sites in the 3′-untranslated region of several MMPs (44) and miRNAs can participate in MMP
regulation at the posttranscriptional level and change the
expression of MMP genes (45).
miR-21 promotes upregulation of MMP2 and MMP9 in human
hepatocellular and pancreatic carcinoma cells (46,47).
It would be worth studying a possible involved of miR-21 in the
regulation of MMP20 and KLK4. In general, the association of genes
that play a role in tooth development being modulated by miR-21
might be the basis for future research.
The results presented here demonstrate that the
molars and the respective alveolar bone in the mandible are
significantly smaller in the miR-21 knockout mice compared to the
wild-type controls. Shape changes were not found but a reduced
asymmetry for either of the anatomical sites. It will now be
critical to determine the molecular and cellular mechanisms how
miR-21 affects tooth growth.
Acknowledgements
The authors would like to thank Ms. Astrid Fabry,
(Center of Biomedical Research, Department for Laboratory Animal
Science and Genetics, Medical University of Vienna, Austria) for
excellent assistance with animal care. The authors would also like
to acknowledge Professor Eric N. Olson (UT Southwestern, Dallas,
TX, USA) for providing miR-21 knockout animals.
Funding
Yuxin Ni received a Scholarship from the
Eurasia-Pacific Uninet. The present study was supported by the
Osteology Foundation (grant no. YG 15-244), the Austrian Science
Fund (grant no. 4072-B28) and Herzfelder'sche Familienstiftung
(grant nos. P30623 and I2514-B28).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
UYS, YN, JG and RG made substantial contributions to
conception and design. UYS, YN, YZ, LTZ, MS, MH and JG acquired the
landmark data. UYS, YN and RG analyzed and interpreted the data.
UYS, YN and RG drafted the manuscript. UYS, YN, YZ, LTZ, MS, MH, JG
and RG critically revised the manuscript for important intellectual
content. UYS, YN, YZ, LTZ, MS, MH, JG and RG gave final approval of
the version to be published. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the local animal
welfare committee and the Austrian Federal Ministry for Science
(approval no. GZ BMWFW-66.009/0080-WF/V/3b/2017).
Patient consent for publication
Not applicable.
Competing interests
JG and MH are co-founders of TAmiRNA GmbH. The
remaining authors declare that they have no competing
interests.
References
|
1
|
Griffiths-Jones S, Grocock RJ, van Dongen
S, Bateman A and Enright AJ: miRBase: microRNA sequences, targets
and gene nomenclature. Nucleic Acids Res. 34:D140–D144. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng YH, Wu CL, Tsao CJ, Chang JG, Lu PJ,
Yeh KT, Uen YH, Lee JC and Shiau AL: Deregulated expression of
sprouty2 and microRNA-21 in human colon cancer: Correlation with
the clinical stage of the disease. Cancer Biol Ther. 11:111–121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fulci V, Chiaretti S, Goldoni M, Azzalin
G, Carucci N, Tavolaro S, Castellano L, Magrelli A, Citarella F,
Messina M, et al: Quantitative technologies establish a novel
microRNA profile of chronic lymphocytic leukemia. Blood.
109:4944–4951. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng YH and Tsao CJ: Emerging role of
microRNA-21 in cancer. Biomed Rep. 5:395–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li H, Yang F, Wang Z, Fu Q and Liang A:
MicroRNA-21 promotes osteogenic differentiation by targeting small
mothers against decapentaplegic 7. Mol Med Rep. 12:1561–1567. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weilner S, Skalicky S, Salzer B, Keider V,
Wagner M, Hildner F, Gabriel C, Dovjak P, Pietschmann P,
Grillari-Voglauer R, et al: Differentially circulating miRNAs after
recent osteoporotic fractures can influence osteogenic
differentiation. Bone. 79:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kagiya T and Nakamura S: Expression
profiling of microRNAs in RAW264.7 cells treated with a combination
of tumor necrosis factor alpha and RANKL during osteoclast
differentiation. J Periodontal Res. 48:373–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugatani T, Vacher J and Hruska KA: A
microRNA expression signature of osteoclastogenesis. Blood.
117:3648–3657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Y, Xu L, Huang S, Hou Y, Liu Y, Chan
KM, Pan XH and Li G: mir-21 overexpressing mesenchymal stem cells
accelerate fracture healing in a rat closed femur fracture model.
BioMed Res Int. 2015:4123272015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strauss FJ, Stähli A, Kobatake R, Tangl S,
Heimel P, Apaza Alccayhuaman KA, Schosserer M, Hackl M, Grillari J
and Gruber R: miRNA-21 deficiency impairs alveolar socket healing
in mice. J Periodontol. May 12–2020.(Epub ahead of print). doi:
10.1002/JPER.19-0567. View Article : Google Scholar
|
|
12
|
Wang H, Wang H, Li X, Zhang Z, Zhao X,
Wang C and Wei F: MicroRNA-21 promotes bone reconstruction in
maxillary bone defects. J Oral Rehabil. Sep 25–2019.(Epub ahead of
print). doi: 10.1111/joor.12896 2019.
|
|
13
|
Zhou W, Su L, Duan X, Chen X, Hays A,
Upadhyayula S, Shivde J, Wang H, Li Y, Huang D, et al: MicroRNA-21
down-regulates inflammation and inhibits periodontitis. Mol
Immunol. 101:608–614. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu L, Su Y, Lin F, Zhu S, Wang J, Hou Y,
Du J, Liu Y and Guo L: MicroRNA-21 promotes orthodontic tooth
movement by modulating the RANKL/OPG balance in T cells. Oral Dis.
26:370–380. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen N, Sui BD, Hu CH, Cao J, Zheng CX,
Hou R, Yang ZK, Zhao P, Chen Q, Yang QJ, et al: MicroRNA-21
Contributes to Orthodontic Tooth Movement. J Dent Res.
95:1425–1433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barnett RE, Conklin DJ, Ryan L, Keskey RC,
Ramjee V, Sepulveda EA, Srivastava S, Bhatnagar A and Cheadle WG:
Anti-inflammatory effects of miR-21 in the macrophage response to
peritonitis. J Leukoc Biol. 99:361–371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu CH, Sui BD, Du FY, Shuai Y, Zheng CX,
Zhao P, Yu XR and Jin Y: miR-21 deficiency inhibits osteoclast
function and prevents bone loss in mice. Sci Rep. 7:431912017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin K, Hacia JG, Zhong Z and Paine ML:
Genome-wide analysis of miRNA and mRNA transcriptomes during
amelogenesis. BMC Genomics. 15:9982014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klingenberg CP: Size, shape, and form:
Concepts of allometry in geometric morphometrics. Dev Genes Evol.
226:113–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gómez-Robles A, Martinón-Torres M,
Bermúdez de Castro JM, Margvelashvili A, Bastir M, Arsuaga JL,
Pérez-Pérez A, Estebaranz F and Martínez LM: A geometric
morphometric analysis of hominin upper first molar shape. J Hum
Evol. 53:272–285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gunz P and Mitteroecker P: Semilandmarks:
A method for quantifying curves and surfaces. Hystrix. 24:103–109.
2013.
|
|
22
|
Gómez-Robles A, Martinón-Torres M,
Bermúdez de Castro JM, Prado L, Sarmiento S and Arsuaga JL:
Geometric morphometric analysis of the crown morphology of the
lower first premolar of hominins, with special attention to
Pleistocene Homo. J Hum Evol. 55:627–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Badawi-Fayad J and Cabanis EA:
Three-dimensional Procrustes analysis of modern human craniofacial
form. Anat Rec (Hoboken). 290:268–276. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caskenette D, Penuela S, Lee V, Barr K,
Beier F, Laird DW and Willmore KE: Global deletion of Panx3
produces multiple phenotypic effects in mouse humeri and femora. J
Anat. 228:746–756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parsons TE, Weinberg SM, Khaksarfard K,
Howie RN, Elsalanty M, Yu JC and Cray JJ Jr: Craniofacial shape
variation in Twist1+/− mutant mice. Anat Rec (Hoboken).
297:826–833. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ledevin R, Chevret P, Ganem G,
Britton-Davidian J, Hardouin EA, Chapuis JL, Pisanu B, da Luz
Mathias M, Schlager S, Auffray JC, et al: Phylogeny and adaptation
shape the teeth of insular mice. Proc Biol Sci. 283:2832016.
|
|
27
|
Gómez Cano AR, Hernández Fernández M and
Alvarez-Sierra MA: Dietary ecology of Murinae (Muridae, Rodentia):
A geometric morphometric approach. PLoS One. 8:e790802013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paradis MR, Raj MT and Boughner JC: Jaw
growth in the absence of teeth: The developmental morphology of
edentulous mandibles using the p63 mouse mutant. Evol Dev.
15:268–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zurowski C, Jamniczky H, Graf D and
Theodor J: Deletion/loss of bone morphogenetic protein 7 changes
tooth morphology and function in Mus musculus: Implications for
dental evolution in mammals. R Soc Open Sci. 5:1707612018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bookstein FL: Measuring and Reasoning:
Numerical Inference in the Sciences. University Press; Cambridge:
2014, View Article : Google Scholar
|
|
31
|
Coquerelle M, Bookstein FL, Braga J,
Halazonetis DJ, Weber GW and Mitteroecker P: Sexual dimorphism of
the human mandible and its association with dental development. Am
J Phys Anthropol. 145:192–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mardia kV, Bookstein FL and Moreton IJ:
Statistical assessment of bilateral symmetry of shapes. Biometrika.
87:285–300. 2000. View Article : Google Scholar
|
|
33
|
Sehic A, Tulek A, Khuu C, Nirvani M, Sand
LP and Utheim TP: Regulatory roles of microRNAs in human dental
tissues. Gene. 596:9–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schwarze UY, Dobsak T, Gruber R and
Bookstein FL: Anatomical similarity between the Sost-knockout mouse
and sclerosteosis in humans. Anat Rec. 303:2295–2308. 2020.
View Article : Google Scholar
|
|
35
|
Keller JM, Allen DE, Davis CR and Leamy
LJ: 2,3,7,8-Tetrachlorodibenzo-p-dioxin affects fluctuating
asymmetry of molar shape in mice, and an epistatic interaction of
two genes for molar size. Heredity. 98:259–267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klingenberg CP, Leamy LJ, Routman EJ and
Cheverud JM: Genetic architecture of mandible shape in mice:
Effects of quantitative trait loci analyzed by geometric
morphometrics. Genetics. 157:785–802. 2001.PubMed/NCBI
|
|
37
|
Schaefer K, Lauc T, Mitteroecker P, Gunz P
and Bookstein FL: Dental arch asymmetry in an isolated Adriatic
community. Am J Phys Anthropol. 129:132–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wilkins AS: Canalization: A molecular
genetic perspective. BioEssays. 19:257–262. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gene Ontology C; Gene Ontology Consortium:
Gene Ontology Consortium: Going forward. Nucleic Acids Res.
43D:D1049–D1056. 2015.
|
|
40
|
Krivanek J, Soldatov RA, Kastriti ME,
Chontorotzea T, Herdina AN, Petersen J, Szarowska B, Landova M,
Matejova VK, Holla LI, et al: Dental cell type atlas reveals stem
and differentiated cell types in mouse and human teeth. Nat Commun.
11:48162020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Beertsen W, Holmbeck K, Niehof A, Bianco
P, Chrysovergis K, Birkedal-Hansen H and Everts V: On the role of
MT1-MMP, a matrix metalloproteinase essential to collagen
remodeling, in murine molar eruption and root growth. Eur J Oral
Sci. 110:445–451. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao W, Dong Y, Wu C, Ma Y, Jin Y and Ji
Y: miR-21 overexpression improves osteoporosis by targeting RECK.
Mol Cell Biochem. 405:125–133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu Y, Smith CE, Richardson AS, Bartlett
JD, Hu JC and Simmer JP: MMP20, KLK4, and MMP20/KLK4 double null
mice define roles for matrix proteases during dental enamel
formation. Mol Genet Genomic Med. 4:178–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dalmay T and Edwards DR: MicroRNAs and the
hallmarks of cancer. Oncogene. 25:6170–6175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu Q, Wang Z, Hu Y, Li J, Li X, Zhou L
and Huang Y: miR-21 promotes migration and invasion by the
miR-21-PDCD4-AP-1 feedback loop in human hepatocellular carcinoma.
Oncol Rep. 27:1660–1668. 2012.PubMed/NCBI
|
|
47
|
Giovannetti E, Funel N, Peters GJ, Del
Chiaro M, Erozenci LA, Vasile E, Leon LG, Pollina LE, Groen A,
Falcone A, et al: MicroRNA-21 in pancreatic cancer: Correlation
with clinical outcome and pharmacologic aspects underlying its role
in the modulation of gemcitabine activity. Cancer Res.
70:4528–4538. 2010. View Article : Google Scholar : PubMed/NCBI
|