Introduction
An increasing number of studies have shown that
vascular endothelial dysfunction is an important pathological
change in hypertension, and is also the initial factor underlying
vascular injury in hypertension (1). Vascular endothelial cell (VEC) damage
is the main cause of hypertension, and its degree of damage is
closely associated with the severity of hypertension (2). In turn, hypertension can also cause
VEC function damage. When hypertension occurs, VECs are stimulated
by the blood flow shear stress and blood flow pulsation, which
induces cell damage, resulting in abnormal synthesis and secretion
of bioactive substances, and finally, abnormal VEC function
(3). Following injury of VECs,
numerous active substances are released, such as thromboxane A2 and
ADP, which can affect the coordination of endothelin-1
(ET-1)/nitric oxide, increase the synthesis of ET-1, destroy the
balance of homeostasis, cause the disorder of vascular tension
regulation, and eventually lead to raised blood pressure (4,5).
Previous studies have demonstrated that VECs can synthesize and
release prostacyclin (PGI2), a vasodilator, which can inhibit
platelet aggregation and regulate vasodilation (6). Moreover, PGI2 can inhibit the
apoptosis of VECs through peroxisome proliferator-activated
receptor (7). Therefore,
identifying the bioactive substances secreted by VECs may provide
novel ideas for the treatment of hypertension.
Peptides are a type of small molecule active
substance composed of <50 amino acids, and are widely involved
in various biological functions, such as vasodilatation (8), oxidative stress (9), cell differentiation (10) and apoptosis (11). Due to the advantages of small
molecular weight (MW), good targeting and easy-to-enter cells,
peptides have attracted increased attention in the field of drug
research (12,13). A growing number of peptides are
being demonstrated to play a vital role in the clinical diagnosis
and treatment of various cardiovascular diseases. For example,
atrial natriuretic peptide and brain natriuretic peptide (BNP) have
been widely used as biomarkers for screening in patients with heart
failure (14), and lyophilized
recombinant human BNP has been used in the clinical treatment of
heart failure (15). Bradykinin,
composed of nine amino acids, not only has vasodilation and
hypotensive effects (16), but has
also been reported to have protective effects against myocardial
ischemia-reperfusion injury (17).
An increasing amount of evidence has demonstrated that Apelin, a
vasoactive peptide of endogenous ligand of the apelin receptor, has
regulatory effects in cardiovascular physiology and
pathophysiology, which makes it a potential target for
cardiovascular drug discovery and development (18). Therefore, it has been speculated
whether VECs may protect against cell injury by secreting certain
peptides under stress.
With the rapid development of mass spectrometry
(MS), the concept of peptidomics has been widely investigated by
researchers in previous years (19). Peptidomics is a novel research
method, which can directly analyze small bioactive peptides in
various biological samples by liquid chromatography tandem MS
(LC-MS/MS) (20,21). In the present study, LC-MS/MS
technology was used to investigate the peptides involved in VEC
protection stimulated by Ang II. A total of 211 peptides were
identified from the cell supernatant, of which, six were
upregulated and 13 were downregulated when compared with the
control group. Subsequently, the present study analyzed the
identified peptides via bioinformatics, and successfully screened a
novel peptide, VMP-19, that can alleviate the apoptosis and
oxidative stress injury of VECs induced by Ang II.
Materials and methods
Cell culture and experimental
design
Human umbilical vein endothelial cells (HUVECs) were
acquired from the Type Culture Collection Committee of Chinese
Academy of Science (cat. no. 3142C0001000001250). HUVECs were
cultured in Endothelial Cell Medium (ScienCell Research
Laboratories, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 1% penicillin-streptomycin
(Wisent, Inc.) at 37°C in an incubator with 95% air and 5%
CO2. When the cell confluency reached 70–80%, the cells
were starved in serum-free medium for 6 h, and then treated with
Ang II (1 µM) for 24 h at 37°C. The peptides (10, 20, 50 or 100 µM)
were administered 2 h before Ang II treatment under the same
conditions.
The cell damage model of HUVECs was induced by Ang
II (Sigma Aldrich; Merck KGaA). For the initial peptidomic
experiments, cells were allocated to two groups: Control group and
Ang II (1 µM) treatment group. For the subsequent cell experiments,
cells were designated to the following groups: i) Scrambled peptide
group (Scr); ii) VMP-19 peptide group (VMP-19); iii) Ang II (1 µM)
and scrambled peptide cotreatment group (Ang II+Scr); and iv) Ang
II (1 µM) and VMP-19 peptide cotreatment group (Ang II+VMP-19).
Peptide extraction
The present study collected the cell supernatant of
three control groups and three Ang II treatment groups for the
peptidomics analysis. The supernatant samples were extracted and
ultrafiltrated to 20 µl with a 10 kDa ultrafiltration tube at
10,000 × g. The aforementioned steps were repeated in order to
collect the filtered liquid and discard the protein and other
macromolecules with a MW >10 kDa in the ultrafiltration tube.
The desalting column was activated with 200 µl 0.1% trifluoroacetic
acid (TFA) and 80% acetonitrile, balanced with 400–600 µl 0.1% TFA
and 1% acetonitrile solution. The sample was dissolved with 200 µl
0.1% TFA aqueous solution and added into the desalting column to
make the sample flow through the desalting column slowly. The
peptides were captured by the desalting column, and other
non-hydrophobic small molecules, such as salt, were discarded.
Then, 200 µl 0.1% TFA and 0.5% acetonitrile solutions were added to
the desalting column to remove the residual salts. Next, 300 µl
0.1% TFA and 80% acetonitrile solution were added to slowly flow
through the desalting column to elute the peptides. The elution
solution was collected with a new EP tube, and then
freeze-dried.
LC-MS/MS and peptide
identification
The nano-LC-MS/MS on a Q Exactive Plus MS (Thermo
Fisher Scientific, Inc.) coupled with a LC1000 was used to identify
peptides. Solvent A buffer [Milli-Q (EMD Millipore) water with 2%
acetonitrile and 0.1% formic acid] and solvent B buffer (90%
acetonitrile and 0.1% formic acid) were utilized for the
chromatographic separation. The samples were resuspended with 20 µl
solvent A, separated via nano-LC and analyzed using online
electrospray tandem MS. A total of 3 µl peptide sample was loaded
onto the analytical column (75×250 µm; Acclaim PepMap C18; Thermo
Fisher Scientific, Inc.). The peptides were eluted with 5% solvent
B for 5 min, 5–40% solvent B for 65 min, 40–80% solvent B for 1
min, 80% solvent B for 4 min and 5% solvent B >20 min at 300
nl/min. The MS spectra were acquired in the mass range of 350–2,000
m/z with a mass resolution of 120K. The AGC target was set to
1×106, and the maximum injection time was 45 msec. In
total, ten sequential high energy collisional dissociation MS/MS
scans with a resolution of 15K were acquired in orbitrap. The
intensity threshold was 50,000, and the maximum injection time was
35 msec. The AGC target was set to 1.0×105, and the
isolation window was 1.6 m/z. Xcalibur™ software (3.1.66.10; Thermo
Fisher Scientific, Inc.) was used to perform 10 sec dynamic
exclusion, automatic peak identification and tandem MS analysis of
the first 20 precursor protein ions at 30% normalized collision
energy.
The intensity of peptides was identified through
label-free quantification, and all MS/MS data were analyzed using
MaxQuant software (version 1.6.6.0; Max-Planck-Institute of
Biochemistry). The Uniprot-SwissProt_homo sapiens database
(https://www.uniprot.org/taxonomy/9606; UniProt release
2019-08-09) was used to search and analyze the data. The mass
tolerance of the parent ion in MaxQuant was 10.0 PPM and the
fragment ion was 0.050 Da. P<0.05 (Student's t-test) and
fold-change >2 were used as the selection criteria for
differentially expressed peptides.
Bioinformatics analysis
The heat map was drawn using MetaboAnalyst 5.0
software (http://www.metaboanalyst.ca/). The MW and isoelectric
point (PI) information of the peptides were analyzed online
(https://web.expasy.org/protparam/).
The precursor proteins of identified peptides were analyzed using
the UniProt database. The potential functions of the precursor
proteins from the identified peptides were investigated according
to the ‘Molecular Function’, ‘Cellular Component’ and ‘Biological
Process’ categories in the Gene Ontology (GO) (22,23)
and Kyoto Encyclopedia of Genes and Genomes (KEGG) (24) pathways using the Functional
Annotation Tool, Database for Annotation, Visualization and
Integrated Discovery (DAVID) Bioinformatics Resource 6.8
(https://david.ncifcrf.gov/). Using the
Search Tool for the Retrieval of Interacting Genes/Proteins
(STRING) database (https://string-db.org/; version, 11.0; medium
confidence 0.400), the protein-protein interaction networks were
generated and analyzed.
Peptide synthesis and
administration
The VMP-19 peptide sequence was LLQDSVDFSLADAINTEFK,
and its Scr was NLQSVDLLIADTKFELFSD. The present study selected the
top six peptides from 19 differential peptides according to the
P-value, and these peptides were synthesized by Shanghai Science
Peptide Biological Technology Co., Ltd. The purity of all
synthesized peptides was >95%. All peptides were administered 2
h before Ang II treatment.
Cell viability analysis
Cell viability was detected using a Cell Counting
Kit-8 (CCK-8) assay according to the manufacturer's protocol. The
HUVECs were seeded in 96-well plates at density of 5×103
cells/well, and upon reaching 70–80% confluency, the cells were
starved in serum-free medium for 6 h, and then treated with Ang II
(1 µM) for 24 h at 37°C. The peptides (10, 20, 50 or 100 µM) were
administered 2 h before Ang II treatment under the same conditions.
After 24 h of culture, 10 µl CCK-8 reagent (cat. no. C0038;
Beyotime Institute of Biotechnology) was added to each well and
cultured away from light in 37°C incubators for 2 h. The intensity
of the light absorption at 450 nm wavelength was measured using a
microplate reader.
Lactate dehydrogenase (LDH)
detection
The LDH Release Assay kit (cat. no. C0017; Beyotime
Institute of Biotechnology) was utilized to detect the level of LDH
release. The reaction working solution was prepared according to
the manufacturer's instructions. The cell supernatant (120 µl/well)
was collected, mixed with the reaction working solution (60
µl/well) and added to the 96-well plate. The 96-well plate was
incubated in the dark at room temperature for 30 min. Finally, the
absorbance was detected at 490 nm wavelength using a microplate
reader.
Reactive oxygen species (ROS),
superoxide dismutase (SOD) and malondialdehyde (MDA) detection
A ROS assay kit (cat. no. S0033M; Beyotime Institute
of Biotechnology) was utilized to determine the levels of
intracellular ROS according to the manufacturer's protocol.
Briefly, the HUVECs were seeded in 6-well plates at a density of
2×105 cells/well, and upon reaching 70–80% confluency,
the cells were starved in serum-free medium for 6 h, and then
treated with Ang II (1 µM) for 24 h at 37°C; the peptides (20 µM)
were administered 2 h before Ang II treatment under the same
conditions. Then, the cells were incubated with serum-free medium
including 0.1% DCFH-DA away from light at 37°C for 20 min, and then
washed with serum-free medium three times. Images were captured
using a fluorescence microscope (BX61; Olympus Corporation) and the
intensity of ROS fluorescence was quantified using ImageJ software
1.26 (National Institutes of Health). The HUVECs were seeded in
6-well plate at a density of 2×105 cells/well. After the
drug treatment, the cells were digested with trypsin, lysed in RIPA
buffer (cat. no. P0013C; Beyotime Institute of Biotechnology) and
centrifuged (12,000 × g, 4°C) for 10 min. Commercial assay kits
were used to detect the activity of SOD (cat. no. A001-3-1; Nanjing
Jiancheng Bioengineering Institute) and the content of MDA (cat.
no. A003-3-1; Nanjing Jiancheng Bioengineering Institute) in cells,
according to the manufacturer's instructions.
Mitochondrial membrane potential (MMP)
detection
The MMP was detected using the JC-1 MMP assay kit
(cat. no. C2006; Beyotime Institute of Biotechnology) according to
the manufacturer's instructions. The HUVECs were seeded in a 6-well
plate at a density of 2×105 cells/well, and treated with
drugs once they reached 70–80% confluency. After 24 h, the cells
were washed with PBS and incubated with JC solution for 10 min at
37°C. Cells were then washed with serum-free medium three times and
analyzed on a Laser Scanning Confocal Microscope.
Western blotting
The proteins of the HUVECs were extracted using
lysate buffer including RIPA buffer (cat. no. P0013K; Beyotime
Institute of Biotechnology) and 1% PMSF (cat. no. ST506; Beyotime
Institute of Biotechnology), and the protein concentration was
determined using a BCA detection kit (cat. no. 23229; Thermo Fisher
Scientific). Following protein extraction, 1X SDS loading buffer
was added to the samples, followed by denaturation by boiling at
95°C for 5 min. After cooling for 5 min on ice, the same amount of
protein sample (20 mg) was separated via SDS-PAGE (10% gel), and
then separated proteins were transferred onto PVDF membranes (EMD
Millipore). The membrane was blocked with 5% skimmed milk for 2 h
at room temperature and incubated with anti-poly (ADP-ribose)
polymerase 1 (PARP; 1:1,000; cat. no. 9542; Cell Signaling
Technology, Inc.), anti-cleaved-PARP (1:1,000; cat. no. 9541; Cell
Signaling Technology, Inc.), anti-Caspase3 (1:1,000; cat. no. 9662;
Cell Signaling Technology, Inc.), anti-cleaved-Caspase3 (1:1,000;
cat. no. 9661; Cell Signaling Technology, Inc.) and anti-β-actin
(1:2,000; cat. no. 4970; Cell Signaling Technology, Inc.) overnight
at 4°C.
After that, the membrane was washed three times for
10 min each time with TBS with 0.1% Tween-20 (cat. no. ST825;
Beyotime Institute of Biotechnology) buffer and incubated with
horseradish peroxidase-conjugated secondary antibodies
(anti-rabbit/mouse; 1:3,000; cat. nos. 7074 and 7076; Cell
Signaling Technology, Inc.) for 1 h at room temperature. Image Lab
6 software (Bio-Rad Laboratories, Inc.) was utilized for the
semi-quantification of protein expression.
Statistical analysis
All data were obtained from ≥3 individual
experiments. GraphPad Prism 8 software (GraphPad Software, Inc.)
was utilized to analyze the experimental data and produce the
statistical graphs. The data are presented as the means ± standard
deviation. An unpaired two-sided Student's t-test or one-way ANOVA
with Bonferroni's correction for multiple comparisons were used to
analyze statistical differences. P<0.05 was considered to
indicate a statistically significant difference.
Results
Process of peptidomics research and
the construction of a vascular endothelial injury model
The present study used HUVECs to construct a
vascular endothelial injury model by treating the cells with Ang II
and then collecting the cellular supernatant to extract peptides
for MS analysis. A schematic diagram is presented in Fig. 1A. To construct a stable vascular
endothelial injury model, the present study evaluated the changes
of cell viabilities in HUVECs treated with Ang II at different
concentrations (0.0, 0.1, 0.5, 1.0, 5.0 and 10.0 µM) for different
times (0, 6, 12, 24 and 36 h) in vitro (Fig. 1B and C). As demonstrated in Fig. 1D, a stable vascular endothelial
injury model was constructed in vitro using 1 µM Ang II
treatment for 24 h. In addition, the cell survival rate was
significantly decreased in the Ang II treatment group (Fig. 1E and F).
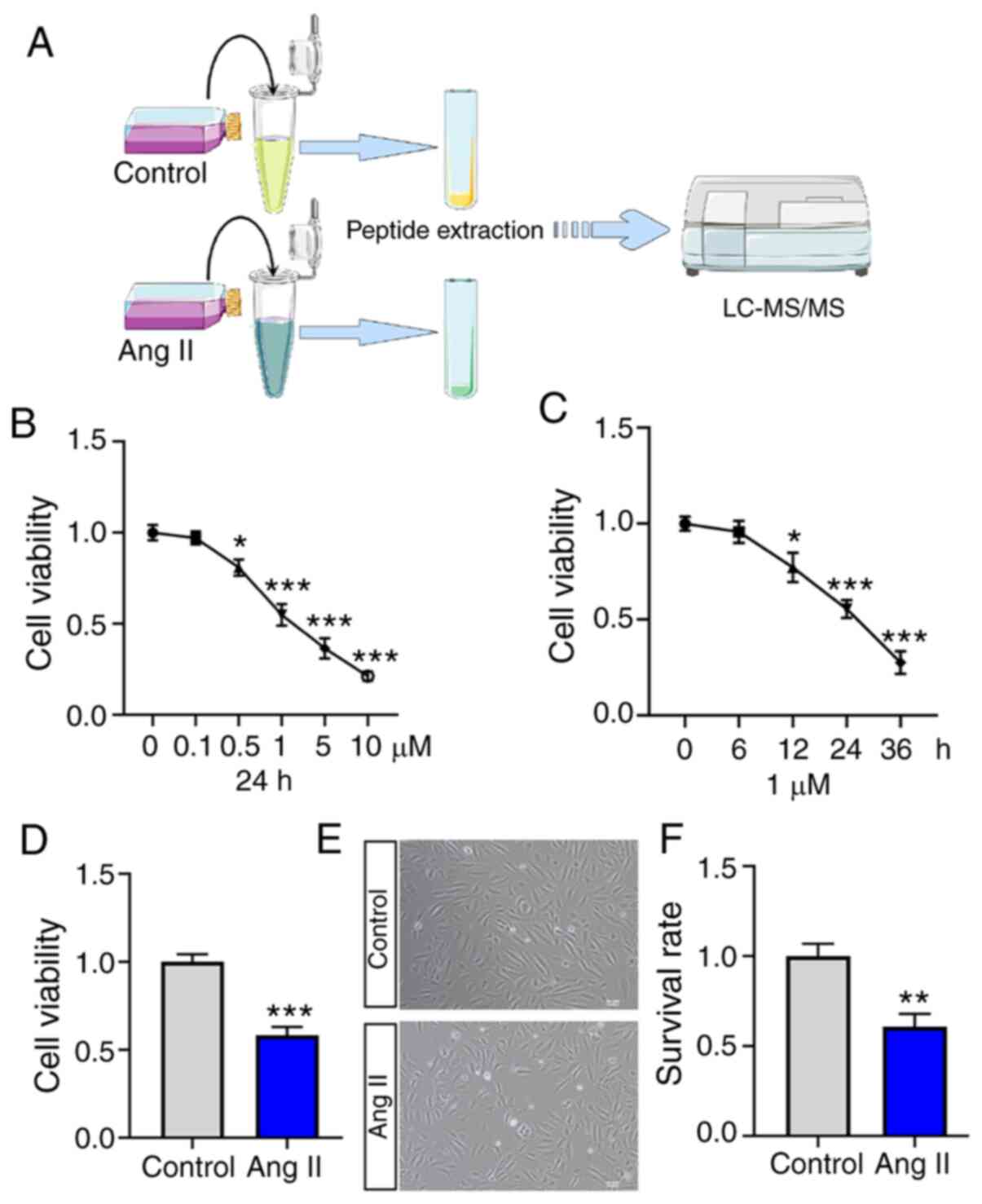 | Figure 1.Process of peptidomics research and
the construction of a vascular endothelial injury model. (A)
Schematic diagram of peptide identification. (B) The changes in
cell viabilities were evaluated in HUVECs treated with Ang II at
different concentrations (0.0, 0.1, 0.5, 1.0, 5.0 and 10.0 µM) for
24 h. (C) The changes in cell viabilities were evaluated in HUVECs
treated with Ang II at 1 µM concentration for different time (0, 6,
12, 24 and 36 h). (D) A stable vascular endothelial injury model
was constructed in vitro using 1 µM Ang II treatment for 24
h. (E) The cell survival rate was markedly decreased in the Ang II
treatment group. (F) Quantitative assessment of cell survival rate.
The data are presented as the means ± SD. *P<0.05 **P<0.01
and ***P<0.001 vs. control group. HUVECs, human umbilical vein
endothelial cells; LC, liquid chromatography; MS, mass
spectrometry. |
Identification of peptide expression
profiles
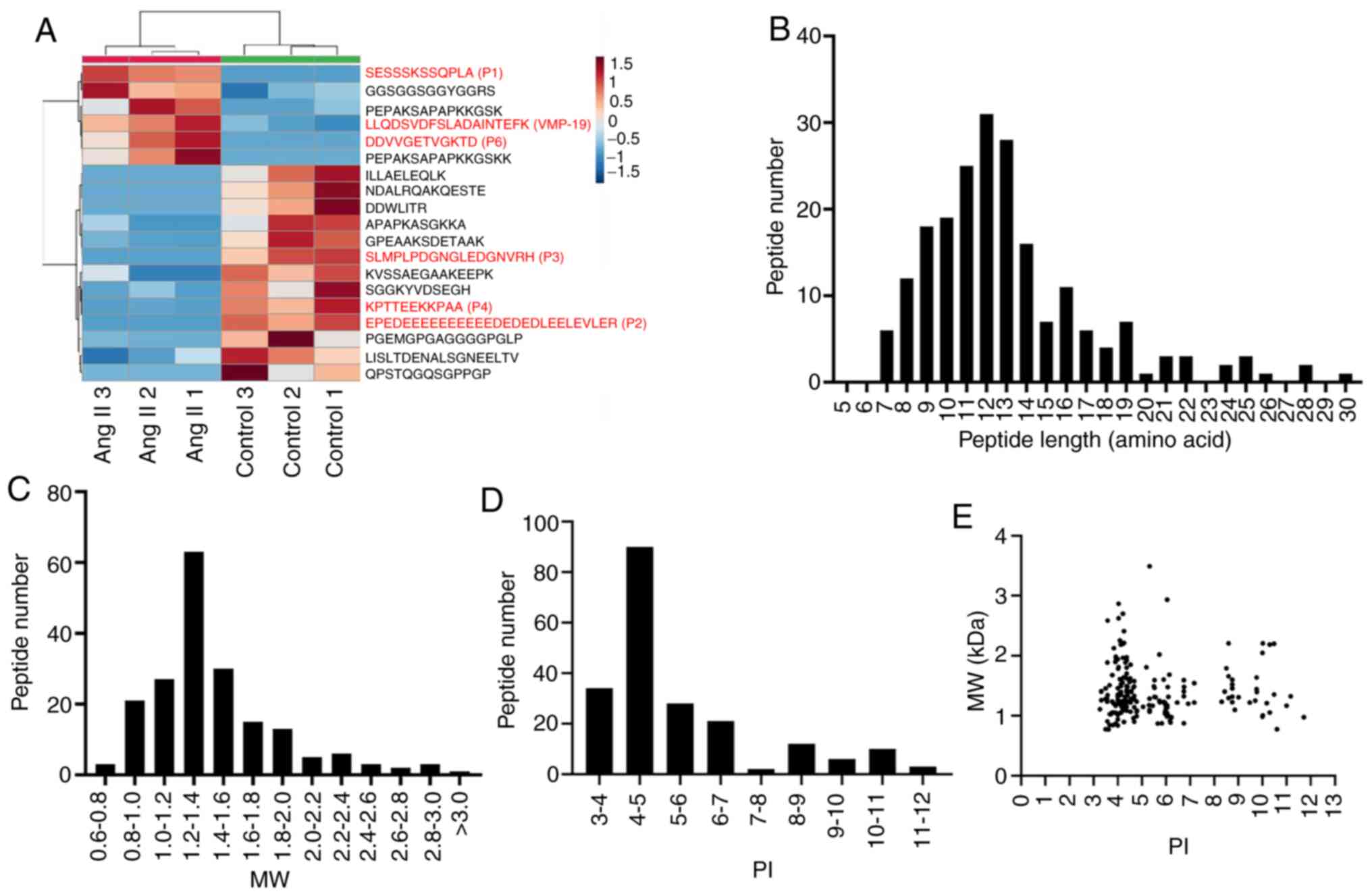
The MS results revealed that a total of 211 peptides
were identified from the cell supernatant, of which, six were
upregulated and 13 were downregulated when compared with the
control group (P<0.05 and fold-change ≥2) (Table I). The heat map shows the
significant differences in the peptide profiles of cell
supernatants treated with Ang II (Fig.
2A). Through the UniProt database analysis, it was revealed
that 57 peptides had no precursor proteins (Table SI). Subsequently, the present study
analyzed the physicochemical properties of the peptides that were
identified. It was revealed that the distribution of the 211
peptides making up the length of the peptide was mainly
concentrated in 9–14 amino acids (Fig.
2B). The present study also analyzed the MW and PI of peptides,
and revealed that the MW of peptides were between 1.2 and 1.4 kDa,
and the PI values were mainly in the PI 4–5 range (Fig. 2C and D). In addition, the present
study investigated the association between the distribution of MW
and PI of peptides. As presented in Fig. 2E, the peptides were mainly clustered
into three groups: Near PI 4, PI 6 and PI 9.
 | Table I.Details of 19 differentially
expressed peptides (Ang II vs. Control). |
Table I.
Details of 19 differentially
expressed peptides (Ang II vs. Control).
| Peptide
sequence | UniProt
accession | Protein name | P-value | Fold-change | Regulation |
|---|
| SESSSKSSQPLA | P17096 | High mobility group
protein |
5.74526×10−5 | 1960.78 | Up |
|
|
| HMG-I
(HMGA1_HUMAN) |
|
|
|
|
EPEDEEEEEEEEEEDEDED | Q9NQC3 | Reticulon-4
(RTN4_HUMAN) |
1.78342×10−4 | 9.15 | Down |
| LEELEVLER |
|
|
|
|
|
|
SLMPLPDGNGLEDGNVRH | Q9ULZ1 | Apelin
(APEL_HUMAN) |
1.74441×10−3 | 16.62 | Down |
| KPTTEEKKPAA | P36578 | 60S ribosomal
protein L4 |
2.99364×10−3 | 69.51 | Down |
|
|
| (RL4_HUMAN) |
|
|
|
|
LLQDSVDFSLADAINTEFK | P08670 | Vimentin
(VIME_HUMAN) |
3.68230×10−3 | 3.82 | Up |
| DDVVGETVGKTD | P27816 |
Microtubule-associated protein 4 |
7.81321×10−3 | 53.48 | Up |
|
|
| (MAP4_HUMAN) |
|
|
|
| GPEAAKSDETAAK | P04792 | Heat shock protein
β-1 |
1.01935×10−2 | 3.91 | Down |
|
|
| (HSPB1_HUMAN) |
|
|
|
| NDALRQAKQESTE | P08670 | Vimentin
(VIME_HUMAN) |
1.22044×10−2 | 26.18 | Down |
| GGSGGSGGYGGRS | P22626 | Heterogeneous
nuclear ribonucleoproteins A2 |
1.36942×10−2 | 4.34 | Up |
|
|
| (ROA2_HUMAN) |
|
|
|
| ILLAELEQLK | P08670 | Vimentin
(VIME_HUMAN) |
1.37267×10−2 | 172.22 | Down |
|
PEPAKSAPAPKKGSKK | O60814 | Histone H2B type
1-K |
1.56135×10−2 | 42.81 | Up |
|
|
| (H2B1K_HUMAN) |
|
|
|
| KVSSAEGAAKEEPK | P05114 | Non-histone
chromosomal protein HMG-14 |
1.74828×10−2 | 5.38 | Down |
|
|
| (HMGN1_HUMAN) |
|
|
|
| DDWDLITR | Q9UBU8 | Mortality factor
4-like protein 1 |
1.83306×10−2 | 68.25 | Down |
|
|
| (MO4L1_HUMAN) |
|
|
|
|
LISLTDENALSGNEELTVK | P14625 | Endoplasmin
(ENPL_HUMAN) |
1.95552×10−2 | 4.30 | Down |
|
PEPAKSAPAPKKGSK | O60814 | Histone H2B type
1-K |
2.49500×10−2 | 15.44 | Up |
|
|
| (H2B1K_HUMAN) |
|
|
|
| APAPKASGKKA | P50914 | 60S ribosomal
protein L14 |
2.60081×10−2 | 8.82 | Down |
|
|
| (RL14_HUMAN) |
|
|
|
| SGGKYVDSEGH | Q03135 | Caveolin-1
(CAV1_HUMAN) |
2.80822×10−2 | 7.99 | Down |
| QPSTQGQSGPPGP | / | / |
4.29166×10−2 | 14.72 | Down |
|
PGEMGPGAGGGGPGLP | Q96JN8 | Neuralized-like
protein 4 |
4.81683×10−2 | 40.51 | Down |
|
|
| (NEURL4_HUMAN) |
|
|
|
Bioinformatics analysis
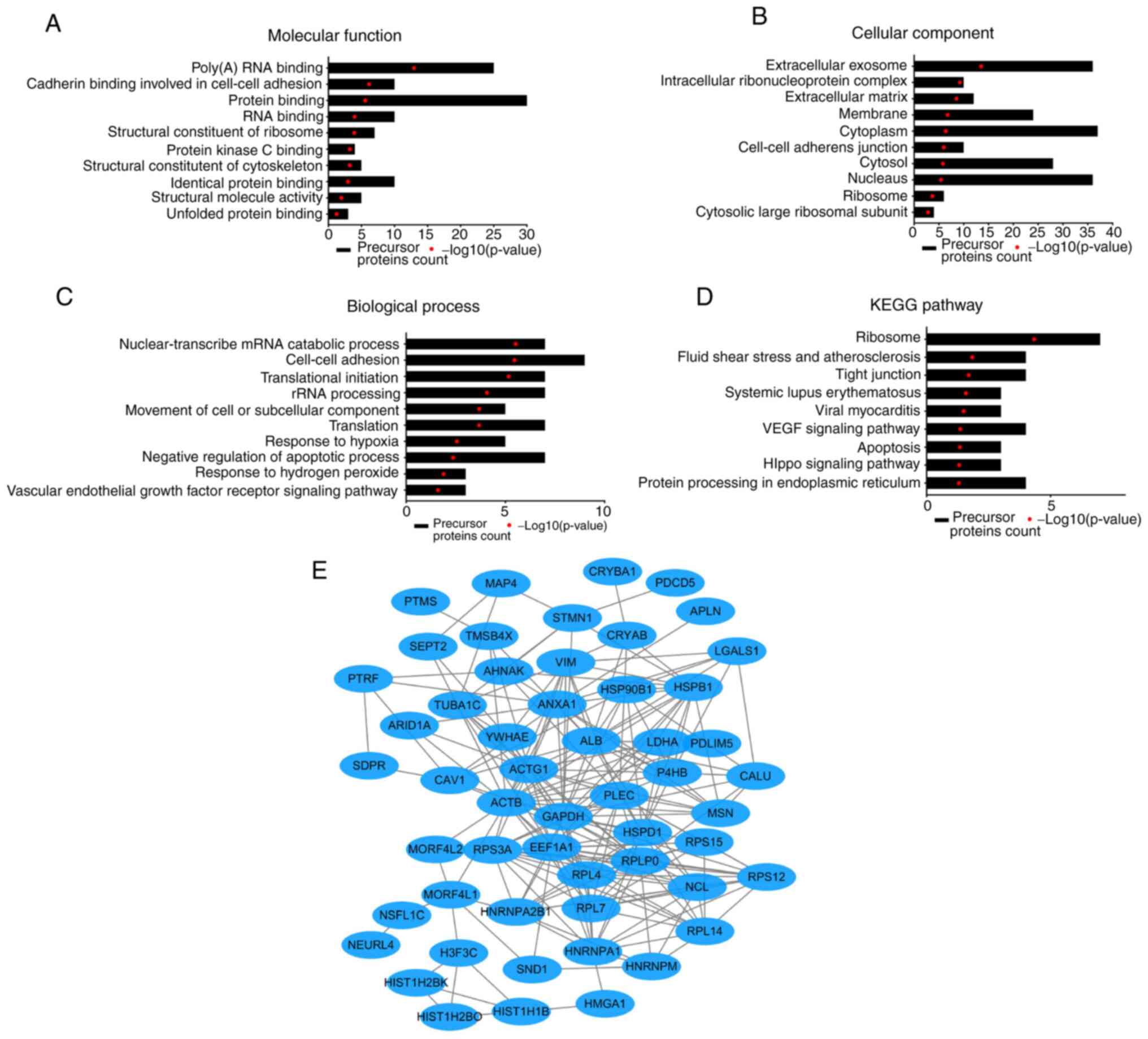
Through the UniProt database analysis, a total of
154 peptides were derived from 62 precursor proteins, and 57
peptides were found to have no precursor proteins. To predict the
potential function of these identified peptides, the present study
performed GO and KEGG pathway analyses on their precursor proteins.
The GO analysis results revealed that the 10 ‘Molecular function’
categories most enriched with these peptides were ‘Poly(A) RNA
binding’, ‘Cadherin binding involved in cell-cell adhesion’,
‘Protein binding’, ‘RNA binding’, ‘Structural constituent of
ribosome’, ‘Protein kinase C binding’, ‘Structural constituent of
cytoskeleton’, ‘Identical protein binding’, ‘Structural molecule
activity’ and ‘Unfolded protein binding’ (Fig. 3A). The top 10 most enriched
subcellular localization categories were ‘Extracellular exosome’,
‘Intracellular ribonucleoprotein complex’, ‘Extracellular matrix’,
‘Membrane’, ‘Cytoplasm’, ‘Cell-cell adherens junction’, ‘Cytosol’,
‘Nucleus’, ‘Ribosome’ and ‘Cytosolic large ribosomal subunit’
(Fig. 3B). The ‘Biological
function’ categories most enriched with these precursors were
mainly associated with ‘Nuclear-transcribed mRNA catabolic
process’, ‘Cell-cell adhesion’, ‘Translational initiation’, ‘rRNA
processing’, ‘Movement of cell or subcellular component’,
‘Translation’, ‘Response to hypoxia’, ‘Negative regulation of
apoptotic process’, ‘Response to hydrogen peroxide’ and ‘Vascular
endothelial growth factor receptor signaling pathway’ (Fig. 3C). The KEGG pathway analysis showed
that the precursor proteins were mainly associated with ‘Ribosome’,
‘Fluid shear stress and atherosclerosis’, ‘Tight junction’,
‘Systemic lupus erythematosus’, ‘Viral myocarditis’, ‘VEGF
signaling pathway’, ‘Apoptosis’, ‘Hippo signaling pathway’ and
‘Protein processing in endoplasmic reticulum’ (Fig. 3D). In addition, the present study
also analyzed the interaction network of these peptide precursor
proteins using the STRING website. A typical STRING network
interaction diagram is presented in Fig. 3E.
Preliminary functional investigation
of peptides
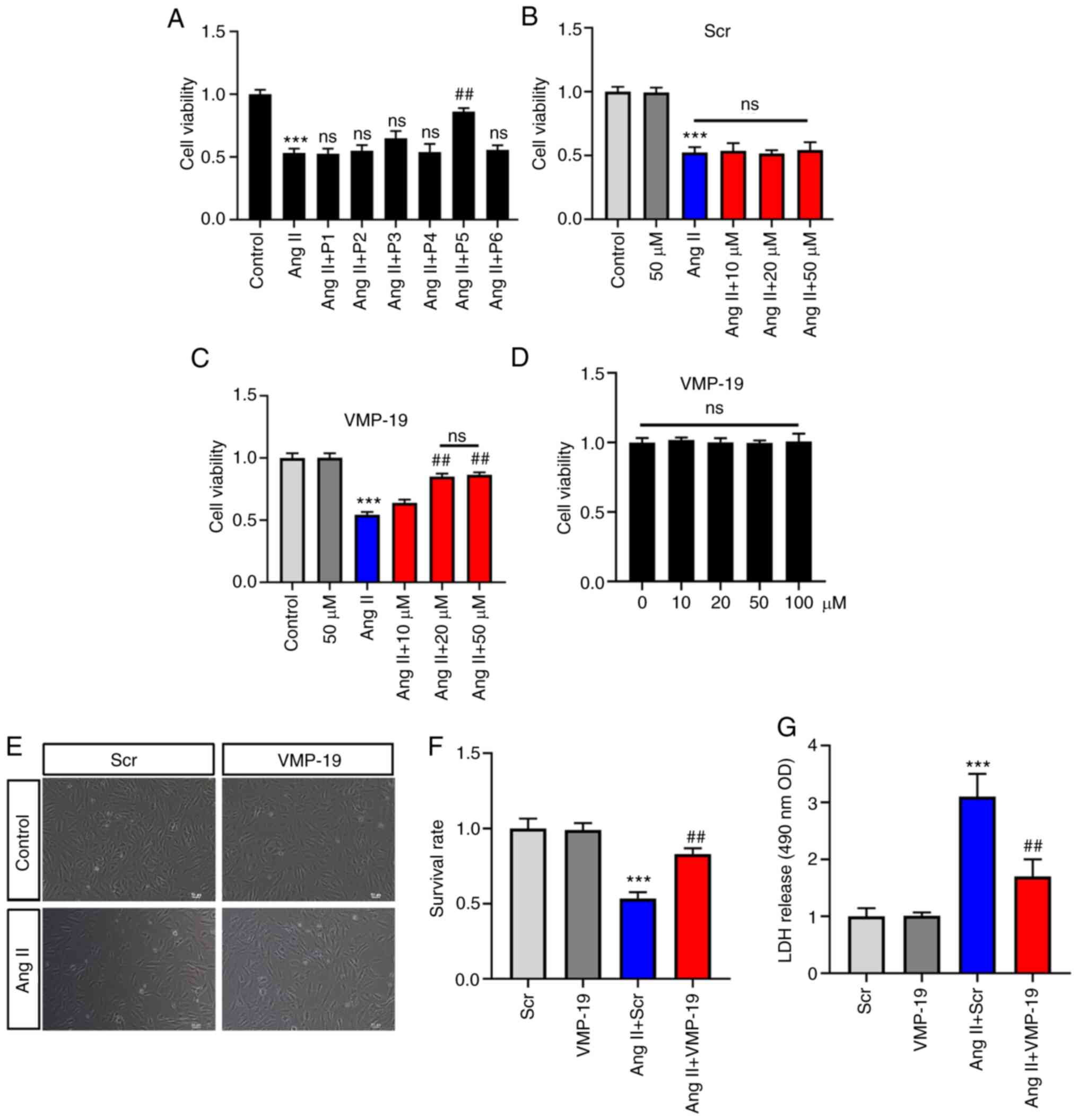
To identify the peptides with vascular endothelial
protection function, the present study selected the top six
peptides from 19 differential peptides according to the P-value.
The sequences of the six peptides are presented in Table SII. The results revealed that the
peptide with the LLQDSVDFSLADAINTEFK sequence could significantly
increase the cell viability induced by Ang II (Fig. 4A). According to the name of its
precursor protein and the number of amino acids, this peptide was
named VMP-19 (a peptide derived from Vimentin, 19 amino acids) in
the present study. In addition, the present study assessed the
effects of different concentrations of VMP-19 and its Scr on cell
viability. As a control peptide, there was no significant
difference in the cell viability between the Scr and Ang II
treatment (Fig. 4B). However,
VMP-19 could significantly increase the cell viability induced by
Ang II in a concentration-dependent manner, and the effect was most
obvious at 20 µM (Fig. 4C). The
present study also evaluated the effects of the VMP-19 peptide
itself on the cell viability of HUVECs. The results revealed that
VMP-19 had no toxic effect at high concentrations in HUVECs
(Fig. 4D). Cell survival was also
evaluated, and it was revealed that 20 µM VMP-19 significantly
increased the survival rate of HUVECs with treatment of Ang II
(Fig. 4E and F). LDH is an
important indicator of cell damage. In the present study, 20 µM
VMP-19 could significantly decrease the release of LDH caused by
Ang II treatment (Fig. 4G). The
aforementioned results indicated that VMP-19 exerts protection in
Ang II-induced HUVEC damage in a dose-dependent manner, and the
effect was most obvious at 20 µM.
VMP-19 peptide attenuates oxidative
stress induced by Ang II
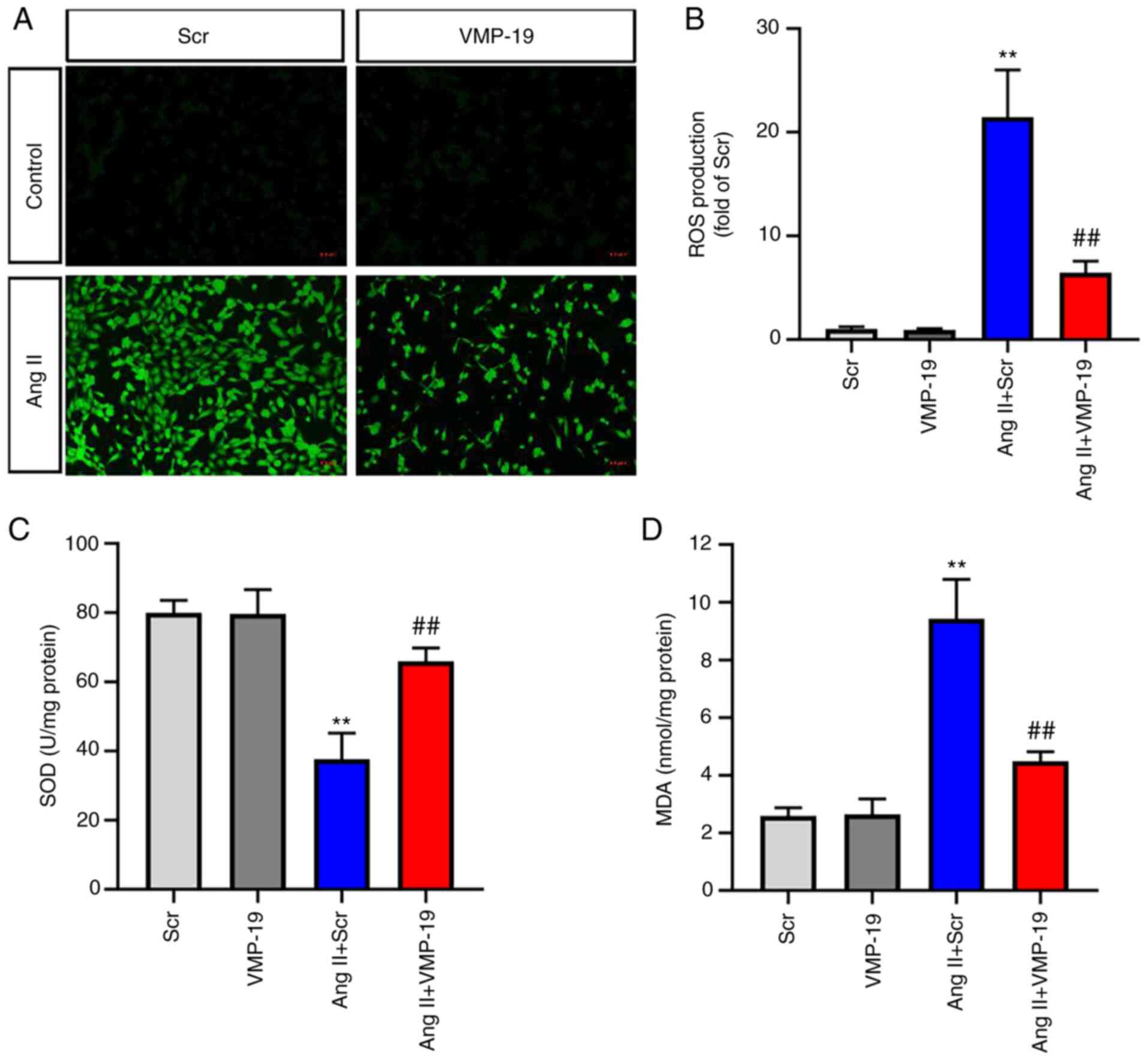
Considering that the excessive production of ROS is
the main biological event of Ang II-induced HUVECs injury, ROS were
examined via DCFH-DA staining. As presented in Fig. 5A and B, 20 µM VMP-19 significantly
decreased the production of ROS after Ang II treatment in HUVECs.
In addition, the present study also measured the activities of
major antioxidant enzymes: SOD and the content of MDA. A dose of 20
µM VMP-19 peptide significantly increased the activities of SOD and
decreased the content of MDA after Ang II treatment in HUVECs
(Fig. 5C and D).
VMP-19 peptide attenuates apoptosis
induced by Ang II
Apoptosis of VECs is a vital event in the
development of endothelial dysfunction. The decrease of MMP is a
sign of early apoptosis. In the present study, VMP-19 significantly
improved the decrease of MMP by Ang II in HUVECs, as evidenced by
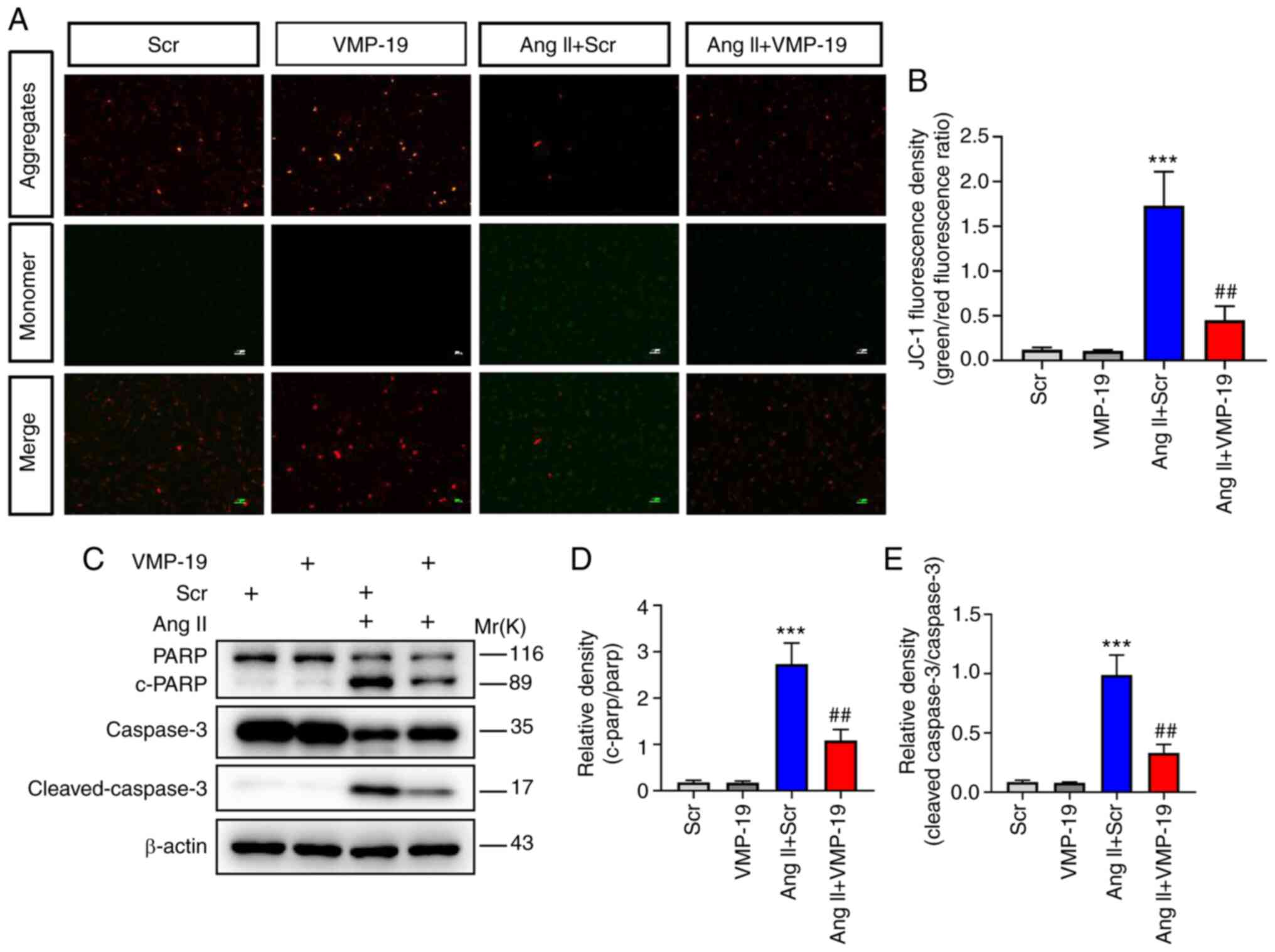
the ratio of aggregated JC-1 monomer (Fig. 6A and B). In order to further
investigate the effect of VMP-19 on Ang II-induced HUVEC apoptosis
injury, the proteins associated with apoptosis were evaluated. The
activation of PARP and caspase3 was inhibited following VMP-19
peptide treatment in Ang II-induced cell damage (Fig. 6C-E). Hence, the present study
confirmed that VMP-19 peptide could protect HUVECs from apoptosis
induced by Ang II.
Discussion
It is already well-established that VECs are
important metabolic and endocrine organs that play an important
role in regulating vascular function, and vascular endothelial
injury is closely associated with the development and progression
of various cardiovascular diseases, particularly hypertension
(25). Therefore, focusing on the
alleviation of vascular endothelial injury will help to prevent and
treat hypertension. However, at present, there is no effective
prevention and treatment strategy for vascular endothelial injury.
In the present study, proteomics methods were used to identify the
peptides secreted by HUVECs under Ang II stimulation. A total of
211 secreted peptides were identified, of which 19 were
significantly differentially expressed. Through further
experiments, a VMP-19 peptide was identified to alleviate the
apoptosis and oxidative stress injury in HUVECs. The results
indicated that VMP-19 peptide may be a candidate molecule for the
treatment of vascular endothelial injury.
As HUVECs exhibit a similar human physiological
state and have similar biological characteristics to VECs, no
species difference, rich sources and ethical advantages, they are
widely used as cell models to simulate hypertension injury
(26). A large number of studies
have revealed that Ang II, as the main effector peptide of the
renin-angiotensin-aldosterone system, is closely associated with
the occurrence and development of hypertension (27,28).
Therefore, stimulation of HUVECs with Ang II is a reliable model to
simulate vascular endothelial injury in vitro. In previous
studies, the concentration and dose time of Ang II in an in
vitro model were different (29,30).
As presented in Fig. 1B-D, the
present study used HUVECs to construct a stable vascular
endothelial model with 1 µM Ang II treatment for 24 h in
vitro. Similarly, the same in vitro model method was
used to verify the protective effect of E3 ubiquitin-protein ligase
NEDD4 on Ang II-induced vascular endothelial cell injury by
regulating exportin-1-mediated nuclear export (31).
The present study identified a total of 211 peptides
from the cell supernatant. The length of these peptides was mainly
concentrated in 9–14 amino acids, and the MW was distributed
between 1.2 and 1.4 kDa, and <3.0 kDa, which suggested that the
peptides identified in the present study were valid. Among the
identified peptides, a number of them were derived from the same
precursor protein. It is already well-known that the majority of
peptides are produced by protein cleavage into fragments, and
proteases play a vital role in the protein cleavage process by
recognizing the cleavage site specifically (32). Notably, the present study revealed
that the precursor proteins of 57 of the identified peptides were
not found in the UniProt database. A previous study shown that some
non-coding RNAs with short open reading frames have been verified
to possess coding ability and can exert their functions via
encoding peptides (33). For
instance, a peptide encoded by a putative lncRNAHOXB-AS3 suppresses
colon cancer growth through competitively binding to the arginine
residues in the RGG motif of heterogenous nuclear ribonucleoprotein
A1 (34). In addition, the
LINC00961-encoded SPAR peptide could regulate mTORC1 and muscle
regeneration (35). Therefore, the
present study speculated that these peptides may also be encoded by
some non-coding RNAs, which will be clarified in future
research.
The underlying mechanism of vascular endothelial
injury mainly involves endothelial cell apoptosis, inflammation and
oxidative stress injury (36,37).
The present study focused on the role of peptides in VEC apoptosis
and oxidative stress injury, and successfully identified a peptide
named VMP-19 with the protective functions of VECs. The results
demonstrated that the VMP-19 peptide could alleviate the apoptosis
and oxidative stress injury of VECs induced by Ang II, as indicated
by a decreased level of the proteins associated with apoptosis and
production of ROS. Some precursor proteins and their derived
peptides may play the same role in certain diseases. The VMP-19
peptide is derived from the Vimentin protein, located at amino acid
79–97. A previous study revealed that Vimentin not only maintains
cell integrity, but also regulates apoptosis, inflammation and
immune response (38). Kueper et
al (39) found that Vimentin
cleavage is a common phenomenon in the process of apoptosis.
Combined with the findings of the present study, we speculated that
the Vimentin protein may cleave into a shorter peptide to protect
VECs from apoptosis and oxidative stress injury.
Although a novel peptide was identified in the
present study, which was named VMP-19, which could protect HUVECs
from apoptosis and oxidative stress injury, there were still some
limitations to the study. For example, whether different
modification methods could affect the function of VMP-19 requires
further verification. In addition, the specific mechanism
underlying the VMP-19 peptide in the protection of VECs remains to
be verified. Therefore, in future work, the effect of the VMP-19
peptide under different modification conditions will be
investigated in order to clarify the specific mechanism of its
vascular endothelial protection.
In summary, to the best of our knowledge, the
present study was the first to use peptidomics to analyze the
peptide spectrum of supernatants secreted by HUVECs and identified
that a novel peptide, VMP-19, could protect HUVECs from apoptosis
and oxidative stress injury. The results of the present study could
provide novel insights into the treatment of hypertension.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the Health
Commission of Changning District, Shanghai, China (grant no.
YXMZK009) and the National Natural Science Foundation of China
(grant no. 81873540).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZX, JD and LZ performed the experiments and wrote
the manuscript. XF and JZ performed the bioinformatics analysis. XS
and HL performed some of the in vitro experiments. XL and LQ
contributed to the study design and concept, and supervised the
project. ZX and JD confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Humbert M, Montani D, Perros F, Dorfmuller
P, Adnot S and Eddahibi S: Endothelial cell dysfunction and cross
talk between endothelium and smooth muscle cells in pulmonary
arterial hypertension. Vascul Pharmacol. 49:113–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Konukoglu D and Uzun H: Endothelial
dysfunction and hypertension. Adv Exp Med Biol. 956:511–540. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cahill PA and Redmond EM: Vascular
endothelium-Gatekeeper of vessel health. Atherosclerosis.
248:97–109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martelli A, Testai L, Anzini M, Cappelli
A, Di Capua A, Biava M, Poce G, Consalvi S, Giordani A, Caselli G,
et al: The novel anti-inflammatory agent VA694, endowed with both
NO-releasing and COX2-selective inhibiting properties, exhibits
NO-mediated positive effects on blood pressure, coronary flow and
endothelium in an experimental model of hypertension and
endothelial dysfunction. Pharmacol Res. 78:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lankhorst S, Kappers MH, van Esch JH,
Danser AH and van den Meiracker AH: Hypertension during vascular
endothelial growth factor inhibition: Focus on nitric oxide,
endothelin-1, and oxidative stress. Antioxid Redox Signal.
20:135–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang PW, Yu CL, Wang YZ, Luo SF, Sun LS
and Li RS: Influence of
3,4′,5-trihydroxystibene-3-beta-mono-D-glucoside on vascular
endothelial epoprostenol and platelet aggregation. Zhongguo Yao Li
Xue Bao. 16:265–268. 1995.PubMed/NCBI
|
|
7
|
Gizard F and Bruemmer D: Transcriptional
control of vascular smooth muscle cell proliferation by peroxisome
proliferator-activated receptor-gamma: Therapeutic implications for
cardiovascular diseases. PPAR Res. 2008:4291232008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carney EF: Hypertension: New non-RAS
peptide modulates the vasoregulatory effects of angiotensin II. Nat
Rev Nephrol. 11:3172015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ceriello A, Novials A, Ortega E, Canivell
S, La Sala L, Pujadas G, Esposito K, Giugliano D and Genovese S:
Glucagon-like peptide 1 reduces endothelial dysfunction,
inflammation, and oxidative stress induced by both hyperglycemia
and hypoglycemia in type 1 diabetes. Diabetes Care. 36:2346–2350.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chaturvedi LS and Basson MD: Glucagonlike
peptide 2 analogue teduglutide: Stimulation of proliferation but
reduction of differentiation in human Caco-2 intestinal epithelial
cells. JAMA Surg. 148:1037–1042. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dang LT, Feric NT, Laschinger C, Chang WY,
Zhang B, Wood GA, Stanford WL and Radisic M: Inhibition of
apoptosis in human induced pluripotent stem cells during expansion
in a defined culture using angiopoietin-1 derived peptide QHREDGS.
Biomaterials. 35:7786–7799. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fosgerau K and Hoffmann T: Peptide
therapeutics: Current status and future directions. Drug Discov
Today. 20:122–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaspar AA and Reichert JM: Future
directions for peptide therapeutics development. Drug Discov Today.
18:807–817. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anand IS, Fisher LD, Chiang YT, Latini R,
Masson S, Maggioni AP, Glazer RD, Tognoni G and Cohn JN; Val-HeFT
Investigators, : Changes in brain natriuretic peptide and
norepinephrine over time and mortality and morbidity in the
Valsartan heart failure trial (Val-HeFT). Circulation.
107:1278–1283. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Armstrong PW and Rouleau JL: A canadian
context for the acute study of clinical effectiveness of nesiritide
and decompensated heart failure (ASCEND-HF) trial. Can J Cardiol.
24 (Suppl B):30B–32B. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cerrato BD, Carretero OA, Janic B, Grecco
HE and Gironacci MM: Heteromerization between the bradykinin B2
receptor and the angiotensin-(1–7) mas receptor: Functional
consequences. Hypertension. 68:1039–1048. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koid SS, Ziogas J and Campbell DJ:
Aliskiren reduces myocardial ischemia-reperfusion injury by a
bradykinin B2 receptor- and angiotensin AT2 receptor-mediated
mechanism. Hypertension. 63:768–773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mughal A and O'Rourke ST: Vascular effects
of apelin: Mechanisms and therapeutic potential. Pharmacol Ther.
190:139–147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dallas DC, Guerrero A, Parker EA, Robinson
RC, Gan J, German JB, Barile D and Lebrilla CB: Current
peptidomics: Applications, purification, identification,
quantification, and functional analysis. Proteomics. 15:1026–1038.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Slavoff SA, Mitchell AJ, Schwaid AG,
Cabili MN, Ma J, Levin JZ, Karger AD, Budnik BA, Rinn JL and
Saghatelian A: Peptidomic discovery of short open reading
frame-encoded peptides in human cells. Nat Chem Biol. 9:59–64.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rubakhin SS, Churchill JD, Greenough WT
and Sweedler JV: Profiling signaling peptides in single mammalian
cells using mass spectrometry. Anal Chem. 78:7267–7272. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ashburner M, Ball CA, Black JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology: The Gene
ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gene Ontology Consortium: The Gene
Ontology resource: Enriching a GOld mine. Nucleic Acids Res.
49:D325–D334. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanehisa M: Toward pathway engineering: A
new database of genetic and molecular pathways. Sci Technol Japan.
59:34–38. 1996.
|
|
25
|
Liu S, Yi F, Cheng W, Qu X and Wang C:
Molecular mechanisms in vascular injury induced by hypertension:
Expression and role of microRNA-34a. Exp Ther Med. 14:5497–5502.
2017.PubMed/NCBI
|
|
26
|
Reichlin T, Wild A, Durrenberger M,
Daniels AU, Aebi U, Hunziker PR and Stolz M: Investigating native
coronary artery endothelium in situ and in cell culture by scanning
force microscopy. J Struct Biol. 152:52–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Biancardi VC, Bomfim GF, Reis WL,
Al-Gassimi S and Nunes KP: The interplay between Angiotensin II,
TLR4 and hypertension. Pharmacol Res. 120:88–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cha SA, Park BM and Kim SH:
Angiotensin-(1–9) ameliorates pulmonary arterial hypertension via
angiotensin type II receptor. Korean J Physiol Pharmacol.
22:447–456. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Y, Tian T, Wang Y, Li Z, Xing K and
Tian G: SIRT6 protects vascular endothelial cells from angiotensin
II-induced apoptosis and oxidative stress by promoting the
activation of Nrf2/ARE signaling. Eur J Pharmacol. 859:1725162019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song J, Huang S, Wang K, Li W, Pao L, Chen
F and Zhao X: Long Non-coding RNA MEG3 attenuates the angiotensin
II-induced injury of human umbilical vein endothelial cells by
interacting with p53. Front Genet. 10:782019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu J, Sheng Z, Li F, Wang S, Yuan Y, Wang
M and Yu Z: NEDD4 protects vascular endothelial cells against
Angiotensin II-induced cell death via enhancement of XPO1-mediated
nuclear export. Exp Cell Res. 383:1115052019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li F, Wang Y, Li C, Marquez-Lago TT, Leier
A, Rawlings ND, Haffari G, Revote J, Akutsu T, Chou KC, et al:
Twenty years of bioinformatics research for protease-specific
substrate and cleavage site prediction: A comprehensive revisit and
benchmarking of existing methods. Brief Bioinform. 20:2150–2166.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu P, Mo Y, Peng M, Tang T, Zhong Y, Deng
X, Xiong F, Guo C, Wu X, Li Y, et al: Emerging role of
tumor-related functional peptides encoded by lncRNA and circRNA.
Mol Cancer. 19:222020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang JZ, Chen M, Chen D, Gao XC, Zhu S,
Huang H, Hu M, Zhu H and Yan GR: A peptide encoded by a putative
lncRNA HOXB-AS3 suppresses colon cancer growth. Mol Cell.
68:171–184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsumoto A, Pasut A, Matsumoto M,
Yamashita R, Fung J, Monteleone E, Saghatelian A, Nakayama KI,
Clohessy JG and Pandolfi PP: mTORC1 and muscle regeneration are
regulated by the LINC00961-encoded SPAR polypeptide. Nature.
541:228–232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ellinsworth DC: Arsenic, reactive oxygen,
and endothelial dysfunction. J Pharmacol Exp Ther. 353:458–464.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Q, Zhang M, Ding Y, Wang Q, Zhang W,
Song P and Zou MH: Activation of NAD(P)H oxidase by
tryptophan-derived 3-hydroxykynurenine accelerates endothelial
apoptosis and dysfunction in vivo. Circ Res. 114:480–492. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang L, Tang L, Dai F, Meng G, Yin R, Xu X
and Yao W: Raf-1/CK2 and RhoA/ROCK signaling promote TNF-α-mediated
endothelial apoptosis via regulating vimentin cytoskeleton.
Toxicology. 389:74–84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kueper T, Grune T, Prahl S, Lenz H, Welge
V, Biernoth T, Vogt Y, Muhr GM, Gaemlich A, Jung T, et al: Vimentin
is the specific target in skin glycation. Structural prerequisites,
functional consequences, and role in skin aging. J Biol Chem.
282:23427–23436. 2007. View Article : Google Scholar : PubMed/NCBI
|