Introduction
Sepsis is defined as a syndrome characterized by
‘life-threatening organ dysfunction caused by the dysregulated host
response to an infection,’ with emphasis on multiple organ
dysfunction syndromes (MODS) (1).
In China, sepsis affects one-fifth of patients admitted to
intensive care units in mainland China with a 90-day mortality rate
of 35.5% (2). In 2015, a total of
1,937,299 deaths occurred across the 605 mainland Chinese disease
surveillance points and the standardized sepsis-related mortality
rate was 66.7 deaths per 100,000 population (3). The immunological responses in patients
with sepsis are complex, comprising concurrent pro- and
anti-inflammatory responses (4).
Compelling experimental and clinical evidence has indicated that
immunosuppression is the cause of the aggravation, complicated with
MODS and even mortality of patients with sepsis (5).
Immune activation is accompanied by functional
impairment of innate and adaptive immune cells, such as apoptosis
of a large number of immune cells, CD4+ T cell anergy
and enhancement of negative immunomodulatory cells (5,6). It
has become more evident that the majority of patients with sepsis
succumb not to the early, overwhelming pro-inflammatory response,
but to the immunosuppression-related complications that occur later
in their disease trajectory (7,8).
Sepsis-induced immunosuppression prevents patients from removing
pathogenic microorganisms and increases their susceptibility to
secondary infections, especially caused by opportunistic pathogens
(9). CD4+ T cell anergy
is defined by three features in sepsis-induced immunosuppression:
Apoptosis-induced depletion leading to a decrease in the number of
CD4+ T cells, a decrease in the CD4+ T cell
proliferative response and even immunological paralysis and a shift
from a helper T cells (Th)1 to a Th2 cell profile, defined as Th2
immune polarization (10).
Semaphorin 3A (Sema3A) is a ligand of Neuropilin-1
(Nrp-1) and serves a key role in neuraxon development (11). Using lipopolysaccharide
(LPS)-induced acute kidney injury model, Sema3A has been identified
in tubular epithelial cells (12).
Anti-Sema3A antibodies have been reported to be effective in
improving the survival rate in LPS-induced sepsis in mouse models
(13). By contrast, the suppression
of Sema-3A in tumor cells with a small interfering (si)RNA augments
T cell activation (14). Thus, the
objective of the present study was to investigate the effect of
Sema3A on the CD4+ T cell anergy. This information will
provide a potential novel target for the study of immune regulation
in sepsis. The present study demonstrated that inhibition of Sema3A
significantly relieved CD4+ T cell anergy via regulation
of the NF-κB signaling pathway in sepsis.
Materials and methods
Animals and ethics statement
A total of 120 inbred male C57BL/6J mice (obtained
from the Laboratory Animal Center of the Chinese Academy of Medical
Sciences, no. SCXK-Jing-2014-0004), 6–8 weeks old, 20±2 g were used
for the in vivo study. Mice received standard care under a
12-h dark/light cycle (25°C with an atmosphere of 60%) and given
free access to food and water, in accordance with the animal care
guidelines of the Tianjin Medical University General Hospital
(Tianjin, China). Before the experiment, adaptive feeding was
carried out for 1 week. Before surgery, food and water was not
given for 12 h. All experimental procedures complied with the
National Institute of Health Guidelines for the Care and Use of
Laboratory Animals (15) and were
approved by the scientific investigation review board of Tianjin
Medical University General Hospital (approval no.
ZYY-DWFL-IRB-001F-01).
Sepsis model
After being anesthetized, a 0.5-cm incision was made
on the abdomen of mice and the cecum was exposed. Briefly, mice
were anesthetized with isoflurane inhalation at the concentration
of 2.5% for anesthetic induction and then at 1% for anesthetic
maintenance until the end of the cecal ligation and perforation
(CLP). The diameter of the puncture needle was 0.6 mm and it was
used to induce CLP in the experiment. The mice were given a
subcutaneous injection of 0.9% sterile saline solution in a volume
of 40 ml/kg body weight following CLP.
Experimental design
The mice were randomly divided into six groups
(n=10): Control, sham, CLP and the different
epigallocatechin-3-gallate (EGCG; cat. no. 989-51-5; Beijing
Solarbio Science & Technology Co., Ltd.)-treatment groups. In
the control group, the mice were anesthetized, but no surgery was
carried out. For mice in the sham group, the cecum was exteriorized
without ligation and puncture. In the CLP group, a midline
laparotomy was performed according to the sepsis model described
above. After CLP was obtained, the different EGCG-treatment groups
were immediately administered increasing concentrations (25, 50 and
100 mg/kg, intravenously) of EGCG, a strong inhibitor of Sema3A
activity.
Isolation and culture of splenic
lymphocytes
The mice were sacrificed by cervical dislocation.
Spleens were harvested and prepared as single-cell suspensions and
then subjected to Ficoll-Paque density gradient centrifugation
(cat. no. LTS1092P; Tianjin TBD Science) at 4°C, 1,500 × g for 15
min. Lymphocytes [CD4+ T cells,
CD4+CD25+ regulatory T cells (Tregs)], B
cells and natural killer (NK) cells were isolated from mononuclear
cells using a CD4+ T cell Isolation kit, mouse (cat. no.
130-104-454), a CD4+CD25+ regulatory T cell
Isolation kit, mouse (cat. no. 130-091-041), a Pan B Cell Isolation
kit II, mouse (cat. no. 130-104-443), and a NK Cell Isolation kit,
mouse (cat. no. 130-115-818; all from Miltenyi Biotec GmbH) and
autoMACS® Columns (Miltenyi Biotec GmbH) according to
the manufacturer's instructions. Cells of the myeloid lineage,
including macrophage (Mø) cells and dendritic cells (DC), were
isolated from the abdominal cavity and femur, respectively, using a
Mø Isolation kit (Peritoneum), mouse (cat. no. 130-110-434) and a
Pan DC Isolation kit, mouse (cat. no. 130-100-875; both from
Miltenyi Biotec GmbH) and autoMACS® Columns (Miltenyi
Biotec GmbH) according to the manufacturer's instructions. Isolated
cells were cultured in RPMI-1640 medium (Nanjing KeyGen Biotech
Co., Ltd.) supplemented with 10% fetal bovine serum (FBS;
Sigma-Aldrich; Merck KGaA) and incubated at 37°C with 5%
CO2, following treatment with anti-CD3e (5 µg/ml; cat.
no. 564378; BD Pharmingen; BD Biosciences) and anti-CD28 (2 µg/ml;
cat. no. 562767; BD Pharmingen; BD Biosciences) antibodies for
polyclonal activation of T cells.
In vitro studies
LPS (100 ng/ml; Escherichia coli 0111:B4;
cat. no. L2630; Sigma-Aldrich; Merck KGaA) induction was used for
septic simulation. Subsequently, splenic CD4+ T cells
were seeded on 96-well cell-culture plates (2×105
cells/well) and treated with anti-CD3e and anti-CD28 antibodies for
polyclonal activation of T cells and treated with different doses
of recombinant Sema3A polyclonal antibody (anti-Sema3A antibody,
0.5, 2 and 4 µg/ml; cat. no. 201253-T10; Sino Biological, Inc.),
recombinant Sema3A (rSema3A, 10, 100 and 1,000 ng/ml; cat. no.
7201; BioVision, Inc.) and EGCG (1, 10 and 100 µmol/l) for 24 h.
The proliferative activity, apoptotic rate, cytokine secretion
(including IFN-γ and IL-4) and Foxp-3 expression of CD4+
T cells were determined. The serum levels of aspartate
aminotransferase (AST), alanine transaminase (ALT) and creatinine
(Cr), as well as arterial blood gas (ABG), were determined by The
Central Laboratory of Tianjin Medical University General
Hospital.
Immunofluorescence analysis
The expression of phosphorylated (p)-ikkβ/ikkβ and
p-P65/P65, the main molecules of the NF-κB signaling pathway, were
examined by immunofluorescence microscopy (Olympus Corporation).
CD4+ T cells were suspended in 1 ml
fixation/permeabilization solution (cat. no. 88-8824-00;
eBioscience; Thermo Fisher Scientific, Inc.) at 25°C for 30 min,
and seeded on glass coverslips, washed with phosphate buffer saline
(PBS), fixed in 4% paraformaldehyde (Nanjing KeyGen Biotech Co.,
Ltd.) at 25°C for 15 min, and then blocked with 10% normal goat
serum (cat. no. SL038; Beijing Solarbio Science & Technology
Co., Ltd.) at 25°C for 30 min. Following which, cells were
incubated at 4°C for 12 h with rabbit anti-mouse p-ikkβ (1:100;
cat. no. ab194845), ikkβ (1:100; cat. no. ab97406), p-P65 (1:100;
cat. no. ab222494) and P65 (1:100; cat. no. ab32536) antibodies
(all purchased from Abcam). The cells were subsequently washed and
incubated at 25°C for 60 min with FITC/APC-conjugated goat
anti-rabbit IgG (1:200; cat. no. 4030-02; SouthernBiotech).
Immunofluorescence was assessed by fluorescence microscopy. Data
were collected and processed with ImageJ (v1.51j8; National
Institutes of Health).
Flow cytometric analysis
To investigate the expression of Foxp-3,
CD4+ T cells (1×106 cells/ml) were obtained
post-treatment according to the flow cytometry kit protocol.
CD4+ T cells were suspended in 1 ml
fixation/permeabilization solution and incubated at 25°C for 30
min. After washing cells with fixation/permeabilization buffer
twice, CD4+ T cells were stained with FITC-conjugated
anti-mouse/rat-Foxp-3 (cat. no. MA1-41628; eBioscience; Thermo
Fisher Scientific, Inc.) and incubated at 25°C for 15 min. Cells
were analyzed using a flow cytometer (BD FACSCalibur™; cat. no.
342975; BD Biosciences) and FlowJo software (version 7; FlowJo
LLC).
To investigate CD4+ T cell apoptosis,
cells (1×106 cells/ml) were obtained post-treatment
according to the flow cytometry kit protocol. CD4+ T
cells were suspended in 200 µl binding buffer, followed by
FITC-conjugated Annexin V and propidium iodide (cat. no. CA1020;
Beijing Solarbio Science & Technology Co., Ltd.) in the dark
according to the manufacturer's protocol, and then subjected to
flow cytometric analysis using a flow cytometer (BD FACSCalibur)
and FlowJo software (version 7).
Enzyme-linked immunosorbent assay
(ELISA)
Supernatants or serum were collected for the
measurement of Sema3A (cat. no. 011592), IFN-γ (cat. no. HS4595)
and IL-4 (cat. no. BH-E0800) levels, using ELISA kits (Shanghai Xin
Fan Biotechnology Co., Ltd.), according to the protocols specified
by the manufacturer. The absorbance was read in a microplate reader
(Spectra MR; Dynex Technologies) at a wavelength of 450 nm.
Terminal deoxynucleotidyl transferase
(TdT) dUTP nick-end labeling (TUNEL) assay
The present study used a one-step TUNEL Apoptosis
Assay kit (cat. no. T2190; Beijing Solarbio Science &
Technology Co., Ltd.) according to the manufacturer's instructions.
CD4+ T cells were suspended in 1 ml
fixation/permeabilization solution at 25°C for 30 min, and seeded
on glass coverslips, washed with PBS, fixed in 4% paraformaldehyde
at 25°C for 15 min, and then incubated with 20 µg/ml Proteinase K
(cat. no. P9460; Beijing Solarbio Science & Technology Co.,
Ltd.) at 37°C for 30 min. Cells were then blocked with 10% normal
goat serum at 25°C for 30 min. Apoptotic cells were observed and
the images from five random fields were analyzed by
immunofluorescence microscopy (Olympus Corporation) at ×200
magnification. The emission wavelength of the green fluorescence
was 525 nm. Data were collected and processed using ImageJ software
(v1.51j8; National Institutes of Health).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The MTT assay (Ameresco, Inc.) was used to evaluate
the proliferative ability of CD4+ T cells. Growth medium
was removed and replaced with fresh medium. The assays were
initiated by adding 10 ml MTT reagent (10 mg/ml) to each well and
incubating the cells for 4 h at 25°C. Finally, the medium was
removed and 100 µl DMSO (Ameresco, Inc.) was added to each well.
The absorbance was read in a microplate reader (Spectra MR; Dynex)
at a wavelength of 490 nm.
Statistical analysis
Data are presented as the mean ± standard deviation.
All experiments were performed at least four times. Data were
analyzed by one-way ANOVA and Tukey's post hoc test using SPSS
version 24 (IBM Corp.). Unpaired Student's t-test was used to
evaluate significance between two groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Sema3A exacerbates sepsis-induced T
cell immunosuppression and MODS
In vivo, the serum concentration of Sema3A
was significantly enhanced in CLP-induced sepsis by >4-fold at
24 h after CLP (P<0.01; Fig.
1A). CLP was immediately followed by intravenous administration
of EGCG at different concentrations. The 50 mg/kg dose, in
particular, had a clear ability to improve sepsis-induced T cell
immunosuppression and MODS compared with the CLP-induced sepsis
group. The serum concentrations of IFN-γ and IL-4 produced by
CD4+ T cells were measured to identify the polarization
of Th1/Th2 cells (Fig. 1B).
Following treatment with 25 and 50 mg/kg EGCG, IFN-γ levels were
significantly enhanced (P<0.01), while IL-4 levels were
significantly lowered (P<0.01), but the serum concentrations of
IFN-γ and IL-4 were both significantly reduced following treatment
with 100 mg/kg EGCG (P<0.01). The early apoptotic rate of
CD4+ T cells was significantly decreased when animals
were treated with 50 mg/kg EGCG (P<0.01) from 12 to 24 h, but
100 mg/kg EGCG significantly increased the early apoptotic rate of
CD4+ T cells, especially at the 24 h time point, with
the early apoptotic rate reaching >90% (P<0.01), there were
no significant differences in early and late apoptosis between the
control and 50 mg/kg EGCG groups (P>0.05) at the 12 to 24 h time
points (Fig. 1C-F). The
proliferative ability of CD4+ T cells was significantly
enhanced with 50 mg/kg EGCG treatment compared with the CLP group
(P<0.01) and was similar to that of the control group.
CD4+ T cell proliferation of the 100 mg/kg EGCG
treatment group was significantly lower than that of the
LPS-treatment group and even of the control group (P<0.01;
Fig. 1G). CLP-induced sepsis
impaired organ function, as indicated by the significantly
increased serum levels of AST (Fig.
1H), ALT (Fig. 1H), Cr
(Fig. 1I) and Lac (Fig. 1L), but the levels of PCO2
(Fig. 1J) and PO2
(Fig. 1K) were significantly
decreased (P<0.01), administration of 50 mg/kg EGCG following
CLP significantly improved the appellate quotas of organ function
(P<0.01), however, 100 mg/kg EGCG aggravated organ dysfunction
when compared with the CLP group (P<0.01).
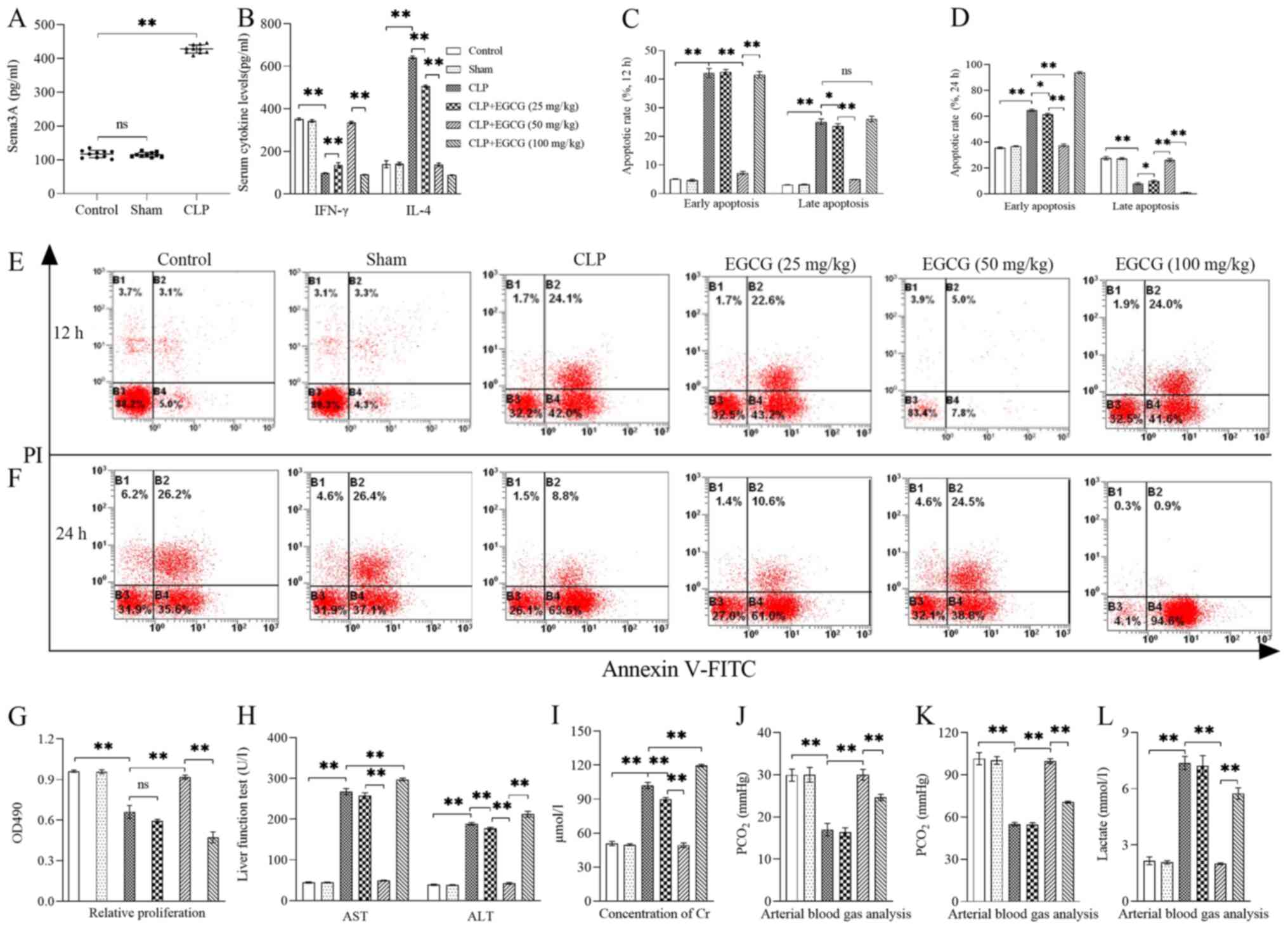 | Figure 1.Treatment with EGCG improves
sepsis-induced T cell immunosuppression and MODS. (A) The serum
concentration of Sema3A was significantly enhanced in CLP-induced
sepsis (n=10). (B) Serum concentrations of IFN-γ and IL-4.
Statistical analysis and representative flow cytometry images of
proportions of early and late apoptotic stage CD4+ T
cells from (C and E) 12 h to (D and F) 24 h. PI-Annexin
V-FITC+ represent early apoptosis, while
PI+Annexin V-FITC+ represent late apoptosis.
(G) The proliferation of CD4+ T cells. Serum
concentrations of (H) AST and ALT, (I) Cr, (J) PCO2, (K)
PO2 and (L) Lactate. Data are presented as the mean ±
standard deviation, n=4 per group. One-way ANOVA was performed for
data analysis. *P<0.05, **P<0.01. EGCG,
(−)-epigallocatechin-3-gallate; MODS, multiple organ dysfunction
syndromes; ns, not significant; CLP, cecal ligation and
perforation; AST, aminotransferase; ALT, alanine transaminase; Cr,
creatinine; Sema3A, semaphorin 3A. |
Lymphoid and myeloid lineages secrete
high concentration of Sema3A during LPS-induced sepsis
Cells of the lymphoid lineage (including T cells,
Tregs, B cells and NK cells) were isolated from normal spleen
tissue and cultured under LPS-induced (100 ng/ml) septic conditions
for 2–24 h. LPS treatment significantly upregulated the
concentration of Sema3A in T cells (Fig. 2A), Tregs (Fig. 2B), B cells (Fig. 2C) and NK cells (Fig. 2D), especially after 24 h (P<0.05
or 0.01). Tregs did not secrete Sema3A significantly until 8 h
(P<0.01). Cells of the myeloid lineage, including Mø cells and
DC, were isolated and cultured under LPS-induced (100 ng/ml) septic
conditions from 2 to 24 h. Compared with cells of the lymphoid
lineage, the time required for the myeloid lineage to secrete high
concentrations of Sema3A was significantly delayed by LPS-induced
sepsis, Mø cells did not secrete Sema3A significantly until after 6
h (P<0.01; Fig. 2E). DC did not
secrete Sema3A significantly until after 6 h of LPS induction
(P<0.01; Fig. 2F).
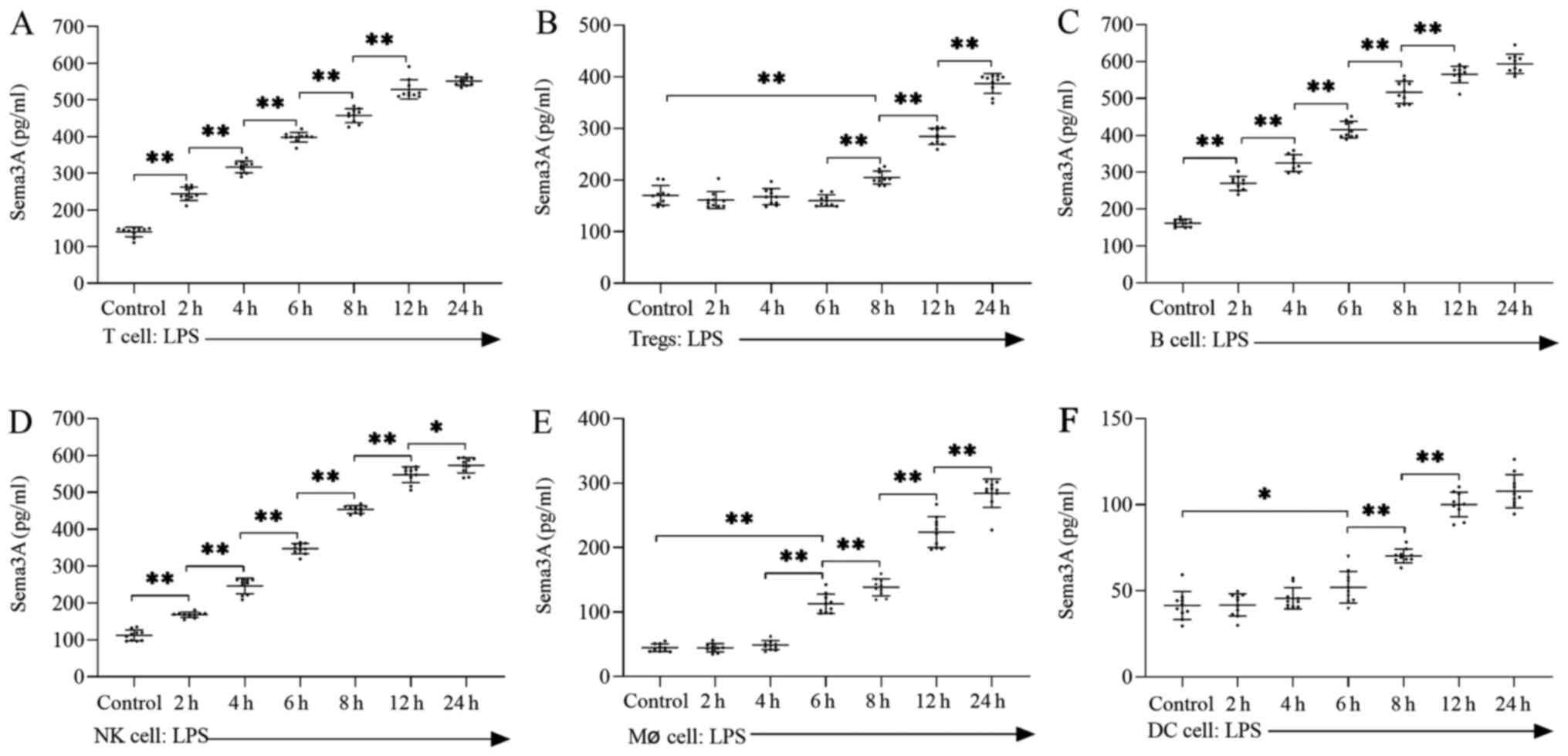 | Figure 2.LPS increases the concentration of
Sema3A in both lymphoid and myeloid lineages. Secretion levels of
Sema3A from (A) T cells, (B) Tregs, (C) B cells, (D) NK cells, (E)
Mø cells and (F) DC. Data are presented as the mean ± standard
deviation, n=10 per group. One-way ANOVA was performed for data
analysis. *P<0.05, **P<0.01. LPS, lipopolysaccharide; Tregs,
regulatory T cells; Mø, macrophage; DC, dendritic cells; NK,
natural killer; Sema3A, semaphorin 3A. |
Inhibition of Sema3A-mediates
autocrine loop-alleviated T cell immune dysfunction during
LPS-induced sepsis
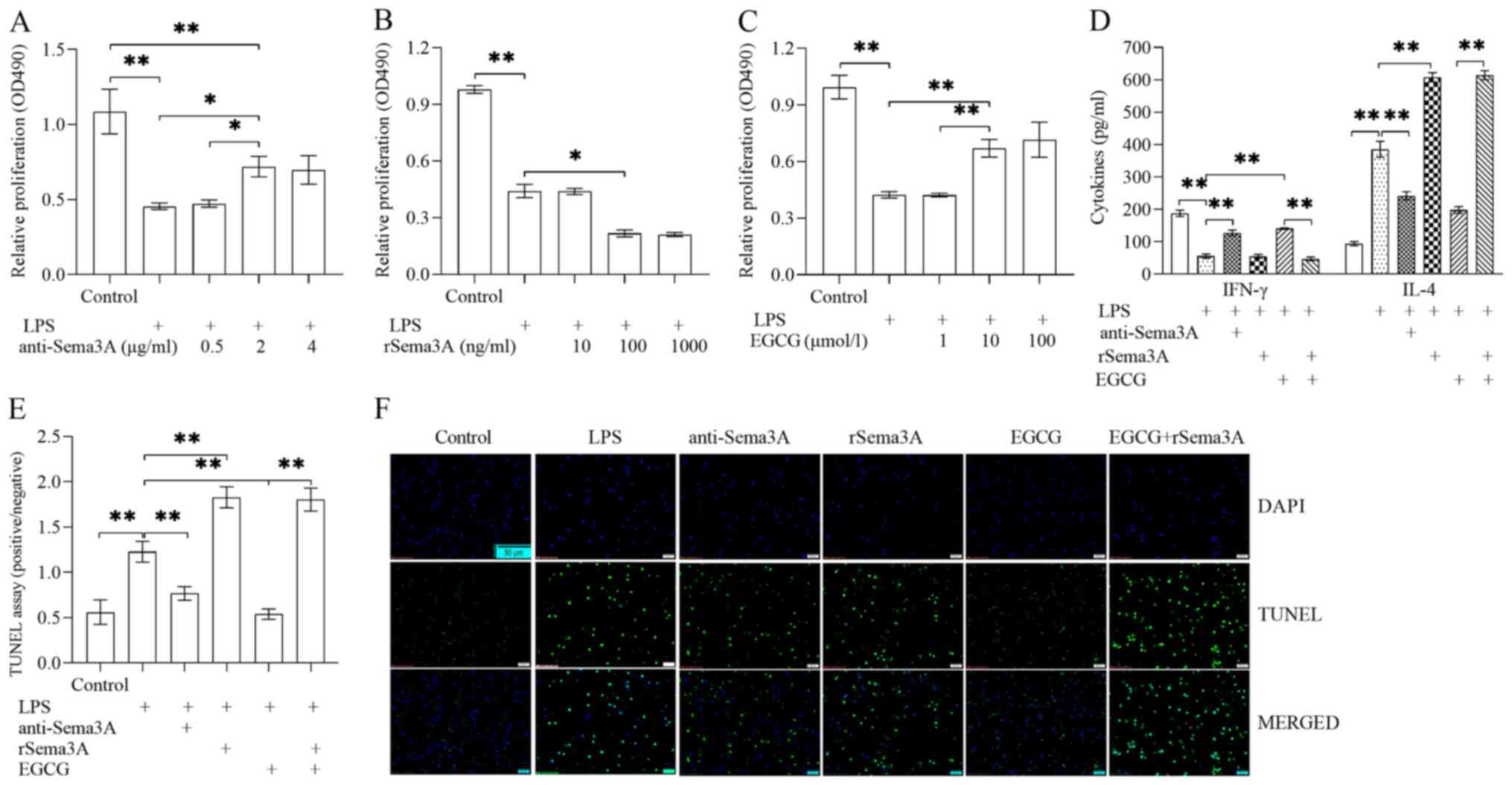
T cells were isolated, treated with LPS (100 ng/ml)
to induce sepsis and further exposed to anti-Sema3A antibody,
rSema3A and/or EGCG for 24 h. Compared with LPS induction alone,
the proliferative ability of CD4+ T cells was enhanced
following anti-Sema3A antibody treatment (P<0.05; Fig. 3A) and was significantly enhanced
with EGCG treatment (P<0.01; Fig.
3C), buT cells were unable to reach the level of the control
group. The proliferative ability of CD4+ T cells was
further inhibited following treatment with rSema3A (P<0.05;
Fig. 3B). Compared with LPS
induction alone, treatment with anti-Sema3A antibody or EGCG could
alleviate the polarization of Th1/Th2 cells (Fig. 3D) and apoptosis (Fig. 3E and F) of T cells, specifically
promoting IFN-γ secretion (P<0.01; Fig. 3D), as well as inhibiting IL-4
secretion (P<0.01; Fig. 3D) and
apoptosis (P<0.01; Fig. 3E and
F). Compared with LPS induction alone, Sema3A could further
impair polarization of Th1/Th2 cells and apoptosis of T cells,
including inhibiting IFN-γ secretion (P<0.01; Fig. 3D), as well as promoting IL-4
secretion (P<0.01; Fig. 3D) and
apoptosis (P<0.01; Fig. 3E and
F) until they reached the levels of the LPS induction
alone.
NF-κB signaling pathway is involved in
Sema3A-mediated autocrine loop aggravating T cell immune
dysfunction during LPS-induced sepsis
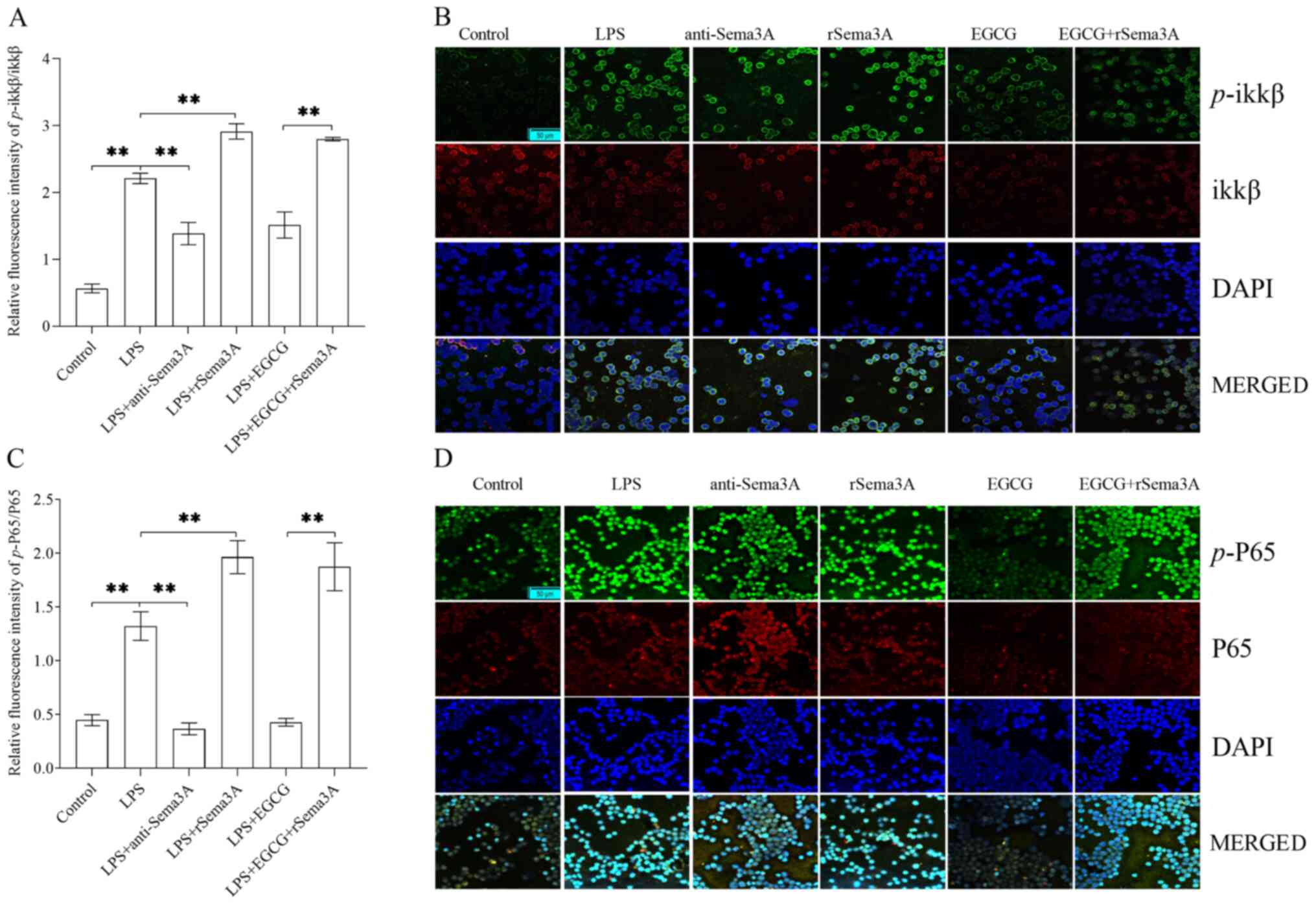
In vitro, CD4+ T cells were
treated with LPS (100 ng/ml)-induced sepsis for 24 h, the ratio of
p-ikkβ/ikkβ (Fig. 4A and B) and
p-P65/P65 (Fig. 4C and D) were
significantly enhanced when compared with the control group
(P<0.01). The ratio of p-ikkβ/ikkβ and p-P65/P65 were
significantly decreased with anti-Sema3A or EGCG treatment when
compared with the LPS-induced sepsis group (P<0.01), but were
unable to reach the level of the control group. The ratio of
p-ikkβ/ikkβ and p-P65/P65 were further significantly enhanced
following rSema3A with or without EGCG treatment when compared with
the LPS induction alone group (P<0.01).
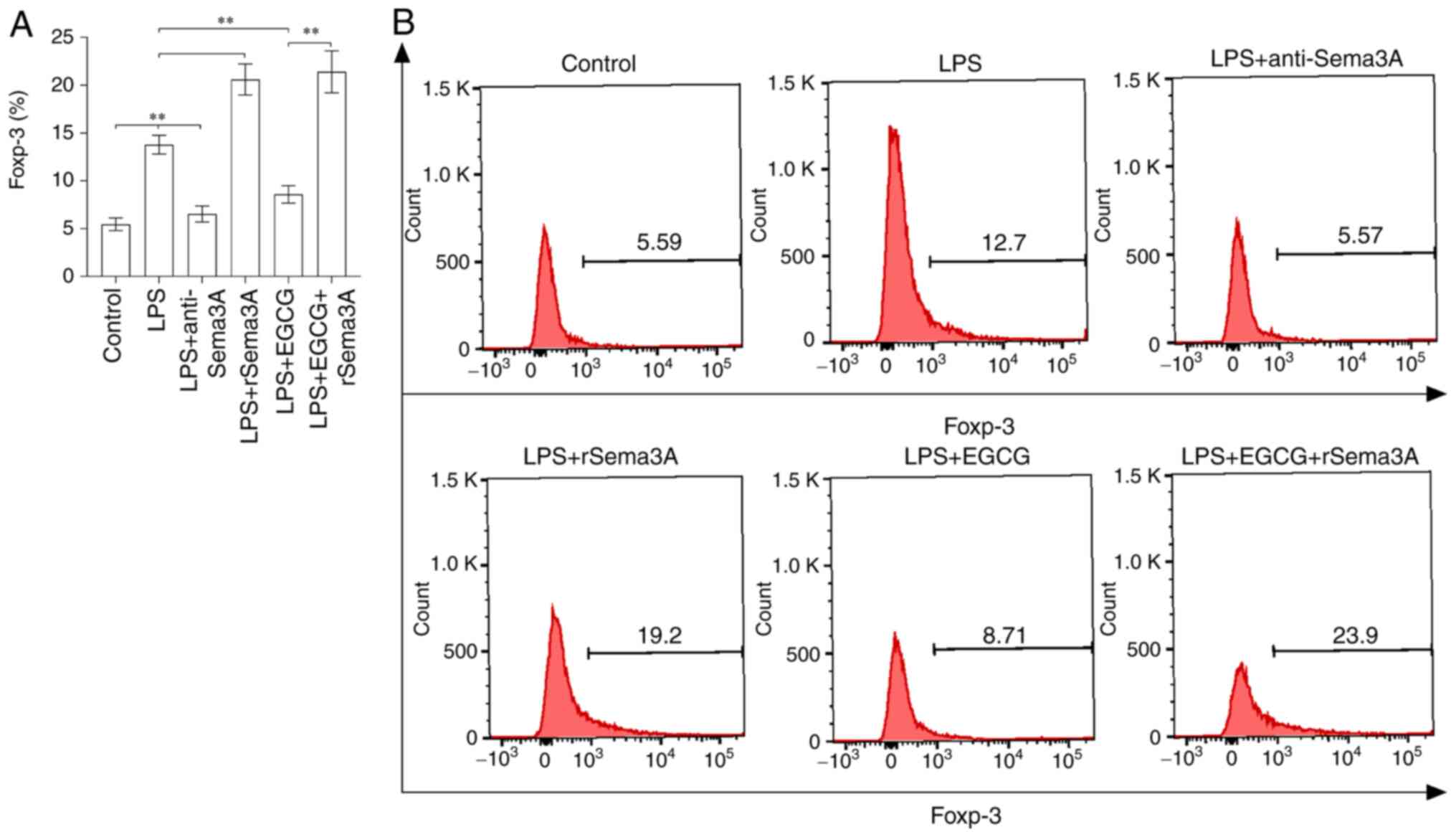
Sema3A-mediated autocrine loop
enhances the expression of Foxp-3 during LPS-induced sepsis
In vitro, CD4+ T cells were
treated with LPS (100 ng/ml)-induced sepsis for 24 h, the
expression of Foxp-3 (Fig. 5A and
B) was significantly enhanced when compared with the control
group (P<0.01). The expression of Foxp-3 was significantly
decreased with anti-Sema3A antibody or EGCG treatment when compared
with the LPS-induced sepsis group (P<0.01), but was unable to
reach the level of the control group, especially following exposure
to EGCG. The expression of Foxp-3 was further significantly
enhanced following treatment with rSema3A with or without EGCG
treatment when compared with the LPS induction alone
(P<0.01).
Discussion
Sepsis is a life-threatening disease that causes
severe MODS and leads to mortality in the majority of patients with
severe sepsis-induced MODS (1).
Yamashita et al (13)
generated a specific Sema3A monoclonal antibody that could
successfully neutralize Sema3A activity and showed that
administration of this specific antibody before LPS injection
significantly increased the survival rate of LPS-treated mice
(16). Treatment with inhibitor
neutralization approaches to reduce Sema3A, such as exposure to
EGCG or siRNA interference, has been shown to alleviate acute organ
damage (12,17). In the current study, it was
demonstrated that administration of EGCG markedly improved
sepsis-induced MODS, as evidenced by a reduction in the serum
levels of AST, ALT, Cr and Lac and an increase in the levels of
PCO2 and PO2. Pasterkamp et al
(18) showed that the selective
monooxygenase inhibitor EGCG, is not a specific Sema3A inhibitor,
but attenuated the repellant effects of Sema3A and Sema3F in
vitro in a dose-dependent manner. E. coli sepsis is
associated with a marked upregulation of sema3A and downregulation
of Sema3F expression (19). Thus,
EGCG was used to perform in vivo experiments that may have
some limitations, such as the role of Sema3F may be further
weakened.
Fresh specimens from liver, kidney and lung, as well
as the cells in the circulatory system of septic patients who
succumb in intensive care units, show a progressive, profound,
apoptosis-induced loss of adaptive immunocytes and especially a
rapid decrease of T-lymphocytes levels, resulting in an decreased
ability to clear life-threatening pathogens (8,20,21).
The highest level of Sema3a mRNA can be observed in T, B and NK
cells of the lymphoid lineage (13,14,16,22,23).
Myeloid and monocytic cells also express Sema3a, but at a lower
level (22,23). The in vitro findings of the
present study showed that both lymphoid and myeloid lineages
secreted high concentrations of Sema3A during LPS-induced sepsis.
The concentrations of Sema3A of the T, B and NK cells were
significantly upregulated with more rapidity compared with Tregs,
Mø cells and DC. In vivo, the serum concentrations of Sema3A
were significantly enhanced in CLP-induced sepsis. Administration
of EGCG had the ability to improve the immune dysfunction of
CD4+ T cells.
Toll-like receptor (TLR) engagement can induce
Sema3A expression, thus completing an autocrine loop in LPS-induced
sepsis (23). Sema3A is expressed
by activated T cells and downmodulated T cell activation in
vitro (22). Nrp-1, primarily
regarded as a receptor for Sema proteins (such as Sema3A) and as a
co-receptor for vascular endothelial growth factor family proteins,
is expressed by neuronal and endothelial cells and has been
reported to serve an essential role in the establishment of both
the nervous system and the endothelial network during embryogenesis
(24). NP-1 is also involved in
initial cell-cell contact between T cells and antigen presenting
cells (25). Tumor and T cells both
express NP-1 and the cluster formation between tumor and T cells
increases with reduced Sema-3A expression in tumor cells; this
effect is rescued by adding a blocking anti-NP-1 antibody (14). The present study demonstrated that
administration of the inhibitor alleviated T cell anergy during
LPS-induced sepsis. The proliferative ability, polarization of
Th1/Th2 cells and apoptosis of CD4+ T cells was improved
with anti-Sema3A and EGCG treatment, but they were unable to
recover normal levels. Using a mouse model of collagen-induced
arthritis, Sema3A can increase the proportion of
CD4+NP-1+ T cells and this group of cells is
able to inhibit the growth of CD4+ T cells as well as
Tregs (26), a full-time negative
immunomodulatory type of the CD4+ T lymphocyte
subpopulation (27,28). The characteristic transcription
factor, Foxp-3, is certainly an crucial determinant for the
stability of Tregs (29). Previous
studies have demonstrated that increased expression of Foxp-3 in
Tregs is positively associated with the mortality of septic mice
(6,21,23,29).
The present study demonstrated that the Foxp-3 expression of
CD4+ T cells was impaired by rSema3A treatment, while
anti-Sema3A or EGCG treatment inhibited the expression of Foxp-3,
especially EGCG. Catalano et al (26) showed that treatment of Sema3A
increases IL-10 concentration in serum. IL-10 serves a key role in
induced immunosuppression, including sepsis. Therefore, IL-10 may
also be involved, whereby rSema3A may enhance the stability of
Tregs by increasing the secretion of IL-10.
TLR engagement by pathogen-associated molecular
pattern is a key mechanism of cellular immune responses requiring
NF-κB activation (30). Previous
studies have demonstrated that the NF-κB signaling pathway and its
transcription factors [in particular, the canonical signaling
subunits, ikkβ, c-Rel and P65 (RelA)], occupied a critical role in
CD4+ T cell biology (31,32).
PlexinA4, as a negative regulator in CD4+ T cell
activation, is a receptor of Sema3A that guides the development of
the nervous system and regulates the function of the immune system
(23). Plexins resemble TLRs in
their evolutionary conservation from flies to mammals, a plexina4
knockouT cell model exhibits defective inflammatory cytokine
production upon activation by a spectrum of TLR agonists and
bacteria (23). Sema3A-plexinA4
interaction affects the activation of Rac1, which is known to
promote NF-κB activation and cytokine production in TLR-stimulated
macrophages (33). Unfortunately,
the present study did not delve into specific receptors on the cell
surface.
The present study demonstrated that the NF-κB
signaling pathway is involved in a Sema3A-mediated autocrine loop,
aggravating T cell immune dysfunction during LPS-induced sepsis.
In vitro, the ratios of p-ikkβ/ikkβ and p-P65/P65 of
CD4+ T cells were significantly enhanced when treated
with LPS and decreased with anti-Sema3A antibody or EGCG treatment.
The phosphorylation levels should be detected as early as possible,
especially within 24 h or even a few minutes after the test. The
present study conducted a preliminary 24 h phosphorylation
detection and future studies will explore the phosphorylation level
in depth.
Acknowledgements
The authors would like to thank Professor Shu-Zhang
Cui of the Emergency Department of Tianjin Medical University
General Hospital (Tianjin, China) for help with the experimental
design.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81701931 and 81871593) and
the National Natural Science Foundation of Tianjin (grant no.
18JCQNJC10500).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG, CW and YC designed the study, wrote the
protocol, collected the data, performed statistical analyses and
contributed to writing the manuscript. ZW and WL performed the
technical work. ZW, YL and SS helped with data collection, study
design and coordinated the sstudy. YC participated in the study
design and helped to critically revise the manuscript. YG and YC
were responsible for confirming the authenticity of the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were undertaken following the
National Institute of Health Guide for the Care and Use of
Laboratory Animals and approved by the scientific investigation
board, Tianjin Medical University General Hospital (approval no.
ZYY-DWFL-IRB-001F-01; Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Raith EP, Udy AA, Bailey M, McGloughlin S,
MacIsaac C, Bellomo R and Pilcher DV; Australian New Zealand
Intensive Care Society (ANZICS) Centre for Outcomes and Resource
Evaluation (CORE), : Prognostic accuracy of the SOFA score, SIRS
criteria, and qSOFA score for in-hospital mortality among adults
with suspected infection admitted to the Intensive Care Unit. JAMA.
317:290–300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xie J, Wang H, Kang Y, Zhou L, Liu Z, Qin
B, Ma X, Cao X, Chen D, Lu W, et al CHinese Epidemiological Study
of Sepsis (CHESS) Study Investigators, : The epidemiology of sepsis
in Chinese ICUs: A National Cross-Sectional Survey. Crit Care Med.
48:e209–e218. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weng L, Zeng XY, Yin P, Wang LJ, Wang CY,
Jiang W, Zhou MG and Du B; China Critical Care Clinical Trials
Group (CCCCTG), : Sepsis-related mortality in China: A descriptive
analysis. Intensive Care Med. 44:1071–1080. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taeb AM, Hooper MH and Marik PE: Sepsis:
current definition, pathophysiology, diagnosis, and management.
Nutr Clin Pract. 32:296–308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stolk RF, Kox M and Pickkers P:
Noradrenaline drives immunosuppression in sepsis: Clinical
consequences. Intensive Care Med. 46:1246–1248. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van der Poll T, van de Veerdonk FL,
Scicluna BP and Netea MG: The immunopathology of sepsis and
potential therapeutic targets. Nat Rev Immunol. 17:407–420. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hotchkiss RS, Monneret G and Payen D:
Sepsis-induced immunosuppression: From cellular dysfunctions to
immunotherapy. Nat Rev Immunol. 13:862–874. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boomer JS, To K, Chang KC, Takasu O,
Osborne DF, Walton AH, Bricker TL, Jarman SD II, Kreisel D,
Krupnick AS, et al: Immunosuppression in patients who die of sepsis
and multiple organ failure. JAMA. 306:2594–2605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Otto GP, Sossdorf M, Claus RA, Rödel J,
Menge K, Reinhart K, Bauer M and Riedemann NC: The late phase of
sepsis is characterized by an increased microbiological burden and
death rate. Crit Care. 15:R1832011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Delano MJ, Scumpia PO, Weinstein JS, Coco
D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S,
Al-Quran SZ, et al: MyD88-dependent expansion of an immature
GR-1(+)CD11b(+) population induces T cell suppression and Th2
polarization in sepsis. J Exp Med. 204:1463–1474. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morita A, Yamashita N, Sasaki Y, Uchida Y,
Nakajima O, Nakamura F, Yagi T, Taniguchi M, Usui H, Katoh-Semba R,
et al: Regulation of dendritic branching and spine maturation by
semaphorin3A-Fyn signaling. J Neurosci. 26:2971–2980. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian X, Gan H, Zeng Y, Zhao H, Tang R and
Xia Y: Inhibition of semaphorin-3a suppresses
lipopolysaccharide-induced acute kidney injury. J Mol Med (Berl).
96:713–724. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamashita N, Jitsuki-Takahashi A, Ogawara
M, Ohkubo W, Araki T, Hotta C, Tamura T, Hashimoto S, Yabuki T,
Tsuji T, et al: Anti-Semaphorin 3A neutralization monoclonal
antibody prevents sepsis development in lipopolysaccharide-treated
mice. Int Immunol. 27:459–466. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Catalano A, Caprari P, Moretti S, Faronato
M, Tamagnone L and Procopio A: Semaphorin-3A is expressed by tumor
cells and alters T-cell signal transduction and function. Blood.
107:3321–3329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
16
|
Liu LN, Li XM, Ye DQ and Pan HF: Emerging
role of semaphorin-3A in autoimmune diseases. Inflammopharmacology.
26:655–665. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Körner S, Böselt S, Wichmann K,
Thau-Habermann N, Zapf A, Knippenberg S, Dengler R and Petri S: The
Axon Guidance Protein Semaphorin 3A Is Increased in the Motor
Cortex of Patients With Amyotrophic Lateral Sclerosis. J
Neuropathol Exp Neurol. 75:326–333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pasterkamp RJ, Dai HN, Terman JR, Wahlin
KJ, Kim B, Bregman BS, Popovich PG and Kolodkin AL: MICAL
flavoprotein monooxygenases: Expression during neural development
and following spinal cord injuries in the rat. Mol Cell Neurosci.
31:52–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Loron G, Olivier P, See H, Le Saché N,
Angulo L, Biran V, Brunelle N, Besson-Lescure B, Kitzis MD, Pansiot
J, et al: Ciprofloxacin prevents myelination delay in neonatal rats
subjected to E. coli sepsis. Ann Neurol. 69:341–351. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaukonen KM, Bailey M, Pilcher D, Cooper
DJ and Bellomo R: Systemic inflammatory response syndrome criteria
in defining severe sepsis. N Engl J Med. 372:1629–1638. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Darcy CJ, Minigo G, Piera KA, Davis JS,
McNeil YR, Chen Y, Volkheimer AD, Weinberg JB, Anstey NM and
Woodberry T: Neutrophils with myeloid derived suppressor function
deplete arginine and constrain T cell function in septic shock
patients. Crit Care. 18:R1632014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lepelletier Y, Moura IC, Hadj-Slimane R,
Renand A, Fiorentino S, Baude C, Shirvan A, Barzilai A and Hermine
O: Immunosuppressive role of semaphorin-3A on T cell proliferation
is mediated by inhibition of actin cytoskeleton reorganization. Eur
J Immunol. 36:1782–1793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wen H, Lei Y, Eun SY and Ting JP:
Plexin-A4-semaphorin 3A signaling is required for Toll-like
receptor- and sepsis-induced cytokine storm. J Exp Med.
207:2943–2957. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gu Z, Ueno M, Klinefelter K, Mamidi M,
Yagi T and Yoshida Y: Skilled movements in mice require inhibition
of corticospinal axon collateral formation in the spinal cord by
semaphorin signaling. J Neurosci. 39:8885–8899. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tordjman R, Lepelletier Y, Lemarchandel V,
Cambot M, Gaulard P, Hermine O and Roméo PH: A neuronal receptor,
neuropilin-1, is essential for the initiation of the primary immune
response. Nat Immunol. 3:477–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Catalano A: The neuroimmune semaphorin-3A
reduces inflammation and progression of experimental autoimmune
arthritis. J Immunol. 185:6373–6383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rubtsov YP, Niec RE, Josefowicz S, Li L,
Darce J, Mathis D, Benoist C and Rudensky AY: Stability of the
regulatory T cell lineage in vivo. Science. 329:1667–1671. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ramsdell F and Rudensky AY: Foxp3: A
genetic foundation for regulatory T cell differentiation and
function. Nat Immunol. 21:708–709. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao YL, Chai YF, Dong N, Han S, Zhu XM,
Zhang QH and Yao YM: Tuftsin-derived T-peptide prevents cellular
immunosuppression and improves survival rate in septic mice. Sci
Rep. 5:167252015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gelman AE, Zhang J, Choi Y and Turka LA:
Toll-like receptor ligands directly promote activated
CD4+ T cell survival. J Immunol. 172:6065–6073. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zaph C, Troy AE, Taylor BC, Berman-Booty
LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, et al:
Epithelial-cell-intrinsic IKK-beta expression regulates intestinal
immune homeostasis. Nature. 446:552–556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kilpinen S, Henttinen T, Lahdenpohja N,
Hulkkonen J and Hurme M: Signals leading to the activation of
NF-kappa B transcription factor are stronger in neonatal than adult
T lymphocytes. Scand J Immunol. 44:85–88. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arbibe L, Mira JP, Teusch N, Kline L, Guha
M, Mackman N, Godowski PJ, Ulevitch RJ and Knaus UG: Toll-like
receptor 2-mediated NF-kappa B activation requires a Rac1-dependent
pathway. Nat Immunol. 1:533–540. 2000. View
Article : Google Scholar : PubMed/NCBI
|