Introduction
Atopic dermatitis (AD, also known as atopic eczema)
is a chronic inflammatory skin disease that affects 2–20% of the
general population, and is the most significant non-fatal health
burden caused by skin disease (1,2). AD
results in major social and psychological burdens for patients and
their relatives, impacting the social functioning and psychological
well-being of patients (3). Current
approaches for preventing and treating AD focus on antibiotics,
corticosteroids, immunomodulators, immunosuppressants, skincare and
lifestyle changes; however, the effects of current treatments are
not ideal, and an optimal AD treatment needs to be developed to
improve patient prognosis, and reduce the burden on patients and
their families (4–6). AD is typically associated with type I
allergic diseases, including allergic rhinitis and asthma (7). A major marker of the disease is
elevated serum total immunoglobulin E (IgE) levels, which are
observed in ~80% of patients with AD (8). In fact, patients with AD develop IgE
antibody responses to a variety of environmental allergens and
autoantigens (3).
It has been proposed that bitter taste receptors
(TAS2Rs) (9) only exist on the
tongue, and that their activation enables the perception of
bitterness (10). However, a
previous study has found that TAS2Rs are also present in the
respiratory system (11). Children
with severe asthma exhibit increased TAS2R expression in
leukocytes, which are associated with mast cells (12). Administration of a TAS2R agonist can
inhibit IgE-dependent mast cell activation, thereby reducing IgE
expression (13).
Quinine is a TAS2R agonist that was purified in 1820
(14). A large number of medical
cases have reported that quinine is a drug specific for malarial
fever (15,16). However, quinine has been reported to
treat or alleviate numerous nonmalarial diseases, including ulcers,
hemorrhoids and gastric inflammation (17). A TAS2R agonist was found to reduce
IgE levels in mice with allergic asthma and to reduce clinical
symptoms (18). Shaw et al
(19) also detected TAS2R
expression in human skin. In the present study, quinine was used to
treat AD-like mice. ELISAs, western blotting, immunohistochemistry
and reverse transcription-quantitative PCR (RT-qPCR) were used to
investigate the effect and mechanism of quinine in the treatment of
AD.
Materials and methods
Reagents and drugs
The quinine (purity, >99%) employed in the
present study was purchased from MedChemExpress (cat. no.
HY-D0143), the 2,4-dinitrochlorobenzene (DNCB) was supplied by
Sigma-Aldrich; Merck KGaA (cat. no. 237329). TRIzol®
reagent was purchased from Invitrogen; Thermo Fisher Scientific,
Inc.. The reverse transcription kit (PrimeScript™ RT-PCR kit) and
the SYBR-Green kit (both Takara Bio, Inc.) were employed for
RT-qPCR analysis.
Induction of the atopic
dermatitis-like mice and treatment of quinine in mice
A total of 60 male BALB/c mice (age, 5–6 weeks;
weight, 17–20 g; n=5/group) were purchased from the Guangdong
Province Medical Experimental Center. AD-like skin lesions in the
mice were induced as previously described (20,21).
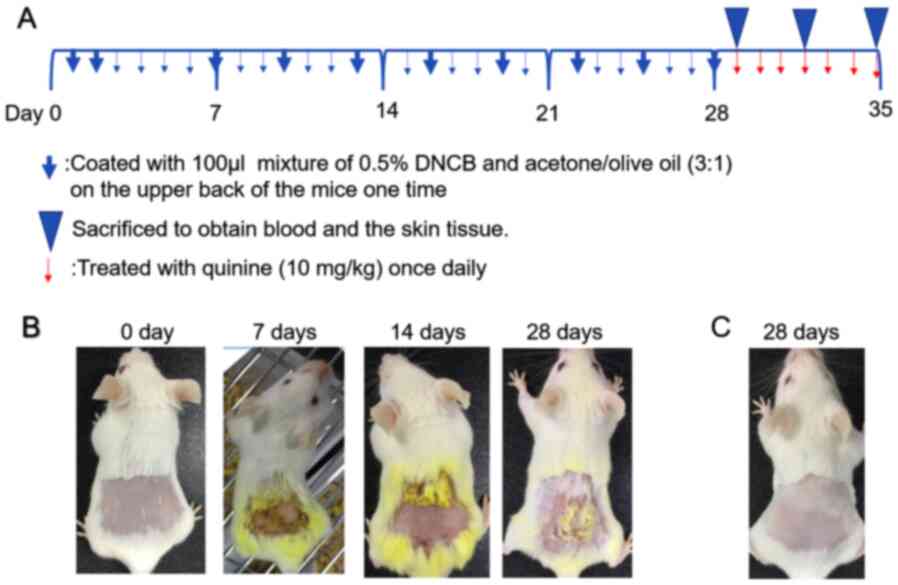
The experimental schedule is presented in Fig. 1A. Specifically, the procedure was as
follows: Before the experiment, the back hair above the hind legs
was shaved over an area of 2.5×2.5 cm. On days 1 and 2, the mice
were coated with a 100-µl mixture of 0.5% DNCB and acetone/olive
oil (3:1) on the shaved areas. From day 3 to 6, nothing was
applied. On day 7, and then every 2 days afterwards until day 28,
the mice were coated with 100 µl of the above mixture to induce an
AD-like phenotype (Fig. 1B). The
skin severity was evaluated based on four symptoms
(erythema/hemorrhage, edema, excoriation/erosion and dryness) and
defined as a sum of the individual scores (0, no symptoms; 1, mild;
2, moderate; 3, severe) (22).
In a control group of mice, the acetone/olive oil
mixture was applied to the back area with the same time and
quantity of application as the model group (Fig. 1C). All mice were divided into three
groups: i) 1 Day group (A); ii) 4 days group (B); and iii) 7 days
group (C). Each group was comprised of four sub-groups: i) Normal
control group (control); ii) atopic dermatitis (AD) group; iii) AD
group treated with quinine (AD + Q); and iv) control group treated
with quinine (C + Q). For treatment, 0.9% NaCl (100 µl) and quinine
(100 µl;10 mg/kg) were daubed once a day, and the daubed position
of the four groups was the same, taking the dermatitis lesion skin
position of the AD group as a reference (Table I). At the end of the study period,
animals were anesthetized with ether, and blood was collected from
the retro-orbital plexus (500 µl) prior to euthanasia via cervical
dislocation. Skin samples were then collected for analyses. All
procedures on the mice complied with the Guide for the Care and Use
of Laboratory Animals of the National Institutes of Health
(23). The Institutional Animal
Care and Use Committee of Shenzhen University Health Science Center
approved the study protocol (permit no. SCXK-2018-0002).
 | Table I.Experimental design, treatment groups
and analyses. |
Table I.
Experimental design, treatment groups
and analyses.
| Day | Group | Sub-Group | Intervention | Analyses |
|---|
| 1 | A | Control | 0.9% NaCl (100
µl) | Blood (ELISA) and
skin tissue |
|
|
| AD | 0.9% NaCl (100
µl) | (RT-qPCR, H&E
and ELISA) |
|
|
| AD + Q | Quinine (100 µl;10
mg/kg) |
|
|
|
| C + Q | Quinine (100 µl;10
mg/kg) |
|
| 4 | B | Control | 0.9% NaCl (100
µl) | Blood (ELISA) and
skin tissue |
|
|
| AD | 0.9% NaCl (100
µl) | (RT-qPCR, WB, IHC,
H&E and ELISA) |
|
|
| AD + Q | Quinine (100 µl;10
mg/kg) |
|
|
|
| C + Q | Quinine (100 µl;10
mg/kg) |
|
| 7 | C | Control | 0.9% NaCl (100
µl) | Blood (ELISA) and
skin tissue |
|
|
| AD | 0.9% NaCl (100
µl) | (RT-qPCR, H&E
and ELISA) |
|
|
| AD + Q | Quinine (100 µl;10
mg/kg) |
|
|
|
| C + Q | Quinine (100 µl;10
mg/kg) |
|
Histopathological analysis
Dorsal skin samples were separated and fixed in 10%
neutral buffered formaldehyde for 24 h at room temperature and
embedded in paraffin. Paraffin-embedded samples were cut into 4-µm
thick serial sections and subjected to H&E staining for general
histopathological analysis. Briefly, paraffin sections were
deparaffinized with xylene at room temperature and rehydrated with
a descending series (100, 95, 80 and 70%) of ethanol. Then,
sections were stained with hematoxylin for 5 min, stained with 5%
acetic acid for 1 min and stained with eosin for 1 min (all at room
temperature). Sections were subsequently dehydrated with an
ascending series (70, 80, 95 and 100%) of ethanol at room
temperature. Stained sections were observed under a light
microscope (magnification, ×100; Olympus BX51; Olympus
Corporation). The status of the epidermis in skin lesions was
analyzed using Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc.).
Immunohistochemical detection
Tissue sections (which were prepared as described
above up to the staining steps) were boiled in citrate buffer for
15 min for antigen retrieval. The slices were then incubated with
3% hydrogen peroxide for 5 min to block the endogenous peroxidase
activity at room temperature. Subsequently, the sections were
blocked with 5% bovine serum albumin for 30 min at 37°C. The
sections were incubated overnight at 4°C with diluted antibody
against FLG (1:1,000; cat. no. HPA030189; Sigma-Aldrich; Merck
KGaA) and KLK7 (1:1,000; cat. no. HPA062126; Sigma-Aldrich; Merck
KGaA). Following the primary antibody incubation, the tissues
slides were washed with PBS and incubated with reaction enhancer
solution (cat. no. PV-9001; OriGene Technologies, Inc.) at 37°C for
60 min, washed with PBS and then incubated with goat anti-rabbit
IgG polymer (1:1; cat. no. PV-9001; OriGene Technologies, Inc.) at
room temperature for 20 min, prior to being washed with PBS.
Immunoreactivity was visualized by DAB for 1–5 min at room
temperature, washed in water and re-stained with hematoxylin. Light
microscopy was performed to observe the histological profiles of
dorsal skin sections (magnification, ×100; Eclipse E100; Nikon
Corporation).
Quantification of cytokines in dorsal
skin tissue and IgE in serum
Dorsal skin samples (100 mg) were homogenized in 1
ml T-PER Tissue Protein Extraction reagent. Blood samples were
obtained from each treatment group after 1, 4 or 7 days of quinine
treatment (24) and centrifuged at
2,000 × g for 20 min at 4°C to obtain serum. The cytokine levels of
IL-4 (cat. no. ab100710; Abcam), IL-5 (cat. no. ab204523; Abcam),
IL-13 (cat. no. ab219634; Abcam), TNF-α (cat. no. ab208348; Abcam)
and IL-1β (cat. no. ab197742; Abcam) in skin tissue and IgE (cat.
no. ab157718; Abcam) in serum were measured via ELISAs, according
to the manufacturers' protocols.
Quantitative analysis of gene
expression in the NF-κB signaling pathway
RT-qPCR was conducted to detect the mRNA expression
levels of IκBα, IκB kinase α (IKKα) and NF-κB. The tissues samples
were harvested in TRIzol® reagent for total RNA
extraction. RT of the total RNA was performed with random hexamers
using a PrimeScript™ RT-PCR kit according to the manufacturer's
protocol. qPCR was then performed to determine the expression
levels of the target genes using specific primers (Table II) and SYBR-Green kit according to
the manufacturer's protocols. The following thermocycling
conditions were used for the qPCR: 94°C for 30 sec; followed by 40
cycles of 95°C for 5 sec and 58°C for 30 sec. The expression levels
of IκBα, IκB kinase α (IKKα) and NF-κB were normalized to the
endogenous control GAPDH using the 2−∆∆Cq method
(25).
 | Table II.Sequences of primers used in the
study. |
Table II.
Sequences of primers used in the
study.
| Gene | Sequence
(5′→3′) |
|---|
| IκBα | F:
GGCAAGATGTAGAGGGGTATTT |
|
| R:
ATGGAAGTCATTGGTCAGGTG |
| IKKα | F:
TCTGGAAACACTCAAGATAGACAC |
|
| R:
AAGCACAACAATGCAGGTACA |
| NF-κB | F:
GTTATCGTTCAGTTGGTCACA |
|
| R:
ATATGCCGTCCTCACAGT |
| GAPDH | F:
ACCCATCACCATCTTCCAGGAG |
|
| R:
GAAGGGGCGGAGATGATGAC |
Western blotting analysis
The back skin tissue samples (100 mg) of the mice
were lysed using RIPA lysis buffer (Beijing Solarbio Science &
Technology Co., Ltd.), homogenized in a tissue grinder and
centrifuged at 12,000 × g at 4°C for 30 min to obtain the proteins
in the supernatant. Each sample was run in duplicate, and a BCA
protein assay kit (cat. no. PC0020; Solarbio) was subsequently used
to quantify total protein content. Equal quantities (30 µg/lane) of
protein from each sample were resolved via 12% SDS-PAGE and
transferred to PVDF membranes. Following blocking in 5% skimmed
milk at room temperature for 1 h, membranes were incubated
overnight with primary antibodies against KLK7 (1:1,000; cat. no.
DF7384; Affinity Biosciences), FLG (1:500; cat. no. DF13653;
Affinity Biosciences) and GAPDH (1:1,000; cat. no. 5174T; Cell
Signaling Technology, Inc.). The membranes were then incubated with
secondary antibodies (1:5,000; cat. no. 7074S; Cell Signaling
Technology, Inc.) for 1 h at room temperature. Protein bands were
visualized using an enhanced chemiluminescent western blotting kit
(cat. no. P0018AS-2; Beyotime Institute of Biotechnology) according
to the manufacturer's protocols. Densitometry was performed using
Quantity One software (version 4.0; Bio-Rad Laboratories,
Inc.).
Statistical analysis
The results are presented as the mean ± standard
deviation, and the statistical comparisons of the different groups
were performed using one-way ANOVA followed by Tukey's post hoc
test. The Mann-Whitney test was used for comparison of two groups.
α=0.05 was the statistical benchmark, and all statistical tests
were two-tailed. Analyses were performed and graphs were generated
using SPSS 23.0 (IBM Corp.) and GraphPad Prism 7.0 (GraphPad
Software, Inc.), and all experiments were performed at least 3
times unless otherwise specified. P<0.05 was considered to
indicate a statistically significant difference.
Results
Quinine improves the condition of
AD-like skin lesions
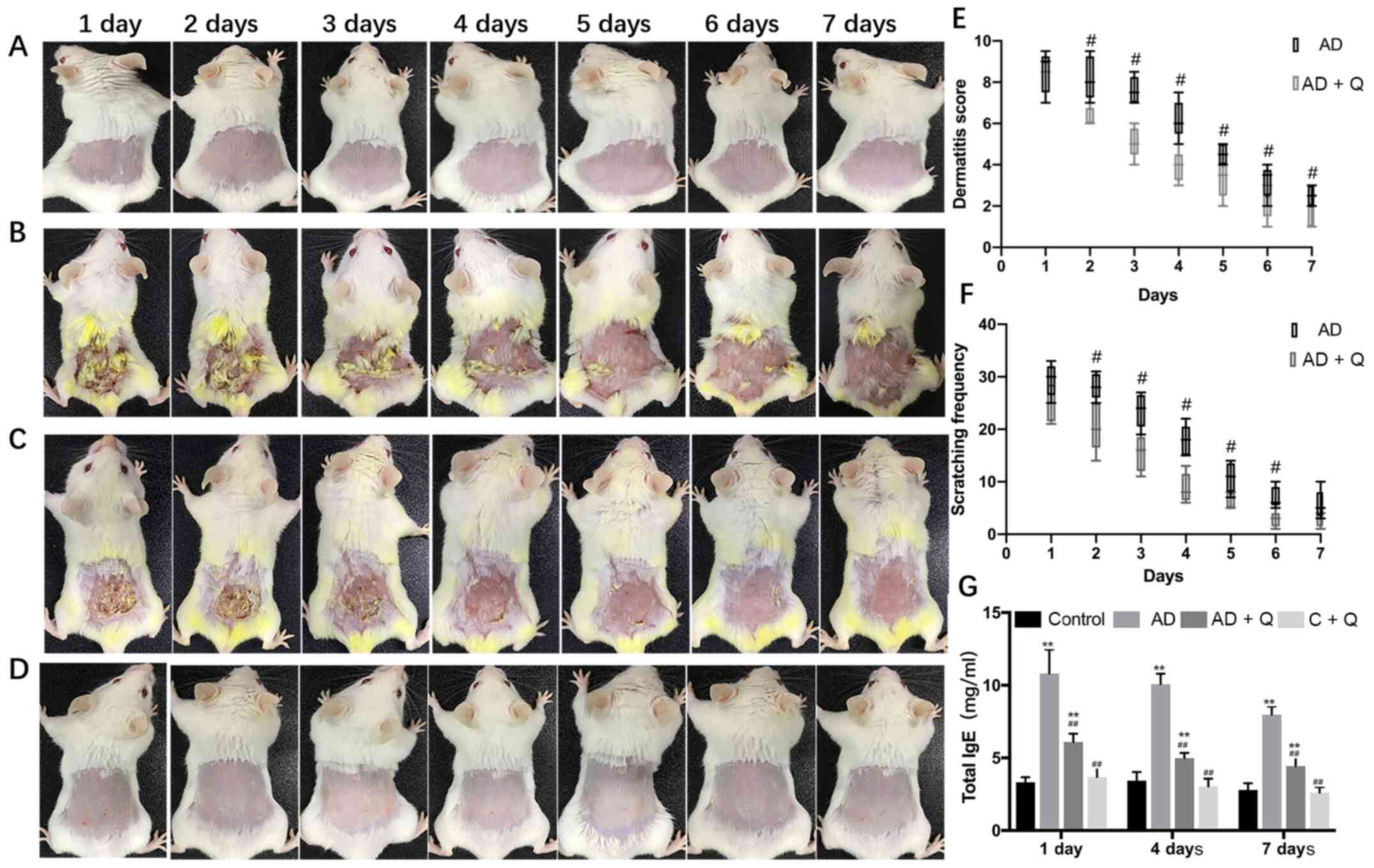
To study the effect of quinine treatment on AD-like
symptoms, the effects of quinine on AD-like mice were evaluated
using a dermatitis severity score and the amount of scratching by
the mice in a 10-min period. When treated with quinine, AD-like
symptoms, including dryness, erythema, edema and excoriation, were
ameliorated following quinine treatment, and quinine did not cause
notable skin abnormalities in the C + Q group (Fig. 2A-D). The dermatitis severity scores
and scratching frequency were significantly reduced in the AD + Q
group compared with the AD-like mice (Fig. 2E and F). These results indicated
that quinine relieved the clinical symptoms of AD without causing
notable irritation or itching to healthy skin.
Quinine treatment reduces serum IgE
levels
A major marker of AD is elevated serum total IgE
levels, which are observed in ~80% of patients with AD (8). After measuring the serum IgE levels of
each mouse group, it was found that the IgE levels in the AD-like
mice were increased, and that these levels decreased significantly
after treatment with quinine (Fig.
2G).
Quinine reduces pathological damage to
skin
As presented in Fig.
3A-C, compared with healthy mice, the epidermis in skin lesions
induced by DNCB in the AD-like mice thickened, and dermal
inflammatory cells had infiltrated. After quinine treatment, these
pathological changes were alleviated, as DNCB-induced local
thickening in the AD + Q group was significantly decreased; there
were no notable changes in epidermal thickness in the C + Q group
compared with the healthy controls (Fig. 3D). These indicated that quinine
reduced inflammatory responses in the skin tissue of AD-like mice
without inducing notable changes in healthy tissue.
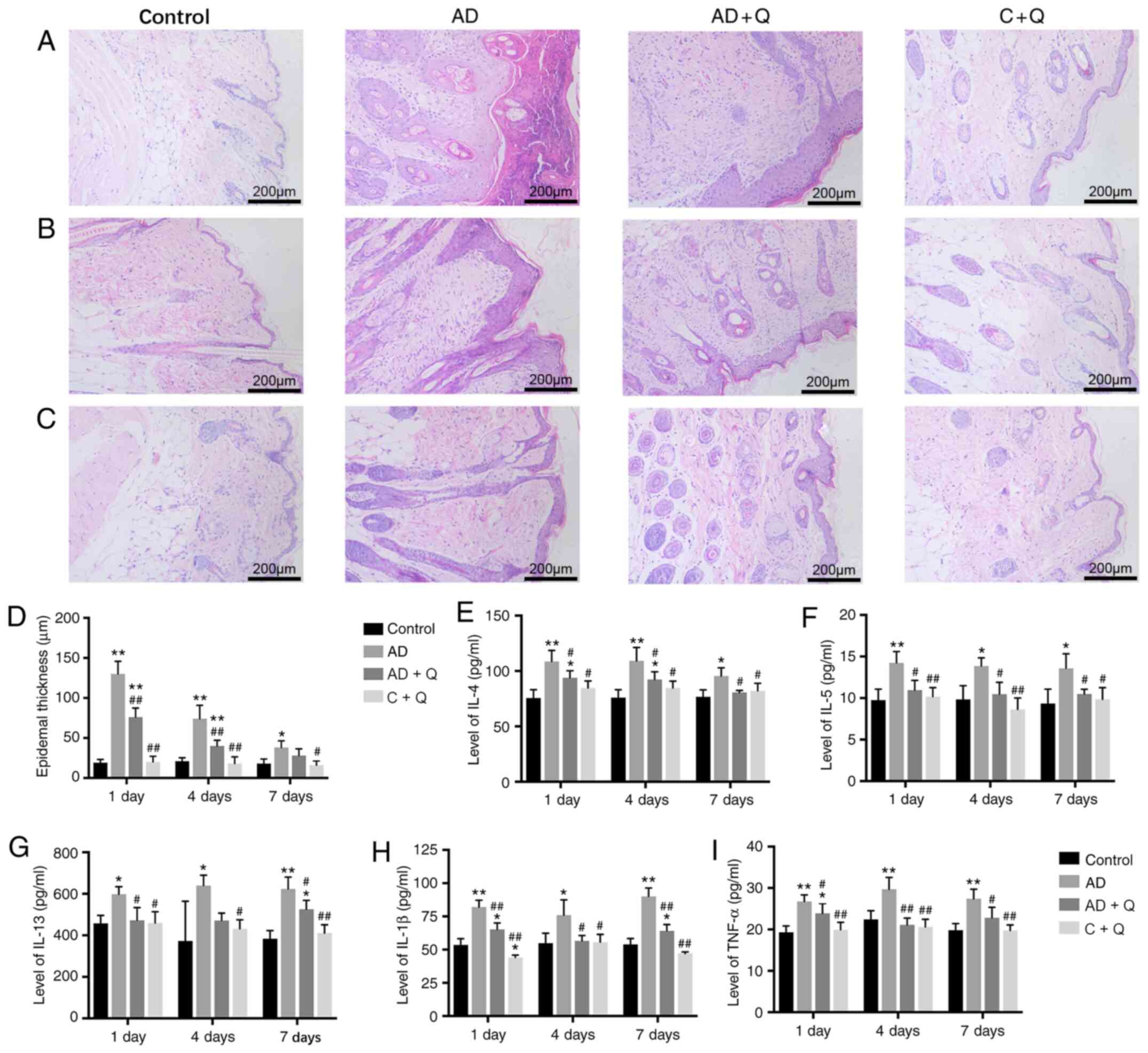 | Figure 3.Histopathological analysis of the
back skin tissues. Dorsal skin sections were stained with H&E
(magnification, ×100). (A) Skin tissues following treatment for 1
day. (B) Skin tissues following treatment for 4 days. (C) Skin
tissues following treatment for 7 days. (D) Epidermal thickness in
the dorsal skin. Levels of (E) IL-4, (F) IL-5, (G) IL-13, (H) IL-1β
and (I) TNF-α in dorsal skin tissues. Data are presented as the
mean ± standard deviation. *P<0.05, **P<0.001 vs. control;
#P<0.05, ##P<0.001 vs. AD. AD, atopic
dermatitis; AD + Q, AD group treated with quinine; C + Q, control
group treated with quinine; IL, interleukin; TNF, tumor necrosis
factor. |
Quinine treatment decreases cytokine
levels in the dorsal skin
The pathophysiology of AD shows that Th1 and Th2
reactions are maladjusted, causing allergic dermatitis symptoms and
leading to the release of numerous cytokines (26). NF-κB signaling pathways have
reported to be involved in the production of proinflammatory
cytokines in patient-derived cells, as well as in the release of
tumor necrosis factor-α (TNF-α) and interleukin (IL)-1β (27). To study whether quinine could alter
the immune response, the effects of quinine on cytokine levels were
evaluated in dorsal skin samples from the experimental mice. The
levels of IL-4, IL-5 and IL-13 (Th2 cytokines) (28), and TNF-α and IL-1β (NF-κB-related
cytokines) (27) in the skin tissue
of AD-like mice were significantly elevated compared with healthy
mice; after treatment with quinine, the levels of IL-4, IL-5,
IL-13, TNF-α and IL-1β were significantly decreased, whereas the
immune response in the skin of the C + Q group was unchanged
(Fig. 3E-I).
Quinine treatment improves skin
barrier function
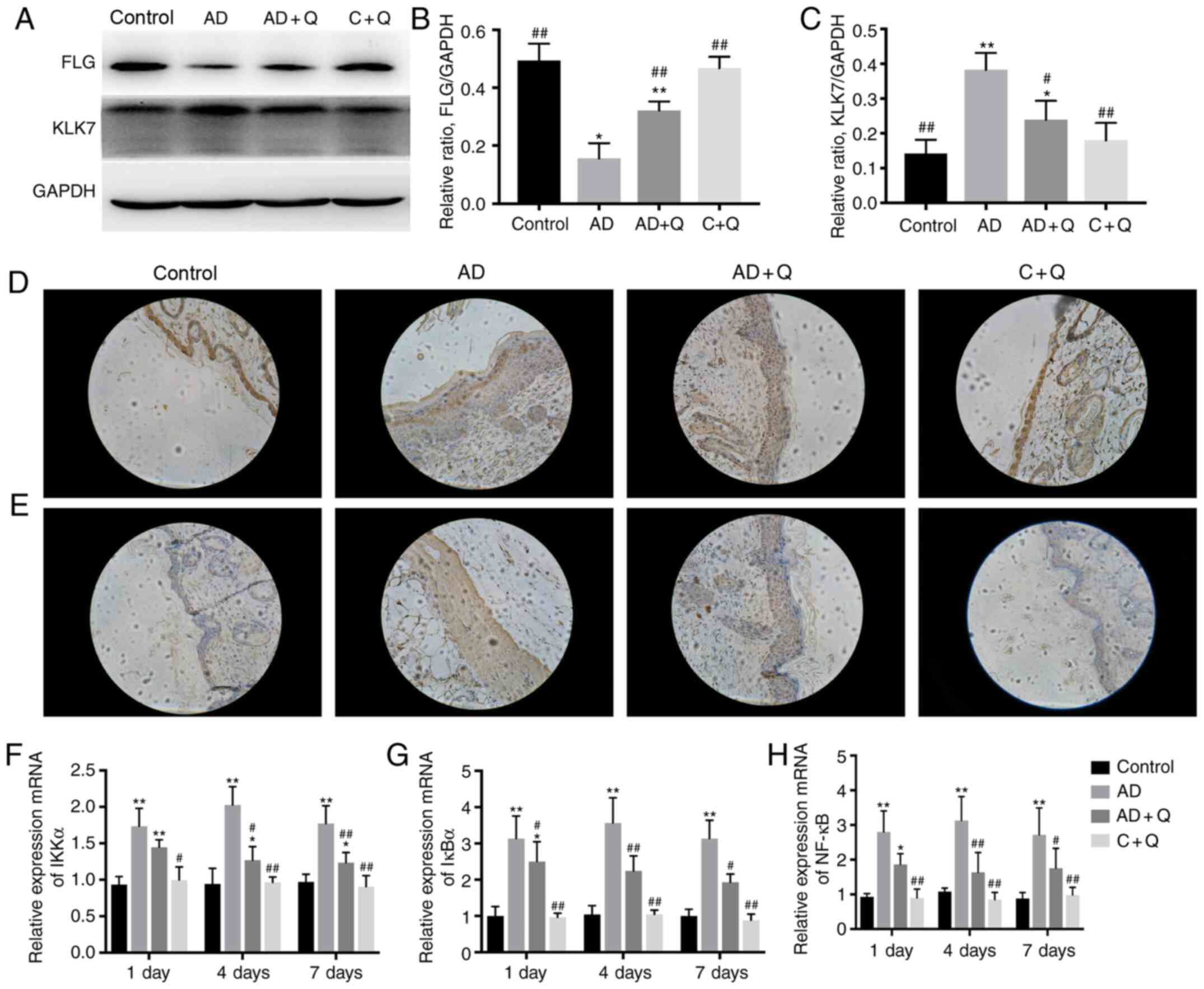
FLG serves the protective function of maintaining
the skin barrier, and KLK7 is a serine protease involved in the
proteolysis of extracellular corneal linker components, which
results in desquamation (29).
Increased KLK7 levels have been shown to induce spontaneous itching
in mice (30). To further
investigate the protective function of quinine on the skin barrier,
western blotting and immunohistochemistry were performed. Reduced
FLG expression and increased KLK7 expression in AD-like mice
suggested that the skin barrier of the AD-like mice was damaged.
Following quinine treatment, FLG expression was increased and KLK7
expression was decreased in the AD + Q group compared with the AD
group, indicating that the skin barrier was restored (Fig. 4A-C). The expression of FLG and KLK7
was also investigated in skin lesions via immunohistochemistry, and
the results were consistent with the western blot analysis
(Fig. 4D and E), suggesting that
quinine exhibited a protective effect on the skin barrier.
Quinine inhibits the expression of
genes involved in the NF-κB signaling pathway
To study the mechanism via which quinine ameliorates
inflammation in AD-like mice, the expression levels of genes
associated with the NF-κB signaling pathway were evaluated,
including IκBα, IKKα and NF-κB. It has been reported that IκBα is a
negative regulator of NF-κB (31);
however, the present results found that the levels of IκBα, IKKα
and NF-κB mRNA expression were significantly increased in AD-like
mice (Fig. 4F-H). After quinine
treatment, the expression of IκBα, IKKα and NF-κB mRNA was
decreased significantly compared with the AD group (Fig. 4F-H), which may be involved in
inhibiting the development of inflammation. These results suggested
that quinine reduced activation of the NF-κB signaling pathway at
the mRNA level, thus serving a potential role in the treatment of
AD.
Discussion
Quinine, an alkaloid derived from the bark of
cinchona in the Andean forests, was the first effective treatment
for malaria (32). Studies have
reported that the TAS2R agonist can inhibit IgE secretion in a
mouse model of asthma, thus relieving the clinical symptoms
(12,13). Therefore, the present study aimed to
evaluate the therapeutic effects of quinine on AD-like mice. The
results demonstrated that quinine treatment effectively attenuated
AD-like symptoms in mice, reducing the dermatitis severity score,
serum IgE levels and the infiltration of inflammatory cells, as
well as alleviating the pathological damage, inhibiting the
expression of genes related to the NF-κB signaling pathway,
reducing the inflammatory response and promoting the expression of
the skin-protective protein FLG. It was also found that quinine
reduced cytokine levels in the AD-like mice. These results
suggested that the topical application of quinine may provide a
novel therapeutic regimen for the treatment AD, as well as
indicating that quinine does not cause notable harm to healthy
skin.
In the present study, it was revealed that quinine
increased the levels of Th2-related factors and a number of
cytokines associated with NF-κB signaling in the dorsal skin
lesions of AD-like mice. When treated with quinine, the cytokine
levels decreased. Previous studies had shown that the
pathophysiology of the skin changes in AD, including dysregulation
of Th1 and Th2 responses, characterized by IL-4, IL-5 and
IL-13-mediated allergic dermatitis (26,28).
Elevated levels of Th2-related cytokines have been detected in the
skin lesions of patients with AD, and these elevated levels have
been associated with elevated circulating IgE levels (33).
AD primarily consists of skin damage. The main
reason for the onset of the disease is skin barrier defects; one of
the important proteins involved is the skin structural protein FLG
(34,35). Naeem et al (36) demonstrated that increasing FLG
expression could alleviate AD-like skin lesions. The present study
demonstrated that FLG expression was reduced in the skin lesions of
AD-like mice and that quinine treatment increased FLG levels, which
may be an important factor underlying the improvement in the skin
lesions. Studies have shown that the skin lesions of patients with
AD contain high KLK levels, which may be related to the itching
associated with AD (37).
Experiments with mice have shown that KLK overexpression in the
skin can spontaneously induce AD-like disease (29,38).
In the present study, it was found that the scratching frequency of
the AD-like mice was significantly reduced after treatment with
quinine compared with the untreated AD-like mice. To further study
the mechanism, KLK7 expression in the dorsal skin was examined. The
results showed that KLK7 expression decreased in the skin lesions
of AD-like mice following quinine treatment.
To further elucidate the mechanisms underlying the
anti-AD effects of quinine, NF-κB mRNA expression was measured in
the dorsal skin of each group. NF-κB expression was increased in
the AD-like mice; when treated with quinine, this increase was
attenuated. NF-κB is an important transcription factor that serves
central roles in the occurrence and development of acute and
chronic inflammatory diseases (39). NF-κB signaling pathways have been
reported to be involved in the production of proinflammatory
cytokines in patient-derived cells (40). To verify the effect and mechanism of
quinine in AD, we the mRNA expression levels of IκBα, IKKα and
NF-κB, which are all involved in NF-κB signaling pathway, were
investigated. Upon receiving an extracellular signal, which results
in the phosphorylation, ubiquitination, and degradation of IκBα,
NF-κB is permitted to enter the nucleus (31,41).
Newly synthesized IκBα enters the nucleus and strips NF-κB from
DNA, thereby terminating the signal response through an unknown
mechanism, a process in which a transient ternary complex between
NF-κB, its cognate DNA κB sequence and IκBα is observed (31,41,42).
Interestingly, the present results showed that the expression of
IκBα and NF-κB increased simultaneously in the skin lesion of
AD-like mice, and concurrently decreased following administration
of quinine. The present data were inconsistent with previous
reports which indicated that IκBα was a negative regulator of NF-κB
(31,41,42).
However, the present study only investigated mRNA expression
levels; thus, further research will aim to clarify the regulatory
role of IκBα on the expression levels of proteins associated with
the NF-κB signaling pathway in AD.
Taken together, the present results indicated that
quinine suppressed Th2-related cytokines (IL-4, IL-5 and IL-13),
IL-1β and TNF-α by inhibiting the activity of NF-κB signaling
pathways in a DNCB-induced mouse model of AD. Quinine may therefore
be an effective treatment for AD. In future studies, inhibitors of
proteins in the NF-κB signaling pathway will be used to further
determine the mechanisms underlying the effects of quinine in the
treatment of AD.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81902067, 30872281,
81272115 and 81300028), the Natural Science Foundation of Shenzhen
University General Hospital (grant no. SUGH2018QD037), the Shenzhen
Science and Technology Innovation Committee (grant no.
JCY20180305124849781) and the China Postdoctoral Science Foundation
(grant no. 2020M670047ZX).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW and SC conceived and designed this study. QZ,
HJJ, ML, XCL and MRZ performed the in vivo studies and
collected the data. YSL and JKH analyzed the data and interpreted
the results of the experiments. LW and SC confirm the authenticity
of all the raw data. XCL and MRZ prepared the figures. SC and QZ
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The Institutional Animal Care and Use Committee of
Shenzhen University Health Science Center approved the study
protocol (permit no. SCXK-2018-0002).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nutten S: Atopic dermatitis: Global
epidemiology and risk factors. Ann Nutr Metab. 66 (Suppl 1):S8–S16.
2015. View Article : Google Scholar
|
|
2
|
Eichenfield LF, Tom WL, Chamlin SL,
Feldman SR, Hanifin JM, Simpson EL, Berger TG, Bergman JN, Cohen
DE, Cooper KD, et al: Guidelines of care for the management of
atopic dermatitis: Section 1. Diagnosis and assessment of atopic
dermatitis. J Am Acad Dermatol. 70:338–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weidinger S and Novak N: Atopic
dermatitis. Lancet. 387:1109–1122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Benedetto A, Kubo A and Beck LA: Skin
barrier disruption: A requirement for allergen sensitization? J
Invest Dermatol. 132:949–963. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leung DY: New insights into atopic
dermatitis: Role of skin barrier and immune dysregulation. Allergol
Int. 62:151–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leung DY: Atopic dermatitis: New insights
and opportunities for therapeutic intervention. J Allergy Clin
Immunol. 105:860–876. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han H, Roan F and Ziegler SF: The atopic
march: Current insights into skin barrier dysfunction and
epithelial cell-derived cytokines. Immunol Rev. 278:116–130. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kasperkiewicz M, Schmidt E, Ludwig RJ and
Zillikens D: Targeting IgE antibodies by immunoadsorption in atopic
dermatitis. Front Immunol. 9:2542018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeruzal-Świątecka J, Fendler W and
Pietruszewska W: Clinical role of extraoral bitter taste receptors.
Int J Mol Sci. 21:51562020. View Article : Google Scholar
|
|
10
|
Workman AD, Palmer JN, Adappa ND and Cohen
NA: The role of bitter and sweet taste receptors in upper airway
immunity. Curr Allergy Asthma Rep. 15:722015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deshpande DA, Wang WCH, McIlmoyle EL,
Robinett KS, Schillinger RM, An SS, Sham JS and Liggett SB: Bitter
taste receptors on airway smooth muscle bronchodilate by localized
calcium signaling and reverse obstruction. Nat Med. 16:1299–1304.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orsmark-Pietras C, James A, Konradsen JR,
Nordlund B, Söderhäll C, Pulkkinen V, Pedroletti C, Daham K,
Kupczyk M, Dahlén B, et al: Transcriptome analysis reveals
upregulation of bitter taste receptors in severe asthmatics. Eur
Respir J. 42:65–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ekoff M, Choi JH, James A, Dahlén B,
Nilsson G and Dahlén SE: Bitter taste receptor (TAS2R) agonists
inhibit IgE-dependent mast cell activation. J Allergy Clin Immunol.
134:475–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tomic S: L'Analyse chimique des végétaux:
Le cas du quinquina. Ann Sci. 58:287–309. 2001. View Article : Google Scholar
|
|
15
|
Achan J, Talisuna AO, Erhart A, Yeka A,
Tibenderana JK, Baliraine FN, Rosenthal PJ and D'Alessandro U:
Quinine, an old anti-malarial drug in a modern world: Role in the
treatment of malaria. Malar J. 10:1442011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tse EG, Korsik M and Todd MH: The past,
present and future of anti-malarial medicines. Malar J. 18:932019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gachelin G, Garner P, Ferroni E, Tröhler U
and Chalmers I: Evaluating Cinchona bark and quinine for treating
and preventing malaria. J R Soc Med. 110:31–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharma P, Yi R, Nayak AP, Wang N, Tang F,
Knight MJ, Pan S, Oliver B and Deshpande DA: Bitter taste receptor
agonists mitigate features of allergic asthma in mice. Sci Rep.
7:461662017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shaw L, Mansfield C, Colquitt L, Lin C,
Ferreira J, Emmetsberger J and Reed DR: Personalized expression of
bitter ‘taste’ receptors in human skin. PLoS One. 13:e02053222018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang H, Jung EM, Ahn C, Lee GS, Lee SY,
Kim SH, Choi IG, Park MJ, Lee SS, Choi DH and Jeung EB: Elemol from
chamaecyparis obtusa ameliorates 2,4-dinitrochlorobenzene-induced
atopic dermatitis. Int J Mol Med. 36:463–472. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caglayan Sozmen S, Karaman M, Cilaker
Micili S, Isik S, Arikan Ayyildiz Z, Bagriyanik A, Uzuner N and
Karaman O: Resveratrol ameliorates 2,4-dinitrofluorobenzene-induced
atopic dermatitis-like lesions through effects on the epithelium.
PeerJ. 4:e18892016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamamoto M, Haruna T, Yasui K, Takahashi
H, Iduhara M, Takaki S, Deguchi M and Arimura A: A novel atopic
dermatitis model induced by topical application with
dermatophagoides farinae extract in NC/Nga mice. Allergol Int.
56:139–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
National Institutes of Health, . Guide for
the care and use of laboratory animals. 7th edition. Washington DC:
National Academy Press; 1996
|
|
24
|
Jin W, Huang W, Chen L, Jin M, Wang Q, Gao
Z and Jin Z: Topical application of JAK1/JAK2 inhibitor momelotinib
exhibits significant anti-inflammatory responses in DNCB-induced
atopic dermatitis model mice. Int J Mol Sci. 19:39732018.
View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grewe M, Bruijnzeel-Koomen CA, Schöpf E,
Thepen T, Langeveld-Wildschut AG, Ruzicka T and Krutmann J: A role
for Th1 and Th2 cells in the immunopathogenesis of atopic
dermatitis. Immunol Today. 19:359–361. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yatoo MI, Gopalakrishnan A, Saxena A,
Parray OR, Tufani NA, Chakraborty S, Tiwari R, Dhama K and Iqbal
HMN: Anti-inflammatory drugs and herbs with special emphasis on
herbal medicines for countering inflammatory diseases and
disorders-a review. Recent Pat Inflamm Allergy Drug Discov.
12:39–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lambrecht BN, Hammad H and Fahy JV: The
cytokines of asthma. Immunity. 50:975–991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Igawa S, Kishibe M, Minami-Hori M, Honma
M, Tsujimura H, Ishikawa J, Fujimura T, Murakami M and
Ishida-Yamamoto A: Incomplete KLK7 secretion and upregulated LEKTI
expression underlie hyperkeratotic stratum corneum in atopic
dermatitis. J Invest Dermatol. 137:449–456. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo CJ, Mack MR, Oetjen LK, Trier AM,
Council ML, Pavel AB, Guttman-Yassky E, Kim BS and Liu Q:
Kallikrein 7 promotes atopic dermatitis-associated itch
independently of skin inflammation. J Invest Dermatol.
140:1244–1252.e4. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mukherjee SP, Quintas PO, McNulty R,
Komives EA and Dyson HJ: Structural characterization of the ternary
complex that mediates termination of NF-κB signaling by IκBα. Proc
Natl Acad Sci USA. 113:6212–6217. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shanks GD: Historical review: Problematic
malaria prophylaxis with quinine. Am J Trop Med Hyg. 95:269–272.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jeong CW, Ahn KS, Rho NK, Park YD, Lee DY,
Lee JH, Lee ES and Yang JM: Differential in vivo cytokine mRNA
expression in lesional skin of intrinsic vs extrinsic atopic
dermatitis patients using semiquantitative RT-PCR. Clin Exp
Allergy. 33:1717–1724. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Callard RE and Harper JI: The skin
barrier, atopic dermatitis and allergy: A role for Langerhans
cells? Trends Immunol. 28:294–298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jakasa I, de Jongh CM, Verberk MM, Bos JD
and Kezić S: Percutaneous penetration of sodium lauryl sulphate is
increased in uninvolved skin of patients with atopic dermatitis
compared with control subjects. Br J Dermatol. 155:104–109. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Naeem AS, Tommasi C, Cole C, Brown SJ, Zhu
Y, Way B, Willis Owen SA, Moffatt M, Cookson WO, Harper JI, et al:
A mechanistic target of rapamycin complex 1/2 (mTORC1)/V-Akt murine
thymoma viral oncogene homolog 1 (AKT1)/cathepsin H axis controls
filaggrin expression and processing in skin, a novel mechanism for
skin barrier disruption in patients with atopic dermatitis. J
Allergy Clin Immunol. 139:1228–1241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Komatsu N, Saijoh K, Kuk C, Liu AC, Khan
S, Shirasaki F, Takehara K and Diamandis EP: Human tissue
kallikrein expression in the stratum corneum and serum of atopic
dermatitis patients. Exp Dermatol. 16:513–519. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Briot A, Deraison C, Lacroix M, Bonnart C,
Robin A, Besson C, Dubus P and Hovnanian A: Kallikrein 5 induces
atopic dermatitis-like lesions through PAR2-mediated thymic stromal
lymphopoietin expression in Netherton syndrome. J Exp Med.
206:1135–1147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Barnes PJ and Karin M: Nuclear
factor-kappaB: A pivotal transcription factor in chronic
inflammatory diseases. N Engl J Med. 336:1066–1071. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fisher CL, Pineault N, Brookes C, Helgason
CD, Ohta H, Bodner C, Hess JL, Humphries RK and Brock HW:
Loss-of-function additional sex combs like 1 mutations disrupt
hematopoiesis but do not cause severe myelodysplasia or leukemia.
Blood. 115:38–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sue SC, Alverdi V, Komives EA and Dyson
HJ: Detection of a ternary complex of NF-kappaB and IkappaBalpha
with DNA provides insights into how IkappaBalpha removes NF-kappaB
from transcription sites. Proc Natl Acad Sci USA. 108:1367–1372.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Genes Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|