Introduction
Preeclampsia is a pregnancy-specific hypertensive
disorder that may lead to the death of newborns and pregnant women
(1). Patients with preeclampsia are
likely to have placental dysplasia, which seriously affects the
health of pregnant women and fetuses. Pregnant women with chronic
hypertension have a significantly higher risk of developing
preeclampsia compared with healthy pregnant women. For those with a
family history of preeclampsia, the incidence of chronic
hypertension is further greatly increased (2). In addition, the risk of patients with
preeclampsia having chronic hypertension is estimated to be higher
in the future compared with now (3,4).
Researchers confirmed that the ATP2B1 gene is a gene susceptible to
chronic hypertension among different races (5–7).
The ATP2B1 gene, located at 12q21.3, encodes the
ATPase plasma membrane Ca2+ transporting 1 (ATP2B1)
protein, which belongs to the P-type ion transport ATPase family of
proteins and is widely expressed in mammals (8). In 2009, through use of a genome-wide
association study, Levy et al (9) conducted a large cohort study on two
European populations, and found a single nucleotide polymorphism
locus associated with chronic hypertension; the SNPrs2681472 locus
located in the ATP2B1 gene. SNPrs2681472 polymorphism of ATP2B1
gene has been previously reported to be associated with early-onset
preeclampsia among Chinese pregnant women (10). The SNPrs2681472 polymorphism of
ATP2B1 gene may participate in the regulation of hypertension and
preeclampsia by affecting ATP2B1 gene expression levels (5).
In recent years, the effects of microRNAs
(miRNAs/miRs) on hypertension and preeclampsia have been widely
studied. miRNAs are non-coding RNAs 21–25 nucleotides in length,
and are involved in ~30% of the regulation of gene expressions in
the human genomes (11). A previous
study by Wang et al (12)
found that compared with normal pregnant women, the expression
levels of nine miRNAs, namely, miR-223, −195, −17, −18a, −218,
−19b1, −379, −92a1 and miR-411, are reduced in placenta tissues of
patients with severe preeclampsia compared with controls. Moreover,
the expression of another seven miRNAs (miR-30a-3p, −210, −524,
−518b, −18a, −17-3 and −411) are also reduced, and miR-151 and
miR-193b expression levels are increased in the tissues derived
from patients with preeclampsia (13). Previous studies showed that
miR-125b-5p, −100-5p and −199a-5p have a low level of expression in
the peripheral blood of patients with pregnancy-induced
hypertension and preeclampsia (14). Compared with normal pregnant women,
the levels of total miRNAs and hsa-miR-210 in peripheral blood of
pregnant women with hypertensive are significantly increased.
hsa-miR-210 has been seen as a suitable biomarker for indicating
pregnancy-induced hypertension (15). Moreover, miR-210 is involved in the
pathogenesis of preeclampsia (16).
Recently, it has been found that miR-27b-3p participates in various
pathological processes, including in tumor angiogenesis, lipid
metabolism, inflammatory response and the oxidative stress response
(17).
The present study was designed to explore the
relationship between hypertension and preeclampsia, and the role of
miR-27b-3p. miR-27b-3p was found to be upregulated in patients with
hypertension and patients with preeclampsia. The effects of
miR-27b-3p on HUVECs stimulated by the serum of patients with
hypertension were investigated. Moreover, the effects of miR-27b-3p
on HTR-8/SVneo cells co-cultured with HUVECs cells were explored,
following stimulation by the serum of patients with hypertension.
These results confirmed that miR-27b-3p might be a diagnostic
marker for hypertension and preeclampsia.
Materials and methods
Patients
Normal pregnant women (n=21; age 33.5±4; body mass
index 22.3±3 kg/m2; vaginal delivery), women with
preeclampsia (n=21; age 34.1±5; body mass index 24.6±4
kg/m2; vaginal delivery), female patients with
hypertension (n=13; 51.5±4) and female healthy volunteers (n=13;
52.5±4), who attended The Affiliated Hangzhou First People's
Hospital, were included. The study was approved by The Affiliated
Hangzhou First People's Hospital Ethics Committee (Hangzhou,
China). All subjects were informed of the purpose and process of
the study, and signed an informed consent form. The inclusion
criteria of normal pregnant women are: i) Healthy subjects; ii)
delivery after 37 weeks; iii) successful pregnancy without any
complications, normal blood pressure and negative proteinuria. The
diagnostic criteria for preeclampsia are: i) Hypertension after 20
weeks of pregnancy and ii) positive proteinuria. Systolic blood
pressure (>140 mmhg) and/or diastolic blood pressure (>90
mmhg) served as diagnostic criteria for hypertension, with
proteinuria ≥0.3 g/24 h. Patients with a history of diabetes,
chronic kidney disease and/or heart disease were excluded. The
venous blood of pregnant women was collected (3 ml) at birth and
centrifuged at 12,000 × g for 8 min at 4°C. The serums were stored
at −80°C.
Cell culture and treatment
HUVECs and HTR-8/SVneo cells were purchased from The
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. The cells were grown in RPMI-1640 media containing 10%
fetal bovine serum (FBS, Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 ng/ml streptomycin (Sigma-Aldrich;
Merck KGaA) in a humid incubator at 37°C with CO2. When
the density of cells (1×106 cells/ml) reached 70–80%,
the cells either remained untransfected or were transfected with
miR-27b-3p inhibitor control (cat. no. 4464076, Thermo Fisher
Scientific, Inc.,), miR-27b-3p inhibitor (cat. no. 4464084, Thermo
Fisher Scientific, Inc.,) or miR-27b-3p mimics (cat. no. 4464066,
Thermo Fisher Scientific, Inc.,) using Lipofectamine®
RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.). After
recovery in fresh medium for 24 h, the cells transfected with
either the miR-27b-3p inhibitor control or miR-27b-3p inhibitor
were cultured in the medium added with 10% serum from patients with
hypertension for 24 h as Serum + control (C) cells and Serum +
inhibitor (I) cells. The untransfected cells or those transfected
with miR-27b-3p mimics, mimics control (MC) and inhibitor control
(IC) were cultured in fresh medium for 24 h as C cells, mimic (M)
cells, MC and IC cells.
Reveres-transcription-quantitative PCR
(RT-qPCR) assay
Total miRNAs were separated from tissues and cells
using a miRNeasy Mini kit (cat. no. 217004; Qiagen GmbH) following
the manufacturer's instructions, and 1 µg of miRNAs was used to
obtain cDNAs with a miScript II reverse transcription kit (cat. no.
218160; Qiagen GmbH) at 37°C for 15 min and 98°C for 5 min. In
addition, total cellular RNA was isolated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and the SMART
MMLV Reverse Transcriptase (cat. no. 639523; Takara Bio, Inc.) was
used to reverse-transcribe mRNAs into cDNAs at 37°C for 15 min and
98°C for 5 min. Then, the levels of miRNAs were detected using a
miScript SYBR-Green PCR kit (cat. no. 218075; Qiagen GmbH)
according to the manufacturer's instructions (18), and the mRNA levels were measured
using SYBR Premix Ex Taq kit (cat. no. RR820A; Takara Bio, Inv.) in
an ABI7300 Thermocycler (Applied Biosystems; Thermo Fisher
Scientific, Inc.). U6 served as an endogenous control of miRNA, and
GAPDH was used to normalize the mRNAs. The sequences of primers
used are listed in Table I. The
reaction conditions were as follows: Denatured at 95°C for 10 min
followed by 40 cycles at 95°C for 10 sec, at 60°C for 20 sec and at
72°C for 30 sec. Gene expression levels were calculated using
2−ΔΔCq method (19).
 | Table I.Primer base sequences. |
Table I.
Primer base sequences.
| Primer name | Sequence
(5′-3′) |
|---|
| miR-27b-3p | Forward:
CTCAACTGGCGTGGAGTCGGCAATTCAGTTGAGGCAGAACT |
| miR-27b-3p | Reverse:
ACACTCCAGCTGGGTTCACAGTGGCTAAG |
| ATP1B2 | Forward:
TGTGTTGGCAAGAGAGATGAAGA |
| ATP1B2 | Reverse:
GGGTCACATTCAGGAACTTTACA |
| GAPDH | Forward:
CACCCACTCCTCCACCTTTG |
| GAPDH | Reverse:
CCACCACCCTGTTGCTGTAG |
| U6 | Forward:
CTCGCTTCGGCAGCACA |
| U6 | Reverse:
AACGCTTCACGAATTTGCGT |
Western blotting
Total cell proteins were extracted by RIPA buffer
(cat. no. 9806; Cell Signaling Technology, Inc.), and protein
concentration was measured by BCA. A total of 50 µg protein/lane
was separated by 12% SDS-PAGE, and then transferred to PVDF
membrane by electroporation. The membrane was blocked for 1 h using
5% skimmed milk powder dissolved in PBS at room temperature. The
membranes were incubated overnight with anti-ATP1B2 (1:1,000; cat.
no. ab185210; Abcam), anti-vascular endothelial growth factor
(VEGF; 1:1,000 cat. no. ab53465; Abcam), anti- matrix
metalloproteinase (MMP)-2 (1:1,000; cat. no. ab97779; Abcam,),
anti-MMP-9 (1:1,000; cat. no. ab38898; Abcam,) and anti-GAPDH
(1:2,000; cat. no. ab9485; Abcam) at 4°C. Then the membranes were
washed three times with PBST including 0.05% Tween-20, incubated
the HRP-conjugated rabbit anti-mouse IgG H&L antibody (1:2,000;
cat. no. ab6728; Abcam) at room temperature for 1 h, and then
washed three times with PBST. Finally, the membranes were
visualized using ECL detection reagents (Thermo Fisher Scientific,
Inc.) and then semi-quantified using ImageJ software (version 1.48;
National Institutes of Health).
Dual-luciferase reporter gene
assay
The HUVECs were cultured in 24-well plates and
co-transfected with 200 ng/µl miR-27b-3p mimics, miR-27b-3p
inhibitor or miRNA control, and 200 ng of pGL3-ATP2B1-3′UTR or
pGL3-ATP2B1-mutant (mut, 5′-AUGUCUUGGUGAAUCGUCGG-3′)-3′UTR using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
After transfection for 36 h, luciferase activity was measured with
the dual-luciferase assay system (Promega Corporation). Luciferase
activity of genes was normalized to that of the Renilla
luciferase. All analyses and experiments were performed in
triplicate.
Cell Counting Kit (CCK)-8 assay
The HUVECs (5×103 cells/ml) were
inoculated into a 96-well plate. miRNA control, miR-27b-3p
inhibitor or miR-27b-3p mimics was transfected into the cells,
followed by stimulation with 10% serum from patients with
hypertension or in combination with Trifluoperazine (TFP;
MedChemExpress), which is an inhibitor of ATP2B1 (20). After cell culture for 48 h, 10 µl
CCK-8 reagent was added into the cells and incubated together for 4
h. The absorbance value was detected at 490 nm by a microplate
reader (Bio-Rad Laboratories, Inc.).
Cell migration assay
A cell suspension of serum-free medium was prepared
at 1×105 cells/ml, and inoculated into the upper chamber
of a Transwell dish in 100 µl/well, while complete medium including
the 10% FBS was added to the lower chamber. After incubation for 48
h at 37°C, the cells remaining in the upper chamber were wiped off
using a cotton swab, and the invading cells were fixed with 4%
polyformaldehyde at room temperature for 15 min and stained by
crystal violet. Cell migration was calculated from five randomly
selected visual fields with a light microscope at ×200
magnification (XDS-800D; Shanghai Caikon Optical Instrument Co.,
Ltd.). The average number of transplanted cells was counted
manually in five randomly selected fields.
Tube formation assay
The bottom of the culture plate was added with 0.3
ml growth factor-reduced Matrigel and rested for 10 h at 3°C. The
HUVECs were cultured on upper side of the Matrigel for 12 h, then
the culture medium was discarded, and cells were cultured for 24 h
in the complete culture medium with 10% FBS. The formation of
tubules was observed under an inverted light microscope (XDS-800D;
Shanghai Caikon Optical Instrument Co., Ltd.).
Trophoblast invasion assay
For the trophoblast invasion assays, each Transwell
chamber 100 μl of growth factor-reduced Matrigel (placed in a
24-hole plate) was added in the upper chamber, and then solidified
in an incubator at 37°C for 2 h. Trophoblast HTR-8/SVneo cells
(5×103 cells) were seeded into the upper chamber. The
transfected HUVECs (2×104 cells) under the
aforementioned conditions were added into the lower chamber for 24
h at 37°C. Next, the chamber was removed, and the remaining cells
in the upper chamber were wiped off using cotton swabs, while those
which had invaded the lower chamber were fixed and stained by
hematoxylin and eosin at room temperature for 15 min, observed
under an inverted light microscope (XDS-800D; Shanghai Caikon
Optical Instrument Co., Ltd.) at ×200 magnification and images were
captured. Invasion was evaluated by counting the cells in five
randomly selected fields.
Bioinformatics TargetScan7.2 (targetscan.org) predicted that miR-27b-3p could target
the 3′-UTR of ATP2B1.
Statistical analysis
The data were analyzed by GraphPad Prism 5.0
software, and shown as mean ± standard deviation with ≥3
independent experiments. Statistical significance was determined by
unpaired Student's t-test or one-way ANOVA followed by Tukey's post
hoc test according to the number of experimental groups analyzed.
P<0.05 was used to indicate a statistically significant
difference.
Results
miR-27b-3p is upregulated in serum of
patients with hypertension and preeclampsia
The clinical characteristics of patients were
described in Table II. Expression
levels of miR-27b-3p were detected in higher concentrations in the
serum of patients with hypertension compared with those in the
serum of healthy females (Fig. 1A).
Furthermore, miR-27b-3p expression levels were also found to be
higher in the serum of patients with preeclampsia compared with
those in normal pregnant women (Fig.
1B).
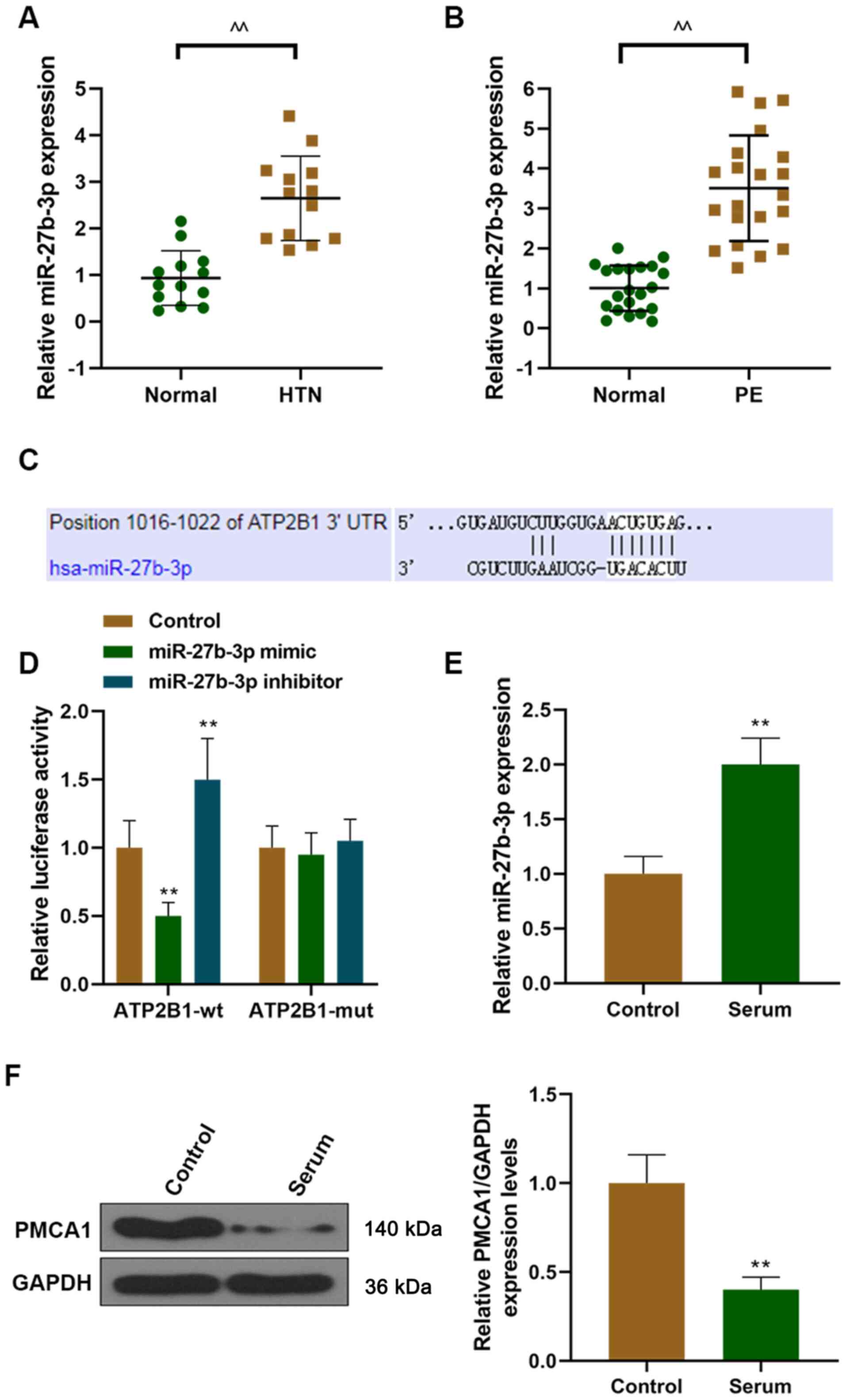 | Figure 1.miR-27b-3p is upregulated in patients
with hypertension and patients with PE, and miR-27b-3p targets
ATP2B1. The level of miR-27b-3p was measured in (A) patients with
hypertension and (B) patients with PE using RT-qPCR. (C) A
predicted target site for miR-27b-3p was identified in the 3′-UTR
of ATP2B1. (D) Luciferase activity was determined using a
dual-luciferase reporter assay kit. (E) After HUVECs treated with
the serum from patients with hypertension, the level of miR-27b-3p
was detected using RT-qPCR. (F) After HUVECs were treated with the
serum of patients with hypertension, the expression of ATP2B1 was
detected using western blotting. ^^P<0.01 vs. normal,
**P<0.01 vs. control. ATP2B1, ATPase plasma membrane
Ca2+ transporting 1; HTN, hypertension; miR, microRNA;
mut, mutant; PE, preeclampsia; RT-qPCR, reverse
transcription-quantitative PCR; UTR, untranslated region; wt,
wild-type; HUVECs, human umbilical vein endothelial cells. |
 | Table II.Clinical characteristics. |
Table II.
Clinical characteristics.
| Variable | Normal pregnant
women, n=21 | Preeclampsia,
n=21 | Hypertension,
n=13 | Healthy volunteers,
n=13 |
|---|
| Maternal age,
years | 33.5±4 | 34.1±5 | – | – |
| Age, years | – | – | 51.5±4 | 52.5±4 |
| Gestational age,
weeks | 37.5±1.6 | 36.8±2.3 | – | – |
| Body mass index,
kg/m2 | 22.3±3 | 24.6±4 | 22.6±3.8 | 21.1±2 |
| Mode of
delivery | Vaginal | Vaginal | – | – |
| Baby birth weight,
kg | 3.2±0.33 | 2.9±0.49 | – | – |
ATP2B1 is a target gene of
miR-27b-3p
ATP2B1 was a hypertensive-susceptible gene, and its
expression was downregulated in preeclampsia women (21). Moreover, downregulation of ATP2B1
could increase blood pressure. TargetScan predicted that miR-27b-3p
could target the 3′-UTR of ATP2B1 (Fig.
1C). Subsequently, luciferase reporter assays were performed,
and demonstrated that the miR-27b-3p mimic reduced luciferase
activity in the ATP2B1 wild-type (wt) group, and that miR-27b-3p
inhibitor increased luciferase activity in the ATP2B1 wt group
compared with the control and mut groups. These results
demonstrated that miR-27b-3p targeted ATP2B1 to reduce its
expression (Fig. 1D).
Expression levels of ATP2B1 and
miR-27b-3p are differentially regulated in HUVECs stimulated with
the serum of patients with hypertension
A previous study demonstrated that hypertension can
induce dysfunction of HUVECs (22).
In the present study, miR-27b-3p and ATP2B1 were predicted to be
involved in the regulation of HUVECs biological functions.
Subsequent results indicated that the miR-27b-3p expression levels
were increased and those of ATP2B1 were reduced when HUVECs were
stimulated with the serum of patients with hypertensive (Fig. 1E and F). The expression of
miR-27b-3p decreased after miR-27b-3p inhibitor transfection, and
increased after miR-27b-3p mimics transfection (Fig. 2A and B). Furthermore, the protein
and mRNA expression levels of ATP2B1 were downregulated in HUVECs
transfected with miR-27b-3p mimics, which was consistent with the
results that HUVECs were stimulated by the serum of patients with
hypertension (Fig. 2C and D). In
addition, adding the serum of patients with hypertension after the
transfection of the miR-27b-3p inhibitor into HUVECs restored the
down-regulation of ATP2B1 caused by the hypertensive serum
stimulation alone (Fig. 2C and D).
The present results therefore revealed that the serum of patents
with hypertension reduced the expression of ATP2B1 in HUVECs.
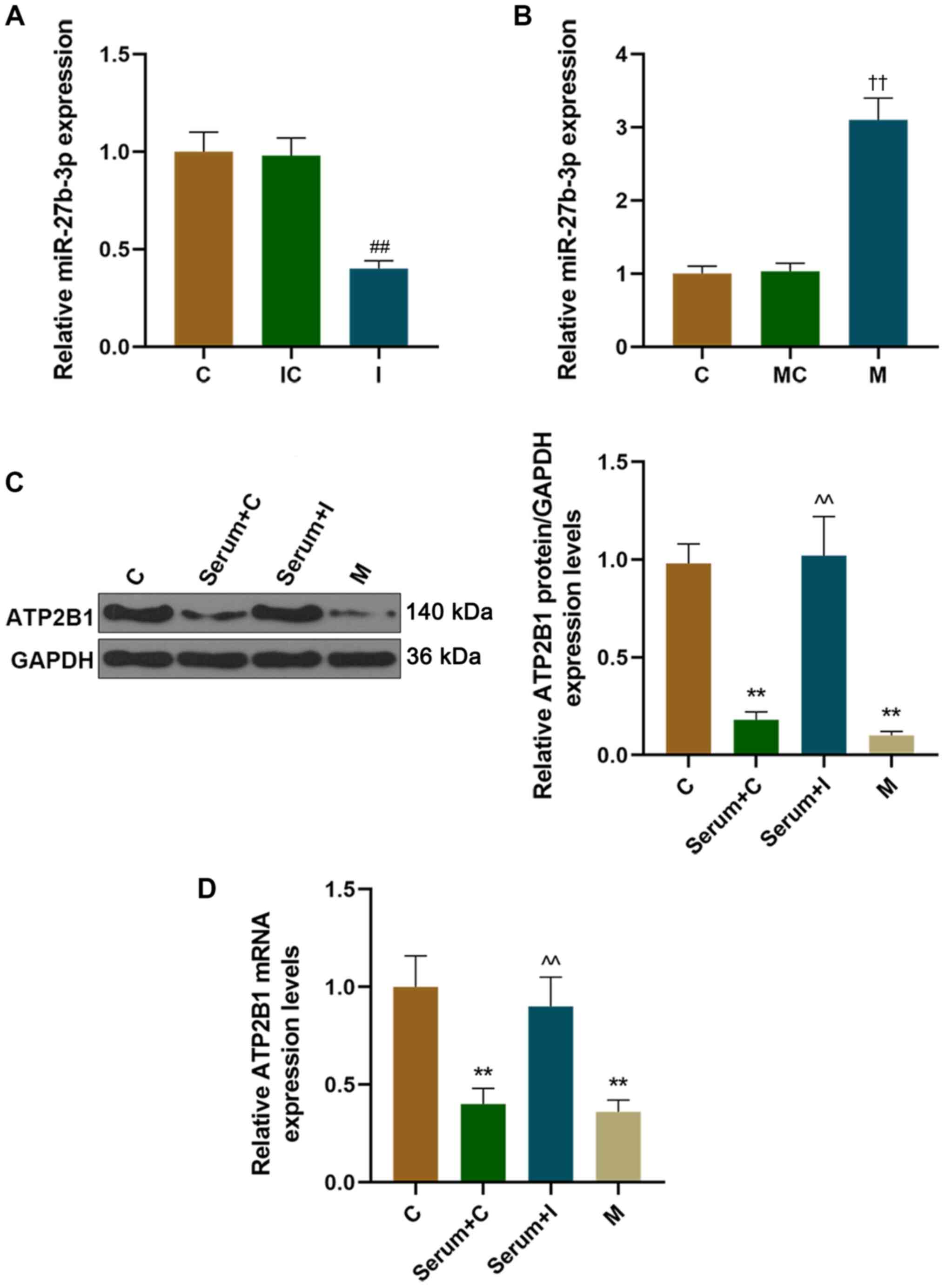 | Figure 2.The expression of ATP2B1 was reduced
in HUVEC cells following treatment with the serum of patients with
hypertension, and miR-27b-3p regulated the expression of ATP2B1.
The transfection efficiency of (A) miR-27b-3p inhibitor or (B)
mimics were detected using RT-qPCR. After HUVECs were treated with
the serum of patients with hypertension and/or miR-27b-3p inhibitor
or mimics, the protein and mRNA expressions of ATP2B1 were detected
using (C) western blotting and (D) RT-qPCR. ††P<0.01
vs. MC, ##P<0.01 vs. IC; **P<0.01 vs. C,
^^P<0.01 vs. Serum+C. ATP2B1, ATPase plasma membrane
Ca2+ transporting 1; C, control; M, mimic; miR,
microRNA; I, inhibitor; MC, mimic control; IC, inhibitor control;
RT-qPCR, reverse transcription-quantitative PCR; HUVECs, human
umbilical vein endothelial cells. |
Effect of miR-27b-3p on angiogenesis
of HUVECs in vitro
The dysfunction of endothelial cells could cause
blockage of angiogenesis, which was the main cause of preeclampsia
(23). The effects of miR-27b-3p on
endothelial angiogenesis were examined in vitro, and it was
observed that serum from hypertensive patients inhibited the
proliferation of endothelial cells. Furthermore, such an effect was
reversed by the transfection of an miR-27b-3p inhibitor into
HUVECs, but the subsequent addition of an ATP2B1 inhibitor, TFP,
alongside the miR-27b-3p inhibitor served to inhibit the
proliferation of endothelial cells again (Fig. 3A). This demonstrated that miR-27b-3p
mimics or TFP treatment of HUVECs inhibited the cell proliferation,
and similar effects were observed on cell migration and tubular
formation (Fig. 3B and C).
Moreover, miR-27b-3p mimics and inhibiting ATP2B1 decreased the
expressions of VEGF, MMP-9 and MMP-2 in HUVECs. miR-27b-3p
inhibitor increased the expressions of VEGF, MMP-9 and MMP-2, while
inhibition of ATP2B1 could reverse the aforementioned effects on
HUVECs (Fig. 3D). These results
indicated that miR-27b-3p inhibited endothelial cell angiogenesis
through targeting ATP2B1.
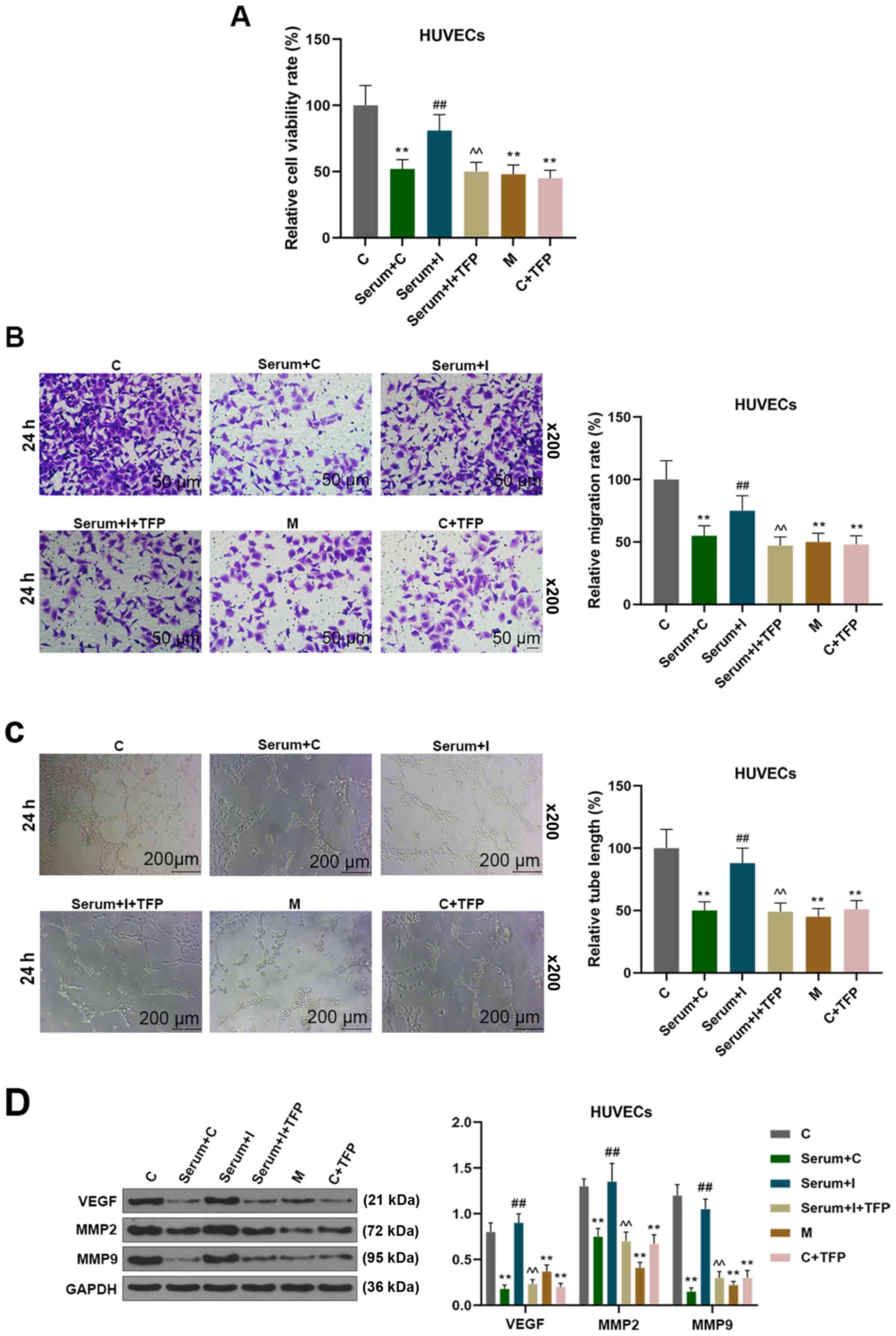 | Figure 3.miR-27b-3p inhibited the process of
angiogenesis of HUVECs. The experimental groups were as follows: i)
HUVECs were transfected with C miRNA; ii) HUVECs transfected with C
were stimulated by the serum of patients with hypertension; iii)
HUVEC cells were transfected with miR-27b-3p I, followed by
stimulation with the serum of patients with hypertension; iv)
HUVECs were transfected with miR-27b-3p I, followed by stimulation
with the serum of patients with hypertension and TFP; v) HUVECs
transfected with miR-27b-3p M; vi) HUVECs transfected with C miRNA
and treated with TFP (A) HUVECs viability was determined by CCK-8.
(B) Cell migration was detected by Transwell assay. (C) Endothelial
cell tube formation was determined on a layer of growth
factor-reduced Matrigel. (D) The expressions of VEGF, MMP-9 and
MMP-2 were detected by western blot analysis. **P<0.01 vs. C,
##P<0.01 vs. Serum+C, ^^P<0.01 vs.
Serum+miR-27b-3p I. C, control; CCK-8, Cell Counting Kit-8; I,
inhibitor; M, mimic; TFP, trifluoperazine; MMP, matrix
metalloprotenase; VEGF, vascular endothelial growth factor; HUVECs,
human umbilical vein endothelial cells. |
miR-27b-3p promotes trophoblast
invasion in a co-culture system of endothelial cells and
trophoblast cells
Endothelial cells have an important role in the
invasion of trophoblast cells, which possess a critical function in
maintaining normal pregnancy, and any disturbance between these two
cells will induce the occurrence of preeclampsia (24). In the present study, the invasion of
trophoblast cells was detected by a co-culture system of
endothelial cells and trophoblast cells. The data revealed that the
invasion of co-cultured trophoblast HTR-8/SVneo cells was inhibited
when HUVECs were treated with the serum from patients with
hypertension, miR-27b-3p mimics or TFP; however, the aforementioned
effects of the hypertensive serum were reversed by an miR-27b-3p
inhibitor. In addition, the invasion of trophoblast HTR-8/SVneo
cells was again inhibited by the addition of TFP (Fig. 4A). In addition, miR-27b-3p mimics
and inhibition of ATP2B1 decreased the expression of VEGF, MMP-9
and MMP-2 in a co-culture of HTR-8/SVneo and HUVEC cells.
miR-27b-3p inhibitor could increase the expressions of VEGF, MMP-9
and MMP-2, and ATP2B1 inhibition was found to reverse the effect in
a co-culture of HTR-8/SVneo and HUVEC cells hypertension model
(Fig. 4B). These results
demonstrated that endothelial miR-27b-3p inhibited the invasion of
trophoblast HTR-8/SVneo cells through targeting ATP2B1.
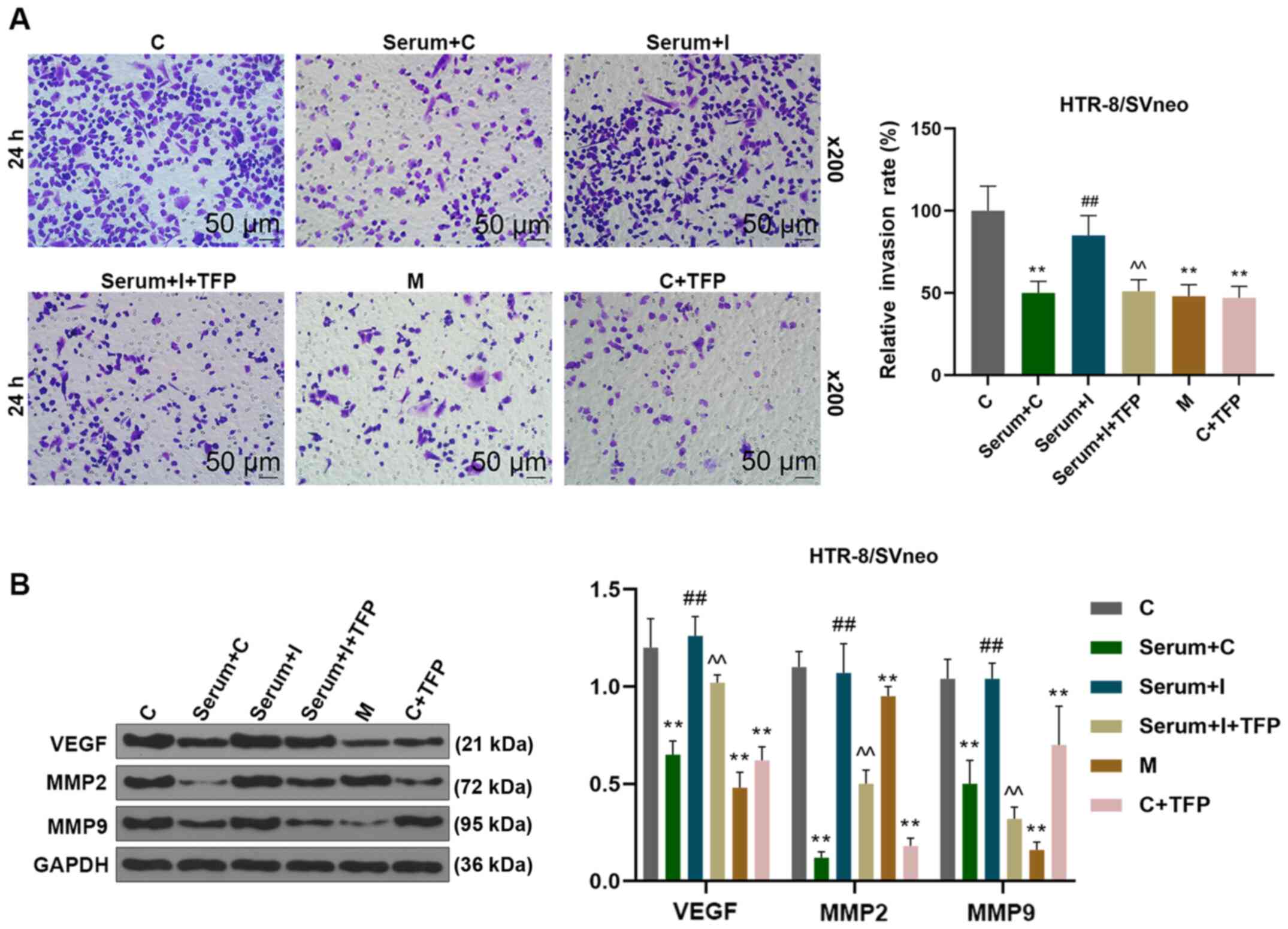 | Figure 4.miR-27b-3p inhibited endothelial cell
tube formation and trophoblast invasion by suppressing the
expression of ATP2B1. The HUVEC experimental groups were as
follows: Transfected with i) C miRNA; ii) C miRNA and stimulated by
serum from patients with hypertension; iii) miR-27b-3p I, and
stimulated with serum from patients with hypertension; iv)
miR-27b-3p I, followed by stimulation with serum from patients with
hypertension and TFP; v) miR-27b-3p M and vi) C miRNA and treated
with TFP. (A) HUVECs were co-cultured with trophoblastic
HTR-8/SVneo cells in Transwell plates for 5 h. HTR-8/SVneo cells
invasion was detected and analyzed using ImageJ software. (B) After
HTR-8/SVneo cells were isolated, the expression levels of VEGF,
MMP-9 and MMP-2 were detected using western blotting. **P< 0.01
vs. C, ##P<0.01 vs. Serum+C, ^^P<0.01
vs. Serum+miR-27b-3p I. miR, microRNA; ATP2B1, ATPase plasma
membrane Ca2+ transporting 1; TFP, trifluoperazine; C,
control; I, inhibitor; M, mimic; MMP, matrix metalloproteinase;
VEGF, vascular endothelial growth factor; HUVECs, human umbilical
vein endothelial cells. |
Discussion
Preeclampsia, which is one of the most important
complications during pregnancy, seriously threatens the health of
mothers and infants (25,26). Studies have showed that endothelial
dysfunction and placental trophoblast invasion play important roles
in the pathogenesis of preeclampsia (27,28).
Preeclampsia is often accompanied by hypertension, and vascular
endothelial cell dysfunction is closely related to hypertension
(29,30).
As miRNAs are highly-expressed under hypertension,
they may have clinical value in early diagnosis of hypertension and
prognosis of complications (31,32).
Detection of the expression levels of miRNAs in peripheral blood
mononuclear cells of healthy individuals and those with
hypertension showed that the expressions of miR-1, miR-21, −208b
and −499 were increased, and the expressions of miR-26b and −133a
were reduced in patients with hypertension (33). Migration and vascularization of the
three types of endothelial cells are regulated by miR-505/FGF18
(34). miR-27b-3p is a
multifunctional miRNA molecule that plays an important role in
numerous physiological and pathological processes (35–37),
and is closely related to cell proliferation, invasion, migration
and apoptosis (38–40). The current study found that
miR-27b-3p expression was elevated in the serum of patients with
hypertension and preeclampsia.
The ATP2B1 gene is known to be a risk gene for
hypertension (41), and
hypertension is a risk factor for preeclampsia (42). The risk of preeclampsia in patients
with chronic hypertension is known to be increased after the
incidence of pregnancy (43).
ATP2B1 acts as a sodium/calcium exchanger to transport
intracellular Ca2+ to the outside of the cells, thereby
maintaining a low intracellular Ca2+ concentration in
the resting state, which then affects the function of endothelial
cells and angiogenesis (44,45).
Higher nitric oxide production and endothelial nitric oxide
synthase activity, which are related with reduced ATP2B1 activity,
could induce endothelial cell injury (46). The present results showed that
ATP2B1 was targeted by miR-27b-3p and low-expression levels in
HUVECs were stimulated by hypertensive patients' serum, which could
help to reduce cell migration and invasion.
Preeclampsia a disease that occurs during pregnancy,
with superficial placenta implantation and uterine spiral artery
remodeling disorder as its main pathological mechanisms (25,47).
This process is an orderly activity, where active trophoblast cells
and endothelial cells form a linkage through cell proliferation,
invasion and tube formation (48,49).
Angiogenesis plays an important role in tissue repair and organ
growth. Imbalance in angiogenesis will easily lead to the
occurrence and development of several diseases, including
preeclampsia (50).
VEGF is a highly specific mitogen that promotes
vascular endothelial cell division, and is closely related to
angiogenesis (51). The high
expression of VEGF can promote vascular endothelial cell division
and the expression of MMP to increase the infiltration and
promotion of cell migration and invasion and angiogenesis of
endothelial cells (52,53). Moreover, degradation of
extracellular matrix by MMPs is known to be a rate-limiting step
for trophoblast invasion (54).
miR-378 has been shown to bind to VEGF and promote angiogenesis
(55). Abnormal expression of
miR-10b and −200c can regulate the expression of soluble Fma-like
tyrosine kinase-1, and induce vascular endothelial injury and
vascular remodeling disorder (56).
Wang et al (12) found that
the expression of miR-16 in decidua-derived mesenchymal stem cells
(dMSCs) of patients with severe preeclampsia is significantly
increased. miR-16 was negatively correlated with cyclin E1 and
VEGF-A in dMSCs from severe preeclampsia. Furthermore, miR-16 is
known to regulate the migration and tube formation of endothelial
cells (12). A recent study has
shown that miR-27b suppressed endothelial cell proliferation and
migration by targeting Smad7 in Kawasaki disease (57).
In the present study, a miR-27b-3p inhibitor was
shown to ameliorate the impairment of endothelial cells known to be
induced by hypertensive serum, angiogenesis and trophoblast
invasion, all of which are characteristics of preeclampsia
(58). Moreover, miR-27b-3p
inhibited the process of angiogenesis of HUVECs and invasion of
trophoblasts with ATP2B1 inhibition, and also inhibited the
expression of VEGF, MMP-9 and MMP-2 in HUVECs. Furthermore, similar
changes to VEGF, MMP-9 and MMP-2 expression were observed when
testing the HTR-8/SVneo trophoblast cells. The results indicated
that serum from patients with hypertension promoted endothelial
dysfunction to induce trophoblast invasion through the
miR-27b-3p/ATP2B1 axis.
The limitation of the present study is that the main
signaling pathways need further study (such as Akt signaling).
Furthermore, in vivo experiments would be beneficial in
order to clarify the effects of miR-27b-3p/ATP2B1 on preeclampsia
in a natural setting and determine if miRNA expression could be
successfully used as a biomarker.
Taken together, expression of miR-27b-3p was
upregulated in patients with hypertension and patients with
preeclampsia. Downregulation of miR-27b-3p could inhibit the
dysfunction of endothelial cells induced by the serum of patients
with hypertension and promote the invasion of trophoblast cells
through upregulating ATP2B1, thus inhibiting the development of
preeclampsia. In addition, the present data revealed that
miR-27b-3p might be a novel predictive risk factor of endothelial
dysfunction and hypertension. The current findings may improve our
understanding of the pathogenesis of preeclampsia.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ made substantial contributions to conception and
design. LZ and ZL authors were involved in the data acquisition,
analysis and interpretation, along with drafting the article or
critically revising it for important intellectual content. LZ and
ZL authors agree to be accountable for all aspects of the work in
ensuring that questions related to the accuracy or integrity of the
work are appropriately investigated and resolved. LZ and ZL authors
approved the final version of the manuscript for publication.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. The present study was approved by The Affiliated
Hangzhou First People's Hospital Ethics Committee (approval no.
YY201802016C). All subjects were informed of the purpose and
process of the study and signed an informed consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ATP2B1
|
ATPase plasma membrane Ca2+
transporting 1
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
FBS
|
fetal bovine serum
|
|
RT-qPCR
|
reverse-transcription quantitative
PCR
|
|
TFP
|
trifluoperazine
|
References
|
1
|
Alrahmani L and Willrich MAV: The
complement alternative pathway and preeclampsia. Curr Hypertens
Rep. 20:402018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steegers EA, von Dadelszen P, Duvekot JJ
and Pijnenborg R: Pre-eclampsia. Lancet. 376:631–644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kelly RS, Giorgio RT, Chawes BL, Palacios
NI, Gray KJ, Mirzakhani H, Wu A, Blighe K, Weiss ST and Lasky-Su J:
Applications of metabolomics in the study and management of
preeclampsia: A review of the literature. Metabolomics. 13:132017.
View Article : Google Scholar
|
|
4
|
Seely EW and Maxwell C: Cardiology patient
page. Chronic hypertension in pregnancy. Circulation.
115:e188–e190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tabara Y, Kohara K and Miki T; Millennium
Genome Project for Hypertension, : Hunting for genes for
hypertension: The Millennium Genome Project for Hypertension.
Hypertens Res. 35:567–573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabara Y, Kohara K, Kita Y, Hirawa N,
Katsuya T, Ohkubo T, Hiura Y, Tajima A, Morisaki T, Miyata T, et al
Global Blood Pressure Genetics Consortium, : Common variants in the
ATP2B1 gene are associated with susceptibility to hypertension: The
Japanese Millennium Genome Project. Hypertension. 56:973–980. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH, Ban
HJ, Yoon D, Lee MH, Kim DJ, Park M, et al: A large-scale
genome-wide association study of Asian populations uncovers genetic
factors influencing eight quantitative traits. Nat Genet.
41:527–534. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kohara K, Tabara Y, Nakura J, Imai Y,
Ohkubo T, Hata A, Soma M, Nakayama T, Umemura S, Hirawa N, et al:
Identification of hypertension-susceptibility genes and pathways by
a systemic multiple candidate gene approach: The millennium genome
project for hypertension. Hypertens Res. 31:203–212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Levy D, Ehret GB, Rice K, Verwoert GC,
Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund
T, et al: Genome-wide association study of blood pressure and
hypertension. Nat Genet. 41:677–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Zhang Y, Li Y, Zhou X, Wang X, Gao
P, Jin L, Zhang X and Zhu D: Common variants in the ATP2B1 gene are
associated with hypertension and arterial stiffness in Chinese
population. Mol Biol Rep. 40:1867–1873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Doridot L, Houry D, Gaillard H, Chelbi ST,
Barbaux S and Vaiman D: miR-34a expression, epigenetic regulation,
and function in human placental diseases. Epigenetics. 9:142–151.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Fan H, Zhao G, Liu D, Du L, Wang
Z, Hu Y and Hou Y: miR-16 inhibits the proliferation and
angiogenesis-regulating potential of mesenchymal stem cells in
severe pre-eclampsia. FEBS J. 279:4510–4524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu P, Zhao Y, Liu M, Wang Y, Wang H, Li
YX, Zhu X, Yao Y, Wang H, Qiao J, et al: Variations of microRNAs in
human placentas and plasma from preeclamptic pregnancy.
Hypertension. 63:1276–1284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hromadnikova I, Kotlabova K, Hympanova L
and Krofta L: Gestational hypertension, preeclampsia and
intrauterine growth restriction induce dysregulation of
cardiovascular and cerebrovascular disease associated microRNAs in
maternal whole peripheral blood. Thromb Res. 137:126–140. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
JR Jr, Alasztics B, Molvarec A, Nagy B and
Biró O: 11 expression analysis of circulating exosomal hsa miR 210
in hypertensive disorders of pregnancy: Biomarkers, prediction of
preeclampsia. Pregnancy Hypertens An Int J Wom Cardiovasc Health.
6:183. 2016.
|
|
16
|
Liu C, Zhou Y and Zhang Z: miR-210: An
important player in the pathogenesis of preeclampsia? J Cell Mol
Med. 16:943–944. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tao J, Zhi X, Zhang X, Fu M, Huang H, Fan
Y, Guan W and Zou C: miR-27b-3p suppresses cell proliferation
through targeting receptor tyrosine kinase like orphan receptor 1
in gastric cancer. J Exp Clin Cancer Res. 34:1392015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruzzo A, Graziano F, Vincenzi B,
Canestrari E, Perrone G, Galluccio N, Catalano V, Loupakis F,
Rabitti C, Santini D, et al: High let-7a microRNA levels in
KRAS-mutated colorectal carcinomas may rescue anti-EGFR therapy
effects in patients with chemotherapy-refractory metastatic
disease. Oncologist. 17:823–829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thongon N, Nakkrasae LI, Thongbunchoo J,
Krishnamra N and Charoenphandhu N: Enhancement of calcium transport
in Caco-2 monolayer through PKCzeta-dependent Cav1.3-mediated
transcellular and rectifying paracellular pathways by prolactin. Am
J Physiol Cell Physiol. 296:C1373–C1382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Little R, Cartwright EJ, Neyses L and
Austin C: Plasma membrane calcium ATPases (PMCAs) as potential
targets for the treatment of essential hypertension. Pharmacol
Ther. 159:23–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gonçalves-Rizzi VH, Possomato-Vieira JS,
Nascimento RA, Caldeira-Dias M and Dias-Junior CA: Maternal
hypertension and feto-placental growth restriction is reversed by
sildenafil: Evidence of independent effects of circulating nitric
oxide levels. Eur J Pharmacol. 822:119–127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan X, Yang Z, Chen Y, Li N, Wang L, Dou
G, Liu Y, Duan J, Feng L, Deng S, et al: Endothelial cells-targeted
soluble human Delta-like 4 suppresses both physiological and
pathological ocular angiogenesis. Sci China Life Sci. 58:425–431.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beltrame JS, Scotti L, Sordelli MS,
Cañumil VA, Franchi AM, Parborell F and Ribeiro ML:
Lysophosphatidic acid induces the crosstalk between the
endovascular human trophoblast and endothelial cells in vitro. J
Cell Physiol. 234:6274–6285. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roberts JM, Taylor RN, Musci TJ, Rodgers
GM, Hubel CA and McLaughlin MK: Preeclampsia: An endothelial cell
disorder. Am J Obstet Gynecol. 161:1200–1204. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huppertz B: Placental origins of
preeclampsia: Challenging the current hypothesis. Hypertension.
51:970–975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brennan L, Morton JS, Quon A and Davidge
ST: Postpartum vascular dysfunction in the reduced uteroplacental
perfusion model of preeclampsia. PLoS One. 11:e01624872016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen L, Diao Z, Sun HX, Yan GJ, Wang Z, Li
RT, Dai Y, Wang J, Li J, Ding H, et al: Up-regulation of CD81
inhibits cytotrophoblast invasion and mediates maternal endothelial
cell dysfunction in preeclampsia. Proc Natl Acad Sci USA.
114:1940–1945. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Granger JP, Alexander BT, Llinas MT,
Bennett WA and Khalil RA: Pathophysiology of hypertension during
preeclampsia linking placental ischemia with endothelial
dysfunction. Hypertension. 38:718–722. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sánchez-Aranguren LC, Prada CE,
Riaño-Medina CE and Lopez M: Endothelial dysfunction and
preeclampsia: Role of oxidative stress. Front Physiol. 5:3722014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McDermott AM, Kerin MJ and Miller N:
Identification and validation of miRNAs as endogenous controls for
RQ-PCR in blood specimens for breast cancer studies. PLoS One.
8:e837182013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cengiz M, Karatas OF, Koparir E, Yavuzer
S, Ali C, Yavuzer H, Kirat E, Karter Y and Ozen M: Differential
expression of hypertension-associated microRNAs in the plasma of
patients with white coat hypertension. Medicine (Baltimore).
94:e6932015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karolina DS, Tavintharan S, Armugam A,
Sepramaniam S, Pek SLT, Wong MTK, Lim SC, Sum CF and Jeyaseelan K:
Circulating miRNA profiles in patients with metabolic syndrome. J
Clin Endocrinol Metab. 97:E2271–E2276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Q, Jia C, Wang P, Xiong M, Cui J, Li
L, Wang W, Wu Q, Chen Y and Zhang T: MicroRNA-505 identified from
patients with essential hypertension impairs endothelial cell
migration and tube formation. Int J Cardiol. 177:925–934. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang Z, Xiao Z, Guo H, Fang X, Liang J,
Zhu J, Yang J, Li H, Pan R, Yuan S, et al: Novel role of the
clustered miR-23b-3p and miR-27b-3p in enhanced expression of
fibrosis-associated genes by targeting TGFBR3 in atrial
fibroblasts. J Cell Mol Med. 23:3246–3256. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu J, Lv Y, Wang F, Kong X, Di W, Liu J,
Sheng Y, Lv S and Ding G: miR-27b-3p inhibition enhances browning
of epididymal fat in high-fat diet induced obese mice. Front
Endocrinol (Lausanne). 10:382019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lv X, Li J, Hu Y, Wang S, Yang C, Li C and
Zhong G: Overexpression of miR-27b-3p targeting Wnt3a regulates the
signaling pathway of Wnt/β-catenin and attenuates atrial fibrosis
in rats with atrial fibrillation. Oxid Med Cell Longev.
2019:57037642019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Cai Q, Bao PP, Su Y, Cai H, Wu J,
Ye F, Guo X, Zheng W, Zheng Y, et al: Tumor tissue microRNA
expression in association with triple-negative breast cancer
outcomes. Breast Cancer Res Treat. 152:183–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen D, Si W, Shen J, Du C, Lou W, Bao C,
Zheng H, Pan J, Zhong G, Xu L, et al: miR-27b-3p inhibits
proliferation and potentially reverses multi-chemoresistance by
targeting CBLB/GRB2 in breast cancer cells. Cell Death Dis.
9:1882018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song C, Yao J, Cao C, Liang X, Huang J,
Han Z, Zhang Y, Qin G, Tao C, Li C, et al: PPARγ is regulated by
miR-27b-3p negatively and plays an important role in porcine oocyte
maturation. Biochem Biophys Res Commun. 479:224–230. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xi B, Tang W and Wang Q: Polymorphism near
the ATP2B1 gene is associated with hypertension risk in East
Asians: A meta-analysis involving 15 909 cases and 18 529 controls.
Blood Press. 21:134–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Drost JT, Maas AH, van Eyck J and van der
Schouw YT: Preeclampsia as a female-specific risk factor for
chronic hypertension. Maturitas. 67:321–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sibai BM: Diagnosis and management of
gestational hypertension and preeclampsia. Obstet Gynecol.
102:181–192. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Daily JW, Kim BC, Liu M and Park S: People
with the major alleles of ATP2B1 rs17249754 increases the risk of
hypertension in high ratio of sodium and potassium, and low calcium
intakes. J Hum Hypertens. 31:787–794. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ryan ZC, Craig TA, Filoteo AG, Westendorf
JJ, Cartwright EJ, Neyses L, Strehler EE and Kumar R: Deletion of
the intestinal plasma membrane calcium pump, isoform 1, Atp2b1, in
mice is associated with decreased bone mineral density and impaired
responsiveness to 1, 25-dihydroxyvitamin D3. Biochem Biophys Res
Commun. 467:152–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Long Y, Chen SW, Gao CL, He XM, Liang GN,
Wu J, Jiang CX, Liu X, Wang F and Chen F: ATP2B1 gene silencing
increases NO production under basal conditions through the
Ca2+/calmodulin/eNOS signaling pathway in endothelial
cells. Hypertens Res. 41:246–252. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Y, Fei M, Xue G, Zhou Q, Jia Y, Li
L, Xin H and Sun S: Elevated levels of hypoxia-inducible
microRNA-210 in pre-eclampsia: New insights into molecular
mechanisms for the disease. J Cell Mol Med. 16:249–259. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou Y, McMaster M, Woo K, Janatpour M,
Perry J, Karpanen T, Alitalo K, Damsky C and Fisher SJ: Vascular
endothelial growth factor ligands and receptors that regulate human
cytotrophoblast survival are dysregulated in severe preeclampsia
and hemolysis, elevated liver enzymes, and low platelets syndrome.
Am J Pathol. 160:1405–1423. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cross JC, Hemberger M, Lu Y, Nozaki T,
Whiteley K, Masutani M and Adamson SL: Trophoblast functions,
angiogenesis and remodeling of the maternal vasculature in the
placenta. Mol Cell Endocrinol. 187:207–212. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang M, Muralimanoharan S, Wortman AC and
Mendelson CR: Primate-specific miR-515 family members inhibit key
genes in human trophoblast differentiation and are upregulated in
preeclampsia. Proc Natl Acad Sci USA:. 113:E7069–E7076. 2016.
View Article : Google Scholar
|
|
51
|
Melincovici CS, Boşca AB, Şuşman S,
Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL and Mihu CM:
Vascular endothelial growth factor (VEGF) - key factor in normal
and pathological angiogenesis. Rom J Morphol Embryol. 59:455–467.
2018.PubMed/NCBI
|
|
52
|
VanKlompenberg MK, Manjarín R, Donovan CE,
Trott JF and Hovey RC: Regulation and localization of vascular
endothelial growth factor within the mammary glands during the
transition from late gestation to lactation. Domest Anim
Endocrinol. 54:37–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Thangapazham RL, Sharma A, Gaddipati JP,
Singh AK and Maheshwari RK: Inhibition of tumor angiogenesis by
Brahma Rasayana (BR). J Exp Ther Oncol. 6:13–21. 2006.PubMed/NCBI
|
|
54
|
Zhong T, Chen J, Ling Y, Yang B, Xie X, Yu
D, Zhang D, Ouyang J and Kuang H: Down-Regulation of neuropathy
target esterase in preeclampsia placenta inhibits human trophoblast
cell invasion via modulating MMP-9 levels. Cell Physiol Biochem.
45:1013–1022. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee DY, Deng Z, Wang CH and Yang BB:
MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis
by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci USA.
104:20350–20355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shen X, Fang J, Lv X, Pei Z, Wang Y, Jiang
S and Ding K: Heparin impairs angiogenesis through inhibition of
microRNA-10b. J Biol Chem. 286:26616–26627. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rong X, Ge D, Shen D, Chen X, Wang X,
Zhang L, Jia C, Zeng J, He Y, Qiu H, et al: miR-27b suppresses
endothelial cell proliferation and migration by targeting Smad7 in
Kawasaki disease. Cell Physiol Biochem. 48:1804–1814. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Adu-Gyamfi EA, Fondjo LA, Owiredu WKBA,
Czika A, Nelson W, Lamptey J, Wang YX and Ding YB: The role of
adiponectin in placentation and preeclampsia. Cell Biochem Funct.
38:106–117. 2020. View Article : Google Scholar : PubMed/NCBI
|


















