Introduction
Lymphedema is caused by an abnormal accumulation of
fluid in interstitial tissues due to impaired fluid transport
through lymphatic vasculature; it is a common form of lymphatic
dysfunction and affects ~140–250 million people worldwide. Acquired
lymphedema is a result of lymphatic failure caused by trauma,
surgery or radiotherapy (1,2). Despite the great progress that has
been made in the development of pharmacological interventions
(sodium selenite, antibiotics or antifungal agents), effective
therapeutic options for the treatment of lymphedema remain limited
(3,4). Recent studies have suggested that
promoting the regeneration of lymphatic endothelial cells (LECs)
and lymphangiogenesis may effectively alleviate lymphedema
(5–7). Therefore, it is essential to elucidate
the physiological and molecular mechanisms of lymphangiogenesis for
the development of novel and effective therapeutic targets for
lymphedema.
Adipose-derived mesenchymal stem cells (ADMSCs) are
mesenchymal stem cells isolated from adipose tissues that can be
obtained in great quantities by minimally invasive techniques.
ADMSCs have a superior multi-differentiation potential, and they
have been applied in the treatment and investigation of various
diseases, including lymphedema (8,9). A
previous study reported that ADMSCs can be successfully induced to
differentiate into LECs expressing high levels of lymphatic vessel
endothelial hyaluronan receptor 1 (LYVE-1), Prospero-related
homeobox 1 (Prox1) and fms-related tyrosine kinase 4 (FLT-4) using
a medium containing VEGF-C156S and bovine fibroblast growth factor
(bFGF) (10). VEGF-C156S is a
selective agonist that binds and activates VEGFR-3. However, the
specific mechanism underlying how ADMSCs are differentiated into
LECs has yet to be fully elucidated.
MicroRNAs (miRNAs) and long non-coding RNAs
(lncRNAs) are the two main types of ncRNAs. miRNAs are small ncRNAs
of ~22 nucleotides (nt) that negatively regulate gene expression by
binding to the 3′ untranslated regions (UTRs) of their target
mRNAs, causing mRNA degradation or translational repression
(11,12). lncRNAs are non-coding transcripts
>200 nt in length that can act as competing endogenous RNAs
(ceRNAs) to regulate the expression levels of targeted genes by
sponging miRNAs, and they are involved in numerous physiological
processes including cell growth, differentiation, cell
proliferation and apoptosis. Previous studies have shown that
lncRNA myocardial infarction-associated transcript (MIAT) has an
important role in the differentiation of bone marrow mesenchymal
stem cells (BM-MSCs) into endothelial cells (ECs) (13). Furthermore, Wang et al
(14) demonstrated that MIAT
regulates VEGF by targeting miR-200a, thereby promoting the
differentiation of BM-MSCs into ECs. However, little is known about
the functional role and mechanism of MIAT in the differentiation of
ADMSC into LECs.
In the present study, MIAT was found to be
upregulated during differentiation of ADMSCs into LECs induced by
VEGF-C156S, and both knockdown of MIAT and overexpression of
miR-495 inhibited the differentiation of ADMSCs into LECs.
Mechanistic studies revealed that MIAT could upregulate Prox1
expression by directly sponging miR-495. Therefore, the present
study has newly identified an MIAT/miR-495/Prox1 axis in the
differentiation of ADMSCs into LECs, thereby providing a novel
avenue for lymphedema treatment.
Materials and methods
Isolation and culture of ADMSCs
All protocols were approved by the Ethics Committee
of the Hunan Cancer Hospital and the Affiliated Cancer Hospital of
Xiangya School of Medicine (Changsha, China). All donors gave their
informed consent for harvesting of their adipose tissue; donors
with malignancies, infections or systemic diseases were not
included in the present study. Samples of human adipose tissue [9
females (age, 29.0±2.3 years) and 9 males (age, 31.0±1.9 years)
recruited from January 2017 to December 2018] were harvested by
biopsy from abdominal subcutaneous fat and washed three times with
PBS. Subsequently, tissues were minced and digested with 0.75% type
I collagenase (Merck KGaA) at 37°C for 1 h. After neutralization
with Gibco® DMEM (Thermo Fisher Scientific, Inc.)
supplemented with 10% Gibco fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.), the suspension was centrifuged at 200 × g for 10
min at room temperature. The precipitate was lysed using red blood
cell lysis buffer (Merck KGaA) for 10 min, followed by
centrifugation at 200 × g for 10 min. The collected cells were
filtered through a 150-µm nylon mesh sieve (Falcon; Corning
Life Sciences) and cultured in DMEM/F12 medium (Invitrogen; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS and 100 U/ml
penicillin and streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C in an atmosphere of 5% CO2. After 3 days,
the non-adherent cells were removed, and the medium was replaced
every 3 days thereafter. Cells after the third passage were used
for the following experiments.
Flow cytometry analysis
ADMSC characterization was determined by flow
cytometric analysis. Cultured cells were digested using 0.25%
EDTA-trypsin and washed three times with PBS. After centrifugation
(4°C, 300 × g for 10 min), 1×104 cells/tube were
incubated in the dark at room temperature for 30 min with a
monoclonal APC-conjugated antibody for CD44 (1:200; clone IM7; cat.
no. 560569) or with FITC-conjugated antibodies for CD105 (1:200;
clone 266; cat. no. 563920), CD31 (1:100; clone WM59; cat. no.
563652) and HLA-DR (1:100; clone G46-6; cat. no. 560743); all
antibodies purchased from BD Biosciences. Subsequently, the cells
were washed with PBS, fixed with 4% formaldehyde and analyzed on a
CytoFLEX flow cytometer and CytExpert Software 2.3 (both Beckman
Coulter, Inc.).
Endothelial differentiation of
ADMSCs
To induce the differentiation, ADMSCs from passage 3
were cultured in endothelial differentiation medium (EGM-2-MV;
Lonza Group, Ltd.) at 37°C supplemented with 100 ng/ml VEGF-C156S
(PeproTech, Inc.) for 2, 5 and 10 days, according to a previous
protocol (15–17). Morphological observations were made
using an inverted light microscope (TE300; Nikon).
Cell transfection
Short hairpin RNA (shRNA) against MIAT (shMIAT) and
Prox1 (shProx1), an miR-495 inhibitor, miR-495 mimics and the
corresponding control mimics (mimics NC; cat. no. B01001) or
control inhibitor (inhibitor NC; cat. no. B03001) were designed and
synthesized by Shanghai GeneChem. For Prox1 overexpression,
full-length human Prox1 cDNA was cloned into the pcDNA3.1 vector
(Invitrogen; Thermo Fisher Scientific, Inc.). Cells transfected
with the empty pcDNA3.1 vector alone were used as a negative
control. Cell transfection was performed at room temperature using
Invitrogen Lipofectamine™ 3000 reagent (Thermo Fisher Scientific,
Inc.), following the manufacturer's recommendations. Mimics (100
nM), inhibitors (100 nM) and shRNAs (1 µg) were added together with
1 µl Lipofectamine 3000. A second transfection was performed 6 days
after the first one, following the same procedure. At day 10, cells
were harvested for further experiments.
Bioinformatics analysis
The interactions between lncRNA MIAT and miR-495
were predicted by starBase v2.0 (starbase.sysu.edu.cn/index.php). The target genes of
miR-495 were predicted by TargetScan Release 3.1
(targetscanmamm31/).
Luciferase reporter assay
Wild-type (WT)-MIAT, mutant type (MUT)-MIAT,
WT-Prox1 and MUT-Prox1 were purchased from Hanbio Biotechnology
Co., Ltd., and cloned into the luciferase reporter plasmid
(psi-CHECK2; Promega Corporation). For the miR-495 and MIAT
dual-luciferase reporter assay, ADMSCs were seeded into 24-well
plates at a density of 1×105 cells/well and cultured at
37°C for 24 h. Subsequently, ADMSCs were co-transfected with
WT-MIAT or MUT-MIAT combined with miR-495 mimics, miR-495 inhibitor
or the respective negative controls using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h, the
luciferase activity was determined using a Dual Luciferase Assay
System (Promega Corporation), according to the manufacturer's
instructions. The interaction between miR-495 and Prox1 was
verified according to the identical procedure as for miR-495 and
MIAT.
Western blot analysis
Total protein was extracted from cultured cells
(5×105 cells) using 500 µl (1 ml/10 µl)
immunoprecipitation cell lysis buffer (cat. no. P0013; Beyotime
Institute of Biotechnology) for 30 min. Afterwards, cell lysate was
centrifuged at 10,000 × g for 5 min at 4°C. Protein quantification
of lysates was determined by the Bradford method (BioRad
Laboratories, Inc.). Samples (30 µg) of protein were separated by
10% SDS-PAGE and transferred onto a PVDF membrane (EMD Millipore).
In order to ensure loading of the same amounts of sample, proteins
of similar molecular weight to the control were not probed on the
same membrane. After washing 3 times with Tris-buffered saline with
0.1% Tween-20 solution (TBST), the membranes were blocked with 5%
skimmed milk for 2 h. Subsequently, membranes were incubated at 4°C
overnight with primary antibodies against the following proteins:
Goat polyclonal antibody against Prox1 (cat. no. AF2727; R&D
Systems, Inc., 1:1,000), rabbit polyclonal antibody against LYVE-1
(cat. no. ab33682; Abcam, 1:1,000), rabbit polyclonal antibody
against VEGFR3 (cat. no. ab27278; Abcam, 1:500), mouse monoclonal
antibody against podoplanin (PDPL; cat. no. ab10288; Abcam,
1:1,000) and mouse monoclonal antibody against GAPDH (cat. no.
ab8245; Abcam, 1:1,000). After washing with TBST, the blots were
incubated with the appropriate horseradish peroxidase
(HRP)-conjugated secondary antibodies (cat. nos. ab97030, ab97200
and ab97100; Abcam; 1:5,000) for 2 h at room temperature. Finally,
membranes were rinsed using TBST and protein bands were
subsequently visualized using chemiluminescence reagents
(Immobilon; cat. no. WBKLS0500; EMD Millipore), and band
densitometry were normalized to GAPDH and quantified using ImageJ
software version 1.52 (National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to isolate total RNA from
cultured cells. Subsequently, cDNA was synthesized from total RNA
using PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.).
The expression levels of MIAT and Prox1 were detected using
SYBR®-Green PCR Master mix (Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. To detect the
expression of miR-495, RT was performed using TaqMan®
MicroRNA Reverse Transcription kit and qPCR was performed using the
TaqMan Universal Master Mix II (Applied Biosystems; Thermo Fisher
Scientific, Inc.) on StepOnePlus Real-Time PCR Systems (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The gene expression
levels were quantified using the 2−ΔΔCq method (18). Thermocycling parameters were 94°C
for 30 sec, 57°C for 30 sec and 72°C for 30 sec for 40 cycles. The
primer sequences used for qPCR were as follows: GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′;
U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; MIAT forward,
5′-ATCCTCGAGACAAAGAGCCCTCTGCACTAG-3′ and reverse,
5′-ATCGGATCCGAGCAAATGGAGACAAAGGAC-3′; miR-495 forward,
5′-GCGAAACAAACATGGTGC-3′ and reverse, 5′-GCAGGGTCCGAGGTATTC-3′; and
Prox1 forward, 5′-CAGATGGAGAAGTACGCAC-3′ and reverse,
5′-CTACTCATGAAGCAGCTCTTG-3′.
Immunofluorescence analysis
ADMSCs were grown for 72 h on glass coverslips and
fixed with 4% paraformaldehyde at room temperature for 30 min. The
cells were rinsed with PBS and permeabilized with 0.25% Triton
X-100 in PBS for 15 min. Subsequently, the cells were incubated
with a Prox1 antibody (1:500; cat. no. AF2727; R&D Systems,
Inc.) resuspended in 2% BSA in PBS overnight at 4°C. After rinsing
three times with PBS (10 min each wash), the cells were incubated
with a secondary fluorochrome-conjugated antibody [donkey anti-goat
IgG H&L (Alexa Fluor® 488); 1:1,000; cat. no.
ab150132; Abcam) for 2 h in the dark. After washing with PBS, the
slides were incubated with DAPI nuclear stain for 15 min. Finally,
the slides were washed again and examined under an Olympus Fluoview
500 Laser Scanning Microscope (Olympus Corporation). Images were
acquired from six fields of view per slide.
Transwell migration assay
Following treatment with VEGF-C156S, ADMSCs were
subjected to 12 h of starvation in DMEM without serum. ADMSCs
(1×105 cells/well) were placed into the upper chambers
of Transwell inserts (8-µm pore size; BD Biosciences). DMEM with
10% FBS was then added to the lower chambers. After 24 h of
incubation at 37°C, non-migrating cells were carefully removed. The
inserts were subsequently fixed with 4% paraformaldehyde and
stained with 0.1% crystal violet for 30 min at room temperature.
After washing with PBS, the number of migrated cells was determined
using a Leica microscope, and cells were counted from five randomly
selected fields using Image-Pro Plus version 6.0 software (Media
Cybernetics, Inc.). Experiments were performed in triplicate.
Tube-formation assay
Twenty four-well plates were coated with ice-cold
Matrigel™ solution (Phenol Red-free; BD Biosciences) and incubated
at 37°C for 30 min to allow the Matrigel to solidify. Subsequently,
treated ADMSCs (7×103 cells/well) were suspended in
DMEM, seeded onto the Matrigel and incubated with normal DMEM/F12
or endothelial differentiation medium at 37°C overnight. Formation
of tube-like structures was observed by phase-contrast microscopy
using a Nikon Eclipse Ts2 microscope (Nikon Corporation), and the
total tube length, number of tubes and area covered by tubes were
quantified using ImageJ software version 1.52 (National Institutes
of Health). Experiments were performed in triplicate.
Statistical analysis
All experiments were performed in triplicate and
repeated three times. The results are presented as the mean ± SD,
and data were analyzed using SPSS 13.0 software (SPSS, Inc.). The
normality of the data was assessed using the Shapiro-Wilk test.
Considering a significance level of P=0.05, no significant
deviations from normality were identified for any of the data
(P>0.05). Statistical analyses between two groups were performed
using Student's t-test, and multiple comparisons were carried out
using one-way ANOVA with Tukey's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
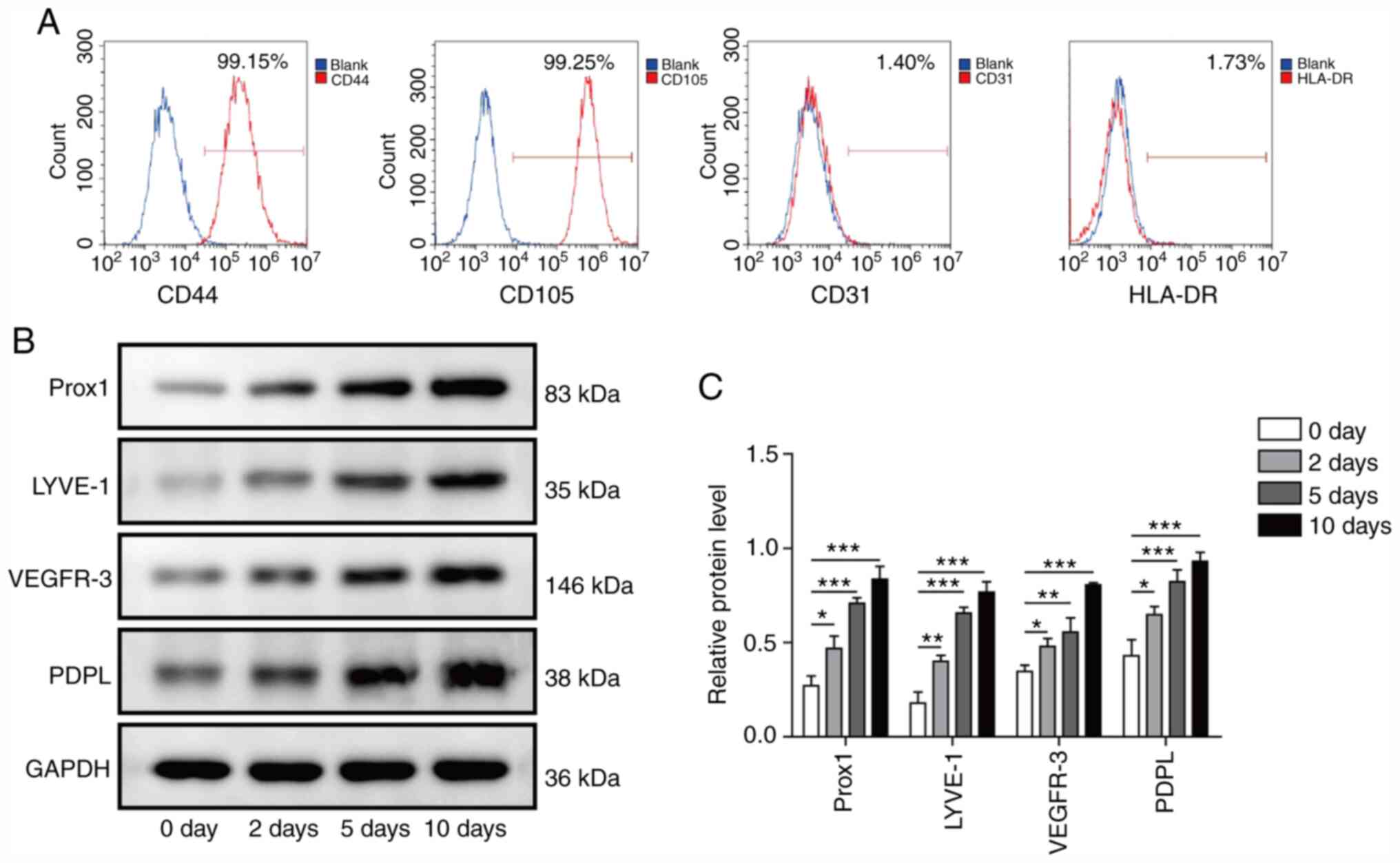
Efficient differentiation of ADMSCs
into LECs by VEGF-C156S treatment
Primary cultures were established from ADMSCs
isolated from abdominal subcutaneous fat. Flow cytometric analysis
was performed to characterize the isolated cells by examining the
percentage of cells expressing four characteristic surface markers,
CD44, CD105, CD31 and HLA-DR (Fig.
1A). The isolated cells were positive for CD44 and CD105
(>95% positive), which are both ADMSC markers. However, the
isolated cells were negative for CD31 and HLA-DR (<2% positive),
which suggested the absence of ECs and lymphocyte cells in the
culture. After identification, to determine whether ADMSCs could be
converted into LECs through VEGF-C156S treatment, ADMSCs were grown
in EGM medium supplemented with 100 ng/ml VEGF-C156S. As early as 2
days after stimulation, the protein expression levels of multiple
LEC markers, including Prox1, LYVE-1, VEGFR-3 and PDPL, were
upregulated in ADMSCs, with the highest expression detected
occurring at day 10 (Fig. 1B and
C). These results suggested that VEGF-C156S treatment may
efficiently convert the ADMSCs into LECs.
Profiling of dysregulated lncRNA-MIAT,
miR-495 and Prox1 in ADMSCs during endothelial differentiation
To determine whether MIAT may be involved in the
differentiation of ADMSCs into LECs, RT-qPCR was performed to
evaluate the expression levels of MIAT, miR-495 and Prox1 in cells
treated with VEGF-C156S for differentiation at different time
points (days 0, 2, 5 and 10). The results showed that the
expression levels of MIAT and Prox1 in ADMSCs were significantly
increased in a time-dependent manner during VEGF-C156S treatment
(Fig. 2A and C). However, the
expression of miR-495 was significantly downregulated during
VEGF-C156S treatment (Fig. 2B).
These RT-qPCR results were further confirmed with
immunofluorescence experiments, which revealed an increase in the
protein expression level of Prox1 during VEGF-C156S stimulation on
days 2, 5 and 10 (Fig. 2D). These
results demonstrated that MIAT may have a key role in the
endothelial differentiation of ADMSCs.
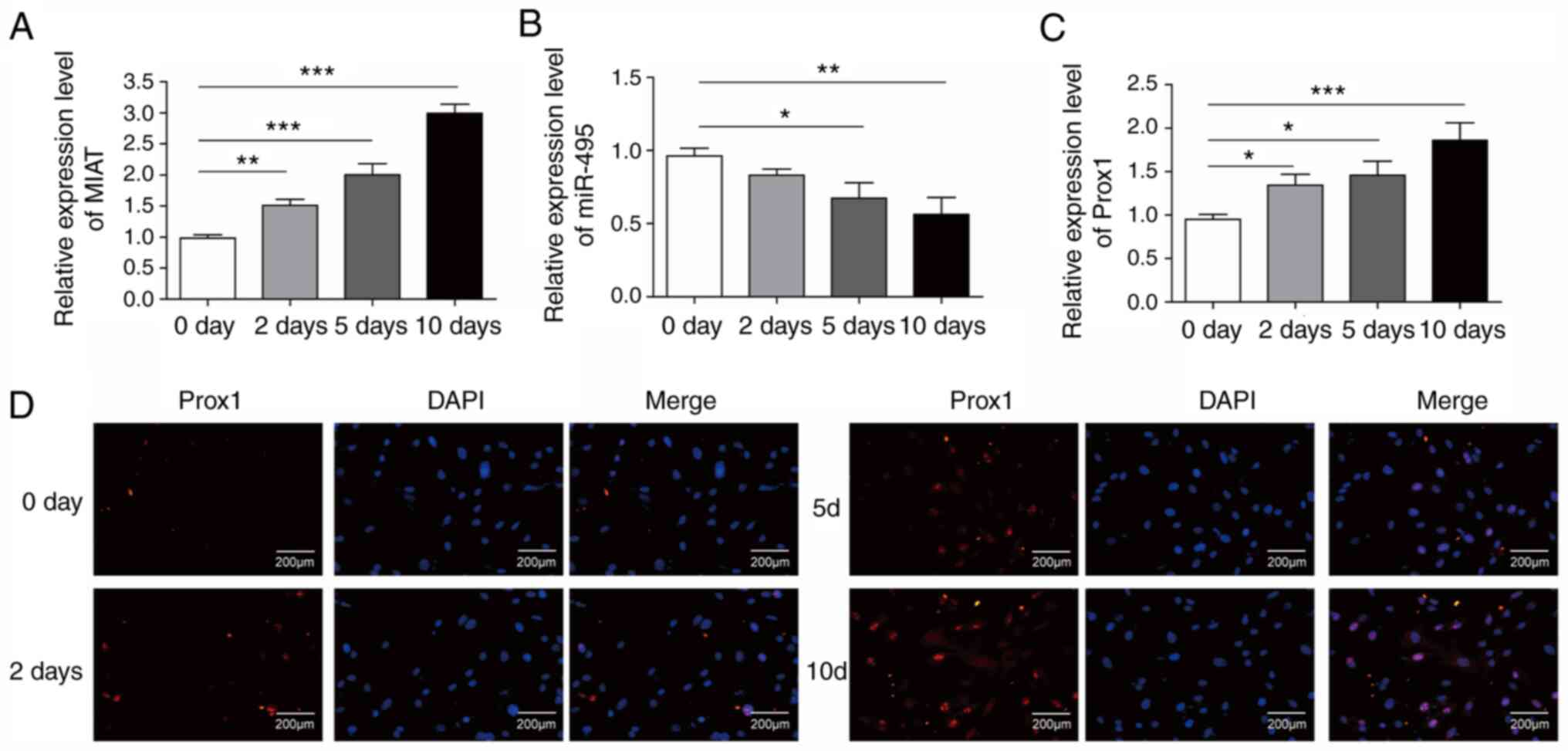 | Figure 2.Expression levels of long non-coding
RNA MIAT and Prox1 in ADMSCs during endothelial differentiation.
The expression levels of (A) MIAT, (B) miR-495 and (C) Prox1 in
ADMSCs were measured by reverse transcription-quantitative PCR at
different time points during VEGF-C156S treatment (0, 2, 5 and 10
days). (D) Immunofluorescence staining of Prox1 (red) and DAPI
nuclear stain (blue) in ADMSCs at different time points during
VEGF-C156S treatment (0, 2, 5 and 10 days). Scale bar, 200 µm. All
results are presented as the means ± SD (n=3) for three different
experiments performed in triplicate. *P<0.05, **P<0.01 and
***P<0.001. ADMSC, adipose-derived mesenchymal stem cell; MIAT,
myocardial infarction-associated transcript; miR, microRNA; Prox1,
Prospero-related homeobox 1. |
lncRNA-MIAT binds with miR-495 to
upregulate Prox1 expression
The majority of lncRNAs function to sponge miRNAs to
regulate the expression level of target genes (19); therefore, the biological
associations between MIAT, miR-495 and Prox1 were further
investigated. As shown in Fig. 3A,
shMIAT transfection significantly decreased the expression levels
of MIAT and Prox1, whereas that of miR-495 was increased compared
with the shNC group. Furthermore, the expression level of miR-495
was significantly increased following transfection with miR-495
mimics and downregulated after transfection with the miR-495
inhibitor (Fig. 3B). By contrast,
the expression level of Prox1 was significantly decreased after
transfection with miR-495 mimics and upregulated after transfection
with the miR-495 inhibitor (Fig.
3B). Moreover, the results of a bioinformatics analysis
demonstrated that there were putative binding sites there were
putative binding sites for miR-495 in MIAT and miR-495 target sites
in the Prox1 3′UTR (Fig. 3C and D).
Furthermore, the interactions between miR-495 and MIAT, as well as
between miR-495 and Prox1, were verified with dual-luciferase
reporter assays. The luciferase activity in the miR-495 mimics +
WT-MIAT group was significantly weaker compared with the mimics NC
+ WT-MIAT group; however, the luciferase activity in the miR-495
inhibitor + WT-MIAT group was significantly higher compared with
that in the inhibitor NC + WT-MIAT group (Fig. 3E). However, no significant
differences in the luciferase activity of MUT-MIAT were identified
following treatment with the miR-495 mimics or the miR-495
inhibitor. In addition, the results of the experiment showing the
interaction between mir-495 and Prox1, as confirmed by the
dual-luciferase reporter assay, were consistent with the above
results (Fig. 3F). Collectively,
these results suggested that MIAT may enhance the expression of
Prox1 by acting as a sponge for miR-495.
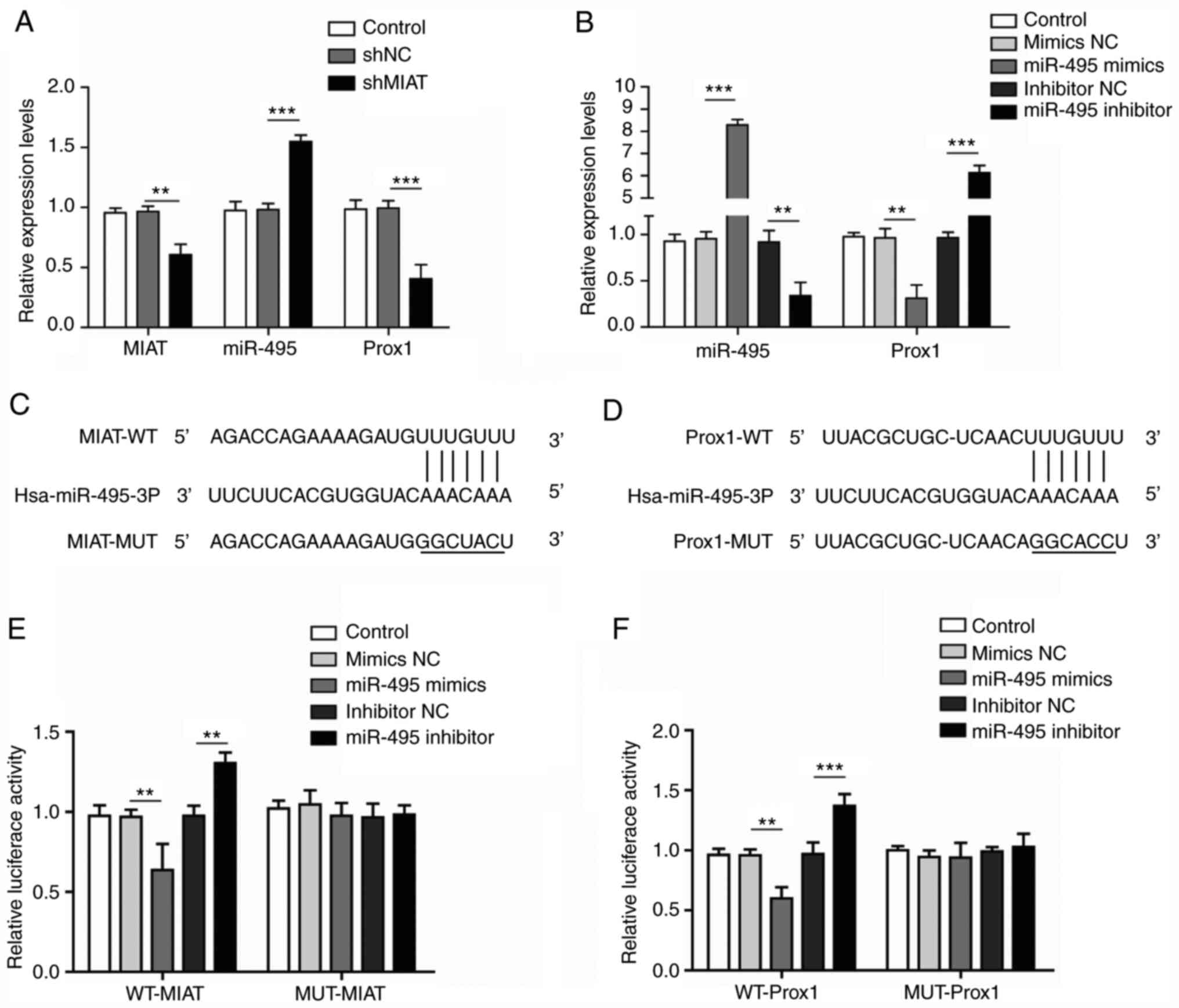 | Figure 3.Long non-coding RNA MIAT negatively
regulates miRNA-495 to enhance the expression of Prox1. (A) ADMSCs
were transfected with shNC or shMIAT, and the expression levels of
MIAT, miR-495 and Prox1 were measured by RT-qPCR. (B) ADMSCs were
transfected with miR-495 mimics, miR-495 inhibitor or their
respective negative controls, and the expression levels of miR-495
and Prox1 were measured by RT-qPCR. (C) StarBase analysis revealed
the potential binding sites for miR-495 and MIAT. (D) StarBase
analysis also revealed the potential target sites between Prox1
3′UTR and miR-495 for miR-495. (E) Luc-WT-MIAT or Luc-MUT-MIAT
plasmids were co-transfected into ADMSCs with miR-495 mimics,
miR-495 inhibitor or their respective negative controls, and
subsequently luciferase activity was measured. (F) Luc-WT-Prox1 or
Luc-MUT-Prox1 plasmids were co-transfected into ADMSCs with miR-495
mimics, the miR-495 inhibitor or their respective NCs, and
luciferase activity was measured. All results are presented as the
mean ± SD (n=3) for three different experiments performed in
triplicate. **P<0.01 and ***P<0.001. ADMSC, adipose-derived
mesenchymal stem cell; luc, luciferase; MIAT, myocardial
infarction-associated transcript; miR, microRNA; MUT, mutant type;
NC, negative control; Prox1, Prospero-related homeobox 1; RT-qPCR,
reverse transcription-quantitative PCR; WT, wild-type. |
Knockdown of MIAT inhibits the
differentiation of ADMSCs into LECs by binding miR-495
To further explore the biological function of MIAT
and miR-495 in endothelial differentiation, ADMSCs exposed to
VEGF-C156S were transfected with shMIAT or miR-495 mimics, or
co-transfected with shMIAT and the miR-495 inhibitor, and
subsequently the protein levels of Prox1, LYVE-1, VEGFR-3 and PDPL
were measured by western blotting. It has previously been reported
that Prox1, LYVE-1, VEGFR-3 and PDPL may be used as markers of
LECs, and these are often used to assess the ability of stem cells
from different sources to differentiate into LECs (20,21).
Increased expression levels of Prox1, LYVE-1, VEGFR-3 and PDPL were
observed in ADMSCs treated with VEGF-C156S (Fig. 4A and B). However, the expression
levels of Prox1, LYVE-1, VEGFR-3 and PDPL in ADMSCs transfected
with shMIAT or the miR-495 mimics were decreased compared with the
control (Fig. 4A and B).
Additionally, the Prox1, LYVE-1, VEGFR-3 and PDPL levels in ADMSCs
inhibited by shMIAT were reversed upon co-transfection with the
miR-495 inhibitor (Fig. 4A and B).
To further confirm the expression of Prox1, immunofluorescence
experiments were performed. Similarly, to the results from the
western blotting analyses, the VEGF-C156S-induced expression of
Prox1 was suppressed by MIAT silencing or miR-495 overexpression,
whereas the effect of shMIAT was reversed by miR-495 knockdown
(Fig. 4C). Taken together, these
results indicated that MIAT silencing inhibited Prox1 expression by
sponging miR-495 levels, leading to inhibited endothelial
differentiation of ADMSCs.
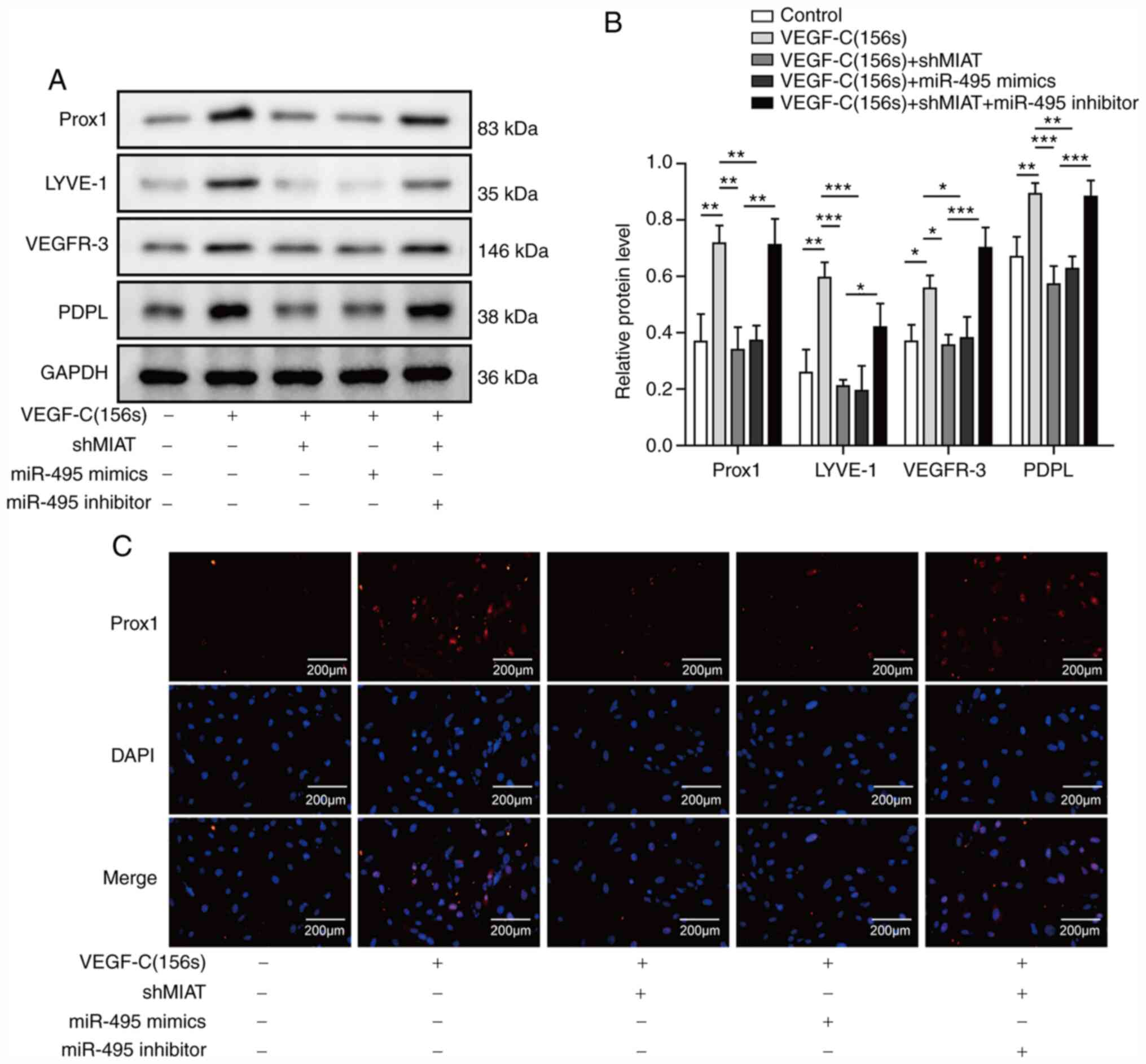 | Figure 4.Long non-coding RNA MIAT knockdown
inhibits LEC marker expression by sponging miR-495. ADMSCs were
transfected with shMIAT or miR-495 mimics, or co-transfected with
shMIAT and miR-495 inhibitor, or with shNC and control inhibitor NC
or mimics NC, and the cells were incubated with VEGF-C156S for 10
days. (A and B) The protein expression levels of Prox1, LYVE-1,
VEGFR-3 and PDPL were detected by western blotting. (C)
Immunofluorescence staining of Prox1 (red) was measured; DAPI
(blue) was used to stain the nuclei; scale bar, 200 µm. All results
are presented as the mean ± SD (n=3) for three different
experiments performed in triplicate. *P<0.05, **P<0.01 and
***P<0.001. ADMSC, adipose-derived mesenchymal stem cell; LEC,
lymphatic endothelial cell; LYVE-1, lymphatic vessel endothelial
hyaluronan receptor 1; MIAT, myocardial infarction-associated
transcript; miR, microRNA; NC, negative control; PDPL, podoplanin;
Prox1, Prospero-related homeobox 1. |
Knockdown of MIAT inhibits migration
and tube formation of ADMSCs by sponging miR-495
The migration capability of the transfected ADMSCs
was measured using Transwell assays. The results indicated that
knockdown of MIAT or overexpression of miR-495 inhibited the cell
migration that was induced by VEGF-C156S, and co-transfection with
the miR-495 inhibitor reversed the inhibition mediated by shMIAT
(Fig. 5A and B). To investigate the
role of MIAT and miR-495 in angiogenesis, tube-formation assays
were performed. As shown in Fig.
5C, ADMSCs treated with VEGF-C156S developed extensive
capillary-like tube structures. Transfection with shMIAT or miR-495
mimics clearly inhibited the tube formation induced by VEGF-C156S
treatment, whereas the inhibitory effect of shMIAT on
VEGF-C156S-induced tube formation was significantly rescued by
transfection of the miR-495 inhibitor (Fig. 5C and D). Collectively, these data
demonstrated that MIAT regulated migration and tube formation of
ADMSCs during endothelial differentiation, likely by targeting
miR-495.
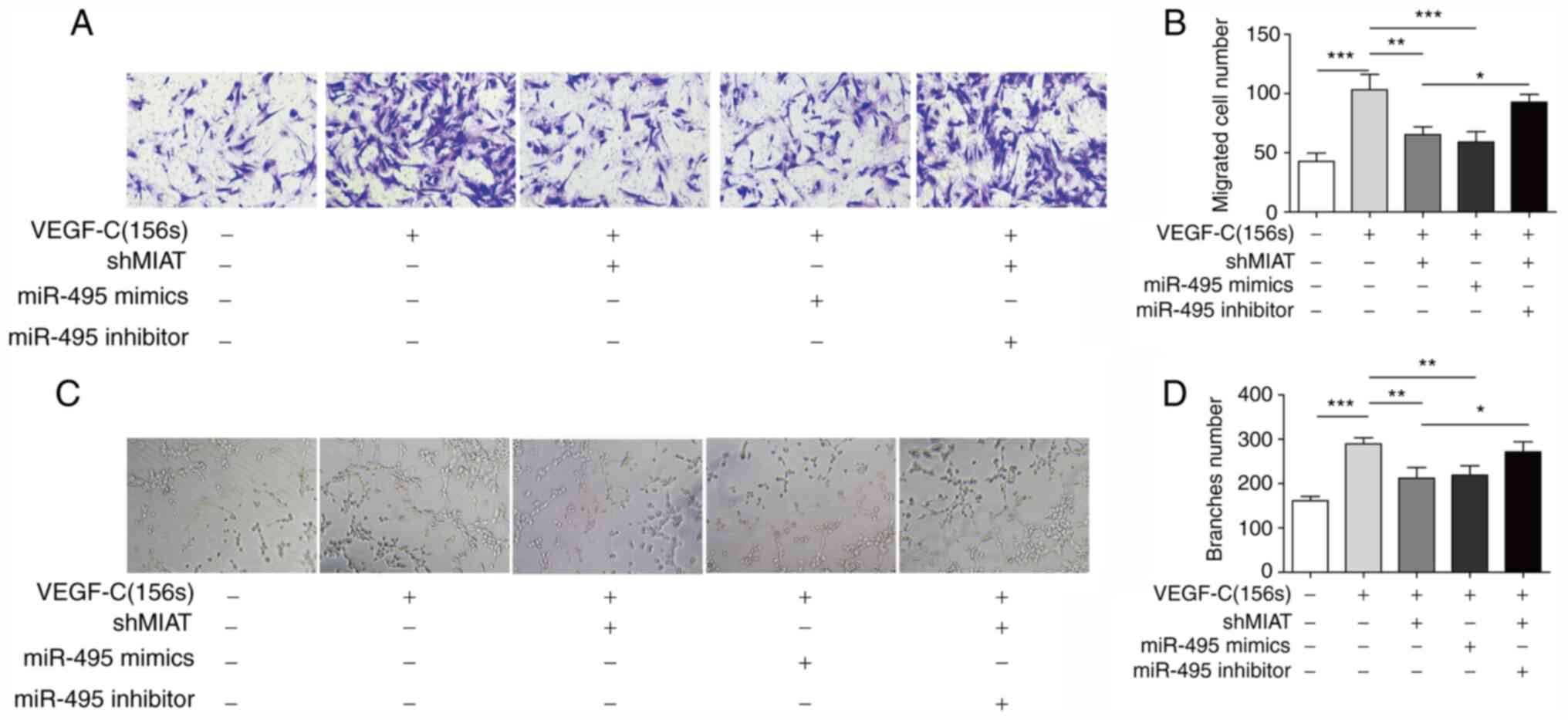 | Figure 5.Long non-coding RNA MIAT knockdown
inhibits migration and formation of capillary structures. ADMSCs
were transfected with shMIAT, miR-495 mimics, or co-transfected
with shMIAT and the miR-495 inhibitor, or with shNC and control
inhibitor NC or mimics NC, and subsequently incubated with
VEGF-C156S. (A and B) The migratory ability of pretreated ADMSCs
was measured by Transwell assay (magnification, ×100). (C and D)
The capillary-like structure formation of pretreated ADMSCs was
examined using tube-formation assay (magnification, ×100). All
results are presented as the mean ± SD (n=3) for three different
experiments performed in triplicate. *P<0.05, **P<0.01 and
***P<0.001. ADMSC, adipose-derived mesenchymal stem cell; MIAT,
myocardial infarction-associated transcript; miR, microRNA; NC,
negative control; sh, short hairpin RNA. |
miR-495 regulates VEGF-C156S-induced
migration and tube formation of ADMSCs by targeting Prox1
To further delineate the effect of the miR-495/Prox1
axis on VEGF-C156S-induced migration and tube formation, a Prox1
overexpression vector and shProx1 were used. RT-qPCR analysis and
western blotting revealed that the Prox1 expression levels in
ADMSCs was increased following transfection with the Prox1 vector,
and decreased Prox1 expression was found after transfection with
shProx1 (Fig. 6A and B).
Furthermore, the increases in ADMSC migration (Fig. 6C and D) and tube formation (Fig. 6E and F) induced by VEGF-C156S were
inhibited by pre-transfection with shProx1. Furthermore, the
inhibition of VEGF-C156S-induced migration (Fig. 6C and D) and tube formation (Fig. 6E and F) in ADMSCs induced by miR-495
mimics was also dramatically attenuated when cells were
co-transfected with the Prox1 vector. Taken together, these results
indicated that miR-495 may suppress the VEGF-C156S-induced
migration and tube formation of ADMSCs by targeting Prox1.
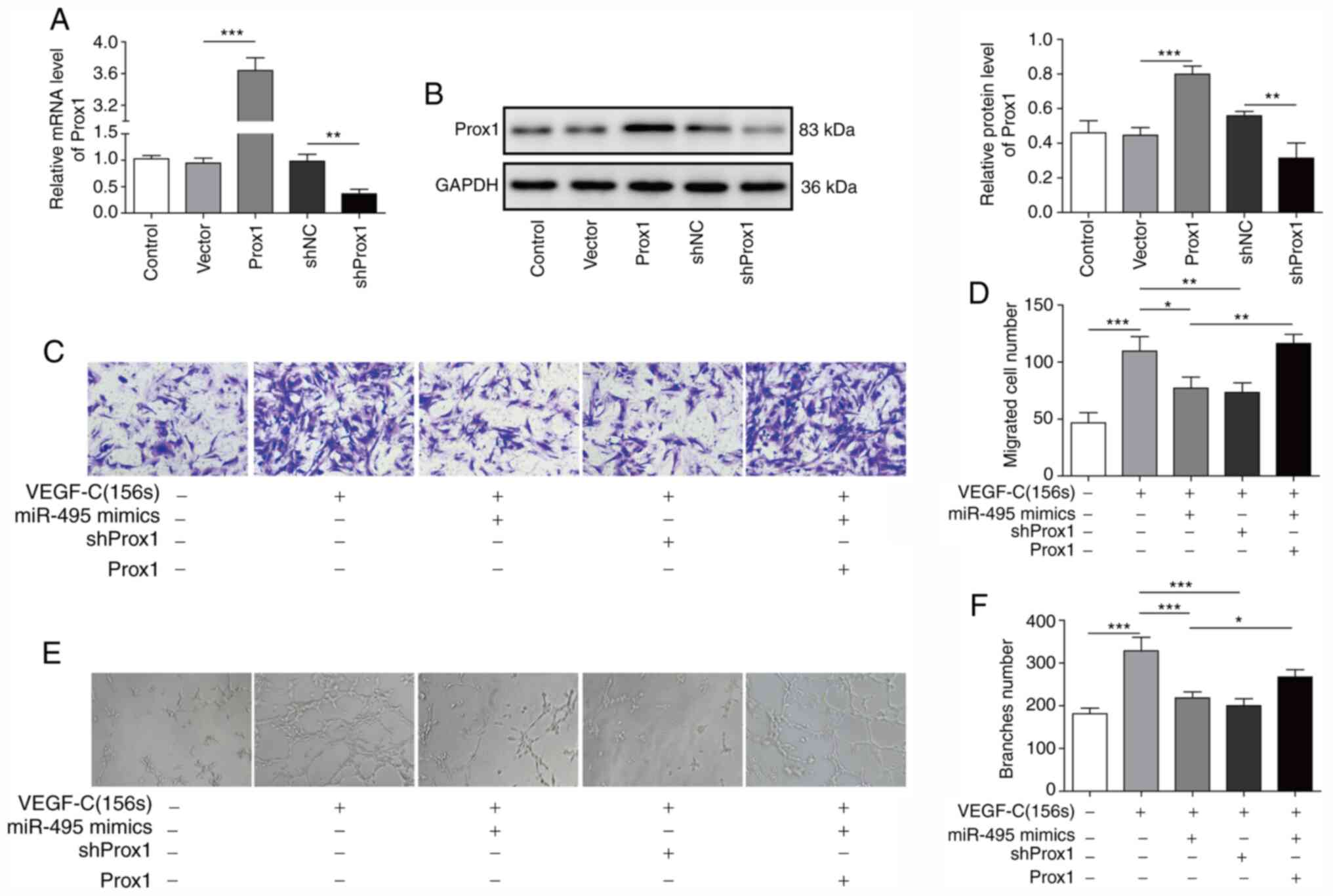 | Figure 6.miR-495 regulates VEGF-C156S-induced
migration and tube formation of ADMSCs by targeting Prox1. ADMSCs
were transfected with the Prox1 overexpression vector, a control
vector, shNC or shProx1, and subsequently the expression level of
Prox1 was measured by (A) reverse transcription-quantitative PCR
and (B) western blotting analysis. ADMSCs were transfected with
miR-495 mimics or shProx1, or co-transfected with miR-495 mimics
and the Prox1 overexpression vector, and subsequently incubated
with VEGF-C156S. (C and D) The migration and (E and F)
capillary-like structure formation capabilities of the cells were
measured by Transwell assay and tube-formation assay, respectively.
Magnification, ×100. All results are presented as the mean ± SD
(n=3) for three different experiments performed in triplicate.
*P<0.05, **P<0.01 and ***P<0.001. ADMSC, adipose-derived
mesenchymal stem cell; miR, microRNA; Prox1, Prospero-related
homeobox 1; sh, short hairpin RNA. |
Discussion
ADMSCs are accessible and abundant cells and have
been used to treat lymphedema (22). It has previously been reported that
VEGF-C156S-pretreated ADMSCs produced a markedly increased
lymphangiogenic response in a mouse Matrigel plug lymphangiogenesis
model (15). A patient treated with
ADMSCs combined with a fat-graft procedure exhibited great
improvements in daily symptoms, and a reduced need for compression
therapy (23). However, the
underlying pathophysiological mechanisms are incompletely
understood. In the present study, evidence was provided to show
that MIAT exerts an important role in the process of
lymphangiogenesis induced by ADMSCs. Importantly, it was shown that
MIAT positively regulated the differentiation of ADMSCs into LECs
by acting as a ceRNA to regulate Prox1 expression via sponging
miR-495.
MSCs are multipotent cells capable of
differentiating into cells of the mesodermal lineage (24). To utilize MSCs in endothelial
regeneration, it is of great importance to investigate the
molecular mechanisms underpinning how the differentiation of MSCs
into EC-like cells is regulated. Recent studied have shown that
lncRNAs may act as regulators in multiple biological processes,
including MSC specification and differentiation (25,26).
MIAT is considered to make important contributions to development
and disease, and has been shown to facilitate the endothelial
differentiation of MSCs (27,28).
For example, a previous study reported that MIAT facilitated BM-MSC
differentiation into ECs by regulated VEGF via targeting miR-200a
(14). In addition, MIAT knockdown
decreased the viability, increased the rate of apoptosis and
inhibited the proliferation of ECs (29). However, the functional role of MIAT
in the differentiation of ADMSCs into LECs had not been elucidated.
In the present study, a marked increase in the expression of MIAT
was identified in ADMSCs treated with VEGF-C156S. Furthermore,
knockdown of MIAT suppressed the expression levels of Prox1 induced
by VEGF-C156S and inhibited the differentiation of ADMSCs into
LECs. These findings suggested that MIAT may perform crucial roles
in the regulation of ADMSC differentiation into LECs.
Recently, interactions between multiple networks of
lncRNA-miRNA have drawn increasing attention. Several studies have
demonstrated that MIAT functions as a ceRNA to regulate cell
proliferation, differentiation and angiogenesis (28,30,31).
One recently published study showed that MIAT inhibited the
upregulation of AKT by miR-150-5p and modulated the function and
survival of human lens epithelial cells (14). It has also been reported that
miR-495 can affect human umbilical vein EC proliferation and
apoptosis, promote the senescence of MSCs, and inhibit the
chondrogenic differentiation of human MSCs (32–34).
In the present study, markedly decreased expression levels of
miR-495 were identified in ADMSCs during VEGF-C156S treatment.
Furthermore, overexpression of miR-495 had an effect similar to
that of MIAT silencing on the differentiation of ADMSCs into LECs.
Therefore, whether MIAT could promote the endothelial
differentiation of ADMSCs through miR-495 was further investigated.
Bioinformatics analysis combined with a dual-luciferase reporter
assay demonstrated a further sustained interaction between MIAT and
miR-495. Furthermore, MIAT-inhibited ADMSCs exhibited an increase
in miR-495 expression, whereas knockdown of miR-495 abolished the
biological effects of shMIAT on ADMSC endothelial differentiation.
These results collectively suggested that MIAT may function as a
ceRNA to regulate the expression of miR-495 to promote the
differentiation of ADMSCs into LECs.
Prox1 exerts a key role in the development of the
mammalian lymphatic vasculature (35). In the absence of Prox1 activity,
LECs were not able to be specified (36). Maintenance of the LEC phenotype
requires the constant expression of Prox1 (37). Moreover, several studies have
confirmed that Prox1 promotes the expression of the VEGF-C
receptor, VEGFR3, and promotes lymphangiogenesis by activating
VEGF-C (38,39). Consistent with these previous
studies, the present study has shown that Prox1 expression was
significantly increased in ADMSCs during VGEF-C156S treatment, and
knockdown of Prox1 inhibited the VEGF-C156S-induced migration and
tube formation of ADMSCs. Previous studies have also suggested that
lymphangiogenesis is regulated by different miRNAs through
post-transcriptional regulation of Prox1 (40,41).
In the present study, it was shown that miR-495 directly reduced
Prox1 protein expression through binding to the 3′-untranslated
region of the Prox1 mRNA. Transfection with miR-495 mimics
repressed VEGF-C156S-induced tube formation and migration of ADMSCs
by targeting Prox1. However, a direct interaction between VEGF-C
and miR-495 was not conclusively demonstrated in the present study,
and this provides a useful basis for future studies. In addition,
functional experiments demonstrated that, through binding to
miR-495, MIAT also indirectly regulated the expression of Prox1 in
ADMSCs. Based on these results, it is possible to propose that MIAT
upregulates Prox1 to promote the differentiation of ADMSCs into
LECs by binding miR-495.
In conclusion, the present study has demonstrated
the role of MIAT in promoting ADMSC endothelial differentiation,
and has also identified a potential network by which MIAT may act
as a sponge for miR-495 to regulate the expression of Prox1 in
ADMSCs. Further investigation of the functional roles of MIAT may
provide novel insights into developing effective treatments for
lymphedema based on ADMSC transplantation.
Acknowledgements
Not applicable.
Funding
The current study was supported by Hunan Provincial
Natural Science Foundation of China (grant no. 2018JJ6028).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
This study was designed by CLL. XWD collected,
analyzed and ascertained the integrity and accuracy of the data. WL
analyzed data and prepared the paper. All authors read approved the
final manuscript.
Ethics approval and consent to
participate
All protocols were approved by the Ethics Committee
of the Hunan Cancer Hospital and the Affiliated Cancer Hospital of
Xiangya School of Medicine (Changsha, China); and all donors gave
their informed consent for the collection of their adipose
tissue.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saito Y, Nakagami H, Kaneda Y and
Morishita R: Lymphedema and therapeutic lymphangiogenesis. Biomed
Res Int. 2013:8046752013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Girgis A, Stacey F, Lee T, Black D and
Kilbreath S: Priorities for women with lymphoedema after treatment
for breast cancer: Population based cohort study. BMJ.
342:d34422011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Szuba A and Rockson SG: Lymphedema:
Classification, diagnosis and therapy. Vasc Med. 3:145–156. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ko DS, Lerner R, Klose G and Cosimi AB:
Effective treatment of lymphedema of the extremities. Arch Surg.
133:452–458. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karkkainen MJ, Saaristo A, Jussila L,
Karila KA, Lawrence EC, Pajusola K, Bueler H, Eichmann A, Kauppinen
R, Kettunen MI, et al: A model for gene therapy of human hereditary
lymphedema. Proc Natl Acad Sci USA. 98:12677–12682. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szuba A, Skobe M, Karkkainen MJ, Shin WS,
Beynet DP, Rockson NB, Dakhil N, Spilman S, Goris ML, Strauss HW,
et al: Therapeutic lymphangiogenesis with human recombinant VEGF-C.
FASEB J. 16:1985–1987. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saito Y, Nakagami H, Morishita R, Takami
Y, Kikuchi Y, Hayashi H, Nishikawa T, Tamai K, Azuma N, Sasajima T
and Kaneda Y: Transfection of human hepatocyte growth factor gene
ameliorates secondary lymphedema via promotion of
lymphangiogenesis. Circulation. 114:1177–1184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koh KS, Oh TS, Kim H, Chung IW, Lee KW,
Lee HB, Park EJ, Jung JS, Shin IS, Ra JC and Choi JW: Clinical
application of human adipose tissue-derived mesenchymal stem cells
in progressive hemifacial atrophy (Parry-Romberg disease) with
microfat grafting techniques using 3-dimensional computed
tomography and 3-dimensional camera. Ann Plast Surg. 69:331–337.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee MJ, Kim J, Kim MY, Bae YS, Ryu SH, Lee
TG and Kim JH: Proteomic analysis of tumor necrosis
factor-alpha-induced secretome of human adipose tissue-derived
mesenchymal stem cells. J Proteome Res. 9:1754–1762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y, Chen XH, Li FG, Chen YX, Gu LQ,
Zhu JK and Li P: In vitro induction of human adipose-derived stem
cells into lymphatic endothelial-like cells. Cell Reprogram.
17:69–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mendell JT: MicroRNAs: Critical regulators
of development, cellular physiology and malignancy. Cell Cycle.
4:1179–1184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang F, Fang ZF, Hu XQ, Tang L, Zhou SH
and Huang JP: Overexpression of miR-126 promotes the
differentiation of mesenchymal stem cells toward endothelial cells
via activation of PI3K/Akt and MAPK/ERK pathways and release of
paracrine factors. Biol Chem. 394:1223–1233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Ding XG, Yang JJ, Li SW, Zheng H,
Gu CH, Jia ZK and Li L: LncRNA MIAT facilitated BM-MSCs
differentiation into endothelial cells and restored erectile
dysfunction via targeting miR-200a in a rat model of erectile
dysfunction. Eur J Cell Biol. 97:180–189. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan A, Avraham T, Zampell JC, Haviv YS,
Weitman E and Mehrara BJ: Adipose-derived stem cells promote
lymphangiogenesis in response to VEGF-C stimulation or TGF-β1
inhibition. Future Oncol. 7:1457–1473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan H, Zhang C, Wang Z, Tu T, Duan H, Luo
Y, Feng J, Liu F and Yan X: CD146 is required for VEGF-C-induced
lymphatic sprouting during lymphangiogenesis. Sci Rep. 7:74422017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu J, Zhang X, Kuzontkoski PM, Jiang S,
Zhu W, Li DY and Groopman JE: Slit2N and Robo4 regulate
lymphangiogenesis through the VEGF-C/VEGFR-3 pathway. Cell Commun
Signal. 12:252014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun X, Huang T, Zhang C, Zhang S, Wang Y,
Zhang Q and Liu Z: Long non-coding RNA LINC00968 reduces cell
proliferation and migration and angiogenesis in breast cancer
through up-regulation of PROX1 by reducing hsa-miR-423-5p. Cell
Cycle. 18:1908–1924. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu JK, Kitajewski C, Reiley M, Keung CH,
Monteagudo J, Andrews JP, Liou P, Thirumoorthi A, Wong A, Kandel JJ
and Shawber CJ: Aberrant lymphatic endothelial progenitors in
lymphatic malformation development. PLoS One. 10:e01173522015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng J, Dai T, Sun Y, Zhang Q, Jiang Z, Li
S and Cao W: Overexpression of Prox1 induces the differentiation of
human adipose-derived stem cells into lymphatic endothelial-like
cells in vitro. Cell Reprogram. 19:54–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hwang JH, Kim IG and Lee JY, Piao S, Lee
DS, Lee TS, Ra JC and Lee JY: Therapeutic lymphangiogenesis using
stem cell and VEGF-C hydrogel. Biomaterials. 32:4415–4423. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Toyserkani NM, Jensen CH, Sheikh SP and
Sørensen JA: Cell-assisted lipotransfer using autologous
adipose-derived stromal cells for alleviation of breast
cancer-related lymphedema. Stem Cells Transl Med. 5:857–859. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uccelli A, Moretta L and Pistoia V:
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
8:726–736. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun X, Luo LH, Feng L and Li DS:
Down-regulation of lncRNA MEG3 promotes endothelial differentiation
of bone marrow derived mesenchymal stem cells in repairing erectile
dysfunction. Life Sci. 208:246–252. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hou J, Wang L, Wu Q, Zheng G, Long H, Wu
H, Zhou C, Guo T, Zhong T, Wang L, et al: Long noncoding RNA H19
upregulates vascular endothelial growth factor A to enhance
mesenchymal stem cells survival and angiogenic capacity by
inhibiting miR-199a-5p. Stem Cell Res Ther. 9:1092018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ishii N, Ozaki K, Sato H, Mizuno H, Saito
S, Takahashi A, Miyamoto Y, Ikegawa S, Kamatani N, Hori M, et al:
Identification of a novel non-coding RNA, MIAT, that confers risk
of myocardial infarction. J Hum Genet. 51:1087–1099. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun C, Huang L, Li Z, Leng K, Xu Y, Jiang
X and Cui Y: Long non-coding RNA MIAT in development and disease: A
new player in an old game. J Biomed Sci. 25:232018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li
YJ, Tao ZF, Song YC, Chen Q and Jiang Q: lncRNA-MIAT regulates
microvascular dysfunction by functioning as a competing endogenous
RNA. Circ Res. 116:1143–1156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang X, Gao Y, Qin J and Lu S: lncRNA
MIAT promotes proliferation and invasion of HCC cells via sponging
miR-214. Am J Physiol Gastrointest Liver Physiol. 314:G559–G565.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou X, Zhang W, Jin M, Chen J, Xu W and
Kong X: lncRNA MIAT functions as a competing endogenous RNA to
upregulate DAPK2 by sponging miR-22-3p in diabetic cardiomyopathy.
Cell Death Dis. 8:e29292017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu D, Zhang XL, Yan CH, Li Y, Tian XX,
Zhu N, Rong JJ, Peng CF and Han YL: MicroRNA-495 regulates the
proliferation and apoptosis of human umbilical vein endothelial
cells by targeting chemokine CCL2. Thromb Res. 135:146–154. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Song Y, Liu D, Zhao J, Xu J, Ren J,
Hu Y, Wang Z, Hou Y and Zhao G: MiR-495 promotes senescence of
mesenchymal stem cells by targeting Bmi-1. Cell Physiol Biochem.
42:780–796. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee S, Yoon DS, Paik S, Lee KM, Jang Y and
Lee JW: microRNA-495 inhibits chondrogenic differentiation in human
mesenchymal stem cells by targeting Sox9. Stem Cells Dev.
23:1798–1808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi I, Lee S and Hong YK: The new era of
the lymphatic system: No longer secondary to the blood vascular
system. Cold Spring Harb Perspect Med. 2:a0064452012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hong YK, Foreman K, Shin JW, Hirakawa S,
Curry CL, Sage DR, Libermann T, Dezube BJ, Fingeroth JD and Detmar
M: Lymphatic reprogramming of blood vascular endothelium by Kaposi
sarcoma-associated herpesvirus. Nat Genet. 36:683–685. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Johnson NC, Dillard ME, Baluk P, McDonald
DM, Harvey NL, Frase SL and Oliver G: Lymphatic endothelial cell
identity is reversible and its maintenance requires Prox1 activity.
Genes Dev. 22:3282–3291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park YL, Myung E, Park SY, Kim N, Oak CY,
Myung DS, Cho SB, Lee WS, Kweon SS, Kim HS and Joo YE: Impact of
prospero homeobox-1 on tumor cell behavior and prognosis in
colorectal cancer. Am J Cancer Res. 5:3286–3300. 2015.PubMed/NCBI
|
|
39
|
Sasahira T, Ueda N, Yamamoto K, Kurihara
M, Matsushima S, Bhawal UK, Kirita T and Kuniyasu H: Prox1 and
FOXC2 act as regulators of lymphangiogenesis and angiogenesis in
oral squamous cell carcinoma. PLoS One. 9:e925342014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu C, Zhang Y, Wang Q, Jiang J, Gao Y, Gao
M, Kang J, Wu M, Xiong J, Ji K, et al: Long non-coding RNA GAS5
controls human embryonic stem cell self-renewal by maintaining
NODAL signalling. Nat Commun. 7:132872016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ramos AD, Andersen RE, Liu SJ, Nowakowski
TJ, Hong SJ, Gertz C, Salinas RD, Zarabi H, Kriegstein AR and Lim
DA: The long noncoding RNA Pnky regulates neuronal differentiation
of embryonic and postnatal neural stem cells. Cell Stem Cell.
16:439–447. 2015. View Article : Google Scholar : PubMed/NCBI
|