Introduction
Hair loss, including alopecia, is a common and
distressing problem for men and women, and as a result there is
considerable interest in developing treatments that can prevent or
reverse this hair loss. Currently, treatment with drugs and hair
transplantation are the conventional treatments for hair loss or
alopecia. However, drug treatments only delay hair loss and cannot
prevent further hair loss (1).
While autologous hair follicle transplantation is an effective
treatment for hair loss, the area for extracting intact hair
follicles (HFs) is limited in a patient's scalp, and so the
available number of hair follicles that can be extracted is also
limited (1,2). Thus, hair follicle regeneration
through bioengineering is now considered to be a promising
alternative strategy for treating hair loss.
In scalp tissue, dermal papilla (DP) cells (DPCs),
which are derived from neural crest stem cells, are specialized
fibroblast-like cells and are hypothesized to play crucial roles in
hair follicle morphogenesis and cycling (3,4).
Therefore, DPCs are essential for the successful construction of
bioengineered HFs to treat hair loss. Although freshly dissociated
fully intact DPCs have hair-inducing characteristics, they rapidly
lose this capability following serial sub-cultivation due to a
process of cell dedifferentiation, which is characterized by
changes in their morphology and a downregulation of their
characteristic genes (5–9). As such, obtaining a large number of
DPCs through modification of ex vivo culture conditions remain a
challenge.
In vivo, DPCs congregate and form a
three-dimensional (3D) DP structure, which is located at the base
of the hair follicle bulb and are surrounded by a dermal sheath and
the hair matrix (7). In addition to
DPCs, other cellular components of the hair bulb include
keratinocytes and melanocytes (5).
Thus, in the bulb, DPCs are closely in contact with each other and
exist in a specialized 3D environment. Furthermore, their cellular
properties are strictly controlled by complex elements, including
cell-cell interactions, the extracellular matrix (ECM) and various
factors, such as growth factors and cytokines that are secreted by
DPCs and surrounding cells (2,10).
According to reports, the DPC phenotype is tightly regulated by
various signaling pathways, including the Wnt/β-catenin,
platelet-derived growth factor (PDGF) and bone morphogenetic
protein (BMP) pathways (11–15).
In particular, DPCs and keratinocytes interact with each other in a
reciprocal manner, suggesting that signals from keratinocytes are
vital for maintaining the biological function of DPCs (2). Therefore, during in vitro
culture, culture conditions that mimic the in vivo
environment should be used in order to provide a more favorable
environment that is able to preserve DPC trichogenicity.
The present study explored new culture conditions
using conditioned media (CM) derived from the supernatant of
cultured HaCaT cells supplemented with SB431542 (SB, an inhibitor
of the TGFβ/Smad pathway), CHIR99021 (CHIR, a GSK3α/β inhibitor and
activator of Wnt signaling), and PDGF-AA. Using this media,
high-passage (P7) DPCs were cultured under both two-dimensional
(2D) and 3D culture conditions and changes in morphology and gene
expression patterns associated with the trichogenic phenotype of
DPCs were examined.
Materials and methods
Isolation of DPCs and cell
culture
Full-thickness skin samples were obtained from the
occipital human scalp of three individuals undergoing corrective
surgery for the treatment of androgenetic alopecia. The
experimental protocol was established according to the ethical
guidelines of the Helsinki Declaration and was approved by the
Human Ethics Committee of China-Japan Union Hospital of Jilin
University (approval no. 2020042606; Changchun, China). Written
informed consent was obtained from individual patients. Follicles
were removed from the fine scalp. Collagen capsules surrounding the
scalp follicles were then removed to expose the follicle bases, and
DPs were dissected using thin needles. Isolated DPs were placed on
the bottom of the cell culture dishes. DPCs were cultured for 10–14
days, harvested using 0.25% trypsin-EDTA (Sigma-Aldrich; Merck
KGaA), and transferred to fresh culture dishes. DPCs were cultured
in DMEM-F12 (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.) and fibroblast growth factor-basic (bFGF; 10 ng/ml;
PeproTech, Inc.). In total, 4×104 cells/ml DPCs were
cultured in T75 culture flasks. Cells were subcultured or harvested
upon reaching 80–90% confluence. The culture medium was exchanged
every three days. The cells were examined under a bright-field
microscope (magnification, ×40) (Olympus Corporation), the cell
aspect ratio (measured as the length of the long axis divided by
that of the narrow axis) and cell areas were analyzed using
cellSens Dimension software (version 1.12; Olympus
Corporation).
Keratinocyte culture
The foreskin of children (discarded tissue after
circumcision) was obtained from China-Japan Union Hospital of Jilin
University. Procedures were explained and written informed consent
was obtained from participants' guardians in accordance with
Declaration of Helsinki guidelines. The experimental procedures
were officially approved by the Human Ethics Committee as described
above (approval no. 2020042606). The epidermis was separated from
the foreskin after overnight incubation with 2 g/ml dispase II
(Gibco; Thermo Fisher Scientific, Inc.) at 4°C, and keratinocytes
were isolated after trypsinization for 7 min by thorough pipetting.
Keratinocytes were cultured at 37°C in Epidermal Keratinocyte
Medium (Gibco; Thermo Fisher Scientific, Inc.). After cells reached
90% confluence, the medium was completely replaced with DMEM-F12
supplemented with 1% FBS (v/v) and cultured for 24 h, followed by
harvesting the media for further analysis.
HaCaT cell culture and HaCaT-CM
preparation
HaCaT cells were obtained from The Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences. Cells were
cultured to 80–90% confluence in T75 culture flasks and treated
with 10% FBS (v/v). Following this, the medium was replenished with
10 ml DMEM-F12 supplemented with different concentrations of FBS
(0, 1 and 10% by volume) at 37°C. For HaCaT-CM preparation, the
cells were cultured at 37°C for 24 and 48 h with 1% FBS (v/v)
before harvesting. The collected supernatant samples were filtered
through a 0.2-µm filter and stored at −20°C. Then, the supernatant
was used to culture DPCs at different concentrations (0, 25, 50 and
100% by volume).
Alkaline phosphatase (ALP)
activity
ALP levels were assessed using the BCIP/NBT Alkaline
Phosphatase Color Development Kit (Beyotime Institute of
Biotechnology), according to the manufacturer's instructions. Human
DPCs were plated in 12-well plates at 8×104 cells per well. After
growing for 24 h, the cells were fixed in 4% paraformaldehyde at
room temperature for 10 min and then washed with phosphate-buffered
saline (PBS). Cells were then incubated in BCIP/NBT buffer at room
temperature for 30 min. The reaction was stopped by washing with
PBS, and the cells were examined under a bright-field microscope
(magnification, ×40). Dark blue staining indicated a positive
signal for ALP.
Reverse transcription (RT)-polymerase
chain reaction (PCR) and RT-quantitative (q)PCR analyses
To examine the expression of genes in DPCs, total
RNA (1 µg) was isolated using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), and first-strand cDNA
was synthesized using SuperScript™ III Reverse Transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.) and oligo-dT (Promega
Corporation), according to the manufacturer's instructions. For
RT-PCR, the amplification included initial denaturation at 95°C for
10 sec, 35 cycles of denaturation at 95°C for 10 sec, and annealing
at 60°C for 30 sec. The GAPDH mRNA level was used for sample
standardization. The PCR products were separated by electrophoresis
on a 1.5% agarose gel (Beyotime Institute of Biotechnology) and
were stained with GelRed® (Beyotime Institute of
Biotechnology), each band was quantified using ImageJ 1.53a
software (National Institutes of Health). For RT-qPCR, a TransStart
Tip Green qPCR SuperMix (Beijing TransGen Biotech Co., Ltd.) was
used, and the thermocycling program was as follows: 94°C for 30
sec, followed by 40 cycles at 94°C for 5 sec, 55°C for 15 sec and
72°C for 10 sec. At least three independent biological replicates
were performed for the RT-qPCR. The amount of PCR product was
calculated relative to the internal control GAPDH, and then
compared between the experimental and control groups using the
2−ΔΔCq method (16).
Primer sequences were as follows: Sox2 sense,
5′-CGGATTATAAATACCGGCCC-3′ and antisense,
5′-GTGTACTTATCCTTCATGAGC-3′; ALP sense,
5′-CAGGTCCCACAAGCCCGCAA-3′ and antisense, 5′-CCCGGTGGGCCACAAAA-3′;
Versican sense, 5′-TGAGCATGACTTCCGTTGGACTGA-3′ and
antisense, 5′-CCACTGGCCATTCTCATGCCAAAT-3′; Wnt3a sense,
5′-AGATTGGCATCCAGGAGTG-3′ and antisense, 5′-CTCCCTGGTAGCTTTGTCC-3′;
Wnt10a sense, 5′-CTAAGGACTTTCTGGACTCCC-3′ and antisense,
5′-TGTTCTCCATCACTGCCTG-3′; Wnt10b sense,
5′-GAGGTCCTGATCGATCTGC-3′ and antisense, 5′-ATTGCTTAGAGCCCGACTG-3′;
EGF sense, 5′-GAGAAACTGTTGGGAGAGGAATC-3′ and antisense,
5′-TCACAGAGTTTAACAGCCCTGC-3′; BMP6 sense,
5′-TCAGCACAGAGACTCTGAC-3′ and antisense, 5′-ATGTCAAATTCCAGCCAGC-3′;
GAPDH sense, 5′-CGCTCTCTGCTCCTGTT-3′ and antisense,
5′-CCATGGTGTCTGAGCGATGT-3′.
Western blot analysis
Cells were lysed in RIPA buffer [150 mM NaCl, 10 mM
Tris, pH 7.2, 0.1% sodium dodecyl sulfate (SDS), 1.0% Triton X-100,
1% sodium deoxycholate, 5 mM EDTA]. Protein concentrations were
determined by the Bradford assay (Beyotime Institute of
Biotechnology). Samples (25 µg/lane) were separated via SDS-PAGE on
10–12% gels, following which, proteins were electrophoretically
transferred onto a nitrocellulose membrane. After blocking in
sterile PBS containing 5% non-fat dry skimmed milk and 0.05% (v/v)
Tween-20 at room temperature for 1 h, the blots were incubated with
the following primary antibodies overnight at 4°C: Sox2 (cat. no.
3579; 1:1,000; Cell Signaling Technology, Inc.), ALP (cat. no.
ab108337; 1:2,000; Abcam), Versican (cat. no. ab19345; 1:1,000;
Abcam), phosphorylated (p)-Smad2/3 (cat. no. 8828; 1:1,000; Cell
Signaling Technology, Inc.), Smad2/3 (cat. no. 5678; 1:1,000; Cell
Signaling Technology, Inc.), β-catenin (cat. no. 8480; 1:1,000;
Cell Signaling Technology, Inc.), lymphoid enhancer-binding factor
1 (Lef-1) (cat. no. 2230; 1:1,000; Cell Signaling Technology, Inc.)
and β-actin (cat. no. AT0001; 1:1,000; Engibody Biotechnology,
Inc.). Horseradish peroxidase-conjugated anti-mouse (cat. no. 7076;
1:1,000; Cell Signaling Technology, Inc.) and anti-rabbit (cat. no.
7074; 1:1,000; Cell Signaling Technology, Inc.) antibodies were
used as secondary antibodies at room temperature for 1 h. The
immune complexes were assayed with an enhanced chemiluminescence
kit (Invitrogen; Thermo Fisher Scientific, Inc.) and the blots were
analyzed with densitometry using ImageJ 1.53a software (National
Institutes of Health).
Cell proliferation assay
The cell proliferation assay was carried out using a
Cell Counting Kit-8 (CCK-8) cell proliferation assay kit (Dojindo
Molecular Technologies, Inc.) according to the manufacturer's
instruction. DPCs were placed into 96-well plates (2×103
cells/well). After culturing for 1, 2, 3, 4, 5, 6 or 7 days, 10 µl
CCK-8 assay solution was added to each well. Subsequently, after
incubation for 2 h, the optical density (OD) at 450 nm was measured
with an enzyme immunoassay analyzer (Thermo Fisher Scientific,
Inc.) to estimate cell proliferation.
Histological and immunocytochemical
analyses
DPC spheres were harvested for histological and
immunocytochemical analyses. The treated spheres were fixed in 4%
paraformaldehyde at room temperature for 24 h, dehydrated with
sucrose and embedded in optimum cutting temperature compound. The
frozen sections (6-µm thick) were visualized using hematoxylin and
eosin (H&E) stain at room temperature and observed under a
bright-field microscope (Olympus Corporation). For
immunocytochemical analysis, cells were washed with PBS, fixed in
4% paraformaldehyde at room temperature for 30 min, and incubated
in PBS for 20 min at 4°C. Subsequently, the cells were
permeabilized using 0.1% Triton X-100 for 15 min, followed by
blocking with 5% FBS at room temperature for 30 min. The frozen
sections were incubated with ALP (cat. no. ab108337; 1:100; Abcam)
and Versican (cat. no. ab19345; 1:100; Abcam) or Sox2 (cat. no.
3579; 1:100; Cell Signaling Technology, Inc.) at 4°C overnight. The
sections were then washed in PBS with 0.05% (v/v) Tween-20 and
incubated with Alexa Fluor® 594-conjugated goat
anti-rabbit IgG (cat. no. R37117; 1:200; Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature for 1 h. The frozen sections
were stained with DAPI (1:2,000; Sigma-Aldrich; Merck KGaA) at room
temperature for 10 min and observed via fluorescence microscopy
(Olympus Corporation).
Hanging drop aggregation assay
DPCs at P7 (3×103 cells/ml; 40 µl) were
disseminated and pipetted into each well of the 96-well hanging
drop dishes (Sigma-Aldrich; Merck KGaA). The medium for the
HaCaT-CM + 3D group was HaCaT-CM supplemented with 10 mM SB (TGF-β
receptor inhibitor; Tocris Bioscience), 3 mM CHIR (GSK3 inhibitor
and activator of the Wnt pathway; Peprotech, Inc.), and 5 ng/ml
growth factor PDGF-AA (Peprotech, Inc.). The medium of the control
group was DMEM-F12 supplemented with 1% FBS (v/v). Culture media
were changed every 24 h. The cultures were placed at 37°C in a
humidified incubator with a 5% CO2 atmosphere.
Subsequently, the cells from both groups were harvested throughout
the culture period of 5 days and evaluated in terms of morphology
and gene expression. The diameter and surface area of DPC spheres
were analyzed using the cellSens Dimension software (version 1.12;
Olympus Corporation).
Statistics
The results are expressed as the mean ± SEM. All
experiments were repeated three times with independent cultures,
and similar results were obtained. Statistical tests were carried
out with SPSS (version 22.0; SPSS, Inc.). Statistical significance
was determined using one-way analysis of variance (ANOVA), followed
by Tukey's post hoc test. For comparisons between two treatment
groups unpaired Student's t-test was used. P<0.05 was considered
to indicate a statistically significant difference.
Results
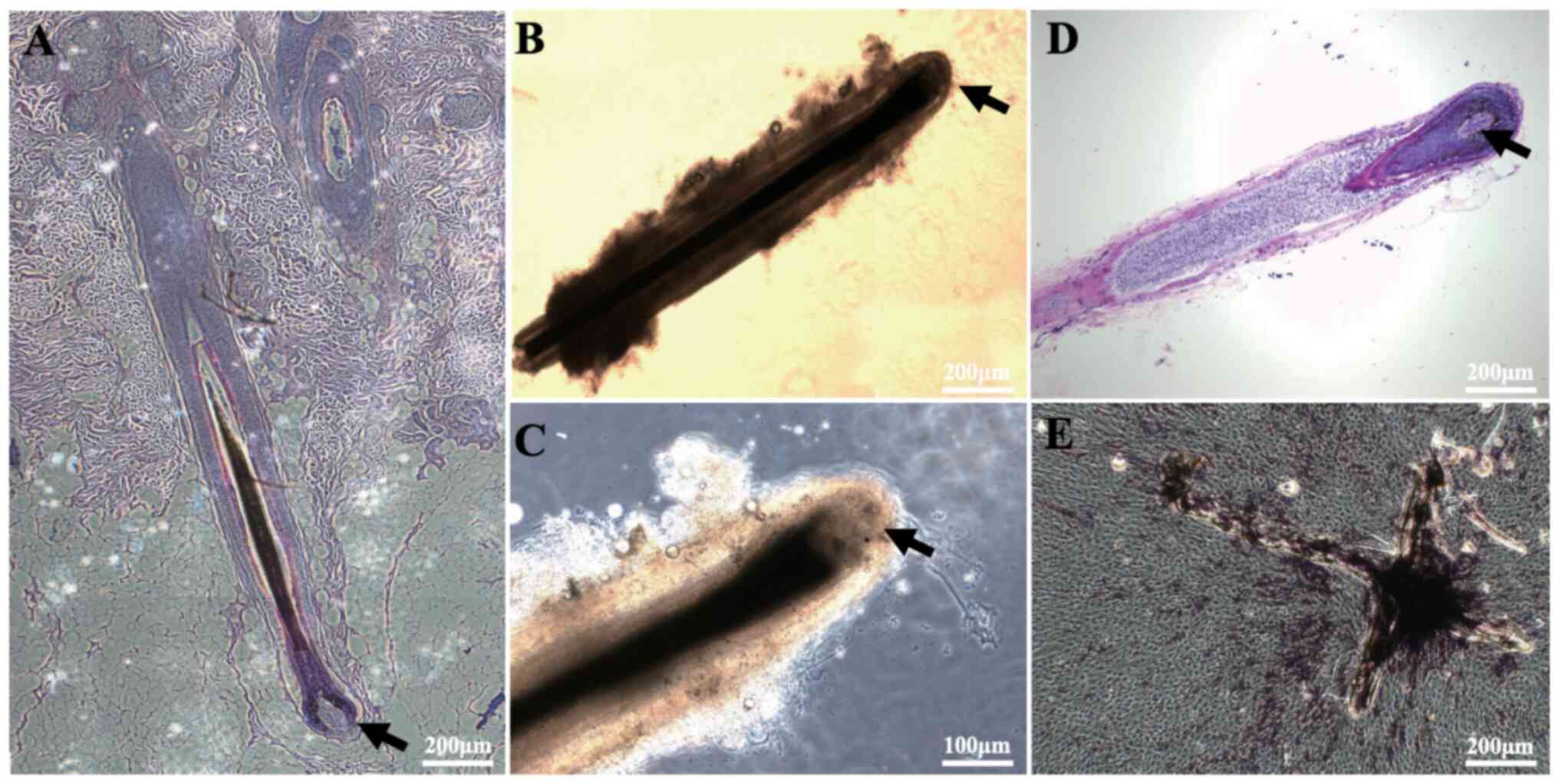
Isolation of human DPs and ALP
staining
First, HFs were separated, and their morphology was
examined under a microscope following histochemical staining. In
addition, DPCs (from micro-dissected DPs) were characterized by ALP
staining. The results showed that DPs were located in the hair bulb
at the bottom of the HFs (Fig. 1A and
B), and that they had a very high cell density in the
follicular growth phase (Fig. 1C and
D). Next, DPs were isolated from the HFs and placed in dishes,
after which the DPCs were observed to spread out and underwent
proliferation after ~14 days (Fig.
1E). It was also observed that there was high ALP activity in
the cultured DPs and DPCs (Fig.
1E).
Morphological observation and specific
ALP staining of subcultured DPCs
Upon observing the subcultured DPCs, the morphology
of DPCs was found to change notably during subculture.
Specifically, the morphology of DPCs gradually shifted from
long-spindle shaped cells to polygonal shaped cells at higher cell
generations (Fig. 2A). Furthermore,
the amount of ALP-stained cells decreased over the culture period
(Fig. 2B and E). A statistical
analysis showed that the aspect ratio of the cells decreased at
higher cell generations, while the cell surface area gradually
increased (Fig. 2C and D).
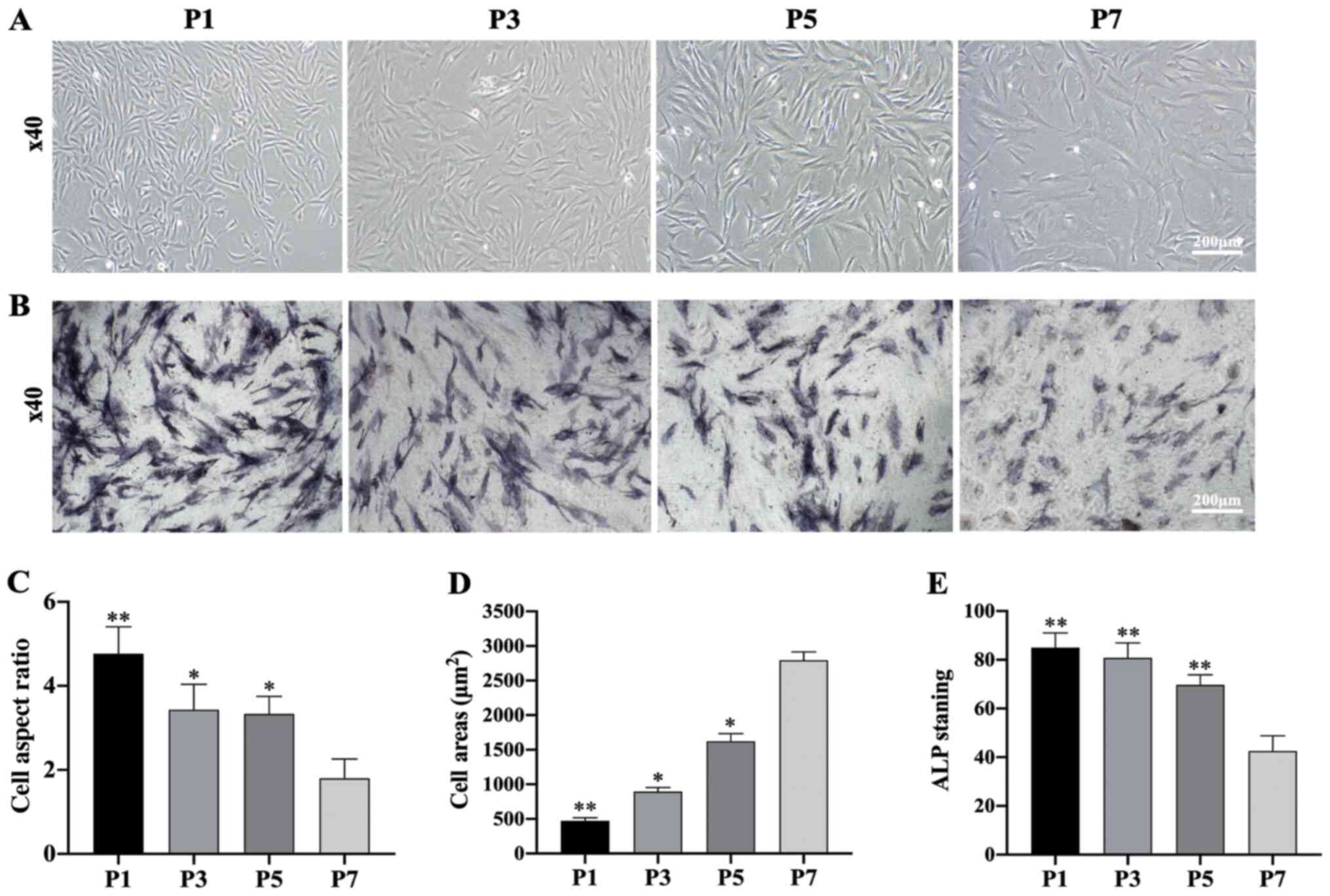 | Figure 2.Morphological observation and
specific ALP staining of DPCs at different passages. (A) Morphology
of DPCs at passages P1, P3, P5 and P7 (scale bar, 200 µm). (B) ALP
staining of DPCs at passages P1, P3, P5 and P7 (scale bar, 200 µm).
(C) Cell aspect ratio of DPCs at P1, P3, P5 and P7; n=20 in each
group. (D) Cell areas of DPCs at passages P1, P3, P5 and P7; n=20
in each group. (E) ALP staining of DPCs at passages P1, P3, P5 and
P7; n=20 in each group. Data represent the mean ± SEM. *P<0.05,
**P<0.01 vs. P7 DPCs. DPC, dermal papilla cells; ALP, alkaline
phosphatase; P, passage. |
Expression of specific genes and
proteins in DPCs during subculture
Changes in the expression of hair induction-related
genes and proteins were examined during DPC culture. The results
showed that the expression levels of hair-inducing genes Sox2,
ALP and Versican were decreased significantly compared
with P1-DPCs (Fig. 3A and B).
Western blotting was used to measure the protein expression levels
of DPCs during subculture, including the hair-inducing proteins
Sox2, ALP and Versican, the Wnt pathway proteins β-catenin and
Lef-1, and the TGF-β/Smad pathway protein p-Smad2/3. The results
showed that Sox2, ALP and Versican expression levels decreased
rapidly during subsequent passaging. Moreover, the expression
levels of β-catenin and Lef-1 also gradually decreased, while the
levels of p-Smad2/3 gradually increased with passaging (Fig. 3C and D).
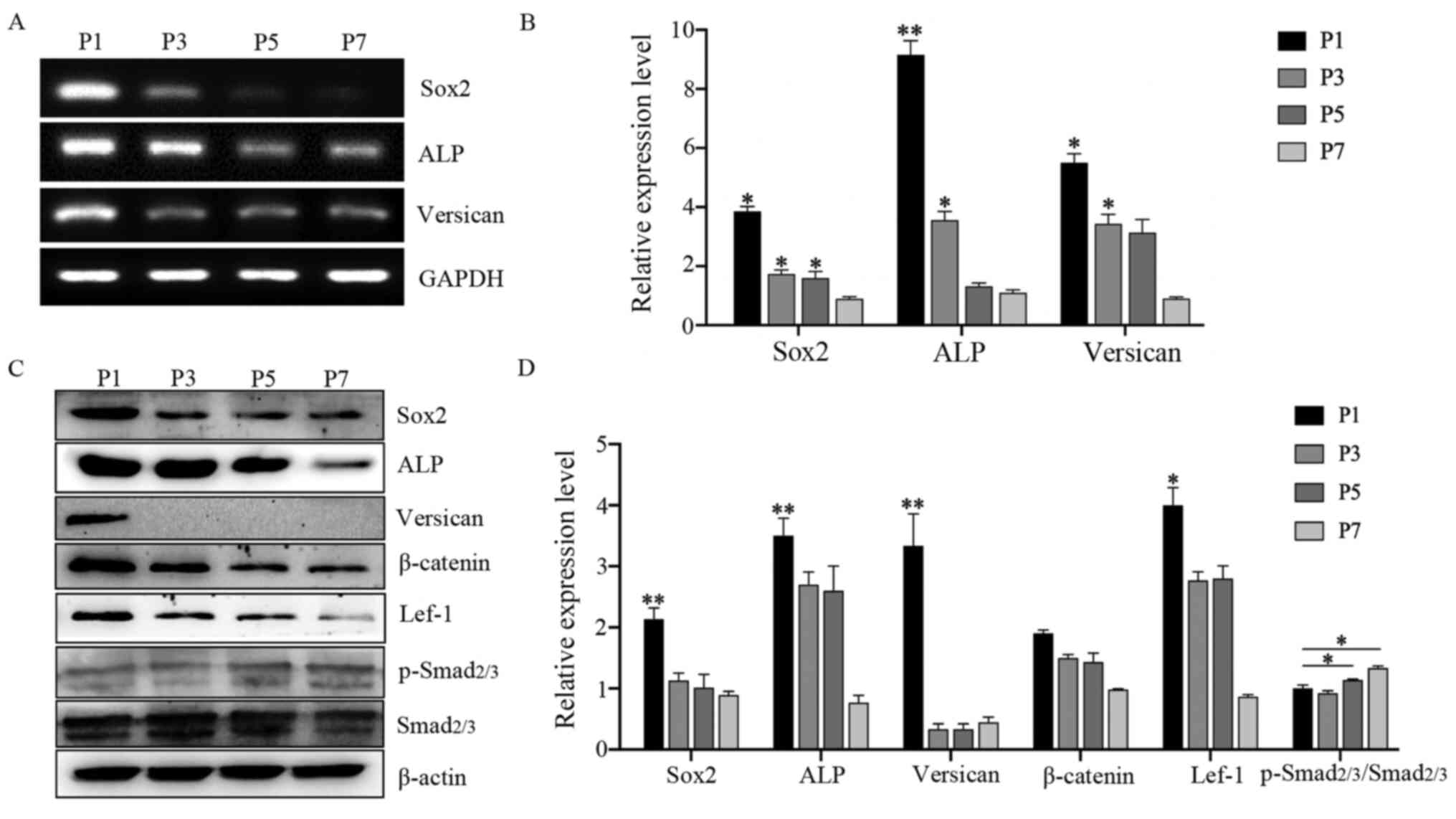 | Figure 3.Expression of characteristic genes
and proteins in DPCs during subculture. (A) RT-PCR analysis of
Sox2, ALP and Versican mRNA levels in DPCs (P1, P3,
P5, P7) subcultured in control medium (DMEM-F12 supplemented with
basic fibroblast growth factor). (B) RT-qPCR analysis of Sox2,
ALP and Versican mRNA levels in DPCs (P1, P3, P5, P7) in
control medium; n=3. (C) Western blot analysis of Sox2, ALP,
Versican, β-catenin, Lef-1, total Smad2/3 and p-Smad2/3 levels in
DPCs (P1, P3, P5, P7) in control medium; (D) Densitometry analysis;
n=3. Data represent the mean ± SEM. *P<0.05, **P<0.01 vs. P7
DPCs. DPC, dermal papilla cells; ALP, alkaline phosphatase; P,
passage; RT-PCR, reverse transcription-quantitative PCR; RT-qPCR,
RT-quantitative PCR; Lef-1, lymphoid enhancer-binding factor 1; p-,
phosphorylated. |
Preparation and evaluation of
HaCaT-CM
HaCaT cells were cultured with different
concentrations of FBS (0, 1 and 10% by volume) and the gene
expression levels of a number of key genes were measured. HaCaT
cells cultured in 0% FBS were found to grow more slowly than those
cultured in 1% or 10% FBS. Cells cultured in the presence of 1% and
10% FBS showed no notable differences in growth rate and morphology
(Fig. 4A). Keratinocytes were also
isolated and cultured (Fig. 4B).
HaCaT cells also showed higher mRNA expression levels of Wnt3a,
Wnt10a, Wnt10b and EGF than DPCs (Fig. 4C and D), as well as higher
expression levels of Wnt3a and Wnt10b than primary
keratinocytes isolated from human foreskin (Fig. 4E and F). To reduce the interference
of exogenous serum cytokines, the supernatant of HaCaT cells
cultured with 1% FBS at different time points (24 or 48 h) were
collected. Subsequently, the supernatant was used to culture DPCs
at different concentrations (0, 25, 50 and 100%). Cell
proliferation and gene expression of the DPCs in each group were
measured and it was found that DPCs had a higher growth rate and
higher expression levels of Sox2 and Versican in a 24
h culture with a 50% volume fraction of the supernatant (Fig. 4G and H). As such, the supernatant
under this condition was designated as HaCaT-CM.
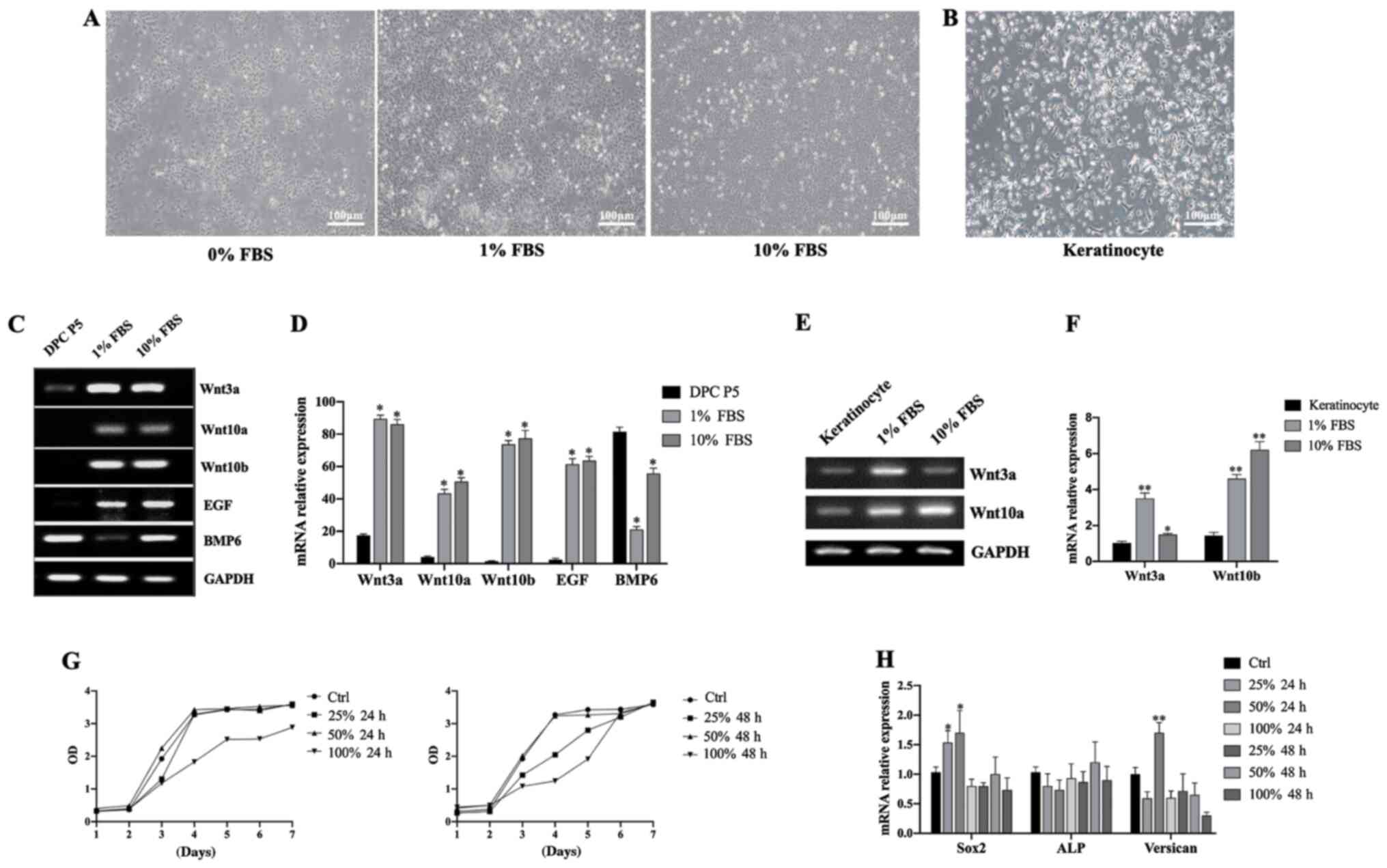 | Figure 4.Preparation and evaluation of
HaCaT-CM. (A) Morphology of HaCaT cells cultured in 0, 1 or 10% FBS
(scale bar, 100 µm). (B) Morphology of keratinocytes isolated from
human foreskin (scale bar, 100 µm). (C) RT-PCR analysis of
Wnt3a, Wnt10a, Wnt10b, EGF and BMP6 mRNA expression
levels in DPCs and HaCaT cells cultured in 0, 1 or 10% FBS. (D)
RT-qPCR analysis of Wnt3a, Wnt10a, Wnt10b, EGF and
BMP6 mRNA levels in DPCs and HaCaT cells cultured in 0, 1 or
10% FBS; n=3. *P<0.05 vs. P5 DPC. (E) RT-PCR analysis of
Wnt3a and Wnt10a mRNA levels in keratinocytes and
HaCaT cells cultured in 1 or 10% FBS. (F) RT-qPCR analysis of
Wnt3a and Wnt10a mRNA levels in keratinocytes and
HaCaT cells cultured in 1 or 10% FBS; n=3. *P<0.05, **P<0.01
vs. keratinocytes. (G) Cell Counting Kit-8 assay in DPCs cultured
with HaCaT-CM under different conditions. (H) RT-qPCR analysis of
Sox2, ALP and Versican mRNA levels in DPCs cultured
in HaCaT-CM under different conditions; n=3. Data represent the
mean ± SEM. *P<0.05, **P<0.01 vs. Ctrl. CM, conditioned
media; FBS, fetal bovine serum; BMP6, bone morphogenetic protein 6;
DPC, dermal papilla cells; ALP, alkaline phosphatase; P, passage;
RT-qPCR, reverse transcription-quantitative PCR; Ctrl, control. |
HaCaT-CM supplemented with SB, CHIR
and PDGF-AA maintains the hair-inducing capacity of DPCs
To further promote the characteristics of DPCs in
vitro and maintain their expression of hair-inducing genes and
proteins, HaCaT-CM was supplemented with small molecule inhibitors
namely 10 mM SB and 3 mM CHIR, as well as 5 ng/ml PDGF-AA, and then
the growth and differentiation of the DPCs at P7 were observed. The
medium components in each group are listed in Fig. 5A. The results showed that treatment
with HaCaT-CM containing SB, CHIR and PDGF-AA significantly
upregulated the mRNA expression levels of Sox2, ALP and
Versican in DPCs compared with other treatment combinations
(Fig. 5B). In addition, a western
blot analysis showed that Sox2, ALP and β-catenin expression levels
were all upregulated after treatment with HaCaT-CM containing SB,
CHIR and PDGF-AA compared with other treatment combinations
(Fig. 5C).
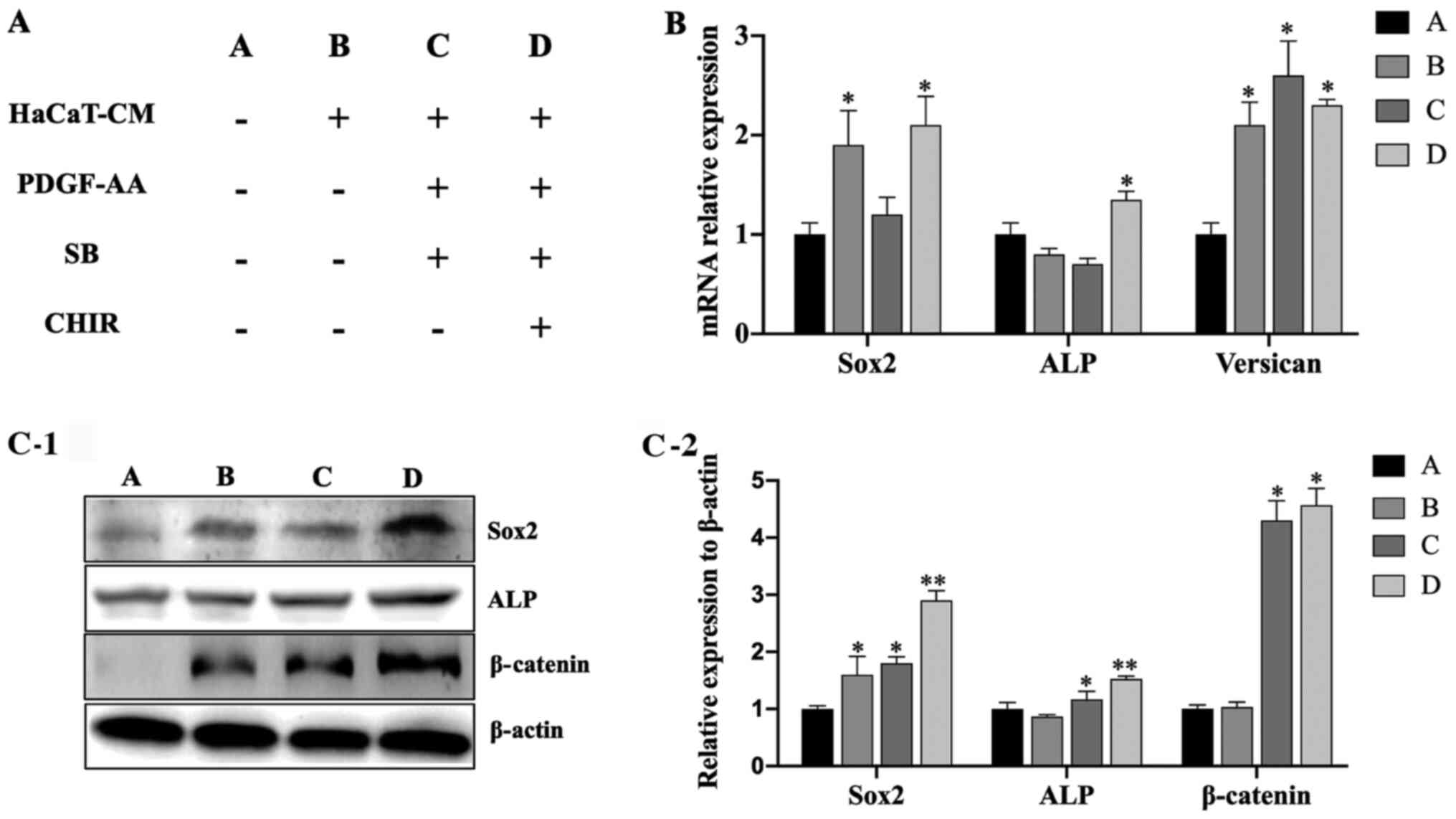 | Figure 5.Culture in HaCaT-CM supplemented with
SB, CHIR and PDGF-AA enhances the hair-inducing capacity of DPCs.
(A) Table showing the small molecule inhibitors used to supplement
HaCaT-CM. (B) Reverse transcription-quantitative PCR analysis of
Sox2, ALP and Versican mRNA levels in DPCs cultured
in HaCaT-CM supplemented with SB, CHIR and PDGF-AA; n=3. (C-1)
Western blot analysis of Sox2, ALP and β-catenin levels in DPCs
cultured in HaCaT-CM supplemented with SB, CHIR and PDGF-AA; (C-2)
densitometry analysis; n=3. Data represent the mean ± SEM.
*P<0.05, **P<0.01 vs. group A. CM, conditioned media; SB,
SB431542; CHIR, CHIR99021; PDGF, platelet-derived growth factor;
DPC, dermal papilla cells; ALP, alkaline phosphatase. |
HaCaT-CM supplemented with SB, CHIR
and PDGF-AA increases DPC characteristics in a hanging drop culture
system
DPCs at P7 were seeded into hanging drop dishes, and
after 7 days of culture, DPC aggregates were observed (Fig. 6A and B). The average diameter and
surface area of DPC sphere cultures in HaCaT-CM supplemented with
SB, CHIR and PDGF-AA were significantly larger than those cultured
in control medium (Fig. 6C and D).
Additionally, the expression levels of Sox2 and ALP
in DPCs cultured in HaCaT-CM supplemented with SB, CHIR and PDGF-AA
in the hanging drop culture were significantly increased compared
with those cultured in control medium (Fig. 6E). Moreover, higher expression
levels of Sox2 and ALP were also found by immunofluorescent
staining following culture in HaCaT-CM supplemented with SB, CHIR
and PDGF-AA compared with those cultured in control medium
(Fig. 6F and G).
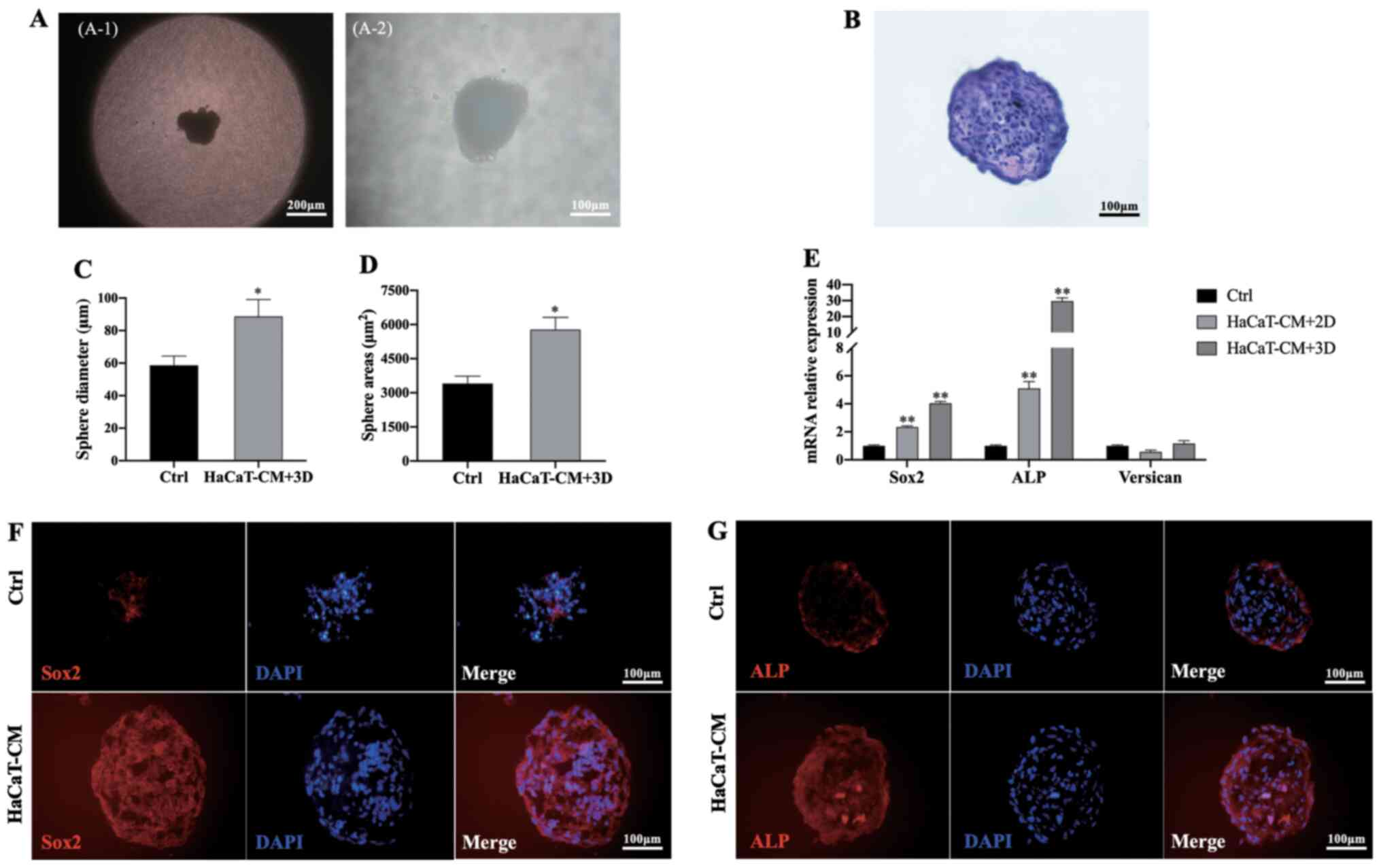 | Figure 6.HaCaT-CM supplemented with SB, CHIR
and PDGF-AA increases DPC characteristics in a hanging drop culture
system. (A) Aggregate of DPCs in hanging drop cultures using
HaCaT-CM supplemented with SB, CHIR and PDGF-AA. (A-1) Scale bar,
200 and (A-2) scale bar, 100 µm. (B) Hematoxylin and eosin staining
of DPC spheres (scale bar, 100 µm). (C) Sphere diameter analysis of
DPC aggregates cultured in HaCaT-CM supplemented with SB, CHIR and
PDGF-AA; n=20. (D) Sphere area analysis of DPC aggregates cultured
in HaCaT-CM supplemented with SB, CHIR and PDGF-AA; n=20. (E)
Reverse transcription-quantitative PCR analysis of Sox2, ALP
and Versican mRNA levels in 3D cultures using HaCaT-CM
supplemented with SB, CHIR and PDGF-AA, and in two-dimensional
cultures in HaCaT-CM supplemented with SB, CHIR and PDGF-AA; n=3.
Immunocytochemical staining for (F) Sox2 and (G) ALP in DPC spheres
from 3D cultures using HaCaT-CM supplemented with SB, CHIR and
PDGF-AA (scale bar, 100 µm). Data represent the mean ± SEM.
*P<0.05, **P<0.01 vs. Ctrl. CM, conditioned media; SB,
SB431542; CHIR, CHIR99021; PDGF, platelet-derived growth factor;
DPC, dermal papilla cells; ALP, alkaline phosphatase; 3D,
three-dimensional; Ctrl, control. |
Discussion
DPs are located in the bulb area of HFs, and it is
here that DPCs receive crucial signals from surrounding
keratinocytes. Numerous studies have proven that keratinocytes are
indispensable cellular components during hair regeneration for
their role of interacting with DPCs (2,6).
Keratinocytes secrete a variety of bioactive molecules that are
necessary for preserving DPC characteristics in vivo
(17,18). Accordingly, we speculated that the
co-culture of DPCs with keratinocytes or using keratinocyte-CM
(containing bioactive molecules) can induce the redifferentiation
of DPCs and preserve their hair formation properties after
long-term 2D subculture. However, co-cultivation with keratinocytes
has some disadvantages, including donor-to-donor variability in the
characteristics of the keratinocyte cells, and the cells
differentiate quickly during cultivation (19), so it is difficult to harvest enough
cells to produce high-quality CM for DPC cultures. As a means of
addressing this, HaCaT cells were used in the present study. These
cells are a spontaneously immortalized human keratinocyte cell line
from adult skin that maintain a stable phenotype during in
vitro passaging, which can proliferate in FBS-supplemented
medium without the addition of growth factors for continuous growth
(19). In the present study, it was
found that the mRNA expression levels of Wnt3a and
Wnt10b in HaCaT cells were significantly higher than in
primary cultured keratinocytes, indicating a potential ability of
these cells to secrete Wnt. Wnt signaling has been demonstrated to
regulate HF induction and promote hair growth. The expression of
Wnt ligands (Wnt1a, 3a, 7a, 10b and 11) has also been reported in
HFs isolated from postnatal skin, suggesting that HaCaT cells could
be utilized to produce CM for DPC culture (11,20–22).
Therefore, unlike normal keratinocytes, a sufficient quantity of CM
with stable quality could be collected from the HaCaT cell line for
long-term cultivation of DPCs in vitro. To the best of our
knowledge, the present study is the first report to explore the
HaCaT cell-CM combined with small molecular compounds to inhibit
the dedifferentiation of DPCs in vitro.
The present study examined changes in gene and
protein expression levels during 2D cell culture of DPCs. The
results showed that there were significant decreases in the levels
of Sox2, Versican and ALP during the normal culture (DMEM-F12 with
10% FBS and 10 ng/ml bFGF). Furthermore, β-catenin expression
levels were also significantly decreased, indicating that the
activity of the Wnt/β-catenin pathway is downregulated in DPC
cultures. These results are consistent with previous reports
(10,11,14),
demonstrating that downregulation of the Wnt/β-catenin pathway can
lead to DPC dedifferentiation.
There are numerous reports that small molecule
compounds can be used to inhibit or activate signaling pathways and
have been extensively used in numerous cell proliferation and
differentiation studies (23–25).
For example, recently, Yoshida et al (24) found that treating DPCs with the
canonical Wnt/β-catenin signaling activator CHIR99021, a potent
inhibitor of GSK3α and GSK3β, significantly enhanced the expression
of DP signature genes associated with their hair-inducing ability
(24). For these reasons, in the
present study, DPCs were cultured in the presence of HaCaT-CM
supplemented with the Wnt signaling activator CHIR. FGF2/bFGF has
also been reported to enhance DPC proliferation (13). Accordingly, in addition to Wnt, bFGF
was added as an elemental component in the DPC culture medium.
PDGF is a potent mitogen for cells of mesenchymal
origin (12,13). It has been suggested that PDGF-AA
expression by immature adipocytes regulates the activity of
follicular stem cells and that PDGF receptor α is activated in the
DP during the anagen phase (13).
Hair reconstitution assays have also revealed that DPCs treated
with both PDGF-AA and FGF2 show an improved ability to maintain
their hair inductive activity compared with those treated with FGF2
alone (12). PDGF has also been
revealed to contribute to the induction and maintenance of the
anagen phase in HFs in vivo (12,13,26).
Based on these findings, PDGF was considered to be essential factor
to promote the growth of DPCs and to maintain hair follicle
inductive ability in vivo. For all of these reasons PDGF was
also added to the HaCaT-CM in the present study.
During the skin healing process that occurs
following skin injury, DPCs dedifferentiate into fibroblast-like
cells and participate in the wound healing process. It has been
reported that TGF-β1 induces DPC dedifferentiation to the
fibroblast-like phenotype (27,28).
In the present study, it was observed that the expression levels of
p-Smad2 and p-Smad3 in the TGF-β/Smad signaling pathway gradually
increased with passage time, suggesting that TGF-β/Smad pathway
activation causes DPC dedifferentiation during DPC sub-cultivation.
SB is a potent small molecule inhibitor of the TGF-β/Smad pathway
that blocks the type I receptor ALK5 (29). For this reason, SB was also included
in the CM to inhibit the TGF-β/Smad pathways.
Culture of DPCs in HaCaT-CM supplemented with SB,
CHIR and PDGF-AA increased the expression levels of Sox2, ALP and
β-catenin more significantly at both the mRNA and protein levels
compared with other culture conditions. The mRNA expression levels
of Versican were also upregulated ~2-fold by culturing in HaCaT-CM
supplemented with SB, CHIR and PDGF-AA compared with those in the
control medium; however, the protein levels did not show a
significant change (data not shown). The reason for this is
unclear, but it does suggest that Versican protein expression is
inhibited at the post-transcriptional stage through an unknown
mechanism. Additionally, the functional role of Versican in hair
follicle development and hair growth is also unclear; however,
several researchers have suggested that Versican functions as an
inhibitor of cell-cell or cell-ECM adhesion (9,30). As
DPCs are densely packed in the postnatal skin, Versican could
selectively prevent the incorporation of dermal fibroblasts
(non-DP-destined cells) into the DP. It is possible that continuous
Versican expression in condensed mesenchymal cells may be required
to exclude the additional surrounding dermal fibroblasts from
condensation, thus maintaining the purity of the induced DPC
population (1). We hypothesized
that without signals or stimulation from fibroblasts or other
dermal components, the Versican levels in DPCs may not rise
significantly. However, this will require further investigation to
uncover the specific underlying mechanism.
In the hair follicle bulb, DPCs are assembled in a
3D organization, where cell-cell and cell-ECM interactions are
crucial for maintaining the biological functions of DPC (4,5,9,18).
However, in vitro, 2D cell culture systems do not reflect
the in vivo environment and may cause DPCs to rapidly lose
their distinctive features and inductive function. Previous studies
have reported that in vitro conditions mimicking in
vivo-like conditions, specifically DPCs grown into a 3D
microtissue, can preserve the natural functions of DPCs and enhance
their hair-inducing potential after in vivo transplantation
(31,32). In both human and murine hair, the DP
size specifies the hair size, shape and cycling (33). Moreover, the DP size mainly depends
on DPC number. The average volume of DP in the human scalp is
~536×103 (µm3), and the total number of DPCs
within the DP is ~1.3×103 (34).
Accordingly, in the present study, the hanging drop technique was
utilized to create cell-aggregated 3D spheroids. It was found that
3×103 cells formed tightly aggregated spheroids in
HaCaT-CM supplemented with SB, CHIR and PDGF-AA, and that the
average volume was ~523×103 (µm3) (data not
shown), which was similar to the size of hair follicle DPs in a
healthy human scalp. Furthermore, increased Sox2 and ALP gene and
protein expression levels were found in the spheroids formed,
indicating that the combination of the hanging drop 3D culture and
the supplemented HaCaT-CM is favorable for reconstructing an
artificial DP structure in vitro. In future studies, we plan
to conduct in vivo transplantation to evaluate the hair
regenerative capacity of these artificial DP structures using these
culture conditions.
To the best of our knowledge, this is the first
study to demonstrate the development of a novel DPC culture medium
comprising HaCaT-CM supplemented with defined small molecules and
growth factors, which has significant synergetic effects in
restoring the expression of signature DPC genes. Furthermore,
combining this with the hanging drop 3D culture was demonstrated to
be more effective in reconstructing DP-like structures with high
expression of hair follicle-inducing proteins than 2D cultures.
In conclusion, cultured and expanded DPCs can change
their morphology and lose their hair-inducing ability. However,
HaCaT-CM was successfully used to reverse the signature gene
expression patterns of high-passage DPCs by culturing in the
presence of small molecule inhibitors, including SB, CHIR, and the
growth factor PDGF-AA. The present study showed that this culture
method could possibly maintain the hair induction ability of DPCs
after several passages in expansion cultures. Therefore, this
strategy could be used to potentially improve DPC culture methods
to provide high-quality and high-quantity DPCs for both the
construction of tissue engineered HFs for hair loss treatment and
for skin wound repair.
Acknowledgements
Not applicable.
Funding
This study was supported by the Projects of
International Cooperation and Exchanges Jilin Province (grant no.
20170414058GH), Science and Technology Research Project of the 13th
five-year plan of Jilin Province Department of Education (grant no.
JJKH20180194KJ) and by the Frontier Interdisciplinary Program of
Norman Bethune Health Science Center of Jilin University (grant no.
2013101002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GC and YH conceived and designed the experiments. DS
and JX performed the experiments. LC, YW and ZH prepared specimens
from the hospital. GC and DS confirmed the authenticity of the raw
data and analyzed the results. GC, YH and DS wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocol was established according
to the ethical guidelines of the Helsinki Declaration and was
approved by the Human Ethics Committee of China-Japan Union
Hospital of Jilin University (approval no. 2020042606). Written
informed consent to participate in this study was provided by the
patients or participants' guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xiao SE, Miao Y, Wang J, Jiang W, Fan ZX,
Liu XM and Hu ZQ: As a carrier-transporter for hair follicle
reconstitution, platelet-rich plasma promotes proliferation and
induction of mouse dermal papilla cells. Sci Rep. 7:11252017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohyama M and Veraitch O: Strategies to
enhance epithelial-mesenchymal interactions for human hair follicle
bioengineering. J Dermatol Sci. 70:78–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Biernaskie J, Paris M, Morozova O, Fagan
BM, Marra M, Pevny L and Miller FD: SKPs derive from hair follicle
precursors and exhibit properties of adult dermal stem cells. Cell
Stem Cell. 5:610–623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu JJ, Zhu TY, Lu YG, Liu RQ, Mai Y, Cheng
B, Lu ZF, Zhong BY and Tang SQ: Hair follicle reformation induced
by dermal papilla cells from human scalp skin. Arch Dermatol Res.
298:183–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohyama M, Kobayashi T, Sasaki T, Shimizu A
and Amagai M: Restoration of the intrinsic properties of human
dermal papilla in vitro. J Cell Sci. 125:4114–4125. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Higgins CA, Chen JC, Cerise JE, Jahoda CAB
and Christiano AM: Microenvironmental reprogramming by
three-dimensional culture enables dermal papilla cells to induce de
novo human hair-follicle growth. Proc Natl Acad Sci USA.
110:19679–19688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Driskell RR, Clavel C, Rendl M and Watt
FM: Hair follicle dermal papilla cells at a glance. J Cell Sci.
124:1179–1182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang CC and Cotsarelis G: Review of hair
follicle dermal cells. J Dermatol Sci. 57:2–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kishimoto J, Ehama R, Wu L, Jiang S, Jiang
N and Burgeson RE: Selective activation of the versican promoter by
epithelial- mesenchymal interactions during hair follicle
development. Proc Natl Acad Sci USA. 96:7336–7341. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Millar SE: Molecular mechanisms regulating
hair follicle development. J Invest Dermatol. 118:216–225. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ouji Y, Nakamura-Uchiyama F and Yoshikawa
M: Canonical Wnts, specifically Wnt-10b, show ability to maintain
dermal papilla cells. Biochem Biophys Res Commun. 438:493–499.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rezza A, Sennett R, Tanguy M, Clavel C and
Rendl M: PDGF signalling in the dermis and in dermal condensates is
dispensable for hair follicle induction and formation. Exp
Dermatol. 24:468–470. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kiso M, Hamazaki TS, Itoh M, Kikuchi S,
Nakagawa H and Okochi H: Synergistic effect of PDGF and FGF2 for
cell proliferation and hair inductive activity in murine vibrissal
dermal papilla in vitro. J Dermatol Sci. 79:110–118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gemayel R and Chenette EJ: β-catenin
signalling in dermal papilla cells leads to a hairy situation. FEBS
J. 283:2820–2822. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soma T, Fujiwara S, Shirakata Y, Hashimoto
K and Kishimoto J: Hair-inducing ability of human dermal papilla
cells cultured under Wnt/β-catenin signalling activation. Exp
Dermatol. 21:307–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moore GP, Du Cros DL, Isaacs K,
Pisansarakit P and Wynn PC: Hair growth induction: roles of growth
factors. Ann N Y Acad Sci. 642:308–325. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inamatsu M, Matsuzaki T, Iwanari H and
Yoshizato K: Establishment of rat dermal papilla cell lines that
sustain the potency to induce hair follicles from afollicular skin.
J Invest Dermatol. 111:767–775. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schürer N, Köhne A, Schliep V, Barlag K
and Goerz G: Lipid composition and synthesis of HaCaT cells, an
immortalized human keratinocyte line, in comparison with normal
human adult keratinocytes. Exp Dermatol. 2:179–185. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong L, Hao H, Liu J, Tong C, Ti D, Chen
D, Chen L, Li M, Liu H, Fu X, et al: Wnt1a maintains
characteristics of dermal papilla cells that induce mouse hair
regeneration in a 3D preculture system. J Tissue Eng Regen Med.
11:1479–1489. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimizu H and Morgan BA: Wnt signaling
through the β-catenin pathway is sufficient to maintain, but not
restore, anagen-phase characteristics of dermal papilla cells. J
Invest Dermatol. 122:239–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kishimoto J, Burgeson RE and Morgan BA:
Wnt signaling maintains the hair-inducing activity of the dermal
papilla. Genes Dev. 14:1181–1185. 2000.PubMed/NCBI
|
|
23
|
Fujimori K, Matsumoto T, Kisa F, Hattori
N, Okano H and Akamatsu W: Escape from pluripotency via inhibition
of TGF-β/BMP and activation of Wnt signaling accelerates
differentiation and aging in hPSC progeny cells. Stem Cell Reports.
9:1675–1691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshida Y, Soma T, Matsuzaki T and
Kishimoto J: Wnt activator CHIR99021-stimulated human dermal
papilla spheroids contribute to hair follicle formation and
production of reconstituted follicle-enriched human skin. Biochem
Biophys Res Commun. 516:599–605. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu J, Liu H, Huang CT, Chen H, Du Z, Liu
Y, Sherafat MA and Zhang SC: Generation of integration-free and
region-specific neural progenitors from primate fibroblasts. Cell
Rep. 3:1580–1591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Osada A, Iwabuchi T, Kishimoto J, Hamazaki
TS and Okochi H: Long-term culture of mouse vibrissal dermal
papilla cells and de novo hair follicle induction. Tissue Eng.
13:975–982. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bin S, Li HD, Xu YB, Qi SH, Li TZ, Liu XS,
Tang JM and Xie JL: BMP-7 attenuates TGF-β1-induced fibroblast-like
differentiation of rat dermal papilla cells. Wound Repair Regen.
21:275–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou-dong L, Bin S, Ying-bin X, Yan S,
Shao-hai Q, Tian-zeng L, Xu-sheng L, Jin-ming T and Ju-lin X:
Differentiation of rat dermal papilla cells into fibroblast-like
cells induced by transforming growth factor β1. J Cutan Med Surg.
16:400–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng Y, Zhao YD, Gibbons M, Abramova T,
Chu PY, Ash JD, Cunningham JM and Skapek SX: Tgfbeta signaling
directly induces Arf promoter remodeling by a mechanism involving
Smads 2/3 and p38 MAPK. J Biol Chem. 285:35654–35664. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lebaron RG: Versican. Perspect Dev
Neurobiol. 3:261–271. 1996.PubMed/NCBI
|
|
31
|
Huang YC, Chan CC, Lin WT, Chiu HY, Tsai
RY, Tsai TH, Chan JY and Lin SJ: Scalable production of
controllable dermal papilla spheroids on PVA surfaces and the
effects of spheroid size on hair follicle regeneration.
Biomaterials. 34:442–451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tan JJY, Common JE, Wu C, Ho PCL and Kang
L: Keratinocytes maintain compartmentalization between dermal
papilla and fibroblasts in 3D heterotypic tri-cultures. Cell
Prolif. 52:e126682019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chi W, Wu E and Morgan BA: Dermal papilla
cell number specifies hair size, shape and cycling and its
reduction causes follicular decline. Development. 140:1676–1683.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elliott K, Stephenson TJ and Messenger AG:
Differences in hair follicle dermal papilla volume are due to
extracellular matrix volume and cell number: Implications for the
control of hair follicle size and androgen responses. J Invest
Dermatol. 113:873–877. 1999. View Article : Google Scholar : PubMed/NCBI
|