Introduction
Adenomyosis (ADS) is an estrogen-dependent disorder
(1) that disturbs the fertility of
reproductive-age women. A recent 10-year (2006–2015)
population-based cohort study in the United States showed a 1%
incidence of ADS among women aged 16–60 years (2). This condition involves endometrial
stroma and glands present in the myometrium of the uterus (3), which lead to a series of clinical
symptoms, including progressive dysmenorrhea, abnormal uterine
bleeding and subfertility. Although this condition has been
recognized for >100 years (4),
its etiology and pathogenesis are yet to be fully elucidated.
The width of junctional zones (JZs) on T2-weighted
images is the main diagnostic factor of ADS (5). Disturbed uterine JZs generate
inordinate peristalsis and impaired uterotubal transport, which may
contribute to the development of ADS (6,7). Some
studies have indicated that abnormal uterine contractions that
originate exclusively from JZs (8)
may be a cause of dysmenorrhea (9).
Gonadotropin-releasing hormone analogs have been reported to reduce
the width of JZs (10,11), indicating that estrogen may regulate
the hyperplasia and hypertrophy of JZ smooth muscle cells (SMCs) to
some extent (12). Our previous
studies also revealed that compared to the outer myometrium, there
are more organelles on the ultrastructure in myocytes of the JZ
(13), and that estrogen can
accelerate SMC proliferation in the JZs of the uterus, which
affects JZ contraction (14,15).
In addition, accumulating evidence has shown that hyperestrogenism
in ADS is similar to that in uterine wound repair (16). It has been reported that estrogen
receptor α (ERα) can regulate oxytocin (OT) and OT receptor (OTR)
to induce peristalsis in the uterine myometrium (17). When OTR is upregulated in the
myometrium of ADS, uterine peristalsis is also active (8). If tissues in the JZ are injured,
inflammation and a series of cascade reactions will occur. First,
elevated IL-1β expression induces cyclooxygenase-2 (COX-2) to
produce prostaglandin E2 (PGE2). Second, P450 aromatase level is
increased, which in turn upregulates steroidogenic acute regulatory
protein (StAR). Finally, StAR induces estradiol to augment the
expression of estrogen receptor β (ERβ), which then accelerates the
development of ADS (18–20).
MicroRNAs (miRNAs/miRs) are non-coding RNAs that
serve a critical role in post-transcriptional gene regulation
(21). miR-let-7 is one of the most
studied miRNAs and includes several variants: let-7a, let-7b,
let-7c, let-7d and let-7e (22).
Lin28 is an RNA-binding protein that can bind to the RNA-binding
domains of let-7 to control cellular function (23). There are two homologous members in
the Lin28 family, Lin28A and Lin28B, and their expression levels
are negatively correlated with let-7 (24). Several studies have reported that
the let-7/Lin28 axis regulates pluripotency, reprogramming and
tumorigenicity in various diseases (25–28),
including neurodegenerative diseases (28), oral squamous carcinoma (29), breast cancer (30) and lung cancer (31). Therefore, this axis may modulate the
proliferation and differentiation of normal and abnormal cells.
Some studies have revealed that women with endometriosis have
several miRNAs (let-7b, miR-135a, let-7c, let-7d, let-7e and
let-7f) that are differentially expressed in sera or cells
(32,33). Moreover, our previous study found
that let-7a was negatively correlated with Lin28B expression in the
JZ in ADS (34).
Based on the aforementioned findings, we
hypothesized that 17β-estradiol may affect the let-a/Lin28B axis to
regulate the proliferation of JZ SMCs, resulting in disordered JZ
contraction and a series of clinical symptoms of ADS. Therefore,
the present study was designed with the following aims: i) To study
how let-7a regulates the proliferation of uterine JZ SMCs in ADS;
ii) to determine whether 17β-estradiol can change the let-7a/Lin28B
axis expression level to accelerate the development of ADS; and
iii) to establish how 17β-estradiol affects the function of the
let-7a/Lin28B axis in the proliferation of JZ SMCs in ADS.
Materials and methods
Sample collection and cell primary
culture
This study was approved by the Ethical Committee of
Clinical Research of Beijing Obstetrics and Gynecology Hospital,
Capital Medical University, China (reference no. 2016-KY-012), and
conducted according to the Declaration of Helsinki. Between July
2019 and January 2020 at the Beijing Obstetrics and Gynecology
Hospital, 36 patients diagnosed with ADS were enrolled as the
experimental group and 34 patients diagnosed with early-stage
cervical cancer, early-stage ovarian cancer or uterine prolapse
without ADS were recruited as the control group. All of these
individuals (age, 30–50 years) were premenopausal women with
regular menses before the hysterectomy operation (lengths ranging
between 21 and 35 days). The inclusion criteria for the
experimental group was patients aged 30–50 years diagnosed with ADS
that had undergone a hysterectomy. in the inclusion criteria for
the control group was patients diagnosed with early-stage cervical
cancer, early-stage ovarian cancer or uterine prolapse, who had
undergone a hysterectomy. The exclusion criteria included
endometriosis, endometrial polyps, fibroids, pelvic inflammation,
endometrial and myometrial cancer, use of hormones or intrauterine
devices within 3 months before surgery, and preoperative
radiotherapy and chemotherapy. All patients signed informed consent
before the hysterectomy. After the surgery, pathological
examination was used to determine if there were also other uterine
pathologies.
After the uterus was removed, it was opened
immediately in a Y shape and multiple 5 mm3 samples were
acquired from the JZ (underneath the endometrium) and then placed
in saline solution at 4°C for laboratory cell culture. The tissues
from the JZ were cut into small pieces and digested with
collagenase type II powder (cat. no. 17101015; Gibco; Thermo Fisher
Scientific, Inc.) and DNase I (cat. no. EN0521; Gibco; Thermo
Fisher Scientific, Inc.) for 4–5 h at room temperature. Then, after
filtration and centrifugation(1,200 × g, 25°C, 10 min), the cells
were incubated in DMEM with 15% FBS (Biological Industries) at 37°C
in a 5% CO2 incubator for subsequent experiments. Due to
bacterial or fungal infection, there were only 20 ADS tissues that
successfully produced primary cells and were used for subsequent
cell functional experiments.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from 20 ADS samples and 20
control samples according to the manufacturer's instructions for
RNAiso Plus (cat. no. 9108; Takara Bio, Inc.), and its quality and
quantity were assessed on a NanoDrop 2000/2000c Spectrophotometer
(Thermo Fisher Scientific, Inc.). Then, for Lin28B detection, RT
(37°C for 1 h, followed by termination at 85°C for 5 min in a
thermal cycler)and qPCR (initial denaturation: 95°C for 10 sec,
followed by 40 cycles of denaturation at 95°C for 10 sec, annealing
and elongation at 60°C for 20 sec) were performed using PrimeScript
RT reagent kit with gDNA Eraser (cat. no. RR047A; Takara Bio, Inc.)
and SYBR Premix Ex Taq II (Tli RNase H Plus; cat. no. RR820A;
Takara Bio, Inc.), respectively, on an ABI 7500 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). For
let-7a detection, Mir-X miRNA First-Strand Synthesis kit (cat. no.
638313; Takara Bio, Inc.) was used for RT (37°C for 1 h, followed
by termination at 85°C for 5 min in a thermal cycler) and TB Green
Advantage qPCR Premix (cat. no. 639676; Takara Bio, Inc.) was used
for qPCR. Two-step qPCR was used for let-7a detection, as follows:
Initial denaturation for 30 sec at 95°C, followed by 40 cycles of
denaturation at 95°Cfor 5 sec, and annealing and elongation at 60°C
for 34 sec. Dissociation curve analysis was conducted at 95°C for
60 sec, 55°C for 30 sec and 95°C for 30 sec. The 2−∆∆Cq
method was used to examine the relative expression levels of let-7a
and Lin28B (35). Since the Mir-X
miRNA First-Strand Synthesis kit supplied the mRQ 3′ primer as the
3′ primer for let-7a detection in qPCR, the entire sequence of
mature let-7a was used as the miRNA-specific 5′ primer. The primer
specific for let-7a was 5′-CCGCGCGCGCTATACAATCTACTGTCT-3′, and U6
was used for normalization with these primers: Forward,
5′-AACGAGACGACGACAGAC-3′ and reverse,
5′-GCAAATTCGTGAAGCGTTCCATA-3′. The primers used for Lin28B were
forward, 5′-AACCAGGTTTCATCAGCCCC-3′ and reverse,
5′-ACTTACAGTGGCCAGTTCCG-3′; and GAPDH was used as an internal
control with the following primers: Forward,
5′-CTCCTCCACCTTTGACGCTG-3′ and reverse,
5′-TCCTCTTGTGCTCTTGCTGG-3′.
Western blotting
RIPA buffer (cat. no. R0010; Beijing Solarbio
Science & Technology Co., Ltd.) was used to lyse cells and
extract total protein from 20 ADS samples and 20 control samples, a
protease inhibitor cocktail (cat. no. P8340; Sigma-Aldrich; Merck
KGaA) was added to inhibit the degradation of proteins. The protein
concentration was measured using an Enhanced BCA Protein Assay kit
(cat. no. P0010; Beyotime Institute of Biotechnology). Then, 10%
SDS-PAGE (cat. no. D1060; Beijing Solarbio Science & Technology
Co., Ltd. was used for electrophoresis, and the protein was
transferred to PVDF membranes (cat. no. IPVH00010; EMD Millipore).
The membranes were blocked for 2 h at room temperature with 5%
non-fat dry milk. Rabbit monoclonal anti-Lin28B (1:500; cat. no.
ab191881; Abcam) and rabbit polyclonal anti-α tubulin (1:2,000;
cat. no. AC007; ABclonal Biotech Co., Ltd.) were added to the
membranes and incubated at 4°C with gentle agitation overnight.
Finally, the membranes were washed three times using Tris-buffered
saline-Tween-20 (cat. no. T1085; Beijing Solarbio Science &
Technology Co., Ltd.) and incubated with HRP goat anti-rabbit IgG
(1:5,000; cat. no. AS029; ABclonal Biotech Co., Ltd.) for 1 h with
gentle agitation at room temperature. Immunoreactive bands were
detected using Chemiluminescent HRP Substrate (cat. no. WBKLS0500;
EMD Millipore). ChemiDoc TM XRS+ and Image Lab software 3.0
(Bio-Rad Laboratories, Inc.) were used to collect imaging
information.
Cell treatments
Primary cells were starved for 24 h and exposed to
17β-estradiol (10 nmol/l; cat. no. 3301; Sigma-Aldrich; Merck KGaA)
or dimethyl sulfoxide (DMSO; cat. no. 276855; Sigma-Aldrich; Merck
KGaA) as the control group for 24 h at 37°C. 17β-estradiol was
dissolved in the DMSO.
Transfection
The empty lentiviral vector (Shanghai Genechem Co.,
Ltd.) was used to infect the ADS group as the control for both
let-7a overexpression and inhibition, the let-7a overexpression
lentiviral vector GV280 (hU6-MCS-Ubiquitin-EGFP-IRES-puromycin;
Shanghai Genechem Co., Ltd.) was used to infect the ADS group as
the Lenti-GV280 group, and the let-7a inhibition lentiviral vector
GV369 (Ubi-MCS-SV40-EGFP-IRES-puromycin; Shanghai Genechem Co.,
Ltd.) was used to infect the ADS group as the Lenti-GV269 group.
Lentiviral vector was mixed with DMEM (Biological Industries) and
infected into the cells at a multiplicity of infection of 10
(1×107 TU/ml) for 12 h at 37°C after cells were grown to
a density of 30–40%. After 72 h, transfection efficiency was
observed using white light microscopy and fluorescence microscopy.
Subsequently, the cells were selected using 1 µg/ml puromycin (cat.
no. P8230; Beijing Solarbio Science & Technology Co., Ltd.)
until there were no dead cells in the culture plates. Transfected
cells were used for western blotting, RT-qPCR and cell functional
assays.
Cell Counting Kit (CCK)-8 assays
A total of 1,000 JZ SMCs/well were seeded in 96-well
plates and incubated at 37°C in 5% CO2. Then, CCK-8
solution (10 µl; cat. no. CK04; Dojindo Molecular Technologies,
Inc.) was added to each well at different time points (12, 24, 36,
48, 60, 72, 84 and 96 h) and incubated for 4 h at 37°C, according
to the manufacturer's instructions. Finally, the absorbance was
measured at 450 nm on a microplate reader
Statistical analysis
GraphPad Prism 8.4 (GraphPad Software, Inc.) was
used for data analysis. All normally distributed data are presented
as the mean ± SD. Unpaired Student's t-test or Wilcoxon signed-rank
test were used to compare differences between two groups. One-way
ANOVA was used to compare ≥3 groups. When means were significantly
different from each group as determined by ANOVA least significant
difference and Student-Newman-Keuls post hoc tests were conducted.
P<0.05 was considered to indicate a statistically significant
difference.
Results
let-7a controls the expression of
Lin28B in JZ SMCs
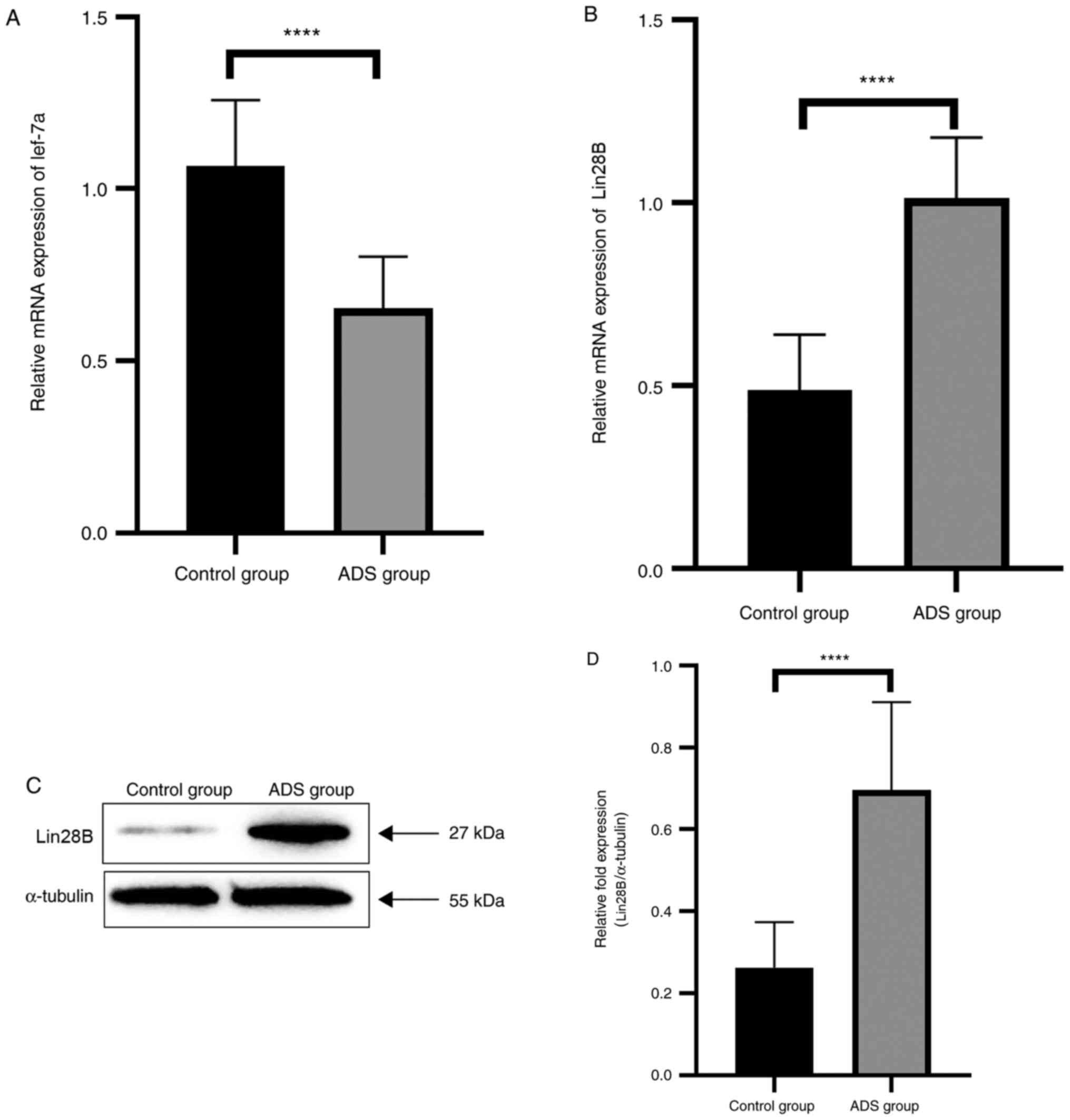
RT-qPCR results demonstrated that let-7a was
downregulated (Fig. 1A) and Lin28B
(Fig. 1B) was upregulated in ADS JZ
SMCs compared with the control JZ SMCs (both P<0.0001). Western
blot analysis also verified that the expression level of Lin28B was
higher in the ADS group compared with in the control group
(P<0.0001; Fig. 1C and D).
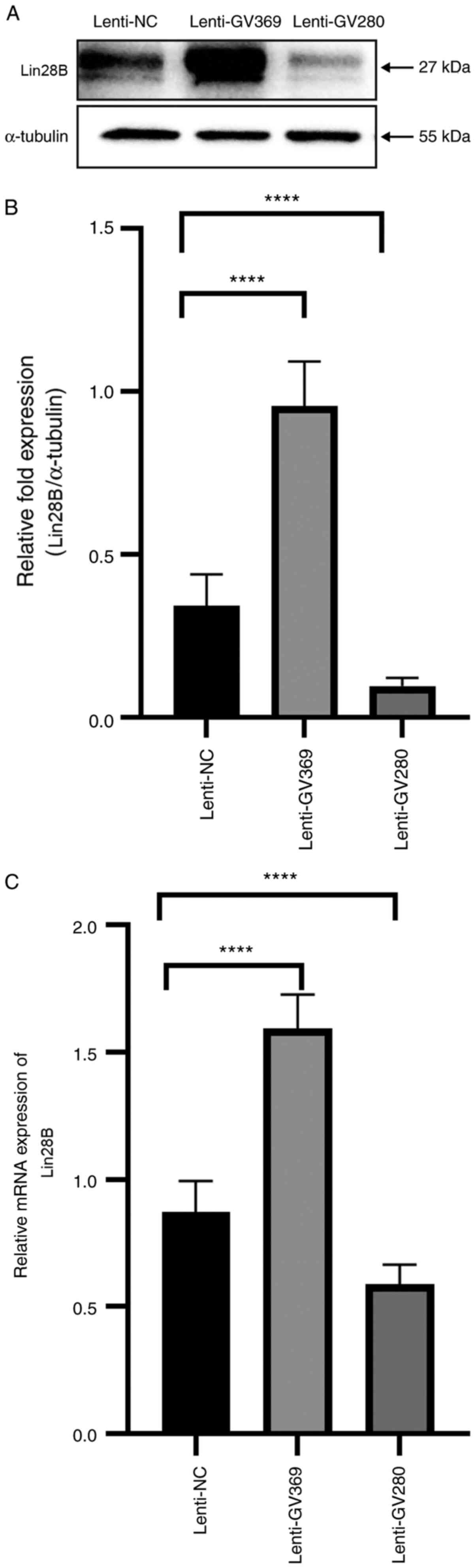
To further examine the relationship between let-7a
and Lin28B, let-7a was overexpressed and knocked down to examine
its effect the expression of Lin28B in the JZ SMCs of the ADS group
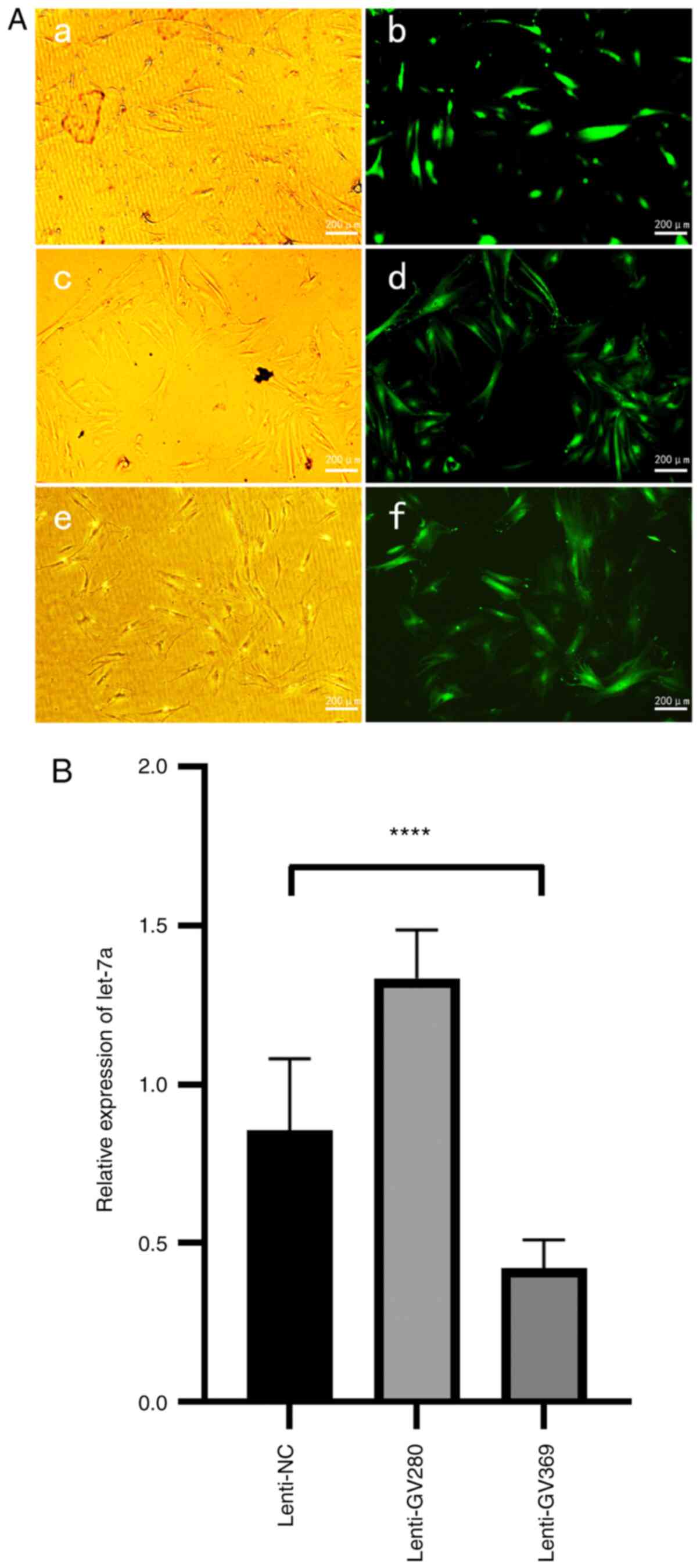
via RT-qPCR and western blotting. Fluorescence microscopy and
RT-qPCR were used to verify the transfection efficiency of the
let-7a overexpression lentiviral vector GV280 and the let-7a
inhibition lentiviral vector GV369 (Fig. 2). The results demonstrated that when
let-7a was overexpressed, Lin28B was downregulated; when let-7a was
downregulated, the expression of Lin28B was upregulated (Fig. 3). Thus, it was suggested that let-7a
controlled the expression of Lin28B in JZ SMCs.
Low expression of let-7a leads to high
proliferation of JZ SMCs
JZ SMCs transfected with the lentiviral null vector,
let-7a inhibition lentiviral vector GV369 and let-7a overexpression
lentiviral vector GV280 were used to investigate how let-7a
participated in the initiation and development of ADS. The CCK-8
results demonstrated that the lenti-GV280 cells had a significantly
lower 450 nm absorbance value compared with the lenti-NC cells
after 36 h (P<0.05; Fig. 4A),
while the lenti-GV369 cells had a higher 450 nm absorbance value
after 48 h (P<0.05 Fig. 4B).
Therefore, low expression of let-7a could accelerate the
proliferation of JZ SMCs in ADS.
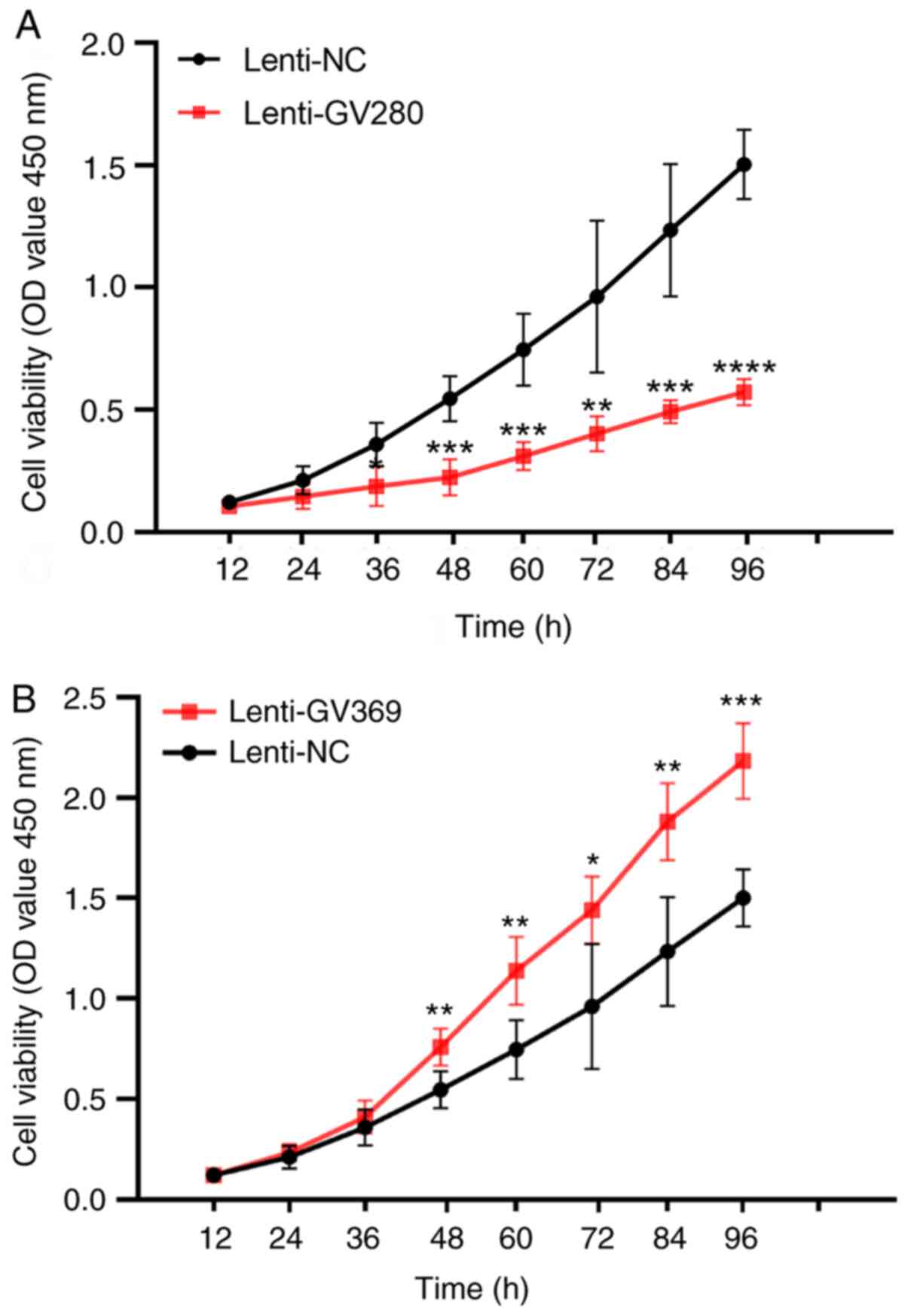 | Figure 4.Proliferation of junctional zone
smooth muscle cells in the ADS group. Cells were transfected with
the lenti-null vector, let-7a inhibition lenti-vector GV369 and
let-7a overexpression lenti-vector GV280 for 72 h, and then plated
in 96-well plates at 1,000 cells/well density and incubated at 37°C
in 5% CO2 for 12, 24, 36 48, 60, 72, 84 and 96 h. Cell
Counting Kit-8 solution (10 µl) was added to each well at different
times and incubated for 4 h. Finally, the 450 nm absorbance value
was measured on a microplate reader. (A) The 450 nm absorbance
between the cells transfected with lenti-NC and lenti-GV280 in the
ADS group. (B) The 450 nm absorbance between the cells transfected
with lenti-NC and lenti-GV369 in the ADS group. These experiments
were performed two times with three replicates in each experiment.
Significance was determined using a Student's t-test. *P<0.05,
**P<0.01, ***P<0.001, ****P<0.0001 vs. Lenti-NC. lenti-,
lentivirus; ADS, adenomyosis; NC, negative control; OD, optical
density. |
Treatment with 17β-estradiol affects
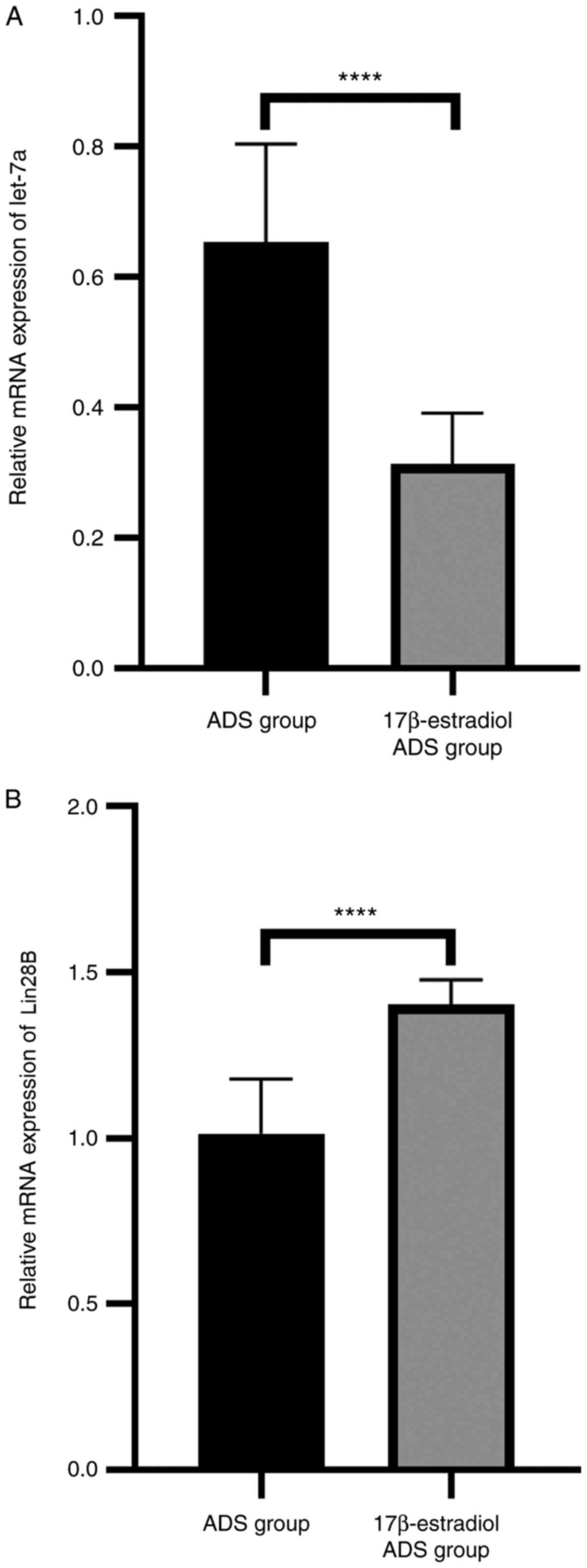
the expression of the let-7a/Lin28B axis in ADS JZ SMCs
let-7a had a lower expression level in the
17β-estradiol ADS group (P<0.0001; Fig. 5A), while the expression level of
Lin28B was higher in the 17β-estradiol ADS group compared with the
ADS group (P<0.0001; Fig. 5B).
These results indicated that 17β-estradiol may affect the
expression of the let-7a/Lin28B axis to accelerate the progression
of ADS.
Treatment with 17β-estradiol affects
the let-7a/Lin28B axis in the proliferation of JZ SMCs
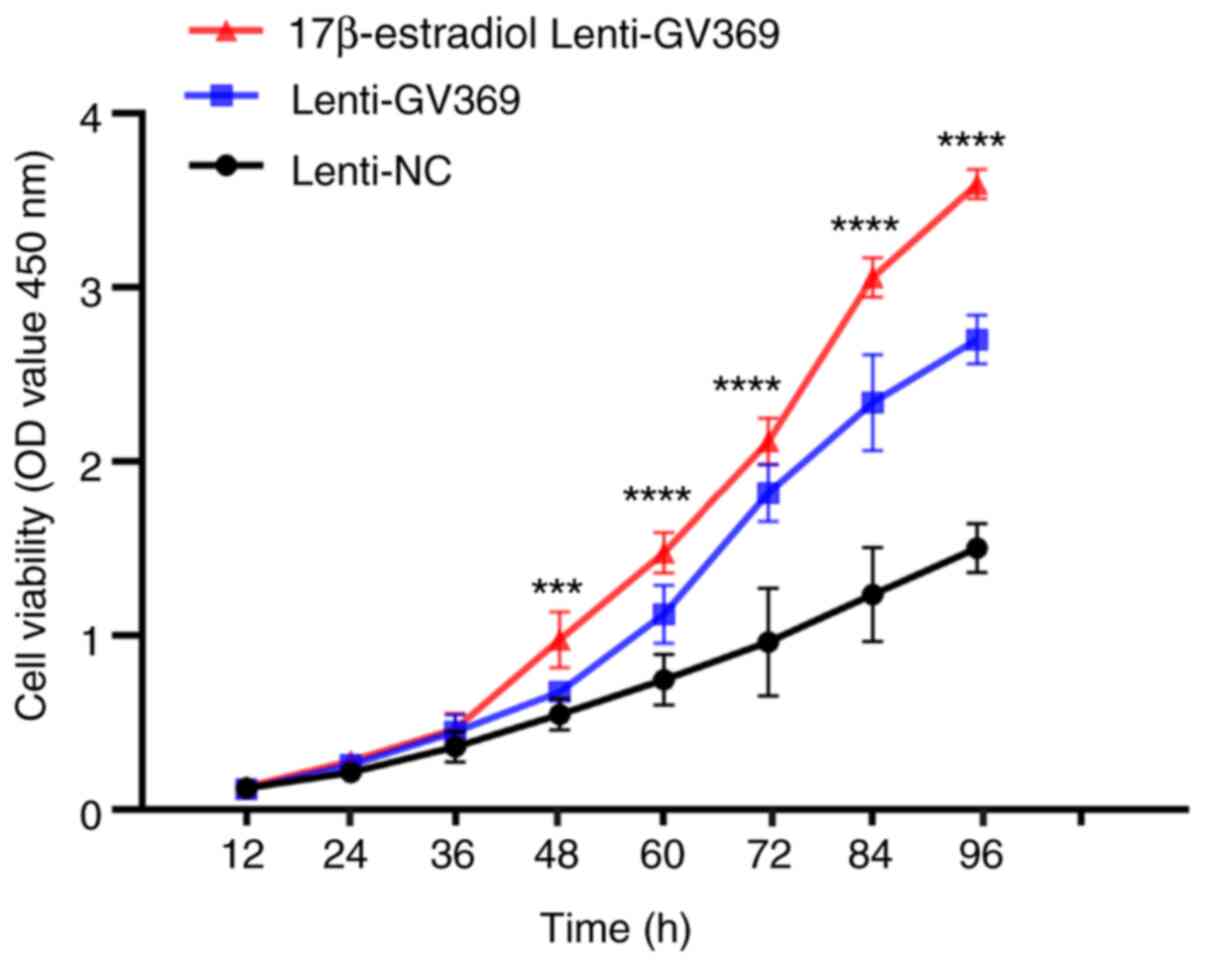
The lenti-GV369 cells had a higher proliferative
ability when exposed to 17β-estradiol compared with the lenti-GV369
cells and the lenti-NC cells; there was a significant difference
among the three groups (P<0.001; Fig. 6). These results indicated that
17β-estradiol promoted the let-7a/Lin28B axis to accelerate the
proliferation of ADS.
Discussion
To the best of our knowledge, the present study
demonstrated for first time that 17β-estradiol affects the
expression level of the let-7a/Lin28B axis to accelerate the
proliferation of JZ SMCs in ADS.
ADS is a common disease with diffuse, homogeneous,
low-signal-intensity thickening of the JZ on MRI; when it is ≥12
mm, it is possible to diagnose ADS in combination with clinical
symptoms (36). de Souza et
al (37) found that subfertile
patients with menorrhagia or dysmenorrhea had an incidence of 54%
for JZ hyperplasia. In the histological diagnosis of ADS,
hyperplastic bundles of SMCs surround the ectopic endometrial gland
stroma, and although endometrial mucosal penetration is a general
phenomenon, JZ thickening is much more extensive (38). In addition, high contraction waves
are found to originate in the JZ of the non-pregnant uterus
(9), and therefore, it is suggested
that hyperplastic SMCs lead to the disruption of JZ architecture,
further resulting in the initiation and development of ADS. The
present study demonstrated that knockdown of let-7a could
accelerate the proliferation of SMCs in the JZ. These findings
preliminarily clarified that miRNAs participate in the development
of ADS.
ADS is influenced by estrogens, and aromatase and
estrogen sulfatase are highly expressed in ADS (1). Local hyperestrogenism is an important
factor that accelerates the development of ADS. Some studies have
shown that estrogen can induce the migration, invasion and
angiogenesis of endometrial epithelial cells (39–41).
Due to the common Müllerian origin, SMCs in JZs have some
functional similarities with endometrial cells. For example, the
expression levels of ER and progesterone receptor show cyclical
changes in the JZ that are similar to that in the endometrium
(42,43). Sun et al (15) noted that E2 induced enhanced
proliferation in JZ SMCs in the ADS group via an ER-dependent
pattern via the ras homolog gene family, member A/Rho-associated
protein kinase signaling pathway. In addition, scholars have used
the oral contraceptive pill or gonadotrophin-releasing hormone
analogs to suppress ovarian activity and then observed an
indistinct appearance of the myometrial layers in postmenopausal
women on a MRI, while typical zonal anatomy reappeared in women
treated with hormone replacement therapy (44). The present study also suggests that
17β-estradiol can accelerate the proliferation of ADS JZ SMCs by
affecting the let-7a/Lin28B axis.
let-7a/Lin28B is a miRNA/protein axis that was first
discovered in the nematode Caenorhabditis elegans, and has
multiple functions in metastasis, tumorigenesis and cancer stem
cell biology (24,45,46).
let-7a has a low expression in numerous types of carcinomas, such
as laryngeal squamous cell carcinoma (47), gastric carcinoma (48), colorectal carcinoma (49) and oral squamous cell carcinoma
(50). The present results are in
accordance with this regulatory mechanism, and the expression level
of let-7a was lower in the ADS group compared with in the control
group. When let-7a was upregulated, the proliferation of the
lenti-GV280 cells was inhibited. Lin28B is an RNA-binding protein
that controls post-transcriptional processes, and has an opposite
expression pattern in embryos and adults (51). Interestingly, it has been reported
that Lin28B is upregulated in various cancer types (52–54).
The present study identified that Lin28B was upregulated in the ADS
group compared with the control group, and its expression level was
negatively correlated with let-7a in JZ SMCs in ADS.
Although ADS is a common gynecological disease, its
pathogenesis remains a difficult problem to solve, and there are
several related theories. In addition to the invagination and
metaplasia theories (55), the
tissue injury and repair (TIAR) theory (20) suggests that endometrial-myometrial
interface (EMI) microtraumatization causes tissue injury, which
subsequently upregulates COX-2 and PGE2, ultimately resulting in
increased local estrogen production. Then, elevated estrogen
activates both ERα and ERβ, leading to the induction of OT/OTR
signaling and subsequent increases in uterine peristalsis,
angiogenesis and proliferation. The increased peristalsis could
further exacerbate uterine hyperperistalsis, and thus, induce TIAR,
causing endometrial invagination and ultimately the formation of
adenomyotic lesions (18). The EMI
disruption theory (4) is a revamp
of the TIAR theory, which accounts for the genesis of ADS arising
from iatrogenic trauma such as dilatation and curettage procedures.
Iatrogenic procedures or uterine hyperperistalsis cause EMI
disruption, which leads to tissue hypoxia and activates TGF-β1,
VEGF, platelet-derived growth factor, COX-2 and stromal
cell-derived factor 1 signaling pathways, leading to enhanced
uterine peristalsis, invasion of endometrial epithelial cells and
ultimately the formation of adenomyotic lesions in the myometrium
(4). All these theories suggest
that ADS is a complicated and multipathogenic disease. Herndon
et al (56) identified 1,024
aberrantly expressed genes, 140 upregulated and 884 downregulated
genes, in ADS via microarray analysis. These genes participate in
numerous cell functions and signaling pathways, such as apoptosis,
extracellular matrix remodeling, oxidative phosphorylation and
mitochondrial dysfunction. Moreover, the present study
preliminarily demonstrated that miRNAs can control the development
of ADS from the perspective of non-coding RNAs, and endocrine
molecules can affect this process.
There are still some limitations in the present
study. First, the current study only observed the SMCs in the JZ of
ADS samples. Second, no further investigations were performed to
determine whether let-7a affects the apoptosis of JZ SMCs in ADS.
Third, for the purpose of minimizing variability, although the
posterior uterine wall is the common place where ADS occurs
(57–60), samples were collected from the
anterior fundal wall. In addition, previous studies have shown that
circular RNAs (circRNAs) act as miRNA sponges and that the
circRNA-miRNA-mRNA network may have effects on cancer-related
pathways (61–63), which the present study did not
examine. Therefore, further cell functional experiments could help
to elucidate the pathogenesis of ADS based on this point of
view.
In conclusion, the present study demonstrated that
let-7a was downregulated, whereas Lin28B was upregulated in ADS. In
addition, let-7a could affect the expression levels of Lin28B; low
expression of let-7a resulted in high Lin28B expression and high
proliferation of JZ SMCs. Treatment with 17β-estradiol also
affected the expression of the let-7a/Lin28B axis and the effects
of this axis on the proliferation of JZ SMCs in ADS. Notably,
17β-estradiol and let-7a/Lin28B axis synergistically induced the
higher proliferation of JZ SMCs in ADS.
Acknowledgements
Not applicable.
Funding
This work was funded by National Natural Science
Foundation of China (grant no. 81571412).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JHH drafted the manuscript, completed the
experiments and performed statistical analysis. HD designed the
experiment and revised the article. SW and YYW helped with
collection of tissue samples, acquisition of data and
interpretation of data. HD and SW confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethical Committee of
Clinical Research of Beijing Obstetrics and Gynecology Hospital,
Capital Medical University, China (reference no. 2016-KY-012), and
conducted according to the Declaration of Helsinki. All patients
signed informed consent before the hysterectomy.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kitawaki J: Adenomyosis: The
pathophysiology of an oestrogen-dependent disease. Best Pract Res
Clin Obstet Gynaecol. 20:493–502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu O, Schulze-Rath R, Grafton J, Hansen K,
Scholes D and Reed SD: Adenomyosis incidence, prevalence and
treatment: United States population-based study 2006–2015. Am J
Obstet Gynecol. 223:94.e1–94.e10. 2020. View Article : Google Scholar
|
|
3
|
Bird CC, McElin TW and Manalo-Estrella P:
The elusive adenomyosis of the uterus - revisited. Am J Obstet
Gynecol. 112:583–593. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo SW: The pathogenesis of adenomyosis
vis-à-vis endometriosis. J Clin Med. 9:4852020. View Article : Google Scholar
|
|
5
|
O'Shea A, Figueiredo G and Lee SI: Imaging
diagnosis of adenomyosis. Semin Reprod Med. 38:119–128. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brosens JJ, de Souza NM and Barker FG:
Uterine junctional zone: Function and disease. Lancet. 346:558–560.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Curtis KM, Hillis SD, Marchbanks PA and
Peterson HB: Disruption of the endometrial-myometrial border during
pregnancy as a risk factor for adenomyosis. Am J Obstet Gynecol.
187:543–544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo SW, Mao X, Ma Q and Liu X:
Dysmenorrhea and its severity are associated with increased uterine
contractility and overexpression of oxytocin receptor (OTR) in
women with symptomatic adenomyosis. Fertil Steril. 99:231–240.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fusi L, Cloke B and Brosens JJ: The
uterine junctional zone. Best Pract Res Clin Obstet Gynaecol.
20:479–491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanos V, Lingwood L and Balami S:
Junctional zone endometrium morphological characteristics and
functionality: Review of the literature. Gynecol Obstet Invest.
85:107–117. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imaoka I, Ascher SM, Sugimura K, Takahashi
K, Li H, Cuomo F, Simon J and Arnold LL: MR imaging of diffuse
adenomyosis changes after GnRH analog therapy. J Magn Reson
Imaging. 15:285–290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan KN, Kitajima M, Hiraki K, Fujishita
A, Nakashima M, Ishimaru T and Masuzaki H: Cell proliferation
effect of GnRH agonist on pathological lesions of women with
endometriosis, adenomyosis and uterine myoma. Hum Reprod.
25:2878–2890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Zhou L, Li TC, Duan H, Yu P and
Wang HY: Ultrastructural features of endometrial-myometrial
interface and its alteration in adenomyosis. Int J Clin Exp Pathol.
7:1469–1477. 2014.PubMed/NCBI
|
|
14
|
Wang S, Duan H, Zhang Y and Sun FQ:
Abnormal activation of RhoA/ROCK-I signaling in junctional zone
smooth muscle cells of patients with adenomyosis. Reprod Sci.
23:333–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun FQ, Duan H, Wang S, Li JJ and FQ S:
17β-estradiol induces overproliferation in adenomyotic human
uterine smooth muscle cells of the junctional zone through
hyperactivation of the estrogen receptor-enhanced RhoA/ROCK
signaling pathway. Reprod Sci. 22:1436–1444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leyendecker G, Kunz G, Wildt L, Beil D and
Deininger H: Uterine hyperperistalsis and dysperistalsis as
dysfunctions of the mechanism of rapid sperm transport in patients
with endometriosis and infertility. Hum Reprod. 11:1542–1551. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kunz G, Noe M, Herbertz M and Leyendecker
G: Uterine peristalsis during the follicular phase of the menstrual
cycle: Effects of oestrogen, antioestrogen and oxytocin. Hum Reprod
Update. 4:647–654. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leyendecker G and Wildt L: A new concept
of endometriosis and adenomyosis: Tissue injury and repair (TIAR).
Horm Mol Biol Clin Investig. 5:125–142. 2011.PubMed/NCBI
|
|
19
|
Liu X, Zou H, Zhao Y, Chen H, Liu T, Wu Z,
Yang C, Li Q and Li Y: Tanshinone inhibits NSCLC by downregulating
AURKA through Let-7a-5p. Front Genet. 11:8382020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leyendecker G, Wildt L and Mall G: The
pathophysiology of endometriosis and adenomyosis: Tissue injury and
repair. Arch Gynecol Obstet. 280:529–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang J, Lee EJ, Gusev Y and Schmittgen
TD: Real-time expression profiling of microRNA precursors in human
cancer cell lines. Nucleic Acids Res. 33:5394–5403. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang S and Baltimore D: RNA-binding
protein Lin28 in cancer and immunity. Cancer Lett. 375:108–113.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balzeau J, Menezes MR, Cao S and Hagan JP:
The LIN28/let-7 Pathway in Cancer. Front Genet. 8:312017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shyh-Chang N and Daley GQ; N S, : Lin28:
Primal regulator of growth and metabolism in stem cells. Cell Stem
Cell. 12:395–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hikasa H, Sekido Y and Suzuki A; H H, :
Merlin/NF2-Lin28B-let-7 is a tumor-suppressive pathway that is
cell-density dependent and hippo independent. Cell Rep.
14:2950–2961. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Farzaneh M, Attari F and Khoshnam SE:
Concise review: LIN28/let-7 signaling, a critical double-negative
feedback loop during pluripotency, reprogramming, and
tumorigenicity. Cell Reprogram. 19:289–293. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shamsuzzama LK, Kumar L, Haque R and Nazir
A: Role of MicroRNA Let-7 in modulating multifactorial aspect of
neurodegenerative diseases: An Overview. Mol Neurobiol.
53:2787–2793. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chien CS, Wang ML, Chu PY, Chang YL, Liu
WH, Yu CC, Lan YT, Huang PI, Lee YY, Chen YW, et al: Lin28B/Let-7
regulates expression of Oct4 and Sox2 and reprograms oral squamous
cell carcinoma cells to a stem-like state. Cancer Res.
75:2553–2565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peng F, Li TT, Wang KL, Xiao GQ, Wang JH,
Zhao HD, Kang ZJ, Fan WJ, Zhu LL, Li M, et al: H19/let-7/LIN28
reciprocal negative regulatory circuit promotes breast cancer stem
cell maintenance. Cell Death Dis. 8:e25692017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yin J, Zhao J, Hu W, Yang G, Yu H, Wang R,
Wang L, Zhang G, Fu W, Dai L, et al: Disturbance of the let-7/LIN28
double-negative feedback loop is associated with radio- and
chemo-resistance in non-small cell lung cancer. PLoS One.
12:e01727872017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cho S, Mutlu L, Grechukhina O and Taylor
HS: Circulating microRNAs as potential biomarkers for
endometriosis. Fertil Steril. 103:1252–60.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho S, Mutlu L, Zhou Y and Taylor HS:
Aromatase inhibitor regulates let-7 expression and let-7f-induced
cell migration in endometrial cells from women with endometriosis.
Fertil Steril. 106:673–680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin SL, Duan H, Wang S and Li JJ; SL L, :
Overexpression of Lin28B promoted the proliferation of adenomyotic
smooth muscle cells of the junctional zone via regulating Let-7a.
Reprod Sci. 27:1156–1163. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Reinhold C, McCarthy S, Bret PM, Mehio A,
Atri M, Zakarian R, Glaude Y, Liang L and Seymour RJ: Diffuse
adenomyosis: Comparison of endovaginal US and MR imaging with
histopathologic correlation. Radiology. 199:151–158. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de Souza NM, Brosens JJ, Schwieso JE,
Paraschos T and Winston RM: The potential value of magnetic
resonance imaging in infertility. Clin Radiol. 50:75–79. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reinhold C, Tafazoli F, Mehio A, Wang L,
Atri M, Siegelman ES and Rohoman L: Uterine adenomyosis:
Endovaginal US and MR imaging features with histopathologic
correlation. Radiographics. 19 (Suppl 1):S147–S160. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Benagiano G, Brosens I and Habiba M:
Structural and molecular features of the endomyometrium in
endometriosis and adenomyosis. Hum Reprod Update. 20:386–402. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen YJ, Li HY, Huang CH, Twu NF, Yen MS,
Wang PH, Chou TY, Liu YN, Chao KC and Yang MH: Oestrogen-induced
epithelial-mesenchymal transition of endometrial epithelial cells
contributes to the development of adenomyosis. J Pathol.
222:261–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang TS, Chen YJ, Chou TY, Chen CY, Li
HY, Huang BS, Tsai HW, Lan HY, Chang CH, Twu NF, et al:
Oestrogen-induced angiogenesis promotes adenomyosis by activating
the Slug-VEGF axis in endometrial epithelial cells. J Cell Mol Med.
18:1358–1371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Daels J: Uterine contractility patterns of
the outer and inner zones of the myometrium. Obstet Gynecol.
44:315–326. 1974.PubMed/NCBI
|
|
43
|
Brosens JJ, Barker FG and de Souza NM:
Myometrial zonal differentiation and uterine junctional zone
hyperplasia in the non-pregnant uterus. Hum Reprod Update.
4:496–502. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
McCarthy S, Tauber C and Gore J: Female
pelvic anatomy: MR assessment of variations during the menstrual
cycle and with use of oral contraceptives. Radiology. 160:119–123.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Slack FJ, Basson M, Liu Z, Ambros V,
Horvitz HR and Ruvkun G: The lin-41 RBCC gene acts in the C.
elegans heterochronic pathway between the let-7 regulatory RNA
and the LIN-29 transcription factor. Mol Cell. 5:659–669. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Moss EG, Lee RC and Ambros V: The cold
shock domain protein LIN-28 controls developmental timing in C.
elegans and is regulated by the lin-4 RNA. Cell. 88:637–646.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Luo C, Zhang J, Zhang Y, Zhang X, Chen Y
and Fan W: Low expression of miR-let-7a promotes cell growth and
invasion through the regulation of c-Myc in oral squamous cell
carcinoma. Cell Cycle. 19:1983–1993. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang Q, Jie Z, Cao H, Greenlee AR, Yang C,
Zou F and Jiang Y: Low-level expression of let-7a in gastric cancer
and its involvement in tumorigenesis by targeting RAB40C.
Carcinogenesis. 32:713–722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Re M, Magliulo G, Gioacchini FM,
Bajraktari A, Bertini A, Çeka A, Rubini C, Ferrante L, Procopio AD
and Olivieri F: Expression levels and clinical significance of
miR-21-5p, miR-let-7a, and miR-34c-5p in laryngeal squamous cell
carcinoma. BioMed Res Int. 2017:39212582017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu TP, Huang CC, Yeh KT, Ke TW, Wei PL,
Yang JR and Cheng YW: Down-regulation of let-7a-5p predicts lymph
node metastasis and prognosis in colorectal cancer: Implications
for chemotherapy. Surg Oncol. 25:429–434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Viswanathan SR and Daley GQ; SR V, :
Lin28: A microRNA regulator with a macro role. Cell. 140:445–449.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
King CE, Cuatrecasas M, Castells A,
Sepulveda AR, Lee JS and Rustgi AK: LIN28B promotes colon cancer
progression and metastasis. Cancer Res. 71:4260–4268. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
West JA, Viswanathan SR, Yabuuchi A,
Cunniff K, Takeuchi A, Park IH, Sero JE, Zhu H, Perez-Atayde A,
Frazier AL, et al: A role for Lin28 in primordial germ-cell
development and germ-cell malignancy. Nature. 460:909–913. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou J, Ng SB and Chng WJ: LIN28/LIN28B:
An emerging oncogenic driver in cancer stem cells. Int J Biochem
Cell Biol. 45:973–978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
García-Solares J, Donnez J, Donnez O and
Dolmans MM: Pathogenesis of uterine adenomyosis: Invagination or
metaplasia? Fertil Steril. 109:371–379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Herndon CN, Aghajanova L, Balayan S,
Erikson D, Barragan F, Goldfien G, Vo KC, Hawkins S and Giudice LC:
Global transcriptome abnormalities of the eutopic endometrium from
women with adenomyosis. Reprod Sci. 23:1289–1303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mijatovic V, Florijn E, Halim N, Schats R
and Hompes P: Adenomyosis has no adverse effects on IVF/ICSI
outcomes in women with endometriosis treated with long-term
pituitary down-regulation before IVF/ICSI. Eur J Obstet Gynecol
Reprod Biol. 151:62–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Levgur M: Diagnosis of adenomyosis: A
review. J Reprod Med. 52:177–193. 2007.PubMed/NCBI
|
|
59
|
Halvorsen TB and Moen MH: The extent and
clinical significance of adenomyotic lesions in the uterine wall. A
quantitative assessment. APMIS. 101:907–913. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bohlman ME, Ensor RE and Sanders RC:
Sonographic findings in adenomyosis of the uterus. AJR Am J
Roentgenol. 148:765–766. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ma HB, Yao YN, Yu JJ, Chen XX and Li HF:
Extensive profiling of circular RNAs and the potential regulatory
role of circRNA-000284 in cell proliferation and invasion of
cervical cancer via sponging miR-506. Am J Transl Res. 10:592–604.
2018.PubMed/NCBI
|
|
62
|
Guo J, Chen M, Ai G, Mao W, Li H and Zhou
J: Hsa_circ_0023404 enhances cervical cancer metastasis and
chemoresistance through VEGFA and autophagy signaling by sponging
miR-5047. Biomed Pharmacother. 115:1089572019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu J, Wang D, Long Z, Liu J and Li W:
CircRNA8924 promotes cervical cancer cell proliferation, migration
and invasion by competitively binding to MiR-518d-5p/519-5p family
and modulating the expression of CBX8. Cell Physiol Biochem.
48:173–184. 2018. View Article : Google Scholar : PubMed/NCBI
|