Introduction
Heart disease is a serious condition that threatens
human life worldwide (1,2). In 2017, the incidence of heart disease
was ~1.8% worldwide (3). Heart
failure is a type of heart disease that leads to body weakness,
such as severe fatigue, swelling of the hands and legs and
difficulty breathing. During heart failure, the blood pumped by the
heart fails to meet the requirements of the organs and tissues of
the body (4–6). Possible causes of heart failure
include coronary artery disease, valvular disease, congenital heart
disease, rhythm disorders and cardiomyopathy (7–11).
Heart transplantation is the optimal therapeutic choice for the
treatment of end-stage heart failure. However, immunological
rejection of heart allografts severely affects the survival of
patients following transplantation (12–14).
Immune tolerance has been recognized as a potential mechanism that
prevents immunological rejection (15,16).
While previous studies have suggested that transplantation
tolerance is can be induced by various stimuli in animal models and
human patients, including regulatory T cells, everolimus and
suppressors of cytokine signaling 1 (13,14,17),
immune rejection remains a major obstacle to the success of heart
transplantation (17,18). Golshayan et al (17) demonstrated that the donor-specific
Treg cells significantly delayed skin graft rejection and promoted
donor-specific transplant tolerance in CBA mice. Therefore,
understanding the molecular mechanisms of immune tolerance
following transplantation would provide an invaluable insight into
novel approaches that could be used to prevent immunological
rejection of heart allografts.
B cells servean important role in adaptive immune
responses by producing antibodies, presenting antigens to T cells
and secreting cytokines (19).
Regulatory T cells (Tregs) promote peripheral immune tolerance by
interacting with other immune cells and secreting anti-inflammatory
cytokines, such as effector T cells and TGF-β. Moreover, a subset
of B cells that exert a regulatory function and promote immune
tolerance have also been identified, and are referred to as
regulatory B cells (Bregs) (20).
Breg dysfunction is associated with autoimmune diseases, chronic
infections, cancer and organ transplant rejection (21–24). A
recent study suggested that Bregs could regulate humoral responses
that lead to immune rejection and exert a negative immunoregulatory
function similar to that of Tregs in the immune system (25). However, the involvement of Bregs in
the maintenance of immune tolerance during heart transplantation
remains poorly understood.
Histone deacetylases (HDACs) are enzymes that
catalyze the removal of acetyl from histones, and serve an
important role in immune cells by altering chromatin structure and
regulating specific transcription factors (26). HDAC inhibitors have been used as
immunomodulatory agents in B-cell-mediated autoimmune diseases, B
cell lymphomas and multiple myelomas can be treated by modulating B
cell function (27). HDAC-8
inhibitors affected the regulation of
reversion-inducing-cysteine-rich protein with kazal motifs and
altered the structure of chromosomes associated with tumorigenesis
in leukemia (28).
Trichostatin A (TSA) is a HDAC inhibitor with a
broad spectrum of epigenetic activities that regulate immune
responses in vitro and in vivo. Krajewski et
al (29) demonstrated that
epigenetic regulation of mast cell activation during immune
responses may occur via TSA modulated histone acetylation. TSA is
an organic compound that selectively inhibits the canonical class I
and II mammalian HDAC families (30). TSA alters gene expression by
interfering with HDAC function via influencing histone acetylation
levels and accessibility of DNA within chromatin to transcription
factors (31). For instance,
previous studies have revealed that TSA could inhibit sirtuin
6-mediated deacetylation of synthetic peptide substrates, as well
as of HDAC2 and HD2-type HDACs (32,33).
TSA treatment can induce the expansion of
CD4+CD25+ forkhead box P3 (Foxp3)+
Treg populations in CBA/J mice (34). Bhat et al (35) reported that HDAC inhibitors could
attenuate the antitumor cytotoxicity of γδ T cells, which
correlated with enhanced expression of the programmed death-1 in γδ
T cells (35). Previous studies
have also observed that HDAC inhibitors can also modulate the
balance of acetylation and deacetylation of non-histone proteins,
and may be responsible for a wide range of immune disorders,
including oncogenes is and immuno-inflammatory disorders (36,37).
Trichloro(dioxoethylene-o,o′)tellurate (AS101) is a
non-toxic immunomodulator that indirectly inhibits the
anti-inflammatory cytokine IL-10 (38). Moreover, AS101 inhibits survivin in
both B and T cell lymphoma via inhibition of the tumor autocrine
IL10/STAT3 signaling pathway (39).
The effect of HDAC inhibitors on B cell function and
immune tolerance following allogeneic heart transplantation are yet
to be elucidated. Thus, the aim of the present study was to
evaluate the effect of HDAC inhibitors on Breg function in a murine
model of heart transplantation. The role of
CD19+CD5+CD1dhigh Bregs in heart
transplantation-induced immune tolerance was specifically examined.
The findings of the present study may provide insights into novel
therapeutic approaches for the prevention of immunological
rejection of heart allografts.
Materials and methods
Mice
A total of 75 male, 7–9-week-old BALB/c
(Haplotype-2; H-2d) and60 C57BL/6 (H-2b) mice
(average weight 23 g) were obtained from the Animal Center of the
Second Military Medical University and housed in a sterile facility
in microisolator cages under conditions as follows: 18–25°C
temperature, 50–70% relative humidity, a 12 h light/dark cycle, and
free access to food and water. All animals received humane care in
compliance with the Principles of Laboratory Animal Care and the
Guide for the Care and Use of Laboratory Animals prepared by the
Institute of Laboratory Animal Resources and published by the
National Institutes of Health (40). All experiments were approved by the
Animal Care Committee of Zhejiang Provincial People's Hospital. A
total of 12BALB/c mice were randomly assigned to two groups: i)
Mice intraperitoneally administered with PBS (control; n=6); and
ii) mice given 1.5 mg/kg TSA intraperitoneally (n=6). TSA was
administered every other day for 4 weeks until sacrifice.
Heart transplantation and
treatment
Heart grafts from the donor C57BL/6 mice were
transplanted into the cervical region of BALB/c mice recipients.
Cessation of heartbeat was defined as the endpoint for all
experiments. Briefly, a 1.5-cm incision was made in the necks of
recipient mice on the right side. The external jugular vein was
exposed and the branches of the vein to the head were ligated. The
head end of the vein was ligated, the proximal end was clipped with
a micro vascular clamp and the vein was cut near the head end
ligature. The artery was ligated at the bifurcation of the neck and
at the external carotid artery. The artery was cut above and below
the ligation, and the lumen was washed with heparinized water. The
donor and recipient mice were intraperitoneally injected with 60
mg/kg sodium pentobarbital for anesthesia and placed on the
operating table. The abdomen was cut, and 1 ml heparin saline was
injected intravenously for 5 sec. The ribs were cut along the
bilateral anterior iliac crest, the diaphragm was cut
longitudinally and the superior atrium was freely ligated. The
distal end of the aorta was cut at the aortic bifurcation, and the
pulmonary artery was cut at the base of the left and pulmonary
bifurcation. The heart was excised free of fat. The recipient mouse
was placed on the operation table and its external jugular vein was
ligated to the donor heart and pulmonary artery, and the looper was
knotted and fixed. The same method was used to fix the recipient
common carotid artery and the donor aorta. Following surgery, the
mice were injected intraperitoneally with 50 U/g penicillin to
minimize infection and inflammation. In total, four mice were
excluded from the study, due to cessation of donor heartbeat within
a day post-transplantation.
A total of 42 mice were randomly assigned to four
groups: i) Recipient mice administered PBS (control; n=10); ii)
recipient mice given 1.5 mg/kg TSA intraperitoneally (n=12); iii)
TSA-treated recipients intraperitoneally injected with 0.5 mg/kg
AS101 (n=10); and iv) TSA-treated recipients intraperitoneally
injected with 10 mg/kg anti-CD20 monoclonal antibody (n=10). TSA,
AS101 and anti-CD20 treatments were administered every other day
post-transplantation until sacrifice. A total of 48 days
post-transplantation, the remaining recipient mice were euthanized
by cervical dislocation. The survival rate was monitored every 5
days and tissue samples were acquired following sacrifice.
Rejection was determined as complete cessation of cardiac
contractility and was confirmed by autopsy as a dark red myocardial
section, as well as expansion and deformation in the heart
cavity.
Reagents and antibodies
Anti-mouse CD3 (cat. no. 561089; PerCP; clone no.
145-2C11), CD19 (cat. no. 561739; PE-Cy7; clone no. 1D3), CD5 (cat.
no. 553020; FITC; clone no. 53-7.3), CD1d (cat. no. 562713;
PerCP-Cy5.5; clone no. 1B1), IL-10 (cat. no. 554468; APC; clone no.
JES5-16E3), CD4 (cat. no. 553051; APC; clone no. RM4-5), CD25 (cat.
no. 553072; FITC; clone no. 7D4) and Foxp3 (cat. no. 560408; PE;
clone no. MF23) antibodies, and brefeldin A (cat. no. 347688) were
obtained from BD Biosciences. Anti-mouse TGF-β1 (cat. no. IC1835P;
PE; clone no. 1D11) antibody was purchased from R&D Systems.
Anti-mouse CD20 (clone no. 5D2) was obtained from Genentech, Inc.
AS101 (cat. no. 2446; Tocris Bioscience) was dissolved in PBS at a
concentration of 50 µg/ml and stored at 4°C. TSA, phorbol myristate
acetate and ionomycin (PIM) were obtained from Sigma-Aldrich; Merck
KGaA.
Splenocyte isolation
Cell suspensions were obtained from the spleen using
a 100-µm nylon mesh. Lymphocytes were separated from the cell
suspensions using Ficoll (GE Healthcare) gradient centrifugation
(805 × g; 20 min) at room temperature. After washing with RPMI-1640
(Invitrogen; Thermo Fisher Scientific, Inc.), cells were
resuspended in RPMI-1640 medium supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.). The
CD19+CD5+CD1dhigh population was
identified via flow cytometry and cells were sorted via
fluorescence-activated cell sorting (FACS), using a FACSCanto II
flow cytometer (BD Biosciences). The FcR was blocked using Fc
Receptor Blocker (Abace Biotechnology) for 15 min at 4°C before
staining. A total of 1×106 cells were suspended in PBS
supplemented with 0.1% sodium azide (Sigma-Aldrich; Merck KGaA) and
2% FBS in 96-well plates and incubated with the following
fluorochrome-tagged antibodies at 4°C for 30 min: CD3(1:200), CD19
(1:200), CD5 (1:200) and CD1d (1:200). Flow cytometry data was
analyzed using FlowJo version 7.6 software (FlowJo, LLC).
Flow cytometry
Blood samples (500 µl per mouse) were collected from
recipient mice eyeballs upon sacrifice. Peripheral blood
mononuclear cells (PBMCs) were incubated in RPMI-1640 medium with
10 µg/ml brefeldin A at 37°C overnight. The cells were labeled with
fluorochrome-conjugated monoclonal antibodies against CD3 (1:200),
CD19 (1:200), CD5 (1:200) and CD1d (1:200) for 30 min at 4°C, fixed
and permeabilized using a Cytofix/Cytoperm kit (BD Biosciences).
The cells were washed and permeabilized in Cytofix/Cytoperm buffer
for 20 min at 4°C. The cells were the nstained with anti-IL-10 and
TGF-β1 antibodies at 4°C for 30 min.
To evaluate the effects of TSA on Tregs in
vitro, PBMCs were isolated and stimulated with 1 µg/ml anti-CD3
monoclonal antibody alone or with 50 nM TSA for 48 h at 37°C. For
CD4+CD25+Foxp3+ Treg staining,
PBMCs were incubated in RPMI-1640 medium with 10 µg/ml brefeldin A
at 37°C overnight. Cells were stained with anti-CD4 and CD25
antibodies at 4°C for 30 min. After being fixed and permeabilized
using Cytofix/Cytoperm solution, the cells were stained
intracellularly with anti-FoxP3 antibody at 4°C for 30 min. Stained
cells were run on a FACSCanto II flow cytometer (BD Biosciences).
The frequency of cytokine-expressing cells was analyzed using
FlowJo version 7.6 software (FlowJo, LLC).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from Bregsusing MiniBEST
universal RNA extraction kit (Takara Bio, Inc.) according to the
manufacturer's protocol. cDNA was synthesized by reverse
transcription at 37°C for 60 min. The expression levels of IL-10
and TGF-β1 were assessed using the SYBR PrimeScript RT-PCR kit
(Takara Bio, Inc.). The thermocycling conditions used for qPCR were
as follow: 50°C for 2 min, 95°C for 10 min followed by 30 cycles of
95°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec and a final
extension at 72°C for 5 min. GAPDH was used as endogenous control.
The following primers were used: IL-10 forward,
5′-CAGAGAAGCATGGCCCAGAA-3′ and reverse, 5′-GCTCCACTGCCTTGCTCTTA-3′;
TGF-β1 forward, 5′-CACTCCCGTGGCTTCTAGTG-3′ and reverse,
5′-GGACTGGCGAGCCTTAGTTT-3′; and GAPDH forward,
5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse,
5′-TGTAGACCATGTAGTTGAGGTCA-3′. All relative expressions were
calculated using the 2−ΔΔCq method and was reported as
fold change (41).
Western blot analysis
Bregs were lysed by incubation in RIPA lysis buffer
(Beyotime Institute of Biotechnology) containing 1.0 mM
phenylmethanesulfonyl fluoride (Sigma-Aldrich; Merck KGaA). Protein
concentrations were quantified for all samples using a BCA Protein
Assay Kit (Beyotime Institute of Biotechnology). A total of 1.5 µg
of protein sample per lane was loaded and separated using SDS-PAGE
on 10% gels, then transferred to a nitrocellulose membrane (Bio-Rad
Laboratories, Inc.). After blocking with 5% non-fat dry milk for 1
h at room temperature, the membranes were incubated with primary
antibodies against HDAC1 (1:1,000; cat. no. 5356; Cell Signaling
Technology, Inc.), IL-10 (1:1,000; cat. no. ab34843; Abcam), TGF-β1
(1:500; cat. no. ab92486; Abcam) antibody, GAPDH (1:500; cat. no.
ab9485; Abcam) at 4°C overnight. Secondary antibodies conjugated
with horseradish peroxidase (1:7,500; cat. no. 7074; Cell Signaling
Technology, Inc.) were incubated at room temperature for 1 h. The
internal reference gene GAPDH was used as control. The protein
bands were developed using enhanced chemiluminescence solution
(cat. no. ECL808-25; Biomiga Inc.). Protein signals were visualized
using the E-Gel™ Imager System with E-Gel™ Adaptor (cat. no.
4466613; Thermo Fisher Scientific, Inc.) and quantified using
ImageJ software (version 1.46; National Institutes of Health).
Carboxyfluorescein succinimidyl ester
(CFSE) staining
FACS-sorted Bregs were resuspended in pre-warmed PBS
+ 0.1% BSA (Sigma-Aldrich; Merck KGaA) at a final concentration of
1×106 cells/ml. CFSE (Invitrogen; Thermo Fisher
Scientific, Inc.) was added at a final concentration of 10 µM and
incubated at 37°C for 10 min. Following incubation, the cells were
washed with PBS and treated with 50 nM TSA or 50 ng/ml phorbol
myristate acetate + 1 µg/ml ionomycin + 10 µg/ml lipopolysaccharide
(LPS; Sigma-Aldrich; Merck KGaA) and incubated at 37°C for 3 days.
LPS + PIM treatment was used as control. CFSE was performed using
the Accuri C6 flow cytometer (BD Biosciences). Cell proliferation
was analyzed using FlowJo 7.6 software (FlowJo, LLC).
Statistical analysis
Data are presented as the mean ± SD of three
independent experiments. Data were analyzed using an unpaired
Student's t-test, or one-way ANOVA followed by Tukey's post hoc
test. The Kaplan-Meier method and a log-rank test were used to
evaluate differences in survival time between different groups.
Statistical analysis was carried out using GraphPad Prismversion
5.0 (GraphPad Software, Inc.). P<0.05 was considered to indicate
a statistically significant difference.
Results
TSA promotes
CD19+CD5+CD1dhigh Breg expansion
in mice
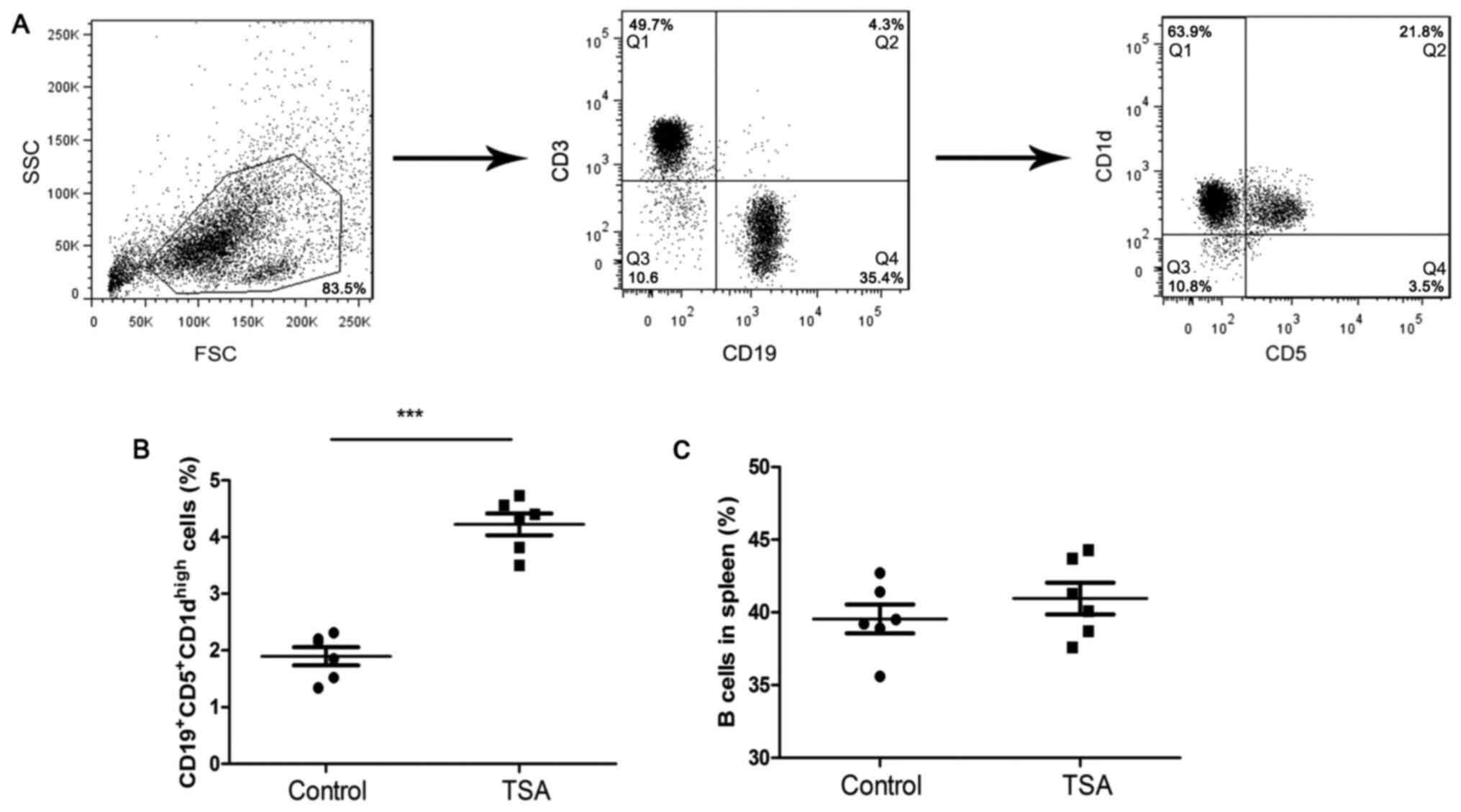
To investigate the effects of HDAC inhibitors on B
cells, mice were treated with TSA for 4 weeks. The gating strategy
for the identification of
CD19+CD5+CD1dhigh Bregs is
presented in Fig. 1A. TSA
significantly increased the frequency of
CD19+CD5+CD1dhigh Bregs, compared
with the control group (Fig. 1B).
However, the frequency of total B cells in remained unchanged
(Fig. 1C).
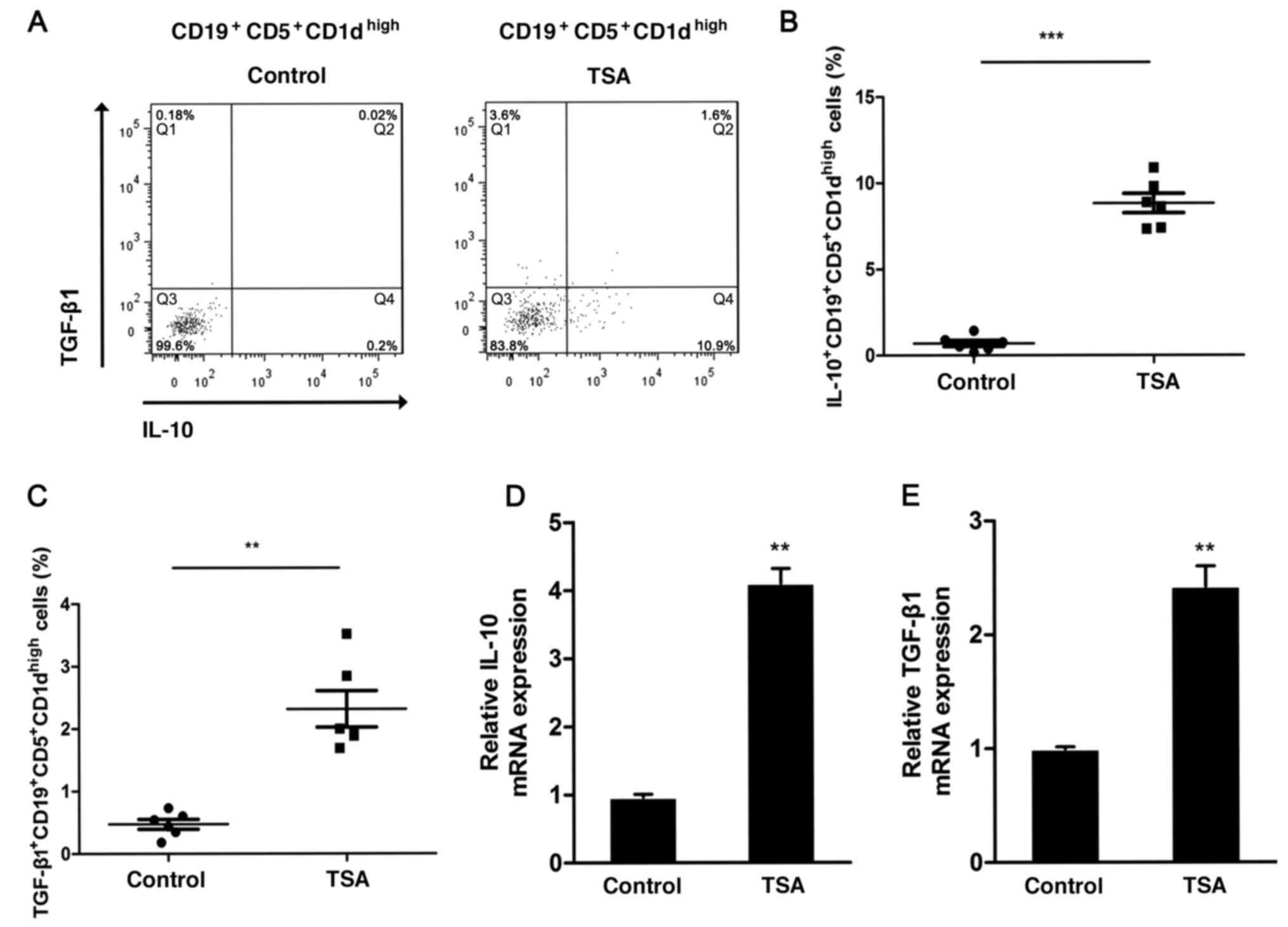
IL-10-producing
CD19+CD5+CD1dhigh Bregs can
promote immune tolerance and prevent immunological rejection
(20,21). To determine whether TSA could affect
IL1-production by Bregs, the frequency of IL-10-positive
CD19+CD5+CD1dhigh Bregs was
analyzed via flow cytometry following treatment (Fig. 2A). TSA significantly increased the
proportion of IL-10-producing
CD19+CD5+CD1dhigh Bregs (Fig. 2B). Furthermore, the expression of
TGF-β1 was also assessed. Similar to IL-10, the frequency of
TGF-β-producing CD19+CD5+CD1dhigh
Bregs increased significantly following treatment with TSA,
compared with the control group (Fig.
2C).
IL-10 mRNA expression was increased nearly 4-fold
following TSA treatment, in comparison with the control group
(Fig. 2D). The relative expression
levels of TGF-β1 also demonstrated a significant increase following
TSA treatment (Fig. 2E).
TSA activates
CD19+CD5+CD1dhigh Bregs in
vitro
In order to investigate the effect of TSA on the
proliferation of CD19+CD5+CD1dhigh
Bregs in vitro, CD19+ B cells were sorted from
total splenocytes via FACS. Relative to controls, treatment with 50
nM TSA significantly increased the proportion of
CD19+CD5+CD1dhigh Bregs in
vitro. This effect was further enhanced by combined treatment
of TSA, LPS and PIM, compared with TSA alone (Figs. 3A and S1). Moreover, the frequency of IL-10 and
TGF-β-expressing CD19+CD5+CD1dhigh
Bregs were increased following treatment with TSA, compared with
controls. Similarly, treatment with TSA, LPS and PIM had a greater
effect compared with TSA alone (Figs.
3B and C and S2). TSA also
promoted the proliferation of Bregs, compared with the control
group. The combination of TSA, LPS and PIM had a greater effect on
Breg proliferation compared with TSA treatment alone (Fig. 3D).
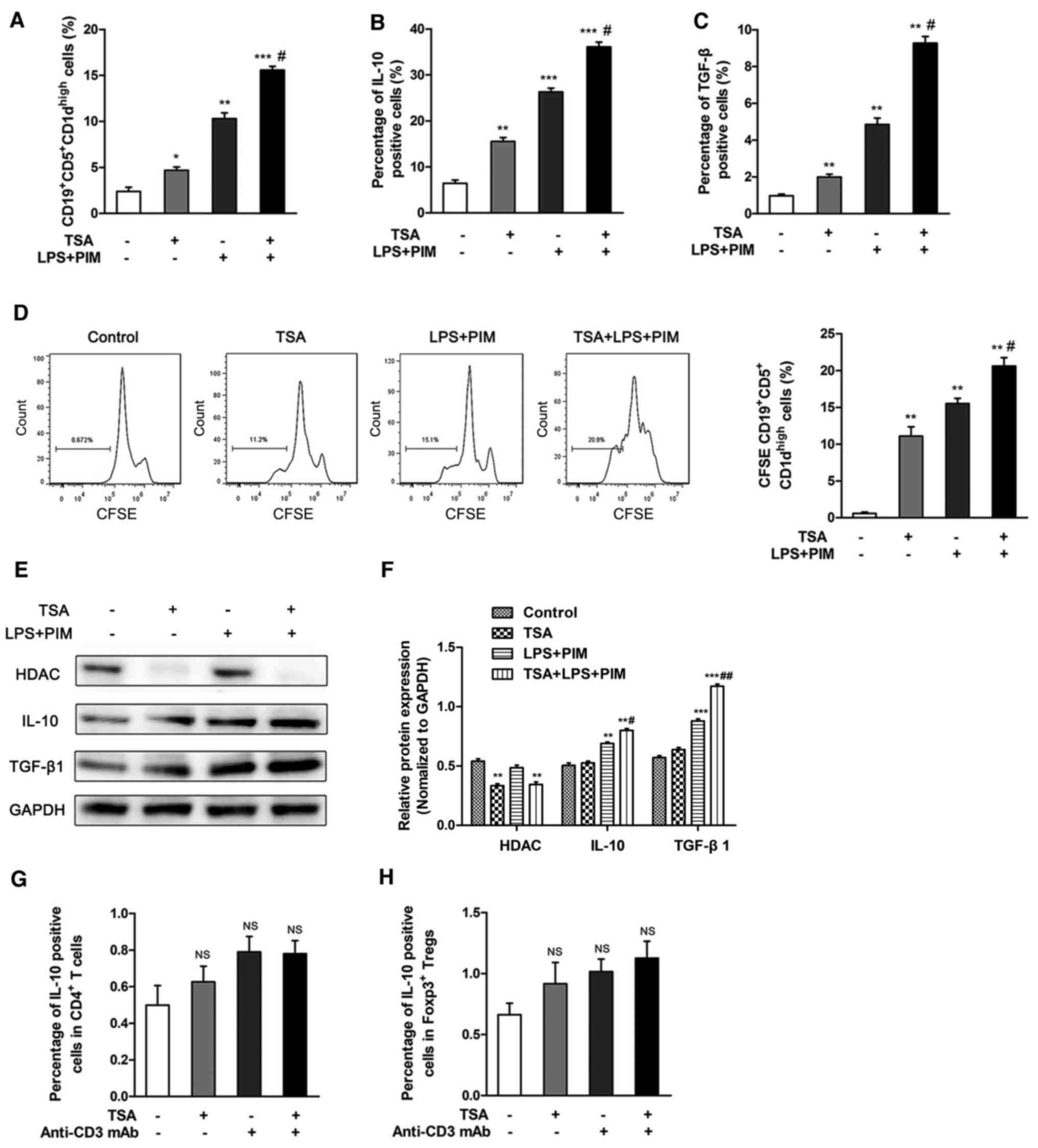 | Figure 3.TSA promotes
CD19+CD5+CD1dhigh Breg cell
proliferation in vitro. CD19+ B cells were
isolated by fluorescence-activated cell sorting, then stimulated
with LPS+PIM and/or TSA for 48 h. (A) Frequencies of
CD19+CD5+CD1dhigh Breg cells was
detected following treatment. Flow cytometry was used to detect the
frequency of (B) IL-10 and (C) TGF-β1-producing Breg cells. (D)
CD19+CD5+CD1dhigh Breg cell
proliferation was quantified using CFSE staining. (E) Protein
expression levels and (F) quantification of HDAC1, IL-10 and
TGF-β1. Peripheral blood mononuclear cells were isolated and
stimulated with anti-CD3 mAb and/or TSA for 48 h in vitro.
Flow cytometry was used to detect the positive cells proportion of
IL-10 in (G) CD4+ T cells and in (H) Foxp3+
Tregs. *P<0.05, **P<0.01, ***P<0.001 vs. control;
#P<0.05, ##P<0.01 vs. TSA. Breg,
regulatory B cell; Treg, regulatory T cell; TSA, trichostatin A;
Foxp3; Forkhead box protein p3; PIM, phorbol myristate acetate and
ionomycin; CFSE, carboxyfluorescein succinimidyl ester; HDAC,
histone deacetylase inhibitor; LPS, lipolysaccharide; mAb,
monoclonal antibody; NS, not significant. |
The protein expression levels of IL-10 and TGF-β1
were notably increased following TSA treatment. It was also
identified that IL-10 and TGF-β1 were significantly up regulated
following combined treatment of LPS and PIM. In addition, HDAC1
expression was significantly reduced following TSA treatment
(Fig. 3E and F).
In order to evaluate the effects of TSA on IL-10
expression in T cells, PBMCs were isolated and stimulated with 1
µg/ml anti-CD3 monoclonal antibody alone or with 50 nM TSA. The
gating strategy used to identify Foxp3+ Tregs is shown
in Fig. S3. TSA treatment, alone
or combined with anti-CD3 crosslinking, did not affect the
frequency of IL10-positive CD4+ T cells and
Foxp3+ Tregs, compared with untreated cells (Figs. 3G and H, S4 and S5). Collectively, these observations
suggested that the effect of TSA on IL-10 expression was specific
to the Breg subset.
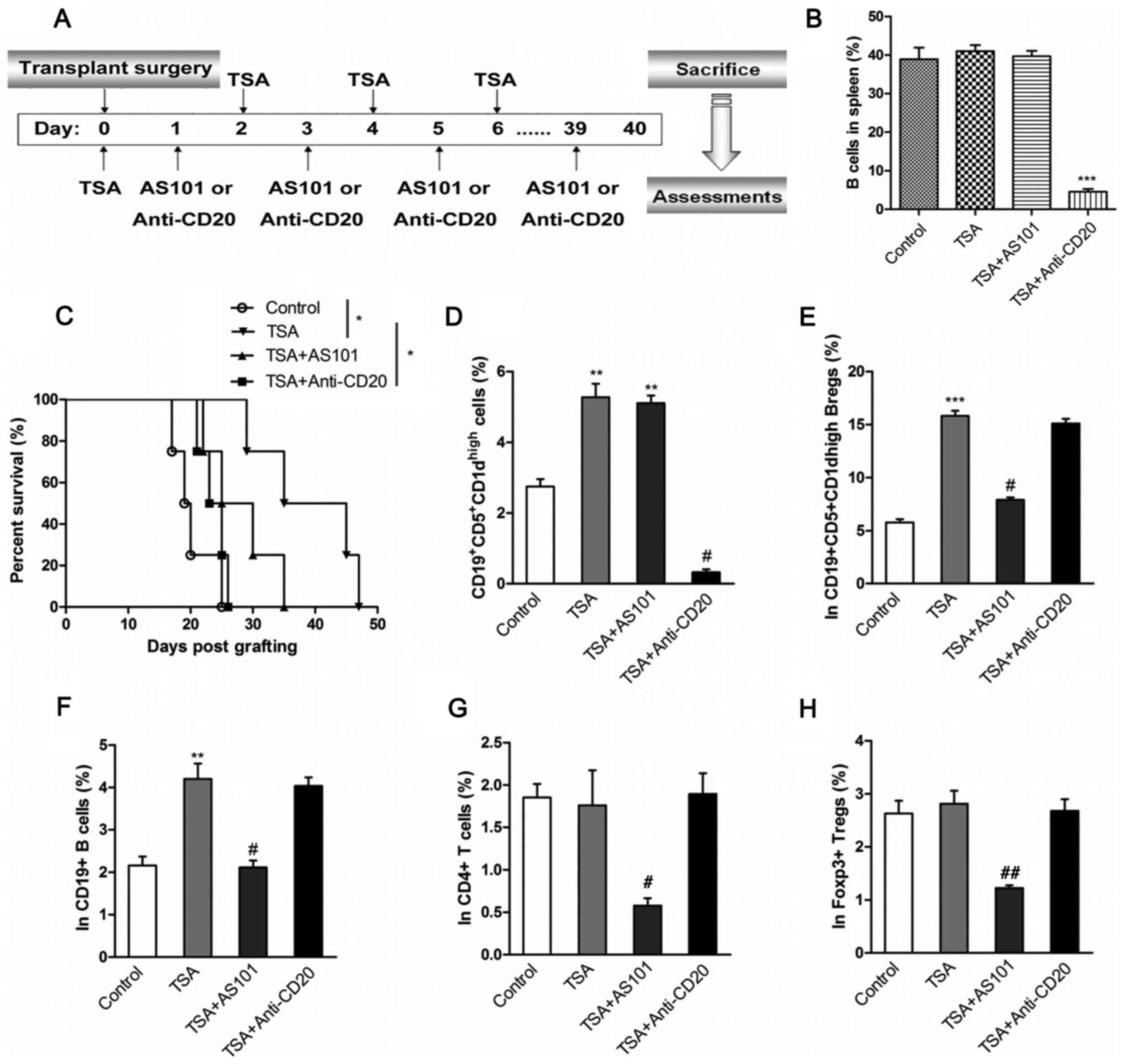
TSA increases the survival rate
following heart transplantation in mice
To further examine the effects of TSA-treated Bregs
on allograft rejection in vivo, a mouse model of heart
transplantation was established. Recipient mice were treated with
an IL10-inhibitor (AS101) or a B cell-depleting antibody
(anti-CD20), as illustrated in Fig.
4A. The effect of the anti-CD20 antibody on B cell numbers was
first assessed via flow cytometry, and it was found that anti-CD20
and TSA treatment reduced proportion of B cells to <5% of
splenocytes compared with control (Fig.
4B). The effect of TSA on survival rates were also recorded.
TSA significantly improved the survival rate of transplanted mice.
By contrast, mice co-treated with AS101 had reduced survival rates
compared with TSA-treated mice. Similarly, B cell depletion also
led to a reduction in survival rates compared with TSA alone. Thus,
the protective effects of TSA on heart allografts appear to be
mediated by B cells.
PBMCs isolated from the transplant recipients were
stimulated with PIM. The frequency of
CD19+CD5+CD1dhigh Bregs was
significantly increased in PBMCs from mice treated with TSA
compared with control mice. The combination of AS101 and TSA
demonstrated no effect on the proportion of
CD19+CD5+CD1dhigh Bregs compared
with TSA treatment alone. However, anti-CD20 and TSA administration
significantly decreased the percentage of
CD19+CD5+CD1dhigh Bregs, compared
with TSA only group (Figs. 4D and
S6). In addition, the frequency of
IL-10-expressing cells was examined in B cells, Bregs,
CD4+ T cells and Foxp3+ Tregs. TSA
significantly increased the proportion of IL-10-producing
CD19+CD5+CD1dhigh Bregs and
CD19+ B cells, compared with controls. However, the
combined administration of AS101 and TSA significantly decreased
the proportion of IL-10-producing cells, compared with TSA
treatment alone (Figs. 4E and F,
S7 and S8). Moreover, TSA, alone or combined with
anti-CD20, had no effect on IL-10 production in CD4+ T
cells and Foxp3+ Tregs (Figs. 4G and H, S9 and S10).
Discussion
The applicability of allogeneic transplantation for
the treatment of heart disease is restricted, partly due to
immunological rejection. Currently, there are no effective methods
that can induce immune tolerance of the transplanted heart
(42,43). Several therapeutic options can
reduce immunological rejection following heart transplantation,
such as the use of mesenchymal stem cells, small hairpin RNA
targeting of CD80, CD86 or Toll-like receptors in dendritic cells,
immunosuppressive drugs, immune checkpoint inhibitors and total
lymphoid irradiation (44–48). However, the clinical benefit of
these treatment strategies remains unknown and serious adverse
effects often develop. The adverse effects of immunosuppressive
regimens with calcineurin inhibitors are linked to increased
morbidity and limit the long-term survival of heart transplant
recipients (49). Therefore, the
investigation of novel therapeutic methods for immune tolerance is
urgently required, which depends on an increased understanding of
the mechanisms occurring during immunological rejection of a
transplanted heart.
B cell responses serve a critical role in
immunological rejection of transplanted organs (50). Dijke et al (51) demonstrated that B cells could
regulate cellular immunity, contribute to the genesis of tolerance
and induce accommodation. Several studies have reported that HDAC
inhibitors could regulate the B cell function in various
immunological disorders (27,52,53).
For instance, HDAC inhibitors could be used as immunomodulatory
agents in order to regulate B cell responses to allogeneic
transplantation (54). However,
despite the prominent regulatory role of HDACs in the immune
system, little is known regarding their function in the context of
heart transplantation. In the present study, the regulatory
mechanisms of the HDAC inhibitor TSA were investigated in
CD19+CD5+CD1dhigh Bregs, together
with its effects on heart transplantation. In heart-transplanted
mice, TSA significantly increased the survival rate and the
percentage of CD19+CD5+CD1dhigh
Bregs. By contrast, antibody-mediated B cell depletion
significantly decreased the survival rate. These results suggested
that HDAC inhibitors may serve an essential role in immune
tolerance by promoting the expansion of
CD19+CD5+CD1dhigh Bregs.
The HDAC inhibitor sodium valproate has been
revealed to increase the expression levels of specific
immunosuppressive cytokines, including IL-10 and TGF-β1, in human
systemic lupus erythematosus (55).
This, in turn, promotes immune tolerance via the alternative
activation of monocyte-derived macrophages in patients with
systemic lupus erythematosus (55).
Consistent with this previous study, an increase in the expression
levels of IL-10 and TGF-β1 was observed in the present study in
TSA-treated mice. Moreover, the proportion of IL-10 and
TGF-β-producing CD19+CD5+CD1dhigh
Bregs was increased following TSA treatment. Thus, TSA could
increase the levels of the immunosuppressive cytokines IL-10 and
TGF-β1 in Bregs. Similarly, TSA increased the proportion of
CD19+CD5+CD1dhigh Bregs, as well
as that of IL-10 and TGF-β-positive Bregs in vitro. This
effect was enhanced following the combined treatment of LPS and
PIM, suggesting TSA could increase LPS- and PIM-induced Bregs.
However, the frequency of IL-10-positive cells remained unchanged
in in CD4+ T and Foxp3+ Tregs.
The IL-10 inhibitor AS101 partially reduced the
frequency of Bregs, including IL-10-producing Bregs in TSA-treated,
heart-transplanted mice, suggesting that TSA stimulation may
promote immune tolerance by enhancing IL-10 expression in Bregs.
Furthermore, in the in vivo heart transplant model, AS101
reduced the survival rate and the percentage of
CD19+CD5+CD1dhigh Bregs.
Similarly, B cell depletion significantly decreased the survival
rate, suggesting that TSA-mediated
CD19+CD5+CD1dhigh Bregs may
promote immune tolerance by enhancing IL-10 expression. Although
the protective effects of TSA on heart transplant survival were
demonstrated to involve IL-10 and TGF-β1 expression in Bregs, the
molecular basis of TSA function requires further investigation.
In conclusion, TSA administration significantly
prolonged the allograft survival in a heart transplant model. The
present study demonstrated that HDAC inhibitors can promote immune
by regulating the expansion of Bregs and promoting the secretion of
immunosuppressive cytokines. The present findings provided a
potential therapeutic strategy for the prevention of immunological
rejection in cardiac transplantation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study work was supported by The Natural Science
Foundation of Zhejiang Province (grant no. LY15H100003).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BZ and YC conceived the project. BZ, FM and CW
performed the experiments. HX and ZL analyzed and interpreted the
data. BZ wrote the article and YC revised the article. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Animal Care
Committee of Zhejiang Provincial People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benjamin EJ, Virani SS, Callaway CW,
Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling
FN, Deo R, et al: Heart disease and stroke statistics-2018 update:
A report from the American heart association. Circulation.
137:e67–e492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parry J: China and Japan face epidemic of
heart disease. BMJ. 329:6432004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu WL, He JX and Shao XB: Incidence and
mortality trend of congenital heart disease at the global,
regional, and national level, 1990–2017. Medicine. 99:e205932020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi
JL, et al: 2013 ACCF/AHA guideline for the management of heart
failure: A report of the American College of Cardiology
Foundation/American Heart Association Task Force on Practice
Guidelines. J Am Coll Cardiol. 62:e147–e239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang WR, Yu CY and Yeh SJ: Fatigue and its
related factors in patients with chronic heart failure. J Clin
Nurs. 19:69–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Corra U, Pistono M, Mezzani A, Braghiroli
A, Giordano A, Lanfranchi P, Bosimini E, Gnemmi M and Giannuzzi P:
Sleep and exertional periodic breathing in chronic heart failure:
Prognostic importance and interdependence. Circulation. 113:44–50.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lala A and Desai AS: The role of coronary
artery disease in heart failure. Heart Fail Clin. 10:353–365. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Donal E, Leclercq C, Linde C and Daubert
JC: Effects of cardiac resynchronization therapy on disease
progression in chronic heart failure. Eur Heart J. 27:1018–1025.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
You H, Li Y, Wang S, Qi L and Du J:
GW28-e0697 Metabolomic profiling in relation to heart failure based
on ischemic cardiomyopathy and dilated cardiomyopathy. J Am Coll
Cardiol. 70:C25–C26. 2017. View Article : Google Scholar
|
|
10
|
Thireau J, Aimond F, Poisson D, Zhang B,
Bruneval P, Eder V, Richard S and Babuty D: New insights into
sexual dimorphism during progression of heart failure and rhythm
disorders. Endocrinology. 151:1837–1845. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vanderlaan RD, Caldarone CA and Backx PH:
Heart failure in congenital heart disease: The role of genes and
hemodynamics. Pflugers Arch. 466:1025–1035. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Popjes ED, Owens AT and Jessup M:
End-stage diastolic and systolic heart failure: Evaluation and
timing of heart transplantation. Springer; London: 2015, View Article : Google Scholar
|
|
13
|
de Weger RA: Immune regulators regulated
to prevent transplant reactions. J Am Coll Cardiol. 63:30–32. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa
J, Mancini D, Valantine-von Kaeppler HA, Starling RC, Sorensen K,
Hummel M, Lind JM, et al: Everolimus for the prevention of
allograft rejection and vasculopathy in cardiac-transplant
recipients. N Engl J Med. 349:847–858. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Waldmann H, Adams E, Fairchild P and
Cobbold S: Infectious tolerance and the long-term acceptance of
transplanted tissue. Immunol Rev. 212:301–313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Divito SJ and Morelli AE: Apoptotic Cells
for Therapy of Transplant Rejection. Springer; Netherlands: 2009,
View Article : Google Scholar
|
|
17
|
Golshayan D, Jiang S, Tsang J, Garin MI,
Mottet C and Lechler RI: In vitro-expanded donor
alloantigen-specific CD4+CD25+ regulatory T
cells promote experimental transplantation tolerance. Blood.
109:827–835. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan
X, Huang X, Markmann JF, Kassaee A, Rosengard BR and Hancock WW:
Homeostatic proliferation is a barrier to transplantation
tolerance. Nat Med. 10:87–92. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoffman W, Lakkis FG and Chalasani G: B
cells, antibodies, and more. Clin J Am Soc Nephrol. 11:137–154.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van de Veen W, Stanic B, Wirz OF, Jansen
K, Globinska A and Akdis M: Role of regulatory B cells in immune
tolerance to allergens and beyond. J Allergy Clin Immunol.
138:654–665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dambuza IM, He C, Choi JK, Yu CR, Wang R,
Mattapallil MJ, Wingfield PT, Caspi RR and Egwuagu CE: IL-12p35
induces expansion of IL-10 and IL-35-expressing regulatory B cells
and ameliorates autoimmune disease. Nat Commun. 8:7192017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gong Y, Zhao C, Zhao P, Wang M, Zhou G,
Han F, Cui Y, Qian J, Zhang H, Xiong H, et al: Role of
IL-10-producing regulatory B cells in chronic Hepatitis B virus
infection. Dig Dis Sci. 60:1308–1314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Biragyn A and Lee-Chang C: A new paradigm
for an old story: The role of regulatory B cells in cancer. Front
Immunol. 3:2062012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chu Z, Zou W, Xu Y, Sun Q and Zhao Y: The
regulatory roles of B cell subsets in transplantation. Expert Rev
Clin Immunol. 14:115–125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berthelot JM, Jamin C, Amrouche K, Le Goff
B, Maugars Y and Youinou P: Regulatory B cells play a key role in
immune system balance. Joint Bone Spine. 80:18–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Witt O, Deubzer HE, Milde T and Oehme I:
HDAC family: What are the cancer relevant targets? Cancer Lett.
277:8–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Winkler R and Kosan C: Effects of HDACi on
Immunological Functions. Springer; New York: 2017, View Article : Google Scholar
|
|
28
|
Amin SA, Adhikari N and Jha T: Is dual
inhibition of metalloenzymes HDAC-8 and MMP-2 a potential
pharmacological target to combat hematological malignancies?
Pharmacol Res. 122:8–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krajewski D, Kaczenski E, Rovatti J,
Polukort S, Thompson C, Dollard C, Ser-Dolansky J, Schneider SS,
Kinney SRM and Mathias CB: Epigenetic regulation via altered
histone acetylation results in suppression of mast cell function
and mast cell-mediated food allergic responses. Front Immunol.
9:24142018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Montagud-Romero S, Cantacorps L and
Valverde O: Histone deacetylases inhibitor trichostatin A reverses
anxiety-like symptoms and memory impairments induced by maternal
binge alcohol drinking in mice. J Psychopharmacol. 33:1573–1587.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, He G, Wang Y, Guan X, Pang X and
Zhang B: MCM-2 is a therapeutic target of Trichostatin A in colon
cancer cells. Toxicol Lett. 221:23–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wood M, Rymarchyk S, Zheng S and Cen Y:
Trichostatin A inhibits deacetylation of histone H3 and p53 by
SIRT6. Arch Biochem Biophys. 638:8–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wójcikowska B, Botor M, Morończyk J,
Wójcik AM, Nodzyński T, Karcz J and Gaj MD: Trichostatin A Triggers
an Embryogenic transition in arabidopsis explants via an
Auxin-related pathway. Front Plant Sci. 9:13532018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Yang J, Yan Y, Zhu Z, Mu Y, Wang
X, Zhang J, Liu L, Zhao F and Chi Y: Effect of adoptive transfer of
CD4+CD25+Foxp3+ Treg induced by
trichostatin A on the prevention of spontaneous abortion. J Reprod
Immunol. 131:30–35. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bhat SA, Vedpathak DM and Chiplunkar SV:
Checkpoint blockade rescues the repressive effect of histone
deacetylases inhibitors on γδ T cell function. Front Immunol.
9:16152018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Frikeche J, Peric Z, Brissot E, Grégoire
M, Gaugler B and Mohty M: Impact of HDAC inhibitors on dendritic
cell functions. Exp Hematol. 40:783–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hull EE, Montgomery MR and Leyva KJ: HDAC
inhibitors as epigenetic regulators of the immune system: Impacts
on cancer therapy and inflammatory diseases. Biomed Res Int.
2016:87972062016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hayun R, Shpungin S, Malovani H, Albeck M,
Okun E, Nir U and Sredni B: Novel involvement of the
immunomodulator AS101 in IL-10 signaling, via the tyrosine kinase
Fer. Ann NY Acad Sci. 1095:240–250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Danoch H, Kalechman Y, Albeck M, Longo DL
and Sredni B: Sensitizing B and T lymphoma cells to
Paclitaxel/Abraxane-induced death by AS101 via inhibition of the
VLA-4/IL-10/survivin axis. Mol Cancer Res. 13:411–422. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
National Institutes of Health Committee on
Care and Use of Laboratory Animals of the Institute of Laboratory
Animal Resources Commission on Life Sciences: Guide for the care
and use of laboratory animals. NIH Publication; pp. 86–23. 1985
|
|
41
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Davis CL and Hricik DE: Transplant:
Immunology and treatment of rejection. Am J Kidney Dis.
43:1116–1137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Suzuki JI, Ogawa M and Isobe M: Murine
Heart Transplantation and Graft Arterial Disease. Springer; Japan:
2016, View Article : Google Scholar
|
|
44
|
Wu GD, Nolta JA, Jin YS, Barr ML, Yu H,
Starnes VA and Cramer DV: Migration of mesenchymal stem cells to
heart allografts during chronic rejection. Transplantation.
75:679–685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hunt SA, Strober S, Hoppe RT and Stinson
EB: Total lymphoid irradiation for treatment of intractable cardiac
allograft rejection. J Heart Lung Transplant. 10:211–216.
1991.PubMed/NCBI
|
|
46
|
Yang Z, Liu Y and Zhou X: Immune
modulation by silencing CD80 and CD86 production in dendritic cells
using small hairpin RNA to reduce heart transplant rejection.
Transpl Immunol. 49:20–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang X, Beduhn M, Zheng X, Lian D, Chen
D, Li R, Siu LK, Marleau A, French PW, Ichim TE and Min WP:
Induction of alloimmune tolerance in heart transplantation through
gene silencing of TLR adaptors. Am J Transplant. 12:2675–2688.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lindenfeld J, Miller GG, Shakar SF, Zolty
R, Lowes BD, Wolfel EE, Mestroni L, Page RL II and Kobashigawa J:
Drug therapy in the heart transplant recipient: Part I: Cardiac
rejection and immunosuppressive drugs. Circulation. 110:3734–3740.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ensor CR, Goehring KC, Iasella CJ, Moore
CA, Lendermon EA, McDyer JF, Morrell MR, Sciortino CM,
Venkataramanan R and Wiland AM: Belatacept for maintenance
immunosuppression in cardiothoracic transplantation: The potential
frontier. Clin Transpl. 32:e133632018. View Article : Google Scholar
|
|
50
|
Chong AS: New insights into the
development of B cell responses: Implications for solid organ
transplantation. Human Immunol. 80:378–384. 2019. View Article : Google Scholar
|
|
51
|
Dijke EI, Platt JL, Blair P, Clatworthy
MR, Patel JK, Kfoury AG and Cascalho M: B cells in transplantation.
J Heart Lung Transplant. 35:704–710. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Locatelli SL, Cleris L, Stirparo GG,
Tartari S, Saba E, Pierdominici M, Malorni W, Carbone A, Anichini A
and Carlo-Stella C: BIM upregulation and ROS-dependent necroptosis
mediate the antitumor effects of the HDACi Givinostat and Sorafenib
in Hodgkin lymphoma cell line xenografts. Leukemia. 28:1861–1871.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
West AC, Smyth MJ and Johnstone RW: The
anticancer effects of HDAC inhibitors require the immune system.
Oncoimmunology. 3:e274142014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ye J, Li J, Zhou M, Xia R, Liu R and Yu L:
Modulation of donor-specific antibody production after organ
transplantation by valproic acid: A histone deacetylase inhibitor.
Transplantation. 100:2342–2351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mohammadi S, Saghaeian-Jazi M, Sedighi S
and Memarian A: Sodium valproate modulates immune response by
alternative activation of monocyte-derived macrophages in systemic
lupus erythematosus. Clin Rheumatol. 37:719–727. 2018. View Article : Google Scholar : PubMed/NCBI
|