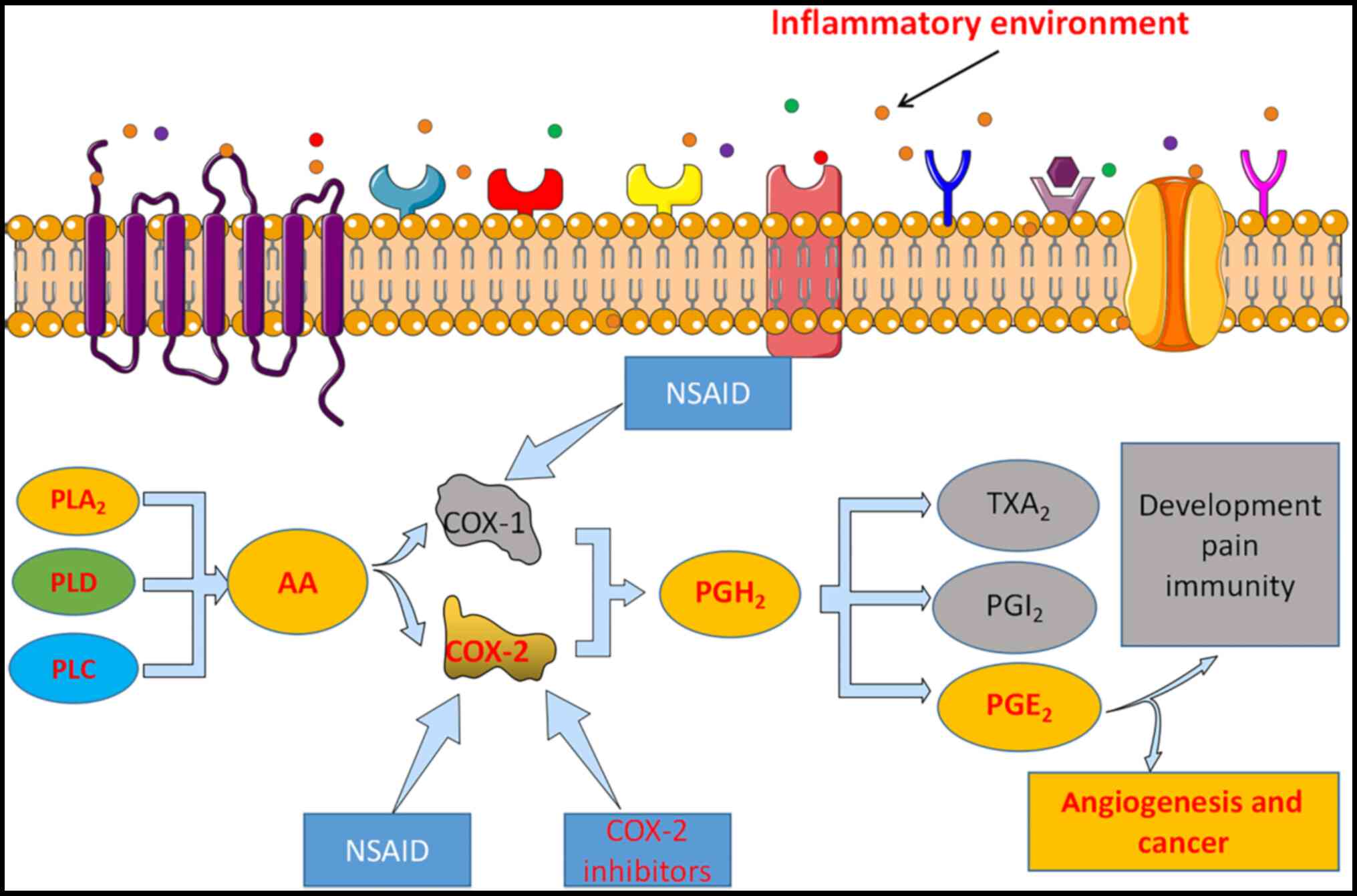
Cancer is the second leading cause of mortality
worldwide, with 18.1 million new cases and 9.6 million mortalities
reported in 2018 (1). Previous
studies have demonstrated that there is a causal association
between inflammation and carcinogenesis (2,3). In
addition, inflammation notably contributes to tumor growth,
progression, metastasis, recurrence and treatment resistance
(2). Cyclooxygenase (COX) is
classified into three isozymes: COX-1, COX-2 and COX-3 (3). COX-1 is predominantly expressed in
most tissues, such as in blood vessels, stomach and kidney, and
acts as a housekeeping enzyme during cellular homeostasis (2,3). COX-2
is constitutively expressed in certain pathological processes and
is required to produce prostaglandin E2 (PGE2), an inflammatory
mediator expressed in different types of cancer (2). COX-3 is predominantly expressed in the
spinal cord and brain (4).
Upregulated COX-2 expression is observed at inflammation sites that
predispose to cancer development (5). However, COX-2 is expressed at
relatively low levels in normal tissues adjacent to tumor tissues
(6). COX-2 is considered an
important therapeutic target for several diseases, including
cancer, autoimmune diseases and gastric inflammation (Fig. 1). Given that COX-2 can exert
pleiotropic effects on cancer development, the role of COX-2 in
tumor growth and metastasis has been investigated using
COX-2-specific microRNAs (miRNAs/miRs) (2,4).
Over the past years, miRNAs have been of great
interest in cancer research due to their prominent role as multiple
gene regulators. miRNAs are involved in every phase of malignant,
premalignant and inflammatory processes, including cytokine
production and immunity activation (7–15).
miRNAs are a group of small non-coding RNAs that control mRNA
stability and translation, and also regulate transcription
(7). Upon complementary binding to
the 3′-untralsated region (UTR) of the target mRNA, miRNAs can
translationally inhibit and degrade mRNAs, which in turn decreases
protein expression (7). Several
studies have reported that a single miRNA can bind to >200
target genes with diverse functions, and up to one third of human
mRNAs are regulated by miRNAs (8,9).
Currently, several miRNAs have been identified in humans, which are
expressed in a tissue-dependent manner (10). Active RNA-induced silencing complex
utilizes the guide strands to cleave the target mRNAs, thereby
inducing translational repression or degradation (11) and affects various cellular processes
(12). miRNAs are important
regulators of target genes responsible for cell proliferation,
apoptosis, and/or differentiation (13). Several miRNAs, such as miR-101, have
been reported to play key roles in cancer development and
progression (14). Abnormal
expression of miRNAs has been observed in different types of human
tumors, and each tumor has a distinct miRNA signature (15). Recent studies suggest that
dysregulated miRNA expression may exert detrimental effects on cell
survival, particularly in cancer cells (9,13).
The present review discusses the role of COX-2 in
cancer by targeting upregulated or downregulated miRNAs to provide
insight on the future of molecular cancer therapy.
In addition to cancer cells, the networks of
vascular cells, lymphatic endothelial cells, immune cells, stromal
cells, endothelial cells and cancer-associated fibroblasts are
alternatives but basic choices for tumor cells to invade and
survive (16). Tumor-associated
inflammation and aberrantly expressed biomarkers have been
demonstrated to play crucial roles in the cancer microenvironment.
COX-2 is released by macrophage type II cells, cancer-associated
fibroblasts and tumor cells to the cancer microenvironment
(16). In a healthy state, COX-2 is
involved in the maintenance of cellular homeostasis; however, when
homeostasis is perturbed by certain diseases, it may respond to
homeostatic dysregulation and lead to the development of cancer
(17). Several factors affect COX-2
expression (18), and its
overexpression may be explained by the mechanisms of
transcriptional and/or post-transcriptional regulation (19). The experimental results of cells and
animal models have demonstrated that overexpression of COX-2 can
inhibit tumor cell apoptosis, enhance cellular adhesion to achieve
an invasive phenotype and promote tumor-induced angiogenesis
(20,21). These theories have been confirmed in
different types of tumors, including gastric (4), lung (22), pancreas (23), bladder (24), head and neck (25) and breast cancer (26). The present review discusses the
effect of overexpressing COX-2 on the regulation of tumor growth
and carcinogenesis.
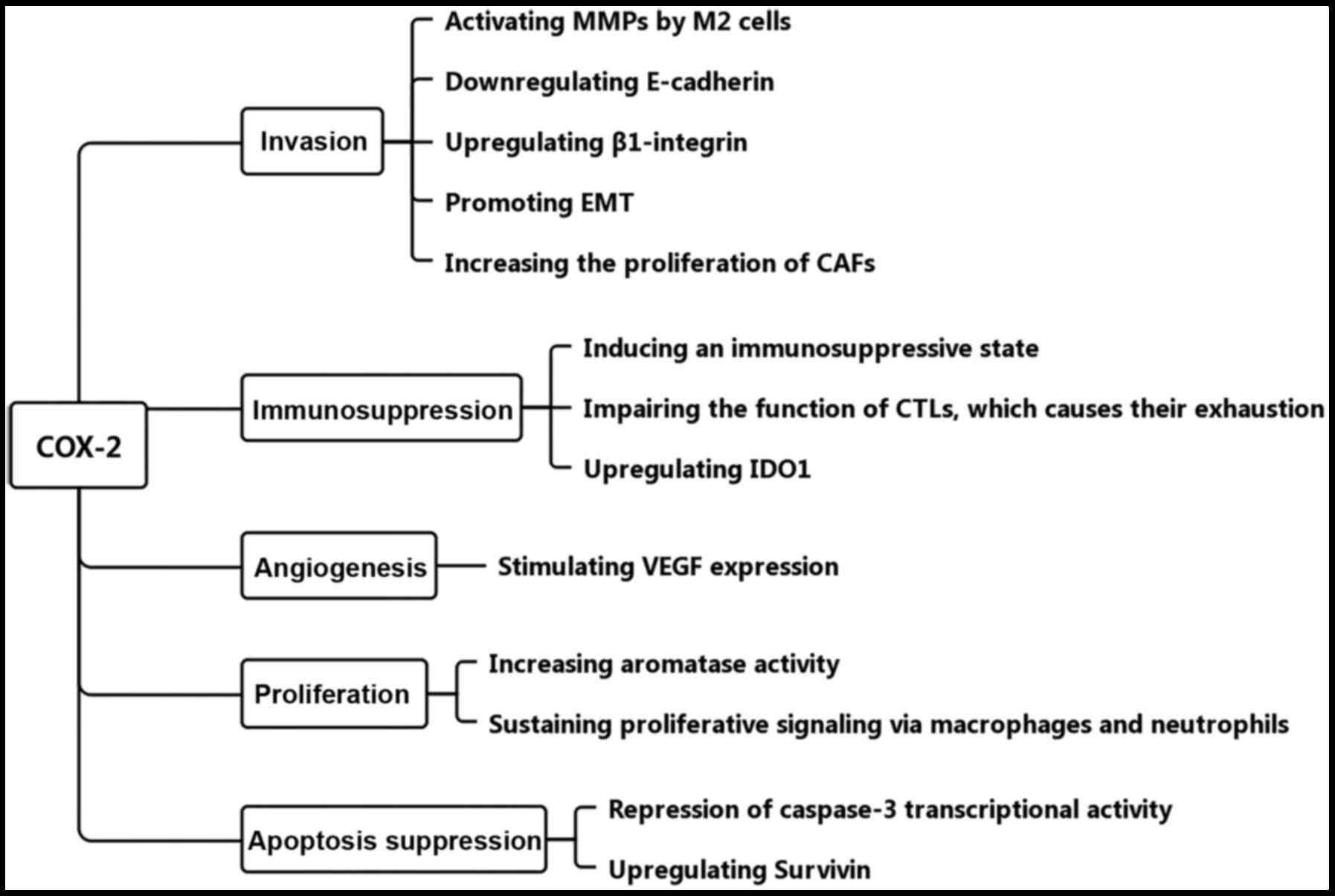
Tumors use multiple mechanisms to avoid recognition
and destruction by the immune system (41–43).
In addition to tumor development and progression, COX-2 has the
potential to alter the phenotype of tumor cells into an
immunosuppressed milieu (41), in
favor of cancer cell activation (42). In addition, COX-2/PGE2 released from
tumor cells into this milieu impairs the immune responses against
tumor-associated antigens by impairing cytotoxic T lymphocytes
(CTLs) effector functions and causing CTLs exhaustion (43). Macrophage type 2 cells, by releasing
COX-2, are involved in tumor angiogenesis, invasion and metastasis
(44). In addition, COX-2 can
modulate the actions of the immune system by constitutively
upregulating indoleamine 2,3-dioxygenase 1 expression in human
tumor cells (45). However, COX-2
knockdown significantly suppresses the degree of differentiation in
genetically modified mice bearing cutaneous cancer (46) and esophageal cancer (47). Notably, the lack of differentiation
is an important hallmark of cancer cells (17). A recent study demonstrated that
COX-2 can initiate the formation of aggressive cancer cells from
tumor-prone stem cells in mouse skin, and is involved in the
occurrence and progression of epithelial cancer cells (17).
Based on previous studies, COX-2 is considered an
inducer of different types of cancer, which exerts multiple
functions (31–45) (Fig.
2). Thus, it is essential to assess the effects of COX-2 on the
tumor microenvironment to implement effective prevention measures
for cancer. Notably, an improved understanding of the regulatory
mechanisms of COX-2 is required to facilitate the development of
novel antitumor therapies, particularly with the concomitant use of
other chemotherapeutic agents.
Cancer is a complex, multifactorial disease
characterized by uncontrolled proliferation of abnormal cells,
mainly due to oncogenes or tumor suppressor genes (48). Recent studies have highlighted the
importance of miRNAs in the development and progression of cancer,
and deregulated miRNA expression has been observed in different
types of cancer, including hepatocellular carcinoma, gastric cancer
and colorectal cancer (9,13). A significant association has been
reported between miRNAs and cancer incidence (48). miRNAs bind to their target oncogene
or tumor suppressor gene (13). As
a target of oncogenes, miR-21 is highly expressed in different
types of cancer cells (49,50) and is strongly associated with
immune-inflammatory responses (51). Conversely, as a target of tumor
suppressor genes, miR-101 inhibits the development of cancer and is
typically downregulated in tumor cells (52,53).
However, some miRNAs are often cancer specific, whereby the miRNA
is overexpressed in a specific type of tumor but is suppressed in
other types of cancer (54).
Dysregulated expression of miRNAs is associated with
increased cancer incidence, which are considered oncomiRs or
anti-oncomiRs (48). Downregulated
or upregulated expression of miRNAs can regulate carcinogenesis and
affect cell proliferation by interfering with cell cycle regulators
(48). During tumorigenesis, miRNAs
control programmed cell death in cancer cells, which in turn
affects the survival of these cells (7). It is speculated that upregulated
expression of miRNAs may inhibit different tumor suppressor genes
in cancer cells, while downregulated expression of miRNAs may
suppress oncogenic transformation in healthy tissues. In addition,
epigenetic mechanisms, such as DNA methylation and histone
modifications, can modulate the expression of miRNAs (48). Loss of transcription factor,
extragenic suppression and gene deletion can also inhibit the
expression of tumor suppressive miRNAs in cancer cells (55). However, whether abnormal miRNA
expression can induce the development of cancer, or whether it is a
consequence of this pathological state remains largely unknown.
From the therapeutic point of view, miRNAs exhibit
unique features of multi-target and effective regulation, which
holds a great promise for the development of novel antitumor drugs.
miRNAs are small and stable, and are not easily degradable by
endogenous ribonuclease when extracted from blood and feces. This
allows them to be used as promising biomarkers for early diagnosis
and prognosis, with potential reflection of treatment outcome
(56–58). miRNAs may be therapeutically
targeted in vivo (56). For
example, miR-9 serves as a promising non-invasive marker for
patients with breast cancer, which is detectable in blood, urine
and bile samples (57,58). A meta-analysis revealed that
overexpression of miR-125b predicts poor prognosis in patients with
non-small cell lung cancer (NSCLC) and prostate cancer (59), suggesting that miR-125b acts as a
potential biomarker for predicting poor clinical outcomes in
patients with cancer.
Several intracellular pathways are responsible for
the increase/decrease in COX-2 protein expression in cancer cells.
For example, miR-101 negatively modulates COX-2 protein expression,
which in turn decreases the proliferative ability of cancer cells
(60). Studies have demonstrated
that the COX-2 gene comprises several putative miRNA binding sites,
and its expression is associated with miRNA-mediated translational
repression (53,60) (Table
I). Previous studies have reported that miRNAs can bind to the
3′-UTR of the COX-2 gene, which in turn decreases its expression
(60). In addition, miR-101 exerts
suppressive effects on different types of cancer cells, and its
expression is downregulated in glioblastoma (61), esophageal squamous cell carcinoma
(62), lung cancer (63) and gastric cancer (64). Similarly, miR-101 re-expression
inhibits angiogenesis and cell proliferation of aggressive
endometrial carcinoma via COX-2 activation (53). Given that miR-101 has low or null
toxicity (60), it can serve as a
novel class of COX-2 selective inhibitor for the treatment and
prevention of cancer.
Increasing evidence suggest that COX-2 plays an
important role in gastrointestinal tumors (65–72).
miRNAs exert either oncogenic or tumor suppressive roles in
gastrointestinal tumors by regulating their target genes (66,67). A
previous study reported that miR-143 expression is markedly
downregulated in gastric cancer (GC), which is positively
associated with GC progression (68). Further analysis revealed that
miR-143 can bind to the 3′-UTR of COX-2, and COX-2 protein
expression was downregulated following transfection with miR-143
(69). Furthermore, the results of
a dual-luciferase reporter assay demonstrated that miR-144 directly
targets and suppresses COX-2 expression, thus inhibiting the
proliferation of GC cells (70).
Taken together, these findings suggest that miR-143 and miR-144 may
serve as potential diagnostic biomarkers and therapeutic targets
for patients with GC. In addition, both in vivo and in
vitro experimental results have demonstrated that miR-30a-3p
inhibits the proliferation and migration of Helicobacter
pylori-infected GC cells by targeting COX-2 mRNA (71). Furthermore, miR-137 suppresses COX-2
expression, and upregulated expression may decrease the aggressive
properties of cancer cells (72).
Collectively, these findings suggest a strong association between
miR-137, miR-143, miR-144, miR-101, miR-30a-3p and COX-2 expression
in GC, which can help further understand the role of miRNAs in GC
by targeting COX-2.
In most cases, chronic liver inflammation and the
inflammation-associated microenvironment can promote the initiation
and progression of hepatocellular carcinoma (HCC) (76). The COX-2/PGE2 pathway plays an
essential role in mediating the pathophysiology of liver diseases,
including cirrhosis and HCC (76).
A previous study demonstrated that miR-16 directly silences COX-2
expression in HCC cells and indirectly through downregulation of
human antigen R (77). In addition,
miR-16 suppresses cell proliferation and induces cell apoptosis in
HCC cell lines by downregulating COX-2 expression (77). Notably, there is no significant
association between miR-101 and COX-2 expression in HCC. This may
be due to the tumor tissue-specific expression of miRNAs. The
latest report indicates that miR-136 expression is markedly
downregulated in HCC cells and tissues, and negatively associated
with COX-2 mRNA expression (78).
Taken together, these results suggest that miR-136 plays a key role
in regulating HCC cell proliferation and metastasis by targeting
COX-2.
Aberrant angiogenesis is associated with cancer
progression and metastasis, and is mediated by miR-101. Upregulated
miR-101 expression can slow tumor growth, the effects of which are
reversed following downregulation of miR-101 expression (53). A previous study reported that
miR-101 regulates abnormal angiogenesis in endometrial cancer via
COX-2 (53). COX-2 expression has
been observed in nearly 40% of patients with primary breast cancer,
at both pre-invasive and invasive stages of the disease. In
addition, COX-2 expression is significantly associated with breast
cancer progression (84,85). Through downregulation of phosphatase
and tensin homolog deleted on chromosome 10, miR-221/222 induces
AKT phosphorylation and subsequently activates the COX-2 gene,
which increases COX-2 expression in cancer cells (84). Thus, miR-221/222 induces tumor
growth and maintains breast cancer stem-like characteristics by
upregulating COX-2 expression (84). Notably, overexpression of COX-2
upregulates the expression levels of miR-526b and miR-655 in breast
cancer cell lines (85).
Furthermore, miR-526b expression is upregulated via the COX-2 and
EP4 pathways in high-grade primary breast tumors (39). According to the gonadotropin theory,
ovarian cancer commonly occurs in postmenopausal women, mainly due
to the high levels of follicle-stimulating hormone and luteinizing
hormone resulting from the negative feedback of estrogen (86). Follicle-stimulating hormone
increases miR-27a expression, which in turn increases the
expression levels of COX-2, survivin and vascular endothelial
growth factor via the ZBTB10-specificity protein pathway (87).
Glioma is the most common type of brain tumor.
Downregulated miR-128/26b expression and upregulated COX-2
expression have been detected in glioma tissues (88,89).
Transfection with miR-128 and miR-26b mimics decreases the
luciferase activity associated with the 3′-UTR of COX-2, and
miR-128 notably decreases the stability of COX-2 mRNA. Conversely,
transfection with miR-128 inhibitor markedly increases COX-2 mRNA
expression, as well as the protein expression levels of ki67 and
MMP9, while promoting the growth of glioma tumors (88,89).
The direct association between COX-2 and miR-128/26b has been
verified by publicly available data (88,89).
Retinoblastoma is the most common type of eye cancer, which
accounts for high mortality rates in young children (90). Bioinformatic analysis has
demonstrated that miR-137 negatively modulates COX-2 expression and
PGE2 production in retinoblastoma cells (91). Targeted COX-2 knockdown inhibits the
invasion and increases the proliferation of retinoblastoma cells,
while inhibiting the synthesis of PGE2 (91). Bladder cancer is a common malignancy
of the urinary tract and the fifth most diagnosed cancer type in
Western countries (92). The latest
report suggests that miR-143 negatively regulates COX-2 expression
in bladder carcinoma T24 cells (93). In addition, it has been reported
that the role of miR-143 in tumor growth and migration is mainly
due to its involvement in the COX-2 pathway (93). Laryngeal cancer is the most common
type of cancer in the head and neck area (94). A previous study revealed that
miR-203 expression was notably downregulated in laryngeal squamous
cell carcinoma tissues (94).
Collectively, this finding suggest that miR-203 acts as a tumor
suppressor in laryngeal squamous cell carcinoma, partially by
regulating COX-2 expression.
Currently, there are three methods used to inhibit
COX-2 expression, post-transcriptional control, inhibitory
transcription factors and COX-2 inhibitors. Celecoxib was the first
COX-2 inhibitor approved by the FDA. This drug has been used for
over 20 years as an anti-inflammatory, analgesic and antipyretic
agent (95). Given the role of
inflammation in carcinogenesis, celecoxib has gained a novel
opportunity for its application (95). The antitumor and chemoprevention
effects of celecoxib on colon carcinogenesis were first
demonstrated in rats (96), and
later in different in vivo experimental models (97).
Celecoxib inhibits the migration, invasion and EMT
of bladder cancer cells, partially by regulating the
miR-145/TGFBR2/Smad3 pathway (98).
In addition, the concomitant use of celecoxib and miR-145 mimic
notably inhibits the migration and invasion of bladder cancer cells
(98). The results of other
experiments also suggest that celecoxib increases miR-146a
expression in high-risk human papillomavirus (HPV) (99). Another miRNA investigated in this
experiment was miR-150. miR-150 is positively mediated by NF-kB
(99), a common transcription
factor expressed in HPV-related cancer types (100). In this experiment, celecoxib also
downregulated the NF-kB pathway via miR-150. Taken together, these
findings suggest that the antitumor effect of celecoxib against
HPV-induced lesions is partly mediated by upregulating miR-146a and
miR-150 expression (101).
Furthermore, miRNA microarray analysis has demonstrated that
miR-29c expression is markedly higher in GC tissues compared with
normal gastric mucosa, and celecoxib can promote miR-29c expression
in GC cells (102). In addition,
Mcl-1 is a target of miR-29, which encodes Bcl-2-like antiapoptotic
proteins (103). A previous study
reported that miR-29 regulates cell apoptosis by targeting Mcl-1
(103). Celecoxib increases
miR-29c expression and inhibits its target oncogene, Mcl-1,
resulting in the apoptosis of GC cells (102). In addition, miR526b/miR655
expression is significantly higher in breast tumors, and the
interplay between COX-2 and hypoxia has been demonstrated to
promote tumor aggression (104).
Previous studies have demonstrated that celecoxib regulates
hypoxia-enhanced function in breast cancer cells by downregulating
miR526b/miR655 expression (104,105).
Parecoxib is another important selective COX-2
inhibitor, with high postoperative pain control and less side
effects (106). Treatment with
parecoxib has exhibited a promising anticancer role in different
types of human cancer (106,107). The latest research suggests that
parecoxib inhibits the proliferation, migration and invasion of
glioblastoma cells by upregulating miR-29c expression (108).
Despite the extensive use of COX-2 inhibitors in the
treatment of cancer, their application is limited due to the
associated adverse events. The most reported side effect of
celecoxib is the increased frequency of cardiovascular disorders
following its long-term use (109). Considering that
post-transcriptional control may exert a more appreciable effect,
it has attracted great interest. Although the expression patterns
of miRNAs are not yet fully understood in human cells, their
functional roles represent one of the most exciting topics for
elucidating the molecular mechanisms underlying the therapeutic
effects of COX-2 inhibitors (Table
II).
Based on the current literature regarding
miRNA-mediated COX-2 regulation, a novel COX-2 selective inhibitor
may be developed with miRNAs. It was suggested that anti-COX-2
miRNAs may serve as novel targets for the treatment of cancer. In
addition, small interfering (si)RNAs, instead of miRNAs, may also
be used to inhibit COX-2 expression via similar molecular
mechanisms (97). However,
off-target effects may exist due to mRNA destabilization by
identical siRNA sequences, thus suppressing other mRNAs with
partial complementarity (96).
Taken together, the results discussed here suggest that miRNA-based
strategies hold great promise for inhibiting COX-2 expression, and
thus may be used to treat cancer types overexpressing COX-2.
Not applicable.
The present review was supported by the Chinese
National Science Foundation (grant no. 81172210) and the China
Postdoctoral Science Foundation (grant no. 2012M521528).
Not applicable.
WGH and BYW conceived the present review. ZXG
performed the literature review and revised the manuscript for
important intellectual content. NL prepared the figures. SKL, WJL
and QZ interpreted the table data. ZXG and BYW confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piotrowski I, Kulcenty K and Suchorska W:
Interplay between inflammation and cancer. Rep Pract Oncol
Radiother. 25:422–427. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beeghly-Fadiel A, Wilson AJ, Keene S,
Ramahi M, Xu S, Marnett LJ, Fadare O, Crispens MA and Khabele D:
Differential cyclooxygenase expression levels and survival
associations in type I and type II ovarian tumors. J Ovarian Res.
11:172018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ayiomamitis GD, Notas G, Vasilakaki T,
Tsavari A, Vederaki S, Theodosopoulos T, Kouroumalis E and
Zaravinos A: Understanding the Interplay between COX-2 and hTERT in
colorectal cancer using a multi-omics analysis. Cancers (Basel).
11:15362019. View Article : Google Scholar
|
|
5
|
Pollock JK, Greene LM, Nathwani SM,
Kinsella P, O'Boyle NM, Meegan MJ and Zisterer DM: Involvement of
NF-κB in mediating the anti-tumour effects of combretastatins in T
cells. Invest New Drugs. 36:523–535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gurram B, Zhang S, Li M, Li H, Xie Y, Cui
H, Du J, Fan J, Wang J and Peng X: Celecoxib conjugated fluorescent
probe for identification and discrimination of cyclooxygenase-2
enzyme in cancer cells. Anal Chem. 90:5187–5193. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O'Brien J, Hayder H, Zayed Y and Peng C:
Overview of MicroRNA biogenesis, mechanisms of actions, and
circulation. Front Endocrinol (Lausanne). 9:4022018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esquela-Kerscher A and Slack FJ: Oncomirs
microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun X, Ge X, Xu Z and Chen D:
Identification of circular RNA-microRNA-messenger RNA regulatory
network in hepatocellular carcinoma by integrated analysis. J
Gastroenterol Hepatol. 35:157–164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Griffiths-Jones S, Saini HK, van Dongen S
and Enright AJ: miRBase: Tools for microRNA genomics. Nucleic Acids
Res. 36:D154–D158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim B, Jeong K and Kim VN: Genome-wide
mapping of DROSHA cleavage sites on primary MicroRNAs and
noncanonical substrates. Mol Cell. 66:258–269.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Babaei K, Shams S, Keymoradzadeh A, Vahidi
S, Hamami P, Khaksar R, Norollahi SE and Samadani AA: An insight of
microRNAs performance in carcinogenesis and tumorigenesis; an
overview of cancer therapy. Life Sci. 240:1170772020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J,
Li J, Li F and Tan HB: Immune cells within the tumor
microenvironment: Biological functions and roles in cancer
immunotherapy. Cancer Lett. 470:126–133. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moon H, White AC and Borowsky AD: New
insights into the functions of Cox-2 in skin and esophageal
malignancies. Exp Mol Med. 52:538–547. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han F, Ren J, Zhang J, Sun Y, Ma F, Liu Z,
Yu H, Jia J and Li W: JMJD2B is required for Helicobacter
pylori-induced gastric carcinogenesis via regulating COX-2
expression. Oncotarget. 7:38626–38637. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Borchert GL, Surazynski A and Phang
JM: Proline oxidase, a p53-induced gene, targets COX-2/PGE2
signaling to induce apoptosis and inhibit tumor growth in
colorectal cancers. Oncogene. 27:6729–6737. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hashemi Goradel N, Najafi M, Salehi E,
Farhood B and Mortezaee K: Cyclooxygenase-2 in cancer: A review. J
Cell Physiol. 234:5683–5699. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Montezuma MAP, Fonseca FP, Benites BM,
Soares CD, do Amaral-Silva GK, de Almeida OP, Soares FA, Pagano RL
and Fregnani ER: COX-2 as a determinant of lower disease-free
survival for patients affected by ameloblastoma. Pathol Res Pract.
214:907–913. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han L, Fang S, Li G, Wang M and Yu R:
Total flavonoids suppress lung cancer growth via the COX-2-mediated
Wnt/β-catenin signaling pathway. Oncol Lett. 19:1824–1830.
2020.PubMed/NCBI
|
|
23
|
Conejo-Garcia JR: Breaking barriers for T
cells by targeting the EPHA2/TGF-β/COX-2 axis in pancreatic cancer.
J Clin Invest. 129:3521–3523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bourn J, Pandey S, Uddin J, Marnett L and
Cekanova M: Detection of tyrosine kinase inhibitors-induced COX-2
expression in bladder cancer by fluorocoxib A. Oncotarget.
10:5168–5180. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu Y, Shi C, Zeng L, Liu G, Jiang W,
Zhang X, Chen S, Guo J, Jian X, Ouyang J, et al: High COX-2
expression in cancer-associated fibiroblasts contributes to poor
survival and promotes migration and invasiveness in nasopharyngeal
carcinoma. Mol Carcinog. 59:265–280. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng Y, Wang Y, Tang N, Sun D, Lan Y, Yu
Z, Zhao X, Feng L, Zhang B, Jin L, et al: Andrographolide inhibits
breast cancer through suppressing COX-2 expression and angiogenesis
via inactivation of p300 signaling and VEGF pathway. J Exp Clin
Cancer Res. 37:2482018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Y, Zhu J, Gou H, Cao D, Jiang M and
Hou M: Clinical significance of Cox-2, Survivin and Bcl-2
expression in hepatocellular carcinoma (HCC). Med Oncol.
28:796–803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Garrido MP, Hurtado I,
Valenzuela-Valderrama M, Salvatierra R, Hernández A, Vega M, Selman
A, Quest AFG and Romero C: NGF-enhanced vasculogenic properties of
epithelial ovarian cancer cells is reduced by inhibition of the
COX-2/PGE2 signaling Axis. Cancers (Basel). 11:19702019. View Article : Google Scholar
|
|
29
|
Hosseini F, Mahdian-Shakib A,
Jadidi-Niaragh F, Enderami SE, Mohammadi H, Hemmatzadeh M, Mohammed
HA, Anissian A, Kokhaei P, Mirshafiey A and Hassannia H:
Anti-inflammatory and anti-tumor effects of α-l-guluronic acid
(G2013) on cancer-related inflammation in a murine breast cancer
model. Biomed Pharmacother. 98:793–800. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Janakiraman H, House RP, Talwar S,
Courtney SM, Hazard ES, Hardiman G, Mehrotra S, Howe PH, Gangaraju
V and Palanisamy V: Repression of caspase-3 and RNA-binding protein
HuR cleavage by cyclooxygenase-2 promotes drug resistance in oral
squamous cell carcinoma. Oncogene. 36:3137–3148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raj V, Bhadauria AS, Singh AK, Kumar U,
Rai A, Keshari AK, Kumar P, Kumar D, Maity B, Nath S, et al: Novel
1,3,4-thiadiazoles inhibit colorectal cancer via blockade of
IL-6/COX-2 mediated JAK2/STAT3 signals as evidenced through
data-based mathematical modeling. Cytokine. 118:144–159. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krishnamachary B, Stasinopoulos I, Kakkad
S, Penet MF, Jacob D, Wildes F, Mironchik Y, Pathak AP, Solaiyappan
M and Bhujwalla ZM: Breast cancer cell cyclooxygenase-2 expression
alters extracellular matrix structure and function and numbers of
cancer associated fibroblasts. Oncotarget. 8:17981–17994. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Esbona K, Yi Y, Saha S, Yu M, Van Doorn
RR, Conklin MW, Graham DS, Wisinski KB, Ponik SM, Eliceiri KW, et
al: The presence of cyclooxygenase 2, tumor-associated macrophages,
and collagen alignment as prognostic markers for invasive breast
carcinoma patients. Am J Pathol. 188:559–573. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Esbona K, Inman D, Saha S, Jeffery J,
Schedin P, Wilke L and Keely P: COX-2 modulates mammary tumor
progression in response to collagen density. Breast Cancer Res.
18:352016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hull MA, Cuthbert RJ, Ko CWS, Scott DJ,
Cartwright EJ, Hawcroft G, Perry SL, Ingram N, Carr IM, Markham AF,
et al: Paracrine cyclooxygenase-2 activity by macrophages drives
colorectal adenoma progression in the ApcMin/+ mouse
model of intestinal tumorigenesis. Sci Rep. 7:60742017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Watanabe Y, Imanishi Y, Ozawa H, Sakamoto
K, Fujii R, Shigetomi S, Habu N, Otsuka K, Sato Y, Sekimizu M, et
al: Selective EP2 and Cox-2 inhibition suppresses cell migration by
reversing epithelial-to-mesenchymal transition and Cox-2
overexpression and E-cadherin downregulation are implicated in neck
metastasis of hypopharyngeal cancer. Am J Transl Res. 12:1096–1113.
2020.PubMed/NCBI
|
|
37
|
Sorski L, Melamed R, Matzner P, Lavon H,
Shaashua L, Rosenne E and Ben-Eliyahu S: Reducing liver metastases
of colon cancer in the context of extensive and minor surgeries
through beta-adrenoceptors blockade and COX2 inhibition. Brain
Behav Immun. 58:91–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Soto MS, O'Brien ER, Andreou K, Scrace SF,
Zakaria R, Jenkinson MD, O'Neill E and Sibson NR: Disruption of
tumour-host communication by downregulation of LFA-1 reduces COX-2
and e-NOS expression and inhibits brain metastasis growth.
Oncotarget. 7:52375–52391. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Majumder M, Landman E, Liu L, Hess D and
Lala PK: COX-2 elevates oncogenic miR-526b in breast cancer by EP4
activation. Mol Cancer Res. 13:1022–1033. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pan J, Yang Q, Shao J, Zhang L, Ma J, Wang
Y, Jiang BH, Leng J and Bai X: Cyclooxygenase-2 induced β1-integrin
expression in NSCLC and promoted cell invasion via the
EP1/MAPK/E2F-1/FoxC2 signal pathway. Sci Rep. 6:338232016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lang S, Picu A, Hofmann T, Andratschke M,
Mack B, Moosmann A, Gires O, Tiwari S and Zeidler R: COX-inhibitors
relieve the immunosuppressive effect of tumor cells and improve
functions of immune effectors. Int J Immunopathol Pharmacol.
19:409–419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Höing B, Kanaan O, Altenhoff P, Petri R,
Thangavelu K, Schlüter A, Lang S, Bankfalvi A and Brandau S:
Stromal versus tumoral inflammation differentially contribute to
metastasis and poor survival in laryngeal squamous cell carcinoma.
Oncotarget. 9:8415–8426. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mortezaee K: Immune escape: A critical
hallmark in solid tumors. Life Sci. 258:1181102020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miao J, Lu X, Hu Y, Piao C, Wu X, Liu X,
Huang C, Wang Y, Li D and Liu J: Prostaglandin E 2 and PD-1
mediated inhibition of antitumor CTL responses in the human tumor
microenvironment. Oncotarget. 8:89802–89810. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hennequart M, Pilotte L, Cane S, Hoffmann
D, Stroobant V, Plaen E and Van den Eynde BJ: Constitutive IDO1
expression in human tumors is driven by cyclooxygenase-2 and
mediates intrinsic immune resistance. Cancer Immunol Res.
5:695–709. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Moon H, Kim D, Donahue LR and White AC:
Phenotypic plasticity of cutaneous squamous cell carcinoma mediated
by cyclooxygenase-2. J Invest Dermatol. 140:1665–1669, e1665. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moon H, Zhu J, Donahue LR, Choi E and
White AC: Krt5+/Krt15+ foregut basal
progenitors give rise to cyclooxygenase-2-dependent tumours in
response to gastric acid stress. Nat Commun. 10:22252019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xiang Y, Tian Q, Guan L and Niu SS: The
dual role of miR-186 in cancers: Oncomir battling with tumor
suppressor miRNA. Front Oncol. 10:2332020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bai J, Xu J, Zhao J and Zhang R: LncRNA
NBR2 suppresses migration and invasion of colorectal cancer cells
by downregulating miRNA-21. Hum Cell. 33:98–103. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang W, Chen J and He G, Xu W and He G:
Impact of mirna-21 on survival prognosis in patients with
pancreatic cancer: A protocol for systematic review and
meta-analysis. Medicine (Baltimore). 99:e220452020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bascuñán KA, Pérez-Bravo F, Gaudioso G,
Vaira V, Roncoroni L, Elli L, Monguzzi E and Araya M: A miRNA-based
blood and mucosal approach for detecting and monitoring celiac
disease. Dig Dis Sci. 65:1982–1991. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Irimie AI, Braicu C, Sonea L, Zimta AA,
Cojocneanu-Petric R, Tonchev K, Mehterov N, Diudea D, Buduru S and
Berindan-Neagoe I: A looking-glass of non-coding RNAs in oral
cancer. Int J Mol Sci. 18:26202017. View Article : Google Scholar
|
|
53
|
Liu Y, Li H, Zhao C and Jia H:
MicroRNA-101 inhibits angiogenesis via COX-2 in endometrial
carcinoma. Mol Cell Biochem. 448:61–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pop-Bica C, Pintea S, Cojocneanu-Petric R,
Del Sal G, Piazza S, Wu ZH, Alencar AJ, Lossos IS, Berindan-Neagoe
I and Calin GA: MiR-181 family-specific behavior in different
cancers: a meta-analysis view. Cancer Metastasis Rev. 37:17–32.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ruan K, Fang X and Ouyang G: MicroRNAs:
Novel regulators in the hallmarks of human cancer. Cancer Lett.
285:116–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Villadsen SB, Bramsen JB, Ostenfeld MS,
Wiklund ED, Fristrup N, Gao S, Hansen TB, Jensen TI, Borre M,
Ørntoft TF, et al: The miR-143/-145 cluster regulates plasminogen
activator inhibitor-1 in bladder cancer. Br J Cancer. 106:366–374.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Aldebasi YH, Rahmani AH, Khan AA and Aly
SM: The effect of vascular endothelial growth factor in the
progression of bladder cancer and diabetic retinopathy. Int J Clin
Exp Med. 6:239–251. 2013.PubMed/NCBI
|
|
58
|
Li X, Zeng Z, Wang J, Wu Y, Chen W, Zheng
L, Xi T, Wang A and Lu Y: MicroRNA-9 and breast cancer. Biomed
Pharmacother. 122:1096872020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Stiegelbauer V, Perakis S, Deutsch A, Ling
H, Gerger A and Pichler M: MicroRNAs as novel predictive biomarkers
and therapeutic targets in colorectal cancer. World J
Gastroenterol. 20:11727–11735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hao Y, Gu X, Zhao Y, Greene S, Sha W,
Smoot DT, Califano J, Wu TC and Pang X: Enforced expression of
miR-101 inhibits prostate cancer cell growth by modulating the
COX-2 pathway in vivo. Cancer Prev Res (Phila). 4:1073–1083. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Smits M, Nilsson J, Mir SE, van der Stoop
PM, Hulleman E, Niers JM, de Witt Hamer PC, Marquez VE, Cloos J,
Krichevsky AM, et al: miR-101 is down-regulated in glioblastoma
resulting in EZH2-induced proliferation, migration, and
angiogenesis. Oncotarget. 1:710–720. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shao Y, Li P, Zhu ST, Yue JP, Ji XJ, He Z,
Ma D, Wang L, Wang YJ, Zong Y, et al: Cyclooxygenase-2, a potential
therapeutic target, is regulated by miR-101 in esophageal squamous
cell carcinoma. PLoS One. 10:e01406422015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lv P, Zhang P, Li X and Chen Y: Micro
ribonucleic acid (RNA)-101 inhibits cell proliferation and invasion
of lung cancer by regulating cyclooxygenase-2. Thorac Cancer.
6:778–784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
He XP, Shao Y, Li XL, Xu W, Chen GS, Sun
HH, Xu HC, Xu X, Tang D, Zheng XF, et al: Downregulation of miR-101
in gastric cancer correlates with cyclooxygenase-2 overexpression
and tumor growth. FEBS J. 279:4201–4212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nagaraju GP and El-Rayes BF:
Cyclooxygenase-2 in gastrointestinal malignancies. Cancer.
125:1221–1227. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang J, Ding Y, Wu Y and Wang X:
Identification of the complex regulatory relationships related to
gastric cancer from lncRNA-miRNA-mRNA network. J Cell Biochem.
121:876–887. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu G and Li B: Role of miRNA in
transformation from normal tissue to colorectal adenoma and cancer.
J Cancer Res Ther. 15:278–285. 2019.PubMed/NCBI
|
|
68
|
Takagi T, Iio A, Nakagawa Y, Naoe T,
Tanigawa N and Akao Y: Decreased expression of microRNA-143 and
−145 in human gastric cancers. Oncology. 77:12–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wu XL, Cheng B, Li PY, Huang HJ, Zhao Q,
Dan ZL, Tian DA and Zhang P: MicroRNA-143 suppresses gastric cancer
cell growth and induces apoptosis by targeting COX-2. World J
Gastroenterol. 19:7758–7765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yao Q, Gu A, Wang Z and Xue Y:
MicroRNA-144 functions as a tumor suppressor in gastric cancer by
targeting cyclooxygenase-2. Exp Ther Med. 15:3088–3095.
2018.PubMed/NCBI
|
|
71
|
Liu X, Ji Q, Zhang C, Liu X, Liu Y, Liu N,
Sui H, Zhou L, Wang S and Li Q: miR-30a acts as a tumor suppressor
by double-targeting COX-2 and BCL9 in H. pylori gastric
cancer models. Sci Rep. 7:71132017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cheng Y, Li Y, Liu D, Zhang R and Zhang J:
miR-137 effects on gastric carcinogenesis are mediated by targeting
Cox-2-activated PI3K/AKT signaling pathway. FEBS Lett.
588:3274–3281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen P, Wang BL, Pan BS and Guo W:
MiR-1297 regulates the growth, migration and invasion of colorectal
cancer cells by targeting cyclo-oxygenase-2. Asian Pac J Cancer
Prev. 15:9185–9190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang D, Li Y, Zhang C, Li X and Yu J:
MiR-216a-3p inhibits colorectal cancer cell proliferation through
direct targeting COX-2 and ALOX5. J Cell Biochem. 119:1755–1766.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chakraborty C, Sharma AR, Sharma G and Lee
SS: The interplay among miRNAs, major cytokines, and cancer-related
inflammation. Mol Ther Nucleic Acids. 20:606–620. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yang YM, Kim SY and Seki E: Inflammation
and liver cancer: Molecular mechanisms and therapeutic targets.
Semin Liver Dis. 39:26–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Agra Andrieu N, Motiño O, Mayoral R,
Llorente Izquierdo C, Fernández-Alvarez A, Boscá L, Casado M and
Martín-Sanz P: Cyclooxygenase-2 is a target of microRNA-16 in human
hepatoma cells. PLoS One. 7:e509352012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jia H, Wang H, Yao Y, Wang C and Li P:
miR-136 inhibits malignant progression of hepatocellular carcinoma
cells by targeting cyclooxygenase 2. Oncol Res. 26:967–976. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li J, Lu X, Zou X, Jiang Y, Yao J, Liu H,
Ni B and Ma H: COX-2 rs5275 and rs689466 polymorphism and risk of
lung cancer: A PRISMA-compliant meta-analysis. Medicine
(Baltimore). 97:e118592018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zago M, Rico de Souza A, Hecht E, Rousseau
S, Hamid Q, Eidelman DH and Baglole CJ: The NF-κB family member
RelB regulates microRNA miR-146a to suppress cigarette
smoke-induced COX-2 protein expression in lung fibroblasts. Toxicol
Lett. 226:107–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Xia M, Duan ML, Tong JH and Xu JG: MiR-26b
suppresses tumor cell proliferation, migration and invasion by
directly targeting COX-2 in lung cancer. Eur Rev Med Pharmacol Sci.
19:4728–4737. 2015.PubMed/NCBI
|
|
82
|
Kwon Y, Kim Y, Eom S, Kim M, Park D, Kim
H, Noh K, Lee H, Lee YS, Choe J, et al:
MicroRNA-26a/-26b-COX-2-MIP-2 loop regulates allergic inflammation
and allergic inflammation-promoted enhanced tumorigenic and
metastatic potential of cancer cells. J Biol Chem. 290:14245–14266.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wu C, Li X, Zhang D, Xu B, Hu W, Zheng X,
Zhu D, Zhou Q, Jiang J and Wu C: IL-1β-mediated Up-regulation of
WT1D via miR-144-3p and their synergistic effect with
NF-κB/COX-2/HIF-1α pathway on cell proliferation in LUAD. Cell
Physiol Biochem. 48:2493–2502. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li B, Lu Y, Yu L, Han X, Wang H, Mao J,
Shen J, Wang B, Tang J, Li C and Song B: miR-221/222 promote cancer
stem-like cell properties and tumor growth of breast cancer via
targeting PTEN and sustained Akt/NF-κB/COX-2 activation. Chem Biol
Interact. 277:33–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Majumder M, Dunn L, Liu L, Hasan A,
Vincent K, Brackstone M, Hess D and Lala PK: COX-2 induces
oncogenic micro RNA miR655 in human breast cancer. Sci Rep.
8:3272018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Liao H, Zhou Q, Gu Y, Duan T and Feng Y:
Luteinizing hormone facilitates angiogenesis in ovarian epithelial
tumor cells and metformin inhibits the effect through the mTOR
signaling pathway. Oncol Rep. 27:1873–1878. 2012.PubMed/NCBI
|
|
87
|
Lai Y, Zhang X, Zhang Z, Shu Y, Luo X,
Yang Y, Wang X, Yang G, Li L and Feng Y: The microRNA-27a:
ZBTB10-specificity protein pathway is involved in follicle
stimulating hormone-induced VEGF, Cox2 and survivin expression in
ovarian epithelial cancer cells. Int J Oncol. 42:776–784. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lin Y and Wu Z: MicroRNA-128 inhibits
proliferation and invasion of glioma cells by targeting COX-2.
Gene. 658:63–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chen ZG, Zheng CY, Cai WQ, Li DW, Ye FY,
Zhou J, Wu R and Yang K: miR-26b mimic inhibits glioma
proliferation in vitro and in vivo suppressing COX-2 expression.
Oncol Res. 27:147–155. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Shields CL and Shields JA: Retinoblastoma
management: Advances in enucleation, intravenous chemoreduction,
and intra-arterial chemotherapy. Curr Opin Ophthalmol. 21:203–212.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang J, He J and Zhang L: The
down-regulation of microRNA-137 contributes to the up-regulation of
retinoblastoma cell proliferation and invasion by regulating
COX-2/PGE2 signaling. Biomed Pharmacother. 106:35–42. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yu Q, Zhang K, Wang X, Liu X and Zhang Z:
Expression of transcription factors snail, slug, and twist in human
bladder carcinoma. J Exp Clin Cancer Res. 29:1192010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Song T, Zhang X, Wang C, Wu Y, Dong J, Gao
J, Cai W and Hong B: Expression of miR-143 reduces growth and
migration of human bladder carcinoma cells by targeting
cyclooxygenase-2. Asian Pac J Cancer Prev. 12:9292011.PubMed/NCBI
|
|
94
|
Xu L, Shen B, Chen T and Dong P: miR-203
is involved in the laryngeal carcinoma pathogenesis via targeting
VEGFA and Cox-2. Onco Targets Ther. 9:4629–4637. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Tołoczko-Iwaniuk N, Dziemiańczyk-Pakieła
D, Nowaszewska BK, Celińska-Janowicz K and Miltyk W: Celecoxib in
cancer therapy and prevention-review. Curr Drug Targets.
20:302–315. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jackson AL, Bartz SR, Schelter J,
Kobayashi SV, Burchard J, Mao M, Li B, Cavet G and Linsley PS:
Expression profiling reveals off-target gene regulation by RNAi.
Nat Biotechnol. 21:635–637. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
97
|
Strillacci A, Griffoni C, Valerii MC,
Lazzarini G, Tomasi V and Spisni E: RNAi-based strategies for
cyclooxygenase-2 inhibition in cancer. J Biomed Biotechnol.
2010:8280452010. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Liu X, Wu Y, Zhou Z, Huang M, Deng W, Wang
Y, Zhou X, Chen L, Li Y, Zeng T, et al: Celecoxib inhibits the
epithelial-to-mesenchymal transition in bladder cancer via the
miRNA-145/TGFBR2/Smad3 axis. Int J Mol Med. 44:683–693.
2019.PubMed/NCBI
|
|
99
|
Ghose J and Bhattacharyya NP:
Transcriptional regulation of microRNA-100, −146a, and −150 genes
by p53 and NFκB p65/RelA in mouse striatal STHdh(Q7)/Hdh(Q7) cells
and human cervical carcinoma HeLa cells. RNA Biol. 12:457–477.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
DA Costa RM, Bastos MM, Medeiros R and
Oliveira PA: The NFkB signaling pathway in papillomavirus-induced
lesions: Friend or foe? Anticancer Res. 36:2073–2083.
2016.PubMed/NCBI
|
|
101
|
DA Costa RMG, Araújo R, Santos JMO,
Fernandes M, Neto T, Sousa H, Ribeiro J, Bastos MMSM, Oliveira PA,
Carmo D, et al: Regulation of miRNA-146a and miRNA-150 Levels by
celecoxib in premalignant lesions of K14-HPV16 mice. Anticancer
Res. 37:2913–2918. 2017.PubMed/NCBI
|
|
102
|
Saito Y, Suzuki H, Imaeda H, Matsuzaki J,
Hirata K, Tsugawa H, Hibino S, Kanai Y, Saito H and Hibi T: The
tumor suppressor microRNA-29c is downregulated and restored by
celecoxib in human gastric cancer cells. Int J Cancer.
132:1751–1760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Mott JL, Kobayashi S, Bronk SF and Gores
GJ: mir-29 regulates Mcl-1 protein expression and apoptosis.
Oncogene. 26:6133–6140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hunter S, Nault B, Ugwuagbo KC, Maiti S
and Majumder M: Chemicall induced hypoxia enhances miRNA functions
in breast cancer. Cancers (Basel). 12:20082020. View Article : Google Scholar
|
|
105
|
Najafi M, Farhood B, Mortezaee K,
Kharazinejad E, Majidpoor J and Ahadi R: Hypoxia in solid tumors: A
key promoter of cancer stem cell (CSC) resistance. J Cancer Res
Clin Oncol. 146:19–31. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Xiong W, Li WH, Jiang YX, Liu S, Ai YQ,
Liu R, Chang L, Zhang M, Wang XL, Bai H, et al: Parecoxib: An
enhancer of radiation therapy for colorectal cancer. Asian Pac J
Cancer Prev. 16:627–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zagani R, Hamzaoui N, Cacheux W, de
Reyniès A, Terris B, Chaussade S, Romagnolo B, Perret C and
Lamarque D: Cyclooxygenase-2 inhibitors down-regulate osteopontin
and Nr4A2-new therapeutic targets for colorectal cancers.
Gastroenterology. 137:1358–1366.e1-3. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Li LY, Xiao J, Liu Q and Xia K: Parecoxib
inhibits glioblastoma cell proliferation, migration and invasion by
up-regulating miRNA-29c. Biol Open. 6:311–316. 2016. View Article : Google Scholar
|
|
109
|
Nissen SE, Yeomans ND, Solomon DH, Lüscher
TF, Libby P, Husni ME, Graham DY, Borer JS, Wisniewski LM, Wolski
KE, et al: Cardiovascular safety of celecoxib, naproxen, or
ibuprofen for arthritis. N Engl J Med. 375:2519–2529. 2016.
View Article : Google Scholar : PubMed/NCBI
|