Introduction
Cervical cancer is the 2nd most common malignancy
and is the leading cause of cancer mortality in women in the United
States (1). Cisplatin (DDP)-based
chemotherapy is a standard treatment for cervical cancer. While DDP
has shown efficacy for treating cervical cancer, numerous patients
present with resistance to available chemotherapeutics prior to
mortality, which is due to widespread metastasis and tumor relapse
(2). Therefore, it is important to
identify the molecular mechanisms underlying cervical cancer DDP
resistance.
Hypoxia is a common phenomenon in the majority of
solid tumors (3). Furthermore,
hypoxia is associated with aggressive tumor progression and
resistance to chemotherapy and radiation (4). Hypoxia-inducible factor-2 α (HIF-2α)
is the oxygen-regulated α-subunit of HIF (5). HIF-2α has the ability to upregulate
drug resistance-related gene expression and causes chemotherapy
resistance in numerous tumors (6,7).
However, the relationship between HIF-2α and chemotherapy
resistance in cervical cancer cells remains elusive.
MicroRNAs (miRNAs/miRs), which are non-coding RNAs,
are shown to post-transcriptionally regulate gene expression by
binding to the seed region and sequences in the 3′-untranslated
region of the target mRNA (8).
Previous studies have revealed that miRNAs have a number of roles
such as regulating the cell cycle, cell proliferation, migration,
invasion, adhesion, angiopoiesis and apoptosis, as well as having
an important role in cervical cancer occurrence and progression
(9,10). Recent studies have shown that
miR-519-3p regulates cell proliferation, migration and invasion in
multiple cancer cells (11–13). The authors' previous study revealed
that in cervical cancer, under hypoxic conditions miR-519d-3p plays
an important role in the regulation of HIF-2α expression (14). However, the function and regulatory
mechanisms of the miR-519d-3p/HIF-2α axis in cervical cancer DDP
resistance remains unknown.
Therefore, the present study assessed the expression
level of miR-519d-3p in cervical cancer cells and examined the
effects of miR-519d-3p overexpression on DDP resistance in cervical
cancer cells. Moreover, the present study analyzed the potential
regulatory mechanism of the miR-519d-3p/HIF-2α axis in cervical
cancer DDP resistance under hypoxic conditions.
Materials and methods
Cell culture and hypoxic exposure
Human cervical cancer cell lines, CaSki and HeLa,
were purchased from the American Type Culture Collection.
DDP-resistant cervical cancer cell lines, HeLa/DDP and CaSki/DDP,
were purchased from Shenglong Biological Corporation. CaSki, HeLa,
Hela/DDP and CaSki/DDP cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.), and supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin G
and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA). Cells were
maintained at 37°C in a humidified 5% CO2 incubator. To
expose cells to hypoxic conditions, the cells were cultured for 24
h in a Billups-Rothenburg chamber with 94% N2, 1%
O2 and 5% CO2 at 37°C.
miRNA mimic synthesis, HIF-2α
overexpression plasmid construction and cell transfection
miR-519d-3p mimics (5′-CAAAGUGCCUCCCUUUAGAGUG-3′)
and negative-control (NC) miRNA mimics
(5′-UUCUCCGAACGUGUCACGUTT-3′) were purchased from Shanghai
GenePharma Co., Ltd. To study the function of miR-519d-3p, HeLa/DDP
and CaSki/DDP cells were plated into 96 well plates
(4×104 cells/well) or 6-well plates (5×105
cells/well), and subsequently incubated at 37°C for 6 h. Cells were
then transfected with 50 nM miR-519d-3p or 50 nM NC using
Lipofectamine 2000 transfection reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. To
examine the relationship between miR-519d-3p and HIF-2α, the
full-length HIF-2α (NM_001430.5) was cloned and inserted into the
pcDNA3.1 expression plasmid (Promega Corporation), referred to as
‘pcDNA-HIF-2α’. Empty pcDNA3.1 plasmid was used as a control. Cells
were plated into 96-well plates (4×104 cells/well) or
6-well plates (5×105 cells/well), and co-transfected
with 50 nM miR-519d-3p, and 2 µg/ml empty pcDNA3.1 plasmid
(miR-519d-3p + pcDNA) or pcDNA-HIF-2α (miR-519d-3p + pcDNA-HIF-2α),
using Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). After transfection for 48 h, MTT assays and
western blotting were performed.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and RT to cDNA
was performed using a miRcute miRNA first-strand cDNA synthesis kit
at 42°C for 60 min (Tiangen Biotech Co., Ltd.) and PrimeScript RT
Reagent kit with gDNA Eraser (Tiangen Biotech Co., Ltd.). mRNA
expression level was detected using qPCR with a miRcute miRNA qPCR
detection kit (SYBR® Green; Tiangen Biotech Co., Ltd.).
U6 was used as an internal reference. qPCR was performed on a 7500
RT PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
under the following conditions: Initial denaturation at 95°C for 5
min, followed by 40 cycles at 95°C for 15 sec and 60°C for 60 sec.
Gene expression was measured in triplicate using the
2−ΔΔCq method (15). The
product length of miR-519d-3p for the RT-qPCR was 73 bp and was
amplified using the following primers: Forward,
5′-ACACTCCAGCTGGGCAAAGTGCCTCCCTTT-3′ and reverse,
5′-CTCAACTGGTGTCGTGGA-3′. The product length of U6 for the RT-qPCR
was 94 bp and was amplified using the following primers: Forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′.
MTT assay
After 24 h of seeding cells into 96-well plates
(5,000 cells per well), cells were transfected with miR-519d-3p,
miR-519d-3p + pcDNA, or miR-519d-3p + pcDNA-HIF-2α. Cells were
treated with DDP (Merck KGaA) (0, 1, 2, 4, 6, 8, 16 or 32 µg/ml) at
24 h post-transfection 37°C (16).
Then, 48 h after transfection, 20 µl MTT (5 mg/ml; Sigma-Aldrich;
Merck KGaA) was added and the cells were incubated for another 4 h
in a humidified incubator. After the supernatant was discarded, 200
µl DMSO was added to dissolve the formazan. The optical density
(OD) at 570 nm was measured. The growth inhibition rate was
calculated as follows: Growth inhibition rate (%)=(average
OD570 nm of the control group-average
OD570 nm value of the experimental group)/average
OD570 nm value of the control group ×100%. The
IC50 was calculated based on growth inhibition rate
using GraphPad Prism software, version 7.0 (GraphPad Software,
Inc.).
Western blotting
Total cellular protein was extracted using
pre-cooled RIPA buffer (Beyotime Institute of Biotechnology) with a
protease inhibitor. Protein concentrations were determined using
BCA Protein Assay kit (Beyotime Institute of Biotechnology). Equal
amounts (30 µg) of protein samples were separated using 10%
SDS-PAGE and transferred to PVDF membranes. After blocking in PBST
(0.1% Tween-20) containing 5% non-fat milk at 25°C for 2 h, the
membrane was incubated with the corresponding primary antibody
overnight at 4°C. After washing with PBST, the membranes were
incubated with the secondary antibody (1:10,000; Goat Anti-Mouse
IgG1, Human ads-HRP; cat. no. 1070-05; SouthernBiotech) at 25°C for
2 h. The membranes were rinsed with PBST and protein bands were
visualized using an SuperSignal™ West Pico PLUS (Thermo Fisher
Scientific, Inc.). GAPDH was used as the loading control. Membranes
were incubated with the following primary antibodies: Anti-HIF-2α
mouse monoclonal antibody (1:2,000; cat. no. sc-13596, Santa Cruz
Biotechnology, Inc.), anti-human PI3K p85 polyclonal antibody
(1:10,000; cat. no. sc-1637, Santa Cruz Biotechnology, Inc.),
anti-human phosphorylated (p)-AKT1 polyclonal antibody (1:10,000;
cat. no. sc-52940, Santa Cruz Biotechnology, Inc.), anti-human
total (t)-AKT1 polyclonal antibody (1:4,000; cat. no. sc-5298,
Santa Cruz Biotechnology, Inc.), anti-human p-mTOR polyclonal
antibody (1:5,000; cat. no. sc-293133, Santa Cruz Biotechnology,
Inc.), anti-human mTOR polyclonal antibody (1:5,000; cat. no.
sc-517464, Santa Cruz Biotechnology, Inc.) and anti-human GAPDH
polyclonal antibody (1:10,000; cat. no. ab8245, Abcam). The
quantification of specific bands was performed using Image Pro-Plus
6.0 software (Media Cybernetics, Inc.). The relative expression of
p-AKT1 or p-mTOR was referenced to both t-protein and GAPDH. The
relative expression of HIF-2α, AKT1 or mTOR was referenced to
GAPDH.
Statistical analysis
All experiments were repeated three times. All
statistical analysis was performed using SPSS version 19.0 software
(IBM Corp.). Continuous variables are presented as the mean ± SD.
An unpaired t-test was used to compare the differences between the
two groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
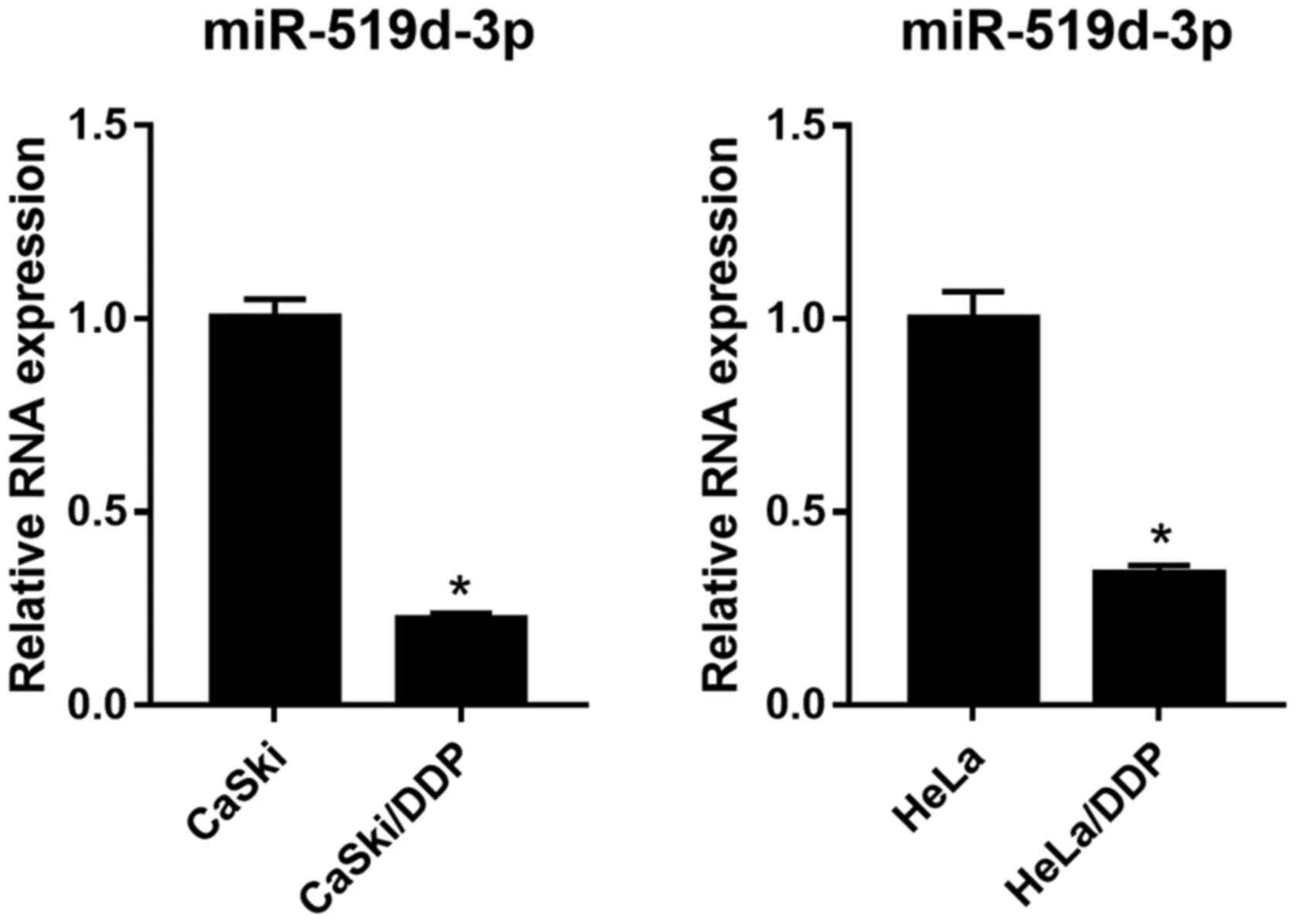
miR-519d-3p expression decreases in
CaSki/DDP and HeLa/DDP cells under hypoxic conditions
RT-qPCR was used to detect the expression level of
miR-519d-3p in CaSki, HeLa, CaSki/DDP and HeLa/DDP cells under
hypoxic conditions. It was found that miR-519d-3p expression level
was significantly decreased in both CaSki/DDP and HeLa/DDP cells,
compared with CaSki and HeLa cells under hypoxic conditions
(Fig. 1).
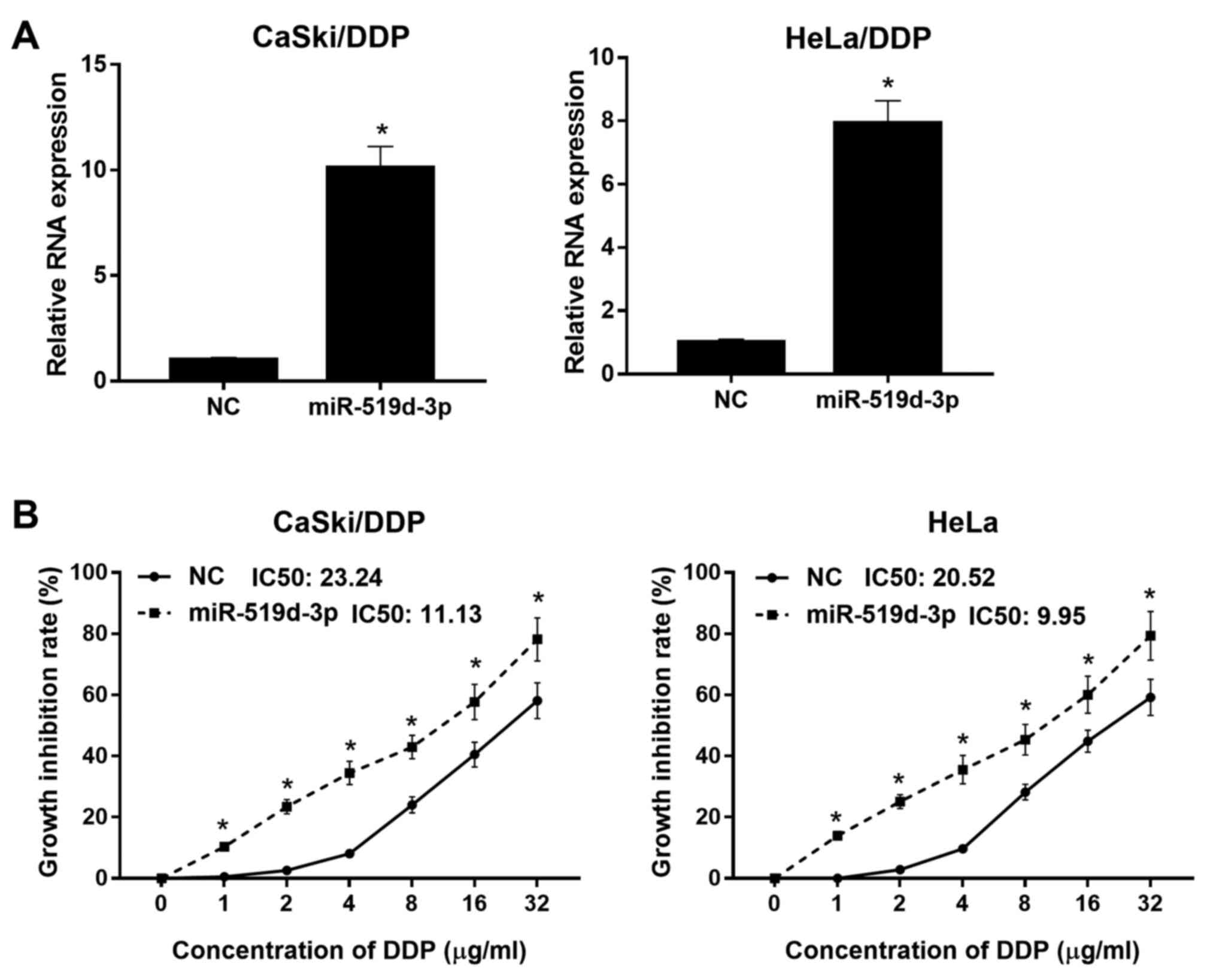
miR-519d-3p overexpression decreases
DDP resistance in HeLa/DDP and CaSki/DDP cells
To investigate the effect of miR-519d-3p
overexpression on DDP-resistant cells, miR-519d-3p mimic or NC were
transfected into HeLa/DDP and CaSki/DDP cells. RT-qPCR was used to
evaluate the mRNA expression level of miR-519d-3p in HeLa/DDP and
CaSki/DDP cells after transfection. It was demonstrated that
miR-519d-3p expression levels were significantly increased in
HeLa/DDP and CaSki/DDP cells transfected with miR-519d-3p mimics
compared with the NC groups, under hypoxic conditions (Fig. 2A). Thus, the present results
indicated that miR-519d-3p was successfully overexpressed in
HeLa/DDP and CaSki/DDP cells. To examine the effect of miR-519d-3p
overexpression on DDP resistance in HeLa/DDP and CaSki/DDP cells
under hypoxic conditions, miR-519d-3p mimic or NC-transfected
HeLa/DDP and CaSki/DDP cells were treated with different DDP doses.
The growth inhibition rate of HeLa/DDP and CaSki/DDP cells
transfected with miR-519d-3p mimic was increased compared with
cells transfected with NC, under hypoxic conditions after DPP
treatment (Fig. 2B). It was
demonstrated that the DDP IC50 of HeLa/DDP and CaSki/DDP
cells transfected with miR-519d-3p mimic was 9.95 and 11.13 µg/ml,
respectively. Furthermore, the DDP IC50 of HeLa/DDP and
CaSki/DDP cells transfected with NC was 20.52 and 23.24 µg/ml,
respectively. Therefore, the present results suggested that
miR-519d-3p overexpression decreased DDP resistance in HeLa/DDP and
CaSki/DDP cells.
Overexpression of miR-519d-3p inhibits
HIF-2α protein expression levels and PI3K/AKT signaling pathways
under hypoxic conditions
Western blotting results showed that the protein
expression levels of HIF-2α, PI3K p85, p-AKT1 and p-mTOR were
significantly decreased in HeLa/DDP and CaSki/DDP cells transfected
with miR-519d-3p mimic compared with the NC group (Fig. 3). However, the protein expression
levels of AKT1 and mTOR were not significantly different (Fig. 3).
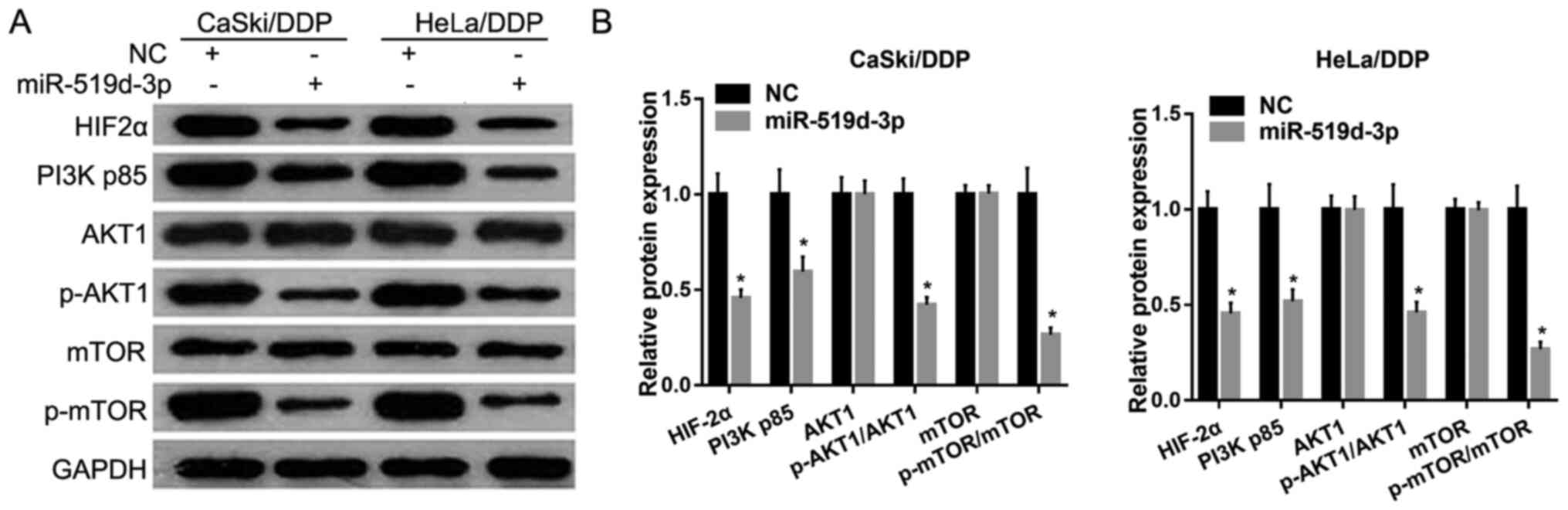 | Figure 3.Overexpression of miR-519d-3p inhibits
HIF-2α protein expression and the PI3K/AKT signaling pathway under
hypoxic conditions. (A) Protein expression levels of HIF-2α, PI3K
p85, p-AKT1, AKT1, mTOR and p-mTOR was measured by western blotting
after transfection with miR-519d-3p mimics or NC mimics. (B)
Relative protein expression levels of HIF-2α, PI3K p85, p-AKT1,
AKT1, mTOR and p-mTOR. Data are presented as the mean ± SD.
*P<0.05. miR, microRNA; DDP, cisplatin; NC, negative control;
p-, phosphorylated; HIF-2α, hypoxia-inducible factor-2 α. |
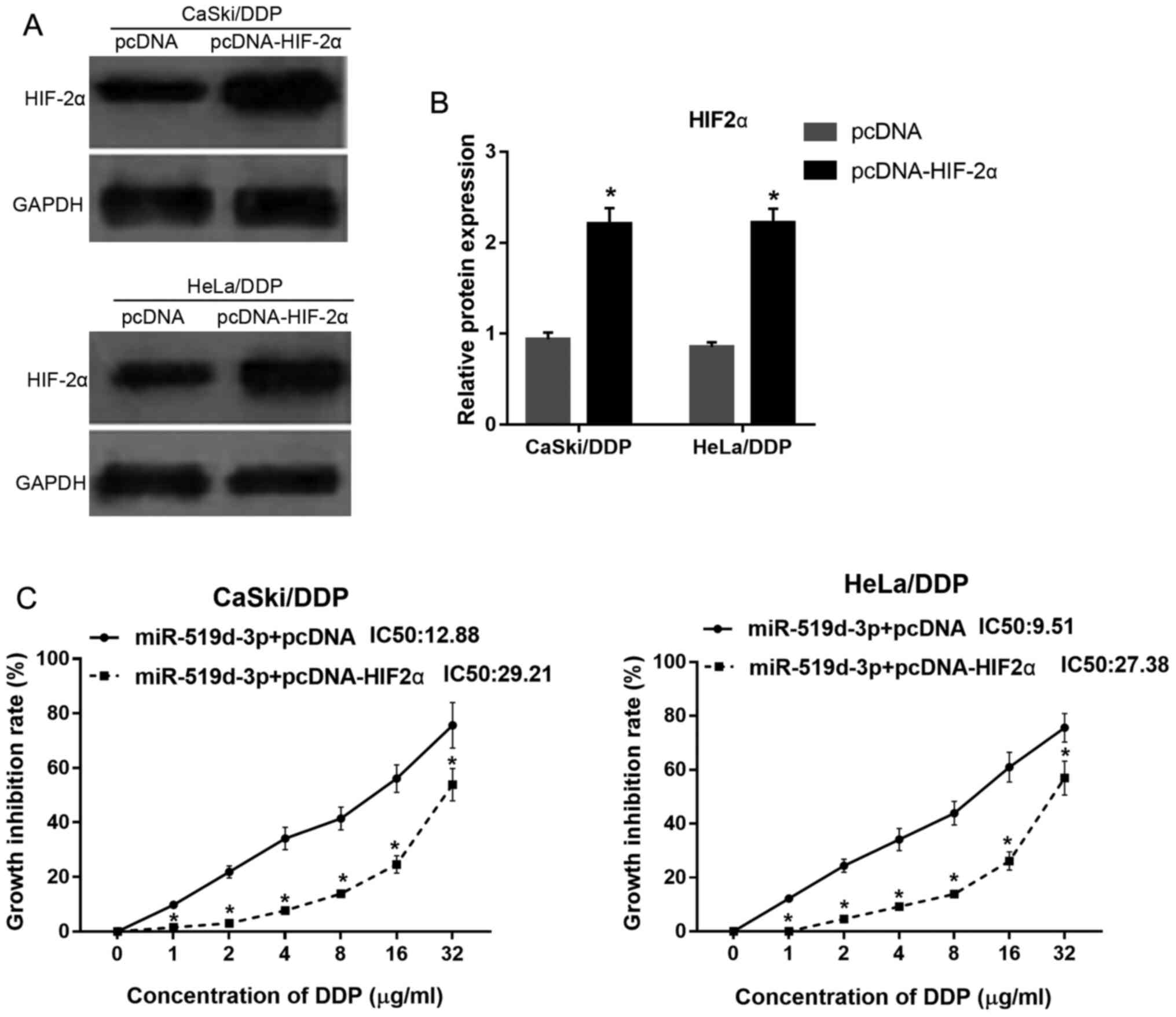
HIF-2α overexpression weakens the
effect of miR-519d-3p overexpression on HeLa/DDP and CaSki/DDP
cells under hypoxic conditions
Western blotting was used to assess HIF-2α
expression in HeLa/DDP and CaSki/DDP cells transfected with pcDNA
or pcDNA-HIF-2α. The present results indicated that HIF-2α
expression was significantly increased both in HeLa/DDP and
CaSki/DDP cells transfected with pcDNA-HIF-2α, compared with cells
transfected with pcDNA, under hypoxic conditions (Fig. 4A and B).
To investigate whether HIF-2α overexpression reduces
the effect of miR-519d-3p overexpression on DDP resistance,
HeLa/DDP and CaSki/DDP cells were co-transfected with miR-519d-3p,
and either pcDNA or pcDNA-HIF-2α. It was found that the growth
inhibition rate of HeLa/DDP and CaSki/DDP cells transfected with
miR-519d-3p + pcDNA-HIF-2α was lower compared with cells
transfected with miR-519d-3p + pcDNA, under hypoxic conditions
after DDP treatment (Fig. 4C).
Moreover, the DDP IC50 of HeLa/DDP and CaSki/DDP cells
transfected with miR-519d-3p + pcDNA-HIF-2α was 27.38 and 29.21
µg/ml, respectively. The DDP IC50 of HeLa/DDP and
CaSki/DDP cells transfected with miR-519d-3p + pcDNA was 9.51 and
12.88 µg/ml, respectively. Therefore, the present results indicated
that HIF-2α overexpression could reduce the effect of miR-519d-3p
overexpression on DDP resistance in HeLa/DDP and CaSki/DDP cells,
under hypoxic conditions.
To examine whether HIF-2α overexpression reduces the
effect of miR-519d-3p overexpression on the PI3K/AKT signaling
pathway, HeLa/DDP and CaSki/DDP cells were co-transfected with
miR-519d-3p, and either pcDNA or pcDNA-HIF-2α. Western blotting
results demonstrated that the protein expression levels of HIF-2α,
PI3K p85, p-AKT1 and p-mTOR were significantly increased in
HeLa/DDP and CaSki/DDP cells transfected with miR-519d-3p +
pcDNA-HIF-2α, compared with cells transfected with miR-519d-3p +
pcDNA (Fig. 5). Furthermore, the
present results suggested that the protein expression levels of
AKT1 and mTOR were not significantly different (Fig. 5).
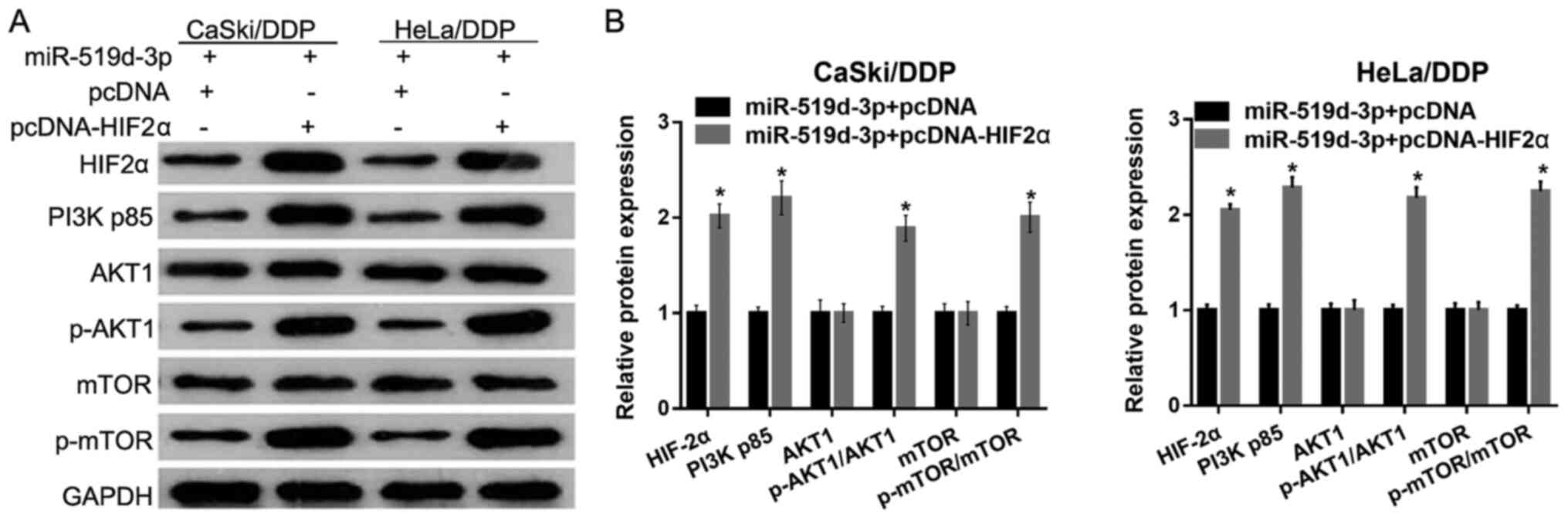 | Figure 5.HIF-2α overexpression reduces the
effect of miR-519d-3p overexpression on the PI3K/AKT signaling
pathways under hypoxic conditions. (A) Protein expression levels of
HIF-2α, PI3K p85, p-AKT1, AKT1, mTOR and p-mTOR were measured by
western blotting after co-transfection with miR-519d-3p, and pcDNA
or pcDNA-HIF-2α. (B) Relative protein expression levels of HIF-2α,
PI3K p85, p-AKT1, AKT1, mTOR and p-mTOR are presented as the mean ±
SD. *P<0.05. miR, microRNA; DDP, cisplatin; NC, negative
control; p-, phosphorylated; HIF-2α, hypoxia-inducible factor-2 α;
pcDNA, empty pcDNA3.1 plasmid; pcDNA-HIF-2α, HIF-2α overexpressing
pcDNA3.1 plasmid. |
Discussion
While DDP chemotherapy is one of the most widely
used drugs for the treatment of cervical cancer, it eventually
results in drug resistant cervical cancer cells, which leads to
poor clinical outcomes (17,18).
Therefore, improving the understanding of the underlying
chemoresistance mechanism may facilitate the development of
strategies to reverse drug resistance in cervical cancer and
improve overall survival of patients with cervical cancer.
miRNAs can regulate multiple pathways involved in
the cellular response to DDP (19).
Wang et al (20) showed that
miR-214 reduces cell survival and enhances DDP-induced cytotoxicity
via downregulation of Bcl-2-like protein 2 in cervical cancer
cells. Moreover, Lei et al (21) revealed that upregulated miR-155
reverses the epithelial-mesenchymal transition induced by epidermal
growth factor and increases chemosensitivity to DDP in human CaSki
cells. Several previous studies have investigated the mechanisms of
drug resistance in human cervical cancer cells (17). However, evidence on the role of
miR-519d-3p in cervical cancer DDP resistance is limited. Previous
studies have demonstrated that miR-519d-3p functions as a tumor
suppressor in several tumors (11–13).
In gastric cancer, miR-519d-3p suppresses cell proliferation and
invasion by downregulating B-cell lymphoma 6 (11). Furthermore, in colorectal cancer,
miR-519d-3p inhibits oncogenicity and promotes apoptosis by
targeting trophinin associated protein (12). The authors' previous study found
that miR-519d-3p inhibits proliferation and promotes apoptosis by
targeting HIF-2α in cervical cancer cells under hypoxic conditions
(14). In the present study, it was
found that miR-519d-3p expression was lower in CaSki/DDP and
HeLa/DDP cells, compared with CaSki and HeLa cells under hypoxic
conditions. Moreover, it was demonstrated that miR-519d-3p
overexpression decreased the IC50 value in CaSki/DDP and
HeLa/DDP cells. Therefore, the present results suggested that
miR-519d-3p may be correlated with DDP resistance.
Hypoxia plays an important role in tumor
chemoresistance (3). HIF-2α is
associated with drug resistance-related gene expression and leads
to chemotherapy resistance in numerous tumors (6,7). In
the present study, miR-519d-3p overexpression was shown to inhibit
HIF-2α protein expression. However, HIF-2α overexpression reduced
the effect of miR-519d-3p overexpression on DDP resistance. Thus,
the present results indicated that the miR-519d-3p/HIF-2α axis may
be involved in DDP resistance regulation in cervical cancer cells.
Therefore, the miR-519d-3p/HIF-2α axis could be a potential target
for cervical cancer treatment in DDP resistance.
Previous studies have revealed that aberrant
activation of the PI3K/AKT signaling pathway plays a pivotal role
in malignant transformation and chemoresistance in cancer cells
(22,23). The present study identified that
miR-519d-3p overexpression decreased the expression levels of
HIF-2α, PI3K p85, p-AKT1 and p-mTOR. However, HIF-2α overexpression
reversed the effect of miR-519d-3p overexpression on the PI3K/AKT
signaling pathway. Moreover, western blotting results demonstrated
that HIF-2α overexpression increased PI3K p85, p-AKT1 and p-mTOR
expression levels. Thus, the present results suggested that the
miR-519d-3p/HIF-2α axis may regulate the PI3K/AKT signaling
pathway.
However, there are certain limitations of the
present study, which will need to be overcome in future studies.
Firstly, miR-519d-3p expression was not detected in solid cervical
cancer tissues from patients with DDP sensitivity or resistance. In
addition, the function of miR-519d-3p and its regulatory mechanism
were not investigated in an animal model.
In conclusion, it was found that miR-519d-3p was
expressed at a low level in CaSki/DDP and HeLa/DDP cells.
Furthermore, miR-519d-3p overexpression decreased DDP resistance in
HeLa/DDP and CaSki/DDP cells, and inhibited HIF-2α protein
expression and the PI3K/AKT signaling pathway. It was also
demonstrated that HIF-2α overexpression reduced the effect of
miR-519d-3p overexpression on CaSki/DDP and HeLa/DDP cells.
Collectively, the present results suggested that the
miR-519d-3p/HIF-2α axis is important in chemoresistance development
and may be a novel target for human cervical cancer treatment.
However, further studies, including both in vivo models and
clinical trials, are required to verify the present results.
Acknowledgements
Not applicable.
Funding
The present study is supported by the National
Natural Science Foundation of China (grant no. 81760472) and the
Natural Science Foundation of Jiangxi (grant no.
20171BAB205066).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ was involved in study design and preparation of
the manuscript. LJ, SS and FL performed the experiments. QS and TZ
revised the manuscript and performed the western blotting. HZ and
YX performed the cell culture. All the authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pectasides D, Kamposioras K, Papaxoinis G
and Pectasides E: Chemotherapy for recurrent cervical cancer.
Cancer Treat Rev. 34:603–613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li SH, Shin DH, Chun YS, Lee MK, Kim MS
and Park JW: A novel mode of action of YC-1 in HIF inhibition:
Stimulation of FIH-dependent p300 dissociation from HIF-1{alpha}.
Mol Cancer Ther. 7:3729–3738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lou JJ, Chua YL, Chew EH, Gao J, Bushell M
and Hagen T: Inhibition of hypoxia-inducible factor-1alpha
(HIF-1alpha) protein synthesis by DNA damage inducing agents. PLoS
One. 5:e105222010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wiesener MS, Jurgensen JS, Rosenberger C,
Scholze CK, Hörstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei
UA, Pugh CW, et al: Widespread hypoxia-inducible expression of
HIF-2alpha in distinct cell populations of different organs. FASEB
J. 17:271–273. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao ZJ, Yuan WD, Yuan JQ, Yuan K and Wang
Y: Downregulation of HIF-2α reverse the chemotherapy resistance of
lung adenocarcinoma a549 cells to cisplatin. Med Sci Monit.
24:1104–1111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Xue M and Chung DC: c-Myc is
regulated by HIF-2α in chronic hypoxia and influences sensitivity
to 5-FU in colon cancer. Oncotarget. 7:78910–78917. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pereira DM, Rodrigues PM, Borralho PM and
Rodrigues CM: Delivering the promise of miRNA cancer therapeutics.
Drug Discov Today. 18:282–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li S, Yang F, Wang M, Cao W and Yang Z:
miR-378 functions as an onco-miRNA by targeting the
ST7L/Wnt/β-catenin pathway in cervical cancer. Int J Mol Med.
40:1047–1056. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang H, Luo R, Chen X, Zhao Y and Tan A:
miR-187 inhibits the growth of cervical cancer cells by targeting
FGF9. Oncol Rep. 38:1977–1984. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li YY, Shao JP, Zhang SP, Xing GQ and Liu
HJ: miR-519d-3p inhibits cell proliferation and invasion of gastric
cancer by downregulating B-cell lymphoma 6. Cytogenet Genome Res.
154:12–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ye X and Lv H: MicroRNA-519d-3p inhibits
cell proliferation and migration by targeting TROAP in colorectal
cancer. Biomed Pharmacother. 105:879–886. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma L and Li J: MicroRNA-519d-3p inhibits
cell proliferation and cell cycle G1/S transition in glioma by
targeting CCND1. Biosci Biotechnol Biochem. 84:297–304. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang L, Shi S, Shi Q, Zhang H, Xia Y and
Zhong T: MicroRNA-519d-3p inhibits proliferation and promotes
apoptosis by targeting HIF-2α in cervical cancer under hypoxic
conditions. Oncol Res. 26:1055–1062. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng Y, Zou W, Hu C, Li G, Zhou S, He Y,
Ma F, Deng C and Sun L: Modulation of CASC2/miR-21/PTEN pathway
sensitizes cervical cancer to cisplatin. Arch Biochem Biophys.
623-624:20–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu H, Luo H, Zhang W, Shen Z, Hu X and
Zhu X: Molecular mechanisms of cisplatin resistance in cervical
cancer. Drug Des Devel Ther. 10:1885–1895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Small W Jr, Bacon MA, Bajaj A, Chuang LT,
Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR,
Viswanathan AN and Gaffney DK: Cervical cancer: A global health
crisis. Cancer. 123:2404–2412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Drayton RM: The role of microRNA in the
response to cisplatin treatment. Biochem Soc Trans. 40:821–825.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang F, Liu M, Li X and Tang H: MiR-214
reduces cell survival and enhances cisplatin-induced cytotoxicity
via down-regulation of Bcl2l2 in cervical cancer cells. FEBS Lett.
587:488–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lei C, Wang Y, Huang Y, Yu H, Huang Y, Wu
L and Huang L: Up-regulated miR155 reverses the
epithelial-mesenchymal transition induced by EGF and increases
chemo-sensitivity to cisplatin in human Caski cervical cancer
cells. PloS One. 7:e523102012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma Q, Chang Z, Wang W and Wang B:
Rapamycin-mediated mTOR inhibition reverses drug resistance to
adriamycin in colon cancer cells. Hepatogastroenterology.
62:880–886. 2015.PubMed/NCBI
|
|
23
|
Marklein D, Graab U, Naumann I, Yan T,
Ridzewski R, Nitzki F, Rosenberger A, Dittmann K, Wienands J,
Wojnowski L, et al: PI3K inhibition enhances doxorubicin-induced
apoptosis in sarcoma cells. PLoS One. 7:e528982012. View Article : Google Scholar : PubMed/NCBI
|