Introduction
Endometriosis (EM) is a common gynecological disease
characterized by the growth of endometrial tissue outside the
uterine cavity. EM affected ~10% of women of reproductive age and
5–50% of infertile women worldwide in 1997 (1). As a common gynecological disease, EM
results in a number of clinical symptoms, including chronic pelvic
pain, dysmenorrhea, dyspareunia, menorrhagia and mental suffering,
which affect the quality of life of patients (2,3). At
present, the primary treatment strategy for EM involves relieving
pain and other symptoms, but does not involve curing EM (4). Therefore, identifying the molecular
mechanism underlying EM is important for the development of novel
effective therapeutic strategies.
MicroRNAs (miRNA/miR) are non-coding short RNAs that
modulate diverse life processes via regulating target genes at the
post-transcriptional level (5).
Previous studies identified abnormal expression of miRNAs in the
eutopic endometrium, which indicated that miRNAs serve a key role
in modulating the progression of EM (6–8).
miR-143-3p is involved in cell proliferation, apoptosis, adhesion,
invasion and other cellular processes (9,10).
Previous studies have reported that miR-143-3p was markedly
dysregulated in EM (11–13).
Autophagy is a complex process, which is crucial for
cellular self-regulation, and dysfunction of autophagy is
associated with multiple human diseases, including cardiovascular
diseases, neurodegeneration, metabolic diseases, infectious
diseases and cancer (14–16). Several studies have demonstrated
abnormal activation of autophagy in ovarian EM (17,18).
Therefore, the present study aimed to identify the functions and
mechanisms underlying miR-143-3p in EM.
Materials and methods
Clinical samples, and cell isolation
and culture
The present study was approved by the Protection of
Human Subjects Committee of Shanghai Shuguang Hospital. All
patients provided written informed consent. Ectopic (n=10), eutopic
(n=10) and normal endometrial (n=10) tissues from patients with or
without EM (mean age, 43.2 years; age range, 28–51 years) who had
undergone a laparoscopy and uterine curettage were obtained from
the Shanghai Shuguang Hospital Affiliated with Shanghai University
of Traditional Chinese Medicine between November 2017 and March
2019. Endometriotic stromal cells (ESCs) were isolated from
endometriotic tissues as previously described (19). Normal endometrial stromal cells
(NESCs) were isolated from eutopic endometrial tissues obtained
from four individuals without EM (age range, 30–53 years) who
underwent a hysterectomy procedure. Briefly, the endometrium was
minced and digested with collagenase type I (Gibco; Thermo Fisher
Scientific, Inc.) for 45 min at room temperature. After filtrating
through a stainless wire mesh (200 µm) and gentle centrifugation at
250 × g for 25 min at room temperature, ESCs or NESCs were isolated
via passing over a stainless wire mesh (400 µm). Subsequently,
resuspended cells were layered over Ficoll (Beijing Solarbio
Science & Technology Co., Ltd.), centrifuged at 2,000 × g for
25 min at room temperature and cells in the middle layer were
collected. ESCs or NESCs were cultured in DMEM/F-12 (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (HyClone; GE
Healthcare Life Sciences) with 5% CO2 at 37°C.
EM mouse model, and isolation of ESCs
and NESCs
The EM mouse model was established using C57BL/6
female mice (age, 8 weeks; weight, 25–30 g; n=10; n=5 per group;
Shanghai Model Organisms Center, Inc.). Mice were divided into two
groups (n=5 per group): i) EM and ii) Control. All experimental
procedures were approved by the Ethics Committee for Animal
Experimentation of Shanghai Shuguang Hospital. Mice were housed in
a barrier unit in a sterile environment at 22°C with 40–80%
humidity, 12-h light/dark cycles, and ad libitum access to
food and water. Mice were anesthetized with an intraperitoneal
injection of chloral hydrate (350 mg/kg). Animals did not exhibit
signs of peritonitis following the administration of chloral
hydrate. Endometrial pieces (1 mm3) isolated from
ovarian endometriotic samples from patients with EM were suspended
in saline and 400 µl suspension was injected into the peritoneal
cavity of the mice. At 3 weeks after model establishment, mice were
euthanized by cervical dislocation. Sections of endometrial tissue
were isolated from control mice and EM model mice. Tissue sections
were washed twice with PBS and cultured in DMEM/F12 medium
supplemented with 20% FBS at 37°C with 5% CO2. The
culture medium was changed with the appearance of a large number of
desquamated endothelial cells (every 3 days). Non-adherent cells
were removed carefully. At 80–90% confluence, the culture medium
was removed, cells were washed twice with PBS and then treated with
0.25% trypsin. Subsequently, the cell concentration was adjusted to
1.0×106/ml using DMEM medium supplemented with 10% FBS.
Cells were cultured in DMEM/F12 supplemented with 10% FBS at 37°C
with 5% CO2.
Transfection
miR-143-3p mimic (5′-UGAGAUGAAGCACUGUAGCUC-3′),
2′-O-methyl-modified anti-miR-143-3p (5′-GAGCUACAGUGCUUCAUCUCA-3′),
miR-143-3p mimic negative control (NC;
5′-UCACAACCUCCUAGAAAGAGUAGA-3′) and anti-miR-143-3p NC
(5′-UACUCUUUCUAGGAGGUUGUGAUU-3′) were obtained from Shanghai
GenePharma Co., Ltd. Cells (1×106) were transfected with
20 ng/ml miR-143-3p mimic, anti-miR-143-3p, miR-143-3p mimic NC or
anti-miR-143-3p NC using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h at 37°C. At
48 h post-transfection, cells were collected for subsequent
experiments.
Total RNA was extracted from 293T cells using
TRIzol® reagent (Takara Bio, Inc.). Total RNA was
reverse transcribed into cDNA using the PrimeScript™ RT reagent kit
(Takara Bio, Inc.). The full-length cDNA of Autophagy-related 2B
(ATG2B) was cloned into the pcDNA3.1 vector (Invitrogen; Thermo
Fisher Scientific, Inc.). The sequences of the primers used to
amplify ATG2B were as follows: forward, 5′-GGAGCCACTCTCCAGCATAG-3′
and reverse, 5′-GTGCACAGCTCCAAAGATGA-3′. The following
thermocycling conditions were used: Incubation at 50°C for 2 min;
95°C for 2 min; followed by 40 cycles of 95°C for 15 sec and 60°C
for 32 sec. NESCs were seeded into multiple-well plates at ~80%
confluence. Cells were transfected with recombinant plasmids (1.5
µg per well) using Lipofectamine® 2000 (Invitrogen) at
room temperature for 6 h according to the manufacturer's protocol.
At 48 h post-transfection, subsequent experiments were performed.
pcDNA3.1 was used as a negative control.
Luciferase reporter assay
To confirm the potential target genes of miR-143-3p
in ESCs, the present study searched for candidate genes using
TargetScan (version 7.1; www.targetscan.org/vert_71) and miRBase22 (www.mirbase.org) databases. A 3′-untranslated region
(UTR) luciferase reporter vector of ATG2B containing the predicted
binding sites of miR-143-3p was produced by cloning the 3′-UTR of
the corresponding mRNA into the pGL3-promoter vector (Promega
Corporation). Subsequently, 293T cells (1×104) were
co-transfected with 1 ng pRL-TK (Promega Corporation), 100 ng
pGL3-ATG2B-3′-UTR-wild-type (WT) or pGL3-ATG2B-3′-UTR-mutant (mut)
and 20 nM miR-143-3p mimic or miR-143-3p mimic NC using
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.). At
72 h post-transfection, luciferase activities were measured using a
Dual-Luciferase Reporter Assay System (Promega Corporation)
according to the manufacturer's protocol. Firefly luciferase
activity was normalized to Renilla luciferase activity.
Immunofluorescence
Autophagy vacuoles were assessed using an Autophagy
Detection kit (cat. no. ab139484; Abcam) according to the
manufacturer's protocol. A coverslip was placed into each well of a
24-well plate. ESCs were seeded (2×104) into the 24-well
plate and incubated at 37°C for 1 day. Following washing three
times with PBS, cells were fixed with 4% cold paraformaldehyde for
15 min and then washed three times with PBS. After blocking with 1%
BSA (Sigma-Aldrich; Merck KGaA) for 30 min at room temperature, the
fluorescent dyes for nuclei staining and autophagy detection were
added and incubated for 30 min at room temperature. After washing
three times using PBS, the slides were observed using a
FV1000s-SIM/IX81 confocal laser scanning microscope (Olympus
Corporation). The ratio of green to blue fluorescence was
calculated to assess the degree of autophagy.
RNA isolation and reverse
transcription quantitative PCR (qPCR)
Total RNA was extracted from ESCs and NESCs using
TRIzol® reagent (Takara Bio, Inc.). Total RNA was
reverse transcribed into cDNA using the PrimeScript™ RT reagent kit
(Takara Bio, Inc.) at 37°C. Subsequently, qPCR was performed using
SYBR-Green PCR Master Mix (Takara Bio, Inc.) on an ABI Step One
Plus™ real-time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following thermocycling conditions were used
for qPCR: 95°C for 10 min; followed by 35 cycles of 95°C for 10
sec, 58°C for 15 sec and 72°C for 20 sec; and final extension at
72°C for 20 min. The following primers were used for qPCR:
miR-143-3p forward, 5′-CTGGCGTTGAGATGAAGCAC-3′ and reverse,
5′-CAGAGCAGGGTCCGAGGTA-3′; miR-125b-5p forward,
5′-TCCCTGAGACCCTAACTTGTGA-3′ and reverse,
5′-AGTCTCAGGGTCCGAGGTATTC-3′; miR-150-5p forward,
5′-TCGGCGTCTCCCAACCCTTGTAC-3′ and reverse,
5′-GTCGTATCCAGTGCAGGGTCCGAGGT-3′; ATG2B forward,
5′-TCCTTCAGGAAGAACAAAGCA-3′ and reverse,
5′-AAGCCTTACACGTGTGTCCA-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′; and GAPDH forward,
5′-ATTCCACCCATGGCAAATTC-3′ and reverse,
5′-TGGGATTTCCATTGATGACAAG-3′. miRNA and mRNA expression levels were
quantified using the 2−ΔΔCq method (20) and normalized to the internal
reference genes U6 and GAPDH, respectively.
Western blotting
Total protein was extracted from ESCs using RIPA
buffer (Cell Signaling Technology, Inc.). Total protein was
quantified using a BCA kit (Takara Bio, Inc.). Proteins (20 µg)
were separated via 12% SDS-PAGE and transferred to PVDF membranes.
After blocking with 5% BSA (in TBS/0.05% Tween-20 buffer) for 2 h
at room temperature, the membranes were incubated at 4°C overnight
with primary antibodies (all purchased from Abcam) targeted against
the following: ATG2B (1:5,000; cat. no. ab226832), p62 (1:1,000;
cat. no. ab109012) and microtubule associated protein 1 light chain
3α (LC3)-I/II (1:2,000; cat. no. ab128025) and actin (1:5,000; cat.
no. ab8226). Following primary incubation, the membranes were
incubated with a Goat Anti-Mouse IgG H&L (HRP) secondary
antibody (1:2,000; cat. no. ab205719; Abcam) for 1 h at room
temperature. Protein bands were visualized using an ECL kit
(Pierce; Thermo Fisher Scientific, Inc.). Protein expression levels
were semi-quantified using the Odyssey Infrared Imaging System
(version 3.0; LI-COR Biosciences) with actin as the loading
control.
Transwell assays
A Transwell assay was performed to assess cell
invasion. The Transwell insert (pore size, 8 µm; Corning Life
Sciences) was precoated with Matrigel for 4 h at 37°C. ESCs were
seeded (5×104) into the upper chamber in serum-free
medium and DMEM/F-12 medium supplemented with 10% FBS was plated
into the lower chamber. Cells were incubated at 37°C with 5%
CO2 for 1 day. Subsequently, invading cells were fixed
with 4% paraformaldehyde for 40 min at room temperature and stained
with 1% crystal violet staining solution for 15 min at room
temperature. Stained cells were quantified by counting the number
of cells in five randomly selected fields of view using a light
microscope.
Cell proliferation assay
Cell proliferation was assessed by conducting a Cell
Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.)
according to the manufacturer's protocol. Briefly, cells were
seeded (5×103 cells/well) into 96-well plates.
Subsequently, at 0, 24, 48 and 72 h, 10 µl CCK-8 reagent was added
to each well and incubated for 4 h. The absorbance was measured at
a wavelength of 450 nm using a VersaMax microplate reader
(Molecular Devices, LLC).
Statistical analysis
Data are presented as the mean ± SD. All experiments
were repeated at least three times. Statistical analyses were
performed using SPSS software (version 15; SPSS, Inc.). Comparisons
between two groups were analyzed using the Mann-Whitney U test
(Fig. 1) or unpaired Student's
t-test (Figs. 2, 3, and 4B, C
and F). Comparisons among multiple groups were analyzed using
one-way ANOVA followed by Scheffé's post hoc test (Fig. 4D and E). P<0.05 was considered to
indicate a statistically significant difference.
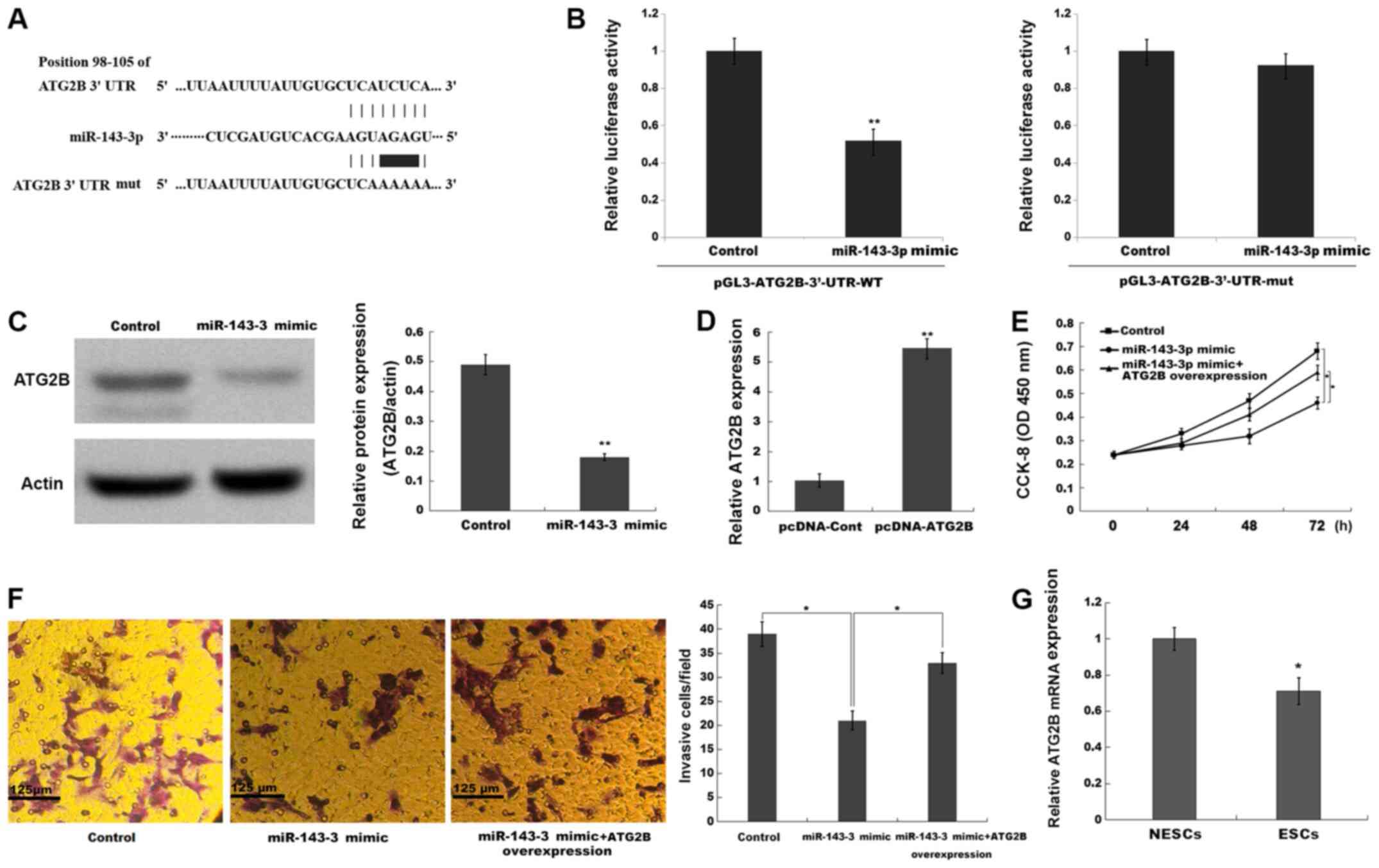 | Figure 4.miR-143-3p inhibits ESC proliferation
and invasion by repressing ATG2B. (A) The binding sites between
miR-143-3p and the 3′-UTR of ATG2B. (B) The luciferase reporter
assay was performed in ESCs co-transfected with miR-143-3p mimic or
negative control, pGL3-ATG2B-3′-UTR-WT or pGL3-ATG2B-3′-UTR-mut and
pRL-TK. **P<0.001 vs. control. (C) ATG2B protein expression
levels were determined via western blotting and semi-quantified.
(D) Transfection efficiency of pcDNA-ATG2B. Compared with the
control group, miR-143-3p overexpression repressed ESC (E)
proliferation and (F) invasion, whereas ATG2B overexpression
reversed miR-143-3p-mimic mediated effects. (G) ATG2B mRNA
expression levels in ESCs and NESCs isolated from endometriosis
model mice. *P<0.05 and **P<0.01 vs. control or NESCs. miR,
microRNA; ESC, endometriotic stromal cell; ATG2B, autophagy-related
2B; UTR, untranslated region; WT, wild-type; mut, mutant; CCK-8,
Cell Counting Kit-8; OD, optical density; NESC, normal endometrial
stromal cell; cont, control. |
Results
miR-143-3p is markedly upregulated in
ESCs
A number of studies have demonstrated that miRNAs
participate in the development and progression of EM via multiple
mechanisms (21,22). Abdel-Rasheed et al (23) reported that 32 miRNAs were
significantly dysregulated in EM and Cosar et al (13) demonstrated that there are 10 miRNAs
that may serve as diagnostic markers of EM. The present study
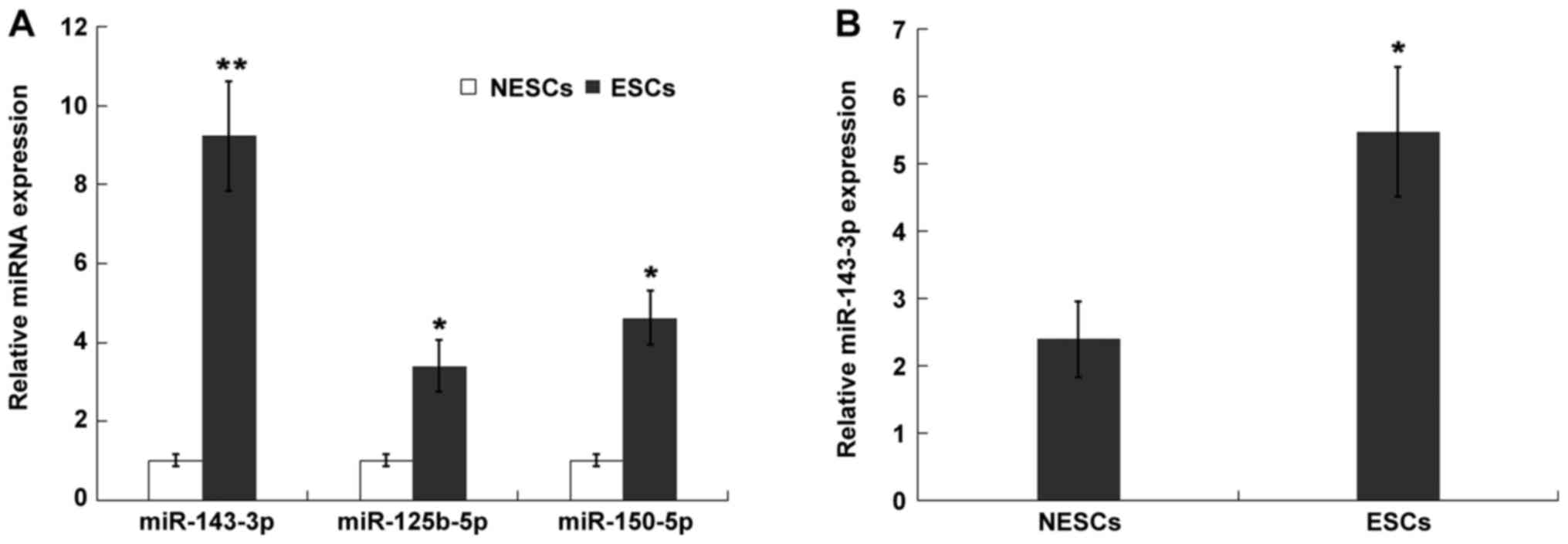
re-analyzed the expression level of three upregulated miRNAs
(miR-125b-5p, miR-150-5p and miR-143-3p) that appeared in both
datasets (Fig. S1A). The result
was verified in ESCs and NESCs. The expression levels of
miR-125b-5p, miR150-5p and miR-143-3p were significantly increased
in ESCs compared with NESCs (Fig.
1A). In the present study, as miR-143-3p was the most
upregulated miRNA among the three miRNAs in ESCs, miR-143-3p was
further investigated. Previous studies have also demonstrated that
miR-143-3p is upregulated in ectopic endometrial tissues compared
with eutopic endometrial tissues (7,24). To
further verify the in vitro results, the present study
established an EM mouse model and assessed miR-143-3p expression
levels in isolated ESCs. miR-143-3p expression levels were
significantly increased in ESCs compared with NESCs (Fig. 1B).
miR-143-3p inhibits ESC proliferation
and invasion
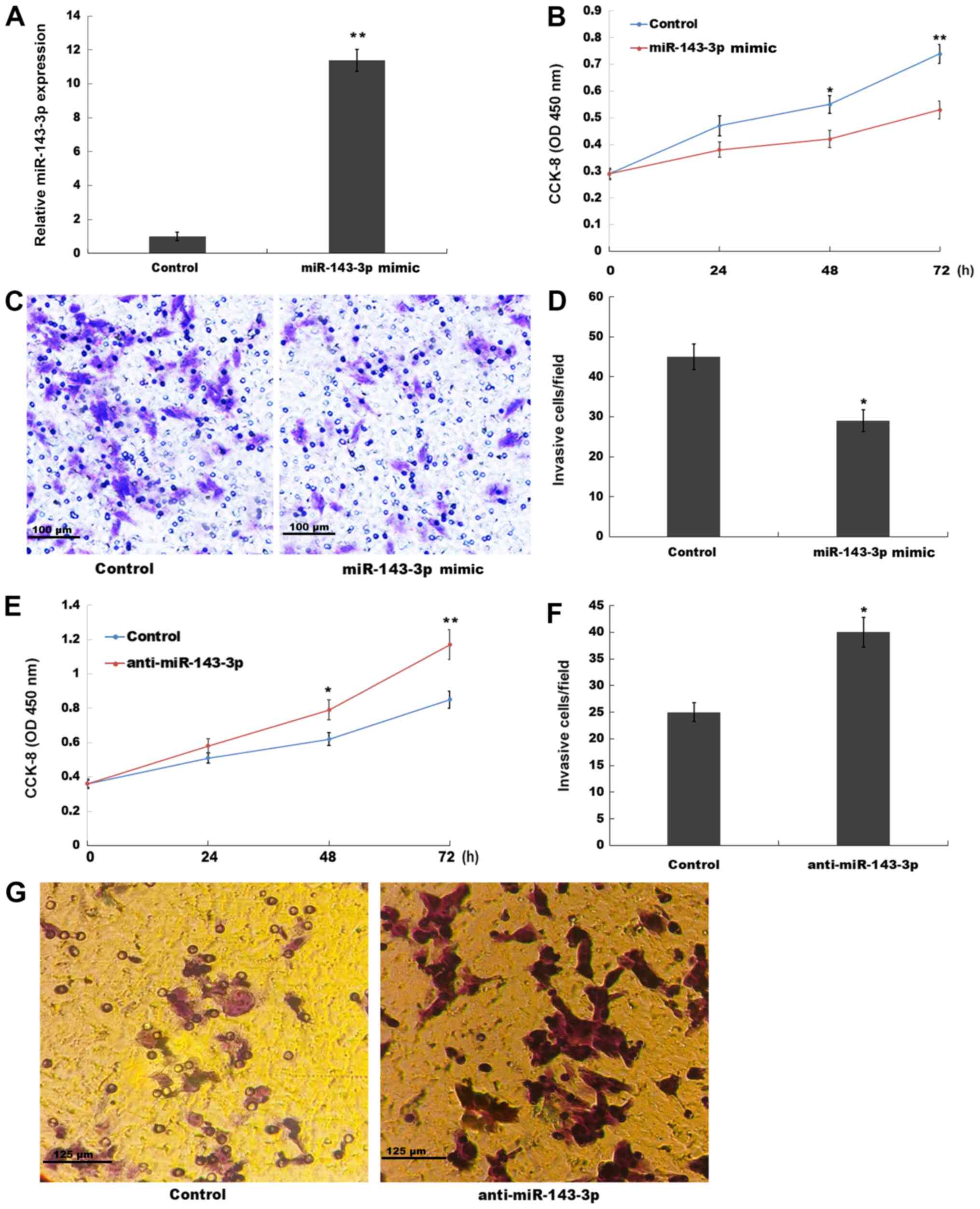
The role of miR-143-3p in ESC proliferation and
invasion was investigated in the present study. miR-143-3p was
overexpressed in ESCs and subsequently, cell proliferation and
invasion were assessed by performing CCK-8 and Transwell invasion
assays, respectively. miR-143-3p mimic significantly increased
miR-143-3p expression levels in ESCs compared with the control
group (Fig. 2A). The CCK-8 assay
results indicated that miR-143-3p overexpression also significantly
suppressed ESC proliferation at the 48 and 72 h time points
compared with the control group (Fig.
2B). The present study also assessed the role of miR-143-3p
overexpression in ESC invasion. The Transwell invasion assay
results indicated that miR-143-3p overexpression significantly
inhibited ESC invasion compared with the control group (Fig. 2C and D). By contrast, compared with
the control group, miR-143-3p knockdown significantly enhanced ESC
proliferation at 48 and 72 h, and significantly increased ESC
invasion (Figs. S1B and 2E-G). The aforementioned results suggested
that miR-143-3p overexpression inhibited EM progression.
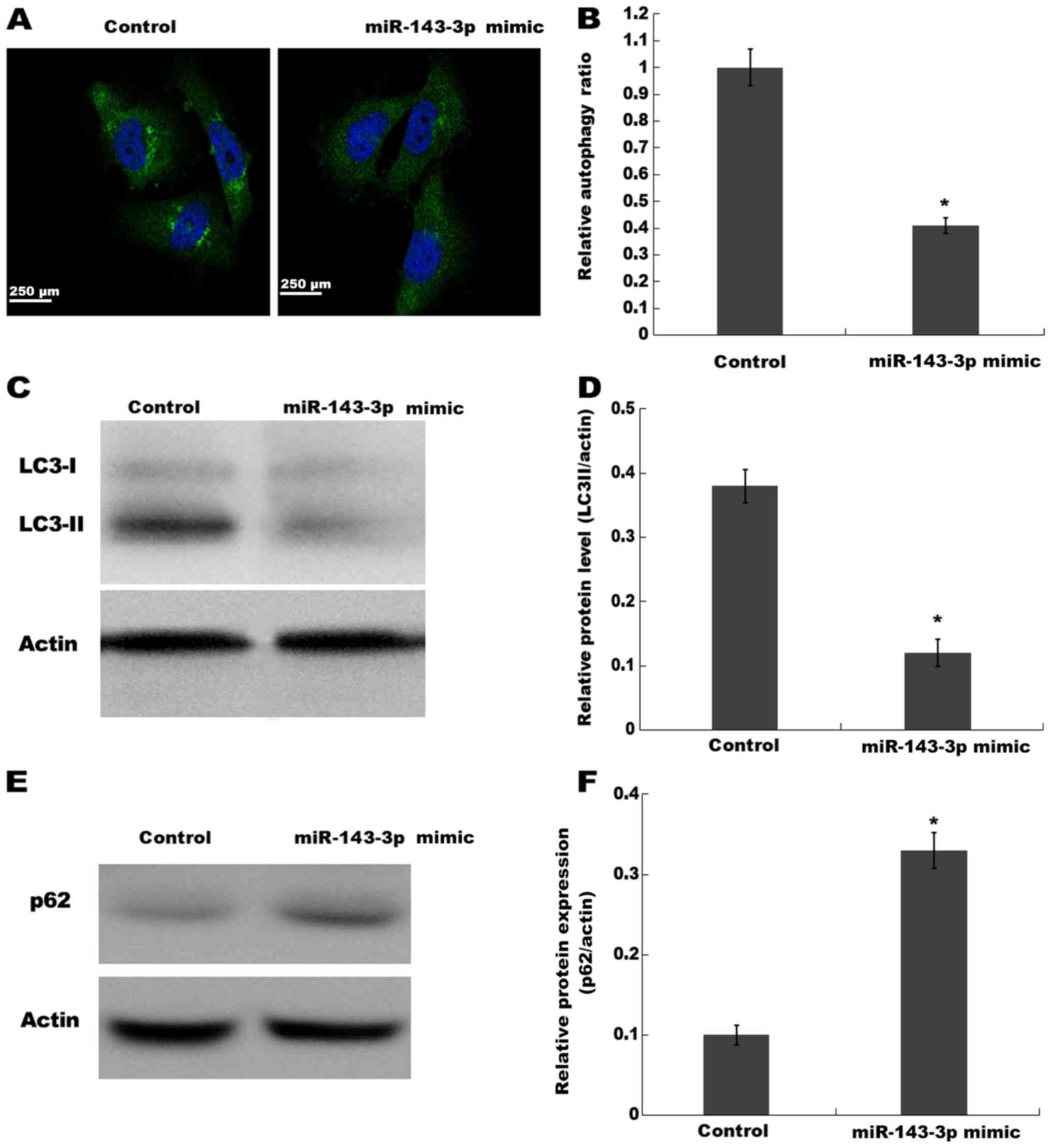
miR-143-3p suppresses autophagy
activation in ESCs
Previous studies have observed autophagy activation
in ectopic endometrium of patients with ovarian endometriosis
(17,25,26).
Based on the finding that miR-143-3p displays the potential to
regulate autophagy in other diseases (27,28),
the present study investigated whether miR-143-3p inhibited ESC
proliferation and invasion via inactivating autophagy. The
activation of autophagy was analyzed using an autophagy detection
kit and western blotting. Compared with the control group,
miR-143-3p overexpression significantly decreased the autophagy
ratio, as evidenced by a decreased number of green puncta, which
represented autophagic vacuoles (Fig.
3A and B). miR-143-3p overexpression also significantly
decreased LC3-II expression levels (a reliable indicator of
autophagy) in ESCs compared with the control group, indicating
inhibition of autophagy (Fig. 3C and
D). Furthermore, miR-143-3p overexpression significantly
increased p62 (an autophagy substrate) protein expression levels in
ESCs compared with the control group (Fig. 3E and F). The results indicated that
miR-143-3p overexpression inhibited autophagy activation in
ESCs.
miR-143-3p suppresses ESC
proliferation and invasion by repressing ATG2B
To confirm the potential target genes of miR-143-3p
in ESCs, the present study searched for candidate genes using
TargetScan (version 7.1; www.targetscan.org/vert_71) and miRBase22 (www.mirbase.org) databases. Bioinformatics analysis
using TargetScan and miRBase identified 487 potential target genes.
Among the identified target genes, ATG2B was the only gene
associated with the autophagy signaling pathway (29). The bioinformatics analysis indicated
that miR-143-3p directly targeted the 3′-UTR of the ATG2B gene, an
essential regulator of autophagy activation (30) (Fig.
4A). To verify whether miR-143-3p directly bound to the 3′-UTR
of ATG2B and repressed its expression, the present study
constructed a luciferase reporter vector containing the 3′-UTR of
ATG2B. The results indicated that miR-143-3p mimic significantly
inhibited the luciferase activity of pGL3-ATG2B-3′-UTR-WT compared
with the control group, whereas miR-143-4p mimic displayed no
significant effect on pGL3-ATG2B-3′-UTR-mut compared with the
control group (Fig. 4B).
Furthermore, miR-143-3p overexpression significantly decreased the
protein expression levels of ATG2B in ESCs compared with the
control group (Fig. 4C). In
addition, compared with the control group, miR-143-3p
overexpression significantly inhibited ESC proliferation and
invasion, whereas ATG2B overexpression significantly alleviated
miR-143-3p mimic-mediated effects (Fig.
4D-F). The present study also assessed the expression levels of
ATG2B in ESCs derived from the EM mouse model. The results
indicated that ATG2B expression levels were significantly decreased
in ESCs compared with NESCs (Fig.
4G). Collectively, the aforementioned results suggested that
miR-143-3p overexpression inhibited EM progression by repressing
ATG2B expression, thus inactivating autophagy.
Discussion
In the present study, the effect of miRNAs on
regulating EM progression was investigated. miR-143-3p expression
was significantly upregulated in ESCs compared with NESCs.
miR-143-3p overexpression markedly decreased ESC cell proliferation
and invasion compared with the control group. In addition, compared
with the control group, miR-143-3p overexpression significantly
decreased LC3-II expression levels and increased p62 expression
levels, indicating that miR-143-3p may serve as an inhibitor of
autophagy activation in ESCs. Furthermore, the present study
verified that miR-143-3p directly targeted the 3′-UTR of ATG2B, and
miR-143-3p overexpression significantly decreased the protein
expression levels of ATG2B compared with the control group. ATG2B
overexpression partially reversed miR-143-3p mimic-mediated effects
on ESC proliferation and invasion.
As a gynecological disease, EM frequently results in
infertility and chronic pelvic pain (31). Immune disorders affect ectopic
endometrial lesions. Following dysfunction of the immune system,
the number of immune cells increases, and various growth factors,
cytokines, non-specific immunoglobulins and proinflammatory
mediators are present in the peritoneum (32–34). A
theory of EM is the local hypoxia microenvironment, whereby the
first indicator of EM is the topical shifted hypoxic
microenvironment, when the shed endometrial fragments retrograde to
the peritoneal cavity (35–37).
Autophagy is a highly conserved cellular process,
which is activated by various factors, such as hypoxia (38). For the past few years, research has
focused on autophagy progression in tumorigenesis (39,40).
EM displays certain biological characteristics of tumor diseases,
such as hyperproliferation and metastasis (41) Increasing evidence has demonstrated
that autophagy is dysregulated in the uterine horns and eutopic
endometria of EM model mice (42),
and is correlated with endometrial regulation and the
pathophysiology of EM (43,44). However, the role of autophagy in EM
is controversial, thus whether autophagy in EM is beneficial or
detrimental remains to be elucidated. Several studies have
demonstrated that the expression levels of autophagy-related genes
(for example, Beclin-1 and LC3-II) are markedly decreased and
autophagy activation is downregulated in endometrial stromal cells
of patients with EM compared with healthy controls (45–47).
Functionally, autophagy inhibition contributes to endometrial cell
invasion, whereas autophagy activation represses ESC proliferation,
colony formation and invasion (48). By contrast, other studies have
reported that the expression levels of autophagy-associated genes
[for example, Beclin-1, autophagy-related (ATG)14, ATG7 and LC3-II]
are increased and autophagy activation is upregulated in ESCs in EM
(17). Liu et al (44) further demonstrated that HIF-1α
facilitates endometrial stromal cell invasion by activating
autophagy, whereas autophagy inhibition alleviates hypoxia-induced
cell invasion. The present study demonstrated that miR-143-3p
overexpression inhibited ESC proliferation and invasion by
regulating ATG2B in EM, whereas ATG2B overexpression partially
reversed miR-143-3p overexpression-mediated effects, indicating
that autophagy may be beneficial in EM. Although the effect of
miR-143-3p on inhibiting autophagy has been verified in Crohn's
disease (27), the present study
aimed to investigate the association of miR-143-3p with ATG2B and
autophagy in EM. The present study further clarified the function
of miR-143-3p in EM. However, a key limitation of the present study
was that the role of miR-143-3p in NESC autophagy was not
investigated.
A previous study demonstrated that miRNAs are
crucial modulators in the occurrence and development of various
diseases (49–52). Several studies have implicated that
the aberrant expression of miRNAs is a potential regulator of EM
pathogenesis (11,23). It has been verified that the
expression levels of HIF-1α and VEGF were elevated in ectopic
endometrial tissues compared with eutopic endometrial tissues,
which was induced by hypoxia stress. miR-17-5p/20a is a regulator
of HIF-1α and VEGF via directly targeting the 3′-UTR, and further
regulates downstream hypoxic stress-associated proteins (53–55).
The present study investigated the function of miR-143-3p in EM and
demonstrated that miR-143-3p overexpression notably inhibited ESC
proliferation and invasion in EM compared with the control group.
In hepatocellular carcinoma, upregulated miR-143-3p promotes cancer
cell migration and invasion by repressing fibronectin type III
domain containing 3B (56).
Therefore, the results of the present study and the aforementioned
previous studies suggested that miR-143-3p may serve various
regulatory roles in different biological processes or diseases.
In summary, miR-143-3p was significantly upregulated
in ESCs compared with NESCs. miR-143-3p regulated the phenotype of
EM, suppressing autophagy activation in ESCs, and inhibiting ESC
proliferation and invasion by directly targeting ATG2B. The results
of the present study may further the current understanding of the
role of miR-143-3p in the pathogenesis of EM.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81704108).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HY and LQ made substantial contributions to the
conception and design of the present study. TH, PH, CQ and LQ
collected, analyzed and interpreted the data. PH, CQ and LQ drafted
the work and made critical modifications to the manuscript. All
authors agreed to be accountable for the work in ensuring that
questions related to the integrity of any part of the work are
appropriately investigated and resolved. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Protection of
Human Subjects Committee of Shanghai Shuguang Hospital and the
Ethics Committee for Animal Experimentation of Shanghai Shuguang
Hospital (approval no. 2018-618-47-01). All patients or their legal
guardians provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
miRNAs
|
microRNAs
|
|
EM
|
endometriosis
|
|
ESCs
|
endometriotic stromal cells
|
|
NESCs
|
normal endometrial stromal cells
|
|
ATG2B
|
autophagy-related 2B
|
References
|
1
|
Eskenazi B and Warner ML: Epidemiology of
endometriosis. Obstet Gynecol Clin North Am. 24:235–258. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simoens S, Dunselman G, Dirksen C,
Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL,
DeLeire T, et al: The burden of endometriosis: Costs and quality of
life of women with endometriosis and treated in referral centres.
Hum Reprod. 27:1292–1299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shafrir AL, Farland LV, Shah DK, Harris
HR, Kvaskoff M, Zondervan K and Missmer SA: Risk for and
consequences of endometriosis: A critical epidemiologic review.
Best Pract Res Clin Obstet Gynaecol. 51:1–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Friend DR: Drug delivery for the treatment
of endometriosis and uterine fibroids. Drug Deliv Transl Res.
7:829–839. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vishnoi A and Rani S: miRNA biogenesis and
regulation of diseases: An overview. Methods Mol Biol. 1509:1–10.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arora S, Rana R, Chhabra A, Jaiswal A and
Rani V: miRNA-transcription factor interactions: A combinatorial
regulation of gene expression. Mol Genet Genomics. 288:77–87. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Filigheddu N, Gregnanin I, Porporato PE,
Surico D, Perego B, Galli L, Patrignani C, Graziani A and Surico N:
Differential expression of microRNAs between eutopic and ectopic
endometrium in ovarian endometriosis. J Biomed Biotechnol.
2010:3695492010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao L, Gu C, Ye M, Zhang Z, Li L, Fan W
and Meng Y: Integration analysis of microRNA and mRNA paired
expression profiling identifies deregulated microRNA-transcription
factor-gene regulatory networks in ovarian endometriosis. Reprod
Biol Endocrinol. 16:42018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi H, Shen H, Xu J, Zhao S, Yao S and
Jiang N: MiR-143-3p suppresses the progression of ovarian cancer.
Am J Transl Res. 10:866–874. 2018.PubMed/NCBI
|
|
10
|
Deng L, Blanco FJ, Stevens H, Lu R,
Caudrillier A, McBride M, McClure JD, Grant J, Thomas M, Frid M, et
al: MicroRNA-143 Activation regulates smooth muscle and endothelial
cell crosstalk in pulmonary arterial hypertension. Circ Res.
117:870–883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teague EM, Print CG and Hull ML: The role
of microRNAs in endometriosis and associated reproductive
conditions. Hum Reprod Update. 16:142–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng B, Xue X, Zhao Y, Chen J, Xu CY and
Duan P: The differential expression of microRNA-143,145 in
endometriosis. Iran J Reprod Med. 12:555–560. 2014.PubMed/NCBI
|
|
13
|
Cosar E, Mamillapalli R, Ersoy GS, Cho S,
Seifer B and Taylor HS: Serum microRNAs as diagnostic markers of
endometriosis: A comprehensive array-based analysis. Fertil Steril.
106:402–409. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bravo-San Pedro JM, Kroemer G and Galluzzi
L: Autophagy and mitophagy in cardiovascular disease. Circ Res.
120:1812–1824. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Menzies FM, Fleming A, Caricasole A, Bento
CF, Andrews SP, Ashkenazi A, Füllgrabe J, Jackson A, Jimenez
Sanchez M, Karabiyik C, et al: Autophagy and neurodegeneration:
Pathogenic mechanisms and therapeutic opportunities. Neuron.
93:1015–1034. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
White E, Mehnert JM and Chan CS:
Autophagy, metabolism, and cancer. Clin Cancer Res. 21:5037–5046.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allavena G, Carrarelli P, Del Bello B,
Luisi S, Petraglia F and Maellaro E: Autophagy is upregulated in
ovarian endometriosis: A possible interplay with p53 and heme
oxygenase-1. Fertil Steril. 103:1244–51.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neshkov NS: Use of ultrasound in therapy
of neuroreceptor forms of impotence. Vopr Kurortol Fizioter Lech
Fiz Kult. 35:2701970.(In Russian). PubMed/NCBI
|
|
19
|
Shi YL, Luo XZ, Zhu XY, Hua KQ, Zhu Y and
Li DJ: Effects of combined 17beta-estradiol with TCDD on secretion
of chemokine IL-8 and expression of its receptor CXCR1 in
endometriotic focus-associated cells in co-culture. Hum Reprod.
21:870–879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mari-Alexandre J, Sanchez-Izquierdo D,
Gilabert-Estelles J, Barcelo-Molina M, Braza-Boils A and Sandoval
J: miRNAs regulation and its role as biomarkers in endometriosis.
Int J Mol Sci. 17:932016. View Article : Google Scholar
|
|
22
|
Okamoto M, Nasu K, Abe W, Aoyagi Y, Kawano
Y, Kai K, Moriyama M and Narahara H: Enhanced miR-210 expression
promotes the pathogenesis of endometriosis through activation of
signal transducer and activator of transcription 3. Hum Reprod.
30:632–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abdel-Rasheed M..Nour Eldeen G..Mahmoud
M..El Hefnawi M..Abu-Shahba N..Reda M..Elsetohy K..Nabil
M..Elnoury, et al: MicroRNA expression analysis in endometriotic
serum treated mesenchymal stem cells. EXCLI journal. 16:852–867.
2017.PubMed/NCBI
|
|
24
|
Ohlsson Teague EM, Van der Hoek KH, Van
der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, Print CG and
Hull LM: MicroRNA-regulated pathways associated with endometriosis.
Mol Endocrinol. 23:265–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He R, Liu X, Zhang J, Wang Z, Wang W, Fu
L, Fan Y, Sun S, Cao Y, Zhan L, et al: NLRC5 inhibits inflammation
of secretory phase ectopic endometrial stromal cells by
up-regulating autophagy in ovarian endometriosis. Front Pharmacol.
11:12812020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding Y, Zhu Q, He Y, Lu Y, Wang Y, Qi J,
Wu H, Xu R, Li J, Li X, et al: Induction of autophagy by Beclin-1
in granulosa cells contributes to follicular progesterone elevation
in ovarian endometriosis. Transl Res. 227:15–29. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma W, Ding F, Wang X, Huang Q, Zhang L, Bi
C, Hua B, Yuan Y, Han Z, Jin M, et al: By Targeting Atg7
MicroRNA-143 mediates oxidative stress-induced autophagy of
c-Kit+ mouse cardiac progenitor cells. EBioMedicine.
32:182–191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin XT, Zheng XB, Fan DJ, Yao QQ, Hu JC,
Lian L, Wu XJ, Lan P and He XS: MicroRNA-143 targets ATG2B to
inhibit autophagy and increase inflammatory responses in Crohn's
disease. Inflamm Bowel Dis. 24:781–791. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei J, Ma Z, Li Y, Zhao B, Wang D and Jin
Y and Jin Y: miR-143 inhibits cell proliferation by targeting
autophagy-related 2B in non-small cell lung cancer H1299 cells. Mol
Med Rep. 11:571–576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao S, Wang K and Wang X: miR-375
targeting autophagy-related 2B (ATG2B) suppresses autophagy and
tumorigenesis in cisplatin-resistant osteosarcoma cells. Neoplasma.
67:724–734. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanbo T and Fedorcsak P:
Endometriosis-associated infertility: Aspects of pathophysiological
mechanisms and treatment options. Acta Obstet Gynecol Scand.
96:659–667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahn SH, Monsanto SP, Miller C, Singh SS,
Thomas R and Tayade C: Pathophysiology and immune dysfunction in
endometriosis. BioMed Res Int. 2015:7959762015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Králíčková M and Vetvicka V: Immunological
aspects of endometriosis: A review. Ann Transl Med.
3:1532015.PubMed/NCBI
|
|
34
|
Olovsson M: Immunological aspects of
endometriosis: An update. Am J Reprod Immunol. 66 (Suppl
1):101–104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Filippi I, Carrarelli P, Luisi S, Batteux
F, Chapron C, Naldini A and Petraglia F: Different expression of
hypoxic and angiogenic factors in human endometriotic lesions.
Reprod Sci. 23:492–497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhan L, Wang W, Zhang Y, Song E, Fan Y and
Wei B: Hypoxia-inducible factor-1alpha: A promising therapeutic
target in endometriosis. Biochimie. 123:130–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsuzuki T, Okada H, Cho H, Tsuji S,
Nishigaki A, Yasuda K and Kanzaki H: Hypoxic stress simultaneously
stimulates vascular endothelial growth factor via hypoxia-inducible
factor-1α and inhibits stromal cell-derived factor-1 in human
endometrial stromal cells. Hum Reprod. 27:523–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Daskalaki I, Gkikas I and Tavernarakis N:
Hypoxia and selective autophagy in cancer development and therapy.
Front Cell Dev Biol. 6:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu W, Meng Y, Zong C, Zhang S and Wei L:
Autophagy and tumorigenesis. Adv Exp Med Biol. 1207:275–299. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
White E: The role for autophagy in cancer.
J Clin Invest. 125:42–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Leyendecker G, Kunz G, Noe M, Herbertz M
and Mall G: Endometriosis: A dysfunction and disease of the
archimetra. Hum Reprod Update. 4:752–762. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ruiz A, Rockfield S, Taran N, Haller E,
Engelman RW, Flores I, Panina-Bordignon P and Nanjundan M: Effect
of hydroxychloroquine and characterization of autophagy in a mouse
model of endometriosis. Cell Death Dis. 7:e20592016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhan L, Li J and Wei B: Autophagy in
endometriosis: Friend or foe? Biochem Biophys Res Commun.
495:60–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu H, Zhang Z, Xiong W, Zhang L, Xiong Y,
Li N, He H, Du Y and Liu Y: Hypoxia-inducible factor-1alpha
promotes endometrial stromal cells migration and invasion by
upregulating autophagy in endometriosis. Reproduction. 153:809–820.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pei T, Huang X, Long Y, Duan C, Liu T, Li
Y and Huang W: Increased expression of YAP is associated with
decreased cell autophagy in the eutopic endometrial stromal cells
of endometriosis. Mol Cell Endocrinol. 491:1104322019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sui X, Li Y, Sun Y, Li C, Li X and Zhang
G: Expression and significance of autophagy genes LC3, Beclin1 and
MMP-2 in endometriosis. Exp Ther Med. 16:1958–1962. 2018.PubMed/NCBI
|
|
47
|
Mei J, Zhou WJ, Zhu XY, Lu H, Wu K, Yang
HL, Fu Q, Wei CY, Chang KK, Jin LP, et al: Suppression of autophagy
and HCK signaling promotes PTGS2high FCGR3- NK cell differentiation
triggered by ectopic endometrial stromal cells. Autophagy.
14:1376–1397. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Luo X, Cheng W, Wang S, Chen Z and Tan J:
Autophagy suppresses invasiveness of endometrial cells through
reduction of Fascin-1. BioMed Res Int. 2018:86154352018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao Y and Srivastava D: A developmental
view of microRNA function. Trends Biochem Sci. 32:189–197. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Stolzenburg LR and Harris A: The role of
microRNAs in chronic respiratory disease: Recent insights. Biol
Chem. 399:219–234. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Soroosh A, Koutsioumpa M, Pothoulakis C
and Iliopoulos D: Functional role and therapeutic targeting of
microRNAs in inflammatory bowel disease. Am J Physiol Gastrointest
Liver Physiol. 314:G256–G262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu MH, Chen KF, Lin SC, Lgu CW and Tsai
SJ: Aberrant expression of leptin in human endometriotic stromal
cells is induced by elevated levels of hypoxia inducible
factor-1alpha. Am J Pathol. 170:590–598. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hsiao KY, Lin SC, Wu MH and Tsai SJ:
Pathological functions of hypoxia in endometriosis. Front Biosci
(Elite Ed). 7:309–321. 2015.PubMed/NCBI
|
|
55
|
Donnez J, Smoes P, Gillerot S,
Casanas-Roux F and Nisolle M: Vascular endothelial growth factor
(VEGF) in endometriosis. Hum Reprod. 13:1686–1690. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang X, Liu S, Hu T, Liu S, He Y and Sun
S: Up-regulated microRNA-143 transcribed by nuclear factor kappa B
enhances hepatocarcinoma metastasis by repressing fibronectin
expression. Hepatology. 50:490–499. 2009. View Article : Google Scholar : PubMed/NCBI
|