Introduction
Nasopharyngeal carcinoma (NPC), an epithelial
carcinoma originating from the nasopharyngeal mucosal tissue, is
widely distributed in Southeast Asia, and especially in southern
China (1–3). The incidence of NPC in southern China
is 60 per 100,000 individuals, and the corresponding mortality in
2015 was 34 per 100,000 individuals (4,5).
Metastasis usually occurs in the early stage of NPC and is the main
cause of patient mortality (6).
Therefore, it is urgent for researchers to examine the underlying
mechanism of the development and metastasis of NPC and to identify
potential therapeutic targets for NPC.
Galectin-3 (gal-3), a member of the
β-galactoside-binding protein family, is characterized by a
specific chimeric structure containing a unique carbohydrate
recognition domain and multifunctional N-terminal domain (7). Gal-3 is involved in cellular
homeostasis, organogenesis, angiogenesis, tumor invasion and
metastasis (8). Research has
indicated that the upregulation of gal-3 promotes neoplastic
transformation and contributes to the phenotype maintenance of
malignant breast cells (9,10). In addition, patients with tumor
metastasis exhibited significantly higher concentrations of
circulating gal-3 compared with healthy individuals (11). Gal-3 was also found to induce the
formation of new capillaries in vivo and sustain the
angiogenic capability of tumor-associated macrophages (12,13).
Thus, it was hypothesized that gal-3 may be associated in the
development and metastasis of NPC. ERK1/2 and Akt signaling
pathways are crucial regulators in the development and metastasis
of NPC (14,15). However, whether gal-3 is involved in
the activation of ERK1/2 and Akt signaling pathways remains unknown
and requires further investigation.
In the present study, tumor tissue samples from
patients with NPC and nasopharyngeal tissues from patients with
chronic rhinitis (CR) were collected, and the difference in the
expression levels of gal-3 was investigated. Subsequently, the NPC
cell lines, 5-8F and 6-10B, and the nasopharyngeal epithelium cell
line, NP69, were used to examine the function and potential
mechanism of gal-3 in NPC.
Materials and methods
Collection of human samples
The study was approved by the Ethics Committee of
Xiangya Hospital Central South University. In total, 40 tumor
specimens and 37 serum samples (5 ml) were obtained from 40
patients with NPC, and 15 nasopharyngeal tissues and 26 serum
samples were collected from 26 patients with CR in Xiangya Hospital
Central South University between January 2015 and January 2018.
Written informed consent was obtained from all patients prior to
inclusion in the study. Characteristics of the patients, including
sex, age and TNM stage, were recorded. The tissues were fixed in 4%
formalin at room temperature for 24 h and embedded in paraffin for
subsequent histological experiments, and the serum was stored at
−80°C.
Cell culture
The NPC cell line, 5-8F, was kindly provided by the
Cancer Research Institute of Central South University. The NPC cell
line, 6-10B, and the immortalized nasopharyngeal epithelium cell
line, NP69, were purchased from Hunan Fenghui Biotechnology Co.,
Ltd. 5-8F and 6-10B cells were cultured in high-glucose DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin. NP69 cells were cultured in keratinocyte
serum-free medium (Invitrogen; Thermo Fisher Scientific, Inc.). All
cells were cultured in a 37°C incubator with 5% CO2.
Gal-3 short hairpin (sh)RNA (1,000 ng/µl) and
control shRNA (1,000 ng/µl) (Shanghai Genechem Co., Ltd.) were
transfected into cells at room temperature for 15 min using the
FuGENE HD transfection reagent (Promega Corporation), according to
the manufacturer's recommendation. After a month and a half of
screening, subsequent experiments were performed. The sequences of
the gal-3 shRNA and control shRNA are presented in Tables SI and SII.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated using RNAiso Plus reagent
(Takara Bio, Inc.) and reverse transcribed using the PrimeScript RT
Reagent kit with gDNA Eraser (Takara Bio, Inc.). The temperature
and duration of reverse transcription was: 37°C for 15 min, 85°C
for 5 sec. Relative gene expression levels were measured using SYBR
Premix Ex Taq II (Takara Bio, Inc.). The thermocycling conditions
were: Holding stage, 95°C for 30 sec; PCR stage, 95°C for 5 sec,
58°C for 30 sec, 40 cycles; melt curve stage, 95°C for 15 sec, 60°C
for 60 sec and 95°C for 15 sec. The results were quantitatively
analyzed according to the 2−ΔΔCq method (16). The sequences of the primers used for
RT-qPCR analysis are presented in Table SIII.
Immunoblotting
For immunoblotting, whole cell lysates were prepared
in RIPA buffer (Sigma-Aldrich; Merck KGaA) containing protease
inhibitor and phosphatase inhibitor (Roche Diagnostics). Protein
concentration was determined using a BCA protein assay kit (Thermo
Fisher Scientific, Inc.). Then, protein samples (20 µg) were
separated by SDS-PAGE and subsequently transferred to PVDF
membranes (EMD Millipore). The membranes were blocked in 5% milk
for 1 h at 37°C before incubation with primary mouse monoclonal
anti-gal 3 antibody (1:1,000; cat. no. 60207-1-lg; ProteinTech
Group, Inc.), rabbit monoclonal anti-phosphorylated (p)-Akt (S473)
antibody (1:1,000; cat. no. BS9913M; Bioworld Technology, Inc.),
rabbit polyclonal anti-Akt antibody (1:1,000; cat. no. BS1810;
Bioworld Technology, Inc.), rabbit monoclonal anti-p-ERK1/2
antibody (1:1,000; cat. no. 4370; Cell Signaling Technology, Inc.),
rabbit monoclonal anti-ERK1/2 antibody (1:1,000; cat. no. 4695;
Cell Signaling Technology, Inc.) and rabbit polyclonal anti-GAPDH
antibody (1:5,000; cat. no. 10494-1-AP; ProteinTech Group, Inc.)
overnight at 4°C. Then, the membranes were incubated with
HRP-coupled secondary antibodies (Goat Anti-Rabbit IgG(H+L), HRP
conjugate, 1:10,000; cat. no. SA00001-2 and Goat Anti-mouse
IgG(H+L), HRP conjugate, 1:10,000; cat. no. SA00001-2; ProteinTech
Group, Inc.) for 1 h at 37°C. After washing with PBS, the membranes
were visualized using ECL reagent (cat. no. p10100; NCM Biotech)
and imaged using X-ray film. ImageJ software was used for
densitometry (version number: 1.51v, http://imagej.net/downloads).
ELISA
The concentration of gal-3 in serum was determined
using the Human Galectin-3 ELISA kit (cat. no. E-EL-H1470c;
Elabscience, Inc.) according to the manufacturer's
recommendation.
Immunohistochemical assay
Paraffin-embedded sections (tissues fixed in 4%
formalin at room temperature for 24 h; section thickness, 4 µm)
were deparaffinized and hydrated (100, 95, 80 and 70%). After
antigen retrieval, sections were blocked using normal goat serum
(cat. no. ZLI-9022; OriGene Technologies, Inc.) at room temperature
for 30 min and then were incubated with primary antibodies against
gal-3 (1:500; cat. no. 60207-1-lg; ProteinTech Group, Inc.), IL-6
(1:200, cat. no. ab6672; Abcam) and IL-8 (1:400; cat. no.
27095-1-AP; ProteinTech Group, Inc.). Then, sections were stained
using a detection kit (cat. no. PV9000; OriGene Technologies, Inc.)
at room temperature for 60 sec and hematoxylin at room temperature
for 30 sec (both from OriGene Technologies, Inc.). Images (10
fields for each section) were obtained using light microscope with
a digital camera (Olympus Corporation) at ×400 magnification.
According to the method described by Hara and Okayasu (17), the present study blindly analyzed
the expression levels of gal-3, IL-6 and IL-8 in tissues. The
immunohistochemistry score was determined as follows: Staining
percentage: No positive cells, 0; ≤25% positive cells, 1; 26–50%
positive cells, 2; 51–75% positive cells, 3; and >75% positive
cells, 4; and staining intensity: No staining, 0; weak staining, 1;
moderate staining, 2; and dark staining, 3. Comprehensive
score=staining percentage + staining intensity. Comprehensive
scores of gal-3, IL-6 and IL-8 ≥4 were classified as high
expression; scores of <4 were classified as low expression.
Cell proliferation
5-8F and 6-10B cells were seeded in 96-well plates
at a density of 4,000 cells per well. After 24 h, 20 µl MTT
solution was added. Then, 4 h later, MTT was removed, 100 µl DMSO
was added and the absorbance at 490 nm was measured (Varioskan
Flash; Thermo Scientific, Inc.).
Hoechst staining
5-8F and 6-10B cells (1×103) were
cultured in 96-well plates and incubated at 37°C with 5%
CO2 for 24 h. The cells were washed twice with PBS and
incubated at room temperature with glacial acetic acid/methanol
mixture (glacial acetic acid: Methanol=1:3) for 30 min. After
washing with PBS, the cells were incubated with 1 µg/ml Hoechst
33258 solution for 10 min in the dark at 37°C. Images were then
acquired using a fluorescent PerkinElmer Operetta CLS system
(PerkinElmer, Inc.) at ×400 magnification; six fields were randomly
chosen.
5-Ethynyl-2′-deoxyuridine (Edu)
labeling Assay
5-8F and 6-10B cells (1×103) were
cultured in 96-well plates and incubated at 37°C with 5%
CO2 for 24 h. Cells were incubated with EdU solution (50
µM; B8010; Beijing Solarbio Science & Technology Co., Ltd.) for
2 h at room temperature and then was fixed using 4%
paraformaldehyde at room temperature for 30 min. Cells were
incubated with glycine (50 µl; 2 mg/ml; cat. no. ST085; Beyotime
Institute of Biotechnology) for 5 min at room temperature and
washed with PBS thrice. After that, cells were permeated with 0.5%
Triton X-100 (cat. no. ST797; Beyotime Institute of Biotechnology)
for 10 min at room temperature and stained with Apollo staining
solution (cat. no. CA1170; Beijing Solarbio Science &
Technology Co., Ltd.) for 30 min at room temperature. Subsequently,
cells were washed with PBS thrice and stained with Hoechst 33342
solution (cat. no. B8040; Beijing Solarbio Science & Technology
Co., Ltd.) for 30 min at room temperature. Images were then
acquired using a fluorescent PerkinElmer Operetta CLS system
(PerkinElmer, Inc.) at ×400 magnification; six fields were randomly
chosen.
Scratch assay
5-8F and 6-10B cells were seeded in 6-well plates at
a density of 2×105/ml and incubated at 37°C with 5%
CO2. For the scratch assay, cells were scratched using
10-µl pipette tips. During cultivation, the medium was replaced
with high-glucose DMEM containing 1% FBS. Images of cells were
taken at ×100 magnification after 0, 24 and 48 h (Olympus
Corporation). The wound closure rate [(initial wound area-wound
area at 24 h)/initial wound area] was analyzed using Image Pro Plus
6.0 software (Media Cybernetics, Inc.) (18).
Migration assay
Cells were collected and resuspended in serum-free
DMEM. Then, ~5×104 cells in 200 µl serum-free DMEM were
seeded in the upper chamber, while the lower chamber was filled
with 600 µl DMEM containing 15% FBS. After 24 h incubation at 37°C
with 5% CO2, the cells on the upper face were wiped off,
while those that migrated to the lower face were fixed using 4%
paraformaldehyde at room temperature for 20 min and stained with
crystal violet solution (cat. no. C0121; Beyotime Institute of
Biotechnology) for 5 min. Images were obtained using a microscope
(Olympus Corporation). The number of migrated cells was counted in
three random fields (×200 magnification).
Statistical analysis
All experiments were repeated three times. All
analyses were performed using SPSS 18.0 (SPSS, Inc.) and GraphPad
Prism 8 (GraphPad Software, Inc.). The numerical data are presented
as the mean ± SEM, and were analyzed using an unpaired Student's
t-test or one-way ANOVA followed by Tukey's multiple comparisons
test post hoc test. The Fisher's exact test and Pearson's
correlation were used to analyze the difference in the expression
levels of gal-3 and the relationship between gal-3 and inflammation
cytokines. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of gal-3 are
upregulated in patients with NPC and in NPC cell lines
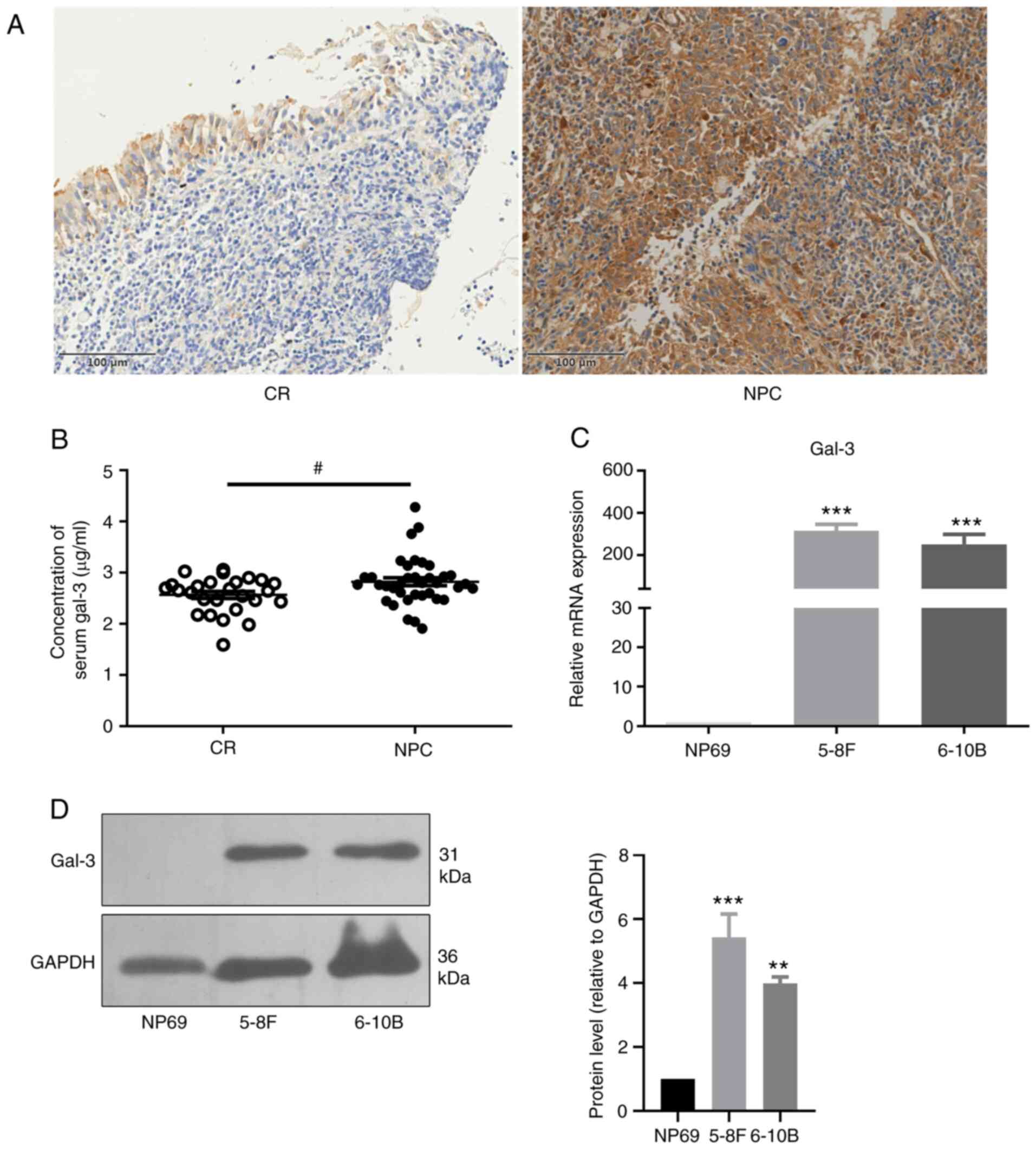
The expression levels of gal-3 in tumor tissues and
nasopharyngeal tissues were determined via immunochemistry. The
results demonstrated that gal-3 was mainly expressed in the
cytoplasm and was significantly upregulated in patients with NPC
compared with patients with CR (Fig.
1A; Table I). Furthermore, the
results of the ELISA showed that the concentration of circulating
gal-3 in patients with NPC was significantly higher compared with
that of patients with CR (Fig. 1B).
The mRNA expression levels of gal-3 in the NPC cell lines, 5-8F and
6-10B, were significantly higher compared with those of NP69, a
nasopharyngeal epithelium cell line (Fig. 1C). In addition, immunoblotting
demonstrated that the expression levels of gal-3 protein in 5-8F
and 6-10B cells were significantly upregulated compared with those
of NP69 cells (Fig. 1D). However,
there was no correlation between the expression levels of gal-3 in
tumor tissues and the TNM stage of patients with NPC (Table SIV).
 | Table I.Expression levels of gal-3 in
patients with NPC compared with patients with CR. |
Table I.
Expression levels of gal-3 in
patients with NPC compared with patients with CR.
|
|
| Expression levels
of gal-3 |
|
|---|
|
|
|
|
|
|---|
| Group | Cases (n) | High
expression | Low expression | P-value |
|---|
| CR | 15 | 7 | 8 | 0.0015 |
| NPC | 40 | 36 | 4 |
|
Knockdown of gal-3 inhibits the
proliferation and migration of NPC cell cells and induces their
apoptosis
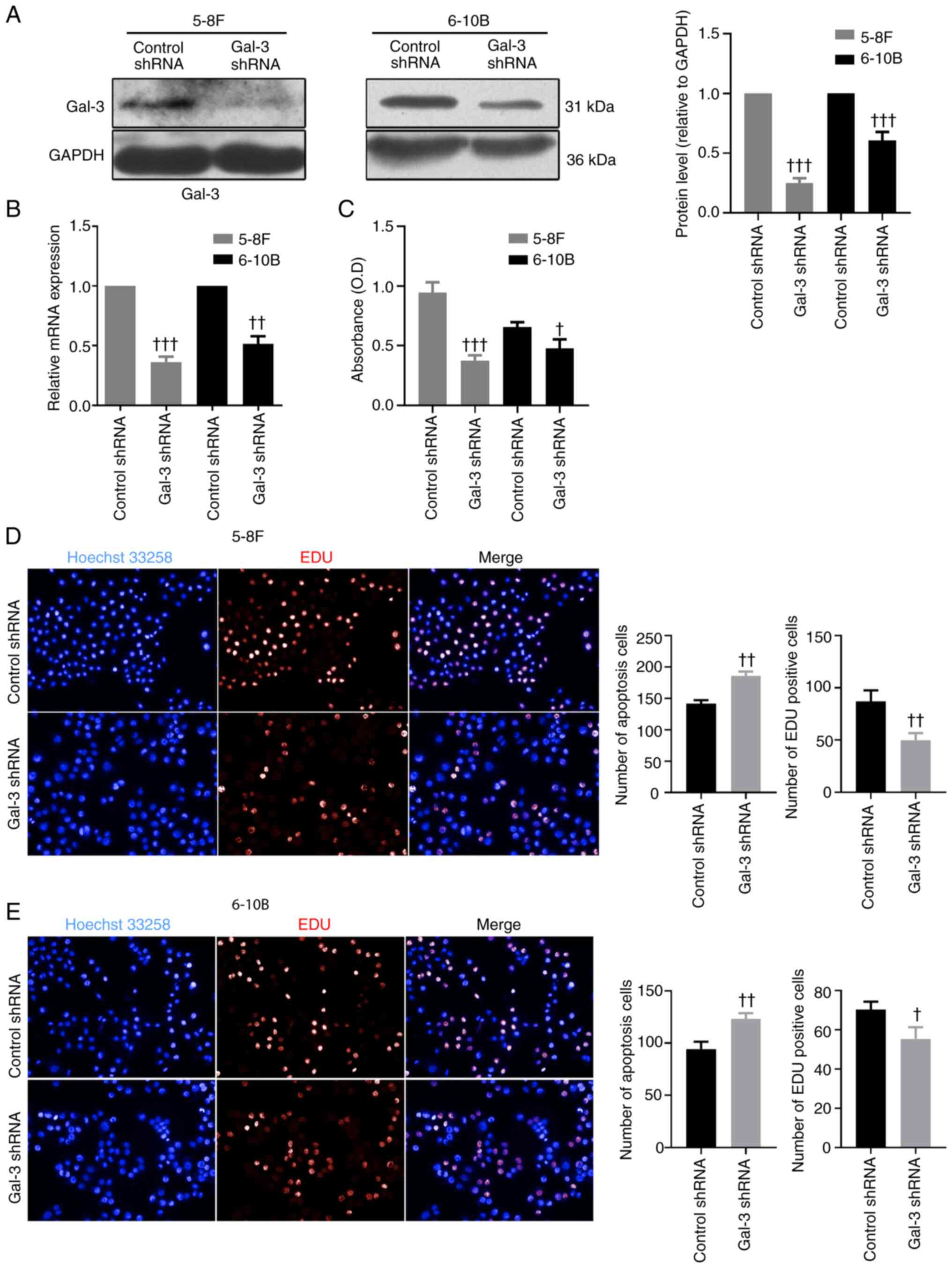
Transfection of gal-3 shRNA was capable of
downregulating the protein and mRNA expression levels of gal-3 in
5-8F and 6-10B cells (Fig. 2A and
B). The MTT proliferation assay demonstrated that, after
transfection with gal-3 shRNA, 5-8F and 6-10B cells exhibited
significantly impaired proliferative capabilities compared with
cells transfected with control shRNA (Fig. 2C). The results of EdU staining
indicated that knockdown of gal-3 significantly inhibited the
proliferation of 5-8F and 6-10B cells, compared with cells
transfected with control shRNA, while the results of Hoechst 33258
staining suggested that knockdown of gal-3 promoted apoptosis in
5-8F and 6-10B cells (Fig. 2D and
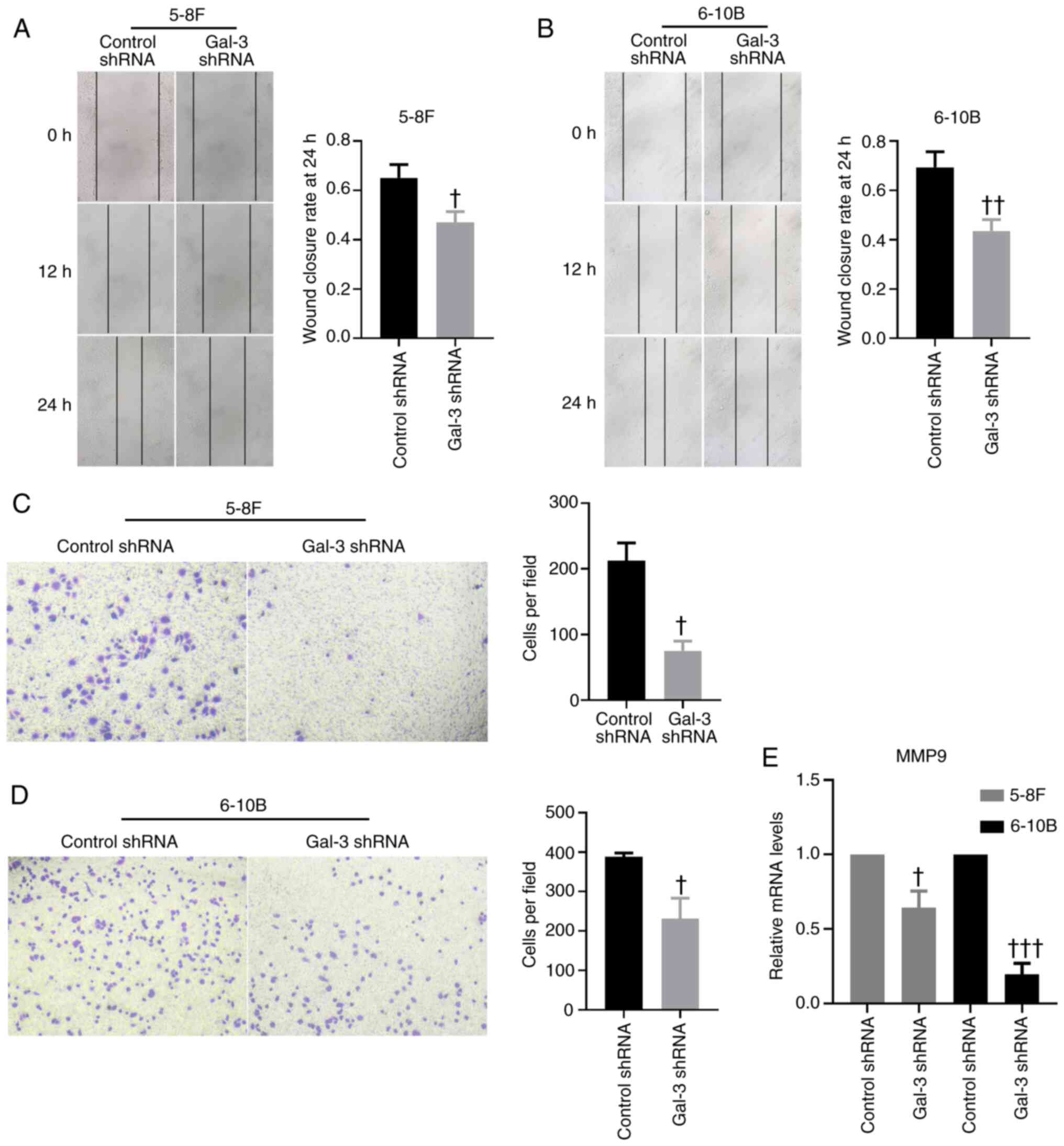
E). On the other hand, based on the scratch assay used to
determine the mobility of 5-8F and 6-10B cells, knockdown of gal-3
in NPC cells significantly decreased their migratory capability
compared with the results of cells transfected with control shRNA
(Fig. 3A and B). A Transwell
migration assay further validated these findings (Fig. 3C and D). In addition, the mRNA
expression levels of MMP-9 in 5-8F and 6-10B cells were
significantly downregulated after transfection with gal-3 shRNA
(Fig. 3E).
Gal-3 is associated with the
inflammatory state of NPC
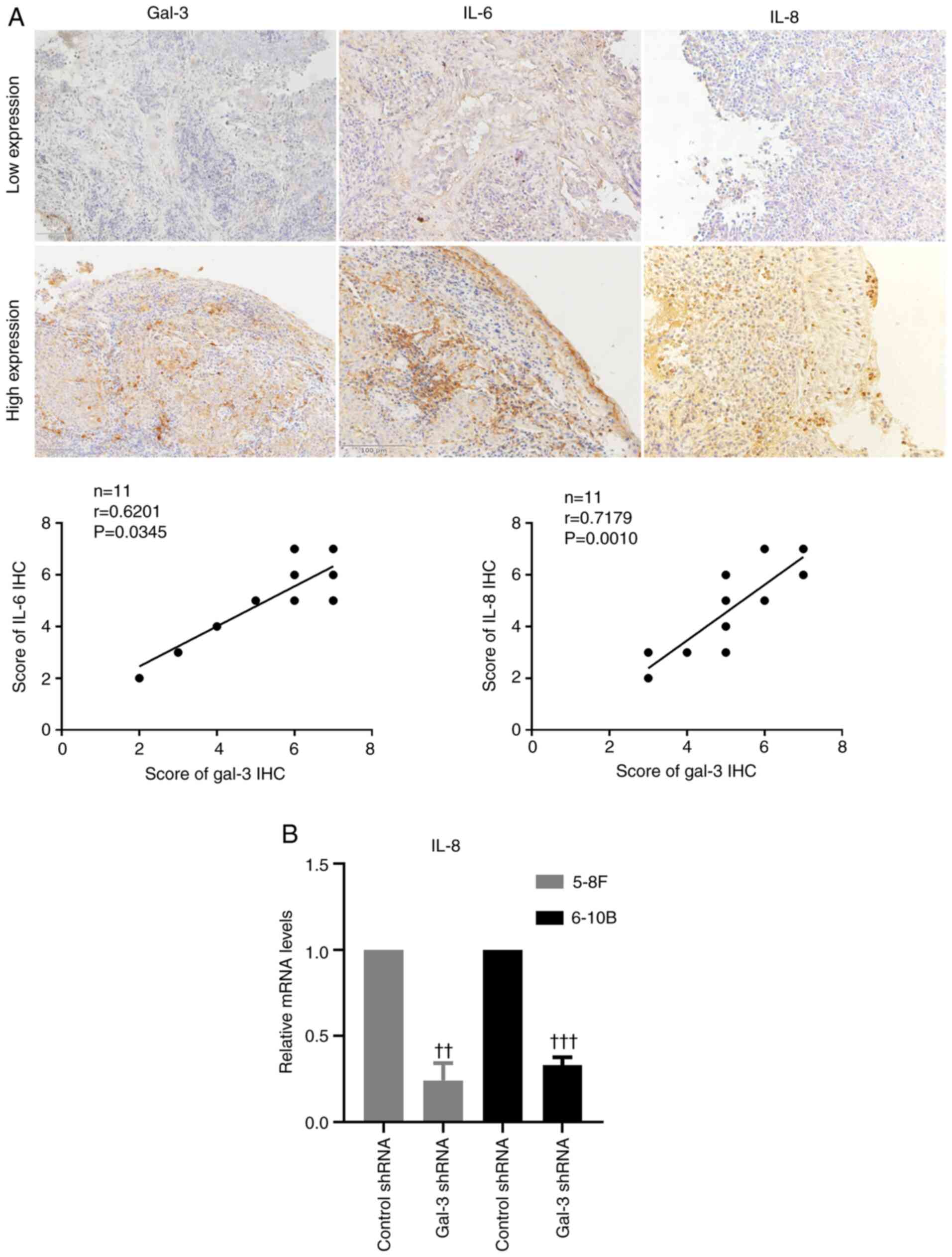
The expression levels of the inflammatory cytokines,
IL-6 and IL-8, were measured using a immunohistochemical assay to
assess the inflammatory state of the tumor tissue. The results
suggested that IL-6 and IL-8 were mainly expressed in the cytoplasm
and extracellular matrix. The relationship between gal-3 and the
inflammatory cytokines was analyzed, and the results demonstrated
that the expression level of gal-3 was positively correlated with
the expression levels of IL-6 and IL-8, which suggests that gal-3
was associated with the inflammatory state of NPC (Fig. 4A). Furthermore, 5-8F and 6-10B cells
transfected with gal-3 shRNA exhibited significantly lower
expression levels of IL-8 compared with cells transfected with
control shRNA (Fig. 4B).
Knockdown of gal-3 suppresses the
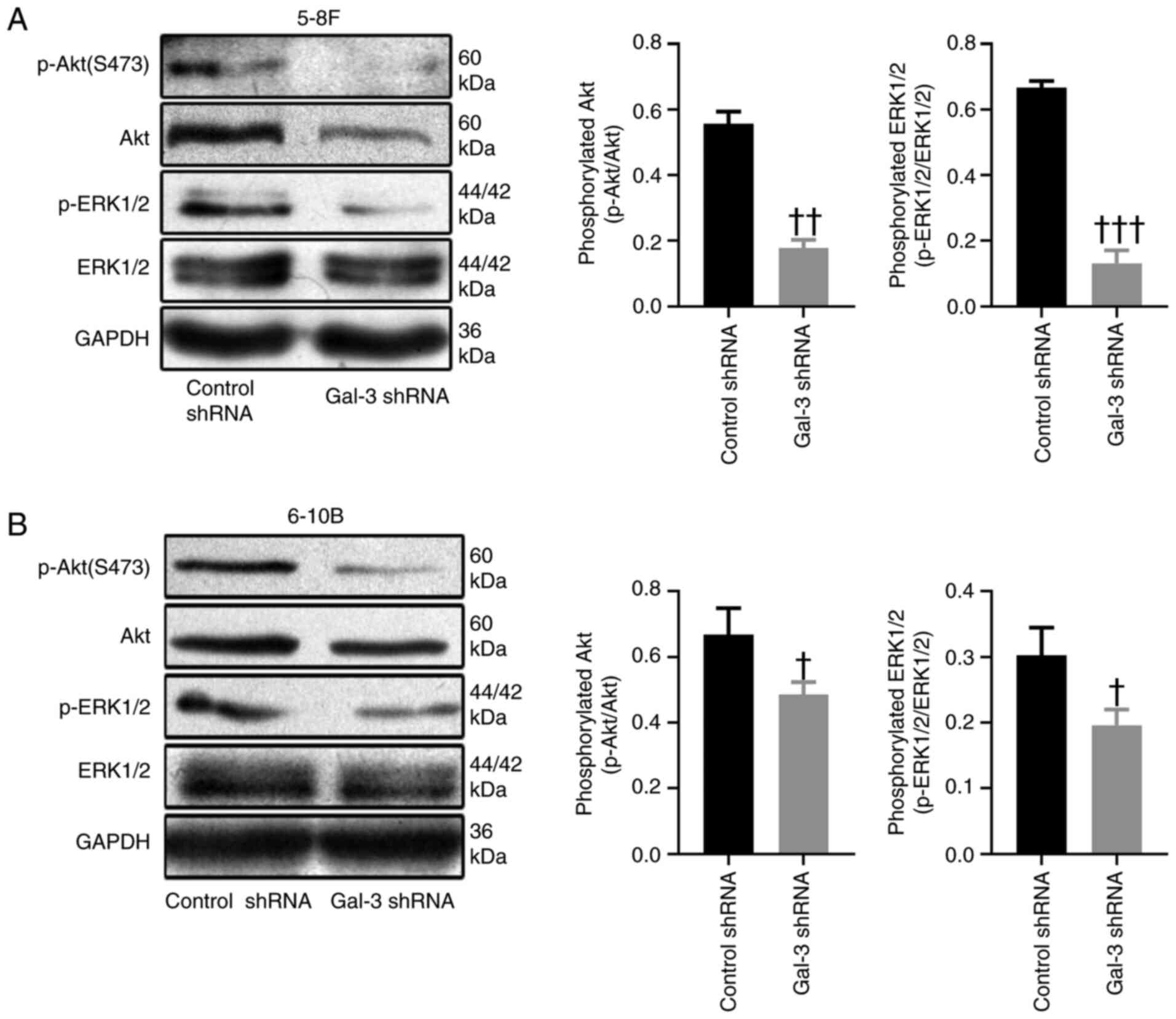
ERK1/2 and Akt signaling pathways
The ERK1/2 and Akt signaling pathways are involved
in the processes of tumor survival and migration (19). After transfection with gal-3 shRNA,
the phosphorylation levels of ERK1/2 and Akt were significantly
decreased in 5-8F and 6-10B cells, compared with cells transfected
with control shRNA (Fig. 5A and
B).
Discussion
Compared with other types of cancer, NPC is
relatively rare, accounting for only 0.7% of all cancer types in
2018 (20). However, >70% of new
NPC cases are diagnosed in Southeast Asia, indicating an extremely
unbalanced global distribution (20). In addition, NPC is one of the most
aggressive cancer types and is characterized by frequent lymph node
metastasis (21). Therefore, most
patients with NPC are diagnosed at the advanced stage (22). Radiotherapy and chemotherapy are the
conventional treatments for NPC, but the prognosis for patients at
the advanced stage remains poor (23). Under such circumstances, it is
important to investigate the underlying mechanism of occurrence and
development of NPC.
Gals are carbohydrate-binding proteins, and members
of the galectin family serve important roles in different types of
cancer (24). Using proteomic
analysis, our previous study reported that gas-1 acted as a
biomarker for NPC (25). Gal-3
exhibits a specific chimeric structure, and is associated with the
development of tumors and metastasis (8). Gal-3 is also significantly upregulated
in Caco2 and DLD-1 cells, which are both human colon cancer cell
lines, and the inhibition of gal-3 can suppress the mobility of
these cells (26). Under hypoxic
conditions, the secretion of gal-3 is significantly upregulated in
tumor-associated macrophages, and the upregulation of gal-3 can
result in the progression of breast cancer (27). Moreover, forkhead box D1 and gal-3
form a positive regulatory loop to modulate the aggressiveness of
lung cancer (28). In head and neck
adenoid cystic carcinoma, gal-3 was found to have a close
relationship with distant metastasis (29). Thus, it was suggested that gal-3 may
be associated with the development and metastasis of NPC.
In the present study, tumor tissues and
nasopharyngeal tissues were obtained from patients with NPC and CR,
respectively, and the immunochemistry results indicated that gal-3
was significantly upregulated in the tumor tissues from patients
with NPC compared with the nasopharyngeal tissues from patients
with CR. Furthermore, the concentration of circulating gal-3 in
patients with NPC was significantly higher compared with that of
patients with CR. These results implied that gal-3 may be involved
in the development and metastasis of NPC. The NPC cell lines, 5-8F
and 6-10B, and immortalized nasopharyngeal epithelium cell line
NP69 were also used to examine the role of gal-3 in NPC. Consistent
with the expression level of gal-3 in vivo, 5-8F and 6-10B
cells exhibited significantly greater expression levels of gal-3
compared with NP69.
In the subsequent experiment, gal-3 shRNA was
established to investigate the roles of gal-3 in NPC cells. After
transfection with gal-3 shRNA, the proliferation and migration of
5-8F and 6-10B cells were significantly inhibited, while apoptosis
in 5-8F and 6-10B cells was significantly activated. In addition,
the expression level of MMP-9 was upregulated in cells transfected
with gal-3 shRNA. MMPs are involved in the modulation of cell-cell
interaction, cell-matrix interaction and extracellular matrix
remodeling, and are associated with tumor metastasis (30). MMP-9 acts as a crucial regulator of
metastasis of NPC and may be a potential therapeutic target for
patients with NPC (31,32). The findings of the present study
suggested that gal-3 served important roles in the proliferation,
migration and apoptosis of NPC cells, and a regulate the
development and metastasis of NPC in vivo. However, analysis
of the correlation between the expression level of gal-3 and the
TNM stage of patients with NPC suggested that there was no
significant correlation between the two (Table SIV). This may be due to the
relatively small number of cases in the present study (40 cases).
Another explanation may be that the majority of the patients were
at TNM stage III–IV (Table
SIV).
The NF-κB signaling pathway regulates the
development of NPC, and the dysregulation of NF-κB is regarded as a
vital component of NPC tumorigenesis (33). NF-κB mediates the inflammatory
response inside tumors and leads to the accumulation of
proinflammatory cytokines in tumor tissues, which contributes to
the tumor microenvironment, and eventually, tumorigenesis (34). ILs are multifunctional inflammatory
cytokines that can regulate inflammatory responses and are
considered important oncogenic mediators (35). Gal-3 is known to provoke an
inflammatory reaction in acute inflammatory diseases, such as
pneumonia, while inducing wound healing and fibrosis in chronic
inflammatory diseases (36,37). The role of gal-3 in the inflammation
state of NPC, however, requires further investigation. In the
present study, the expression levels of IL-6 and IL-8 in tumor
tissue samples from patients with NPC were determined using
immunohistochemistry to assess the inflammatory state of these
tissues. It was found that the expression level of gal-3 in tumor
tissues was positively correlated with the expression levels of
IL-6 and IL-8 in tumor tissues. Furthermore, after transfection
with gal-3 shRNA, the expression level of IL-8 in 5-8F and 6-10B
cells was significantly downregulated compared with that of cells
transfected with control shRNA. These results suggested that gal-3
may be involved in the inflammatory response of NPC.
Finally, the current study sought to define the
underlying mechanism of the effect of gal-3 on NPC cells. The
activation of the ERK1/2 and Akt signaling pathways is involved in
the proliferation, migration, invasion, metastasis and
radiosensitivity of NPC (38–40).
However, whether gal-3 affects the ERK1/2 and Akt signaling
pathways of NPC cell lines remains unknown. Thus, in the present
study, the phosphorylation levels of ERK1/2 and Akt were
investigated in 5-8F and 6-10B cells transfected with gal-3 shRNA
or control shRNA, and the results of immunoblotting indicated that
gal-3 knockdown leads to the suppression of the ERK1/2 and Akt
signaling pathways in 5-8F and 6-10B cells. These results suggest
that activation of the ERK1/2 and Akt signaling pathways may be the
potential mechanism of the effect of gal-3 on NPC cells.
In conclusion, the present study demonstrated that
the expression level of gal-3 was significantly upregulated in
patients with NPC compared with patients with CR, and that the
expression level of gal-3 was associated with the inflammatory
state of tumor tissues from patients with NPC. Knockdown of gal-3
in 5-8F and 6-10B cells using gal-3 shRNA led to the inhibition of
cell proliferation and migration, promotion of cell apoptosis and
downregulation of MMP-9 and IL-8 expression levels. Moreover,
knockdown of gal-3 suppressed the phosphorylation of ERK1/2 and
Akt, which may highlight the underlying mechanism of gal-3 in NPC.
Therefore, gal-3 may be a potential therapeutic target for NPC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81974112), the National
Natural Science Foundation of China (grant no. 81873494), the
National Natural Science Foundation of China (grant no. 81922012),
the Hunan Natural Science Foundation (grant no. 2018JJ2665) and the
Hunan Natural Science Foundation (grant no. 2017JJ2396).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML and YBC completed the experiments and wrote the
manuscript. FL, JQQ and LCR collected the tissues, conducted the
immunohistochemical assay, evaluated the expression levels of
gal-3, IL-6, and IL-8 in tissues, and recorded the patients' data.
JC and CET designed the study, confirmed the authenticity of all
the raw data and helped finalize the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethic Committee of
Xiangya Hospital Central South University. Written informed consent
was obtained from all patients prior to inclusion into the
study.
Patient consent for publication
Patients agreed with the publication of this
study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen YP, Chan ATC, Le QT, Blanchard P, Sun
Y and Ma J: Nasopharyngeal carcinoma. Lancet. 394:64–80. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao Y: EBV based cancer prevention and
therapy in nasopharyngeal carcinoma. NPJ Precis Oncol. 1:102017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu M, Li X, Li X and Li G: Signaling
transduction network mediated by tumor suppressor/susceptibility
genes in NPC. Curr Genomics. 10:216–222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kontny U, Franzen S, Behrends U, Bührlen
M, Christiansen H, Delecluse H, Eble M, Feuchtinger T, Gademann G,
Granzen B, et al: Diagnosis and treatment of nasopharyngeal
carcinoma in children and adolescents - recommendations of the
GPOH-NPC study group. Klin Padiatr. 228:105–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Newlaczyl AU and Yu LG: Galectin-3-a
jack-of-all-trades in cancer. Cancer Lett. 313:123–128. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sciacchitano S, Lavra L, Morgante A,
Ulivieri A, Magi F, De Francesco GP, Bellotti C, Salehi LB and
Ricci A: Galectin-3: One molecule for an alphabet of diseases, from
A to Z. Int J Mol Sci. 19:3792018. View Article : Google Scholar
|
|
9
|
Elad-Sfadia G, Haklai R, Balan E and Kloog
Y: Galectin-3 augments K-Ras activation and triggers a Ras signal
that attenuates ERK but not phosphoinositide 3-kinase activity. J
Biol Chem. 279:34922–34930. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Honjo Y, Nangia-Makker P, Inohara H and
Raz A: Down- regulation of galectin-3 suppresses tumorigenicity of
human breast carcinoma cells. Clin Cancer Res. 7:661–668.
2001.PubMed/NCBI
|
|
11
|
Iurisci I, Tinari N, Natoli C, Angelucci
D, Cianchetti E and Iacobelli S: Concentrations of galectin-3 in
the sera of normal controls and cancer patients. Clin Cancer Res.
6:1389–1393. 2000.PubMed/NCBI
|
|
12
|
Nangia-Makker P, Honjo Y, Sarvis R,
Akahani S, Hogan V, Pienta KJ and Raz A: Galectin-3 induces
endothelial cell morphogenesis and angiogenesis. Am J Pathol.
156:899–909. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Méndez-Huergo SP, Blidner AG and
Rabinovich GA: Galectins: Emerging regulatory checkpoints linking
tumor immunity and angiogenesis. Curr Opin Immunol. 45:8–15. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han YY, Liu K, Xie J, Li F, Wang Y and Yan
B: LINC00114 promoted nasopharyngeal carcinoma progression and
radioresistance in vitro and in vivo through regulating ERK/JNK
signaling pathway via targeting miR-203. Eur Rev Med Pharmacol Sci.
24:2491–2504. 2020.PubMed/NCBI
|
|
15
|
Lv B, Li F, Liu X and Lin L: The
tumor-suppressive role of microRNA-873 in nasopharyngeal carcinoma
correlates with downregulation of ZIC2 and inhibition of AKT
signaling pathway. Cancer Gene Ther. 28:74–88. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hara A and Okayasu I: Cyclooxygenase-2 and
inducible nitric oxide synthase expression in human astrocytic
gliomas: Correlation with angiogenesis and prognostic significance.
Acta Neuropathol. 108:43–48. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Liu F, Han F, Lv L, Tang CE, Xie Z
and Luo F: Omentin-1 is associated with atrial fibrillation in
patients with cardiac valve disease. BMC Cardiovasc Disord.
20:2142020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moloudizargari M, Moradkhani F, Hekmatirad
S, Fallah M, Asghari MH and Reiter RJ: Therapeutic targets of
cancer drugs: Modulation by melatonin. Life Sci. 267:1189342021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bruce JP, Yip K, Bratman SV, Ito E and Liu
FF: Nasopharyngeal cancer: Molecular landscape. J Clin Oncol.
33:3346–3355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang LL, Chen WQ, Xue WQ, He YQ, Zheng RS,
Zeng YX and Jia WH: Global trends in incidence and mortality of
nasopharyngeal carcinoma. Cancer Lett. 374:22–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee AW, Ng WT, Chan YH, Sze H, Chan C and
Lam TH: The battle against nasopharyngeal cancer. Radiother Oncol.
104:272–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thijssen VL, Heusschen R, Caers J and
Griffioen AW: Galectin expression in cancer diagnosis and
prognosis: A systematic review. Biochim Biophys Acta. 1855:235–247.
2015.PubMed/NCBI
|
|
25
|
Tang CE, Tan T, Li C, Chen ZC, Ruan L,
Wang HH, Su T, Zhang PF and Xiao ZQ: Identification of Galectin-1
as a novel biomarker in nasopharyngeal carcinoma by proteomic
analysis. Oncol Rep. 24:495–500. 2010.PubMed/NCBI
|
|
26
|
Wu KL, Kuo CM, Huang EY, Pan HM, Huang CC,
Chen YF, Hsiao CC and Yang KD: Extracellular galectin-3 facilitates
colon cancer cell migration and is related to the epidermal growth
factor receptor. Am J Transl Res. 10:2402–2412. 2018.PubMed/NCBI
|
|
27
|
Wang L, Li YS, Yu LG, Zhang XK, Zhao L,
Gong FL, Yang XX and Guo XL: Galectin-3 expression and secretion by
tumor-associated macrophages in hypoxia promotes breast cancer
progression. Biochem Pharmacol. 178:1141132020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li CH, Chang YC, Hsiao M and Liang SM:
FOXD1 and Gal-3 form a positive regulatory loop to regulate lung
cancer aggressiveness. Cancers (Basel). 11:18972019. View Article : Google Scholar
|
|
29
|
Teymoortash A, Pientka A, Schrader C,
Tiemann M and Werner JA: Expression of galectin-3 in adenoid cystic
carcinoma of the head and neck and its relationship with distant
metastasis. J Cancer Res Clin Oncol. 132:51–56. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chien MH, Lin CW, Cheng CW, Wen YC and
Yang SF: Matrix metalloproteinase-2 as a target for head and neck
cancer therapy. Expert Opin Ther Targets. 17:203–216. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang X and Zhuang R: Dione-thiophene
conjugate inhibits proliferation and metastasis of nasopharyngeal
carcinoma cells through calcium binding protein-P down-regulation.
Eur J Med Chem. 168:199–206. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lan YY, Chang FH, Tsai JH and Chang Y:
Epstein-Barr virus Rta promotes invasion of bystander tumor cells
through paracrine of matrix metalloproteinase 9. Biochem Biophys
Res Commun. 503:2160–2166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li YY, Chung GT, Lui VW, To KF, Ma BB,
Chow C, Woo JK, Yip KY, Seo J, Hui EP, et al: Exome and genome
sequencing of nasopharynx cancer identifies NF-kappaB pathway
activating mutations. Nat Commun. 8:141212017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xia Y, Shen S and Verma IM: NF-kappaB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang X, Yang J, Bian Z, Shi D and Cao Z:
Long noncoding RNA DANCR promotes nasopharyngeal carcinoma
progression by interacting with STAT3, enhancing IL-6/JAK1/STAT3
signaling. Biomed Pharmacother. 113:1087132019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Farnworth SL, Henderson NC, Mackinnon AC,
Atkinson KM, Wilkinson T, Dhaliwal K, Hayashi K, Simpson AJ, Rossi
AG, Haslett C and Sethi T: Galectin-3 reduces the severity of
pneumococcal pneumonia by augmenting neutrophil function. Am J
Pathol. 172:395–405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang L, Friess H, Zhu Z, Frigeri L,
Zimmermann A, Korc M, Berberat PO and Büchler MW: Galectin-1 and
galectin-3 in chronic pancreatitis. Lab. Invest. 80:1233–1241.
2000.
|
|
38
|
Li Y, Lv Y, Cheng C, Huang Y, Yang L, He
J, Tao X, Hu Y, Ma Y, Su Y, et al: SPEN induces miR-4652-3p to
target HIPK2 in nasopharyngeal carcinoma. Cell Death Dis.
11:5092020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng P, Chen X, Xie J, Chen X, Lin S, Ye
L, Chen L, Lin J, Yu X and Zheng M: Capn4 is induced by and
required for Epstein-Barr virus latent membrane protein 1 promotion
of nasopharyngeal carcinoma metastasis through ERK/AP-1 signaling.
Cancer Sci. 111:72–83. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Z, Liu G, Mao J, Xie M, Zhao M, Guo
X, Liang S, Li H, Li X and Wang R: IGF-1R inhibition suppresses
cell proliferation and increases radiosensitivity in nasopharyngeal
carcinoma cells. Mediators Inflamm. 2019:54974672019. View Article : Google Scholar : PubMed/NCBI
|