Introduction
Diabetes mellitus (DM) is a chronic metabolic
disease characterized by prolonged hyperglycemia due to impaired
insulin secretion or loss of insulin-producing islet β cells
(1). Investigation of DM in humans
and animal models demonstrates that long-term hyperglycemia induces
nephropathy, retinopathy, neuropathy and angiopathy (2). Recent studies demonstrate that DM also
causes male infertility through testicular cell apoptosis,
downregulation of testosterone level and reduction of libido
(3,4). Diabetic testicular dysfunction is one
of the most common complications in male patients with DM, ~90%
having varying degrees of reproductive dysfunction (5). With an increase in DM cases and the
general delay in childbearing, the fertility problems caused by
diabetic testicular dysfunction are increasing (6). Therefore, it is important to explore
the molecular mechanisms of diabetic testicular dysfunction and to
develop effective treatment strategies to improve the fertility and
quality of life in male patients with DM.
Studies have demonstrated that oxidative stress and
inflammation are the main causes of testicular dysfunction in DM
(7,8). However, few studies have paid
attention to testicular interstitial fibrosis in DM. Testicular
interstitial fibrosis destroys the spermatogenic environment of the
testis, which impairs testosterone secretion and spermatogenesis
resulting in male infertility and sexual dysfunction (9,10). The
oxidative stress and inflammatory reactions in testicular tissues
induced by DM can be inhibited by highly effective exogenous
antioxidants and anti-inflammatory drugs (11,12).
However, testicular interstitial fibrosis caused by long-term high
glucose levels is difficult to repair (9,10).
Testicular interstitial fibrosis is a necessary process for the
development of diabetic testicular dysfunction characterized by
irreversible oligozoospermia and persistent poor sperm motility
(10,13). There are no reported strategies for
the prevention or treatment of diabetic testicular interstitial
fibrosis. Hence, researchers have a difficult task in developing
reliable methods for treating diabetic testicular interstitial
fibrosis.
Islet transplantation (IT) is currently the most
effective method for clinical treatment of various chronic
complications of DM (14). Previous
studies have demonstrated that IT can ameliorate and even reverse
diabetic complications, including nephropathy, retinopathy and
neuropathy in the early stages (15–17).
Research has also revealed that IT improves testicular injury in
diabetic rats through antioxidant stress and anti-inflammatory
effects (18). However, restoration
or reversal of DM-induced testicular interstitial fibrosis by IT
remains to be elucidated.
TGF-β1 is a ubiquitous cytokine that regulates cell
growth and evidence suggests that TGF-β1 is implicated in
reproductive dysfunction through the activation of testicular
fibroblasts and induction of sperm apoptosis (19,20).
The activation of TGF-β1 signal transduction mainly depends on the
phosphorylation of the Smad protein (21). TGF-β1 activates type I
receptor-phosphorylated (p-) Smad2, which interacts with Smad3 and
Smad4 and then translocate to the nucleus for active transcription
of fibrotic related genes, such as Collagen Type I α 1 Chain
(COL1A1), Collagen Type III α 1 Chain (COL3A1), connective tissue
growth factor (CTGF) and fibronectin (22,23).
In addition, CTGF and α-smooth muscle actin (α-SMA) are important
characteristics in fibrosis formation. CTGF, which is upregulated
by activation of TGF-β1, is a vital mediator in fibroblasts
activation including differentiation, proliferation, adhesion and
extracellular matrix (ECM) synthesis (24). High expression of α-SMA is present
in fibroblasts activation and promotes the deposition of Col1a1 and
Col3a1, leading to interstitial fibrosis (25,26).
The present study investigated the reversal of
testicular interstitial fibrosis in rats treated with IT at an
advanced diabetic stage and the underlying mechanisms. It was
demonstrated that IT could restore testicular interstitial
fibrosis, Leydig cells apoptosis, testosterone deficiency and sperm
motility in the rat model of type 1 diabetes, which had a close
association with the recovery of testicular structure and function
injury. The present study also discussed that the impact of IT in
the testis of diabetic rats by reducing diabetic-induced testicular
interstitial fibrosis and Leydig cells apoptosis may be through
inhibiting the TGF-β1/Smad2 signaling pathway.
Materials and methods
Animals
A total of 42 healthy, clean grade, 8-week-old male
Wistar rats weighing 200–220 g were purchased from the Experimental
Animal Center of Wenzhou Medical University. All rats were housed
with a 12 h light/dark cycle at 24±1°C with 50–60% humidity and fed
ad libitum for 1 week before the study began. All animal
experiments were performed according to the regulations of the
Animal Experimental Ethical Inspection of Laboratory Animal Centre
of Wenzhou Medical University (ID no. wydw-2017-0008) and were
performed following the ‘Guide for the Care and Use of Laboratory
Animals’ (27).
Diabetic models and groups
DM was induced by a single intraperitoneal injection
of streptozotocin (STZ; Sigma-Aldrich; Merck KGaA; 50 mg/kg body
weight) in sodium citrate buffer (pH=4.5). After 3 days, tail vein
blood was collected for the detection of plasma blood glucose
levels using an Accu-Check Active glucometer (Roche Diagnostics).
Successful establishment of experimental diabetic rat models was
identified as a non-fasted blood glucose concentration ≥16.67
mmol/l recorded for 3 consecutive days (17,20).
Then, 12 weeks after the diabetic models were established, the rats
were divided into four groups. The first group comprised normal
control (NC) rats (n=6). The second group comprised DM rats (n=6).
The third group comprised INS rats (n=6) that were treated with
insulin (WanBang Biopharmaceuticals, Co., Ltd.) at a dose of 3U per
injection given at 9 a.m. and 9 p.m. every day. In the fourth group
(n=6), IT was performed. The remaining 18 rats were used as IT
donors and three donor rats matched one recipient rat. Sham
operations were also performed at the same time IT was performed in
the NC, untreated DM and the INS groups. After 4 weeks, all rats
were anesthetized with isoflurane (3.5% for induction and 2.5% for
maintenance). Afterwards, large amounts of arterial blood was
quickly taken from the abdominal aorta of rats (~5–7 ml per rat)
and then rats were sacrificed in the form of an immediate removal
of the heart and arterial blood and testicular tissues were
collected for detection.
Islet transplantation
IT was performed using a previously described
procedure (28). Briefly, the donor
rats were anesthetized with isoflurane (3.5% for induction and 2.5%
for maintenance) and then sacrificed with the heart removed
immediately after their arterial blood was rapidly taken from the
abdominal aorta (~5–7 ml for each rat). Afterwards, the pancreas
was exposed and injected with 8 ml collagenase V (Sigma-Aldrich;
Merck KGaA; 0.8 mg/ml, dissolved in Hank's solution) through the
common bile duct. The pancreas was then separated from the
surrounding tissues and digested with 2 ml collagenase V at 37°C.
The islets were then washed, purified and centrifuged for 5 min
with the speed of 200 × g at room temperature and transferred to a
black glass culture dish for manual selection. The final purified
islets were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% FBS, 2 mM L-glutamate and 100 U/ml penicillin
and streptomycin. Fluorescein diacetate-propidium iodide (FDA-PI)
staining (Sigma-Aldrich; Merck KGaA) was used to evaluate the
activity of the purified islets, which were stained for 5 min at
room temperature and then observed under a fluorescence microscope
(Nikon ECLIPSE; Nikon Corporation; original magnification ×200).
Prior transplantation, the recipient rats were anesthetized with
isoflurane (3.5% for induction and 2.5% for maintenance). Then, the
kidney of the recipient rats was also exposed and the islets were
transferred slowly and carefully through the kidney capsule. The
incision was then sutured layer-by-layer. Subsequently, 4 weeks
after IT, immunochemistry and hematoxylin and eosin (H&E)
staining were used to assess insulin secretion.
Detection of sperm count and sperm
motility
The right epididymis of the rats was obtained and
placed in a culture dish. The epididymis was obtained by gently
cutting from the tail with ophthalmic scissors and was then diluted
with 3 ml of 37°C fertilization medium (cat. no. ART-1021; Sage In
Vitro Fertilization, Inc.; CooperSurgical Company). The culture
dish containing the epididymis was placed in an incubator with a
constant temperature of 37°C for 10 min to enable sperm diffusion
to obtain a suspension. Sperm suspension (50 µl) was taken and
diluted with 3 ml of fertilization medium at 37°C. Then, 10 µl of
the diluted suspension was put on a counting board and sperm count
and motility analysis were performed under a light microscope
(DM750; Leica Microsystems, Inc.; original magnification ×400).
Western blot analysis
Western blotting was performed as previously
described (29). Briefly, proteins
were extracted from the testicular tissue using RIPA buffer
(Beyotime Institute of Biotechnology) complemented with 10%
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA) and
quantified by BCA protein assay (Beyotime Institute of
Biotechnology). A total of 60 µg proteins were separated by 10 or
12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to polyvinylidene difluoride membranes (0.45 µl) for 90
min at 300 mA and then blocked with 5% non-fat milk for 2 h at room
temperature. After washing with Tris-buffered saline and 0.1% Tween
20 (TBS-T), the membrane was incubated with the following primary
antibodies overnight: TGF-β1 (1:1,000; Abcam; ab215715; Rabbit
monoclonal), p-Smad2 (1:1,000; Abcam; ab184557; Rabbit monoclonal),
Smad2 (1:1,000; Abcam; ab40855; Rabbit monoclonal), connective
tissue growth factor (CTGF; 1:1,000; Abcam; ab227180; Rabbit
polyclonal) and α-SMA (1:1,000; Abcam; ab5694; Rabbit polyclonal).
After washing with TBS-T, the membrane was then incubated with a
horseradish peroxidase-conjugated secondary antibody (1:2,000;
Santa Cruz Biotechnology, Inc.; cat. no. sc-2004; Goat anti-rabbit
IgG-HRP) for 2 h at room temperature. Finally, the bands were
visualized using enhanced chemiluminescence (Bio-Rad Laboratories,
Inc.; cat. no. 1705040) and quantified with Image-Pro Plus 6.0
software (Media Cybernetics, Inc.).
Measurement of serum testosterone,
luteinizing hormone (LH) and follicle-stimulating hormone (FSH)
levels
Blood samples were collected following sacrifice of
rats and centrifuged with the speed of 2,000 × g for 8 min at 4°C
and the serum separated. Testosterone, LH and FSH levels in serum
were assessed using ELISA kits (Shanghai Puji Biotechnology Co.,
Ltd.; Testosterone, cat. no. BP-E30610; LH, cat. no. BP-E920995;
FSH, cat. no. BP-E30597) according to the manufacturers'
protocols.
TUNEL analysis
TUNEL assay was utilized to detect apoptosis in the
testes tissues according to the instructions of the TUNEL kit
(Roche Applied Science). Briefly, testis sections (5 µm) were
rehydrated in a 100–70% ethanol gradient after dewaxing in xylene
for 30 min and then incubated in 10 mg/ml proteinase K for antigen
retrieval at 37°C for 30 min. Endogenous peroxidase was inhibited
using 10% hydrogen peroxide-methanol solution for 10 min.
Subsequently, the testis sections were incubated in 1% Triton X-100
for permeabilizing the cell membrane at room temperature for 20
min. Finally, the sections were blocked with fluorescein-labelled
dUTP and TDT-enzyme in proportion for 2 h at 37°C and then
incubated with converter-POD peroxidase (HRP labelled fluorescein
antibody) for 30 min at 37°C, after 30 min of blockage with 10%
goat serum (OriGene Technologies, Inc.) at room temperature. The
sections were washed three times with PBS for 5 min each step.
TUNEL-positive cells were counted under the Nikon fluorescence
microscope (Eight fields were randomly selected to count the number
of positive cells on each slide and the average value of them was
calculated; original magnification ×400) to compare the degree of
apoptosis between different experimental groups.
Histological and immunohistochemical
examinations
Testicular tissues were fixed using 4% formalin for
3 days at 4°C. After gradient dehydration in ethanol solution and
transparency in xylene for 30 min, the tissues were embedded in
paraffin and sliced to 5 µm thickness. The slides were incubated in
an oven at 65°C overnight and then rehydrated in a graded ethanol
series after 30 min of deparaffinization in xylene for histological
examination. The slides were treated with H&E (Beijing Solarbio
Science & Technology Co., Ltd.; Hematoxylin for 10 min; and
Eosin for 15 sec at room temperature). To detect the ratio of
testicular stroma collagen, testicular tissue slides were stained
with Masson's trichrome stain according to manufacturers' protocols
(Beijing Solarbio Science & Technology Co., Ltd.). For
immunohistochemical staining, the testis sections (5 µm) were
incubated with 3% H2O2 at 37°C for 10 min to inhibit endogenous
peroxidase activity. Subsequently, the sections were blocked with
5% normal goat serum (OriGene Technologies, Inc.) for 30 min. After
washing, the samples were incubated with primary antibodies TGF-β1
(1:100; Abcam; ab215715; Rabbit monoclonal), CTGF (1:200; Abcam;
ab227180; Rabbit polyclonal) and insulin (1:1,0000; Abcam;
ab181547; Rabbit monoclonal) overnight at 4°C. The slices were then
incubated with secondary antibody (1:200; cat. no. A0277; Beyotime
Institute of Biotechnology; Goat anti-rabbit IgG-HRP), visualized
with diaminobenzidine (brown color; OriGene Technologies, Inc.) and
analyzed with Image-Pro Plus 6.0 software (Media Cybernetics,
Inc.).
Statistical analysis
All data were presented as mean ± standard
deviation. Statistical significance was determined using one-way
ANOVA for comparison of ≥3 experimental conditions and Tukey's test
was used as a post hoc test following ANOVA. All analyses were
performed using SPSS v19.0 (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
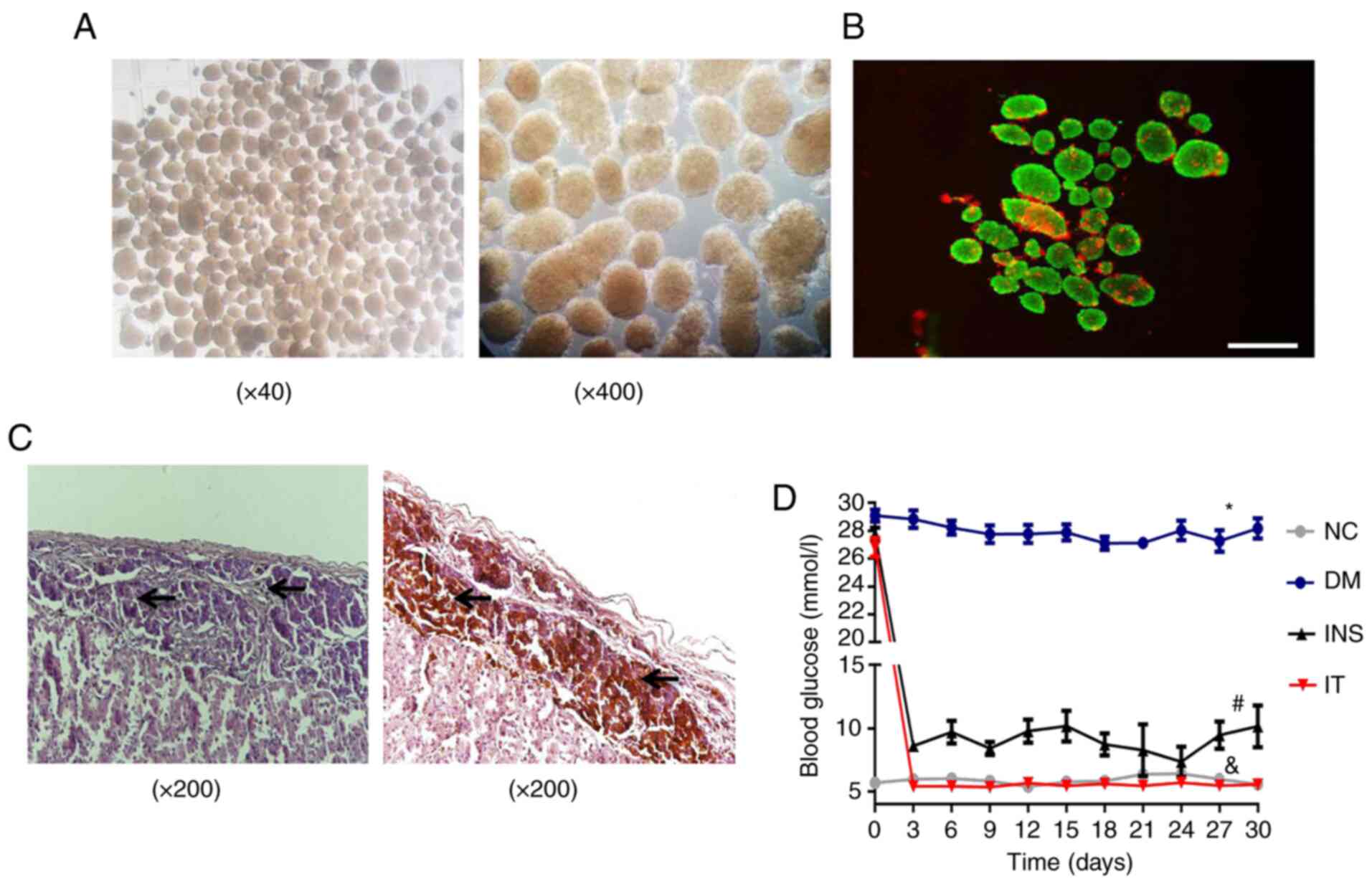
Assessment of purity, activity and
function of isolated islets and blood glucose levels in diabetic
rats following IT
Islet cells were isolated from donor rat pancreas
and used for transplantation. The purity of isolated islet cells
was evaluated by microscopic observation. Results demonstrated the
isolated islet cells had high purity (Fig. 1A). A high level of activity of the
isolated islets was also revealed by FDA-PI staining (Fig. 1B). H&E and immunohistochemical
staining was performed four weeks after transplantation and
demonstrated that the islets were well colonized under the renal
capsule and exhibited a stable insulin-secreting function (Fig. 1C). Blood glucose levels of the rats
in all groups were monitored (Fig.
1D). Diabetic rats treated with insulin or IT demonstrated a
significant decrease in blood glucose levels. However, rats in the
INS group demonstrated a considerable fluctuation in blood glucose
levels compared with the IT group. The blood glucose levels in the
IT group were consistently stable in the normal ranges, suggesting
that IT was improved in lowering and stabilizing blood glucose
levels compared with INS.
IT increased sperm count and motility
as well as testosterone, FSH and LH levels in DM rats
Sperms in the epididymis were isolated and collected
for counting and determination of motility. The sperm count and
motility in the DM group were noted to be lower compared with those
in the NC group. However, IT and INS treatment significantly
improved the sperm count and motility with IT showing improved
results (Fig. 2A and B). To show
the effect of IT on the hypothalamic-pituitary-gonadal axis, FSH,
LH and testosterone levels in the serum were measured (Fig. 2C-E). FSH, LH and testosterone levels
were significantly reduced in the DM group. However, IT and INS
significantly increased the levels of these hormones, with IT
showing greater improvement. No significant differences were
observed in the hormone levels between the IT and NC groups.
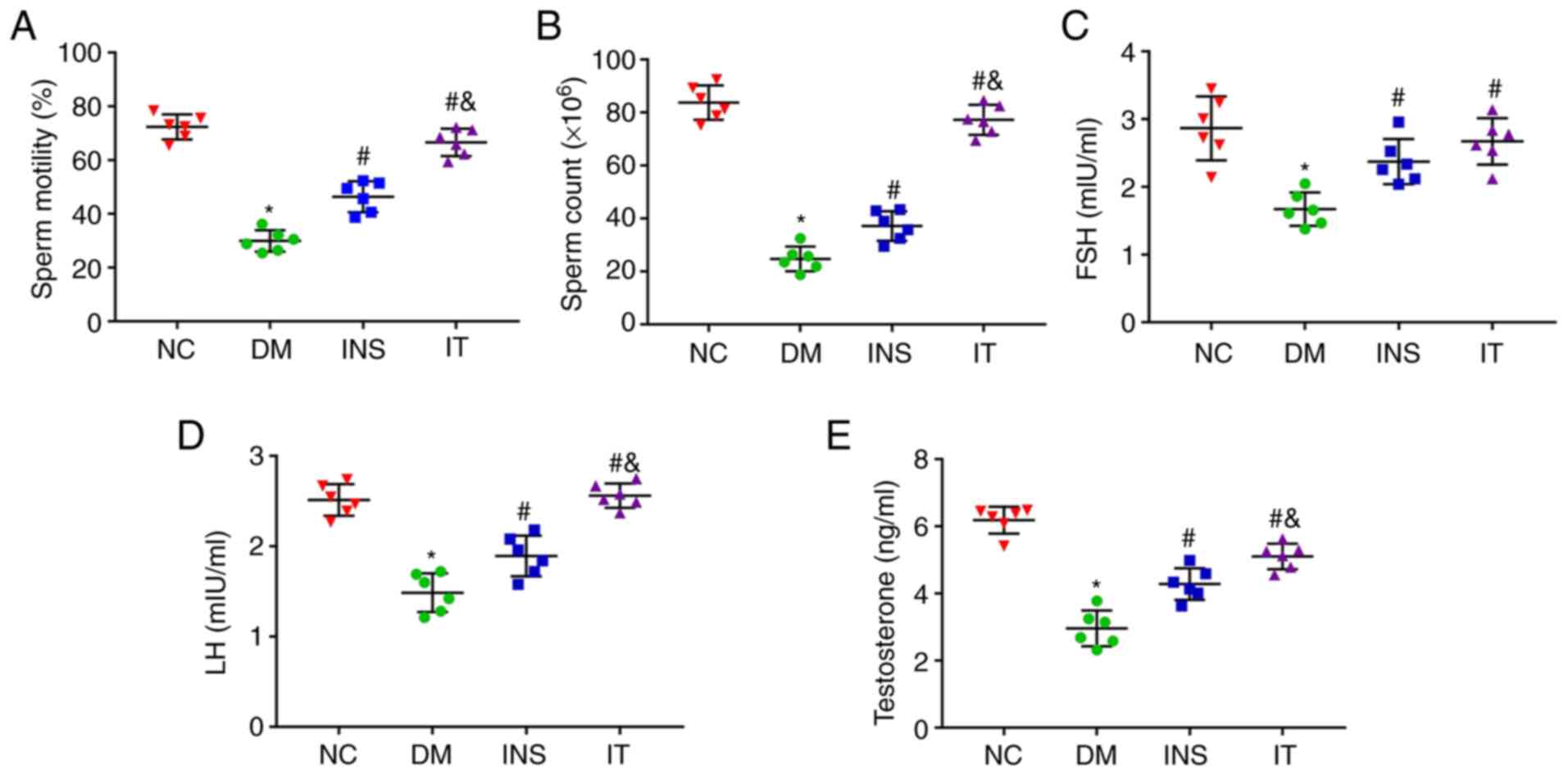 | Figure 2.IT improved sperm count and sperm
motility and function in diabetic rats. (A and B) Sperm count and
sperm motility were measured in each group (n=6 for each group).
The sperm count and the sperm motility were significantly reduced
in DM rats. INS and IT treatments increased the sperm count and
sperm motility with IT nearly restoring these parameters to normal
levels. (C-E) Quantitative detection of (C) FSH, (D) LH and (E)
testosterone expression levels in serum, respectively (n=6 for each
group). DM-induced testicular structural damage was accompanied by
significant decreases in FSH, LH and testosterone expression
levels, which were elevated by INS and IT. Levels of testosterone
and LH increased more with IT treatment. *P<0.05 vs. NC;
#P<0.05 vs. DM; &P<0.05 vs. INS.
FSH, follicle-stimulating hormone; LH, luteinizing hormone; NC,
normal control; DM, diabetes mellitus; INS, insulin treatment; IT,
islet transplantation. |
IT treatment alleviated pathological
lesions in diabetic rat testes
H&E staining demonstrated abatement of Leydig
cells, disruption of seminiferous tubules and fewer intraluminal
spermatozoa in the DM group. INS was able to reverse the structural
abnormalities in the testes. However, IT treatment had more
favorable results than INS (Fig.
3A). These results suggest that IT treatment is more effective
in improving the histological architecture of the testis than
INS.
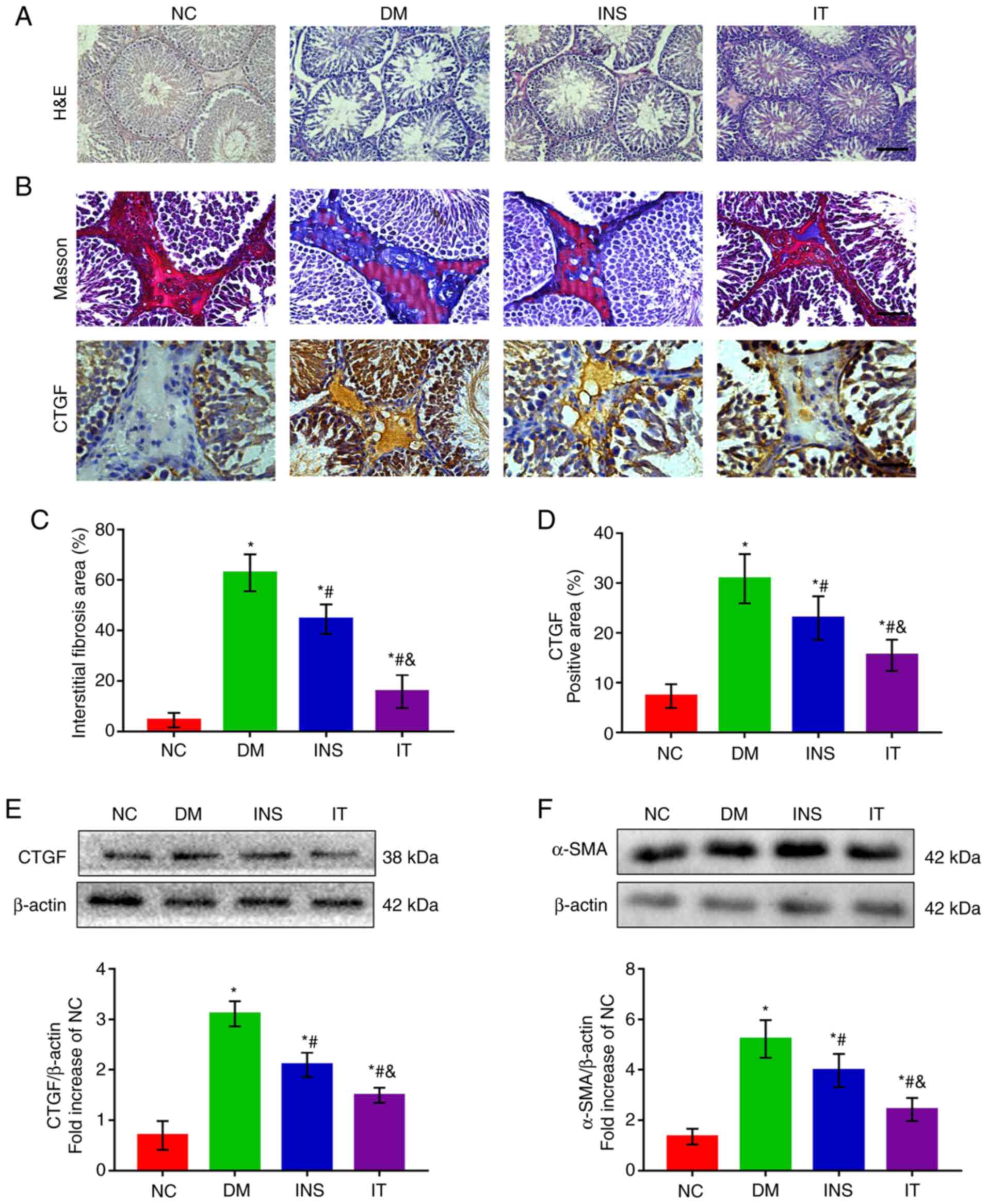 | Figure 3.IT attenuated testicular structural
damage and reduced DM-induced testicular interstitial fibrosis. (A)
hematoxylin and eosin staining of testicular sections in each group
(n=6 for each group; scale bar = 25 µm). Testicular sections of the
NC group demonstrated normal seminiferous tubules and interstitial
structure, including a high number of germ cells, including
spermatogonia, spermatocytes and sperm cells. DM group testicular
sections demonstrated severe destruction of seminiferous tubules,
atrophy of interstitials, decreased Leydig cells and severely
reduced germ cells. The INS and IT groups demonstrated recovery and
improvement of testicular structure. The IT group had a marked
improvement. (B) Masson trichrome staining and CTGF
immunohistochemical staining in testicular stroma (n=6 for each
group; scale bar = 25 µm). (C) Quantitative analysis of the
fibrotic area as reflected by Masson's trichrome staining in the
testicular stroma. The proportion of collagen in the testicular
stroma of DM group was significantly upregulated and IT played a
significant protective effect against testicular interstitial
fibrosis than INS. (D) Quantifications of CTGF-positive area in the
testicular stroma. The positive area of CTGF was larger in the
testicular stroma of the DM group but was significantly reduced in
the INS group. IT lowered the positive area of CTGF more than INS.
(E) Representative western blotting images and quantitative
analysis of CTGF protein expression in testis tissues. DM-induced
protein expression of CTGF was markedly increased. However, IT
significantly attenuated the expression of CTGF compared with INS.
(F) Protein expression and quantitative analysis of α-SMA in testis
tissues. α-SMA expression significantly increased in the DM group.
INS reduced the α-SMA levels with IT showing the lowest levels.
*P<0.05 vs. NC. #P<0.05 vs. DM.
&P<0.05 vs. INS. NC, normal control; DM, diabetes
mellitus; INS, insulin treatment; IT, islet transplantation; CTGF,
connective tissue growth factor; α-SMA, α-smooth muscle actin. |
IT inhibited DM-induced testicular
interstitial fibrosis
Masson staining demonstrated that the ratio of
collagen in the testicular interstitium significantly increased in
untreated diabetic rats compared with the NC group. INS also
markedly increased the collagen as compared with the DM group that
was left untreated. However, IT was more effective in reducing
testicular interstitial collagen deposition than INS (Fig. 3B and C). In addition,
immunohistochemical staining and western blot analysis demonstrated
that DM induced high expression of CTGF in the testicular stroma
(Fig. 3B-E) and these results
revealed the abnormal deposition of ECM in the testicular stroma.
INS significantly attenuated the increase in CTGF with IT producing
a marked reduction in CTGF levels. Additionally, western blot
analysis demonstrated the abnormally elevated α-SMA levels in the
DM group. IT was more effective than INS in reducing α-SMA levels
(Fig. 3F), suggesting that IT could
significantly inhibit the differentiation of fibroblasts in the
testicular stroma.
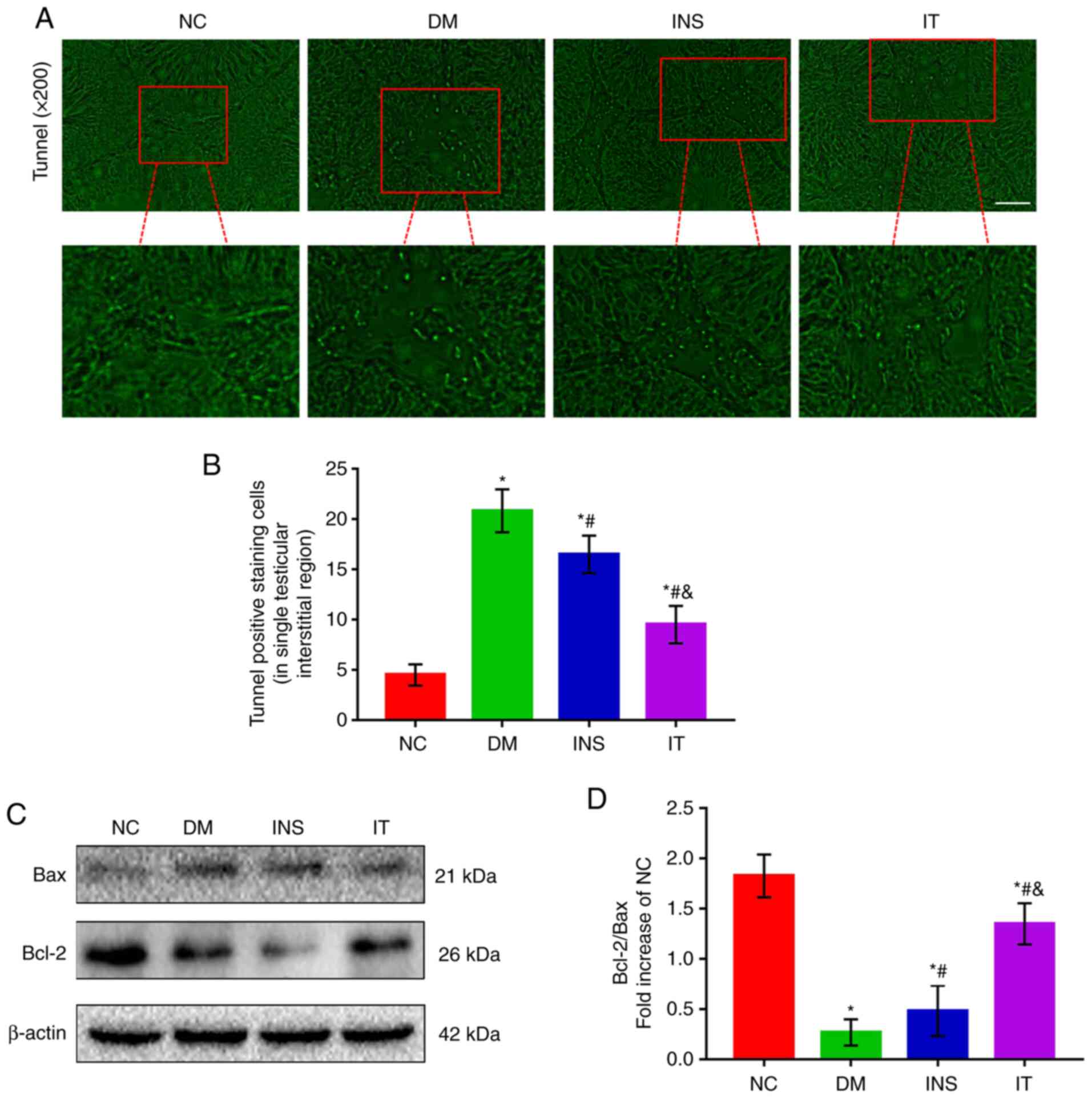
IT treatment alleviated DM-induced
Leydig cells apoptosis in rat testes
Testicular interstitial fibrosis can lead to
apoptosis of Leydig cells, which is closely related to pathological
progression of testicular structure and function changes caused by
DM (30). Therefore, TUNEL staining
was used to detect cell apoptosis (Fig.
4A and B). Apoptosis was observed to be mainly localized to the
Leydig cells of testicular stroma. A significant increase was
observed in the number of TUNEL-positive cells in the DM group
compared with the other groups. Although the number of
TUNEL-positive cells in the treatment groups was higher compared
with the NC group, a significant difference was noted between the
IT and INS groups. To confirm the results, the expression levels of
Bax and Bcl-2 in the testis of diabetic rats was determined
(Fig. 4C and D). The results
demonstrated that the Bcl-2/Bax ratio was significantly reduced in
the DM group compared with the NC group, whereas in the INS group
the ratio was increased compared with the DM group. IT further
attenuated the abnormal expression levels of Bcl-2 and Bax.
IT treatment inhibited the DM-induced
activation of the TGF-β1/ Smad2 signaling pathway
The TGF-β1/Smad2 signaling pathway is highly
correlated with testicular function and testicular fibrosis
(31,32). Therefore, the effects of IT on the
modulation of TGF-β1 and Smad2 expression levels in rat testis
tissues were evaluated. The expression levels of TGF-β1 and p-Smad2
markedly decreased after IT or INS treatment as compared with the
none treated DM group (Fig. 5A-D).
Treatment with IT demonstrated more favorable results than INS.
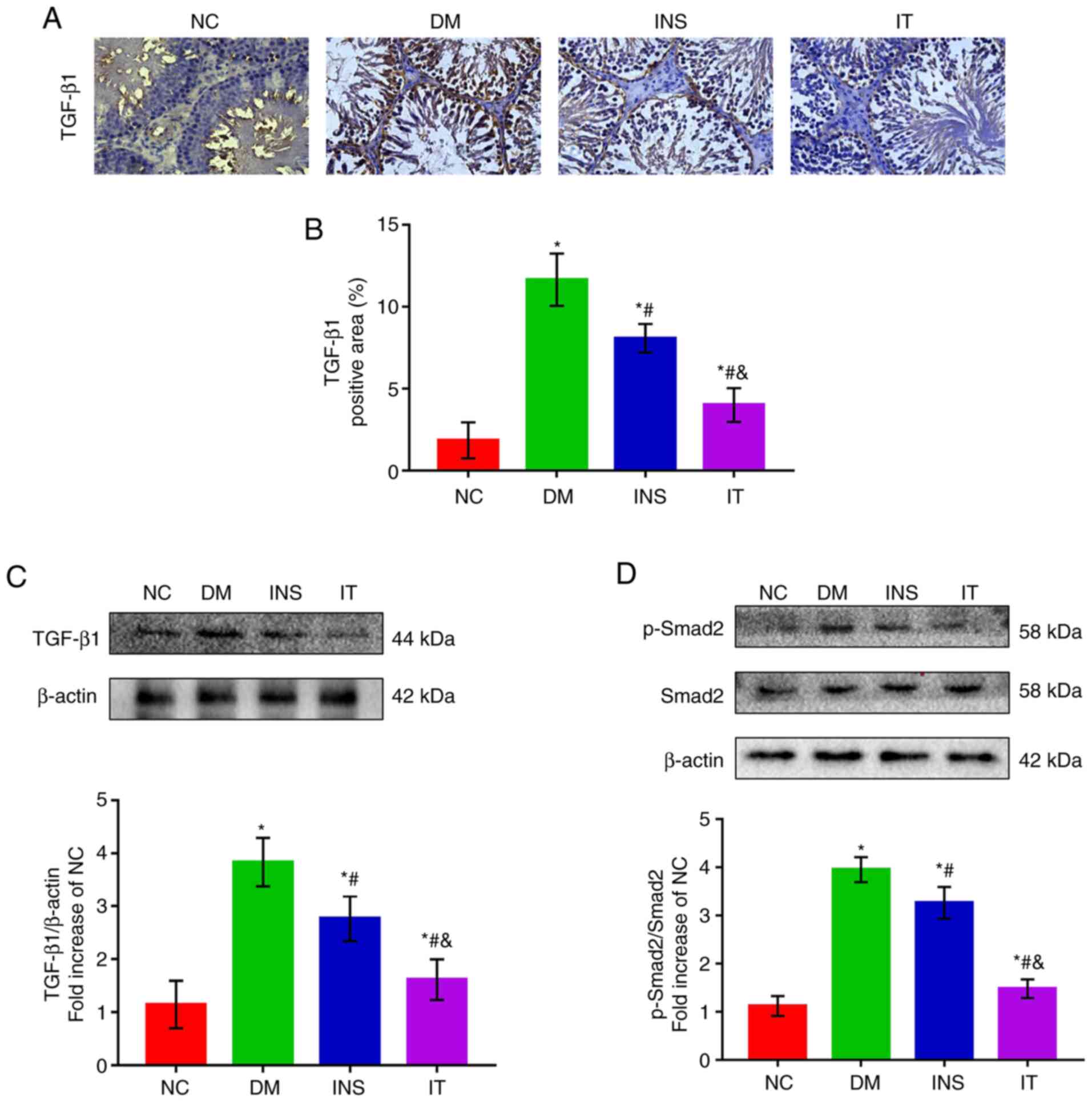 | Figure 5.IT downregulated the activation of
the TGF-β1/Smad2 signaling pathway in the testis tissues of DM
rats. (A) Immunohistochemical staining of TGF-β1 and (B)
quantitative analysis of positive regions in testicular sections,
respectively (n=6 for each group; scale bar = 25 µm). The positive
region of TGF-β1 was largest in the NC group. INS and IT reduced
the positive region of TGF-β1, with IT showing the highest
reduction. (C) Representative western blot images of TGF-β1 and
quantifications of its expression levels, respectively. DM induced
a marked increase in TGF-β1 protein expression in rat testicular
tissue. IT group significantly alleviated TGF-β1 expression
compared with INS. (D) Representative western blot images of
p-smad2, Smad2 and quantitative analysis of their expression
levels, respectively. p-Smad2 was significantly elevated in the DM
group, whereas INS reduced the expression level of Smad2. IT
demonstrated a stronger capability to reduce p-Smad2 compared with
INS. No change was observed in the expression of total Smad2 across
the groups. *P<0.05 vs. NC; #P<0.05 vs. DM;
&P<0.05 vs. INS. IT, islet transplantation; DM,
diabetes mellitus; NC, normal control; INS, insulin treatment; p-,
phosphorylated. |
Discussion
The present study provided a novel insight into the
molecular mechanisms of islet transplantation in improving
testicular dysfunction in diabetic rats. The results demonstrated
that islet transplantation was superior to insulin therapy in
improving blood glucose levels in diabetic rats, which may be
attributed to the accurate and real-time insulin secretion of
transplanted islets. The present study also illustrated that islet
transplantation effectively reversed diabetic-induced testicular
interstitial fibrosis, Leydig cells apoptosis, testosterone
deficiency and sperm motility. IT demonstrated improved reversal
results compared with insulin therapy. These findings suggested
that islet transplantation is a reliable clinical cure for DM and
offers hope in male diabetic patients with testicular dysfunction
especially those with refractory testicular interstitial fibrosis.
The present study also demonstrated that these protective
properties were associated with the inhibition of the TGF-β1/Smad2
signaling pathway.
A number of studies have demonstrated that
DM-induced testicular dysfunction is closely interrelated to
oxidative stress, inflammatory reaction, apoptosis, angiopathy and
other factors (12,33). However, the pathophysiological
mechanism remains to be elucidated. Changes in testicular function
and structure caused by oxidative stress and inflammation can be
easily reversed by effective exogenous antioxidants. However,
testicular interstitial fibrosis, caused by long-term high glucose
stimulation is difficult to alleviate or reverse (34). Testicular fibrosis impairs the
normal structure of the testis and is characterized by the
reduction and hardening of the testis (35). Testicular interstitial fibrosis also
induces apoptosis of Leydig cells thus impairs secretion of
testosterone necessary for maintenance of the number and activity
of germ cells (36). Therefore, it
is particularly important to study DM-induced testicular
interstitial fibrosis. There have been no effective methods for
treating or preventing testicular fibrosis and previous research
has not fully focused on testicular interstitial fibrosis.
Tight glycemic control by IT has been demonstrated
to reverse testicular structural injury through anti-inflammatory
and anti-oxidative stress in a diabetic rat model by previous
studies (18). However, restoration
of the testicular structure does not equate to the restoration of
testicular function. Testicular interstitial fibrosis is closely
related to decreased testosterone production and poor sperm
motility (37). Damaged testicular
interstitium also decreases spermatogenic function (38). The present study revealed that IT
was effective in improving the testicular interstitial fibrosis in
diabetic rats, which manifested as decreased synthesis of ECM and
reduced expression of α-SMA in the testicular interstitium. IT also
effectively inhibited apoptosis of Leydig cells caused by
interstitial fibrosis, thereby restoring the secretion of
testosterone and normal spermatogenic ability of testis.
In clinical settings, insulin is a common strategy
in the control of blood glucose. Previous studies have demonstrated
that insulin prevents the development of diabetic complications in
the early stages but does not improve or reverse complications in
the advanced or late stages (39,40).
Previous studies have demonstrated that blood glucose levels of
STZ-induced DM in rats fluctuate considerably with insulin therapy
(41,42). In addition, insulin in
advanced-stage diabetic rats does not reverse the myocardial
fibrosis process (28). Consistent
with previous studies, the present study revealed that testicular
interstitial fibrosis and apoptosis of Leydig cells in the INS
group were significantly higher compared with those in the NC and
IT groups. The superior effects of IT in retarding diabetic-induced
testicular interstitial fibrosis and impairment of spermatogenesis
may be attributed to the restoration of β cell function and
improved blood glucose regulation.
Tissue fibrosis is considered a reparative process
in response to cell loss or direct hyperglycemic insult. Excessive
fibrosis is inversely correlated with low testosterone and reduced
sperm production (35,43). The TGF-β1/Smad2 pathway is a
well-established molecular mechanism in testicular fibrosis
(22). The present study
demonstrated that DM had a significant effect on the activation of
the TGF-β1/Smad2 pathway in rat testis. The TGF-β1/Smad2 pathway
induced expression of CTGF and α-SMA, which are important mediators
in fibroblast activation (44). IT
was demonstrated to inhibit the activity of the TGF-β1/Smad2
pathway and suppress the expression of CTGF and α-SMA in diabetic
testis, resulting in lower testicular fibroblasts activation and
ECM deposition. In addition, IT exerted a more significant
inhibition of this signaling pathway compared with INS.
The present study had some limitations. First, IT
was demonstrated to improve testicular fibrosis after twelve weeks
of DM induction. Effects of IT need to be investigated in prolonged
DM-induced damage. Second, the present study did not evaluate other
non-classical islet peptides, such as C-peptide, GLP-1and GIP that
are also secreted by islet cells. These hormones play important
roles in regulating the secretion of insulin and have great
potential in the treatment of diabetic complications (45). Therefore, additional studies are
required to determine the effect of IT via these peptides in
improving fibrosis in testis tissues of diabetic rats.
The present study provided novel insights into the
molecular mechanisms underlying the protective effects of IT in the
testis of diabetic rats. IT can reverse the apoptosis of Leydig
cells and restore testosterone production in advanced-stage
diabetic rats. Additionally, IT can inhibit testicular interstitial
fibrosis associated with the downregulation of the TGF-β1/Smad2
pathway. IT is now more widely used in clinics and may replace
insulin as the primary method of managing DM (46). Islet transplantation demonstrated a
superior role in insulin therapy in improving testicular
interstitial fibrosis and restoring testicular spermatogenesis.
These results provide a theoretical basis in the treatment of
testicular interstitial fibrosis in the advanced stage of DM and
offer hope to male diabetic patients to restore fertility and
improve their quality of life. Promotion and recognition of islet
transplantation needs to be further enhanced.
Acknowledgements
Not applicable.
Funding
This project was supported by grants from the
National Natural Science Foundation of China (grant no. 80216096),
Natural Science Foundation of Zhejiang province (grant no.
84119040G) and Research Incubation Project of The First Affiliated
Hospital of Wenzhou Medical University (grant no. FHY2019058).
Availability of data and materials
All data used or analyzed during the present study
are available from the corresponding author on reasonable
request.
Authors' contributions
YCZ, YHW, LJK, MSZ, MMW and CYL performed the
experiments. HCW and HWW conceived and designed the research. HWW,
YLF and YCZ analyzed the data and drafted the manuscript. HWW and
HCW assessed and confirmed the authenticity of all the raw data.
CYL reviewed and edited the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All animal experiments were performed according to
the regulations of the Animal Experimental Ethical Inspection of
Laboratory Animal Centre of Wenzhou Medical University (ID no.
wydw-2017-0008) and were performed following the established Guide
for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang L, Gao P, Zhang M, Huang Z, Zhang D,
Deng Q, Li Y, Zhao Z, Qin X, Jin D, et al: Prevalence and ethnic
pattern of diabetes and prediabetes in China in 2013. JAMA.
317:2515–2523. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chung WK, Erion K, Florez JC, Hattersley
AT, Hivert MF, Lee CG, McCarthy MI, Nolan JJ, Norris JM, Pearson
ER, et al: Precision medicine in diabetes: A consensus report from
the American Diabetes Association (ADA) and the European
Association for the Study of Diabetes (EASD). Diabetes Care.
43:1617–1635. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tatone C, Di Emidio G, Barbonetti A, Carta
G, Luciano AM, Falone S and Amicarelli F: Sirtuins in gamete
biology and reproductive physiology: Emerging roles and therapeutic
potential in female and male infertility. Hum Reprod Update.
24:267–289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maresch CC, Stute DC, Alves MG, Oliveira
PF, de Kretser DM and Linn T: Diabetes-induced hyperglycemia
impairs male reproductive function: A systematic review. Hum Reprod
Update. 24:86–105. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nazmy WH, Elbassuoni EA, Ali FF and Rifaai
RA: Proinsulin C-peptide as an alternative or combined treatment
with insulin for management of testicular dysfunction and fertility
impairments in streptozotocin-induced type 1 diabetic male rats. J
Cell Physiol. 234:9351–9357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glenn DR, McClure N and Lewis SE: The
hidden impact of diabetes on male sexual dysfunction and fertility.
Hum Fertil (Camb). 6:174–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghosh S, Chowdhury S, Das AK and Sil PC:
Taurine ameliorates oxidative stress induced inflammation and ER
stress mediated testicular damage in STZ-induced diabetic Wistar
rats. Food Chem Toxicol. 124:64–80. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khosravi Z, Sedaghat R, Baluchnejadmojarad
T and Roghani M: Diosgenin ameliorates testicular damage in
streptozotocin-diabetic rats through attenuation of apoptosis,
oxidative stress, and inflammation. Int Immunopharmacol. 70:37–46.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aksglaede L and Juul A: Testicular
function and fertility in men with Klinefelter syndrome: A review.
Eur J Endocrinol. 168:R67–R76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shiraishi K, Takihara H and Naito K:
Quantitative analysis of testicular interstitial fibrosis after
vasectomy in humans. Aktuelle Urol. 34:262–264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eid AH, Gad AM, Fikry EM and Arab HH:
Venlafaxine and carvedilol ameliorate testicular impairment and
disrupted spermatogenesis in rheumatoid arthritis by targeting
AMPK/ERK and PI3K/AKT/mTOR pathways. Toxicol Appl Pharmacol.
364:83–96. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arab HH, Gad AM, Fikry EM and Eid AH:
Ellagic acid attenuates testicular disruption in rheumatoid
arthritis via targeting inflammatory signals, oxidative
perturbations and apoptosis. Life Sci. 239:1170122019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kolettis PN and Sabanegh ES: Significant
medical pathology discovered during a male infertility evaluation.
J Urol. 166:178–180. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shapiro AM, Pokrywczynska M and Ricordi C:
Clinical pancreatic islet transplantation. Nat Rev Endocrinol.
13:268–277. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He Y, Zhang M, Wu Y, Jiang H, Fu H, Cai Y,
Xu Z, Liu C, Chen B and Yang T: Aberrant activation of Notch-1
signaling inhibits podocyte restoration after islet transplantation
in a rat model of diabetic nephropathy. Cell Death Dis. 9:9502018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Preguica I, Alves A, Nunes S, Gomes P,
Fernandes R, Viana SD and Reis F: Diet-induced rodent models of
diabetic peripheral neuropathy, retinopathy and nephropathy.
Nutrients. 12:2502020. View Article : Google Scholar
|
|
17
|
Fensom B, Harris C, Thompson SE, Al
Mehthel M and Thompson DM: Islet cell transplantation improves
nerve conduction velocity in type 1 diabetes compared with
intensive medical therapy over six years. Diabetes Res Clin Pract.
122:101–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu X, Guo F, Tang H, Huang C, Xie G,
Huang T, Li Y, Liu C, Wang H and Chen B: Islet transplantation
attenuating testicular injury in type 1 diabetic rats is associated
with suppression of oxidative stress and inflammation via
Nrf-2/HO-1 and NF-κB pathways. J Diabetes Res. 2019:87124922019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salama N, Tsuji M, Tamura M and Kagawa S:
Transforming growth factor (beta1) in testes of aged and diabetic
rats: Correlation with testicular function. Arch Androl.
47:217–226. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kabel AM: Zinc/alogliptin combination
attenuates testicular toxicity induced by doxorubicin in rats: Role
of oxidative stress, apoptosis and TGF-β1/NF-κB signaling. Biomed
Pharmacother. 97:439–449. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-β/Smad signaling in
tissue fibrosis. Chem Biol Interact. 292:76–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tzavlaki K and Moustakas A: TGF-β
signaling. Biomolecules. 10:4872020. View Article : Google Scholar
|
|
23
|
ten Dijke P and Hill CS: New insights into
TGF-beta-Smad signalling. Trends Biochem Sci. 29:265–273. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramazani Y, Knops N, Elmonem MA, Nguyen
TQ, Arcolino FO, van den Heuvel L, Levtchenko E, Kuypers D and
Goldschmeding R: Connective tissue growth factor (CTGF) from basics
to clinics. Matrix Biol. 68-69:44–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hinz B, Celetta G, Tomasek JJ, Gabbiani G
and Chaponnier C: Alpha-smooth muscle actin expression upregulates
fibroblast contractile activity. Mol Biol Cell. 12:2730–2741. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuan X, Pan J, Wen L, Gong B, Li J, Gao H,
Tan W, Liang S, Zhang H and Wang X: MiR-144-3p enhances cardiac
fibrosis after myocardial infarction by targeting PTEN. Front Cell
Dev Biol. 7:2492019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th
edition. The National Academies Press; Washington, DC: 2011
|
|
28
|
Wang HW, Chen YH, Chen YY, Huang W, Zhu
XD, Ni FB, Wu GD, Xu ZQ, Huang ZQ, Chen BC, et al: Islet
transplantation attenuates cardiac fibrosis in diabetic rats
through inhibition of TGF-β1/Smad3 pathway. Am J Transl Res.
10:2445–2456. 2018.PubMed/NCBI
|
|
29
|
Wu Z, Wang H, Ni F, Jiang X, Xu Z, Liu C,
Cai Y, Fu H, Luo J, Chen W, et al: Islet transplantation improved
penile tissue fibrosis in a rat model of type 1 diabetes. BMC
Endocr Disord. 18:492018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kilarkaje N, Al-Hussaini H and Al-Bader
MM: Diabetes-induced DNA damage and apoptosis are associated with
poly (ADP ribose) polymerase 1 inhibition in the rat testis. Eur J
Pharmacol. 737:29–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kolettis PN: Evaluation of the subfertile
man. Am Fam Physician. 67:2165–2172. 2003.PubMed/NCBI
|
|
32
|
Sun T, Xin Z, Jin Z, Wu Y and Gong Y:
Effect of TGF-beta/Smad signaling on sertoli cell and possible
mechanism related to complete sertoli cell-only syndrome. Mol Cell
Biochem. 319:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li M, Liu Z, Zhuan L, Wang T, Guo S, Wang
S, Liu J and Ye Z: Effects of apocynin on oxidative stress and
expression of apoptosis-related genes in testes of diabetic rats.
Mol Med Rep. 7:47–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shiraishi K, Takihara H and Naito K:
Influence of interstitial fibrosis on spermatogenesis after
vasectomy and vasovasostomy. Contraception. 65:245–249. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bhanmeechao C, Srisuwatanasagul S and
Ponglowhapan S: Age-related changes in interstitial fibrosis and
germ cell degeneration of the canine testis. Reprod Domest Anim. 53
(Suppl 3):37–43. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hoffman WH, Kovacs KT, Gala RR, Keel BA,
Jarrell TS, Ellegood JO and Burek CL: Macroorchidism and testicular
fibrosis associated with autoimmune thyroiditis. J Endocrinol
Invest. 14:609–616. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang F, Liu W, Jiang Q, Gong M, Chen R, Wu
H, Han R, Chen Y and Han D: Lipopolysaccharide-induced testicular
dysfunction and epididymitis in mice: A critical role of tumor
necrosis factor alpha. Biol Reprod. 100:849–861. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kangawa A, Otake M, Enya S, Yoshida T and
Shibata M: Histological Changes of the Testicular Interstitium
during Postnatal Development in Microminipigs. Toxicol Pathol.
47:469–482. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cellek S, Foxwell NA and Moncada S: Two
phases of nitrergic neuropathy in streptozotocin-induced diabetic
rats. Diabetes. 52:2353–2362. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Choi WS, Kwon OS, Cho SY, Paick JS and Kim
SW: Effect of chronic administration of PDE5 combined with glycemic
control on erectile function in streptozotocin-induced diabetic
rats. J Sex Med. 12:600–610. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Poradzka A, Wroński J, Jasik M, Karnafel W
and Fiedor P: Insulin replacement therapy in patients with type 1
diabetes by isolated pancreatic islet transplantation. Acta Pol
Pharm. 70:943–950. 2013.PubMed/NCBI
|
|
42
|
Beltrán del Río M, Georgiev GI, Cercone R,
Tiwari M and Rilo HL: Continuous glucose monitoring analysis as
predictor of islet yield and insulin requirements in autologous
islet transplantation after complete pancreatectomy. J Diabetes Sci
Technol. 8:1097–1104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Oka S, Shiraishi K and Matsuyama H:
Effects of human chorionic gonadotropin on testicular interstitial
tissues in men with non-obstructive azoospermia. Andrology.
5:232–239. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lv W, Zhang L, Cheng X, Wang H, Qin W,
Zhou X and Tang B: Apelin Inhibits Angiotensin II–Induced Atrial
Fibrosis and Atrial Fibrillation via TGF-β1/Smad2/α-SMA Pathway.
Front Physiol. 11:5835702020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu F and Kong Y: GLP-1 receptor agonist
on cardiovascular complications of diabetes mellitus. Exp Ther Med.
19:2259–2265. 2020.PubMed/NCBI
|
|
46
|
Roep BO: Improving clinical islet
transplantation outcomes. Diabetes Care. 43:698–700. 2020.
View Article : Google Scholar : PubMed/NCBI
|