Introduction
X-ray is a type of ionizing radiation that is widely
used in medicine, including for radiotherapy of malignant tumors
and imaging examinations. It is considered that any dose of
ionizing radiation, even a very low dose, is harmful, and the
detrimental effects increase linearly with increasing doses. The
effects on bone tissues mainly comprise osteonecrosis, delayed
union of fracture, non-union and osteoporosis (1,2).
Previous studies have found that radiation can destroy the dynamic
balance between osteoblasts and osteoclasts, and inhibit
proliferation and differentiation of osteoblasts. In addition, it
can also reduce bone matrix deposition (3,4),
impair the circulation around the bone tissues and callus, and
directly destroy osteoblasts and osteoblast progenitor cells
(5). This conclusion is based on
research using medium and high doses of ionizing radiation. In
1982, Luckey (6) suggested for the
first time that a low level of radiation may have beneficial
effects on humans, known as an excitatory effect. The excitatory
effect has been shown to enhance immune function and reduce the
incidence of cancer (7). Previous
findings demonstrated that the excitatory effect induced by
low-dose ionizing radiation may be associated with the antioxidant
defense mechanism of free radicals and DNA double-strand breaks
(DSBs) induced by low-dose ionizing radiation (8). Low-dose ionizing radiation can also
inhibit inflammation and production of oxygen-free radicals in
macrophages (9,10).
Low-dose radiation has markedly attracted scholars'
attention in nerve stimulation and anti-tumor therapy (11–13);
however, few studies have evaluated the effects of low-dose X-ray
irradiation on orthopedic diseases. It has been reported that
low-dose irradiation can increase the serum level of alkaline
phosphatase (ALP) and promote the secretion of VEGF, leading to an
increase in the number of mineralized nodules in osteoblasts
(14,15). In a previous study, it was
demonstrated that low-dose ionizing radiation can significantly
increase the number of trabeculae in the distal femur of mice and
improve the microstructure of trabecular bone (16). A relatively low-dose X-ray
irradiation can activate osteoclast production, which was closely
associated with bone loss (17).
Our previous study revealed that low-dose X-ray irradiation could
promote callus formation and mineralization in a rat fracture model
(18). The results of an in
vitro study also showed that X-ray irradiation promoted
osteoblast differentiation (19).
Using microarray analysis, previous findings have indicated that
low-dose X-ray irradiation increases the expression of
cytoskeleton-associated genes, which may promote the proliferation
and differentiation of osteoblasts through changes in the
extracellular matrix (ECM), local adhesion, and actin cytoskeleton
(18).
The cytoskeleton is a complex, dynamic network of
interlinking protein filaments present in the cytoplasm of all
cells, including bacteria and archaea; in eukaryotes, it is
composed of three main components: Microfilaments, intermediate
filaments, and microtubules. Moreover, the cytoskeleton plays a
significant role in maintaining cell morphology, as well as
participating in cell migration and cell differentiation (20,21).
The cytoskeleton is sensitive to ionizing radiation. Indeed,
ionizing radiation was closely associated with the changes of
cytoskeleton (12,13,22).
RhoA is a member of the Rho family of GTPases, which
is closely associated with the organization of the actin
cytoskeleton (23). RhoA is
activated via phosphorylation, leading to subsequent loading of Rho
GTPases with GTP. Activated Rho GTPases transmit signals to
Rho-associated protein kinase (ROCK). ROCK can phosphorylate and
activate LIMK1 and LIMK2 (24).
Eventually, LIMK2 phosphorylates Cofilin, causing Cofilin to lose
its ability to depolymerize actin (24). Previous studies have shown that
X-ray irradiation could induce a rearrangement of the actin
cytoskeleton and cause an increase in stress fibers (25,26).
As a result, the RhoA/ROCK signaling pathway is activated (25,26).
RhoA/ROCK signaling is involved in a variety of cellular processes,
such as cell differentiation and migration (20,27).
RhoA can also promote differentiation of osteoblasts by activating
the Wnt signaling pathway (28). As
for osteoblasts, whether RhoA/ROCK can mediate the reorganization
of actin cytoskeleton and changes in cell function caused by X-ray
irradiation remains unknown. Therefore, in the present study, the
effects of low-dose X-ray irradiation on differentiation of
osteoblasts and reorganization of the cytoskeleton were
investigated, with the aim of providing a theoretical basis for
improved understanding of the biological effects of low-dose X-ray
irradiation.
Materials and methods
Cell culture
MC3T3-E1 cells were purchased from the Cell Bank of
Type Culture Collection of Chinese Academy of Sciences (Shanghai,
China). The cells were cultured in an α-minimum essential medium
(MEM) (HyClone; Cytiva) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin, and 100 mg/ml streptomycin
at 37°C, 5% CO2 and changed every three days. During the
induction of osteogenic differentiation, the osteogenic
conditioning medium (containing MEM, 10% FBS, 5 mM
β-glycerophosphate, 50 µg/ml ascorbic acid and 100 nM
dexamethasone) was replaced. The medium was replaced after low-dose
X-ray irradiation, and changed every 3 days.
Treatments
The ROCK inhibitor Y27632 was purchased from EMD
Millipore. Cells were divided into four groups: i) Group 1, blank
control group, no Y27632 pretreatment and no irradiation; ii) group
2, pretreatment with Y27632 without irradiation; iii) group 3, 0.5
Gy X-ray irradiation without Y27632 pretreatment; and iv) group 4;
pretreatment with Y27632 followed by 0.5-Gy X-ray irradiation.
Cells were pretreated with 10 µM Y27632 for 1 h
before X-ray irradiation in groups 2 and 4. Third-generation
MC3T3-E1 cells-were used, and the cell density was observed daily.
When the cells reached 70% confluency, they were irradiated with 0
and 0.5 Gy X-ray at a rate of 200 cGy/min using a medical linear
accelerator with a 6 MV radiation source (Siemens Primus).
F-actin staining
MC3T3-E1 cells (5×104) in each group were
washed twice with PBS 2, 24, 36, or 120 h after irradiation. After
washing, the cells were fixed with 4% paraformaldehyde for 25 min
at room temperature, and then treated with 0.1% Triton X-100 for 10
min at room temperature. After washing with PBS three times, the
cells were blocked with 5% bovine serum albumin (Beyotime Institute
of Biotechnology) for 30 min at room temperature, then incubated
with fluorescein isothiocyanate (FITC)-conjugated phalloidin
(Sigma-Aldrich; Merck KGaA) for 1 h at 37°C. The cell nuclei were
additionally counterstained with 100 nM
4′,6-diamidino-2-phenylindole (DAPI) for 10 min. After washing,
cells on coverslips were visualized using a fluorescence microscope
(Carl Zeiss AG). The aforementioned processes were performed in the
dark.
ImageJ 1.8.0 software (National Institutes of
Health) was used to analyze the images of six cells per field of
view on the slide, and the average fluorescence intensity of each
cell (average fluorescence intensity=fluorescence intensity/cell
area) was measured accordingly (Table
I). Area represents the total area of the cells counted. ‘Mean
± SD’ expresses the average fluorescence intensity of the cells
measured. ‘Min’ denotes the lowest fluorescence intensity of the
cells. ‘IntDen’ indicates the total fluorescence intensity of the
cells measured.
 | Table I.Fluorescence intensity of F-actin
staining of cells in each group. |
Table I.
Fluorescence intensity of F-actin
staining of cells in each group.
| Group | Area (pixel) | Mean ± SD | Min | Inthen |
|---|
| 2 h 0 Gy | 272,308 | 37.36±4.91 | 15 | 7,479,102 |
| 0.5 Gy | 272,308 | 33.60±1.93 | 12 | 6,342,378 |
| 0 Gy+Y27632 | 272,308 | 37.12±3.31 | 12 | 6,710,960 |
| 0.5 Gy+Y2762 | 272,308 | 27.77±2.64 | 22 | 8,075,451 |
| 1 d 0 Gy | 272,308 | 27.57±2.47 | 16 | 10,052,197 |
| 0.5 Gy | 272,308 | 35.26±3.66 | 12 | 7,362,259 |
| 0 Gy+Y27632 | 272,308 | 28.18±4.45 | 10 | 9,419,783 |
| 0.5 Gy+Y2762 | 272,308 | 30.57±5.14 | 15 | 9,811,449 |
| 3 d 0 Gy | 190,825 | 27.97±6.07 | 12 | 10,295,261 |
| 0.5 Gy | 190,825 | 38.48±4.78 | 13 | 4,343,367 |
| 0 Gy+Y27632 | 252,417 | 25.00±4.50 | 7 | 6,640,082 |
| 0.5 Gy+Y2762 | 232,329 | 35.08±4.73 | 15 | 7,266,554 |
| 5 d 0 Gy | 260,815 | 20.64±2.62 | 13 | 6,912,119 |
| 0.5 Gy | 238070 | 32.76±2.63 | 10 | 7,227,973 |
| 0 Gy+Y27632 | 284,840 | 21.44±1.95 | 13 | 9,183,526 |
| 0.5 Gy+Y2762 | 284,334 | 27.90±2.89 | 8 | 8,617,311 |
Western blot assay
MC3T3-E1 cells (1×106) were lysed on ice
with RIPA buffer containing protease and phosphatase inhibitors
(Beyotime Institute of Biotechnology) after being cultured for 1, 3
or 5 days. The supernatant was collected by centrifugation at
12,000 × g for 15 min at 4°C, and the protein concentration was
quantified using a bicinchoninic acid (Beyotime Institute of
Biotechnology) assay kit. Protein samples (30 µg/lane) were
resolved using 10% SDS-PAGE, then transferred onto PVDF membranes
(EMD Millipore). Membranes were blocked with 5% non-fat dried milk
in Tris-buffered saline with 0.1% Tween 20 (TBST) for 2 h at room
temperature. Membranes were incubated overnight at 4°C with the
following antibodies: Rabbit anti-Cofilin (cat. no. 3312; Cell
Signaling Technology, Inc.; dilution 1:1,000), rabbit anti-ROCK
(cat. no. 4035; Cell Signaling Technology, Inc.; dilution 1:2,000),
rabbit anti-phosphorylated (phospho)-LIM domain kinase 2 (LIMK2;
cat. no. 3845, Cell Signaling Technology, Inc.; dilution 1:2,000),
rabbit anti-phospho-Cofilin (cat. no. 3311; Cell Signaling
Technology, Inc.; dilution 1:2,000), rabbit anti-Runx2 (cat. no.
ab76956; Abcam; dilution 1:2,000), rabbit anti-Osterix (cat. no.
ab209484; Abcam; dilution 1:2,000), rabbit anti-Collagen Type 1
(COL1; cat. no. ab96723, Abcam; dilution 1:2,000), rabbit
anti-osteocalcin (OCN; cat. no. ab93876, Abcam; dilution 1:1,000),
rabbit anti-alkaline phosphatase (ALP; cat. no. ab95462, Abcam;
dilution 1:2,000), and β-actin (cat. no. ab8227, Abcam; dilution
1:2,000). Following washing with TBST three times, the membranes
were incubated with a goat anti-rabbit IgG HRP-conjugated secondary
antibody (cat. no. ab97200, Abcam; dilution 1:2,000) for 1 h at
room temperature. Immunoreactive bands were visualized by using
enhanced chemiluminescence detection reagent (EMD Millipore) and
images were captured using a chemiluminescence imaging system
(Kodak). The data were quantified using Image J software (version
1.8.0; National Institutes of Health).
RhoA activation assay
MC3T3-E1 cells (1×106) were harvested on
days 1, 3, and 5 following X-ray irradiation and lysed with RIPA
buffer. A total of 30 µl of the supernatant was used to determine
the expression of total RhoA. The remaining supernatant was used to
isolate GTP-bound RhoA using an Active GTPase Pull-down kit (cat.
no. 16116; Thermo Fisher Scientific, Inc.), in which the
glutathione S-transferase-Rhotekin Rho binding domain was used,
according to the manufacturer's protocol. The eluted proteins were
then separated using 15% SDS-PAGE, transferred onto PVDF membranes
(EMD Millipore), and detected using specific anti-RhoA antibodies
(Santa Cruz Biotechnology, Inc.). Total RhoA protein was detected
via western blot analysis as described in the previous section.
Immunoreactive bands were visualized using ECL, and band intensity
was quantified using ImageJ software.
Cell proliferation assay
MC3T3-E1 cells were inoculated into 96-well plates
at a density of 3×103 cells/well. After the
aforementioned treatment (X-ray irradiation and/or Y27632
pretreatment), the cells were cultured for 1–7 days. Cell viability
was measured using Cell Counting Kit-8 (CCK-8; Beyotime Institute
of Biotechnology). The specific procedure was carried out according
to the manufacturer's instructions, in which 10 µl CCK-8 solution
was added for a 100-µl volume of medium, and incubated at 37°C for
2 h. Subsequently, the optical density value of each well was
determined using an enzyme-labeling instrument (BioTek Instruments,
Inc.) at 450-nm wavelength.
ALP staining and ALP activity
MC3T3-E1 cells were cultured in 24-well plates at a
density of 1×104 cells/well. Cells were then treated
with 0.5 Gy X-ray irradiation and/or Y27632. The medium was
discarded on day 7. ALP staining was carried out using the ALP
assay kit (cat. no. P0321; Beyotime Institute of Biotechnology) at
room temperature for 30 min according to the manufacturer's
protocol, and visualization was undertaken using an inverted light
microscope (magnification, ×10; Olympus Corporation).
The activity of ALP, a marker of early
differentiation of osteoblasts, was detected using an ALP assay
kit. Osteoblasts were inoculated into 96-well plates at a density
of 3×103 cells/ml in triplicate wells. The 96-well
plates were placed in an incubator at a constant temperature (37°C)
and continued to be cultured until day 7 or day 10. A total of 30
µl culture medium was collected from each well. The absorbance was
detected at 520 nm in each group according to the manufacturer's
protocol, and the relative ALP activity in each group was
calculated according to the measured data.
Alizarin red staining
The mineralization degree of MC3T3-E1 cells
(1.0×105/ml) in each group was determined by Alizarin
red staining. A total of 21 days after irradiation, osteoblasts
were fixed in 4% paraformaldehyde for 1 h at room temperature. The
cells were then washed with deionized water and stained with 40 mM
Alizarin red (pH 4.2) for 30 min at 37°C. After staining, the cells
were washed with deionized water to remove the non-specific
Alizarin red dye. After drying, the formation of calcium nodules in
each group was observed under an inverted phase-contrast
microscope. Orange to red staining indicated mineralized calcium
nodules.
Statistical analysis
All experiments were repeated three times
independently. Data are presented as the mean ± SD. Differences
between the groups were analyzed using one-way ANOVA followed by
Tukey's post hoc test using SPSS 18.0 software (SPPS, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Low-dose X-ray irradiation induced
cytoskeleton reorganization in MC3T3 cells
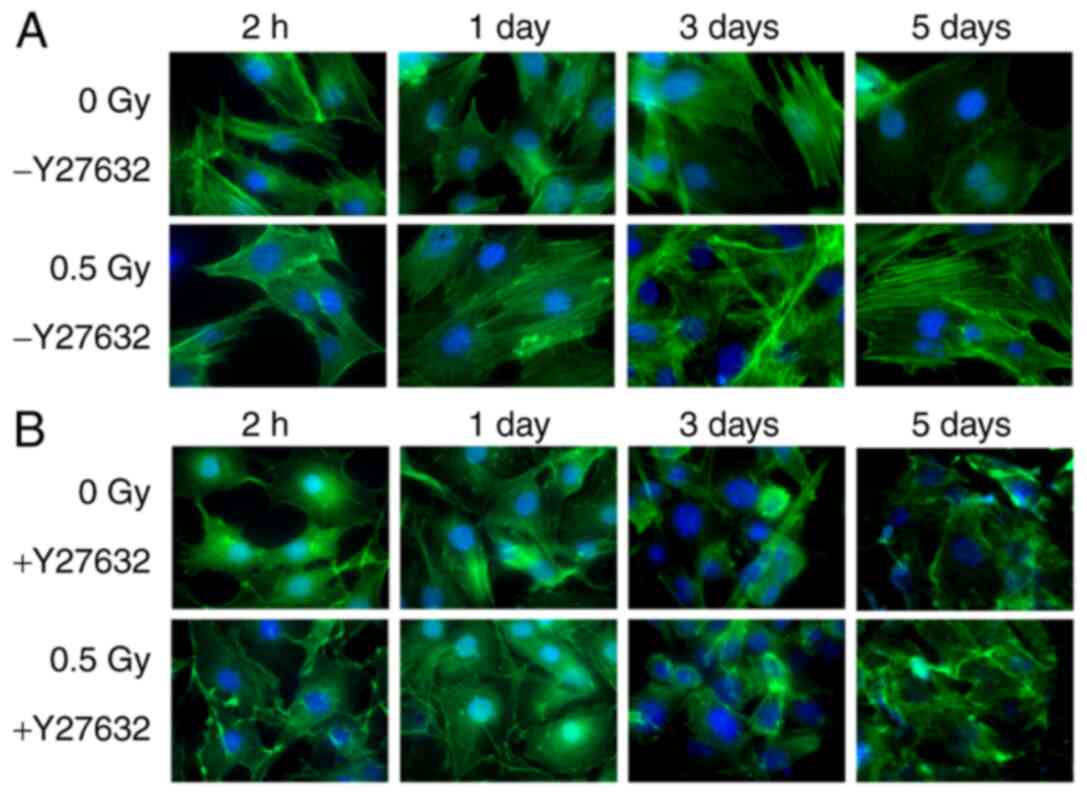
The cells in 0 Gy-Y27632 group were stretched, and
the actin cytoskeleton were clear, complete and neatly arranged,
forming a dense network. After 2 h of X-ray irradiation, the cells
shrank and the formation of actin tension fibers decreased. In
addition, the arrangement was discontinuous, and the green
fluorescence intensity was weakened. After 24 h, the F-actin in the
0.5 Gy group began to increase, and the green fluorescence of the
actin cytoskeleton was significantly improved. The fluorescence
intensity of F-actin in the 0.5 Gy group was markedly higher than
that in other groups at day 3 after irradiation. However, the
fluorescence intensity of F-actin in the 0.5 Gy X-ray irradiation
group tended to be normal on the day after irradiation (Fig. 1A). Cells treated with Y-27632 were
unable to induce the formation of new actin filaments, indicating
that ROCK is highly essential for actin reorganization by 0.5 Gy
X-ray irradiation (Fig. 1B).
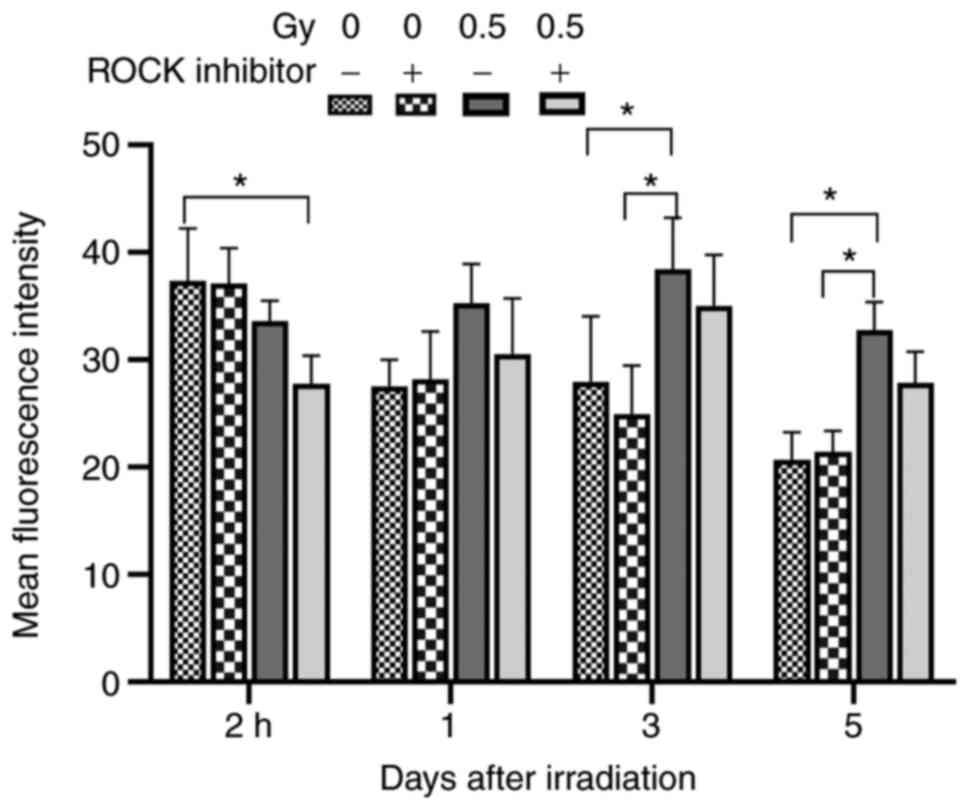
Table I and Fig. 2 are the summary data of Fig. 1. At 2 h following X-ray irradiation,
the fluorescence intensity of cells in the 0.5 Gy-Y27632 group and
0.5 Gy + Y27632 group was lower than that in the non-irradiated
group. The fluorescence intensity of cells in 0.5 Gy +Y27632 group
was significantly lower than that in the 0 GY-Y27632 group
(P<0.05). At 1 day after X-ray irradiation of Y27632-pretreated
cells, no significant difference was detected in the fluorescence
intensity between each irradiated group and the 0 Gy-Y27632 group.
Three days after X-ray irradiation, the fluorescence intensity of
the cells in the 0.5 Gy group was elevated compared with
non-irradiated group. The fluorescence intensity of cells in the
0.5 Gy-Y27632 group was higher than that in other groups
(P<0.05). On day 5, the intracellular F-actin gradually returned
to normal, and the fluorescence intensity of the cells in the 0.5
Gy group was stronger compared with non-irradiated group
(P<0.05; Table I; Fig. 2). These results demonstrated that
after 2 h X-ray irradiation, the F-actin depolymerized and the
fluorescence intensity decreased. At 24 h after X-ray irradiation,
the cytoskeleton was reorganized and the fluorescence intensity was
enhanced. The fluorescence intensity of F-actin reached a peak
value after 3 days X-ray irradiation. On the 5th day after X-ray
irradiation, the fluorescence intensity of F-actin decreased and
returned to normal, indicating that cytoskeleton reorganization
caused by X-ray irradiation was reversible.
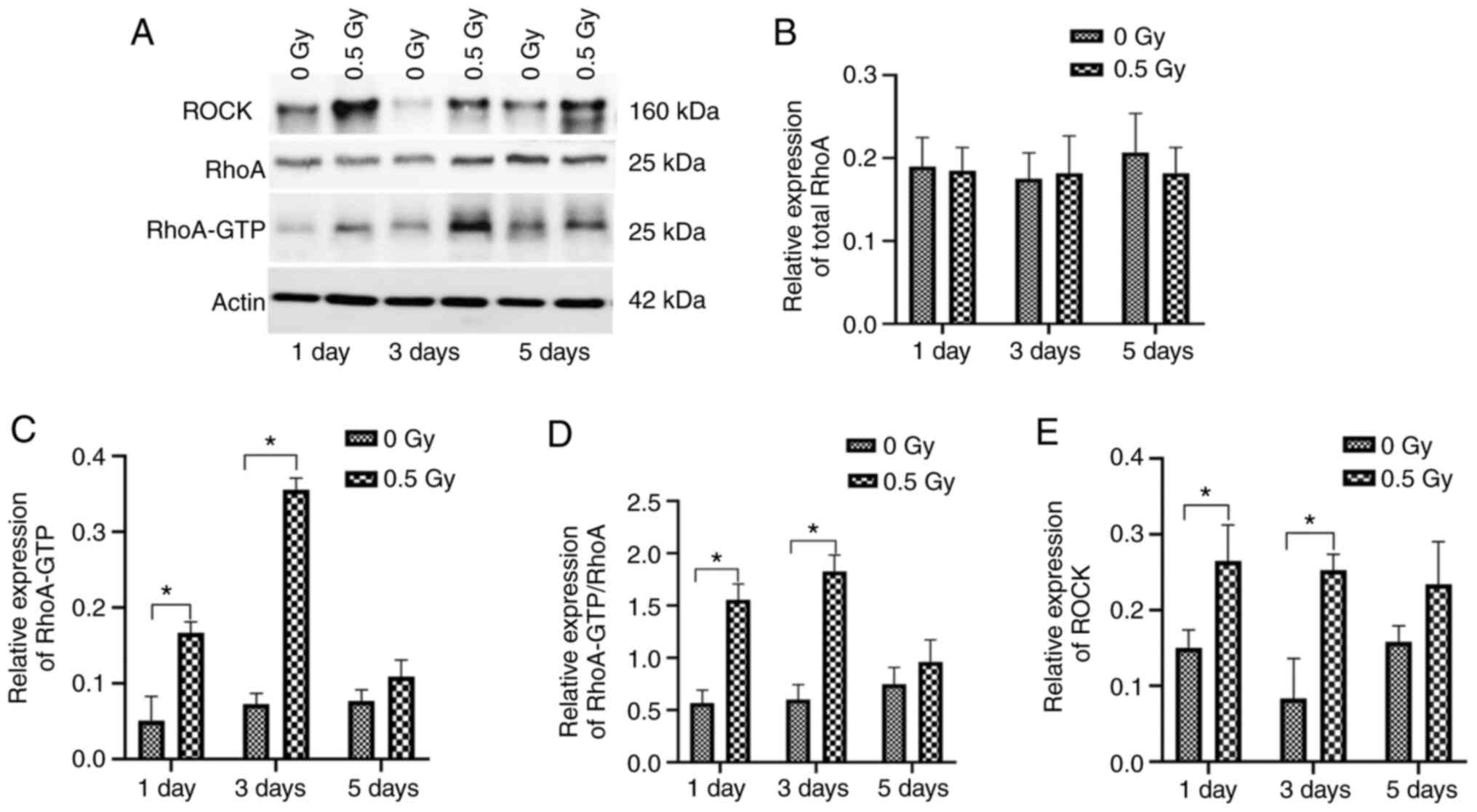
RhoA and ROCK are activated by 0.5 Gy
X-ray irradiation of MC3T3-E1 cells
Accumulating evidence indicates that the RhoA/ROCK
signaling pathway plays a significant role in regulating actin
reorganization through various effectors (24,25).
As the RhoA/ROCK signaling pathway triggers the formation of stress
fibers, it was hypothesized that these proteins could be activated
by 0.5 Gy X-ray irradiation of MC3T3 cells. The activation of RhoA
was analysed in X-ray-irradiated osteoblasts. Cells were treated
with 0 and 0.5 Gy X-ray irradiation, then analysed at different
time points. The protein levels of GTP-RhoA and ROCK were detected
in each group (Fig. 3A). Both RhoA
and ROCK were activated by 0.5-Gy X-ray irradiation in MC3T3-E1
cells. The levels of GTP-RhoA were elevated at day 1 after 0.5-Gy
X-ray irradiation, reached maximum level at day 3 after
irradiation, and decreased to baseline at day 5 (Fig. 3B-D). ROCK exhibited similar results
after 0.5-Gy irradiation (Fig. 3E).
The aforementioned results indicated that 0.5-Gy X-ray irradiation
could induce rapid activation of RhoA/ROCK signaling in MC3T3-E1
cells, which was in agreement with the actin stress fiber formation
in these cells (Fig. 1).
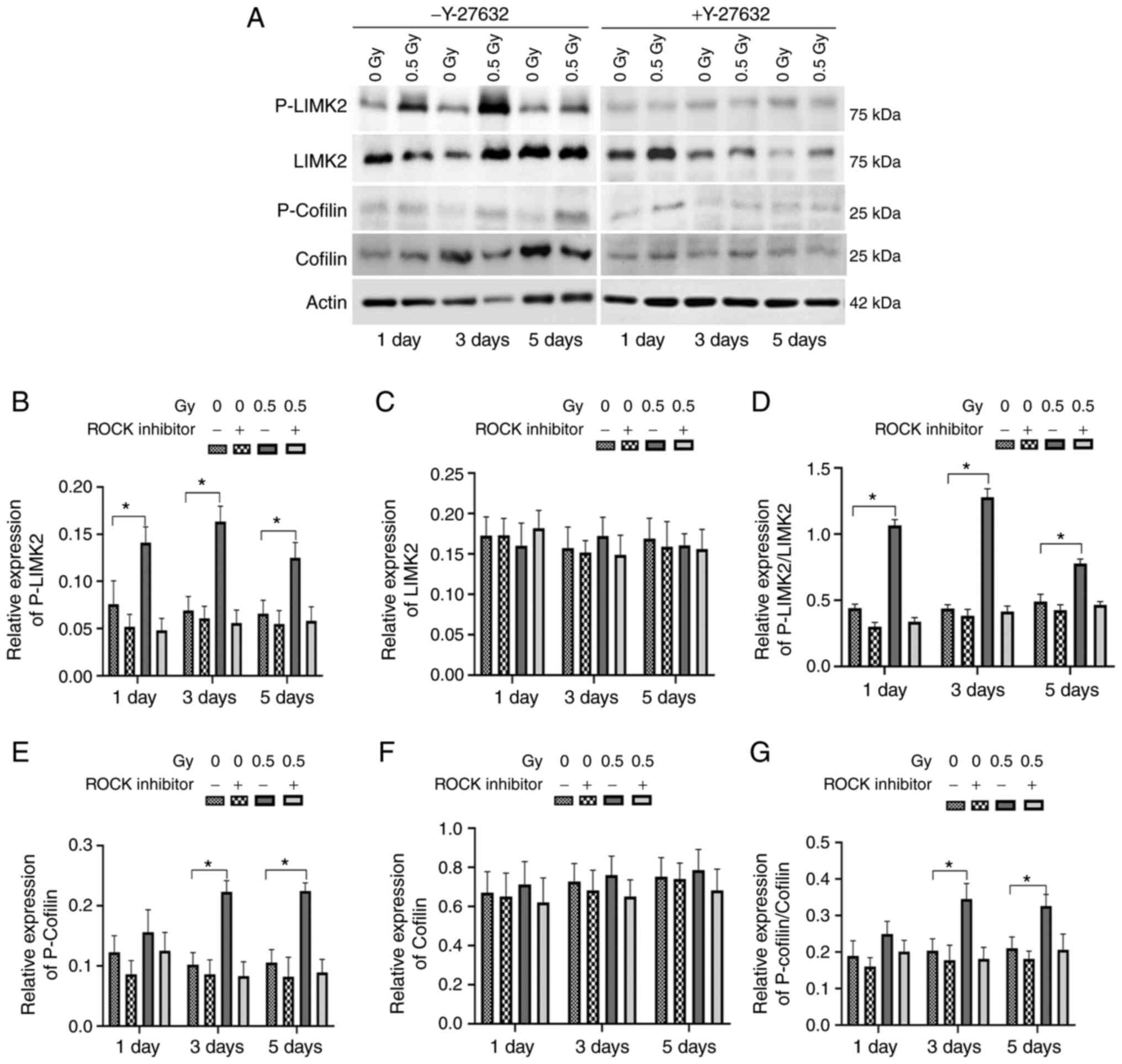
ROCK mediates LIMK2 activation and
actin cytoskeleton reorganization following 0.5-Gy X-ray
irradiation of MC3T3-E1 cells
As LIMK2 is a downstream molecule of the RhoA/ROCK
signaling pathway (24), it was
hypothesized that ROCK could be involved in X-ray
irradiation-induced phosphorylation of LIMK2. As before, cells were
pretreated with Y27632, followed by 0.5-Gy X-ray irradiation, and
western blotting was undertaken to detect the levels of
phospho-LIMK2 and phospho-Cofilin in each group at various time
points (Fig. 4A). The levels of
phospho-LIMK2 and phospho-Cofilin in the 0.5 Gy-Y27632 group were
significantly elevated compared with those in the other groups.
However, there was no change in the levels of phospho-LIMK2 and
phospho-Cofilin between 0.5 Gy + Y27632 and 0 Gy + Y27632 groups,
indicating that ROCK-mediated 0.5 Gy X-ray irradiation could induce
phosphorylation of LIMK2 (Fig.
4B-G).
Effects of RhoA on cell
proliferation
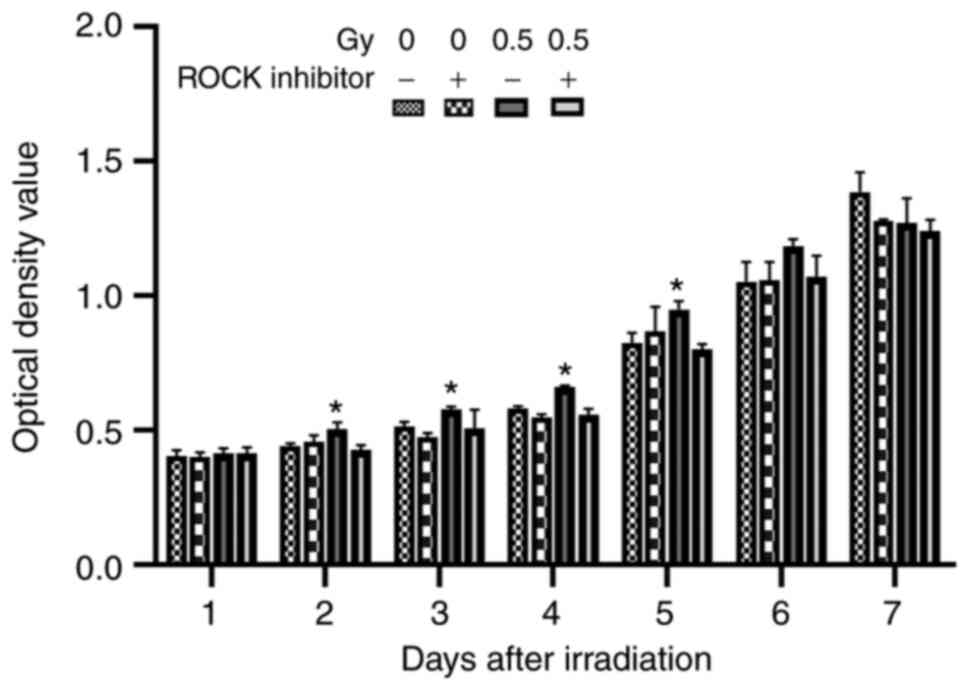
The effects of Y27632 and low-dose irradiation on
cell proliferation were then assessed. The results showed that on
the first day after irradiation, there was no significant
difference in CCK-8 activity in MC3T3-E1 cells between the
irradiated group and the non-irradiated group (Fig. 5). However, the cells irradiated with
low-dose (0.5 Gy) showed an increase in cell proliferation from
days 2–5 (P<0.05). When Y27632 was used before irradiation, the
cell proliferation was decreased compared with the 0.5-Gy
irradiation group. There was no significant difference on days 6
and 7 between the groups, which may be attributed to the stable
growth state of MC3T3-E1 cells in each group. These results
demonstrated that low-dose X-ray irradiation promoted the
proliferation of osteoblasts.
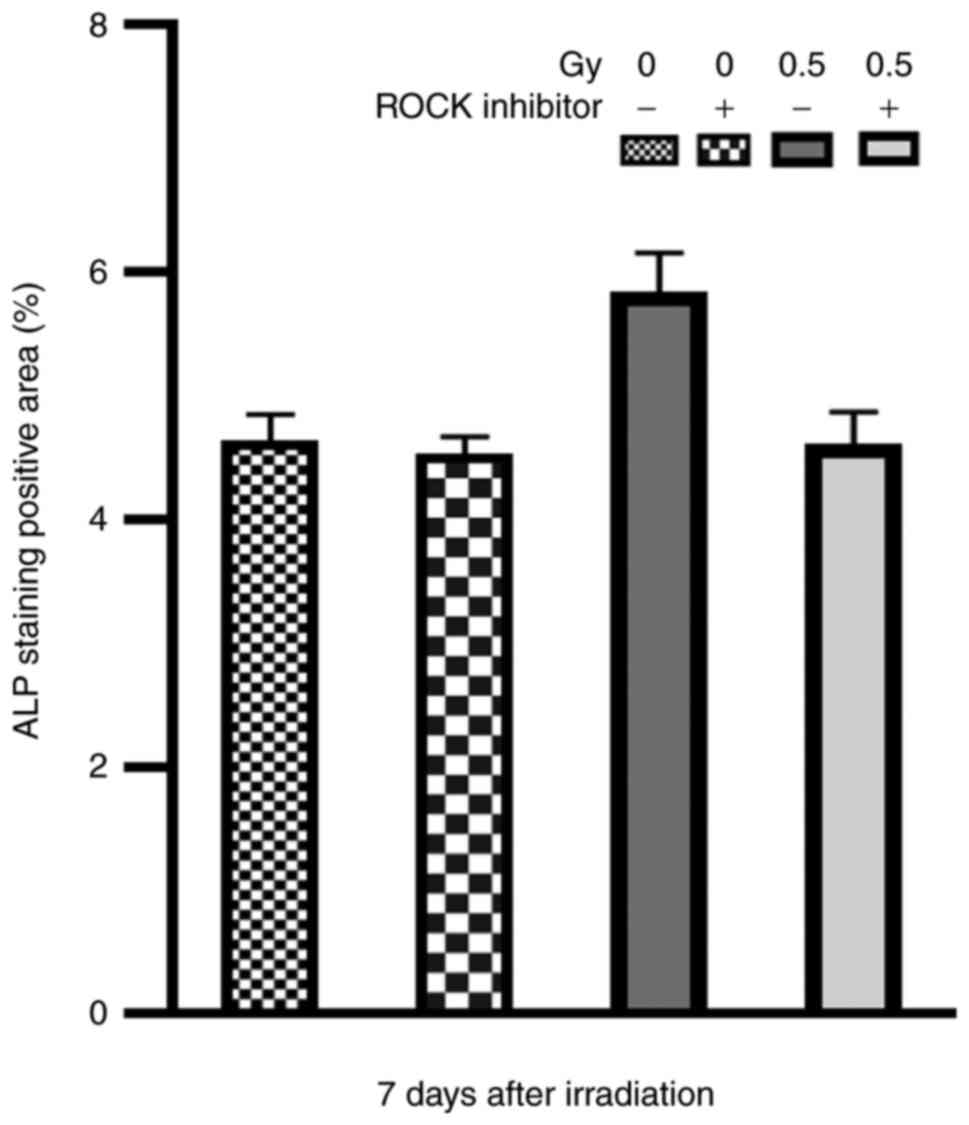
Effects of low-dose X-ray irradiation
on cell differentiation: ALP staining and activity
ALP is one of the most important markers of
osteoblasts, which increases inorganic phosphate concentration and
promotes mineralization of bone formation (18). In the present study, following ALP
staining, the cytoplasm of osteoblasts was purple granular, and the
staining was positive (Fig. 6). The
intensity of staining in 0.5-Gy group was higher than that in other
groups (Fig. 7).
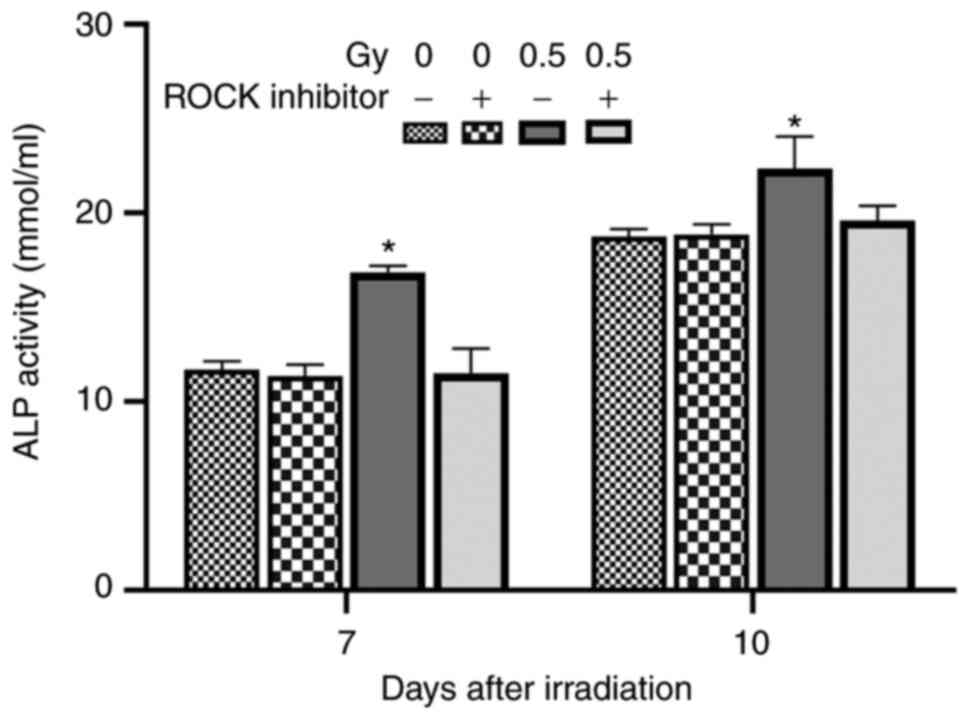
On the day 7 and 10 after irradiation, ALP activity
was detected in MC3T3-E1 cells. The results showed that 0.5-Gy
irradiation without Y27632 pretreatment significantly enhanced the
ALP activity of osteoblasts, which was notably significantly than
that in the other three groups (P<0.05). However, there was no
significant difference in ALP activity between the remaining three
groups (Table II; Fig. 8).
 | Table II.Effects of 0.5 Gy X-ray irradiation
on extracellular ALP activity. |
Table II.
Effects of 0.5 Gy X-ray irradiation
on extracellular ALP activity.
| A, Day 7 |
|---|
|
|---|
| Group | ALP activity
(U/l) |
|---|
| - Y27632 0 Gy | 11.69±0.4518 |
| + Y27632 0 Gy | 11.37±0.6026 |
| - Y27632 0.5
Gy | 16.89±0.3145 |
| + Y27632 0.5
Gy | 11.49±1.3162 |
|
| B, Day
10 |
|
| Group | ALP activity
(U/l) |
|
| - Y27632 0 Gy | 18.75±0.4217 |
| + Y27632 0 Gy | 18.9±0.5201 |
| - Y27632 0.5
Gy | 22.37±1.6963 |
| + Y27632 0.5
Gy | 19.6±0.7915 |
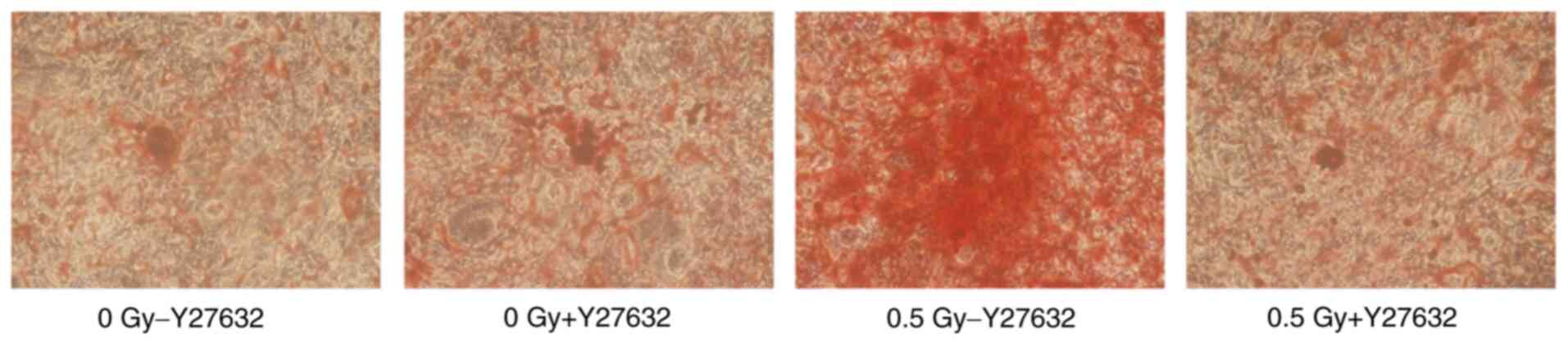
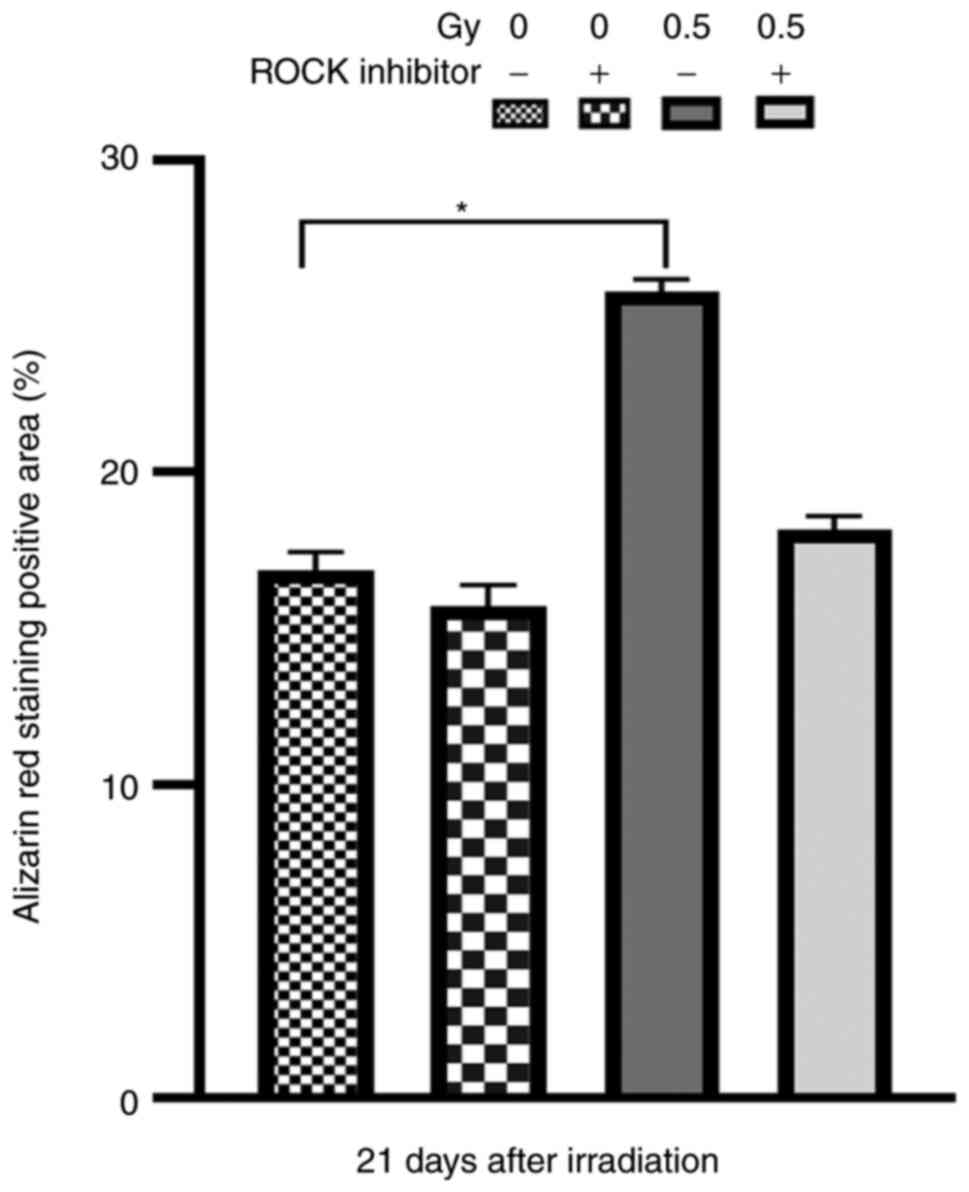
Alizarin red staining
The number of mineralized nodules indicates the
osteogenic capacity of osteoblasts. The results of Alizarin red
staining revealed that at 21 days after X-ray irradiation, the
degree of red staining in the 0.5-Gy group without pretreatment was
higher than that of the other three groups (Fig. 9). Moreover, the Alizarin red
staining positive area was markedly greater than that of the
+Y27632 0.5 Gy and non-irradiated groups (Fig. 10). The aforementioned findings
showed that 0.5-Gy X-ray irradiation promoted the mineralization
and maturation of osteoblasts.
Expression levels of osteogenic
markers induced by 0.5 Gy X-ray irradiation
The expression levels of OCN, Runx2, ALP, COL1, and
Osterix in MC3T3 cells were detected by western blotting (Fig. 11). The results showed that the
expression level of ALP was significantly elevated 10 days after
0.5-Gy irradiation, and the difference was statistically
significant compared with the other three groups (P<0.05;
Fig. 11A). There was no
significant difference in the expression of any of the analyzed
markers at day 7. However, at day 10 after irradiation, Runx2
(Fig. 11B), Osterix (Fig. 11C), COL1 (Fig. 11D) and OCN levels (Fig. 11E) in the 0.5-Gy without Y27632
pretreatment was significantly elevated, compared with the
untreated group (all P<0.05). However, when MC3T3-E1 cells
pretreated with Y27632 were exposed to 0.5-Gy X-ray irradiation,
the expression levels of osteogenic markers were markedly reduced,
compared with irradiated cells that did not receive Y27632
pretreatment (P<0.05). The aforementioned findings suggested
that the RhoA/ROCK signaling pathway was involved in
differentiation of osteoblasts induced by low-dose X-ray
irradiation.
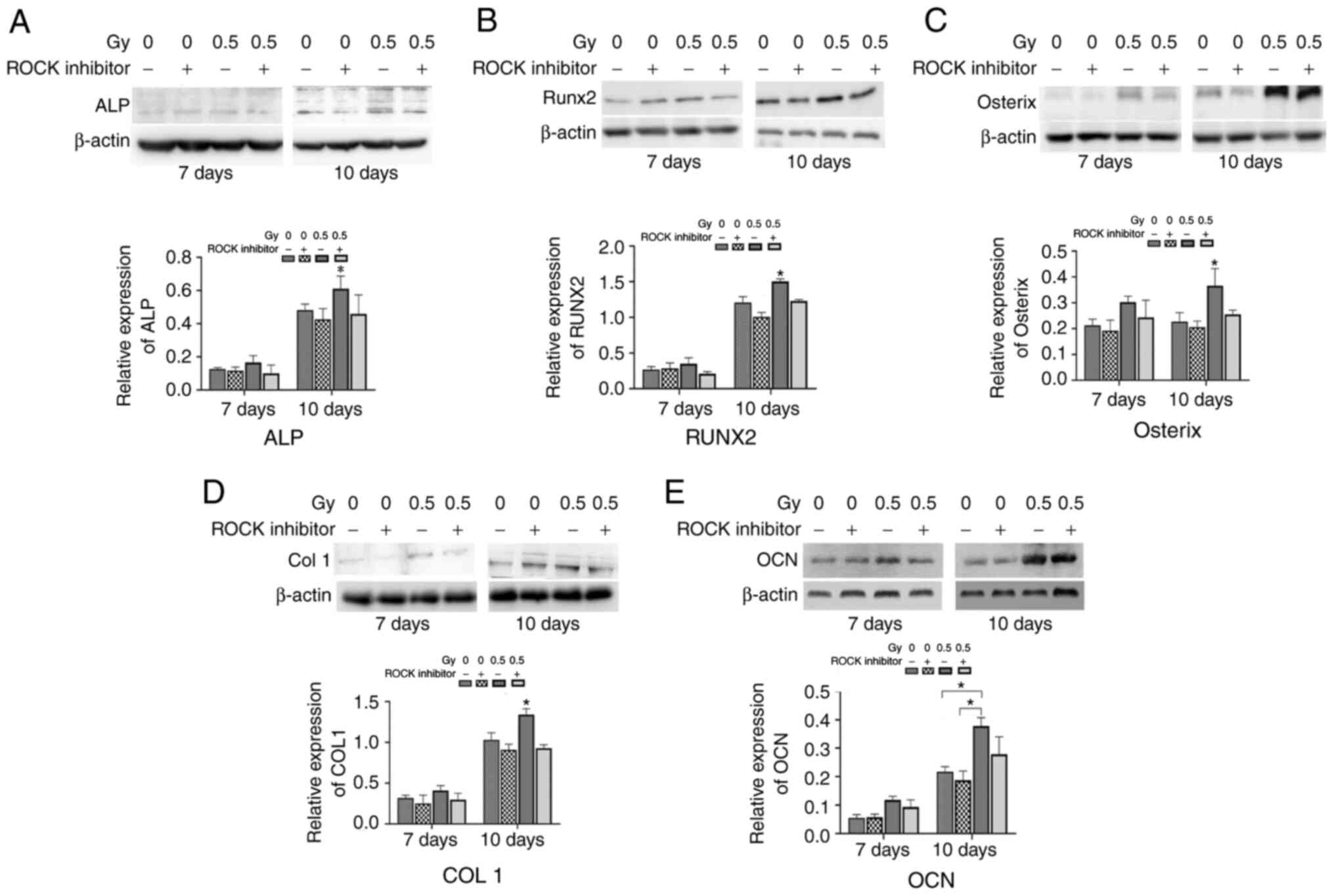 | Figure 11.Effects of X-ray irradiation on the
expression levels of osteogenic markers. The expression levels of
ALP, Runx2, Osterix, COL1 and OCN were detected by western blotting
at 7 and 10 days after irradiation. Protein expression levels of
(A) ALP, (B) Runx2, (C) Osterix, (D) COL1- and (E) OCN proteins,
respectively. *P<0.05. ALP, alkaline phosphatase; OCN,
osteocalcin; COL1, Collagen Type 1. |
Discussion
In the current study, fluorescence microscopy was
used to observe the changes of actin cytoskeleton in MC3T3-E1 cells
after low-dose X-ray irradiation and to examine the mechanism of
cytoskeleton remodeling induced by low-dose X-ray irradiation. At 2
h after X-ray radiation, the cells became wrinkled. The actin
arrangement became discontinuous, and the green fluorescence
intensity decreased. These results indicated that the cellular
microfilament network was destroyed soon after X-ray
irradiation.
The cause of the changes in the cytoskeleton may be
associated with direct damage from ionizing radiation. After 24 h
of X-ray irradiation, the actin cytoskeleton was neatly arranged,
the fluorescence intensity was enhanced in the 0.5 Gy group,
indicating that the cytoskeleton was reorganized. It was
hypothesized that the remodeling of actin fibers after X-ray
irradiation may be related to the activation of injury repair after
X-ray irradiation, which may activate DNA-damage repair response.
It also increased the expression of related growth factors, and
activated downstream signaling pathways by binding to the
corresponding receptors, thereby causing cytoskeleton
rearrangement.
It was hypothesized that low-dose X-ray exposure
caused activation of the Rho/ROCK pathway. The results showed that
the levels of phospho-LIMK2 and phospho-Cofilin were significantly
increased on day 3 and 5 after X-ray irradiation. However, the
levels of phospho-LIMK2 and phospho-cofilin were decreased after
pretreatment with ROCK inhibitors, suggesting that the
RhoA/ROCK/LIMK2/Cofilin pathway was involved in cytoskeleton
remodeling induced by low-dose X-ray irradiation, but the specific
mechanism of cytoskeleton changes caused by ionizing radiation
remains to be elucidated (29,30).
These changes were apparent on day 3 after irradiation, indicating
that RhoA/ROCK signaling pathway was activated, which is consistent
with Murata et al findings (31). In the present experiment, the
expression level of P-Cofilin in the 0.5 Gy group increased
compared with that in the non-irradiated group. It was hypothesized
that 0.5-Gy X-ray irradiation caused activation of the Rho/ROCK
pathway, and the intracellular-synthesized Cofilin was more
converted into P-Cofilin. Other studies have reported similar
results. For instance, Gabryś et al (26) found that radiation could cause rapid
rearrangement of actin in capillary endothelial cells, which
activated the RhoA/ROCK signaling pathway. The initial factors of
RhoA activation caused by ionizing radiation have not been fully
clarified. Cells produce a large amount of reactive oxygen species
(ROS) after radiation (32), which
may activate the RhoA/ROCK signaling pathway. It has been found
that RhoA is the target protein of ROS (33).
The RhoA/ROCK signaling pathway plays a pivotal
role, not only in regulating the cytoskeleton, but also in cell
proliferation and differentiation under various stimuli (20,34–38).
RhoA and ROCK are key signaling molecules in respond to various
stimuli (such as vasoactive substances, shear force, angiogenic
factors, and oxidative stress). The RhoA/ROCK signaling pathway
regulates a variety of cellular functions, such as permeability,
migration, adhesion (39), cell
survival, and apoptosis (40–42).
In the current study, MC3T3-E1 cell proliferation
increased after exposure to 0.5-Gy X-ray irradiation. In addition,
low-dose X-ray irradiation elevated the expression levels of Runx2,
Osterix, ALP, OCN and COLI in MC3T3 cells. These results suggested
that low-dose X-ray irradiation may promote the proliferation and
differentiation of MC3T3 cells through the RhoA/ROCK signaling
pathway. Additionally, 0.5-Gy X-ray irradiation may activate the
RhoA/ROCK signaling pathway and promote osteogenic
differentiation.
The differentiation process of osteoblasts can be
divided into two stages, ECM maturation and ECM mineralization. ALP
is one of the appropriate markers for early-stage of osteoblast
differentiation (43). RUNX2 is an
important transcription factor for osteoblast differentiation and
bone formation, which is also very significant for regulating the
rate of bone matrix deposition. OCN is secreted by mature
osteoblasts during matrix calcification (44). In the current study, the activity of
ALP was analyzed, which showed early osteogenic differentiation
potential of MC3T3 cells. ALP activity in 0.5 Gy X-ray irradiation
group reached the maximum on the 10th day after irradiation, and it
was stronger than that of the other three groups. These results
indicated that MC3T3 cells showed stress response to 0.5 Gy X-ray
irradiation and their early differentiation ability was improved.
Osteogenic differentiation requires a complete actin network. The
activation of ROCK can maintain a complete and robust actin
cytoskeleton, which is indispensable for gene expression caused by
low-dose X-ray irradiation.
Thus, identifying the effector molecules of the
RhoA/ROCK signaling pathway may be of great significance. Previous
studies have shown that Akt, PI3K, P38 phosphorylation and ERK1/2
in MAPK pathway were all associated with RhoA (45–47).
Moreover, the ECM regulates bone formation by affecting downstream
MAPK signaling pathway and RhoA/ROCK signaling pathway (46). Bone sialic acid glycoprotein is
expressed in several types of cells (such as osteoblasts,
osteocytes, chondrocytes, fibroblasts, and endothelial cells)
(48). Its synthesis can be
regulated by the PI3K and MAPK pathways. To some extent, PI3K/MAPK
signaling pathway could be mediated by RhoA (49).
The RhoA/ROCK signaling pathway induces PI3K
activation in a variety of cells, mediating myocardial protection
(50), and proliferating mouse
prostate cancer cells (51). In the
present study, Y27632 blocked osteogenic differentiation induced by
0.5-Gy X-ray irradiation. The possible mechanism was that Y27632
inhibited the activation of RhoA/ROCK signaling pathway induced by
low-dose X-ray irradiation, thereby inhibiting the phosphorylation
of ERK1/2, p38, and Akt, and could ultimately reduce the synthesis
of osteogenic differentiation proteins. Therefore, it can be
concluded that RhoA/ROCK signaling pathway can be involved in
regulating osteoblast differentiation induced by 0.5 Gy X-ray
irradiation.
In addition, Lumetti et al (28) found that RhoA could activate the Wnt
signaling pathway and promote the differentiation of osteoblasts.
Rossol-Allison et al (52)
suggested that, RhoA activation is indispensable in the process of
osteogenic differentiation of mesenchymal stem cells stimulated by
Wnt signaling pathway. RhoA inhibition can significantly inhibit
the transcription of target genes depending on Wnt3A-β-catenin
pathway. Activation or inhibition of RhoA can affect the nuclear
transport of β-catenin and subsequent osteogenic differentiation
(53). These results suggest that
RhoA activation is highly essential for osteogenic differentiation
by Wnt3A/β-catenin signaling pathway.
In conclusion, the results of the present study
suggested that low-dose X-ray irradiation could regulate
cytoskeleton reorganization and promote the proliferation and
differentiation of osteoblasts. This effect may be mediated by
activation of the RhoA/ROCK signaling pathway. However, multiple
signaling pathways may be involved in the promotion of
proliferation and differentiation of osteoblasts by low-dose X-ray
irradiation, while the specific mechanism remains to be further
clarified.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the National
Natural Science Foundation of China (grant no. 81874008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QH and HC carried out the experiments, participated
in collecting data, and drafted the manuscript. SW performed the
statistical analysis and participated in its design. YS and WX
participated in acquisition, analysis, or interpretation of data
and drafting the manuscript. QH and WX confirmed the authenticity
of the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of
interest.
References
|
1
|
Oh D and Huh SJ: Insufficiency fracture
after radiation therapy. Radiat Oncol J. 32:213–220. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Michel G, Blery P, Pilet P, Guicheux J,
Weiss P, Malard O and Espitalier F: Micro-CT analysis of
radiation-induced osteopenia and bone hypovascularization in rat.
Calcif Tissue Int. 97:62–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zou Q, Hong W, Zhou Y, Ding Q, Wang J, Jin
W, Gao J, Hua G and Xu X: Bone marrow stem cell dysfunction in
radiation-induced abscopal bone loss. J Orthop Surg Res. 11:32016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schreurs AS, Shirazi-Fard Y, Shahnazari M,
Alwood JS, Truong TA, Tahimic CG, Limoli CL, Turner ND, Halloran B
and Globus RK: Dried plum diet protects from bone loss caused by
ionizing radiation. Sci Rep. 6:213432016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Curi MM, Cardoso CL, de Lima HG, Kowalski
LP and Martins MD: Histopathologic and histomorphometric analysis
of irradiation injury in bone and the surrounding soft tissues of
the jaws. J Oral Maxillofac Surg. 74:190–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luckey TD: Physiological benefits from low
levels of ionizing radiation. Health Phys. 43:771–789. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Large M, Hehlgans S, Reichert S, Gaipl US,
Fournier C, Rödel C, Weiss C and Rödel F: Study of the
anti-inflammatory effects of low-dose radiation: The contribution
of biphasic regulation of the antioxidative system in endothelial
cells. Strahlenther Onkol. 191:742–749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu SZ: Biological effects of low level
exposures to ionizing radiation: Theory and practice. Hum Exp
Toxicol. 29:275–281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schaue D, Marples B and Trott KR: The
effects of low-dose X-irradiation on the oxidative burst in
stimulated macrophages. Int J Radiat Biol. 78:567–576. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Yao ZY, She C, Li J, Ten B, Liu C,
Lin SB, Dong QR and Ren PG: Effects of low-dose X-ray irradiation
on activated macrophages and their possible signal pathways. PLoS
One. 12:e01858542017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kempf SJ, Buratovic S, von Toerne C,
Moertl S, Stenerlöw B, Hauck SM, Atkinson MJ, Eriksson P and Tapio
S: Ionising radiation immediately impairs synaptic
plasticity-associated cytoskeletal signalling pathways in HT22
cells and in mouse brain: An in vitro/in vivo comparison study.
PLoS One. 9:e1104642014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sabanero M, Azorín-Vega JC,
Flores-Villavicencio LL, Pedro Castruita-Dominguez J, Vallejo MA,
Barbosa-Sabanero G, Cordova-Fraga T and Sosa-Aquino M: Mammalian
cells exposed to ionizing radiation: Structural and biochemical
aspects. Appl Radiat Isot. 108:12–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Panzetta V, De Menna M, Musella I,
Pugliese M, Quarto M, Netti PA and Fusco S: X-rays effects on
cytoskeleton mechanics of healthy and tumor cells. Cytoskeleton
(Hoboken). 74:40–52. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gerber HP, Vu TH, Ryan AM, Kowalski J,
Werb Z and Ferrara N: VEGF couples hypertrophic cartilage
remodeling, ossification and angiogenesis during endochondral bone
formation. Nat Med. 5:623–628. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song XS, Zhou XZ, Zhang G, Dong QR and Qin
L: Low-dose X-ray irradiation promotes fracture healing through
up-regulation of vascular endothelial growth factor. Med
Hypotheses. 75:522–524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karim L and Judex S: Low level irradiation
in mice can lead to enhanced trabecular bone morphology. J Bone
Miner Metab. 32:476–483. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Wang Z, Wu A, Nie J, Pei H, Hu W,
Wang B, Shang P, Li B and Zhou G: Differences in responses to X-ray
exposure between osteoclast and osteoblast cells. J Radiat Res.
58:791–802. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen M, Huang Q, Xu W, She C, Xie ZG, Mao
YT, Dong QR and Ling M: Low-dose X-ray irradiation promotes
osteoblast proliferation, differentiation and fracture healing.
PLoS One. 9:e1040162014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu W, Xu L, Chen M, Mao YT, Xie ZG, Wu SL
and Dong QR: The effects of low dose X-irradiation on osteoblastic
MC3T3-E1 cells in vitro. BMC Musculoskelet Disord. 13:942012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McBeath R, Pirone DM, Nelson CM,
Bhadriraju K and Chen CS: Cell shape, cytoskeletal tension, and
RhoA regulate stem cell lineage commitment. Dev Cell. 6:483–495.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mathieu PS and Loboa EG: Cytoskeletal and
focal adhesion influences on mesenchymal stem cell shape,
mechanical properties, and differentiation down osteogenic,
adipogenic, and chondrogenic pathways. Tissue Eng Part B Rev.
18:436–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lamers ML, Padilha DM, Bernardi L, da
Silveira HE and Fossati AC: X-ray irradiation alters the actin
cytoskeleton in murine lacrimal glands. Acta Odontol Scand.
72:386–391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wan Q, Cho E, Yokota H and Na S: RhoA
GTPase interacts with beta-catenin signaling in clinorotated
osteoblasts. J Bone Miner Metab. 31:520–532. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jaffe AB and Hall A: Rho GTPases:
Biochemistry and biology. Annu Rev Cell Dev Biol. 21:247–269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rousseau M, Gaugler MH, Rodallec A,
Bonnaud S, Paris F and Corre I: RhoA GTPase regulates
radiation-induced alterations in endothelial cell adhesion and
migration. Biochem Biophys Res Commun. 414:750–755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gabryś D, Greco O, Patel G, Prise KM,
Tozer GM and Kanthou C: Radiation effects on the cytoskeleton of
endothelial cells and endothelial monolayer permeability. Int J
Radiat Oncol Biol Phys. 69:1553–1562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshida T, Clark MF and Stern PH: The
small GTPase RhoA is crucial for MC3T3-E1 osteoblastic cell
survival. J Cell Biochem. 106:896–902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lumetti S, Mazzotta S, Ferrillo S,
Piergianni M, Piemontese M, Passeri G, Macaluso GM and Galli C:
RhoA controls Wnt upregulation on microstructured titanium
surfaces. Biomed Res Int. 2014:4018592014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kazmers NH, Ma SA, Yoshida T and Stern PH:
Rho GTPase signaling and PTH 3–34, but not PTH 1–34, maintain the
actin cytoskeleton and antagonize bisphosphonate effects in mouse
osteoblastic MC3T3-E1 cells. Bone. 45:52–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohashi K, Fujiwara S and Mizuno K: Roles
of the cytoskeleton, cell adhesion and rho signalling in
mechanosensing and mechanotransduction. J Biochem. 161:245–254.
2017.PubMed/NCBI
|
|
31
|
Murata K, Noda SE, Oike T, Takahashi A,
Yoshida Y, Suzuki Y, Ohno T, Funayama T, Kobayashi Y, Takahashi T
and Nakano T: Increase in cell motility by carbon ion irradiation
via the Rho signaling pathway and its inhibition by the ROCK
inhibitor Y-27632 in lung adenocarcinoma A549 cells. J Radiat Res.
55:658–664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kondo H, Yumoto K, Alwood JS, Mojarrab R,
Wang A, Almeida EA, Searby ND, Limoli CL and Globus RK: Oxidative
stress and gamma radiation-induced cancellous bone loss with
musculoskeletal disuse. J Appl Physiol (1985). 108:152–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aghajanian A, Wittchen ES, Campbell SL and
Burridge K: Direct activation of RhoA by reactive oxygen species
requires a redox-sensitive motif. PLoS One. 4:e80452009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Amano M, Nakayama M and Kaibuchi K:
Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell
polarity. Cytoskeleton (Hoboken). 67:545–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sordella R, Jiang W, Chen GC, Curto M and
Settleman J: Modulation of Rho GTPase signaling regulates a switch
between adipogenesis and myogenesis. Cell. 113:147–158. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun H and Kaartinen MT: Transglutaminase
activity regulates differentiation, migration and fusion of
osteoclasts via affecting actin dynamics. J Cell Physiol.
233:7497–7513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iwatake M, Nishishita K, Okamoto K and
Tsukuba T: The Rho-specific guanine nucleotide exchange factor
Plekhg5 modulates cell polarity, adhesion, migration, and podosome
organization in macrophages and osteoclasts. Exp Cell Res.
359:415–430. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wen J, Tan D, Li L, Wang X, Pan M and Guo
J: RhoA regulates Schwann cell differentiation through JNK pathway.
Exp Neurol. 308:26–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
van Nieuw Amerongen GP and van Hinsbergh
VW: Cytoskeletal effects of rho-like small guanine
nucleotide-binding proteins in the vascular system. Arterioscler
Thromb Vasc Biol. 21:300–311. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aznar S and Lacal JC: Rho signals to cell
growth and apoptosis. Cancer Lett. 165:1–10. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kumar A, Al-Sammarraie N, DiPette DJ and
Singh US: Metformin impairs Rho GTPase signaling to induce
apoptosis in neuroblastoma cells and inhibits growth of tumors in
the xenograft mouse model of neuroblastoma. Oncotarget.
5:11709–11722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang L, Zhou H and Wei G: miR-506
regulates cell proliferation and apoptosis by affecting RhoA/ROCK
signaling pathway in hepatocellular carcinoma cells. Int J Clin Exp
Pathol. 12:1163–1173. 2019.PubMed/NCBI
|
|
43
|
Choi JY, Lee BH, Song KB, Park RW, Kim IS,
Sohn KY, Jo JS and Ryoo HM: Expression patterns of bone-related
proteins during osteoblastic differentiation in MC3T3-E1 cells. J
Cell Biochem. 61:609–618. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Neve A, Corrado A and Cantatore FP:
Osteocalcin: Skeletal and extra-skeletal effects. J Cell Physiol.
228:1149–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hamamura K, Swarnkar G, Tanjung N, Cho E,
Li J, Na S and Yokota H: RhoA-mediated signaling in
mechanotransduction of osteoblasts. Connect Tissue Res. 53:398–406.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Khatiwala CB, Kim PD, Peyton SR and Putnam
AJ: ECM compliance regulates osteogenesis by influencing MAPK
signaling downstream of RhoA and ROCK. J Bone Miner Res.
24:886–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tatsumi E, Yamanaka H, Kobayashi K, Yagi
H, Sakagami M and Noguchi K: RhoA/ROCK pathway mediates p38 MAPK
activation and morphological changes downstream of P2Y12/13
receptors in spinal microglia in neuropathic pain. Glia.
63:216–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Denhardt DT and Noda M: Osteopontin
expression and function: Role in bone remodeling. J Cell Biochem
Suppl. 72:30–31. 92–102. 1998. View Article : Google Scholar
|
|
49
|
McCormick B, Chu JY and Vermeren S:
Cross-talk between Rho GTPases and PI3K in the neutrophil. Small
GTPases. 10:187–195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Del Re DP, Miyamoto S and Brown JH: Focal
adhesion kinase as a RhoA-activable signaling scaffold mediating
Akt activation and cardiomyocyte protection. J Biol Chem.
283:35622–35629. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ghosh PM, Bedolla R, Mikhailova M and
Kreisberg JI: RhoA-dependent murine prostate cancer cell
proliferation and apoptosis: role of protein kinase Czeta. Cancer
Res. 62:2630–2636. 2002.PubMed/NCBI
|
|
52
|
Rossol-Allison J, Stemmle LN,
Swenson-Fields KI, Kelly P, Fields PE, McCall SJ, Casey PJ and
Fields TA: Rho GTPase activity modulates Wnt3a/beta-catenin
signaling. Cell Signal. 21:1559–1568. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Galli C, Piemontese M, Lumetti S,
Ravanetti F, Macaluso GM and Passeri G: Actin cytoskeleton controls
activation of Wnt/β-catenin signaling in mesenchymal cells on
implant surfaces with different topographies. Acta Biomater.
8:2963–2968. 2012. View Article : Google Scholar : PubMed/NCBI
|